Abstract
Mucogingival surgery has the objective to obtain coverage of the recession, with a favorable long-term prognosis, but also to minimize postoperative problems and surgery-related risks. Human amniotic membrane is increasingly employed for periodontal tissue repair in order to promote epithelialization and to reduce pain and scar tissue formation. A 38-year-old female patient reports dental hypersensitivity resulting from gingival recession affecting tooth 4.1. A mucogingival surgical procedure was proposed and a partial-thickness flap of epithelial-connective tissue was harvested from the palate and grafted on to the receiving site. The amniotic membrane was positioned at the donor site to reduce postoperative morbidity and to encourage rapid palatal healing. One week after the application of HAM (human amniotic membrane), the wound was healed and 1 month after the donor site was completely re-epithelialized. The present case report suggests that in the surgical treatment of gingival recession with palatal epithelial-connective tissue graft, HAM promotes rapid epithelialization of the palatal donor site wound with a reduction in morbidity.
Keywords: gingival recession, amniotic membrane, palatal epithelial-connective tissue, human tissue
Introduction
Gingival recession associated with root abrasion and/or caries is found increasingly often in daily clinical practice.1 Patients who turn to the clinician are motivated by ever-greater aesthetic demands and by discomfort closely related to root hypersensitivity.1 It is precisely in this context that mucogingival surgery comes to the aid of the expert clinician attentive to the patient’s needs. Gingival recession is defined as the apical displacement of the gingival margin from its physiological position, 1–2 mm coronal to the cementoenamel junction (CEJ), with pathological exposure of root surface.2 The teeth mainly affected are the canines and upper and lower premolars owing to the presence of muscle insertions near gingival margins or, in the case of involvement of the lower incisors, due to thin cortical bone. The most frequent causes of gingival recession, such as incorrect brushing, superficial frenula or root vestibularization following orthodontic treatment, are often associated with a thin gingival phenotype.3 Once the aetiology of the recession has been identified, whether traumatic, bacterial or mixed, the actual response to the patient’s needs will depend solely on the clinician’s technical expertise and the surgical rationale adopted; the key objective is, therefore, to obtain coverage of the recession, with a favorable long-term prognosis, but also to minimize postoperative problems and surgery-related risks.4
The human amniotic membrane (HAM) is increasingly employed in oral surgery for periodontal tissue repair.5 HAM is the innermost avascular layer of the placenta consisting of five distinct layers: the epithelium, which is composed of a single layer of cells nearest to the fetus and directly in contact with amniotic fluid, the basement membrane, consisting of reticular fibers, the compact layer, the fibroblast layer, and the spongy layer. The spongy layer is gelatinous and allows the separation of the amniotic and chorionic membranes. Since the first clinical application reported in 1910 for skin transplantation,6 several properties of the amniotic membrane have been reported. HAM has anti-inflammatory and anti-microbial properties, as well as low immunogenicity; it promotes epithelialization and reduces pain and scar tissue formation.7–9 Several cytokines and growth factors have been identified in HAM, such as interleukin-10, transforming growth factors (TGF-α, -β1, -β2), epidermal growth factor (EGF), keratinocyte growth factor (KGF), and basic fibroblast growth factor (bFGF).10,11 Moreover, the presence of structural collagen types creates an important substrate for cell ingrowth and wound healing.12–14
In oral surgery, periodontal and peri-implant surgery, cleft palate and mucosal tumor reconstruction are some of the applications of HAM reported in the literature with promising results.5 This report describes a case of Miller class III gingival recession treated with a palatal epithelial-connective tissue autograft and HAM. HAM was used for the reconstruction of the palatal donor site in order to reduce postoperative morbidity. The patient has provided written informed consent for publication of the case details and images. Institutional approval is not required for publication of this case report.
Case Report
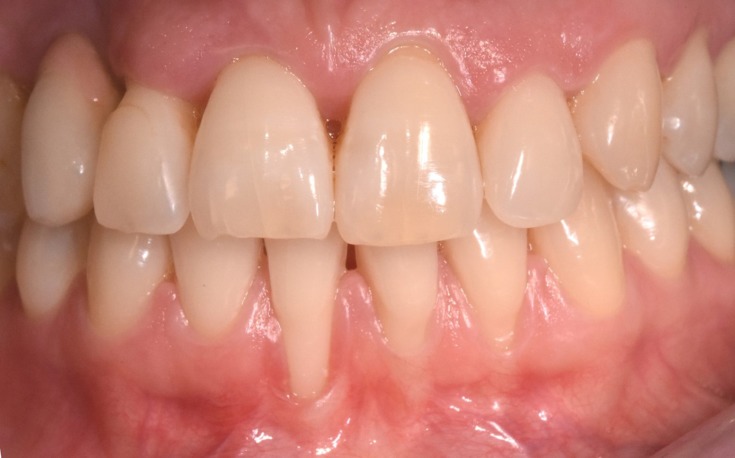
A 38-year-old female patient, non-smoker with good oral hygiene, was referred to the private dental practice of Dr Martelloni M. because of discomfort when smiling (one tooth of the lower arch teeth being longer than the others) and dental hypersensitivity resulting from gingival recession affecting tooth 4.1 (Figure 1), which also caused mild pain during brushing. The recession was caused by brushing and thin phenotype.
Figure 1.
Gingival recession in tooth 4.1.
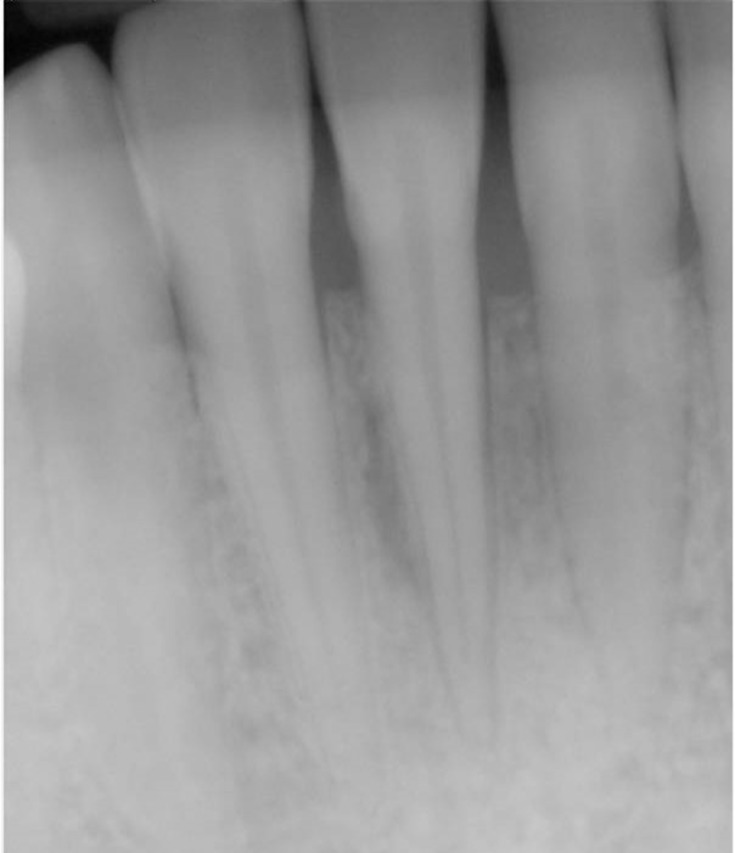
Physical examination using a periodontal probe revealed the probing depth, and slight reabsorption of interproximal bone peaks was noted which was confirmed by intraoral radiography (Figure 2). This led us to define the recession as Miller class III for tooth 4.1 and Miller class I for teeth 3.1 and 3.2.15
Figure 2.
Intraoral dental X-rays showing reabsorption of interproximal bone peaks.
Tooth 4.1 also showed a 5mm recession from the CEJ with a 2mm buccal probing depth, while 3.1 and 3.2 had a 3mm and 2mm recession, respectively, from their CEJ and 1mm probing depth. The tissues were not inflamed but muscular traction was evident by pulling down the lower lip. In view of these considerations, a mucogingival surgical procedure was proposed to the patient. The use of HAM to cover the palatal wound caused by the harvesting of the graft needed to solve the gingival recession was discussed and accepted by the patient.
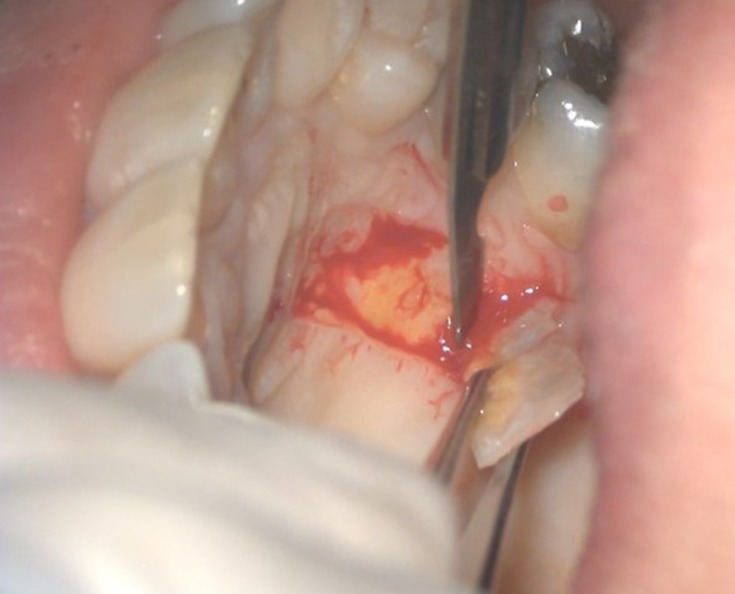
The surgical procedure was performed by means of a free gingival graft on tooth 4.1, i.e. the tooth with its root most exposed and with the complete absence of keratinized tissue. A bilaminar procedure with a coronally advanced flap (CAF) was performed on the adjacent teeth, at the same time releasing muscle insertions. A partial-thickness flap of epithelial-connective tissue measuring approximately 20mm was harvested from the palate (Figure 3) and grafted on to the receiving site16 with Vicryl 5-0 suture absorbable.
Figure 3.
Removal of epithelial-connective tissue from the palate.
The graft was deepithelialised throughout its length except at the point of 4.1 where it was free-grafted (Figure 4).
Figure 4.
Epithelial-connective tissue graft.
Placenta is sourced from donors undergoing cesarean sections and processed shortly after retrieval at “Fondazione Banca dei Tessuti di Treviso”, a tissue bank. In the tissue bank laboratories, HAM is carefully detached from the chorion and rinsed with sterile saline solution to remove residual blood. The membrane is flattened on a nitrocellulose membrane filter (Merck Millipore), with its stromal/mesenchymal side facing down, in contact with the filter. HAM is then immersed in a cocktail of antibiotics including vancomycin 100 µg/mL (Hospira), meropenem 200 µg/mL (Fresenius Kabi Italia), and gentamicin 200 mg/mL (Fisiopharma) at +4°C for 24 hrs in sterile conditions, validated for human tissues.17,18 HAM is then partitioned into 3×3 cm2 patches and cryopreserved in a BASE medium (Alchimia) supplemented with 10% human serum albumin and 10% cryoprotectant dimethyl sulfoxide (DMSO, Cryo Sure, WAKChemie Medical GmbH).
Microbiological analyses are performed at several stages throughout the process and only HAMs without microbial contamination are considered suitable for implants.
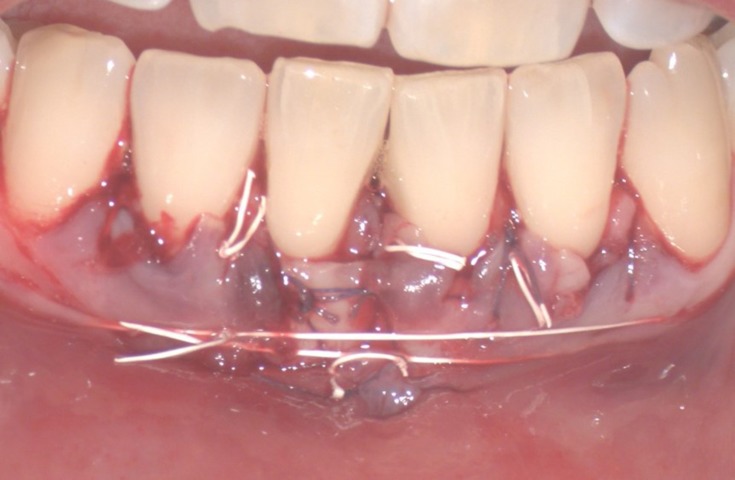
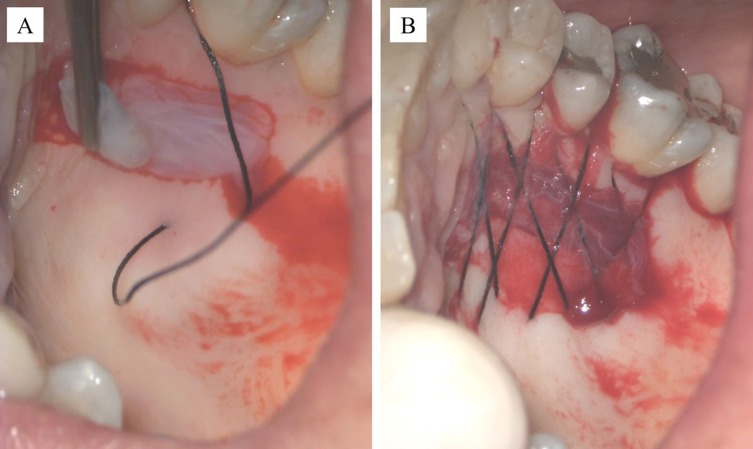
After thawing, HAM mesenchymal side was positioned at the donor site and secured using horizontal mattress sutures with 3–0 silk non-absorbable suture (Figure 5A and B), to reduce postoperative morbidity and to encourage rapid palatal healing.
Figure 5.
Positioning the amniotic membrane at the donor site (A) and horizontal mattress sutures (B).
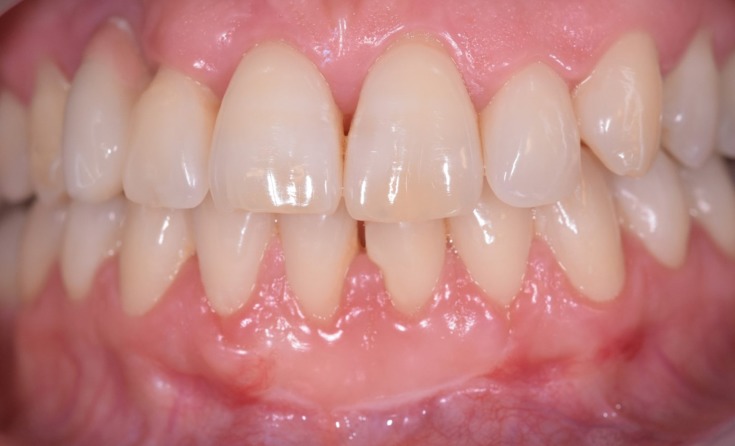
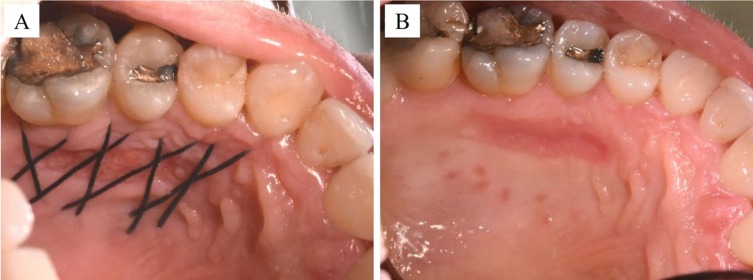
One week after the application of HAM, we notice the closure of the wound as well as no bleeding was detected. Results at 30 days showed not only a positive outcome as regards the resolution of the treated recession (Figure 6), but also the absence of infection and complete reepithelialization of the palate treated with HAM (Figure 7A and B). Furthermore, the patient reported that she had not experienced pain in the days following the procedure and had not taken painkillers.
Figure 6.
Resolution of gingival recession 30 days after the procedure.
Figure 7.
Reepithelialization of the palate 14 days after HAM grafting, before (A) and after (B) removal of stitches.
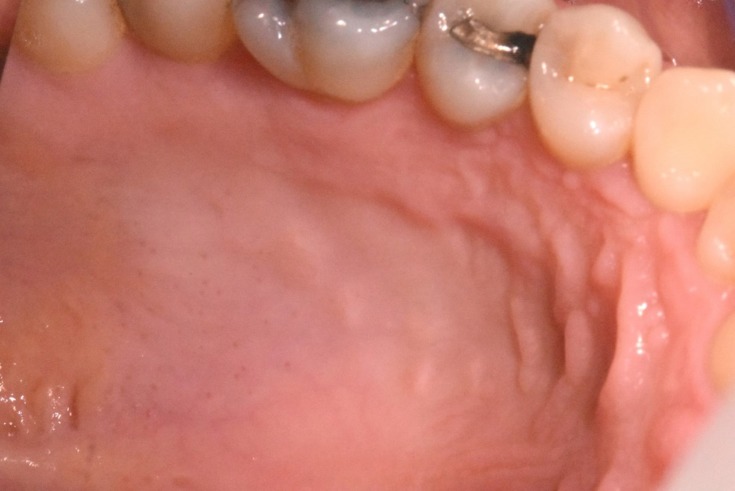
Thirty days after the procedure, the palate was completely reconstructed with no signs of inflammation or infection (Figure 8).
Figure 8.
Appearance of the palate 30 days after the procedure.
The follow-up visits were performed every 2 months until the eighteenth month without relapse.
Discussion
The present case report suggests that in the surgical treatment of gingival recession with palatal epithelial-connective tissue graft, HAM may promote rapid epithelialization of the palatal donor site wound with a reduction in morbidity. The case described was treated with two combined techniques: a bilaminar procedure with CAF and a free gingival graft. Both procedures resulted in a good outcome and a favourable long-term prognosis. We were in fact able to operate on all the incisors at the same time, achieving excellent alignment and with the additional disinsertion of all the relevant fibres of the labial frenulum.
The regenerative properties of HAM have been widely demonstrated in the literature and numerous studies have been conducted on the use of HAM in repairing oral tissue defects. Amnion allograft in conjunction with coronally advanced flap has been used in the management of gingival recession with encouraging results, also in comparison with chorion membrane or platelet-rich fibrin.19–23 The use of amniotic membrane alone was not considered in the present clinical case in the treatment of gingival recession since there was no keratinized tissue, i.e. the fibrous part. To obtain substantial thickening, removal of connective epithelium from the palate was therefore necessary, which can, however, involve patient discomfort and postoperative morbidity.24 Our results showed how the use of amniotic membrane could promote the healing of the palatal wound, reducing post-operational pain. Moreover, the patient did not assume any drugs during the recovery, while in our experience patients undergoing such medical procedure usually ask for the aid of painkillers, sometimes even opioids, for at least 1 week after surgery. This result may be attributed to the properties of HAM, in particular to its content of cytokines and growth factors which contribute to pain reduction.9
The results obtained in this case report suggest conducting a clinical trial comparing the results obtained with and without the use of HAM in reconstructing the palatal donor site.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Susin C, Dalla Vecchia CF, Oppermann RV, Haugejorden O, Albandar JM. Periodontal attachment loss in an urban population of Brazilian adults: effect of demographic, behavioral, and environmental risk indicators. J Periodontol. 2004;75(7):1033–1041. doi: 10.1902/jop.2004.75.7.1033 [DOI] [PubMed] [Google Scholar]
- 2.Patel A, Chapple I. Periodontal Aspects of Esthetic Dentistry: Managing Recession Defects. 2015:10 1016/B978–0–7234–5558–5.00006–3. [Google Scholar]
- 3.Slutzkey S1, Levin L. Gingival recession in young adults: occurrence, severity, and relationship to past orthodontic treatment and oral piercing. Am J Orthod Dentofacial Orthop. 2008;134(5):652–656. doi: 10.1016/j.ajodo.2007.02.054 [DOI] [PubMed] [Google Scholar]
- 4.Oates TW, Robinson M, Gunsolley JC. Surgical therapies for the treatment of gingival recession: a systematic review. Ann Periodontol. 2003;8(1):303–320. doi: 10.1902/annals.2003.8.1.303 [DOI] [PubMed] [Google Scholar]
- 5.Fénelon M, Catros S, Fricain JC. What is the benefit of using amniotic membrane in oral surgery? A comprehensive review of clinical studies. Clin Oral Investig. 2018;22(5):1881–1891. doi: 10.1007/s00784-018-2457-3 [DOI] [PubMed] [Google Scholar]
- 6.Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307. [Google Scholar]
- 7.Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003–1005. doi: 10.1016/S0140-6736(81)91212-5 [DOI] [PubMed] [Google Scholar]
- 8.Kjaergaard N1, Hein M, Hyttel L, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94(2):224–229. doi: 10.1016/S0301-2115(00)00345-6 [DOI] [PubMed] [Google Scholar]
- 9.Mermet I, Pottier N, Sainthillier JM, et al. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen. 2007;15(4):459–464. doi: 10.1111/wrr.2007.15.issue-4 [DOI] [PubMed] [Google Scholar]
- 10.Hao Y. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348–352. doi: 10.1097/00003226-200005000-00018 [DOI] [PubMed] [Google Scholar]
- 11.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20(3):173–177. doi: 10.1076/0271-3683(200003)2031-9FT173 [DOI] [PubMed] [Google Scholar]
- 12.Aplin JD, Campbell S, Allen TD. The extracellular matrix of human amniotic epithelium: ultrastructure, composition and deposition. J Cell Sci. 1985;79:119–136. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of sub-chains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea and conjunctiva. Cornea. 1999;18:73–79. doi: 10.1097/00003226-199901000-00013 [DOI] [PubMed] [Google Scholar]
- 14.Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24(10):299–307. [PubMed] [Google Scholar]
- 15.Miller PD Jr. A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5(2):8–13. [PubMed] [Google Scholar]
- 16.Holbrook T, Ochsenbein C. Complete coverage of the denuded root surface with a one-stage gingival graft. Int J Periodontics Restorative Dent. 1983;3(3):8–27. [PubMed] [Google Scholar]
- 17.Serafini A, Riello E, Trojan D, et al. Evaluation of new antibiotic cocktails against contaminating bacteria found in allograft tissues. Cell Tissue Bank. 2016;17(4):619–628. doi: 10.1007/s10561-016-9581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montagner G, Trojan D, Cogliati E, Manea F, Vantini A, Paolin A. Stability analysis of the antibiotic cocktail used by Treviso Tissue Bank Foundation for tissues decontamination. Cell Tissue Bank. 2018;19:721–726. doi: 10.1007/s10561-018-9725-y [DOI] [PubMed] [Google Scholar]
- 19.Shah R, Sowmya NK, Mehta DS. Amnion membrane for coverage of gingival recession: A novel application. Contemp Clin Dent. 2014;5(3):293–295. doi: 10.4103/0976-237X.137900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborthy S, Sambashivaiah S, Kulal R, Bilchodmath S. Amnion and Chorion Allografts in Combination with Coronally Advanced Flap in the Treatment of Gingival Recession: A Clinical Study. J Clin Diagn Res. 2015;9(9):ZC98–ZC101. doi: 10.7860/JCDR/2015/12971.6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty SS, Chatterjee A, Bose S. Bilateral multiple recession coverage with platelet-rich fibrin in comparison with amniotic membrane. J Indian Soc Periodontol. 2014;18(1):102–106. doi: 10.4103/0972-124X.128261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esteves J, Bhat KM, Thomas B, Varghese JM, Jadhav T. Efficacy of human chorion membrane allograft for recession coverage: a case series. J Periodontol. 2015;86(8):941–944. doi: 10.1902/jop.2014.140025c [DOI] [PubMed] [Google Scholar]
- 23.Agarwal SK, Jhingran R, Bains VK, Srivastava R, Madan R, Rizvi I. Patient-centered evaluation of microsurgical management of gingival recession using coronally advanced flap with platelet-rich fibrin or amnion membrane: a comparative analysis. Eur J Dent. 2016;10(1):121–133. doi: 10.4103/1305-7456.175686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gobbato L, Nart J, Bressan E, Mazzocco F, Paniz G, Lops D. Patient morbidity and root coverage outcomes after the application of a subepithelial connective tissue graft in combination with a coronally advanced flap or via a tunneling technique: a randomized controlled clinical trial. Clin Oral Investig. 2016;20(8):2191–2202. doi: 10.1007/s00784-016-1721-7 [DOI] [PubMed] [Google Scholar]