Abstract
Introduction
Patients with chronic obstructive pulmonary disease (COPD) are at an increased risk of cardiovascular comorbidities such as pulmonary hypertension or heart failure. Impaired cardiovascular function often has a significant impact on patients with COPD. Oxygen pulse (O2P) is a surrogate for stroke volume. However, studies regarding O2P, health-related quality of life (HRQL), and exercise capacity in patients with COPD are lacking. We aimed to confirm the association between O2P, HRQL, exercise capacity, severe exacerbation of COPD, and other parameters in exercise testing.
Materials and methods
This study included 79 patients with COPD who underwent lung function testing, a cardiopulmonary exercise test (CPET), Borg Dyspnea Scale evaluation, completion of the St. George’s Respiratory Questionnaire, and echocardiography. Cardiovascular comorbidities, COPD-related hospitalizations, and emergency room visits were recorded. We compared these parameters between two groups of patients: those with normal peak O2P and those with impaired peak O2P. The relationships of peak O2P with CPET and lung function were analyzed using simple linear regression.
Results
Patients with normal peak O2P had higher exercise capacity (peak oxygen uptake and work rate), better HRQL, lower dyspnea score, lower COPD-related hospitalizations, and higher circulatory and ventilator parameters than patients with impaired peak O2P. According to a simple linear regression analysis, the anaerobic threshold (AT) and forced expiratory volume in one second (FEV1) showed a significant association with peak O2P, and the Pearson correlation coefficients (Pearson’s r) were 0.756 and 0.461, respectively.
Conclusion
Peak O2P has a significant impact on exercise capacity, HRQL, dyspnea, COPD-related hospitalization, and circulatory and ventilatory functions in patients with COPD. The AT and FEV1 have strong and moderate associations with peak O2P, respectively. Therefore, peak O2P is an important indicator of disease severity for patients with COPD.
Keywords: cardiopulmonary exercise test, chronic obstructive pulmonary disease, hospitalization, oxygen pulse, health-related quality of life
Introduction
Chronic obstructive pulmonary disease (COPD) is caused by exposure to noxious particles or gases and characterized by partially reversible airway obstruction with respiratory symptoms including cough, dyspnea, chest tightness, etc.1 In 2015, COPD was responsible for approximately 3 million deaths worldwide. It is also deemed the fourth leading cause of death in the world and is predicted to rank third by 2020.1
With respect to pathophysiology, there is a close interaction between COPD and cardiac function. The principal pathophysiological changes of COPD include airway inflammation, lung emphysema, and pulmonary vascular changes.2 The obstruction and inflammation of COPD cause expiratory flow limitation, air-trapping, and hyperinflation. Airway obstruction and hyperinflation are further associated with impaired left heart diastolic filling.3 The loss of pulmonary capillaries in emphysematous lungs increases the vascular resistance,4 and pulmonary vascular remodeling including intimal and smooth muscle hyperplasia leads to pulmonary hypertension.4
The cardiopulmonary exercise test (CPET) is regarded as the gold standard for evaluating exercise capacity and both ventilatory and circulatory functions of patients with COPD. Oxygen pulse (O2P) derived from CPET is a non-invasive surrogate for stroke volume (SV).5,6 Although pulmonary artery catheterization is considered the gold standard of cardiac output measurement in critical care, it is not widely implemented in stable COPD cases due to its invasive nature and possible complications. CPET provides global assessment of integrative exercise responses including cardiovascular, pulmonary, muscular, and cellular oxidative systems, and its application is more widely utilized in patients with COPD. Therefore, studies investigating the parameters of CPET in patients with COPD are important.
It is important to analyze cardiovascular functions in patients with COPD, and CPET is one of the tools used to investigate the circulatory function in these patients. O2P reflects SV and is an important parameter in patients with COPD. However, studies on the application of O2P in COPD are limited.
Therefore, we aimed to comprehensively analyze the impact of peak O2P on COPD in five domains: exercise capacity, circulatory parameters, ventilatory parameters, dyspnea/health-related quality of life (HRQL) score, and severe exacerbation of COPD. Additionally, we studied the association between peak O2P and other CPET variables.
Methods
Study Cohort
The patients were retrospectively recruited from the outpatient department of the Taipei Tzu Chi Hospital. We collected the clinical data from the medical records. The data included demographic data, laboratory data, data on cardiovascular comorbidities, data from the ABCD assessment tool according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline,1 echocardiography data, and data on the severe exacerbations of COPD. The definition of severe exacerbation was COPD-related hospitalizations or emergency room (ER) visits. The study was approved by the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation Institutional Review Board (Protocol Number: 08-XD-046) in accordance with the Declaration of Helsinki and all patients signed informed consent forms.
The inclusion criteria were: (1) a spirometry diagnosis of COPD based on the GOLD criteria,1 (2) no acute exacerbation of COPD in the last 3 months; and (3) ability to perform exercise on a cycle ergometer independently. The exclusion criteria were: (1) the presence of unstable acute coronary syndrome in the last 3 months or (2) neuromuscular diseases or major surgeries that influenced the exercise test.
Pulmonary Function Test And Respiratory Muscle Power
Pulmonary function tests by CPFS/D USBTM spirometry (Medical Graphics Corporation, St. Paul, MN, USA) were performed according to the American Thoracic Society guidelines.7 The severity of the airway obstruction was categorized according to GOLD stages.1 The lung function variables that were recorded included forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and the calculated FEV1/FVC ratio.
Respiratory muscle power was assessed using a direct dial pressure gauge (Respiratory Pressure Meter, Micro Medical Corp., now Vyaire Medical, Mettawa, IL, USA) to evaluate the maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP).
Cardiopulmonary Exercise Test
All patients were evaluated by CPET on an electronically braked cycle ergometer (Corival; Lode B.V., Groningen, The Netherlands) under the supervision of technicians. Some patients who suffered from severe desaturation (pulse oximeter saturation <85%), intolerable dizziness, or chest tightness withdrew from the examination. During CPET, the air was monitored breath-by-breath using the MGC cardiopulmonary diagnostic system (Breeze suite 6.1; Medical Graphics Corporation, St. Paul, MN, USA). CPET variables were measured at an average of 30 s of the breath-by-breath data evaluation. The respiratory exchange ratio (RER) is the ratio between CO2 production and O2 consumption during metabolism. It is a surrogate marker of a patient’s effort. For the study purposes, qualified exercise required sufficient effort to reach an RER ≥1.1.5 The time of peak exercise was defined as the achievement of peak oxygen uptake (VO2).5 The peak O2P was defined as the O2P value at the time of peak exercise.
The anaerobic threshold (AT) is defined as the level of oxygen consumption at which point the anaerobic metabolism becomes more dominant than the aerobic metabolism, blood lactate accumulates, and the lactate level rises. The AT was identified using the V-slope method according to a previously reported description.5 During the exercise test, the respiratory rate, tidal volume, and minute ventilation (VE) were continuously monitored. The ventilatory equivalent (VEQ) is defined as the ratio of VE to carbon dioxide production (VCO2) at AT. The work efficiency (WE) is the slope of VO2 to work rate during exercise. For comprehensive analysis, other variables including heart rate (HR), blood pressure (BP), partial pressure of carbon dioxide at the end of exhaled breath (PETCO2), and arterial oxygen hemoglobin saturation (SpO2) were also recorded.
O2P is defined as the ratio of VO2 to HR (VO2/HR). According to the Fick equation:
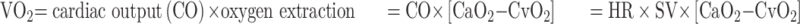 |
Thus, O2P = VO2/HR= SV × [CaO2-CvO2]; where CaO2 is arterial oxygen content, and CvO2 is venous oxygen content.
Since CaO2-CvO2 is assumed to be constant during maximal exercise,8 peak O2P is regarded as a non-invasive parameter of SV.5,6 The normal value of O2P is 80% of the predicted value. In this study, we analyzed the impact of peak O2P on COPD cases. We divided the participants into two groups: Group 1 with normal peak O2P (≥80% of the predicted value) and Group 2 with impaired peak O2P (<80% of the predicted value).
Perceived Dyspnea Measurement And Health-Related Quality Of Life
Borg’s category-ratio 10 scale (Borg CR10) was used for rating perceived dyspnea.9 The Borg CR10 scale is determined at rest and at peak exercise. The scale ranges from 0 to 10, where higher numbers indicate higher degrees of perceived dyspnea.
The St. George’s Respiratory Questionnaire (SGRQ) was used to evaluate the health status of COPD.10 It is a standardized, self-administered questionnaire of 50 items covering four categories: symptoms, physical activities, psychosocial impact, and total scores. The total scores range from 0 to 100, corresponding to the best and worst quality of life, respectively.
Echocardiography
The left ventricular ejection fraction (LVEF) and SV were derived from 2D Simpson’s biplane analysis. Continuous wave Doppler and the simplified Bernoulli equation were used to estimate the tricuspid regurgitation pressure gradient (TRPG).
Statistical Analysis
IBM SPSS Statistics software (version 25; IBM Corp., Armonk, NY, USA) was used for data analysis. Continuous data are expressed as the mean ± standard deviation or number and percentage, while categorical data are expressed as frequencies and percentages. Chi-squared or Fisher’s exact tests (two-tailed) were used to compare categorical variables, independent t-tests were used to compare continuous variables with normal distribution, and Wilcoxon rank-sum tests were used for nonparametric statistics. Relationships between continuous variables were assessed using the Pearson correlation coefficient (Pearson’s r) and simple linear regression analysis. An r-value >0.3 or >0.7 was considered as a moderate or strong relationship, respectively. A p-value <0.05 was established as the level of significance.
Results
Patient Characteristics
The baseline characteristics of all participants are summarized in Table 1. This study enrolled 79 patients with COPD, who were divided into Groups 1 and 2 consisting of 37 and 42 patients, respectively. There were no significant differences detected between the two groups with respect to age, sex, body height, body weight, body mass index, hemoglobin, ABCD assessment tool categorization, smoking history, pharmacologic treatment, cardiovascular comorbidities except hypertension, and echocardiographic parameters. However, the severity of the airway obstruction evaluated under the GOLD criteria was worse in Group 2 than in Group 1 (p < 0.01).
Table 1.
Baseline Characteristics Of The Two Groups
| All (N = 79) |
Group 1 (n = 37) |
Group 2 (n = 42) |
p-value | |
|---|---|---|---|---|
| Age (years) | 70 ± 9 | 71 ± 9 | 69 ± 9 | 0.304 |
| Male/female (n) | 30/7 | 38/4 | 0.331 | |
| BH (cm) | 162 ± 8 | 161 ± 8 | 164 ± 7 | 0.120 |
| BW (kg) | 60 ± 11 | 59 ± 13 | 61 ± 10 | 0.375 |
| BMI (kg/m2) | 23 ± 4 | 23 ± 4 | 24 ± 3 | 0.702 |
| Hemoglobin (mg/dL) | 14 ± 2 | 14 ± 2 | 14 ± 2 | 0.688 |
| GOLD | <0.001 | |||
| I | 3 (4%) | 3 (8%) | 0 (0%) | |
| II | 30 (38%) | 21 (57%) | 9 (21%) | |
| III | 39 (49%) | 13 (35%) | 26 (62%) | |
| IV | 7 (9%) | 0 (0%) | 7 (17%) | |
| ABCD assessment | 0.562 | |||
| A | 7 | 4 | 3 | |
| B | 57 | 27 | 30 | |
| C | 1 | 1 | 0 | |
| D | 14 | 5 | 9 | |
| Smoking | 0.108 | |||
| Non-smoker | 10 (13%) | 7 (19%) | 3 (7%) | |
| Current smoker | 32 (41%) | 11 (30%) | 21 (50%) | |
| Ex-smoker | 37 (47%) | 19 (51%) | 18 (43%) | |
| Pharmacologic Tx | 0.688 | |||
| Nil | 1 | 0 | 1 | |
| SABD | 2 | 1 | 1 | |
| LABA+ICS | 27 | 14 | 13 | |
| LAMA | 9 | 6 | 3 | |
| LABA+LAMA | 10 | 4 | 6 | |
| LABA+LAMA+ICS | 30 | 12 | 18 | |
| CV comorbidities | ||||
| CHF | 12 | 6 | 6 | 0.811 |
| CAD | 3 | 2 | 1 | 0.597 |
| HTN | 23 | 15 | 8 | 0.036 |
| Arrhythmia | 10 | 5 | 5 | 0.830 |
| Valvular disease | 2 | 1 | 1 | 1.000 |
| Echocardiography | ||||
| LVEF (%) | 67 ± 12 | 66 ± 14 | 68 ± 10 | 0.398 |
| Stroke volume (mL) | 65 ± 22 | 64 ± 22 | 66 ± 22 | 0.659 |
| TRPG (mmHg) | 28 ± 12 | 30 ± 14 | 26 ± 10 | 0.055 |
Note: Data are presented as mean ± standard deviation or number (percentage).
Abbreviations: BH, body height; BW, body weight; BMI, body mass index; COPD, chronic obstructive pulmonary disease; Tx, treatment; SABD, short-acting bronchodilator; LABA, long-acting beta2-agonist; ICS, inhaled corticosteroid; LAMA, long-acting antimuscarinic antagonist; CHF, congestive heart failure; CAD, coronary artery disease; CV, cardiovascular; LVEF, left ventricular ejection fraction; TRPG, tricuspid regurgitation pressure gradient; HTN, hypertension.
Impact Of Peak O2P On Exercise Capacity In COPD Cases
Exercise capacity between the two groups is shown in Table 2. Patients with impaired peak O2P had significantly lower exercise capacity as per the assessment of the peak VO2 (p < 0.001) and peak work rate (p < 0.001).
Table 2.
Impact Of Peak Oxygen Pulse (O2P) On Exercise Capacity
| All (N = 79) | Group 1 (n = 37) | Group 2 (n = 42) | p-value | |
|---|---|---|---|---|
| Peak VO2 (mL/min) | 908.6 ± 273.8 | 1060.8 ± 268.2 | 774.5 ± 200.5 | <0.001 |
| Peak VO2 (% predicted) | 64.9 ± 17.2 | 78.9 ± 12.2 | 52.5 ± 9.8 | <0.001 |
| Peak WR (watt) | 62.2 ± 26.7 | 75.1 ± 25.6 | 50.8 ± 22.2 | <0.001 |
| Peak WR (% predicted) | 70.3 ± 28.2 | 89.4 ± 22.9 | 53.4 ± 20.7 | <0.001 |
Note: Data are presented as mean ± standard deviation.
Abbreviations: VO2, oxygen uptake; WR, work rate.
Impact Of Peak O2P On Circulatory Parameters In COPD Cases
Table 3 shows the circulatory parameters and significant differences can be observed between the two groups. The peak O2P was significantly higher in Group 1 (8.69 ± 1.86 mL/beat) than in Group 2 (6.20 ± 1.36 mL/beat; p < 0.001), while the Group 2 patients had significantly lower AT (p = 0.001) and WE (p = 0.015). However, no significant differences were detected in HR and mean BP at rest and at peak exercise between the two groups.
Table 3.
Impact Of Peak Oxygen Pulse (O2P) On Circulatory Parameters
| All (N = 79) | Group 1 (n = 37) | Group 2 (n = 42) | p-value | |
|---|---|---|---|---|
| Peak O2P (mL/beat) | 7.37 ± 2.03 | 8.69 ± 1.86 | 6.20 ± 1.36 | <0.001 |
| Peak O2P (% predicted) | 78.8 ± 19.0 | 96.0 ± 10.4 | 63.6 ± 9.4 | <0.001 |
| AT (mL/min) | 615.9 ± 139.4 | 667.7 ± 134.0 | 568.0 ± 128.1 | 0.001 |
| AT (% predicted) | 44.2 ± 10.0 | 50.4 ± 9.3 | 38.5 ± 6.8 | <0.001 |
| WE (mL/min/watt) | 8.2 ± 1.9 | 8.7 ± 1.9 | 7.7 ± 1.7 | 0.015 |
| MBP rest (mmHg) | 89.5 ± 11.1 | 87.2 ± 11.8 | 91.4 ± 10.0 | 0.088 |
| MBP peak (mmHg) | 107.6 ± 15.1 | 107.5 ± 15.8 | 107.7 ± 14.7 | 0.953 |
| HR rest (beats/min) | 86.5 ± 12.9 | 85.1 ± 13.2 | 87.8 ± 12.6 | 0.363 |
| HR peak (beats/min) | 123.6 ± 16.6 | 122.4 ± 17.0 | 124.7 ± 16.4 | 0.532 |
Note: Data are presented as mean ± standard deviation.
Abbreviations: AT, anaerobic threshold; WE, work efficiency; MBP, mean blood pressure; HR, heart rate.
Impact Of Peak O2P On Ventilatory Parameters In COPD Cases
The ventilatory parameters are summarized in Table 4. Patients with impaired peak O2P (Group 2) showed lower FEV1 (by numerical value and predicted percentage; p = 0.003 and p < 0.001, respectively), lower FEV1/FVC ratio (p < 0.001), lower VE at peak exercise (p = 0.001), and higher VEQ (p = 0.029) than those with normal peak O2P (Group 1). However, there were no significant differences detected in MIP and MEP between the two groups.
Table 4.
Impact Of Peak Oxygen Pulse (O2P) On Ventilatory Parameters
| All (N = 79) | Group 1 (n = 37) | Group 2 (n = 42) | p-value | |
|---|---|---|---|---|
| FVC (L) | 2.21 ± 0.69 | 2.25 ± 0.71 | 2.18 ± 0.67 | 0.650 |
| FVC (% predicted) | 80.0 ± 19.0 | 84.2 ± 19.0 | 76.3 ± 18.4 | 0.065 |
| FEV1 (L/s) | 1.04 ± 0.42 | 1.18 ± 0.47 | 0.91 ± 0.32 | 0.003 |
| FEV1 (% predicted) | 47.9 ± 16.0 | 56.3 ± 15.9 | 40.5 ± 12.0 | <0.001 |
| FEV1/FVC | 0.47 ± 0.11 | 0.53 ± 0.09 | 0.42 ± 0.10 | <0.001 |
| MIP (cm H2O) | 65.1 ± 26.1 | 64.8 ± 23.6 | 65.3 ± 28.4 | 0.934 |
| MIP (% predicted) | 65.8 ± 25.7 | 67.7 ± 25.4 | 64.1 ± 26.1 | 0.538 |
| MEP (cm H2O) | 109.6 ± 38.1 | 108.8 ± 27.7 | 110.3 ± 45.6 | 0.866 |
| MEP (% predicted) | 59.7 ± 20.6 | 61.7 ± 18.5 | 57.9 ± 22.3 | 0.412 |
| VE rest (L/min) | 12.1 ± 3.1 | 12.3 ± 3.1 | 11.9 ± 3.1 | 0.574 |
| VE peak (L/min) | 32.3 ± 10.3 | 36.2 ± 9.7 | 28.8 ± 9.6 | 0.001 |
| RR rest (breath/min) | 19.1 ± 4.6 | 19.4 ± 4.2 | 18.9 ± 5.0 | 0.654 |
| RR peak (breath/min) | 32.3 ± 7.4 | 34.0 ± 6.5 | 30.8 ± 7.8 | 0.056 |
| VT rest (mL) | 652.8 ± 159.4 | 650.7 ± 146.2 | 654.6 ± 171.9 | 0.913 |
| VT peak (mL) | 1020.0 ± 306.6 | 1091.9 ± 326.3 | 956.7 ± 276.8 | 0.05 |
| VE/VCO2 at AT | 37.0 ± 6.1 | 35.4 ± 6.4 | 38.4 ± 5.6 | 0.029 |
| SpO2 rest (%) | 95.9 ± 2.0 | 96.2 ± 1.9 | 95.7 ± 2.1 | 0.252 |
| SpO2 peak (%) | 92.9 ± 3.6 | 93.5 ± 3.3 | 92.4 ± 3.8 | 0.171 |
| PETCO2 rest (mmHg) | 35.2 ± 5.5 | 35.6 ± 5.6 | 35.0 ± 5.4 | 0.648 |
| PETCO2 peak (mmHg) | 41.0 ± 7.1 | 41.2 ± 7.9 | 40.8 ± 6.4 | 0.778 |
Note: Data are presented as mean ± standard deviation.
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; VE, minute ventilation; RR, respiratory rate; VT, tidal volume; VCO2, carbon dioxide production; SpO2, arterial oxygen hemoglobin saturation; PETCO2, partial pressure of carbon dioxide at the end of exhaled breath.
Dyspnea Score And Health-Related Quality Of Life
Table 5 illustrates the dyspnea scores and HRQL of the two groups. There was no significant difference detected in the dyspnea score between the two groups at rest and at peak exercise. The total score of SGRQ of Group 2 was significantly higher than that of Group 1 (p = 0.029) with a mean difference of 9.2 points.
Table 5.
Impact Of Peak Oxygen Pulse (O2P) On Dyspnea Score And Health-Related Quality Of Life
| All (N = 79) | Group 1 (n = 37) | Group 2 (n = 42) | p-value | |
|---|---|---|---|---|
| Borg CR10, rest (points) | 0.47 ± 0.67 | 0.32 ± 0.63 | 0.60 ± 0.69 | 0.073 |
| Borg CR10, peak (points) | 5.48 ± 1.66 | 5.49 ± 1.63 | 5.48 ± 1.70 | 0.978 |
| SGRQ, total (points) | 36.8 ± 18.6 | 31.9 ± 15.8 | 41.1 ± 19.9 | 0.029 |
| SGRQ, symptom (points) | 42.7 ± 22.7 | 36.8 ± 19.8 | 47.9 ± 24.0 | 0.029 |
| SGRQ, activity (points) | 51.9 ± 20.0 | 46.4 ± 18.0 | 56.6 ± 20.6 | 0.023 |
| SGRQ, impact (points) | 27.2 ± 22.2 | 23.2 ± 19.9 | 30.8 ± 23.6 | 0.129 |
Note: Data are presented as mean ± standard deviation.
Abbreviations: Borg CR10, Borg’s category-ratio 10 scale; SGRQ, Saint George’s Respiratory Questionnaire.
Impact Of Peak O2P On Severe Exacerbation Of COPD
There was a clinically relevant impact of peak O2P on severe exacerbation of COPD (Table 6). After enrollment, the number of hospitalizations in the following year for Group 2 was higher than for Group 1 (0.57 vs 0.22; p = 0.009). Group 2 trended higher for the number of ER visits than Group 1 (0.74 vs 0.38; p = 0.112), but the difference did not reach the level of significance.
Table 6.
Impact Of Peak Oxygen Pulse (O2P) On The Number Of Hospitalizations And Emergency Room (ER) Visits In The Following Year After Enrollment
| All (N = 79) | Group 1 (n = 37) | Group 2 (n = 42) | p-value | |
|---|---|---|---|---|
| Hospitalizations | 0.41 ± 0.968 | 0.22± 0.886 | 0.57 ± 1.016 | 0.009a |
| ER visits | 0.57 ± 1.420 | 0.38 ±1.361 | 0.74 ± 1.466 | 0.112a |
Note: Data are presented as mean ± standard deviation.
Abbreviation: ER, emergency room.
Relationship Between AT And FEV1 With Peak O2P
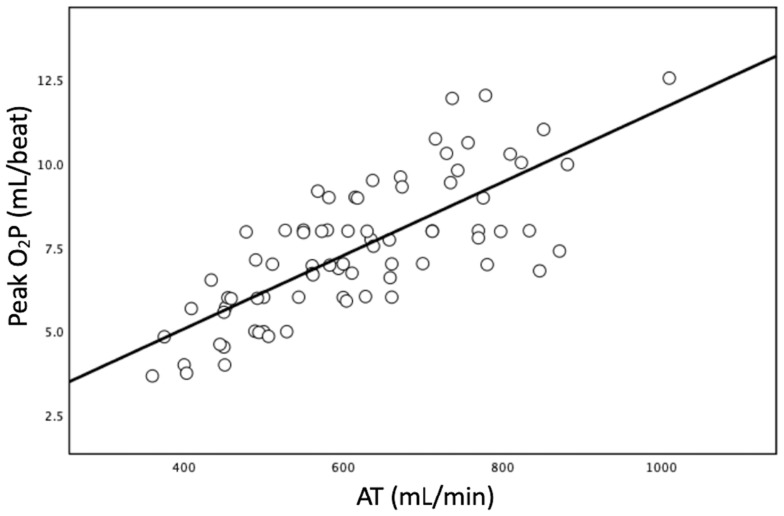
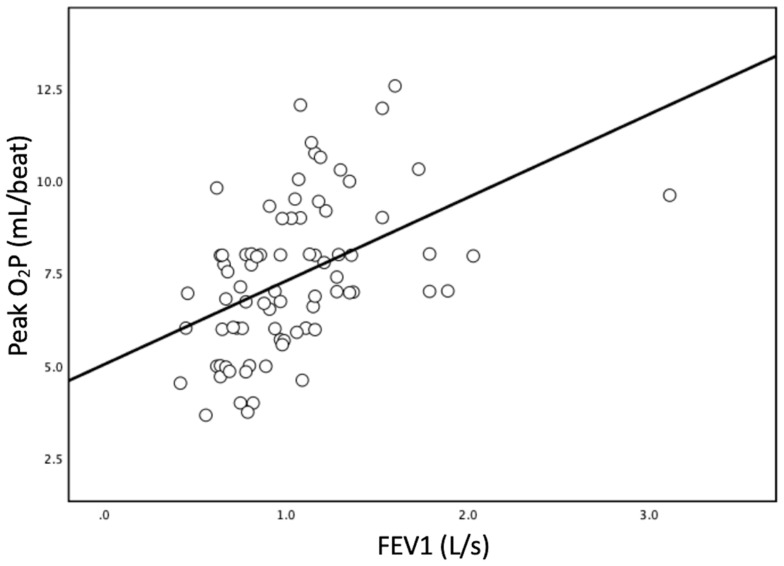
Figure 1 reveals a significant correlation between AT and peak O2P (p < 0.001), showing a strong relationship (Pearson’s r = 0.756). Figure 2 illustrates a significant correlation between FEV1 and peak O2P (p < 0.001), showing a moderate relationship (Pearson’s r = 0.461).
Figure 1.
The simple linear regression between peak oxygen pulse (O2P) and anaerobic threshold (AT). The slant line is calculated using least square regression analysis. (p < 0.001; R2 = 0.566; Pearson’s r = 0.756).
Figure 2.
The simple linear regression between forced expiratory volume in one second (FEV1) and peak oxygen pulse (O2P). The slant line is calculated using least square regression analysis. (p < 0.001; R2 = 0.202; Pearson’s r = 0.461).
Discussion
This study reported some important findings. Patients with impaired peak O2P demonstrated poor exercise capacity, circulatory condition (such as AT or WE), ventilatory condition, HRQL, and COPD-related hospitalizations. The AT (a circulatory parameter) and FEV1 (a ventilatory parameter) exhibited strong and moderate correlations with peak O2P, respectively. Patients with COPD are at an increased risk for cardiovascular dysfunction.11,12 Since O2P is a parameter of SV, it is necessary to understand that cases with impaired peak O2P might be complicated with cardiovascular dysfunction. To the best of our knowledge, this is the first study that comprehensively analyzed the impact of peak O2P on exercise capacity, HRQL, severe exacerbation, and circulatory and ventilator parameters in patients with COPD.
The possible mechanisms of cardiac dysfunction in patients with COPD include lung hyperventilation. Prior published reports have demonstrated the close relationship between lung hyperinflation and O2P.13–15 Hyperinflation leads to elevated intrathoracic pressure and results in reduced venous return, which further leads to impaired left ventricular filling and consequently reduced SV. Moreover, pulmonary hypertension leads to increased resistance to right ventricular (RV) contraction and results in RV dilation. The RV dilation further results in compression of the left ventricle (LV) due to ventricular interdependence. Additionally, the increased pulmonary vascular resistance leads to impaired RV systolic function, and therefore, reduced preload of the left heart. A previous study supported the impact of pulmonary hypertension on cardiac function and showed that low peak O2P in patients with COPD is associated with pulmonary hypertension.11 Finally, there is a high incidence of heart failure and COPD overlap.12 Both conditions share similar risk factors including smoking, aging, and systemic inflammation. Patients with COPD have a 2.57-times higher risk of heart failure as compared to patients without COPD.12 The presence of impaired peak O2P in patients indicates the comorbidity of heart failure in COPD.
Exercise capacity (peak VO2) is an important issue in COPD. Peak VO2 is the most important prognostic factor for patients with COPD as well as heart failure.16 Patients with COPD overlapping with heart failure had poor exercise capacity and higher mortality.17 Since the comorbidity of heart failure is important in COPD, O2P is thus an important parameter in COPD. According to the current study, patients with low peak O2P had poor exercise capacity and HRQL.
This is the first study demonstrating that the circulatory parameter AT has a strong relationship with peak O2P in COPD. AT occurs when the oxygen consumption exceeds the oxygen supply by the cardiovascular system and leads to lactate accumulation and excessive CO2 production.18 Therefore, AT indicates the ability of the cardiovascular system to supply oxygen. It has been reported that a lower AT predicts higher mortality in heart failure patients.19 Since O2P is a marker of SV and impaired O2P suggests inadequate oxygen delivery, it is conceivable that impaired O2P is associated with lower AT.
There are three main impacts of peak O2P on ventilatory parameters. First, lung function is correlated with peak O2P. In this study, patients with impaired peak O2P had lower baseline FEV1. This is the first study demonstrating that FEV1 (a ventilatory parameter) in COPD had a significant correlation with peak O2P (a circulatory parameter), and it was a moderate relationship. This could be explained indirectly by the fact that O2P is inversely associated with lung hyperinflation13-15 and lung hyperinflation is inversely associated with the FEV1 level.20 Second, VE is associated with peak O2P; patients with COPD with impaired peak O2P showed lower VE at peak exercise. This could be inferred from the fact that O2P is inversely associated with hyperinflation,13–15 and progressive dynamic hyperinflation during exercise leads to lower VE during exercise.21 Third, VEQ is associated with peak O2P, i.e., patients with impaired peak O2P have higher VEQ which reflects the patient’s ventilatory efficiency. Since patients with impaired peak O2P had more severe hyperinflation and poor cardiac function,22 they had higher VEQ. In previous studies, patients with higher VEQ had higher mortality.23
HRQL is important in patients with COPD. It is reported that a poor SGRQ score is associated with increased morbidity and mortality in patients with COPD.24 Few studies have investigated the correlation between CPET variables and SGRQ. They have shown that in stable patients with COPD, the FEV1, maximal work rate, and breathing reserve are negatively correlated with SGRQ scores, while maximal VO2 is not significantly associated with SGRQ scores.25 Although it is intuitive to imagine the association between peak O2P and total score of SGRQ, the relationship has not been formally expressed in the literature. A novelty of our study is that the patients with COPD with impaired peak O2P had a higher total score of SGRQ than those with normal peak O2P, and the mean difference was greater than the minimal clinically important difference (4 points). Impairment of O2P in patients with COPD indicates lung hyperinflation and comorbidity with heart failure. Hyperinflation is an important cause of exertional dyspnea in patients with COPD, and it also has a significant impact on exercise capacity, HRQL, and survival.26 Previous studies have shown that the incidence of heart failure in patients with COPD worsened their health status.24,27 These previous reports strengthen the importance of peak O2P measurement in patients with COPD.
Acute exacerbation of COPD leads to significant morbidity and mortality. Prior exacerbation history is a well-established predictor of future exacerbation.1 In the current study, patients with impaired peak O2P had more COPD-related hospitalizations during 1-year follow-up. To our knowledge, peak O2P is a novel predictor of exacerbation. This study established the role of peak O2P in patients with COPD. Patients with impaired peak O2P had more susceptibility to severe exacerbation, thus we should pay more attention to reduce risks such as adjustment of inhaled bronchodilators or corticosteroids. A larger study is needed to validate the role of peak O2P in exacerbation risk.
In the current study, the echocardiographic parameters and cardiovascular comorbidities except hypertension revealed that no significant differences could be detected between the two groups. However, the gold standard of stroke volume and pulmonary artery pressure measurement requires invasive instruments such as a pulmonary artery catheter. In addition, heart failure is a clinical diagnosis and there is no diagnostic tool. In Group 2 patients, the peak O2P may imply the overall conditions of worse hyperinflation, heart failure, and pulmonary hypertension. The cardiopulmonary disorders could result in more COPD-related hospitalizations, worse exercise capacity, HRQL, and circulatory and ventilator parameters.
Limitations Of The Study
There are some limitations in this study. First, we did not measure any lung volume, such as total lung volume, residual volume, etc. in these patients. The hyperinflation could not be shown in the current study. Second, we did not use right heart catheterization in these patients, and the degree of pulmonary hypertension was unavailable. Third, peak O2P indicates SV according to the Fick equation; however, we did not measure the arterial oxygen saturation and mixed venous oxygen saturation, hence the actual oxygen extraction value was unknown. Moreover, a previous study demonstrated that oxygen extraction is constant near peak exercise.8 Therefore, the peak O2P could be a useful parameter of SV near peak exercise. Further studies are required to verify the impact of O2P on mortality, and long-term follow-up of these patients is necessary. Finally, the patients with impaired O2P had poor exercise capacity, HRQL, and more COPD-related hospitalizations. The effect of pulmonary rehabilitation on these patients should be verified.
Conclusions
To the best of our knowledge, this is the first study that comprehensively demonstrated the impact of peak O2P on patients with COPD. Impaired peak O2P was shown to be associated with poor exercise capacity, circulatory function, ventilatory function, HRQL, and COPD-related hospitalizations. The AT and FEV1 showed a significant association with the peak O2P. Additionally, peak O2P is an important parameter in COPD, and could be an indicative parameter of the overall condition of lung hyperinflation, pulmonary hypertension, and heart failure comorbidity.
Acknowledgements
This study was supported by grants from the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCRD-TPE-104-33 and TCRD-TPE-108-RT-4).
Disclosure
The authors have no conflicts of interest in this work.
References
- 1.Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145 [DOI] [PubMed] [Google Scholar]
- 3.Alter P, Watz H, Kahnert K, et al. Airway obstruction and lung hyperinflation in COPD are linked to an impaired left ventricular diastolic filling. Respir Med. 2018;137:14–22. doi: 10.1016/j.rmed.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 4.Blanco I, Piccari L, Barbera JA. Pulmonary vasculature in COPD: the silent component. Respirology. 2016;21(6):984–994. doi: 10.1111/resp.12772 [DOI] [PubMed] [Google Scholar]
- 5.Mezzani A. Cardiopulmonary exercise testing: basics of methodology and measurements. Ann Am Thorac Soc. 2017;14(Supplement_1):S3–S11. doi: 10.1513/AnnalsATS.201612-997FR [DOI] [PubMed] [Google Scholar]
- 6.Murata M, Adachi H, Oshima S, Kurabayashi M. Influence of stroke volume and exercise tolerance on peak oxygen pulse in patients with and without beta-adrenergic receptor blockers in patients with heart disease. J Cardiol. 2017;69(1):176–181. doi: 10.1016/j.jjcc.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 7.Miller MR, Hankinson J, Brusasco, V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 8.Stringer WW, Hansen JE, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol (1985). 1997;82(3):908–912. doi: 10.1152/jappl.1997.82.3.908 [DOI] [PubMed] [Google Scholar]
- 9.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 10.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 11.Thirapatarapong W, Armstrong HF, Bartels MN. Comparing cardiopulmonary exercise testing in severe COPD patients with and without pulmonary hypertension. Heart Lung Circ. 2014;23(9):833–840. doi: 10.1016/j.hlc.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Thomas J, Sadatsafavi M, JM FitzGerald Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. doi: 10.1016/S2213-2600(15)00241-6 [DOI] [PubMed] [Google Scholar]
- 13.Vassaux C, Torre-Bouscoulet L, Zeineldine S, et al. Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur Respir J. 2008;32(5):1275–1282. doi: 10.1183/09031936.00151707 [DOI] [PubMed] [Google Scholar]
- 14.Lammi MR, Ciccolella D, Marchetti N, Kohler M, Criner GJ. Increased oxygen pulse after lung volume reduction surgery is associated with reduced dynamic hyperinflation. Eur Respir J. 2012;40(4):837–843. doi: 10.1183/09031936.00169311 [DOI] [PubMed] [Google Scholar]
- 15.Come CE, Divo MJ, Estépar RS, et al. Lung deflation and oxygen pulse in COPD: results from the NETT randomized trial. Respir Med. 2012;106(1):109–119. doi: 10.1016/j.rmed.2011.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paolillo S, Agostoni P. Prognostic role of cardiopulmonary exercise testing in clinical practice. Ann Am Thorac Soc. 2017;14(Supplement_1):S53–S58. doi: 10.1513/AnnalsATS.201610-818FR [DOI] [PubMed] [Google Scholar]
- 17.Hawkins NM, Virani S, Ceconi C. Heart failure and chronic obstructive pulmonary disease: the challenges facing physicians and health services. Eur Heart J. 2013;34(36):2795–2803. doi: 10.1093/eurheartj/eht192 [DOI] [PubMed] [Google Scholar]
- 18.Wasserman K, Beaver WL, Whipp BJ. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation. 1990;81(1 Suppl):Ii14–Ii30. [PubMed] [Google Scholar]
- 19.Gitt AK, Wasserman K, Kilkowski C, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106(24):3079–3084. doi: 10.1161/01.CIR.0000041428.99427.06 [DOI] [PubMed] [Google Scholar]
- 20.Park J, Lee CH, Lee YJ, et al. Longitudinal changes in lung hyperinflation in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:501–508. doi: 10.2147/COPD.S122909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L, Peng L, Wu B, Bu X, Wang C. Effects of dynamic hyperinflation on exercise capacity and quality of life in stable COPD patients. Clin Respir J. 2016;10(5):579–588. doi: 10.1111/crj.12260 [DOI] [PubMed] [Google Scholar]
- 22.Arbex FF, Alencar MC, Souza A, et al. Exercise ventilation in COPD: influence of systolic heart failure. COPD. 2016;13(6):693–699. doi: 10.1080/15412555.2016.1174985 [DOI] [PubMed] [Google Scholar]
- 23.Ingle L, Sloan R, Carroll S, Goode K, JG Cleland, Clark AL. Abnormalities of the ventilatory equivalent for carbon dioxide in patients with chronic heart failure. Pulm Med. 2012;2012:589164. doi: 10.1155/2012/589164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urff M, Van den Berg JW, Uil SM, Chavannes NH, Damoiseaux RA. Depression and heart failure associated with clinical COPD questionnaire outcome in primary care COPD patients: a cross-sectional study. NPJ Prim Care Respir Med. 2014;24:14066. doi: 10.1038/npjpcrm.2014.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirdamadi M, Rahimi B, Safavi E, Abtahi H, Peiman S. Correlation of cardiopulmonary exercise testing parameters with quality of life in stable COPD patients. J Thorac Dis. 2016;8(8):2138–2145. doi: 10.21037/jtd.2016.07.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanfleteren MJ, Koopman M, Spruit MA, et al. Effectiveness of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease with different degrees of static lung hyperinflation. Arch Phys Med Rehabil. 2018;99(11):2279–2286.e3. doi: 10.1016/j.apmr.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 27.Sundh J, Ställberg B, Lisspers K, Kämpe M, Janson C, Montgomery S. Comparison of the COPD Assessment Test (CAT) and the Clinical COPD Questionnaire (CCQ) in a clinical population. COPD. 2016;13(1):57–65. doi: 10.3109/15412555.2015.1043426 [DOI] [PubMed] [Google Scholar]