Abstract
Background
Evidence on PD-1/PD-L1-directed immune checkpoint inhibitor (ICI) therapy for advanced non-small-cell lung cancer (NSCLC) is mainly based on clinical trials in first- or second-line settings.
Objective
We aimed to investigate response and prognostic factors with special regard to third- or later-line therapy.
Patients and Methods
We retrospectively analyzed all patients who had received ICI monotherapy with nivolumab, pembrolizumab, or atezolizumab for advanced NSCLC. Computed tomography evaluations were analyzed using response evaluation criteria in solid tumors (RECIST, version 1.1). Kaplan–Meier analyses were conducted to calculate progression-free (PFS) and overall (OS) survival; the impact of influencing variables was evaluated using uni- and multivariate Cox-regression analyses.
Results
Among 153 patients (59% men, mean age 66 years), median PFS was 4 months [mo; 95% confidence interval (95% CI) 3–5], OS was 13 mo (10–17), and objective response rate (ORR) was 22%. Therapy line ≥ 3 was associated with significantly inferior PFS (p = 0.003) and OS (p = 0.001). In first-line therapy PFS, OS, and ORR were 7 mo (3–11), 17 mo [9–not evaluable (n.e.)], and 36%; in second-line 4 mo (3–7), 18 mo (13–n.e.) and 19%, and in ≥ third-line 2 mo (1–3), 9 mo (4–12), and 13%. PFS was significantly influenced by PD-L1 expression in first-line therapy (p = 0.006). In ≥ third-line patients, Eastern Cooperative Oncology Group (ECOG) performance status significantly affected PFS and OS (both p < 0.001).
Conclusions
Third- or later-line single-agent anti-PD-1/PD-L1 therapy is less efficacious as compared to first- and second-line treatment. In that setting, ECOG performance status predominates known predictors like PD-L1 expression or presence of an alteration in EGFR or ALK.
Key Points
| Compared to first- or second-line of treatment, NSCLC patients who had received PD-1/PD-L1-directed immune checkpoint inhibitor monotherapy in line ≥ three had significantly inferior PFS and OS. |
| In multivariate analyses among all patients, PFS was significantly influenced by PD-L1 expression and ECOG performance status, for OS only therapy line was shown to have a significant impact. |
| In first-line treated patients, PFS was significantly influenced by PD-L1 expression, while there were no significant multivariate findings for first-line OS and second-line PFS/OS. In ≥ third-line patients, ECOG performance status significantly affected PFS and OS. |
Introduction
Inhibition of programmed death-ligand 1 (PD-L1) or programmed cell death protein 1 (PD-1) has fundamentally changed lung cancer therapy [1]. Currently, literally any patient with advanced non-small-cell lung cancer (NSCLC) without contraindications will—given a sufficiently long survival period—receive immune checkpoint inhibitor (ICI) treatment at some point during the course of disease. Still, only a minority of patients actually benefit from ICI monotherapy, and foreseeing the individual patient’s response is difficult [2].
Since the advent of ICI therapies for NSCLC, ICI monotherapy has been widely applied, especially in second-line settings following progression after first-line chemotherapy [1, 3–6] or in first-line therapy for highly PD-L1-expressing tumors [7]. Recently, combinations of ICI and platinum-based doublet chemotherapy have been established as the first-line standard for stage IV NSCLC [8, 9]. Also, quadruple combinations including bevacizumab have entered daily clinical practice, especially in EGFR (epidermal growth factor receptor) or ALK (anaplastic lymphoma kinase) mutant patients with no more options for tyrosine kinase inhibitor (TKI) therapy [10]. The value of ICI/ICI combination therapy, however, has not yet been fully clarified, but data are promising [11].
Despite these rapidly evolving combination regimens, a considerable percentage of patients will still receive ICI monotherapy, especially heavily pretreated patients in third- or later-line settings who have not received ICI therapy before. Patients in these situations are usually characterized by lower performance status, co-morbidities, and treatment-related toxicities restricting available treatment options. Evidence on known predictors of response like PD-L1 expression, EGFR mutational status, or tumor mutational burden (TMB) is mainly derived from first- or second-line therapy studies [7, 11, 12]. Thus, it appears questionable if those biomarkers have the same prognostic and/or predictive properties in third- or later-line therapy settings.
Our aim was to evaluate whether response to PD-1/PD-L1 ICI monotherapy changes with therapy line and which patient or tumor-related factors are associated with patient outcomes in a real-life setting.
Patients and Methods
One hundred and fifty-three consecutive patients with advanced NSCLC who had received at least one cycle of nivolumab, pembrolizumab, or atezolizumab at the lung cancer unit of Kepler University Hospital Linz and the medical oncology unit of Paracelsus Medical University Salzburg between May 2015 and June 2018 were retrospectively registered.
First-line therapy was defined as primary treatment in a non-curable (e.g., stage IV [13] or not otherwise treatable stage III) setting, not considering previous therapies in potentially curable stages. Patients in stage III disease were eligible to receive ICI therapy and to be included in the study if they were pre-treated by chemo(radio)therapy and another line of chemotherapy was not reasonably feasible. Also, in selected cases upon contraindications to chemotherapy and despite PD-L1 < 50%, a first-line ICI treatment in stage IV disease could be applied after multidisciplinary tumor-board discussion. We excluded patients in clinical trials, on ICI/chemotherapy or ICI/ICI combinations, and those who received ICI for thoracic malignancies other than NSCLC.
Patients were retrospectively followed from ICI therapy initiation to death or censored at the date of the last verified contact. The time of disease progression was retrospectively defined by imaging and death, as well as by the patients’ medical records. In selected cases of considerable clinical benefit, ICI therapy could be applied beyond the determined point of disease progression. Radiological response was routinely assessed by an iodinated contrast medium-enhanced CT scan of the chest and the upper abdomen after four cycles of nivolumab or three cycles of pembrolizumab/atezolizumab, equaling a time interval of 10 or 12 weeks, respectively. Re-staging could be preponed in case of clinical suspicion of disease progression, and additional/alternative imaging modalities like 18F-FDG-PET/CT or cerebral magnetic resonance tomography could be applied if necessary, according to the clinician’s judgment. For this study, radiological response was re-evaluated by an expert thoracic radiologist and graded by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [14] for first and best response (CR—complete remission, PR—partial remission, SD—stable disease, PD—progressive disease). Overall response rate (ORR) was defined as the percentage of patients having reached a best response of CR or PR.
Kaplan–Meier-analyses for PFS and OS were conducted for all patients and according to therapy line (first line, second line, ≥ third line). Results were expressed as median in months [95% confidence interval (CI)], unless otherwise specified. The Kaplan–Meier survival curves were compared statistically using the log rank test, and a p value < 0.05 was regarded as statistically significant.
Evaluation of predictive factors for PFS and OS was conducted applying uni- and multivariate Cox-regression analyses. Variables analyzed in these models were age groups (</≥ 70 years), sex, histological subtype (adeno-, squamous-cell carcinoma), presence of brain metastases, palliative therapy line (1 vs. 2, ≥ 3), ECOG (Eastern Cooperative Oncology Group) performance status (0 vs. 1, 2, 3) and presence of a targetable genetic tumor alteration (ALK, EGFR, ROS). PD-L1 expression on tumor cells was assessed with a 22C3 assay for Autostainer Link 48 by Dako (Agilent Technologies, Santa Clara, CA, USA), whereas a negative PD-L1 status was defined as a proportion of < 1% of viable tumor cells showing membranous staining.
Results
Baseline patient and tumor characteristics for all patients and according to therapy line are shown in Table 1.
Table 1.
Baseline patient and tumor characteristics for all patients and according to therapy line
| All patients (N = 153) | First-line therapy (N = 45) | Second-line therapy (N = 70) | ≥ Third-line therapy (N = 38) | |
|---|---|---|---|---|
| Age (mean, SD) | 66 (11) | 72 (10) | 66 (9) | 61 (12) |
| Age range (years) | 26–90 | 47–90 | 39–85 | 26–81 |
| ECOG status | ||||
| 0 | 45 (29) | 16 (36) | 17 (24) | 12 (29) |
| 1 | 91 (59) | 27 (60) | 44 (63) | 20 (60) |
| 2 | 14 (10) | 1 (2) | 9 (13) | 4 (9) |
| 3 | 3 (2) | 1 (2) | 0 | 2 (2) |
| Sex | ||||
| Female | 62 (41) | 19 (42) | 28 (40) | 15 (40) |
| Male | 91 (59) | 26 (58) | 42 (60) | 23 (60) |
| ICI substance | ||||
| Nivolumab | 80 (52) | 16 (36) | 37 (53) | 27 (71) |
| Pembrolizumab | 58 (38) | 26 (58) | 26 (37) | 6 (16) |
| Atezolizumab | 15 (10) | 3 (7) | 7 (10) | 5 (13) |
| Smoking status | ||||
| Never/≤ 5 py | 20 (13) | 5 (11) | 8 (11) | 7 (18) |
| > 5 py | 123 (80) | 40 (89) | 56 (80) | 27 (71) |
| Unknown | 10 (7) | 6 (9) | 4 (11) | |
| Total py (mean, SD) | 44 (34) | 43 (25) | 48 (39) | 38 (35) |
| Histology | ||||
| Adenocarcinoma | 100 (65) | 19 (42) | 49 (79) | 32 (84) |
| Squamous cell carcinoma | 53 (35) | 26 (58) | 21 (30) | 6 (16) |
| TNM stage | ||||
| III | 19 (12) | 14 (31) | 3 (4) | 2 (5) |
| IV | 134 (88) | 31 (69) | 67 (96) | 36 (95) |
| CNS involvement | 31 (20) | 4 (9) | 16 (23) | 11 (29) |
| Genetic alteration | ||||
| EGFR | 13 (8) | 4 (6) | 9 (24) | |
| ALK | 2 (1) | 2 (5) | ||
| ROS1 | 3 (2) | 3 (4) | ||
| PD-L1 status | ||||
| Not available | 21 (14) | 2 (4) | 10 (14) | 9 (24) |
| Positive | 85 (56) | 30 (67) | 42 (60) | 13 (34) |
| Negative | 47 (31) | 13 (29) | 18 (26) | 16 (42) |
| PD-L1 expression | ||||
| Not available | 24 (16) | 3 (7) | 12 (17) | 9 (24) |
| < 1% | 47 (31) | 13 (29) | 18 (26) | 16 (42) |
| 1–49% | 44 (29) | 13 (29) | 24 (34) | 7 (18) |
| ≥ 50% | 38 (25) | 16 (36) | 16 (23) | 6 (16) |
Figures are given as absolute number and percent within the respective group unless otherwise specified. The numeric discrepancies between PD-L1 status and PD-L1 expression are due to patients with pathologically determined positive PD-L1 status but without exact quantification being reported or with further quantification being impossible (n = 3)
SD standard deviation, ECOG Eastern Cooperative Oncology Group, ICI immune checkpoint inhibitor, py pack years, TNM TNM Classification of Malignant Tumours, CNS central nervous system, EGFR epidermal growth factor receptor, ALK Anaplastic Lymphoma Kinase, ROS1 proto-oncogene tyrosine-protein kinase ROS, PD-L1 Programmed Death-Ligand 1
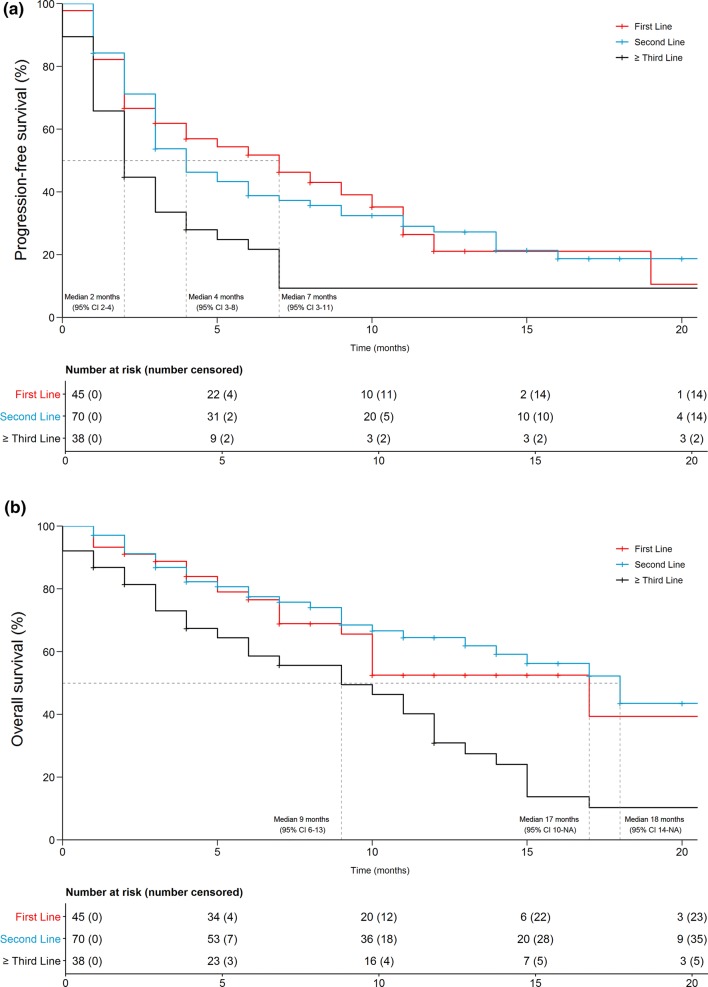
PFS and OS as well as radiological response in all patients and according to the respective therapy line are shown in Table 2. Kaplan–Meier analyses (Fig. 1) showed that therapy line significantly influenced PFS (p = 0.003) and OS (p = 0.001). A univariate Cox-regression analysis confirmed that patients in ≥ third-line treatment had a significant disadvantage concerning PFS (p = 0.005) and OS (p = 0.002). For patients in ≥ third-line therapy, hazard ratio (HR) was 1.97 (1.20–3.21; p = 0.007) for PFS and 1.99 (1.12–3.53; p = 0.019) for OS as compared to first-line therapy. Second-line compared to first-line therapy did not pose a significant risk for inferior PFS [HR 1.02 (0.65–1.60; p = 0.941)] or OS [HR 0.82 (0.46–1.46; p = 0.493)].
Table 2.
Radiological first and best response, objective response rate, progression-free and overall survival in all patients and according to therapy line
| All patients (N = 153) | First-line therapy (N = 45) | Second-line therapy (N = 70) | ≥ Third-line therapy (N = 38) | |
|---|---|---|---|---|
| First response (RECIST) | ||||
| Not available | 34 (22) | 3 (7) | 25 (36) | 6 (16) |
| Complete remission | 1 (1) | 1 (1) | ||
| Partial remission | 25 (16) | 12 (27) | 9 (13) | 4 (11) |
| Stable disease | 40 (26) | 15 (33) | 14 (20) | 11 (29) |
| Progressive disease | 53 (35) | 15 (33) | 21 (30) | 17 (45) |
| Best response (RECIST) | ||||
| Not available | 34 (22) | 3 (7) | 25 (36) | 6 (16) |
| Complete remission | 3 (2) | 1 (2) | 1 (1) | 1 (3) |
| Partial remission | 31 (20) | 15 (33) | 12 (17) | 4 (11) |
| Stable disease | 33 (22) | 11 (24) | 13 (19) | 9 (24) |
| Progressive disease | 52 (34) | 15 (33) | 19 (27) | 18 (47) |
| Objective response (rate in %) | 34 (22) | 16 (36) | 13 (19) | 5 (13) |
| Median progression-free survival (95% CI) | 4 (3, 5) | 7 (3, 11) | 4 (3, 7) | 2 (1, 3) |
| Median overall survival (95% CI) | 13 (10, 17) | 17 (9, –) | 18 (13, –) | 9 (4, 12) |
Figures are given as absolute number and percent within the respective group unless otherwise specified. Objective response rate includes patients with a RECIST best response of complete or partial remission
RECIST Response Evaluation Criteria in Solid Tumors, CI confidence interval
Fig. 1.
Kaplan–Meier curves for progression-free (a) and overall (b) survival according to therapy line. NA not available, CI confidence interval
In the whole patient cohort, the multivariate model (Table 3) revealed that PFS was significantly influenced by PD-L1 status (p = 0.002) and ECOG performance status (p = 0.029). For OS, the only significant variable identified was therapy line (p = 0.025).
Table 3.
Uni- and multivariate analyses for progression-free and overall survival according for all patients
| Variable | Progression-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 70 vs. < 70 years | 0.98 (0.68–1.42) | 0.265 | 1.06 (0.67–1.67) | 0.800 | ||||
| Female vs. male | 0.81 (0.56–1.17) | 0.265 | 0.96 (0.61–1.51) | 0.866 | ||||
| ECOG 1 vs. 0 | 0.85 (0.56–1.29) | 0.441 | 0.81 (0.52–1.27) | 0.359 | 1.25 (0.74–2.10) | 0.407 | ||
| ECOG 2 vs. 0 | 1.88 (1.00–3.52) | 0.049 | 1.01 (0.45–2.24) | 0.987 | 2.66 (1.16–6.11) | 0.021 | ||
| ECOG 3 vs. 0 | 7.38 (2.21–24.67) | 0.001 | 7.13 (1.65–30.73) | 0.008 | 3.51 (0.82–15.09) | 0.092 | ||
| Therapy line 2 vs. 1 | 1.02 (0.65–1.60) | 0.941 | 0.83 (0.47–1.49) | 0.534 | 0.83 (0.47–1.49) | 0.534 | ||
| Therapy line 3 vs. 1 | 2.00 (1.20–3.21) | 0.007 | 2.00 (1.13–3.54) | 0.018 | 2.00 (1.13–3.54) | 0.018 | ||
| PD-L1 status (neg. vs. ≥ 1%) | 1.95 (1.30–2.92) | 0.001 | 2.04 (1.32–3.17) | 0.002 | 1.60 (0.98–2.61) | 0.062 | ||
| Mutational status (pos. vs. neg.) | 1.84 (1.09–3.09) | 0.023 | 1.53 (0.80–2.90) | 0.198 | ||||
| CNS involvement (yes vs. no) | 1.22 (0.79–1.89) | 0.368 | 1.26 (0.74–2.16) | 0.398 | ||||
| Squamous vs. adenocarinoma | 0.88 (0.60–1.29) | 0.495 | 0.93 (0.57–1.50) | 0.750 | ||||
Figures are given as hazard ratio (95% confidence interval), with a ratio > 1 signifying an increased risk of progression/death
Bold values are statistically significant (p < 0.05)
HR hazard ratio, CI confidence interval, ECOG Eastern Cooperative Oncology Group, PD-L1 programmed death-ligand 1, CNS central nervous system
For first-line therapy patients, the multivariate Cox-regression analysis revealed a negative PD-L1 status as significant predictor of PFS (p = 0.006), while for OS no variable was significant. In the second-line setting, no variable had significant impact on either PFS or OS. In third-line therapy, both PFS and OS (both p < 0.001) were significantly determined by ECOG performance status. A significant signal for inferior OS in squamous-cell carcinoma patients in the univariate analysis could not be re-enacted in the multivariate evaluation (Table 4).
Table 4.
Uni- and multivariate analyses for progression-free and overall survival according to therapy line
| First-line therapy | Second-line therapy | Third-line therapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Progression-free survival | ||||||||||||
| Age ≥ 70 vs. < 70 years | 0.99 (0.45–2.14) | 0.985 | 1.27 (0.72–2.23) | 0.404 | 1.09 (0.49–2.45) | 0.045 | ||||||
| Female vs. male | 0.78 (0.37–1.66) | 0.553 | 0.82 (0.47–1.43) | 0.483 | 0.76 (0.38–1.49) | 0.421 | ||||||
| ECOG 1 vs. 0 | 1.55 (0.67–3.60) | 0.305 | 0.71 (0.36–1.38) | 0.310 | 0.83 (0.40–1.75) | 0.629 | 0.83 (0.40–1.75) | 0.629 | ||||
| ECOG 2 vs. 0 | 3.34 (0.40–27.90) | 0.266 | 1.63 (0.69–3.83) | 0.262 | 1.88 (0.58–6.08) | 0.291 | 1.88 (0.58–6.08) | 0.291 | ||||
| ECOG 3 vs. 0 | 4.00 (0.47–33.65) | 0.204 | / | / | 20.7 (2.70–159) | 0.004 | 20.7 (2.70–159) | 0.004 | ||||
| PD-L1 status (neg vs. ≥ 1%) | 3.27 (1.40–7.65) | 0.006 | 3.27 (1.40–7.65) | 0.006 | 1.81 (0.97–3.36) | 0.061 | 1.08 (0.49–2.36) | 0.857 | ||||
| Mutational status (pos. vs. neg.) | / | / | 1.41 (0.60–3.33) | 0.430 | 1.54 (0.72–3.27) | 0.263 | ||||||
| CNS involvement (yes vs. no) | 1.65 (0.57–4.77) | 0.360 | 0.75 (0.39–1.47) | 0.402 | 1.87 (0.89–3.90) | 0.098 | ||||||
| Squamous vs. adenocarinoma | 0.74 (0.35–1.50) | 0.741 | 1.14 (0.64–2.04) | 0.203 | 2.43 (0.98–6.04) | 0.057 | ||||||
| Overall survival | ||||||||||||
| Age ≥ 70 vs. < 70 years | 0.82 (0.32–2.10) | 0.684 | 1.26 (0.59–2.69) | 0.546 | 2.45 (1.01–5.94) | 0.048 | ||||||
| Female vs. male | 1.21 (0.49–2.99) | 0.676 | 1.06 (0.51–2.23) | 0.870 | 0.66 (0.31–1.39) | 0.272 | ||||||
| ECOG 1 vs. 0 | 2.53 (0.83–7.71) | 0.102 | 1.17 (0.47–2.94) | 0.741 | 1.07 (0.48–2.37) | 0.868 | 1.07 (0.48–2.37) | 0.868 | ||||
| ECOG 2 vs. 0 | 3.06 (0.34–27.62) | 0.319 | 2.33 (0.65–8.40) | 0.197 | 6.18 (1.43–26.66) | 0.015 | 6.18 (1.43–26.66) | 0.015 | ||||
| ECOG 3 vs. 0 | / | / | / | / | 58.09 (4.66–724) | 0.002 | 58.09 (4.66–724) | 0.002 | ||||
| PD-L1 status (neg vs. ≥ 1%) | 1.71 (0.66–4.41) | 0.273 | 1.71 (0.74–3.96) | 0.209 | 1.15 (0.50–2.63) | 0.748 | ||||||
| Mutational status (pos. vs. neg.) | / | / | 1.26 (0.38–4.21) | 0.703 | 1.09 (0.48–2.49) | 0.835 | ||||||
| CNS involvement (yes vs. no) | 0.97 (0.22–4.24) | 0.969 | 1.20 (0.51–2.84) | 0.674 | 1.89 (0.77–4.68) | 0.166 | ||||||
| Squamous vs. adenocarinoma | 0.51 (0.20–1.27) | 0.149 | 1.23 (0.57–2.66) | 0.601 | 6.12 (2.14–17.53) | < 0.001 | ||||||
Figures are given as hazard ratio (95% confidence interval), with a ratio > 1 signifying an increased risk of progression/death
Bold values are statistically significant (p < 0.05)
HR hazard ratio, CI confidence interval, ECOG Eastern Cooperative Oncology Group, PD-L1 programmed death-ligand 1, CNS central nervous system
Discussion
Our data suggest that patients receiving single-agent PD-1/PD-L1 ICI therapy in ≥ third-line therapy have significantly inferior PFS and OS compared to first- or second-line treatment. While “traditional” predictive factors like PD-L1 expression are relevant for PFS in first-line treated patients, they seem to have less impact in third- or later-line-treated patients, where only ECOG performance status had significant implications on PFS and OS.
Most phase 3 clinical trials that finally led to the approval of nivolumab, pembrolizumab, and atezolizumab were open for patients with more than one prior line of therapy. As an exception, CheckMate-057 only included second-line patients and CheckMate-017 only allowed for additional TKI therapy or switch maintenance. All those studies had an emphasis on second-line patients, as shown in Table 5 [3–6, 15–20]. Generally, ORR, PFS, and OS decreased with increasing number of previous therapy lines, which is consistent with our findings. Reported outcomes concerning PFS and OS tend to partly surpass our results; however, this may reflect the difference between a real-life cohort and a clinical trial setting. Of note, the mentioned trials included, with very few exceptions, only patients with ECOG performance status 0 or 1. On the contrary, our reported collective comprised 10% of ECOG 2 and 2% of ECOG 3 patients. Thus, an OS of 9 months in a ≥ third-line setting appears to be a very promising result, while the comparatively short PFS of only 2 months requires further considerations: The natural course of disease in a third-line therapy stage IV NSCLC setting has itself never been studied to our knowledge. There was, however, a placebo arm in a study by Shepherd et al. (2005) evaluating erlotinib in chemotherapy-pretreated NSCLC patients, of which nearly 50% were ≥ third-line patients. Patients allocated to the placebo group had an OS of 4.7 months and a PFS of 1.8 months [21]. The question arises how our reported ≥ third-line collective could have witnessed an obviously longer OS, but a PFS not better than placebo. On the one hand, patients who reached such advanced therapy lines tended to be younger, as mean age decreased with therapy line (72 years in first-, 66 years in second-, and 61 years in ≥ third-line patients), whereas ECOG distribution did not show such a trend. On the other hand, the relatively high number of patients with activating alterations in EGFR or ALK in therapy line ≥ 3 (N = 11, 29%) may have influenced outcomes. Surprisingly, however, we did not find a significant association of either PFS or OS with the presence of that known predictive factor in uni- and multivariate analyses. It is likely that patients with such targetable genetic alterations in higher therapy lines may have received less cytotoxic chemotherapies due to available target therapy options and thus still had further and broader treatment options. Of those 11 patients in our collective, 8 (73%) received further therapy, as compared to 11 of 26 (42%) in the non-mutant patient group. As response rates to ICI mono-therapy in more advanced therapy lines [3, 6, 15, 16] as well as in EGFR- or ALK-mutant patients are known to be only modest [12], PFS in ≥ third-line therapy is thus expectedly low. However, in our comparably young and frequently EGFR-mutant ≥ third-line collective, it seems that a considerable OS could still be attained with subsequent chemo- or target therapies after progression on ICI treatment.
Table 5.
Overview of relevant clinical phase 3 trials (framed) as well as trials reporting advanced therapy-line outcomes for single-agent PD-L1/PD-1 directed monotherapy in non-small-cell lung cancer
| Study name | Phase | Substance/comparator | Histological subtype | Advanced therapy line patients (%) | Reported outcomes with emphasis on advanced therapy lines |
|---|---|---|---|---|---|
| CheckMate-017 [4] | 3 | Nivolumab/docetaxel | Squamous NSCLC progressing after first-line chemotherapy | 271 (100%) second-line patients |
Median OS for nivolumab 9.2 mo (7.3–13.3) vs. 6 mo (5.1–7.3) for docetaxel; ORR 20% for nivolumab vs. 9% for docetaxel No patients in therapy line > 2 |
| CheckMate-057 [3] | 3 | Nivolumab/docetaxel | Non-squamous NSCLC after doublet platinum-based chemotherapy | 515 (89%) second line, 66 (11%) third line |
Median OS for nivolumab 12.2 mo (9.7–15), 9.4 mo (8.1–10.7) for docetaxel; ORR 19% for nivolumab vs. 12% for docetaxel Second-line therapy: OS HR for nivolumab vs. docetaxel 0.69 (0.56–0.85); third-line therapy: 1.34 (0.73–2.43) |
| KEYNOTE-010 [5] | 2/3 | Pembrolizumab (2 or 10 mg/kg)/docetaxel | Previously treated PD-L1-positive, advanced NSCLC | 713 (69%) second-line, 210 (20%) third- and 90 (8%) fourth-line patients | Median OS for pembrolizumab 2 mg/kg 10.4 mo (9.4–11.9), 12.7 mo (10.0–17.3) for pembrolizumab 10 mg/kg and 8.5 mo (7.5, 9.8) for docetaxel. No therapy-line specific outcomes reported |
| OAK [6] | 3 | Atezolizumab/docetaxel | Previously treated NSCLC | 210 (25%) third-line patients | OS HR for atezolizumab vs. docetaxel 0.71 (0.59–0.86) in second line and 0.8 (0.57–1.12) in third line. Median OS for atezolizumab 15.2 mo in third line, 12.8 mo in second line |
| KEYNOTE-001 [15] | 1 | Pembrolizumab | Advanced NSCLC | 74 (14.9%) second line, 119 (24%) third line, 106 (21.4%) fourth line, 102 (20.6%) < fourth line |
PD-L1 ≥ 50% previously treated: ORR 43.9%; treatment naïve: ORR 50% PD-L1 1-49% previously treated: ORR 15.6% (8.3–25.6); treatment naïve: ORR 19.2% PD-L1 < 1% previously treated: ORR 9.1%; treatment naïve: ORR 16.7% |
| BIRCH study [16] | 2 | Atezolizumab | Advanced NSCLC | 142 (21%) first line, 271 (41%) second line, 254 (38%) ≥ third line | First-line therapy: ORR 22%, OS 20.1 mo (20.1–n.e.); second-line: ORR 19%, OS 15.5 mo (12.3–n.e.), ≥ third-line: ORR 18%, OS 13.2 mo (10.3–17.5). EGFR or ALK alterations in the subgroups: 11%, 8%, and 7%, respectively |
| POPLAR [17] | 2 | Atezolizumab/docetaxel | Previously treated NSCLC | 189 (66%) second- and 98 (34%) third-line patients | Atezolizumab: 12.6 mo (9.7–16.4), PFS 2.7 mo (0.72–1.23), ORR 15%. Docetaxel: OS 9.7 (8.6–12), PFS 3 mo (0.72–1.23), ORR 15%. Results stratified according to therapy line were not reported |
| MDX1106-03 [18] | 1 | Nivolumab (1, 3, or 10 mg/kg) | Previously treated advanced NSCLC | 59 (45.7%) second line, 70 (54.3%) ≥ third line | Median OS for all patients and doses: 9 mo (2.8–12.4), ORR: 17.1%. ORR for ≥ third-line patients: 21% |
| CheckMate-063 [19] | 2 | Nivolumab | Advanced pretreated (≥ 2 lines) squamous NSCLC | 41 (35%) third line, 52 (44%) fourth line, 24 (21%) ≥ fifth line |
Median OS for all patients: 8.2 mo (6.1–10.9), PFS 1.9 mo (1.8–3.2), ORR: 17.5% No results according to therapy line reported |
| ATLANTIC [20] | 2 | Durvalumab | Advanced NSCLC, ≥ 2 previous therapy lines | 179 (40%) third line, 121 (27%) fourth line, 144 (32%) ≥ fifth line |
EGFR/ALK pos. PD-L1 < 25%: ORR 3.6%, OS 9.9 mo (4.2–13), PFS 1.9 mo (1.8–1.9) EGFR/ALK pos. PD-L1 ≥ 25%: ORR 12.2%, OS 13.3 mo (8.1–), PFS 1.9 mo (1.8–3.6) EGFR/ALK neg. PD-L1 < 25%: ORR 7.5%, OS 9.3 mo (5.9–10.8), PFS 1.9 (1.8–1.9) EGFR/ALK neg. PD-L1 ≥ 25%: ORR 16.4%, OS 10.9 mo (8.6–13.6), PFS 3.3 mo (1.9, 3.7) EGFR/ALK neg. PD-L1 ≥ 90%: ORR 30.9%, OS n.r., PFS 2.4 mo (1.8–5.5) |
NSCLC non-small-cell lung cancer, OS overall survival, M months, OS overall survival, PFS progression-free survival, HR hazard ratio, ORR overall response rate, PD-L1 programmed death-ligand 1, EGFR epidermal growth factor receptor, ALK anaplastic lymphoma kinase
Of interest, the ATLANTIC study on durvalumab evaluated a very similar patient collective of heavily pretreated and frequently EGFR- or ALK-positive patients, and reported results very similar to ours (Table 5) [20]. Also, Lin et al. recently published data on a real-world ICI monotherapy cohort of 74 patients receiving nivolumab or pembrolizumab, of which 41% were EGFR mutant, 49% had an ECOG status ≥ 2, and 69% received ICI therapy in ≥ third-line therapy. The authors reported an ORR of 32%, PFS was 1.8, and OS 7.8 months. In a multivariate Cox-proportional hazards analysis, ECOG ≥ 2 significantly influenced PFS (HR 9.13) and OS (HR 14.72), whereas ICI therapy in ≥ third-line therapy did not significantly impact PFS and OS. Analogously to our reported results, Lin et al. found no meaningful influence of EGFR mutation on the cohort outcomes, with a HR of 2.00 (p = 0.022—univariate) and 1.26 (p = 0.534—multivariate) for PFS and 1.07 for OS (univariate), though there expectedly was a significantly lower treatment response in the EGFR-mutant group with an odds ratio of 0.09 (p = 0.043) [22].
Our study has several limitations. The retrospective design and the relatively small sample size together with numeric differences between the subgroups limited the significance of subgroup analyses. Also, the inclusion criteria may have limited the study results, and a relatively high percentage of stage III patients received first-line mono-ICI treatment and a few patients also received such therapy due to contraindications to chemotherapy despite a PD-L1 expression < 50%. Another possible limitation is that the re-staging schedule differed slightly between nivolumab (10 weeks) and pembrolizumab/atezolizumab (12 weeks). As 71% of patients in ≥ third-line treatment received nivolumab, a shorter PFS could partly be explained by that fact. Besides those methodological limitations, the current therapeutic landscape in NSCLC has clearly shifted away from second- or later-line ICI application, as reported in this cohort, to first-line therapy, either in combination with chemotherapy or alone in highly PD-L1-expressing tumors. In line with these recent developments, our reported results support ICI application earlier rather than later in therapy. Still, advances in molecular or clinical characterization of NSCLC patients may again alter the current therapeutic approach, so that our present data could be of value for future considerations.
We conclude that the efficacy of single-agent PD-1/PD-L1 therapy is lower when applied in more advanced therapy lines (≥ 3). The prognostic value of “traditional” biomarkers like PD-L1 expression or presence of a targetable alteration in EGFR or ALK seems to diminish in third or later therapy lines, where PFS and OS were mainly determined by patient performance status.
Acknowledgements
Open access funding provided by Kepler Universitätsklinikum Linz.
Compliance with Ethical Standards
This study was conducted in accordance with the Declaration of Helsinki and in an entirely retrospective fashion. There was no experimental approach, no additional patient contact, and only patient data assessed in clinical routine were analyzed. Thus, in line with the institutional guidelines, the local ethics committee was not concerned with this study and no written informed consent was obtained from the patients. Patient data were collected in an anonymized fashion and securely electronically stored in a way that only the authors had access to the data. No identifiable patient data have or will ever be published by the authors.
Funding
No external funding was used in the preparation of this article.
Conflict of interest
DL has received travel/accommodation funding from Roche and Merck Sharp & Dohme. FH has received travel/accommodation funding from Bristol-Myers Squibb, Roche, and Merck Sharp & Dohme. GR has served as consultant/advisor to Roche, has received speakers’ honoraria from Bristol-Myers Squibb and Roche, and has received travel/accommodation and research funding from Roche. AH has received travel/accommodation funding from Roche. RW has received speakers’ honoraria and travel/accommodation funding from and served as consultant/advisor to Roche, Merck Sharp & Dohme and Bristol-Myers Squibb. EB has received speakers’ honoraria from and served as consultant/advisor to Roche, Merck Sharp & Dohme, and Bristol-Myers Squibb; he has received travel/accommodation funding from Roche and Merck Sharp & Dohme. GH has served as consultant/advisor to Roche, Merck Sharp & Dohme, and Bristol-Myers Squibb. RG has received speakers’ honoraria and research funding from Roche, Merck Sharp & Dohme, and Bristol-Myers Squibb, he has served as consultant/advisor to Roche and Bristol-Myers Squibb, and has received travel/accommodation funding from Roche. BL has received speakers’ honoraria from and has served as consultant/advisor to Roche, Merck Sharp & Dohme, and Bristol-Myers Squibb. KA, MG, and BK declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Data availability statement
The datasets analyzed during the current study cannot be made publicly available, as they contain possibly identifiable patient data.
Contributor Information
David Lang, Phone: +43 5 7680 83 73797, Email: lunge@kepleruniklinikum.at, Email: david.lang@kepleruniklinikum.at.
Florian Huemer, Email: onkologie.salzburg@salk.at.
Kaveh Akbari, Email: radiologie@kepleruniklinikum.at.
Marcel Granitz, Email: radiologie@salk.at.
Georg Hutarew, Email: pathologie@salk.at.
References
- 1.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 2.Prelaj A, Tay R, Ferrara R, Chaput N, Besse B, Califano R. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer. 2019;106:144–159. doi: 10.1016/j.ejca.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 6.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 10.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 11.Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma. JAMA Oncol. 2018;4:210. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung Cancer. J Thorac Oncol. 2017;12:1109–21. doi: 10.1016/j.jtho.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 16.Peters S, Gettinger S, Johnson ML, Jänne PA, Garassino MC, Christoph D, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH) J Clin Oncol. 2017;35:2781–2789. doi: 10.1200/JCO.2016.71.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 18.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall survival and long-term safety of nivolumab (anti–programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garassino MC, Cho B-C, Kim J-H, Mazières J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19:521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 22.Lin Shu-Yung, Yang Ching-Yao, Liao Bin-Chi, Ho Chao-Chi, Liao Wei-Yu, Chen Kuan-Yu, Tsai Tzu-Hsiu, Hsu Chia-Lin, Hsu Wei-Hsun, Su Kang-Yi, Chang Yih-Leong, Lee Jih-Hsiang, Lin Chia-Chi, Shih Jin-Yuan, Yang James Chih-Hsin, Yu Chong-Jen. Tumor PD-L1 Expression and Clinical Outcomes in Advanced-stage Non-Small Cell Lung Cancer Patients Treated with Nivolumab or Pembrolizumab: Real-World Data in Taiwan. Journal of Cancer. 2018;9(10):1813–1820. doi: 10.7150/jca.24985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study cannot be made publicly available, as they contain possibly identifiable patient data.