Abstract
Purpose
Bronchial thermoplasty is approved in many countries worldwide as a non-pharmacological treatment for severe asthma. This review summarizes recent publications on the selection of patients with severe asthma for bronchial thermoplasty, predictors of a beneficial response and developments in the procedure and discusses specific issues about bronchial thermoplasty including effectiveness in clinical practice, mechanism of action, cost-effectiveness, and place in management.
Results
Bronchial thermoplasty is a treatment option for patients with severe asthma after assessment and management of causes of difficult-to-control asthma, such as nonadherence, poor inhaler technique, comorbidities, under treatment, and other behavioral factors. Patients treated with bronchial thermoplasty in clinical practice have worse baseline characteristics and comparable clinical outcomes to clinical trial data. Bronchial thermoplasty causes a reduction in airway smooth muscle mass although it is uncertain whether this effect explains its efficacy since other mechanisms of action may be relevant, such as alterations in airway epithelial, gland, and/or nerve function; improvements in small airway function; or a placebo effect. The cost-effectiveness of bronchial thermoplasty is greater in countries where the costs of hospitalization and emergency department are high. The place of bronchial thermoplasty in the management of severe asthma is not certain, although some experts propose that bronchial thermoplasty should be considered for patients with severe asthma associated with non-type 2 inflammation or who fail to respond favorably to biologic therapies targeting type 2 inflammation.
Conclusion
Bronchial thermoplasty is a modestly effective treatment for severe asthma after assessment and management of causes of difficult-to-control asthma. Asthma morbidity increases during and shortly after treatment. Follow-up studies provide reassurance on the long-term safety of the procedure. Uncertainties remain about predictors of response, mechanism(s) of action, and place in management of severe asthma.
Keywords: bronchial thermoplasty, severe asthma
Introduction
Difficult-to-control asthma is an umbrella term used to describe people with poorly controlled asthma due to range of aggravating factors, such as nonadherence, poor inhaler technique, comorbidities, under treatment, and other behavioral factors. A subgroup of patients with difficult-to-control asthma have severe refractory disease despite attempts to identify and manage these aggravating factors and who remain symptomatic despite treatment with high-dose inhaled corticosteroids and long-acting β2-agonist. Severe refractory asthma is uncommon, with prevalence rates estimated to be 3–5% of the total population of adults with asthma.1,2 These patients are at increased risk of death from asthma and experience considerable morbidity due to poorly controlled disease and the adverse effects of high-dose corticosteroids.3 The economic burden of severe asthma is high due to the costs of prescription medications, hospital admissions, and time lost from work.4 Severe asthma persists in most patients, particularly in those with more comorbidities or with low socioeconomic status.5 Recently, the concept of targeting treatable traits has been advocated as an effective approach to managing severe asthma.6 The main components of management of severe asthma include providing structured education, instituting non-pharmacological interventions when appropriate, stepping-up or stepping down pharmacological treatments and adding biological therapies for patients with markers of type 2 inflammation. Bronchial thermoplasty is an additional treatment option for severe refractory asthma.7
Bronchial thermoplasty is a non-pharmacological intervention that applies thermal energy to the airways with the aim of reducing the amount of airway smooth muscle and improving asthma control.8 Previously published articles provide detailed information on the preclinical and clinical development of bronchial thermoplasty.8–13 The main evidence for the efficacy and short-term safety of bronchial thermoplasty treatment in patients with moderate to severe asthma is based on the results of three randomized controlled trials,14–16 two of which compared bronchial thermoplasty with usual care, the Asthma Intervention Research (AIR) trial14 and the Research in Severe Asthma (RISA) trial,15 and a third trial (AIR2), which compared bronchial thermoplasty with a sham procedure.16 A Cochrane systematic review of the results of these trials concluded that bronchial thermoplasty produced a modest clinical benefit in asthma quality of life questionnaire (AQLQ) score (mean difference in AQLQ score 0.28, 95% CI 0.07 to 0.50) and based on the results of the AIR2 trial, bronchial thermoplasty was associated with a reduction in the rate of severe exacerbations in the 12 months after treatment.9 A systematic review undertaken by the US Agency for Healthcare and Quality of the three randomized controlled trials with 5-year single-arm follow-up and several descriptive studies published up to April 2017 concluded that bronchial thermoplasty was modestly beneficial in some patients with asthma although the procedure is not without risks, particularly around the time of treatment.17 During the treatment period, defined as the time from pretreatment to 6 weeks after the last (third) treatment, bronchial thermoplasty is associated with an increase in asthma-related symptoms and a 3.5-fold (95% CI 1.26 to 9.68) greater risk of hospital admission for asthma.9,14–16,18 In the posttreatment period, defined as the time from 6 weeks after the last (third) treatment to 1 year, there was no difference in the risk of hospital admission (risk ratio 1.12 (95% CI 0.44 to 2.85).9 Observational follow-up studies on the long-term safety of bronchial thermoplasty for participants to the AIR, RISA and AIR2 trials, from beyond 1 up to 5 years post bronchial thermoplasty treatment,19–21 reported stable lung function and lack of increase in hospital admissions and emergency department visits. A systemic review of the long-term safety of bronchial thermoplasty in the three controlled trials of bronchial thermoplasty14–16 and their respective extension studies19–21 demonstrated no long-term decline in FEV1, no change in the number of emergency room visits or hospital admissions for adverse respiratory events, and found a reduction in the frequency of respiratory adverse events22 BT10+ (bronchial thermoplasty at 10 years follow-up or beyond) observational study assessed the safety and efficacy of the procedure in patients who previously participated in the AIR, RISA and AIR2 trials (ClinicalTrials.gov Identifier: NCT03243292).23 Of the 429 patients enrolled in the clinical trials, 192 had follow-up at >10 years post treatment (137 treated with bronchial thermoplasty; 18 control/sham participants treated with bronchial thermoplasty after the trial; 37 control/sham only). At the >10-year visit after bronchial thermoplasty, the preliminary findings were that AQLQ scores and severe exacerbation rates were comparable with those recorded 1 year after the procedure suggesting that the response to bronchial thermoplasty was sustained over 10 years.24
The interpretation of the evidence for the efficacy and safety of bronchial thermoplasty in severe asthma provided by the published clinical trials has generated considerable controversy. The main criticism of the AIR and RISA trials is that the clinical outcomes in the bronchial thermoplasty group were compared with a control group who did not undergo a sham procedure. In the AIR2 trial, criticisms include that not all patients had severe refractory asthma, that the changes in the primary end point AQLQ were statistically significant, but the clinical relevance was questioned and that inappropriate methods were used to analyze some secondary end points.25 Several AIR2 trial coauthors have provided rebuttals to these criticisms.26 In the long-term follow-up studies of the three trials, the participants treated with bronchial thermoplasty were followed for 5 years, whereas the usual care group was not reviewed after year 1 in the RISA and AIR2 trials, and in the AIR trial, control participants were followed-up only to the end of year 3. The criteria used to obtain regulatory approval of medical devices, such as bronchial thermoplasty, are less stringent than that required for biologics and small-molecule drugs for asthma. Despite reservations in the strength of the evidence from clinical trials on the efficacy and safety of bronchial thermoplasty, the procedure has been approved for the treatment of severe asthma in many countries worldwide and its use is included in national and international asthma guidelines.7,27
This review summarizes recent publications on the criteria used to select patients with severe asthma for bronchial thermoplasty, progress in identifying predictors of a beneficial response and developments in the procedure and discusses specific issues about bronchial thermoplasty including effectiveness in clinical practice, mechanism of action, cost-effectiveness, and place in management.
Patient Selection
Patients being considered for bronchial thermoplasty should undergo systematic evaluation at a specialist asthma clinic to identify and manage causes of difficult-to-control asthma, such as non-adherence or untreated comorbidities. Bronchial thermoplasty is a treatment option for patients with severe refractory asthma that remains uncontrolled despite maximal therapy. Recent reviews provide detailed information on the selection of patients for bronchial thermoplasty.13,28–30 The main criteria used to select patients with severe asthma for bronchial thermoplasty are based on those used in the AIR2 and RISA controlled clinical trials of the procedure (Table 1). Observational studies from North America, the UK, and Australia indicate that patients treated with bronchial thermoplasty in clinical practice have more severe disease than those recruited to AIR14 and AIR2 trials (Table 1).16,31,32 The PAS2 (Post-Food and Drug Administration Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma) study enrolled 284 participants from 2011 at 27 centers in the USA (n=23) and Canada (n=4) of whom 279 patients received at least one bronchial thermoplasty treatment.33 PAS2 study participants33 compared to those recruited to the AIR2 clinical trial were slightly older, more subjects had chronic sinus disease (30.4% vs 18.4%), a larger number had severe asthma (94.7% vs 82.1%), a higher proportion were taking maintenance oral corticosteroids (18.9% vs 4.2%) and more subjects had experienced severe exacerbations (74% vs 52%) and hospitalizations (15.3% vs 4.2%) in the 12 months prior to bronchial thermoplasty. An audit of 126 patients with severe refractory asthma who underwent bronchial thermoplasty in clinical practice in the UK between 2011 and 2016 were on average older, had lower AQLQ scores and worse baseline FEV1 compared with the AIR and AIR2 trial participants, whereas FEV1 values were higher than RISA trial participants.34 A recent case series of consecutive patients with severe asthma included in the Australian Bronchial Thermoplasty Registry reported that the safety and effectiveness of bronchial thermoplasty in patients with an FEV1 less than 50% was similar to that in those with better lung function.35 Contraindications to bronchial thermoplasty include patients with a pacemaker, internal defibrillator, or other implantable electronic device, or who have previously received treatment with bronchial thermoplasty.36 An European consensus statement on bronchial thermoplasty also listed tracheal stenosis and anticoagulation (international normalized ratio >1.5) as contraindications to bronchial thermoplasty.37 The balance of risks and benefits of bronchial thermoplasty treatment should be discussed with patients being considered for the procedure.
Table 1.
Key Eligibility Criteria For Bronchial Thermoplasty Used In Clinical Trials And In Clinical Practice
| Criteria | Key Inclusion And Exclusion Criteria For Bronchial Thermoplasty Used In AIR216 And RISA Trials15a | Eligibility Criteria For Bronchial Thermoplasty Used In Clinical Practice From Selected Case Reports And Case Series |
|---|---|---|
| Age | Inclusion criteria: Adult; age 18–65 years. | Adult; age >65 years53,60,63 |
| Current treatment | Inclusion criteria: Asthma requiring regular maintenance medications that includes inhaled corticosteroids (>1000 μg beclometasone per day or equivalent (AIR2 trial); >750 μg fluticasone propionate per day or equivalent (RISA trial)); and long-acting β2-agonist (≥100 μg salmeterol per day or equivalent), with or without other asthma medications. Oral corticosteroids at a dosage ≤ 10 mg per day (AIR2 trial) or ≤ 30 mg per day (RISA trial). |
Oral corticosteroids at a dosage >10 mg per day33,42,57,63,71 |
| Asthma Quality of Life Questionnaire (AQLQ) score | Inclusion criteria: Baseline AQLQ score 6.25 or lower. | |
| Medical history | Exclusion criteria: chronic sinus disease (AIR2 trial), uncontrolled sinus disease (RISA trial); anticoagulants; use of immunosuppressants other than corticosteroids | Chronic sinus disease (PAS2 study)33 |
| Smoking status | Inclusion criteria: Nonsmoker for 1 year or greater with a less than 10 pack-years smoking history. | Former smokers >10 pack-years total smoking history54 Current smoker, n=160 |
| Lung function | Inclusion criteria: pre-bronchodilator FEV1 ≥60% predicted (AIR2); FEV1 ≥50% predicted (RISA trial); Exclusion criteria: post-bronchodilator FEV1 <55% predicted (RISA trial); diffusing capacity (DLCO) <70% predicted (RISA trial) |
Prebronchodilator FEV1 <60% predicted31,35,53,58,60–63,110 |
| None of the following within the past 12 months | Exclusion criteria i. Three or more lower respiratory tract infections (AIR2 trial); in the last 3 months (RISA trial) ii. Four or more oral corticosteroid pulses for asthma exacerbation iii. Three or more hospitalizations for asthma |
|
| None of the following within the past 24 months | Exclusion criteria History of life-threatening asthma (history of intubation for asthma or intensive care unit admission for asthma) |
History of life-threatening asthma63 |
| Suitability to undergo bronchoscopy | Inclusion criteria Patient considered suitable for bronchoscopy |
Note: aSimilar key exclusion criteria were used in the PAS2 study33 and AIR2 trial16, except for chronic sinus disease, which was an exclusion criterion in AIR2.
Abbreviations: AIR, Asthma Intervention Research; DLCO, diffusing capacity of lung for carbon monoxide; PAS2, Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma; RISA, Research in Severe Asthma.
Predictors Of A Beneficial Response
An important goal for the future use of bronchial thermoplasty in the management of severe asthma is to identify factors that predict a beneficial response.38 Preliminary reports suggest that early-onset atopic severe asthma,39 lower age,34 and increased cough receptor sensitivity to capsaicin40 may be predictive, although further confirmatory evidence is awaited. A history of self-reported allergy21 or bronchodilator reversibility41 is reported not to be predictive. Patients with severe asthma and an FEV1 <80% have more airway smooth muscle mass than those subjects with an FEV1 >80% and they have the greatest reduction in airway smooth muscle mass at 6 months after bronchial thermoplasty.42 In one study, the amount of airway smooth muscle at baseline did not predict a beneficial clinical response to bronchial thermoplasty.43 A study of 24 patients with severe asthma undergoing bronchial thermoplasty found that the number of activations delivered to the airways was higher in responders compared to nonresponders to bronchial thermoplasty (221 ± 45 activations versus 139 ± 11 activations, respectively) and that the improvement in asthma control questionnaire score correlated with a greater number of activations.44 A recent study used human lung specimens and mathematical modeling guided by structural or functional patient data to predict clinical outcomes after bronchial thermoplasty compared with current clinical practice.45 One session of structure-guided treatment produced improvements comparable with three sessions of unguided treatment, whereas function-guided treatment provided no additional benefit compared with current practice.45 Studies underway may provide insights into novel clinical or inflammatory predictors of a therapeutic response to bronchial thermoplasty (ClinicalTrials.gov Identifier: NCT01185275).23 Imaging of the airways by optical coherence tomography,46,47 computed tomography (CT)48 and endobronchial ultrasound are also being investigated as potential noninvasive methods to identify predictors of a beneficial response to bronchial thermoplasty.
Developments In The Procedure
The “Alair” Bronchial Thermoplasty system produces radio-frequency energy that is repeatedly applied for a 10-s period per activation to the medium and large airways via a catheter with an expandable electrode array at its distal end.13,16,49 Bronchial thermoplasty is given over three bronchoscopy sessions at approximately 3-week intervals, one for each lower lobe and one for both upper lobes. Patients receive treatment over three outpatient flexible bronchoscopy sessions under conscious sedation spaced at approximately 3-week intervals, one for each lower lobe and one for both upper lobes. The right middle lobe bronchus is not normally treated, although a case series of 17 patients reported that bronchial thermoplasty treatment to the right middle lobe could be safely performed.50 In some centers, bronchial thermoplasty is performed under general anesthesia. Treatment sessions are typically completed in 45 mins and around 60 radio-frequency activations delivered to the airways. Details of the equipment and procedure have been reviewed in detail previously.13,32 Hyperpolarized xenon (HXe) MRI is being investigated as a biomarker to prioritize the order of airway treatment by bronchial thermoplasty (ClinicalTrials.gov Identifier: NCT01832363; NCT02263794).23 A pilot study reported that one bronchial thermoplasty treatment guided by HXeMRI to select the six most involved airways resulted in improvements in AQLQ score at 3 months with fewer adverse effects (n=15 participants) compared to three treatments with bronchial thermoplasty (n=14 participants).51 An observational study reported that the use of a thinner bronchoscope (outer diameter of 4.2 mm at the distal end) facilitated access to the bronchial tree that resulted in increased radio-frequency treatment delivered during a bronchial thermoplasty session.52 An European consensus statement recommended that patients treated with bronchial thermoplasty should remain under long-term follow-up at the treating center.37
Effectiveness Of Bronchial Thermoplasty Under Real-World Conditions
In the last 6 years, observational studies from Australia,53 Brazil,48 Canada,54,55 Denmark,56 France,43 Ireland,57 Japan,58 Korea,59 Netherlands,42 Spain,60 the UK31,34,61, and the USA33,39,62,63 have reported on the effectiveness and safety of bronchial thermoplasty in clinical practice. The largest report is the PAS2 study, which is a prospective, open-label, multicenter observational post-market study mandated by the FDA to evaluate the durability of the treatment effect and the short- and long-term efficacy and safety of bronchial thermoplasty.33 The interim-analysis of the PAS2 study found similar improvements in efficacy outcomes in the PAS2 population compared to AIR2 participants.33 For example, at 3 years posttreatment, the proportion of people with severe exacerbations and hospitalizations was reduced by 45% and 40% respectively compared to the 12 months prior to treatment, comparable to the reductions of 37% and 25% respectively reported in the AIR2 study.16 In the PAS2 study, post-bronchodilator spirometry was unchanged over the 3 years of follow-up after bronchial thermoplasty. The last patient enrolled in the PAS2 study is expected to complete 5 years of follow-up in January 2020 (ClinicalTrials.gov Identifier: NCT01350336).23 A preliminary report on 4 years of follow-up of PAS2 study participants confirms the efficacy findings reported after 3 years of follow-up.64 The United Kingdom Severe Asthma Registry of 86 patients undergoing bronchial thermoplasty reported clinical significant improvements in AQLQ at 12 months of follow-up and reductions in hospital admissions at 24 months.34 Other observational studies in real-life patients have reported improvement in AQLQ scores, reductions in exacerbations and/or a step-down in treatment in 50–75% of patients undergoing bronchial thermoplasty.32 The Bronchial Thermoplasty Global Registry of 159 patients enrolled at 19 centers in eight countries reported improvements in clinical outcomes, health-care utilization, and medication use at 1 year after bronchial thermoplasty.65
Respiratory-related serious adverse effects during the treatment phase with bronchial thermoplasty (first bronchial thermoplasty treatment to 6 weeks after last procedure) were more frequent in the PAS2 study33 compared to AIR2:16 severe exacerbations, 55.8% versus 40.5%, respectively; emergency room visits, 15.8% versus 5.3%, respectively. Emergency respiratory hospital readmission rates within 30 days of bronchial thermoplasty were similar: 13.2% versus 8.4%, respectively. In observational studies, hospital admission rates during the treatment phase ranged from 10%53 to 30%.31 The United Kingdom Severe Asthma Registry recorded a hospital admission rate of 11.8% in 59 cases undergoing bronchial thermoplasty.61 The reduction in FEV1 after bronchial thermoplasty is transient, occur more often after upper lobe treatment and is associated to the number of radio-frequency activations.66 Acute CT pulmonary peribronchial consolidation frequently occurs shortly after bronchial thermoplasty, but resolves in most patients within 1–6 months.67,68 Case report describes adverse effects associated with bronchial thermoplasty including mild central bronchiectasis developing 1 month after treatment,69 bilateral upper lobe atelectasis associated with the development of a pulmonary cyst and pneumothorax on day 6 after a third procedure,70 atelectasis due to fibrin plug formation within hours of treatment,71 and haemoptysis associated with bronchial nodules showing areas of hemorrhagic necrosis following a second procedure, which resolved by the third session of bronchial thermoplasty.72
Observational studies provide a low-grade of evidence on the effectiveness of bronchial thermoplasty due to weakness in study design, such as lack of data on clinical outcomes of a control group not treated with bronchial thermoplasty and small sample size. The PAS2 study excluded the most severe patients seen in clinical practice, such as those with a baseline FEV1 <60%, more than three hospitalizations, four or more courses of systemic corticosteroids in the last 12 months and oral corticosteroid maintenance dose >10 mg daily. Nevertheless, these studies suggest that bronchial thermoplasty is effective in one half to three-quarters of “real-life” patients with severe asthma and that it can be safely performed in clinical practice although peri-procedure adverse effects occur more often than in clinical trials.
Mechanism Of Action Of Bronchial Thermoplasty
Postulated mechanisms of action of bronchial thermoplasty include alterations in the structure and/or function of airway smooth muscle, epithelial cells, nerves, glands, extracellular matrix components, and/or inflammatory cells.32,73,74 Improvements in small airway function and/or a placebo effect from the procedure could also contribute to better clinical outcomes. The evidence supporting each of these pathways is summarized below, although to date, the precise mode of action of bronchial thermoplasty in asthma is uncertain.
Airway Smooth Muscle
Airway remodeling in severe asthma75,76 includes an increase in airway smooth muscle mass, which is associated with impaired lung function, airway hyperresponsiveness, and poor symptom control.77 Airway smooth muscle remodeling can occur in the absence of inflammation and thus it might not be responsive to anti-inflammatory therapies.78 Bronchial thermoplasty reduces the amount of airway smooth muscle in experimental animals,79 in patients with bronchial carcinoma awaiting lung resection80 and in patients with asthma.42,43,54,55,81–83 Evidence is conflicting to whether reduced airway smooth muscle mass is associated with improvements in clinical outcomes after bronchial thermoplasty.43,54,55 A preliminary report of pre- and post-bronchial thermoplasty data pooled for 99 patients at seven centers reported that airway smooth muscle mass and reticular basement thickness were reduced by bronchial thermoplasty beyond 1 year, but that these improvements in airway remodeling were not associated to improved clinical outcomes.84 A small study in 10 patients with severe asthma reported that CT airway volume increased 4 weeks after bronchial thermoplasty on the treated side compared to untreated airways.85 The improvement in CT airway volume was associated with a nonsignificant trend for an improvement in symptoms indicating the need for a larger study to confirm these preliminary findings.85 Other imaging studies performed up to 2 years after bronchial thermoplasty reported CT airway wall thickness was reduced48,86 and optical coherence tomographic airway wall thickness was decreased in a patient who responded to the procedure and increased in a nonresponder.46 A study involving the in vitro and in silico modeling of the acute effects of bronchial thermoplasty on airway smooth muscle cells concluded that the extent of the reduction in airway smooth muscle mass after bronchial thermoplasty was insufficient to explain the clinical benefits.87 Bronchial thermoplasty has been postulated to target airway smooth muscle function by additional mechanisms to the reduction in airway smooth muscle mass, including decreased contractility of airway smooth muscle by directly disrupting actin–myosin interactions,88 by stiffening the airway wall79 or by reducing the secretion of pro-inflammatory mediators from airway smooth muscle cells.77
Airway Epithelium
A bronchial biopsy study of 33 patients with severe asthma found increased bronchial epithelial cell proliferation at 3 weeks after bronchial thermoplasty that returned to baseline levels at ≥12 months.89 The ASMATHERM (Bicentric Prospective Study, Evaluating Bronchial THERMOPLASTY in Patients Presenting Severe Uncontrolled Asthma) study found no alteration to the histology of the bronchial epithelium at 3 months after the procedure.43 Based on the results of an in vivo study showing increased epithelial integrity after bronchial thermoplasty and in vitro and in silico modeling of the acute effects of bronchial thermoplasty on airway epithelial and smooth muscle cells, Chernyasvsky et al87 postulated that epithelial repair might contribute to the clinical benefits of bronchial thermoplasty. Using bronchoalveolar lavage (BAL) and bronchial epithelial biopsies obtained from patients with asthma both before and up to 12 weeks after bronchial thermoplasty, Sun et al90 reported that BAL fluid and epithelial cell culture supernatant decreased mitochondrial mass and fibroblast proliferation by blocking epithelium-derived heat shock protein 60 (HSP60) secretion and protein arginine methyltransferase 1 (PRMT1) expression in fibroblasts. Based on these findings, the authors hypothesized that bronchial thermoplasty decreases fibroblast remodeling by reducing the function of airway epithelial cells.90
Airway Glands
The ASMATHERM study found no alteration in subepithelial mucous glands or goblet cell hypertrophy or hyperplasia at 1 year after bronchial thermoplasty.43 A recent study reported a decrease in bronchial epithelial Mucin 5AC (MUC5AC) expression after bronchial thermoplasty that correlated with a decrease in expression of interleukin (IL)13.89 The authors speculated that as IL13 is a potent inducer of mucus secretion and goblet cell hyperplasia, bronchial thermoplasty-induced reduction in IL13 may contribute to a decrease in MUC5AC and improvements in clinical outcomes after the procedure.89
Airway Nerves
Several studies have reported an alteration to airway nerves after bronchial thermoplasty,43,83,91 including reduced nerve fibers in the submucosa91 and airway smooth muscle43,91 and a decrease in epithelial neuroendocrine cells.43 In one study, the number of epithelial neuroendocrine cells correlated with the number of severe exacerbations and asthma control test and AQLQ scores at 1 year after bronchial thermoplasty.43 Facciolongo et al91 postulated that the reduction in nerve fibers in the submucosa and airway smooth muscle may attenuate nerve reflexes and contribute to the improvement in clinical outcomes after bronchial thermoplasty.
Airway Extracellular Matrix Components And Airway Vasculature
Bronchial thermoplasty reduces subepithelial basement membrane thickening and type 1 collagen deposition.43,54,55,84 Airway vasculature is reported not to alter after bronchial thermoplasty.43,83
Airway Inflammation
Preliminary studies suggest that bronchial thermoplasty can alter markers of inflammation in asthma. For example, BAL concentrations of TGF-β1 and chemokine (C-C motif) ligand 5 (CCL5) decreased at 6 weeks after bronchial thermoplasty, whereas BAL IL4, IL5, and IL17 concentrations82 and bronchial epithelial expression of TGF- β1 and IL17 were unaltered.83 An observational study reported reductions in peripheral blood eosinophils at 1 year after bronchial thermoplasty,92 although the fall in blood eosinophil count is unlikely to be due to bronchial thermoplasty, since bronchial mucosal eosinophils are not reduced by the procedure.43 Transcriptomic profiling of airway epithelial cells obtained from untreated airways after bronchial thermoplasty found alterations to several gene networks associated with neurophysiological processes linked to asthma.93
Small Airway Function
Severe asthma is associated with small airway dysfunction that may contribute to worse clinical outcomes.94 Whether bronchial thermoplasty treatment to the central airways (<3 mm) can influence the structure or function of the peripheral airways in severe asthma is unclear.95,96 CT image evidence of acute peribronchial consolidations adjacent to airways treated with bronchial thermoplasty is likely to involve the peripheral airways, although it is not known whether this induces long-term structural changes to these airways.67,68 Several small observational studies of the effects of bronchial thermoplasty on lung function and imaging of small airway function in severe asthma have produced conflicting results.48,97,98 In a group of 32 patients with severe asthma, residual volumes (RVs) decreased by 7% at 6 months after bronchial thermoplasty.97 In a subgroup with baseline FEV1 of <60% predicted, gas trapping (RV/TLC ratio) was slightly reduced, a finding which suggests an effect of bronchial thermoplasty on the small airways.97 In contrast to these findings, a study of 43 patients with severe asthma from the same Australian centers found that forced oscillometry, at test used to assess small airway function was unaltered after bronchial thermoplasty.98 Another study in patients with severe asthma showed improvements in CT measures of air trapping at least 1 year after bronchial thermoplasty.48,86 Using a mathematical modeling analysis of human lung specimens from non-asthma, nonfatal asthma, and fatal asthma populations, Donovan et al74 proposed that reductions in airway smooth muscle mass in central airways treated with bronchial thermoplasty cause a cascade of reopening of smaller airways that produce more homogeneous flow patterns in the lung.74 The hypothesis predicts greater efficacy of bronchial thermoplasty with increasing asthma severity and greater agonist stimulation.45,74 In support of these findings, a study of regional lung ventilation in patients with severe asthma using imaging helium 3 MRI and CT imaging demonstrated improved ventilation defects after bronchial thermoplasty.99
Placebo Effect
Patients with uncontrolled asthma can derive considerable clinical benefits from randomization to placebo.100 The sham-controlled arm of the AIR2 trial was associated with increased AQLQ scores16 suggesting that some of the improvement in clinical outcomes clinical from bronchial thermoplasty may be due to a placebo effect.101 Nevertheless, in the AIR2 trial, a larger proportion of bronchial thermoplasty subjects than sham group subjects experienced a clinically meaningful within-subject improvement in AQLQ score of 0.5 or greater.16
Cost-Effectiveness
Bronchial thermoplasty treatment of severe asthma is more cost-effective in health-care systems where the costs of hospitalization and emergency department are high and the costs of the procedure are low.102 For example, in the USA, bronchial thermoplasty is estimated to be cost-effective in patients with severe uncontrolled asthma who have a high risk of exacerbations103 or who have severe allergic asthma,104 whereas in Singapore, the procedure is not cost-effective compared to optimized usual care.102 The threshold for health systems willingness-to-pay per quality-adjusted life years (QALY) influences the cost-effectiveness of the procedure. An indirect comparison of bronchial thermoplasty with omalizumab in patients with moderate-to-severe allergic asthma in the USA reported greater than a 60% chance that bronchial thermoplasty was cost-effective relative to omalizumab and standard therapy at the willingness-to-pay of $100,000 per QALY.104,105
Place In Management Of Bronchial Thermoplasty In Severe Asthma
The Global Initiative for Asthma (GINA) report provides recommendations on the investigation and management of adults and adolescents with difficult-to-treat asthma.7 The initial components include 1) confirmation of the diagnosis; 2) identification of factors contributing to poorly controlled asthma, such as poor inhaler technique, suboptimal adherence, modifiable risk factors and comorbidities; and 3) optimizing management by addressing aggravating contributary factors, considering add-on small-molecule drug therapies and non-pharmacological interventions, and/or considering a trial of high-dose ICS. After 3 to 6 months observation, patients with persistent uncontrolled asthma are assessed whether the diagnosis is severe refractory asthma. The inflammatory phenotype of severe asthma should be determined to identify those individuals with type 2 inflammation, who should be considered for add-on type 2 biologic therapies, such as anti-IgE, anti-IL5/anti-IL5R, or anti-IL4R. Patients with non-type 2 inflammation should be considered for add-on therapies, such as macrolides or low dose oral corticosteroids. GINA considers bronchial thermoplasty as an additional treatment option for patients with non-type 2 inflammation and for individuals who do not obtain a good response to type 2 targeted therapies. Overall, GINA considers that bronchial thermoplasty is a potential treatment option (Evidence grade B) at Step 5 in some countries for selected adults with severe asthma whose asthma remains uncontrolled despite optimized therapy and review by an asthma specialist.7 Longer-term safety follow-up of larger number of active and control patients is required to assess effectiveness and safety.7 Both GINA7 and the International ERS/ATS Guidelines on Definition, Evaluation and Treatment of Severe Asthma2 recommend that bronchial thermoplasty is undertaken in adults with severe asthma only in the context of an Institutional Review Board approved independent systematic registry or a clinical study. The British guideline on the management of asthma states that bronchial thermoplasty can be considered for the treatment of adult patients (aged 18 and over) with severe asthma who have poorly controlled asthma despite optimal medical therapy (Grade B recommendation).27 The guideline recommends that potential candidates for bronchial thermoplasty should have the diagnosis of uncontrolled symptoms due to severe asthma confirmed and that they are considered to be adherent with current therapies.27 Patients should be assessed by an asthma specialist with expertise in bronchial thermoplasty and treated at a center with appropriate resources. Details of all patients undergoing bronchial thermoplasty should be entered in the United Kingdom Severe Asthma Registry.27
Some specialists in bronchial thermoplasty recommend that the procedure should be considered for patients with severe asthma associated with non-type 2 inflammation29,106 or as an alternative therapy for patient unresponsive to currently licensed type 2 therapies. A European Consensus statement on bronchial thermoplasty developed by a panel of five European experts could not agree whether bronchial thermoplasty should be considered before or only after trials of type 2 targeted therapies.37 There are no clinical studies that have directly compared the effectiveness of bronchial thermoplasty with biological type 2 targeted agents. An indirect comparison of clinical outcomes, including severe exacerbations, emergency department visits, and hospital admissions in the AIR2 trial,105 were reported to be broadly similar to those in two placebo-controlled trials of omalizumab (INNOVATE107 and EXTRA105,108). Severe exacerbations were less for omalizumab compared to the total duration of the AIR2 study that included the increase in exacerbations during the peri-treatment period.105
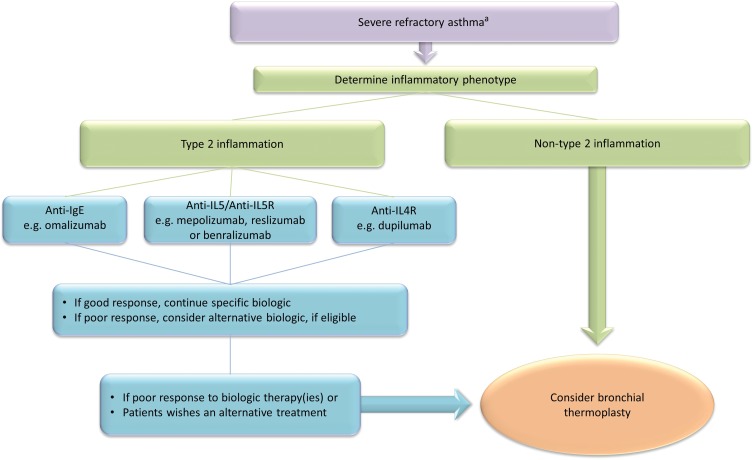
The place of bronchial thermoplasty in the treatment of severe asthma remains to be clearly defined.109 The author's suggestion for the use of bronchial thermoplasty and biologics in management of severe asthma in clinical practice is outlined in Figure 1. Future studies need to assess the effectiveness of bronchial thermoplasty targeted to remodeled airways, compared with biologic therapies or in combination with biologics, and in different inflammatory phenotypes. The high costs and difficulties undertaking comparative clinical studies using treatments developed by different pharmaceutical and device companies might prevent new large clinical trials being undertaken. New data on the effectiveness of bronchial thermoplasty and the identification of predictors of response are likely to come from observational studies in real-world populations of severe asthma.
Figure 1.
Algorithm to guide the selection of patients with severe refractory asthma for treatment with biologics and bronchial thermoplasty.
Note: aBiologics and bronchial thermoplasty are treatment options for patients with severe asthma (Step 5) who have uncontrolled asthma despite high-dose ICS plus long-acting β2-agonist and the long-acting muscarinic antagonist tiotropium and after assessment and management of causes of difficult-to-control asthma, such as nonadherence, poor inhaler technique, comorbidities, under treatment, and other behavioral factors.
Conclusions
Bronchial thermoplasty is approved in many countries worldwide as a non-pharmacological treatment for severe asthma based on the results of a small number of controlled clinical trials. Bronchial thermoplasty modestly improves clinical outcome in severe asthma including asthma quality of life and risk of exacerbations. Asthma morbidity increases during and shortly after treatment. Longer follow-up studies provide reassurance on long-term safety. In clinical practice, patients treated with bronchial thermoplasty have worse baseline characteristics and comparable clinical outcomes compared to trial data. Uncertainties remain about predictors of response, mechanism of action, and place in management of asthma. In the future, greater understanding of the sub-phenotypes of severe asthma and mode of action of bronchial thermoplasty should allow a precision medicine approach to the use of bronchial thermoplasty in the management of severe asthma.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Hekking -P-PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 2.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 3.Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71(4):339–346. doi: 10.1136/thoraxjnl-2015-207630 [DOI] [PubMed] [Google Scholar]
- 4.O’Neill S, Sweeney J, Patterson CC, et al. The cost of treating severe refractory asthma in the UK: an economic analysis from the British Thoracic Society Difficult Asthma Registry. Thorax. 2015;70(4):376–378. doi: 10.1136/thoraxjnl-2013-204114 [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Marra CA, Lynd LD, FitzGerald JM, Zafari Z, Sadatsafavi M. The natural history of severe asthma and influences of early risk factors: a population-based cohort study. Thorax. 2016;71(3):267–275. doi: 10.1136/thoraxjnl-2015-207530 [DOI] [PubMed] [Google Scholar]
- 6.McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: treatable traits down under international workshop report. Eur Respir J. 2019;53(5):1802058. doi: 10.1183/13993003.02058-2018 [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Asthma (GINA). Diagnosis and management of difficult-to-treat and severe asthma in adolescent and adult patients Web site. Available from: http://www.ginasthma.org/ Published 2019 Accessed September10, 2019.
- 8.Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J. 2004;24(4):659–663. doi: 10.1183/09031936.04.00054604 [DOI] [PubMed] [Google Scholar]
- 9.Torrego A, Solà I, Munoz A, et al. Bronchial thermoplasty for moderate or severe persistent asthma in adults. Cochrane Database Syst Rev. 2014;(3):Art. No.: CD009910. doi: 10.1002/14651858.CD009910.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson NC, Bicknell S, Chaudhuri R. Bronchial thermoplasty for severe asthma. Curr Opin Allergy Clin Immunol. 2012;12(3):241–248. doi: 10.1097/ACI.0b013e32835335ca [DOI] [PubMed] [Google Scholar]
- 11.Dombret M-C, Alagha K, Philippe Boulet L, et al. Bronchial thermoplasty: a new therapeutic option for the treatment of severe, uncontrolled asthma in adults. Eur Respir Rev. 2014;23(134):510–518. doi: 10.1183/09059180.00005114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mainardi AS, Castro M, Chupp G. Bronchial thermoplasty. Clin Chest Med. 2019;40(1):193–207. doi: 10.1016/j.ccm.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 13.Tan LD, Yoneda KY, Louie S, Hogarth DK, Castro M. Bronchial thermoplasty: a decade of experience: state of the art. J Allergy Clin Immunol in Pract. 2019;7(1):71–80. doi: 10.1016/j.jaip.2018.08.017 [DOI] [PubMed] [Google Scholar]
- 14.Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Eng J Med. 2007;356(13):1327–1337. doi: 10.1056/NEJMoa064707 [DOI] [PubMed] [Google Scholar]
- 15.Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185–1191. doi: 10.1164/rccm.200704-571OC [DOI] [PubMed] [Google Scholar]
- 16.Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116–124. doi: 10.1164/rccm.200903-0354OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Anci KE, Lynch MP, Leas BF, et al. Effectiveness and safety of bronchial thermoplasty in management of asthma. Comparative Effectiveness Review No. 202. Available from: www.effectivehealthcare.ahrq.gov/reports/final.cfm Published 2017 Accessed September10, 2019.
- 18.Wu Q, Xing Y, Zhou X, Wang D. Meta-analysis of the efficacy and safety of bronchial thermoplasty in patients with moderate-to-severe persistent asthma. J Int Med Res. 2011;39(1):10–22. doi: 10.1177/147323001103900102 [DOI] [PubMed] [Google Scholar]
- 19.Thomson NC, Rubin A, Niven R, et al. Long term (5 Year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011;11(1):8. doi: 10.1186/1471-2466-11-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavord ID, Thomson NC, Niven RM, et al. Safety of bronchial thermoplasty in patients with severe refractory asthma. Ann Allergy Asthma Immunol. 2013;111(5):402–407. doi: 10.1016/j.anai.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295–1302. doi: 10.1016/j.jaci.2013.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou JP, Feng Y, Wang Q, Zhou LN, Wan HY, Li QY. Long-term efficacy and safety of bronchial thermoplasty in patients with moderate-to-severe persistent asthma: a systemic review and meta-analysis. J Asthma. 2016;53(1):94–100. doi: 10.3109/02770903.2015.1065424 [DOI] [PubMed] [Google Scholar]
- 23.U.S. National Institutes of Health. U.S. National Institutes of Health. Available from: https://clinicaltrials.gov/ Published 2019 Accessed September10, 2019.
- 24.Chaudhuri R, Rubin A, Fiterman J, et al. Ten-year follow-up of subjects who received bronchial thermoplasty (BT) in 3 randomized controlled studies (BT10+). Eur Respir Soc Congr. 2019. Madrid. [Google Scholar]
- 25.Iyer VN, Lim KG. Bronchial thermoplasty: reappraising the evidence (or lack thereof). Chest. 2014;146(1):17–21. doi: 10.1378/chest.14-0536 [DOI] [PubMed] [Google Scholar]
- 26.Castro M, Cox G, Wechsler ME, Niven RM. Bronchial thermoplasty: ready for prime time – the evidence is there! Chest. 2015;147(2):e73–e74. doi: 10.1378/chest.14-2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.British Guideline on the Management of Asthma. 2019. Available from: www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/Published Accessed September10, 2019.
- 28.Trivedi A, Pavord ID, Castro M. Bronchial thermoplasty and biological therapy as targeted treatments for severe uncontrolled asthma. Lancet Respir Med. 2016;4(7):585–592. doi: 10.1016/S2213-2600(16)30018-2 [DOI] [PubMed] [Google Scholar]
- 29.Blaiss MS, Castro M, Chipps BE, Zitt M, Panettieri RA Jr., Foggs MB. Guiding principles for use of newer biologics and bronchial thermoplasty for patients with severe asthma. Ann Allergy Asthma Immunol. 2017;119(6):533–540. doi: 10.1016/j.anai.2017.09.058 [DOI] [PubMed] [Google Scholar]
- 30.Bonta PI, Chanez P, Annema JT, Shah PL, Niven R. Bronchial thermoplasty in severe asthma: best practice recommendations from an expert panel. Respiration. 2018;95(5):289–300. doi: 10.1159/000488291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bicknell S, Chaudhuri R, Lee N, et al. Effectiveness of bronchial thermoplasty in severe asthma in ‘real life’ patients compared with those recruited to clinical trials in the same centre. Therap Adv Respir Dis. 2015;9(6):267–271. doi: 10.1177/1753465815601332 [DOI] [PubMed] [Google Scholar]
- 32.Thomson NC. Bronchial thermoplasty as a treatment for severe asthma: controversies, progress and uncertainties. Exp Rev Respir Med. 2018;12(4):269–282. doi: 10.1080/17476348.2018.1444991 [DOI] [PubMed] [Google Scholar]
- 33.Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: a comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:2. doi: 10.1183/13993003.00017-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burn J, Sims AJ, Patrick H, Heaney LG, Niven RM. Efficacy and safety of bronchial thermoplasty in clinical practice: a prospective, longitudinal, cohort study using evidence from the UK Severe Asthma Registry. BMJ Open. 2019;9(6):e026742. doi: 10.1136/bmjopen-2018-026742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langton D, Ing A, Fielding D, et al. Safety and effectiveness of bronchial thermoplasty when FEV1 less than 50%. Chest. 2019; S0012-3692(19)33753–5 Epub ahead of print. doi: 10.1016/j.chest.2019.08.2193 [DOI] [PubMed] [Google Scholar]
- 36.U.S. Food and Drug Administration. Alair bronchial thermoplasty system: Alair catheter and Alair RF controller; 2010. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf8/p080032a.pdf Accessed September10, 2019.
- 37.Niven R, Aubier M, Bonta P, Puente-Maestu L, Facciolongo N, Ryan D. European consensus meeting/statement on bronchial thermoplasty who? Where? How? Respir Med. 2019;150:161–164. doi: 10.1016/j.rmed.2019.02.020 [DOI] [PubMed] [Google Scholar]
- 38.Hall CS, Castro M. Predicting response to bronchial thermoplasty in patients with severe uncontrolled asthma: an elusive goal. Respirology. 2019;24(1):11–12. doi: 10.1111/resp.2019.24.issue-1 [DOI] [PubMed] [Google Scholar]
- 39.Sierra M, Fernandez-Bussy S, Mehta H, et al. Bronchial thermoplasty in severe uncontrolled asthma with different phenotypes. Chest. 2017;152(4, Supplement):A29. doi: 10.1016/j.chest.2017.08.059 [DOI] [Google Scholar]
- 40.Yamamura K, Hara J, Ohkura N, et al. Increased cough receptor sensitivity to capsaicin predicts a positive bronchial thermoplasty response: a single-center retrospective study. J Bronchology Interv Pulmonol. 2019;26(2):137–141. doi: 10.1097/LBR.0000000000000577 [DOI] [PubMed] [Google Scholar]
- 41.Langton D, Ing A, Fielding D, Wang W, Plummer V, Thien F. Bronchodilator responsiveness as a predictor of success for bronchial thermoplasty. Respirology. 2019;24(1):63–67. doi: 10.1111/resp.2019.24.issue-1 [DOI] [PubMed] [Google Scholar]
- 42.d’Hooghe JNS, Goorsenberg AWM, Ten Hacken NHT, et al. Airway smooth muscle reduction after bronchial thermoplasty in severe asthma correlates with FEV1. Clin Exp Allergy. 2019;49(4):541–544. doi: 10.1111/cea.13365 [DOI] [PubMed] [Google Scholar]
- 43.Pretolani M, Bergqvist A, Thabut G, et al. Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: clinical and histopathological correlations. J Allergy Clin Immunol. 2017;139(4):1176–1185. doi: 10.1016/j.jaci.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 44.Langton D, Sha J, Ing A, Fielding D, Thien F, Plummer V. Bronchial thermoplasty: activations predict response. Respir Res. 2017;18(1):134. doi: 10.1186/s12931-017-0617-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donovan GM, Elliot JG, Boser SR, Green FHY, James AL, Noble PB. Patient-specific targeted bronchial thermoplasty: predictions of improved outcomes with structure-guided treatment. J Appl Physiol. 2019;126(3):599–606. doi: 10.1152/japplphysiol.00951.2018 [DOI] [PubMed] [Google Scholar]
- 46.Kirby M, Ohtani K, Lopez Lisbona RM, et al. Bronchial thermoplasty in asthma: 2-year follow-up using optical coherence tomography. Eur Respir J. 2015;46(3):859–862. doi: 10.1183/09031936.00016815 [DOI] [PubMed] [Google Scholar]
- 47.Feroldi F, Willemse J, Davidoiu V, et al. In vivo multifunctional optical coherence tomography at the periphery of the lungs. Biomed Opt Express. 2019;10(6):3070–3091. doi: 10.1364/BOE.10.003070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanon M, Strieder DL, Rubin AS, et al. Use of MDCT to assess the results of bronchial thermoplasty. AJR Am J Roentgenol. 2017;209(4):752–756. doi: 10.2214/AJR.17.18027 [DOI] [PubMed] [Google Scholar]
- 49.Bicknell S, Chaudhuri R, Thomson NC. How to: bronchial thermoplasty in asthma. Breathe. 2014;10(1):48–59. doi: 10.1183/20734735.007813 [DOI] [Google Scholar]
- 50.Eisenmann S, Schütte W, Funke F, Oezkan F, Islam S, Darwiche K. Bronchial thermoplasty including the middle lobe bronchus significantly improves lung function and quality of life in patients suffering from severe asthma. Lung. 2019;197(4):493–499. doi: 10.1007/s00408-019-00240-5 [DOI] [PubMed] [Google Scholar]
- 51.Hall C, Quirk JD, Goss CW, et al. Targeted bronchial thermoplasty guided by 129Xe MRI. Am J Respir Crit Care Med. 2019;A7355–A7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langton D, Gaffney N, Wang WC, Thien F, Plummer V. Utility of a thin bronchoscope in facilitating bronchial thermoplasty. J Asthma Allergy. 2018;11:261–266. doi: 10.2147/JAA.S179359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langton D, Sha J, Ing A, Fielding D, Wood E. Bronchial thermoplasty in severe asthma in Australia. Intern Med J. 2017;47(5):536–541. doi: 10.1111/imj.13372 [DOI] [PubMed] [Google Scholar]
- 54.Chakir J, Haj-Salem I, Gras D, et al. Effects of bronchial thermoplasty on airway smooth muscle and collagen deposition in asthma. Annals ATS. 2015;12(11):1612–1618. [DOI] [PubMed] [Google Scholar]
- 55.Salem IH, Boulet L-P, Biardel S, et al. Long-term effects of bronchial thermoplasty on airway smooth muscle and reticular basement membrane thickness in severe asthma. Annals ATS. 2016;13(8):1426–1428. doi: 10.1513/AnnalsATS.201603-182LE [DOI] [PubMed] [Google Scholar]
- 56.Madsen H, Henriksen DP, Backer V, Siersted HC, Bjerring N, Ulrik CS. Efficacy of bronchial thermoplasty in patients with severe asthma. J Asthma. 2019;1–7. Epub ahead of print. doi: 10.1080/02770903.2019.1678636 [DOI] [PubMed] [Google Scholar]
- 57.O’Reilly A, Browne I, Watchorn D, Egan JJ, Lane S. The efficacy and safety of bronchial thermoplasty in severe persistent asthma on extended follow-up. QJM. 2017;111(3):155–159. doi: 10.1093/qjmed/hcx221 [DOI] [PubMed] [Google Scholar]
- 58.Iikura M, Hojo M, Nagano N, et al. Bronchial thermoplasty for severe uncontrolled asthma in Japan. Allergol Internat. 2017;67(2):273–275. doi: 10.1016/j.alit.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 59.Kang J, Cho YS, Choi D-K, et al. Bronchial thermoplasty in patients with severe uncontrolled asthma: first Korean cases. J Korean Med Sci. 2019;34:15. doi: 10.3346/jkms.2019.34.e120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puente-Maestu L, Llanos Flores M, Benedetti P, et al. Effectiveness and safety of bronchial thermoplasty in severe asthma in clinical practice in Spain. Biomedicine Hub. 2018;3(3):1–9. doi: 10.1159/000492075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burn J, Sims AJ, Keltie K, et al. Procedural and short-term safety of bronchial thermoplasty in clinical practice: evidence from a national registry and Hospital Episode Statistics. J Asthma. 2017;54(8):872–879. doi: 10.1080/02770903.2016.1263652 [DOI] [PubMed] [Google Scholar]
- 62.Doeing DC, Mahajan AK, White SR, Naureckas ET, Krishnan JA, Hogarth DK. Safety and feasibility of bronchial thermoplasty in asthma patients with very severe fixed airflow obstruction: a case series. J Asthma. 2013;50:215–218. doi: 10.3109/02770903.2012.751997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kanaan R, Strange C, Hogarth K, et al. Bronchial thermoplasty is effective and relatively safe in high risk asthma patients. Am J Respir Crit Care Med. 2017;195:A4690. doi: 10.1164/rccm.201701-0150WS [DOI] [Google Scholar]
- 64.Chupp GL, Kline JN, Khatri SB, et al. Long-term efficacy and safety of bronchial thermoplasty (BT): 4 year follow-up results from a large scale prospective study. Am J Respir Crit Care Med. 2019;A2683–A2683. [Google Scholar]
- 65.Torrego A, Herth F, Munoz AM, et al. Bronchial thermoplasty global registry: one year results. Eur Respir J. 2018;52(suppl 62):OA1921. [Google Scholar]
- 66.Langton D, Wang W, Thien F, Plummer V. The acute effects of bronchial thermoplasty on FEV1. Respir Med. 2018;137:147–151. doi: 10.1016/j.rmed.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 67.Debray M-P, Dombret M-C, Pretolani M, et al. Early computed tomography modifications following bronchial thermoplasty in patients with severe asthma. Eur Respir J. 2017;49:3. doi: 10.1183/13993003.01565-2016 [DOI] [PubMed] [Google Scholar]
- 68.d’Hooghe JNS, van den Berk IAH, Annema JT, Bonta PI. Acute radiological abnormalities after bronchial thermoplasty: a prospective cohort trial. Respiration. 2017;94(3):258–262. doi: 10.1159/000477586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu M, Lai Z, Wei S, et al. Bronchiectasis after bronchial thermoplasty. J Thorac Dis. 2018;10(10):E721–E726. doi: 10.21037/jtd.2018.09.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Funatsu A, Kobayashi K, Iikura M, Ishii S, Izumi S, Sugiyama H. A case of pulmonary cyst and pneumothorax after bronchial thermoplasty. Respirol Case Rep. 2018;6(2):e00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Facciolongo N, Menzella F, Lusuardi M, et al. Recurrent lung atelectasis from fibrin plugs as a very early complication of bronchial thermoplasty: a case report. Multidiscipl Respir Med. 2015;10(1):9. doi: 10.1186/s40248-015-0002-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menzella F, Lusuardi M, Galeone C, Montanari G, Cavazza A, Facciolongo N. Heat-induced necrosis after bronchial thermoplasty: a new concern? Allergy Asthma Clin Immunol. 2018;14(1):25. doi: 10.1186/s13223-018-0252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.d’Hooghe JNS, Ten Hacken NH, Weersink EJM, Sterk PJ, Annema JT, Bonta PI. Emerging understanding of the mechanism of action of bronchial thermoplasty in asthma. Pharmacol Therapeut. 2018;181:101–107. doi: 10.1016/j.pharmthera.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 74.Donovan GM, Elliot JG, Green FHY, James AL, Noble PB. Unraveling a clinical paradox: why does bronchial thermoplasty work in asthma? Am J Respir Cell Mol Biol. 2018;59(3):355–362. doi: 10.1165/rcmb.2018-0011OC [DOI] [PubMed] [Google Scholar]
- 75.James AL, Elliot JG, Jones RL, et al. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med. 2012;185(10):1058–1064. doi: 10.1164/rccm.201110-1849OC [DOI] [PubMed] [Google Scholar]
- 76.Pepe C, Foley S, Shannon J, et al. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005;116(3):544–549. doi: 10.1016/j.jaci.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 77.Zuyderduyn S, Sukkar MB, Fust A, Dhaliwal S, Burgess JK. Treating asthma means treating airway smooth muscle cells. Eur Resp J. 2008;32(2):265–274. doi: 10.1183/09031936.00051407 [DOI] [PubMed] [Google Scholar]
- 78.Elliot JG, Noble PB, Mauad T, et al. Inflammation-dependent and independent airway remodelling in asthma. Respirology. 2018;23(12):1138–1145. doi: 10.1111/resp.2018.23.issue-12 [DOI] [PubMed] [Google Scholar]
- 79.Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol. 2004;97(5):1946–1953. doi: 10.1152/japplphysiol.01282.2003 [DOI] [PubMed] [Google Scholar]
- 80.Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest. 2005;127(6):1999–2006. doi: 10.1378/chest.127.6.1999 [DOI] [PubMed] [Google Scholar]
- 81.Gordon I, Husain A, Charbeneau J, Krishnan J, Hogarth D. Endobronchial biopsy: a guide for asthma therapy selection in the era of bronchial thermoplasty. J Asthma. 2013;50(6):634–641. doi: 10.3109/02770903.2013.794239 [DOI] [PubMed] [Google Scholar]
- 82.Denner DR, Doeing DC, Hogarth DK, Dugan K, Naureckas ET, White SR. Airway inflammation after bronchial thermoplasty for severe asthma. Annals Am Thor Soc. 2015;12(9):1302–1309. doi: 10.1513/AnnalsATS.201502-082OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ichikawa T, Panariti A, Audusseau S, et al. Effect of bronchial thermoplasty on structural changes and inflammatory mediators in the airways of subjects with severe asthma. Respir Med. 2019;150:165–172. doi: 10.1016/j.rmed.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 84.Russell R, Aubier M, Pretolani M, et al. Bronchial thermoplasty leads to rapid and persistent improvements in airway remodeling. Eur Respir Soc Congr. 2019. Madrid. [Google Scholar]
- 85.Langton D, Sloan G, Banks C, Bennetts K, Plummer V, Thien F. Bronchial thermoplasty increases airway volume measured by functional respiratory imaging. Respir Res. 2019;20(1):157. doi: 10.1186/s12931-019-1132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Konietzke P, Weinheimer O, Wielpütz MO, et al. Quantitative CT detects changes in airway dimensions and air-trapping after bronchial thermoplasty for severe asthma. Eur J Radiol. 2018;107:33–38. doi: 10.1016/j.ejrad.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 87.Chernyavsky IL, Russell RJ, Saunders RM, et al. In vitro, in silico and in vivo study challenges the impact of bronchial thermoplasty on acute airway smooth muscle mass loss. Eur Respir J. 2018;51:5. doi: 10.1183/13993003.01680-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dyrda P, Tazzeo T, DoHarris L, et al. Acute response of airway muscle to extreme temperature includes disruption of actin-myosin interaction. Am J Respir Cell Mol Biol. 2011;44(2):213–221. doi: 10.1165/rcmb.2009-0259OC [DOI] [PubMed] [Google Scholar]
- 89.Salem IH, Gras D, Joubert P, et al. Persistent reduction of mucin production after bronchial thermoplasty in severe asthma. Am J Respir Crit Care Med. 2019;199(4):536–538. doi: 10.1164/rccm.201811-2064LE [DOI] [PubMed] [Google Scholar]
- 90.Sun Q, Fang L, Roth M, et al. Bronchial thermoplasty decreases airway remodelling by blocking epithelium-derived heat shock protein 60 (HSP60) secretion and protein arginine methyltransferase 1 (PRMT1) in fibroblasts. Eur Respir J. 2019; 1900300 Epub ahead of print. doi: 10.1183/13993003.00300-2019 [DOI] [PubMed] [Google Scholar]
- 91.Facciolongo N, Di Stefano A, Pietrini V, et al. Nerve ablation after bronchial thermoplasty and sustained improvement in severe asthma. BMC Pulm Med. 2018;18(1):29. doi: 10.1186/s12890-017-0554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ryan DM, Fowler SJ, Niven RM. Reduction in peripheral blood eosinophil counts after bronchial thermoplasty. J Allergy Clin Immunol. 2016;138(1):308–310.e302. doi: 10.1016/j.jaci.2015.11.044 [DOI] [PubMed] [Google Scholar]
- 93.Liao S-Y, Linderholm AL, Yoneda KY, Kenyon NJ, Harper RW. Airway transcriptomic profiling after bronchial thermoplasty. ERJ Open Res. 2019;5(1):00123–02018. doi: 10.1183/23120541.00123-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Contoli M, Santus P, Papi A. Small airway disease in asthma: pathophysiological and diagnostic considerations. Curr Opin Pulm Med. 2015;21(1):68–73. doi: 10.1097/MCP.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 95.Chanez P, Pahus L, Charriot J, Bourdin A. Severe asthma treated by bronchial thermoplasty: a success not due to the small airways? Respirology. 2019;24(5):402–403. doi: 10.1111/resp.2019.24.issue-5 [DOI] [PubMed] [Google Scholar]
- 96.Boulet L-P, Laviolette M. Acute effects of bronchial thermoplasty: a matter of concern or an indicator of possible benefit to small airways? Eur Respir J. 2017;49(3):1700029. doi: 10.1183/13993003.00029-2017 [DOI] [PubMed] [Google Scholar]
- 97.Langton D, Ing A, Bennetts K, et al. Bronchial thermoplasty reduces gas trapping in severe asthma. BMC Pulm Med. 2018;18(1):155. doi: 10.1186/s12890-018-0721-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Langton D, Ing A, Sha J, et al. Measuring the effects of bronchial thermoplasty using oscillometry. Respirology. 2019;24(5):431–436. doi: 10.1111/resp.2019.24.issue-5 [DOI] [PubMed] [Google Scholar]
- 99.Thomen RP, Sheshadri A, Quirk JD, et al. Regional ventilation changes in severe asthma after bronchial thermoplasty with (3)He MR imaging and CT. Radiology. 2015;274(1):250–259. doi: 10.1148/radiol.14140080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luc F, Prieur E, Whitmore GA, Gibson PG, Vandemheen KL, Aaron SD. “Placebo effects” in clinical trials evaluating patients with uncontrolled persistent asthma. Annals ATS. 2019;16(9):1124–1130. doi: 10.1513/AnnalsATS.201901-071OC [DOI] [PubMed] [Google Scholar]
- 101.Redberg RF. Sham controls in medical device trials. N Eng J Med. 2014;371(10):892–893. doi: 10.1056/NEJMp1406388 [DOI] [PubMed] [Google Scholar]
- 102.Nguyen HV, Bose S, Mital S, et al. Is bronchial thermoplasty cost-effective as treatment for problematic asthma patients? Singapore’s perspective on a global model. Respirology. 2017;22(6):1102–1109. doi: 10.1111/resp.2017.22.issue-6 [DOI] [PubMed] [Google Scholar]
- 103.Zein JG, Menegay MC, Singer ME, et al. Cost effectiveness of bronchial thermoplasty in patients with severe uncontrolled asthma. J Asthma. 2015;1–7. [DOI] [PubMed] [Google Scholar]
- 104.Zafari Z, Sadatsafavi M, Marra CA, Chen W, FitzGerald JM. Cost-effectiveness of bronchial thermoplasty, omalizumab, and standard therapy for moderate-to-severe allergic asthma. PLoS One. 2016;11(1):e0146003. doi: 10.1371/journal.pone.0146003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Niven RM, Simmonds MR, Cangelosi MJ, Tilden DP, Cottrell S, Shargill NS. Indirect comparison of bronchial thermoplasty versus omalizumab for uncontrolled severe asthma. J Asthma. 2017;55(4):443–451. doi: 10.1080/02770903.2017.1337789 [DOI] [PubMed] [Google Scholar]
- 106.Oberle AJ, Mathur P. Precision medicine in asthma: the role of bronchial thermoplasty. Curr Opin Pulm Med. 2017;23(3):254–260. doi: 10.1097/MCP.0000000000000372 [DOI] [PubMed] [Google Scholar]
- 107.Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi: 10.1111/all.2005.60.issue-3 [DOI] [PubMed] [Google Scholar]
- 108.Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy. Ann Intern Med. 2011;154(9):573–582. doi: 10.7326/0003-4819-154-9-201105030-00002 [DOI] [PubMed] [Google Scholar]
- 109.Siddiqui S, Denlinger LC, Fowler SJ, et al. Unmet needs in severe asthma subtyping and precision medicine trials. Bridging clinical and patient perspectives. Am J Respir Crit Care Med. 2019;199(7):823–829. doi: 10.1164/rccm.201809-1817PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Han X, Zhang S, Zhao W, et al. A successful bronchial thermoplasty procedure in a “very severe” asthma patient with rare complications: a case report. J Asthma. 2019;56(9):1004–1007. doi: 10.1080/02770903.2018.1509992 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global Initiative for Asthma (GINA). Diagnosis and management of difficult-to-treat and severe asthma in adolescent and adult patients Web site. Available from: http://www.ginasthma.org/ Published 2019 Accessed September10, 2019.