Abstract
Background
Exercise‐induced bronchoconstriction (EIB), a strong positive predictor of asthma, becomes progressively less frequent with age. Although asthma tends to become less common only in boys during adolescence, sex differences in EIB, especially in preschoolers, remain unclear. To find EIB for early diagnosis and intervention asthma, mass‐screening tests considering sex differences in preschoolers are needed. In this study, we investigated whether sex differences influence the prevalence and severity of EIB in prepubertal children aged 5–6 years.
Methods
Fifty‐one children aged 5–6 years who were attending a kindergarten in Matsuyama City, Ehime, Japan, were enrolled in this cross‐sectional study. The children underwent a 6‐minute free‐running test in 2015. The peak expiratory flow rate (PEFR) was measured before exercise and 0, 3, 10, and 20 minutes after exercise. The severity of EIB was classified according to the reduction in PEFR, measured as the difference between the postexercise PEFR and the highest pre‐exercise PEFR.
Results
Of the 51 children (23 boys and 28 girls) enrolled, the prevalence of EIB defined as three criteria: a ≥15%, ≥20%, or ≥25% decrease was 54.9% (28/51), 41.2% (21/51), and 25.5% (13/51), respectively. The prevalence of EIB defined as ≥25% decrease was significantly higher in girls than in boys (39.3% vs 8.7%, P = .013). In girls, the mean percentage change in PEFR was significantly higher 20 minutes than 10 minutes postexercise (P = .043).
Conclusions
Sex difference in the prevalence and severity of EIB should be considered when evaluating EIB, even in young, prepubertal children.
Keywords: asthma, children, exercise‐induced bronchoconstriction, peak expiratory flow rate, sex differences
Asthma tends to become less common in boys but more common in girls during the transition from childhood to adolescence, which appears to be influenced by sex hormones; however, sex differences in EIB, especially in preschool children, remain unclear. In this study, we investigated whether sex differences influence the prevalence and severity of EIB in prepubertal children aged 5–6 years.Sex difference in the prevalence and severity of EIB should be considered when evaluating EIB, even in young, prepubertal children.
1. INTRODUCTION
Exercise‐induced bronchoconstriction (EIB) is characterized by transient airway narrowing occurring during and after exercise. It can occur in individuals with and without asthma 1. Estimates of the prevalence of EIB range from 10% to 20% in school‐aged children 2, from 40% to 90% in those with asthma 3, and from 30% to 70% in Olympic or elite‐level athletes. EIB is more common in young children, and the prevalence of EIB progressively decreases with age 4. However, the reported prevalence of EIB is varied, depending on the populations, regions, and methods 1.
Exercise‐induced bronchoconstriction in childhood is a positive predictor of asthma, an important component of asthma, and an early sign of bronchial hyper‐responsiveness 5, 6, On the other hand, asthma‐related death is major in sport‐related death that is a cause of death during or after sporting activity, especially in adolescent among boys 7. Feasible, simple, and effective mass‐screening tests to find EIB in general young children may be helpful to detect subclinical asthma and be able to prevent subsequent asthma‐related deaths in association with sporting activities.
In childhood, wheezing disorders/asthma represent a heterogeneous group of conditions characterized by increased responsiveness to methacholine and increased peak flow variability according to age 8. Thus, the timing to perform is an important issue for a mass‐screening test to find EIB in young children with subclinical asthma. Although recurrent cough and wheeze commonly develop in 2–6‐year‐old children 9, at least three different wheezing phenotypes associated with different risk factors co‐exist during the first 11 years of life 8. “Transient early wheezers” limited to the first 3 years of life and unrelated to increased airway lability; however, part of “nonatopic wheezers” and “IgE‐associated wheezers/asthma” at 4–6 years will continue to have asthma that persists into adult life 8. Our aim was to establish an effective mass screening to find EIB in general young children with subclinical asthma; thus, we chose 5‐ to 6‐year‐old children who might subsequently onset persistent asthma in the near future.
However, the answer to the question “Which test should be used to diagnose EIB in the preschool age group, especially mass survey?” is inconclusive 9. Almost preschool‐aged children have difficulties in performing the forced expiratory maneuvers required for spirometry, because they can either blow “hard” or “long,” but frequently cannot blow both hard and long 9. On the contrary, the PEFR can be measured by a simple instrument—peak expiratory flow meter. The standard ranges of PEFR in normal healthy children are reported in Japanese aged 6 to 18 years and in other races aged more than 4 years 10. Thus, 5–6‐year‐old children can perform peak expiratory flow meter enough. However, in Japanese, the regression equations for PEFR in children aged less than 5 years and a kindergarten‐based general population survey to detect EIB have not been investigated.
For a pulmonary function test in the mass screening, “preschool‐aged child‐friendly” is the utmost importance. These young children must be made to feel comfortable in the survey environment if they are to perform the measurements accurately. Operators to participate in the test have a significant impact on the comfort level of the child, because the children need to be engaged and encouraged by them during the tests. Additionally, safety precautions and hygiene requirements are necessary for preschoolers 9. Thus, for the EIB survey, we chose the 6‐minute free‐running test (6MFRT) with using PEFR meter, because the free‐running in their familial place; a kindergarten makes 5‐ to 6‐year‐old children fun, joyful, and comfortable like playing.
The prevalence of wheezing is stable from birth to adolescence in boys; however, it rapidly decreases from birth to the preschool years and increases from childhood to adolescence in girls 11. Sex hormones appear to exert regulatory effects on human lung development from the neonatal period 12. Moreover, sex‐ and age‐specific differences in lung function and wheezing disorders and associated risk factors have been reported 13, 14. However, sex differences related to the prevalence of EIB in childhood are controversial 4, 15, 16, 17, 18. Furthermore, sex differences in EIB among preschool children are unclear because such young children are usually unable to perform standard spirometry 9. Sex‐ and age‐specific early screening methods for EIB in young children may allow for timely interventions and a more personalized approach to the treatment of EIB and asthma 11, 19
To establish an effective mass‐screening test for early findings of EIB in general young children with subclinical asthma, we performed the first kindergarten‐based preliminary survey for EIB in a general 5–6‐year‐old Japanese children using the 6MFRT. In this survey, we hypothesized that sex differences in EIB exist even since prepubertal children. In this study, we investigated airway reactivity to exercise using peak flow meter measurement in the children and evaluated differences in the prevalence of EIB between boys and girls based on EIB defined as three criteria: a ≥15%, ≥20%, or ≥25% decrease in postexercise PEFR.
2. MATERIALS AND METHODS
2.1. Study design and patients
This epidemiological cross‐sectional study was performed during June 2015 in Matsuyama City, Ehime, Japan. In May 2015, the guardians of 106 children aged 5–6 years, who attended Aiko Kindergarten, were asked permission for their children to perform the 6MFRT and to be included in the study after obtaining written informed consent. Overall, 54.7% (58/106) of the children were enrolled. Children with a fever (n = 1), bone fracture (n = 1), and idiopathic purpura (n = 1) were excluded. Fifty‐five children underwent physical examinations that revealed no evidence of wheezing—they performed the 6MFRT and underwent peak expiratory flow rate (PEFR) measurement. This is the first preliminary mass survey to find EIB in a general kindergarten‐based. Some of the children are supposed to be subclinical asthma or have undiagnosed asthma. In Japan, test for EIB is not commonly performed in children, even in with BA. Because of participating in the 6MFRT is an incentive for children, we performed 6MFRT for all kindergarteners whose gradients allowed the informed consent. Then, four children were subsequently excluded because they either had a diagnosis of asthma or used medication to treat asthma, including inhaled steroids, antihistamines, allergy medications, antibiotics, antitussive drugs, or expectorant drugs. Finally, the data of children were eligible for analysis (Figure 1).
Figure 1.
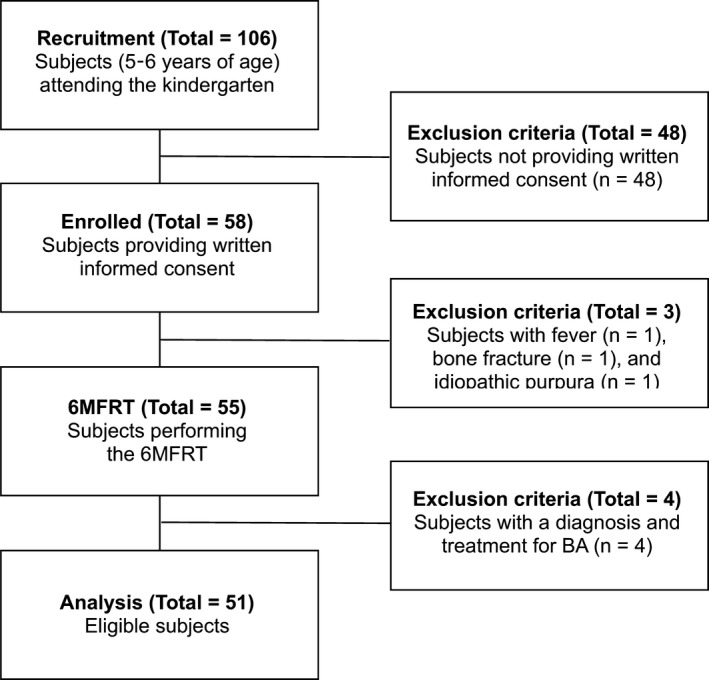
Flow diagram showing the study's recruitment, eligibility, and exclusion process. 6MFRT, 6‐minute free‐running test; BA, bronchial asthma
Age, sex, height, and weight were recorded for all children. Standing heights were measured without shoes or traction. Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m2), rounded to one decimal place. BMI (World Health Organization) z‐scores were calculated. The normality was defined as the z‐score between −3 and 3 20.
The study was approved by the Institutional Review Board at Ehime University in May 2015 (No. 1506014) and was performed according to the tenets of the Helsinki Declaration of 1975, revised in 2008. 106.
2.2. Outcomes
The primary outcome was the sex difference in the prevalence of EIB, defined as three criteria: a ≥15%, ≥20%, or ≥25% decrease in postexercise PEFR. The secondary outcomes were sex differences in the mean PEFR and the response of the mean PEFR percentage to exercise.
2.3. The 6‐minute free‐running test
The 6MFRT was performed in the kindergarten corridors under the supervision of pediatricians and with the assistance of medical students, as previously described 21, 22. The research team constructed by 3–4 pediatrician and 3–5 medical students wearing casual clothes always made the children feel to relax and joy to participate in the 6MFRT like one of the joyful plays in the kindergarten. The children and we had an ice‐breaking time to ask each other about humorous questions about asthma, EIB, and us, because the comfort level of the child has a significant impact on the accuracy of pulmonary tests 9. First, we performed PEFR meter in front of the children, and then, the children practiced the PEFR with us like a play before the 6MFRT. The children performed a 6‐minute free run without a warm‐up period between 10:00 and 11:00am in June 2015. They were asked to run freely back and forth in the 55‐m‐long kindergarten corridor. The mean relative humidity in the corridor was 46%–71%, and the temperature was 24.2–35.4°C. Several adults (pediatricians and medical students) ran with the children to encourage continued running. A portable mobile pulse oximeter monitor (myBeat; Union Tool A, Inc.) measured the heart rate and oxygen saturation continuously throughout the run. The results of the 6MFRT were considered acceptable because exercise was encouraged until the heart rate reached at least 80%–90% of the predicted maximum (calculated as 220—age in years) 23. We categorized the running speed of the children by common running tests in the kindergarten: slow, middle, and fast groups. Then, a child was selected from each categorized group, and a total of three children were measured by the myBeat. The heart rate of the children reached over the target range during the 6MFRT. Coughing, auscultatory wheeze, heart rate, and oxygen saturation, monitored by a portable mobile pulse oximeter monitor (PULSOX‐Lite; Konica Minolta, Inc.), were recorded before exercise and 0, 3, 10, and 20 minutes after exercise. We also prepared administration of an inhaled β2 agonist using a nebulizer, if the children occur wheezing.
2.4. Peak expiratory flow rate measurements
The PEFR was measured before exercise and 0, 3, 10, and 20 minutes after exercise using a Mini‐Wright peak flow meter (Clement Clarke International, Ltd.). For the accuracy and hygiene aspects of PEFR meters, we used new PEFR meters for the study. Before exercise, the children were taught how to exhale into the peak flow meter. The tests were performed standing in a position in which the neck could not be flexed. Maximal expiration (as hard as possible) was performed immediately after full inspiration. The highest PEFR of three correctly performed expirations was recorded and defined as the best PEFR 24. If the values of the highest two of the three acceptable expirations were not within 40 L/min or 5%, one additional expiration was performed to obtain consistent measurements; if this was not achieved, failure to do so was recorded along with the highest reading. Regarding the standard PEFR using Mini‐Wright flow meter, the estimated regression equations in 2614 healthy Japanese aged 6–18 years (1241boysand1,373girls) using Mini‐Wright flow meter are below: boys (L/min) = 64.53 × Ht (m)3 + 0.4795 × Age (y)2 + 77.0 and girls (L/min) = 310.4 × Ht (m) + 6.463 × Age (y) − 209.0 10.
2.5. Exercise‐induced bronchoconstriction criteria
Exercise‐induced changes were quantitated from the PEFR measurements; the following formula was used: EIB = (best pre‐exercise PEFR minus postexercise PEFR/best pre‐exercise PEFR) × 100 17, 25. The severity of EIB was classified according to the magnitude of the reduction in PEFR, as follows: a ≥15%, ≥20%, or ≥25% decrease in postexercise PEFR. The selection of a 15% cutoff for EIB was based on previous studies 16, 21, 22, and the selection of a ≥20% cutoff was based on the Japanese Guideline for Childhood Asthma 25. Previous studies used different normal ranges for the percentage decrease in postexercise PEFR, such as 10%–15% 21, and even <25% 1, 26, 27, 28.
2.6. Statistical analysis
To identify significant differences between girls and boys, the Mann‐Whitney U test was used to analyze differences in age, height, body weight, BMI, heart rate, best PEFR, the mean best PEFR, and the mean percentage change in PEFR. The chi‐square test or Fisher's exact test was used, as appropriate, to assess differences between boys and girls regarding the number of children with EIB defined as three criteria: a ≥15%, ≥20%, or ≥25% decrease in PEFR after the 6MFRT.
All statistical analyses were performed using STATA software (Stata/SE 13.1; Stata Corp.). P‐values < .05 were considered significant.
We also calculated sample sizes by two‐sample comparison of proportions (power 80%) using STATA software.
3. RESULTS
3.1. Characteristics of the participants
We enrolled 23 boys and 28 girls in this study. All children completed the 6MFRT, and none exhibited an asthma attack or audible wheezing after exercise. Their mean age (±standard deviation) was 5.8 ± 0.3 years: range, 5.1–6.2 years. The mean PEFR before exercise was significantly lower in girls than in boys (P = .003) (Table 1).
Table 1.
Characteristics of the 51 participants
| Characteristics | Total | Boys | Girls | P value* |
|---|---|---|---|---|
| (n = 51) | (n = 23) | (n = 28) | ||
| Median (range) | Median (range) | Median (range) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (y) | 5.8 (5.1–6.2) | 5.7 (5.1–6.2) | 5.9 (5.4–6.2) | .162 |
| 5.8 (0.3) | 5.7 (0.3) | 5.9 (0.3) | ||
| Height (cm) | 110.7 (101–123) | 110.5 (101–123) | 111.0 (102.5–118.7) | .571 |
| 110.7 (4.3) | 110.5 (4.9) | 111.0 (3.7) | ||
| Body weight (kg) | 18.8 (15.5–24.5) | 18.9 (15.5–24.0) | 18.7 (16.0–24.5) | .992 |
| 18.8 (2.0) | 18.9 (2.2) | 18.7 (1.8) | ||
| BMI (kg/m2) | 15.3 (13.3–17.6) | 15.4 (13.9–16.9) | 15.2 (13.3–17.6) | .333 |
| 15.3 (1.0) | 15.4 (0.9) | 15.2 (1.0) | ||
| WHO z‐scores | ‐0.03 (−1.42–1.26) | 0.07 (−1.12–1.15) | ‐0.11 (−1.42– 1.26) | .384 |
| (BMI‐for‐age in kg/m2) | −0.03 (0.66) | 0.07 (0.71) | −0.11 (0.61) | |
| Pre‐exercise heart rate (/min) | 91.4 (57–130) | 90.7 (57–120) | 91.9 (71–130) | .985 |
| 91.4 (12.4) | 90.7 (12.5) | 91.9 (12.6) | ||
| Postexercise heart rate (/min) | 118.9 (71–170) | 118.7 (85–140) | 119.0 (71–170) | .865 |
| 118.9 (18.8) | 118.7 (13.8) | 119.0 (22.3) | ||
| Pre‐exercise SpO2 (%) | 97.6 (94–100) | 97.8 (95–99) | 97.5 (94–100) | .337 |
| 97.6 (1.2) | 97.8 (1.1) | 97.5 (1.3) | ||
| Postexercise SpO2 (%) | 97.3 (91–99) | 97.4 (92–99) | 97.2 (91–99) | .738 |
| 97.3 (1.6) | 97.4 (1.6) | 97.2 (1.7) | ||
| Best PEFR (L/min) | 144.4 (90–250) | 160.0 (100–250) | 131.6 (90–190) | .003 |
| 144.4 (33.5) | 160.0 (35.1) | 131.6 (26.5) |
Data expressed as number, the median (range), or the mean (SD).
Abbreviations: BMI, body mass index; PEFR, peak expiratory flow rate; WHO, World Health Organization.
Mann‐Whitney U test.
Associations between the age of the children and variables, such as height and PEFR, related to the standard regression equation of PEFR in Japanese children 10 are shown in Table 2. The ranges of PEFR 6‐year‐old in boys and girls in our study were almost within the estimated range in Japanese. Notably, both associated variables with the standard regression equation of PEFR, such as height and PEFR, were significantly different between the 5‐year‐old and 6‐year‐old girls (P = .019 and P = .004, respectively), except boys (Table 2). In total children, a significant difference between the 5‐year‐old and 6‐year‐old children was found only in their height (P = .007), except the best PEFR.
Table 2.
Associated variables with the regression equation of PEFR in Japanese children in the 51 participants separated by age
| Variables | Total | P value † | Boys | P value † | Girls | P value † | P value ‡ | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | (range) | Median | (range) | Median | (range) | |||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |||||
| Height (cm) | ||||||||||
| 5‐year‐old children | (n = 33) | .007 | (n = 18) | .195 | (n = 15) | .019 | ||||
| 109.8 | (101–123) | 109.8 | (101–123) | 109.7 | (103–119) | .940 | ||||
| 109.8 | (4.5) | 109.8 | (5.1) | 109.7 | (4.0) | |||||
| 6‐year‐old children | (n = 18) | (n = 5) | (n = 13) | |||||||
| 112.5 | (106–118) | 112.6 | (106–118) | 112.4 | (106–118) | .657 | ||||
| 112.5 | (3.2) | 112.6 | (4.3) | 112.4 | (2.9) | |||||
| Best PEFR (L/min) | ||||||||||
| 5‐year‐old children | (n = 33) | .149 | (n = 18) | .793 | (n = 15) | .004 | ||||
| 141.5 | (90–250) | 161.4 | (100–250) | 117.7 | (90–150) | .0005 | ||||
| 141.5 | (38.0) | 161.4 | (39.3) | 117.7 | (17.5) | |||||
| 6‐year‐old children | (n = 18) | (n = 5) | (n = 13) | |||||||
| 149.7 | (100–190) | 155.0 | (145–175) | 147.7 | (100–190) | .654 | ||||
| 149.7 | (23.4) | 155.0 | (12.7) | 147.7 | (26.5) | |||||
Data expressed as number, the median (range), or the mean (SD).
Abbreviation: PEFR, peak expiratory flow rate.
Statistical difference between 5‐ and 6‐year‐old children by Mann‐Whitney U test.
Statistical difference between boys and girls by Mann‐Whitney U test.
3.2. Mean PEFR and changes in the mean percentage PEFR
The nadir time of the mean percentage change in postexercise PEFR was 10 minutes in girls and 3 minutes in boys. At any time point, there were no significant differences in the mean percentage change in PEFR in all children (Figure 2A) and between boys and girls (Figure 2B). The recovery to best PEFR appeared to occur more quickly in girls than in boys at 20 minutes. In girls, the mean percentage change in PEFR was significantly higher at 20 minutes than at 10 minutes after exercise (P = .043) (Figure 2B).
Figure 2.
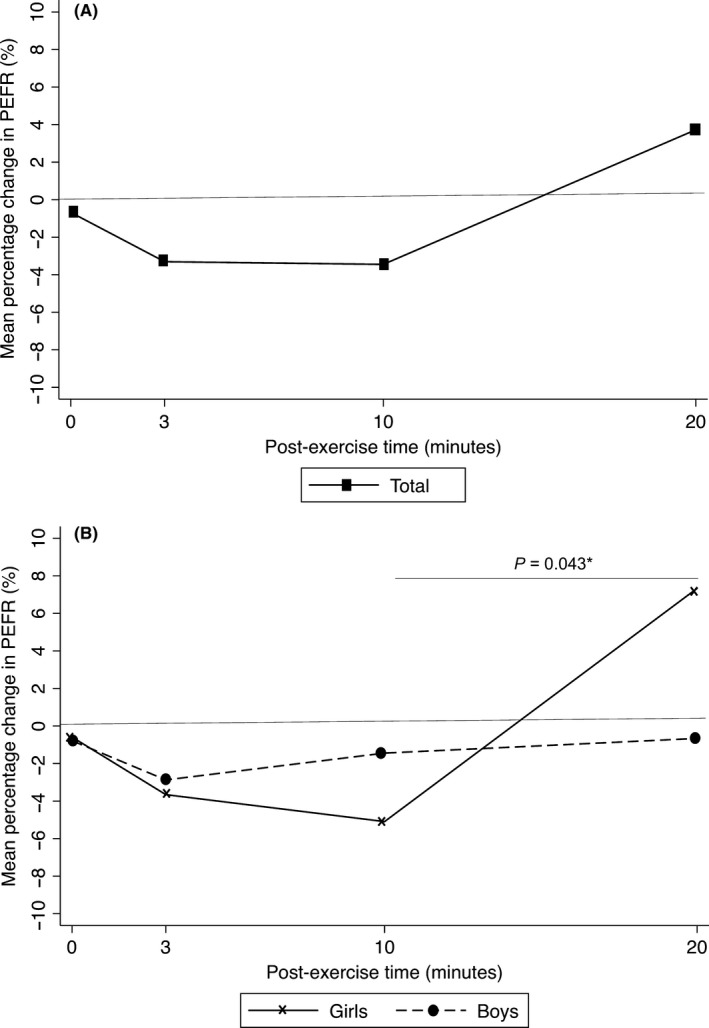
Mean percentage change in PEFR at 0–3, 10, and 20 min postexercise in all participants (A) and stratified by sex (B). Data are shown as mean values. P‐values were calculated using the Mann‐Whitney U test. The P‐values for the mean percentage changes in PEFR in girls vs those in boys at 0, 3, 10, and 20 min postexercise were 0.432, 0.924, 0.378, and 0.099, respectively. PEFR, peak expiratory flow rate
3.3. Sex differences in prevalence of EIB
Overall, the prevalence of EIB defined as a ≥15% and ≥20% decrease in postexercise PEFR was not significantly different between boys and girls, respectively, at any time point (47.8% vs 60.7%, P = .357 and 30.4% vs 50.0%, P = .158, respectively; Figure 3A). However, the prevalence of EIB defined as ≥25% decrease in postexercise PEFR was significantly higher in girls than in boys (39.3% [11/51] vs 8.7% [2/23], P = .013; Figure 3A). For all children and for girls, respectively, the prevalence of EIB defined as a ≥15% and ≥20% decrease in postexercise PEFR peaked 10 minutes after exercise (25.5% [13/51] vs 35.7% [10/28] and 19.6% [10/51] vs 25.0% [7/28], respectively; Figure 3B). The prevalence of EIB defined as ≥25% decrease in postexercise PEFR peaked immediately (0minutes) after exercise in all children (11.8% [6/51]), boys (4.4% [1/23]), and girls (17.9% [5/28]), as shown in Figure 3B.
Figure 3.
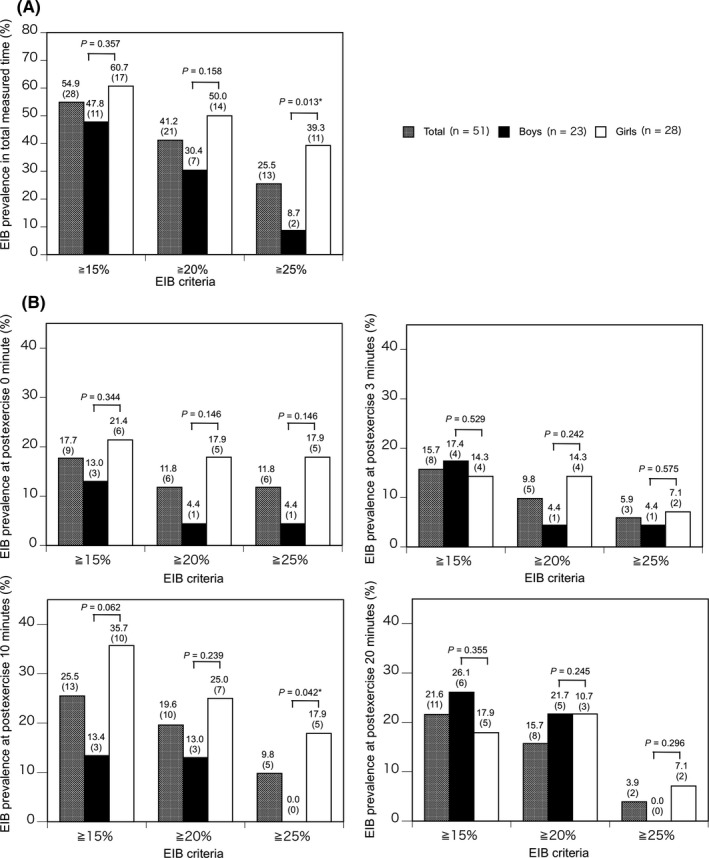
Prevalence of EIB defined as a ≥15%, ≥20%, or ≥25% decrease in PEFR, respectively, in all participants and stratified by sex, during the total measured time (A) and at 0–3, 10, and 20 min after exercise (B). P‐values were calculated using the Chi‐square test or Fisher's exact test, as appropriate. EIB, exercise‐induced bronchoconstriction; 6MFRT, 6‐minute free‐running test; CI, confidence interval; PEFR, peak expiratory flow rate
4. DISCUSSION
In this study, sex differences in the prevalence of EIB and in the pattern of response to exercise were clarified in prepubertal children. The prevalence of EIB (≥25 decrease in postexercise PEFR) was higher in girls than in boys. Additionally, the time to maximal bronchoconstriction was slower and the pattern of recovery after exercise was faster in girls than in boys. Regardless of the severity of EIB, its prevalence was higher in our participants than in those included in previous studies conducted in other countries 2, 14, 23, 28, 29, 30. The younger age 29 and Asian race of the subjects 30, as well as the use of a high‐intensity running test 31, may have contributed to the high prevalence of EIB in our study. Additionally, the policy of the kindergarten recommends physical fitness, and daily exercise might make the physical fitness in children like athletes' level.
In this study, girls had a lower PEFR than boys (Table 1), and the time to maximal bronchoconstriction was slower and the pattern of recovery after exercise was faster in girls than in boys (Figure 2B). Only in the girls, but not in boys, the significant differences in the standard regression equation of PEFR 10‐related variables, height and PEFR, might reflect the sex difference in lung function and growth. For example, in 6‐year‐old children, the standard estimated PEFR is 158.8–205.8 L/min (range 47.0 L/min) in boys (height 1.00–1.20 m) and 124.7–217.8 L/min (range 93.1 L/min) in girls (height 0.95–1.25 m). The range of the estimated PEFR is wider in girls than in boys. In line with the result, interestingly, a recent paper shows that the significant interaction between high peak flow variability and age was significant in females, but not in males, and the diurnal peak flow variability is considered an important measurement of airway lability in the screening and diagnosis of asthma in population‐based studies 32.
On the contrary, in clinical practice using spirometry by preschoolers, forced expiratory volumes at 0.75 second (FEV0.75) or at 0.5 second (FEV0.5) have not been adopted, because children in the preschool age group will not have the chest wall muscle strength to maintain flow limitation to lung volumes as low as 90% of exhaled vital capacity 9. Additionally, morphometry has demonstrated that the lungs of females are generally smaller than those of males throughout the human lifespan 12, 33. Furthermore, airway caliber is thought to be smaller (in proportion to total lung volume) in boys than in girls, predisposing boys to airway obstruction and wheezing in young children 13, 34, 35. The sex differences in the recovery of EIB may be explained by sex differences in airway resistance, airway caliber, and lung function; female lungs have lower specific airway resistance than male lungs 13, and females have larger airways in relation to lung size 36 and higher forced expiratory flow rates controlling for FVC than males do 13. These sex differences in peak flow variability, lung function, and morphometry might cause the sex differences in the recovery of EIB.
In this study, the prevalence of EIB was higher in girls than in boys only when EIB was defined as a ≥25% decrease in postexercise PEFR. When EIB was defined as a ≥15% decrease in postexercise PEFR using a free‐running test in children aged 10–15 years, the prevalence of EIB was higher in girls than in boys in school‐based studies in the USA 16 and Spain 15, whereas the prevalence did not differ in a study from Algeria 17. The discrepancies in study findings may partly be due to different criteria and/or exercise tests used, different methods of measuring postexercise PEFR, and the different ethnic groups examined.
Sex differences in the morphology, maturation, and physiology of the airways begin during gestation 13. Sex hormones appear to exert regulatory effects on fetal lung development and maturation before birth throughout the human lifespan 8, 14, 37, 38. Moreover, most immune cells—including eosinophils, mast cells, and macrophages—express estrogen receptors to varying degrees and can respond to sex hormones 38. Furthermore, a recent study found that testosterone blocks the production of a type of immune cell (group 2 innate lymphoid cell) that triggers allergic asthma 39. These results suggest that sex hormone–related differences in age, together with sex differences in lung size and function and other unknown factors, may contribute to the prevalence and severity of EIB in children.
Our study has both strengths and limitations. First, the main strength of this study was the selection of children. The preschoolers were recruited from a general population in a restricted region; thus, environmental factors such as air pollution, weather, and epidemic infections were similar among the subjects. Second, 6MFRT was performed during a single month in the early summer, which is not the pollen season of the Japanese cedar and cypress trees, and the subjects' lung function was unlikely to have been influenced by pollen. Third, the BMI of all subjects fell within the normal standardized range; thus, obesity had no influence on individual variability of lung function. A limitation of this study was that the temperature and humidity varied during the test period. However, previous studies were also performed outside on different days or during different seasons 16, 21, 22, 25. Second, lung function was measured using only a peak flow meter because during a survey, however, even school‐aged children have difficulty to perform the physiological maneuvers to measure pulmonary function 1, 9, 23, 35, 40. Although previous studies also used free‐running tests in which children performed sustained maximum running for specified, measured periods of time 2, 16, 21, 22, the intensity of 6MFRT might have varied among individual children. Third, the sample size was small; thus, the significant difference in the prevalence of EIB defined as <25% is true. However, the conclusions of the difference in the prevalence of EIB defined as <15% and <20% need further studies in larger sample size. Finally, the results were from Matsuyama City only and cannot be generalized to the whole of Japan or to other countries.
In conclusion, among prepubertal children, the prevalence of EIB defined as a ≥25% decrease in the postexercise PEFR was higher in girls than in boys, and bronchoconstriction recovers more quickly (within 20 minutes) after exercise in girls. The nadir time is within 10 minutes in girls and within 3 minutes in boys. Therefore, even in prepubertal preschool children, the influence of sex should be considered when evaluating the prevalence and severity of EIB in kindergarten‐based surveys to detect EIB using the 6MFRT. To establish more effective and accurate mass‐screening tests for the early finding of EIB in general kindergarten‐based children, further studies are needed.
CONFLICT OF INTEREST
The authors have started explicitly that there are no conflicts of interest in connection with this article.
ACKNOWLEDGEMENTS
We would like to thank all children and guardians who participated in this research. We would like to thank Mr. Ziryou Kawahara, the director of Aiko Kindergarten; Ms. Miyuki Yamauchi and Ms. Ayumi Kita, teachers at Aiko Kindergarten, for assisting with recruitment and the 6‐minute free‐running test; Ms. Yuko Haraoka, Ms. Nagisa Hiranaka, and Ms. Haruna Takeda, medical students at Ehime University Graduate School, for their assistance in recording the data; Dr. Yuhei Hamasaki, Division of Pediatrics, Karatsu Medical and Welfare Center, for his critical supervision of this manuscript, and Dr. Kenji Matsumoto, Department of Allergy and Clinical Immunology, National Research Institute for Child Health and Development, for his valuable supervision of the planning and writing stages of the study.
Shinohara M, Ogawa S, Nakaya T, et al. Sex Differences in the Prevalence and Severity of Exercise‐Induced Bronchoconstriction in Kindergarteners in Japan. J Gen Fam Med. 2019;20:221–229. 10.1002/jgf2.270
REFERENCES
- 1. Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. An official American thoracic society clinical practice guideline: exercise‐induced bronchoconstriction. Am J Respir Crit Care Med. 2013;187(9):1016–27. [DOI] [PubMed] [Google Scholar]
- 2. Austin JB, Russell G, Adam MG, Mackintosh D, Kelsey S, Peck DF Prevalence of asthma and wheeze in the Highlands of Scotland. Arch Dis Child. 1994;71(3):211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Milgrom H, Taussig LM. Keeping children with exercise‐induced asthma active. Pediatrics;1999;104(3):e38. [DOI] [PubMed] [Google Scholar]
- 4. Khan DA. Exercise‐induced bronchoconstriction: burden and prevalence. Allergy Asthma Proc. 2012;33(1):1–6. [DOI] [PubMed] [Google Scholar]
- 5. Hallstrand TS, Moody MW, Aitken ML, Henderson WR Jr. Airway immunopathology of asthma with exercise‐induced bronchoconstriction. J Allergy Clin Immunol. 2005;116(3):586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hallstrand TS. New insights into pathogenesis of exercise‐induced bronchoconstriction. Curr Opin Allergy Clin Immunol. 2012;12(1):42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becker JM, Rogers J, Rossini G, Mirchandani H, D'Alonzo GE Jr Asthma deaths during sports: report of a 7‐year experience. J Allergy Clin Immunol. 2004;113(2):264–7. [DOI] [PubMed] [Google Scholar]
- 8. Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52(11):946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175(12):1304–45. [DOI] [PubMed] [Google Scholar]
- 10. Tsukioka K. Normal healthy Japanese children aged 6 to 18 years. Jpn J Pediatric Allergy Clin Immunol. 2003;17:255–68 [in Japanese]. [Google Scholar]
- 11. Tse SM, Coull BA, Sordillo JE, Datta S, Gold DR. Gender‐ and age‐specific risk factors for wheeze from birth through adolescence. Pediatr Pulmonol. 2015;50(10):955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carey MA, Card JW, Voltz JW, Arbes SJ Jr, Germolec DR, Korach KS, et al. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007;18(8):308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54(12):1119–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dijkstra A, Howard TD, Vonk JM, Ampleford EJ, Lange LA, Bleecker ER, et al. Estrogen receptor 1 polymorphisms are associated with airway hyperresponsiveness and lung function decline, particularly in female subjects with asthma. J Allergy Clin Immunol. 2006;117(3):604–11. [DOI] [PubMed] [Google Scholar]
- 15. DeBaets F, Bodart E, Dramaix‐Wilmet M, VanDaele S, deBilderling G, Masset S, et al. Exercise‐induced respiratory symptoms are poor predictors of bronchoconstriction. Pediatr Pulmonol. 2005;39(4):301–5. [DOI] [PubMed] [Google Scholar]
- 16. Heaman DJ, Estes J. The free‐running asthma screening test: an approach to screening for exercise‐induced asthma in rural Alabama. J Sch Heal. 1997;67(3):83–8. [DOI] [PubMed] [Google Scholar]
- 17. Benarab‐Boucherit Y, Mehdioui H, Nedjar F, Delpierre S, Bouchair N, Aberkane A. Prevalence rate of exercise‐induced bronchoconstriction in Annaba (Algeria) schoolchildren. J Asthma. 2011;48(5):511–6. [DOI] [PubMed] [Google Scholar]
- 18. Bardagi S, Agudo A, Gonzalez CA, Romero PV. Prevalence of exercise‐induced airway narrowing in schoolchildren from a Mediterranean town. Am Rev Respir Dis. 1993;147(5):1112–5. [DOI] [PubMed] [Google Scholar]
- 19. Weiler JM, Hallstrand TS, Parsons JP, Randolph C, Silvers WS, Storms WW, et al. Improving Screening and Diagnosis of Exercise‐Induced Bronchoconstriction: A Call to Action. J Allergy Clin Immunol Pract. 2014;2:275–280.e7. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization https://www.who.int/growthref/who2007_bmi_for_age/en/. Accessed August 1, 2019.
- 21. Burr ML, Eldridge BA, Borysiewicz LK. Peak expiratory flow rates before and after exercise in schoolchildren. Arch Dis Child. 1974;49:923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burr ML, Butland BK, King S, Vaughan‐Williams E. Changes in asthma prevalence: two surveys 15 years apart. Arch Dis Child. 1989;64(10):1452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing‐1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–29. [DOI] [PubMed] [Google Scholar]
- 24. Quanjer PH, Stanojevic S, Stocks J, Hall GL, Prasad KV, Cole TJ, et al. Changes in the FEV(1)/FVC ratio during childhood and adolescence: an intercontinental study. Eur Respir J. 2010;36(6):1391–9. [DOI] [PubMed] [Google Scholar]
- 25. Hamasaki Y, Kohno Y, Ebisawa M, Kondo N, Nishima S, Nishimuta T, et al. Japanese pediatric guideline for the treatment and management of bronchial asthma 2012. Pediatr Int. 2014;56(4):441–50. [DOI] [PubMed] [Google Scholar]
- 26. Cropp GJ. Relative sensitivity of different pulmonary function tests in the evaluation of exercised induced asthma. Pediatrics. 1975;56(5 pt‐2:suppl):860–7. [PubMed] [Google Scholar]
- 27. Pasnick SD, Carlos WG 3rd, Arunachalam A, Celestin FM, Parsons JP, Hallstrand TS, et al. Exercise‐induced bronchoconstriction. Ann Am Thorac Soc. 2014;11(10):1651–2. [DOI] [PubMed] [Google Scholar]
- 28. Godfrey S, Silverman M, Anderson SD. Problems of interpreting exercise‐induced asthma. J Allergy Clin Immunol. 1973;52(4):199–209. [DOI] [PubMed] [Google Scholar]
- 29. Vilozni D, Bentur L, Efrati O, Barak A, Szeinberg A, Shoseyov D, et al. Exercise challenge test in 3‐ to 6‐year‐old asthmatic children. Chest. 2007;132(2):497–503. [DOI] [PubMed] [Google Scholar]
- 30. Jones CO, Qureshi S, Rona RJ, Chinn S. Exercise‐induced bronchoconstriction by ethnicity and presence of asthma in British nine year olds. Thorax. 1996;51(11):1134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Custovic A, Arifhodzic N, Robinson A, Woodcock A. Exercise testing revisited. The response to exercise in normal and atopic children. Chest. 1994;105(4):1127–32. [DOI] [PubMed] [Google Scholar]
- 32. Lombardi E, Stern DA, Sherrill D, Morgan WJ, Wright AL, Garcia‐Aymerich J, et al. Peak flow variability in childhood and body mass index in adult life. J Allergy Clin Immunol. 2019;143(3):1224–1226.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleisher B, Kulovich MV, Hallman M, Gluck L. Lung profile: sex differences in normal pregnancy. Obs Gynecol. 1985;66(3):327–30. [PubMed] [Google Scholar]
- 34. Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63(1):47–57. [DOI] [PubMed] [Google Scholar]
- 35. Wijga A, Tabak C, Postma DS, Kerkhof M, Wieringa MH, Hoekstra MO, et al. Sex differences in asthma during the first 8 years of life: the prevention and incidence of asthma and mite allergy (Piama) birth cohort study. J Allergy Clin Immunol. 2011;127(1):275–7. [DOI] [PubMed] [Google Scholar]
- 36. Thurlbeck WM. Postnatal human lung growth. Thorax. 1982;37(8):564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kimura Y, Suzuki T, Kaneko C, Darnel AD, Akahira J, Ebina M, et al. Expression of androgen receptor and 5alpha‐reductase types 1 and 2 in early gestation fetal lung: a possible correlation with branching morphogenesis. Clin Sci. 2003;105(6):709–13. [DOI] [PubMed] [Google Scholar]
- 38. Keselman A, Heller N. Estrogen signaling modulates allergic inflammation and contributes to sex differences in asthma. Front Immunol. 2015;6:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laffont S, Blanquart E, Savignac M, Cenac C, Laverny G, Metzger D, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017;214(6):1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arets HG, Brackel HJ, van der Ent CK Forced expiratory manoeuvres in children: do they meet ATS and ERS criteria for spirometry? Eur Respir J. 2001;18(4):655–60. [DOI] [PubMed] [Google Scholar]


