Abstract
Purpose:
To assess macular vasculature in healthy infants and children using OCT angiography (OCTA).
Design:
Prospective cross-sectional study.
Participants:
One hundred thirty-five normal maculae of 89 healthy infants and children (mean age, 8.5±5.3 years; range, 9 weeks–17 years) treated at the Duke University Eye Center.
Methods:
We imaged 135 maculae of 89 pediatric patients using the standard Spectralis tabletop and investigational Spectralis with Flex module devices, both equipped with investigational OCTA software (Heidelberg Engineering, Heidelberg, Germany). OCT angiography images of the superficial vascular complex (SVC) and deep vascular complex (DVC) were analyzed for foveal avascular zone (FAZ) area and superficial and deep vessel density. We assessed effects of age, gender, race, axial length (AL), and central subfield thickness on FAZ and vessel density. Patients with both eyes imaged were assessed for agreement between the FAZ and vessel densities of the left and right eyes.
Main Outcome Measures:
The FAZ area, as well as vessel area density (VAD) and vessel length density (VLD) in the SVC and DVC.
Results:
The FAZ varied significantly with race; white patients showed a significantly smaller FAZ than black patients (mean difference, 0.11 mm2; P = 0.004). The FAZ did not vary with age, gender, or AL (P > 0.05). In the SVC, VAD and VLD varied significantly with age (P < 0.001) and AL (R2 = 0.46; P < 0.001) but not gender (P >0.05). The SVC VLD was significantly different between races and ethnicities (P = 0.037), but VAD was not (P < 0.05). In the DVC, VAD and VLD also varied significantly with age (P < 0.001) and AL (R2 = 0.46; P < 0.001) but not gender or race (P > 0.05). There was excellent agreement between the right and left eyes for FAZ (intraclass correlation [ICC], 0.97), SVC VLD (ICC, 1.00), and DVC VLD (ICC, 1.00).
Conclusions:
Quantitative studies of pediatric perifoveal vasculature should consider age, race, and AL. In eyes with unilateral disease, the perifoveal vasculature in the unaffected eye may be used as a control comparison because there is excellent agreement between eyes.
OCT angiography (OCTA) has greatly advanced the study of diseases affecting retinal vasculature1,2 and allows for high-resolution, depth-resolved visualization of areas of retinal ischemia and neovascularization in diseases such as diabetic retinopathy,1,3–7 neovascular age-related macular degeneration,1 and familial exudative vitreoretinopathy.8 Assessment of retinal vascular features such as the foveal avascular zone (FAZ) area are clearly and reproducibly9 quantifiable on OCTA images. The noninvasive, dyeless nature of OCTA may be ideal to image infants and children; it allows frequent monitoring and follow-up and provides information regarding responses to treatment.10 Application of OCTA to infants and children to examine pediatric retinal microvasculature may provide insights on healthy microvascular development and deviations that could indicate disease.
Most studies using OCTA to examine and quantify normal, healthy retinal microvasculature have been performed in adults; a few studies include children, but none have included infants.9,11–20 These studies in adults show that factors such as age15,17,21 and gender17,21 are significant factors when assessing the FAZ and vessel densities in the superficial and deep retinal vascular layers. OCT angiography imaging of adults and older children with a history of preterm birth show persistent foveal vasculature and small or absent FAZ.22,23 Whether these factors manifest as physiologic differences in the perifoveal vascular development in infants and children is currently unknown. Although spectral-domain OCT imaging has been used to study developing infant structural retinal layers,24–26 knowledge of infant retinal vascular development has been limited to a few human histologic studies27 and animal models.28 A previous histologic study by Hendrickson et al25 demonstrated that foveal morphologic features continue to develop until 3.8 years of age and foveal maturity is reached at 13 years of age as soon as cone packing is complete. This infant foveal development was visualized on structural OCT by Vajzovic et al,24,26 Maldonado et al,29 and Lee et al.30 In recent reports, our group demonstrated the feasibility of acquiring depth-resolved OCTA images of retinal microvasculature in healthy, full-term infants31 as well as infants with retinopathy of prematurity,32 retinoblastoma,33 and familial exudative vitreoretinopathy.8,34 To distinguish between pathologic and physiologic retinal vascular development in pediatric patients on OCTA, normative data of healthy eyes of full-term pediatric participants across the range of ages from birth to adulthood without intraocular disease are needed.
In this study, we acquired OCTA images of healthy maculae of pediatric patients born full term, from neonates to children 17 years of age, to develop normative data of healthy eyes and test several hypotheses. We hypothesized that: (1) the FAZ and perifoveal superficial and deep vessel densities would vary with age,19,20,25 (2) the FAZ and perifoveal vasculature would vary based on gender,17,21,35 (3) the FAZ and perifoveal vasculature would vary depending on race or ethnicity because of previously reported racial and ethnic differences in foveal morphologic features,36,37 and (4) the FAZ and perifoveal vascular patterns would show interocular symmetry.35,36
Methods
Study Population
This prospective, cross-sectional study was approved by the Duke University Health System Institutional Review Board and adhered to the Health Insurance Portability and Accountability Act and all tenets of the Declaration of Helsinki. Parents or legal guardians gave written informed consent for study participation. From September 2017 through April 2018, we imaged normal maculae of pediatric patients who were receiving care at the Duke University Eye Center with the standard Spectralis HRA+OCT tabletop and the investigational Spectralis with Flex module (Heidelberg Engineering, Heidelberg, Germany),31 both integrated with investigational OCTA software that has since received 510(k) clearance by the Food and Drug Administration. Patients with clinic appointments were imaged while sitting upright at the tabletop unit, whereas patients undergoing an examination under anesthesia or surgical procedures were imaged with the portable Flex unit while supine under general anesthesia in the operating room. For patients undergoing an examination under anesthesia, the axial length (AL) of the eye was also measured using a Master-Vu A-scan (Sonomed Escalon, Wayne, PA). To reduce the additional time under anesthesia for research imaging in the operating room, only 1 eye was imaged. For patients imaged in the clinic, one or both eyes were imaged depending on patient cooperation.
We included in the study normal eyes of healthy infants and children 0 to 18 years of age with a history of term birth (≥37 weeks’ gestational age), defined as those without any evidence of intraocular disease per ophthalmic examination by a pediatric optometrist or ophthalmologist at the Duke Eye Center. Patients undergoing examination under anesthesia or surgical procedures had diagnoses such as nasolacrimal duct obstruction, strabismus (the nonamblyopic eye was imaged38), chalazion, trauma in the fellow eye (the unaffected healthy eye was imaged), nonpathologic myopia, astigmatism, combined hamartoma in the fellow eye, and a unilateral congenital cataract in the fellow eye without evidence or suspicion of congenital infection or other pathologic features.
Patients were excluded from the study if they were born prematurely23 (<37 weeks’ gestational age) or had any systemic medical or neurologic condition that may impact foveal development or retinal vasculature, such as sickle-cell disease, diabetes,6,39 Down syndrome, albinism, or seizures. Eyes were excluded if they had amblyopia,38,40 had sustained prior trauma or had undergone intraocular surgeries, or had other ocular conditions or diseases including pathologic myopia, Stickler syndrome,41 and uveitis.42 Eyes were also excluded if the fellow eye had Coats disease,43 Sturge-Weber syndrome, or unilateral congenital glaucoma44 because of the potential for vascular impact in the seemingly unaffected eye. Eyes with high refractive errors that prevented the acquisition of focused OCTA images were also excluded.
Imaging and Analysis
All OCTA images of the macula were acquired using the 10°×10°scan angle that included 512 A-scans × 512 B-scans. Motion and fixation artifacts were minimized on the resulting OCTA images with the Spectralis’ TruTrack Active Eye Tracking feature. Segmentation of retinal layers was performed automatically by Spectralis software to generate en face OCTA images of vascular layers, including the superficial vascular complex (SVC) and deep vascular complex (DVC). The OCTA image of the SVC was generated by flow signal between the internal limiting membrane to 17 mm above the lower boundary of the inner plexiform layer. The DVC boundaries were from 17 mm above the lower boundary of the inner plexiform layer to the lower boundary of the outer plexiform layer. The Spectralis’ projection artifact removal algorithm was applied to the DVC OCTA images. An experienced reader (STH) reviewed each image for correct segmentation of the retinal layers and manually corrected any as necessary.
For each OCTA image, we recorded the following data output provided by the Spectralis software: focus setting, OCTA quality, and 1-mm central subfield thickness (CSFT). The focus setting corresponds most closely to refractive error in spherical equivalent.45 The OCTA quality, calculated by the Spectralis software, was the average quality of all the B-scans in the entire volume scan. The CSFT was obtained using Spectralis’ retinal thickness map.
Each OCTA image was also graded for quality (by STH) as great, good, fair, or poor. Images were of great quality if the reader felt confident that she could trace all of the vessels throughout the image; of good quality if the FAZ boundaries were clear, but there was artifact (i.e., because of motion or dry eyes) affecting approximately less than 20% of the image; of fair quality if the FAZ boundaries were clear, but approximately more than 20% of the image was affected by artifact such that the image should be excluded from vessel density analysis; and as poor quality if the image was of such low quality that it should be excluded from both FAZ and vessel density analysis. For any eyes with repeated images obtained at the same imaging session, we chose the best image for analysis based on the quality assigned by the reader (rather than the Spectralis output for OCTA quality, because these were averaged across the entire scan and thus may not reflect patches of signal dropout in an otherwise high-quality image); if the images were of the same quality, then the image with the higher number for the average OCTA quality was used. For analysis involving 1 eye per participant where 2 eyes were imaged, the eye with the higher reader quality grade was used, or if equivalent, then the eye was selected randomly.
To measure the FAZ area in each eye, we used a customized algorithm in MATLAB (MathWorks, Natick, MA; see “Interreader Reproducibility” below) to segment the central avascular area enclosed by the innermost capillary plexus16,18,39 on the en face OCTA image of the DVC16 (Fig 1). Briefly, OCTA images were enhanced first using the Hessian multiscale filtering46 so that vascular and avascular regions could be distinguished more effectively. Then, a point inside the FAZ area was autolocated to use as a seed point for the FAZ area segmentation using a level sets method developed by Li et al.47 All automated FAZ tracings were reviewed and corrected manually as indicated. Calculation of the FAZ area in square millimeters used the scaled x- and y-dimensions provided by the Spectralis software to convert from pixels to square millimeters.
Figure 1.
MATLAB (MathWorks, Natick, MA)-processed OCT angiography images of the superficial vascular complex (SVC) and deep vascular complex (DVC) of a 14-month-old infant undergoing an examination under anesthesia for persistent hyperplastic primary vitreous in the fellow eye. The original images were binarized by comparing the intensity of each pixel against a local threshold that is the average intensity of pixels inside the 7×7-pixel box centered at the pixel of interest. Then the vessel area density (percentage) was measured by calculating the percentage of the overall 10°×10° scan that was occupied by vessels, excluding the foveal avascular zone (FAZ) area. The images were also skeletonized to measure vessel length density per millimeter. The original DVC image was filtered to facilitate automatic measurement of the FAZ.
To assess the vessel density in the SVC and DVC, we measured both the vessel area density (VAD) and vessel length density (VLD) in MATLAB. First, OCTA images were binarized by comparing the intensity of each pixel against a local threshold, defined as the average intensity of pixels inside the 7×7-pixel box centered at the pixel of interest. An advantage of a local threshold in comparison with a global threshold is that it is better in preserving small vessels during the binarization process. The VAD was then measured by calculating the percentage of the overall 10°×10°scan that was occupied by vessels, excluding the FAZ area as measured above (Fig 1). The VLD was then calculated by skeletonizing the OCTA image to obtain the vessel length per millimeter (mm−1; Fig 1).
Interreader Reproducibility
We assessed the reproducibility of manual segmentation of the FAZ area between 2 readers (L.V. and S.T.H.) on a subset of the OCTA images in the study (n = 30). For each image, each reader independently traced the boundary of the FAZ on the en face OCTA image of the DVC, where the FAZ boundaries were most defined, using the freehand tool in FIJI (available at: https://imagej.net/Fiji).48 Agreement between the manual segmentation of the FAZ area between the readers was assessed using the intraclass correlation coefficient (ICC). Then, we validated the customized MATLAB algorithm for automatic FAZ segmentation using the same subset of images (n = 30). We assessed the reproducibility of FAZ area segmented by the gold standard of manual segmentation by one of the readers (STH) and the automatic segmentation using the customized MATLAB algorithm. Agreement between the manual and automatic segmentation was assessed using the ICC.
Statistical Analysis
Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC) and JMP software version 13.0 (SAS Institute). Analysis to assess for significance of age, gender, race and ethnicity, AL, CSFT, focus setting, and OCTA quality on the OCTA measurements for FAZ area and vessel density (SVC VAD, SVC VLD, DVC VAD, and DVC VLD) used images of 1 eye of each patient. Multivariate analysis was used to assess for race, age, and interaction in models for FAZ area and vessel density. The relationships between continuous predictor variables and the continuous outcomes were assessed using linear regression. For vessel density, both linear and quadratic fits were applied in the regression model. In a model for FAZ area, race (black and white only, because smaller sample sizes and other races and ethnicities were excluded from this subanalysis between groups) and age were included, and an interaction term for race and age was tested for significance. The relationships between categorical variables and the outcomes were assessed using the 2-sample t test and Wilcoxon rank-sum test, respectively. For the subset of patients who had both eyes imaged during the same visit, we compared right and left eyes of each patient. Agreement between the eyes for FAZ area, SVC VAD, SVC VLD, DVC VAD, and DVC VLD was assessed using a paired t test and ICC.
Results
We successfully acquired OCTA images of 135 healthy eyes of 89 infants and children (mean age, 8.5±5.3 years; range, 9 weeks–17 years; females, 54%; white, 43%; black, 36%; Hispanic, 10%; Asian, 2%; other race or ethnicity, 4%; Table 1). No manual correction of the automated retinal layer segmentations was needed for any of the images. Of the 89 OCTA images (1 eye per patient), all underwent FAZ analysis, and 8 were excluded from vessel density analysis because of artifacts affecting image quality. Interreader reproducibility of manual FAZ segmentation was excellent (ICC, 1.00; 95% confidence interval, 0.99–1.00). When compared with manual FAZ segmentation, the customized MATLAB algorithm for automatic segmentation showed excellent agreement (ICC, 0.99; 95% confidence interval, 0.98–1.00).
Table 1.
Foveal Avascular Zone Area of Pediatric Patients
| Foveal Avascular Zone Area (mm2) | ||||||
|---|---|---|---|---|---|---|
| Race or Ethnicity | No. (%) | Age (yrs), Mean ± Standard Deviation | Mean ± Standard Deviation | Median | 95% Confidence Interval | Range |
| White | 42 (46) | 7.5±5.4 | 0.289±0.108 | 0.280 | 0.255–0.322 | 0.05–0.63 |
| Black | 32 (43) | 10.6±4.6 | 0.398±0.217 | 0.335 | 0.320–0.477 | 0.12–1.17 |
| Hispanic | 9 (10) | 7.0±6.7 | 0.381 ±0.150 | 0.420 | 0.266–0.496 | 0.07–0.59 |
| Asian | 2 (2) | 8.0±5.7 | 0.410±0.212 | 0.410 | — | 0.26–0.56 |
| Other | 4 (4) | 5.9±5.7 | 0.433±0.099 | 0.430 | 0.275–0.590 | 0.33–0.54 |
| Overall | 89 (100) | 8.5±5.3 | 0.347±0.168 | 0.320 | 0.311–0.382 | 0.05–1.17 |
Mean FAZ area of 1 eye from each of the 89 patients was 0.35±0.17 mm2; Table S1 (available at www.aaojournal.org) shows the FAZ area mean, median, and range split for various age categories (0–2 years, 3–6 years, 7–9 years, 10–12 years, 13–15 years, and 16–18 years). The FAZ area varied significantly among the different races and ethnicities (P = 0.008, Wilcoxon rank-sum test; Table 1). Patients who identified as white showed a significantly smaller FAZ than those who identified as black (mean difference, 0.11 mm2;P = 0.004, Wilcoxon rank-sum test). Central subfield thickness was significantly predictive of FAZ area (slope, −0.004; P < 0.001; R2 = 0.49, linear regression); the thinner the CSFT, the larger the FAZ. Factors that were not significant predictors of FAZ area included age, gender, and AL (P > 0.05). Furthermore, assessment of whether the relationship between age and FAZ area was significantly different for black and white patients showed no significance (P = 0.52 for interaction). In the final regression model of FAZ area with age and race, race was significant (P = 0.02), but age was not (P = 0.06).
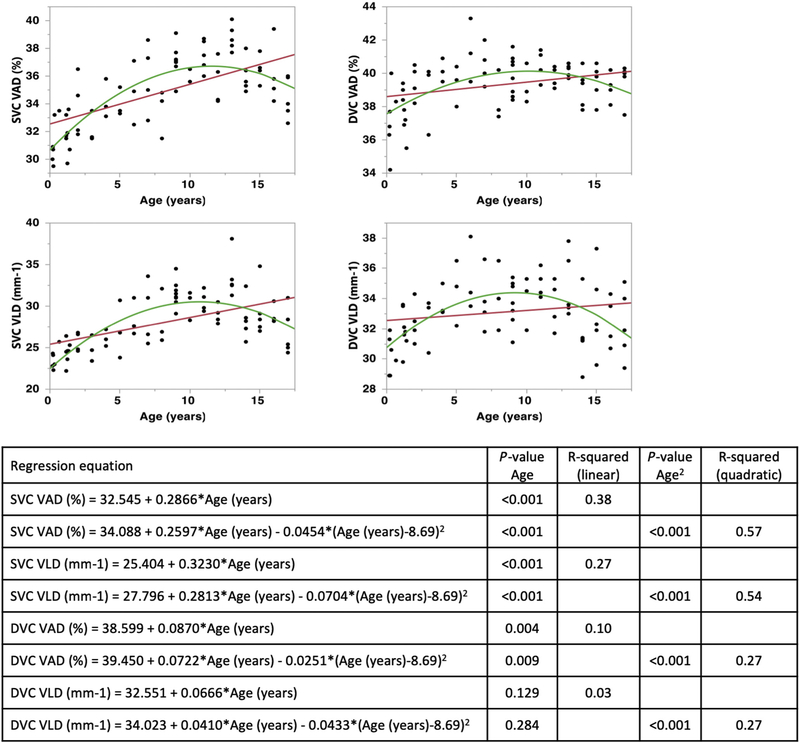
Vessel density in the SVC, measured as both VAD (percentage) and VLD (vessel length per millimeter), was predicted by age (Fig 2), AL, and race or ethnicity (Table 2). The relationship between age and vessel density was fit better to a quadratic, rather than linear, model; VAD and VLD increased with age until 10 to 15 years of age, after which vessel density started to decrease. Further subgroup linear regression analysis of the SVC VAD and VLD for 0 to 7 years of age and 8 to 18 years of age showed that age was a significant predictor in children 0 to 7 years of age for SVC VAD and VLD (P < 0.001; R2 = 0.45 and R2 = 0.50, respectively); although age was also a significant predictor of SVC VLD in children 8 to 18 years of age, it did not explain most of the variation in this linear model (P = 0.017; R2 = 0.12; Fig S1, available at www.aaojournal.org). In the patients from whom we acquired an AL measurement (age range, 9 weeks–5 years; n = 25 eyes; AL mean, 21.1±1.24 mm; AL median, 21.2 mm; AL range, 18.7–23.2 mm), AL was a significant predictor of VAD and VLD (n = 23; P < 0.001; VAD R2 = 0.45; VLD R2 = 0.43). The SVC VLD was significantly different between races (P = 0.036), but VAD was not. The SVC vessel densities were not significantly different between males and females (P > 0.05).
Figure 2.
Graphs showing vessel density in the superficial vascular complex (SVC) and deep vascular complex (DVC) reported in terms of both vessel area density (VAD [percentage]) and vessel length density (VLD [per millimeter]) in linear (red line) and quadratic (green line) regression models with age. For the SVC, both linear and quadratic models were significantly predictive of VAD and VLD (P < 0.001 for all). The relationship between age and vessel density (VAD and VLD) was better fit when using the quadratic (adjusted R2 = 0.57 and adjusted R2 = 0.54, respectively) rather than linear model (R2 = 0.38 and R2 = 0.27, respectively). For the DVC, the linear and quadratic regression models were a poor fit for both VAD (linear R2 = 0.10, quadratic adjusted R2 = 0.27) and VLD (linear R2 = 0.03, quadratic adjusted R2 = 0.27), indicating that although age significantly predicted the DVC VAD and VLD (P < 0.001, quadratic fit), most of the variation in the data was not explained by age.
Table 2.
Vessel Area Density and Vessel Length Density of the Superficial and Deep Vascular Complexes in Pediatric Patients
| Superficial Vascular Complex | Deep Vascular Complex | |||
|---|---|---|---|---|
| Race or Ethnicity | Vessel Area Density (%) | Vessel Length Density (mm−1) | Vessel Area Density (%) | Vessel Length Density (mm−1) |
| White | ||||
| No. | 38 | 38 | 38 | 38 |
| Mean±SD | 34.4±2.7 | 27.5±3.4 | 39.3±1.7 | 33.1±2.2 |
| Median | 34.6 | 27.0 | 39.8 | 33.0 |
| 95% CI | 33.5–35.3 | 26.4–28.6 | 38.7–39.9 | 32.4–33.8 |
| Minimum–maximum | 29.5–39.3 | 22.2–34.8 | 34.2–43.4 | 28.9–38.1 |
| Black | ||||
| No. | 28 | 28 | 28 | 28 |
| Mean±SD | 36.1±2.1 | 29.6±3.1 | 39.4±1.1 | 33.2±2.0 |
| Median | 36.4 | 30.0 | 39.8 | 33.5 |
| 95% CI | 35.3–36.9 | 28.4–30.8 | 39.0–39.9 | 32.5–34.0 |
| Minimum–maximum | 31.5–40.1 | 23.0–38.1 | 37.4–41.1 | 29.4–37.8 |
| Hispanic | ||||
| No. | 9 | 9 | 9 | 9 |
| Mean±SD | 34.8±2.8 | 28.2±3.4 | 39.3 ±2.1 | 33.7±1.8 |
| Median | 35.1 | 28.2 | 39.4 | 33.6 |
| 95% CI | 32.7–37.0 | 25.6–30.8 | 37.7–40.9 | 32.3–35.0 |
| Minimum–maximum | 30.7–38.6 | 24.5–33.6 | 35.5–42.0 | 31.2–36.6 |
| Asian | ||||
| No. | 2 | 2 | 2 | 2 |
| Mean±SD | 35.1±1.8 | 27.6±2.3 | 40.3±0.2 | 34.3±1.5 |
| Median | 35.1 | 27.6 | 40.3 | 34.3 |
| 95% CI | — | — | — | — |
| Minimum–maximum | 33.8–36.3 | 26.0–29.2 | 40.1–40.4 | 33.2–35.3 |
| Other | ||||
| No. | 4 | 4 | 4 | 4 |
| Mean±SD | 33.8±1.0 | 25.5±1.4 | 38.9±0.8 | 31.0±2.0 |
| Median | 33.4 | 25.5 | 38.9 | 31.1 |
| 95% CI | 32.2–35.4 | 23.2–27.8 | 37.67–40.1 | 27.8–34.2 |
| Minimum–maximum | 33.1–35.3 | 23.8–27.3 | 38.1–39.6 | 28.8–33.1 |
| Overall | ||||
| No. | 81 | 81 | 81 | 81 |
| Mean±SD | 35.0±2.5 | 28.2±3.3 | 39.4±1.5 | 33.1±2.1 |
| Median | 35.3 | 28.2 | 39.7 | 33.2 |
| 95% CI | 34.5–35.6 | 27.5–28.9 | 39.0–39.7 | 32.7–33.6 |
| Minimum–maximum | 29.5–40.1 | 22.2–38.1 | 34.2–43.3 | 28.8–38.1 |
CI = confidence interval; SD = standard deviation.
For the DVC vessel density, age and AL were significant predictors. Age significantly predicted the DVC VAD and VLD (P < 0.001, quadratic fit); however, the linear and quadratic regression models were a poor fit for both VAD (linear R2 = 0.10, quadratic adjusted R2 = 0.25) and VLD (linear R2 = 0.03, quadratic adjusted R2 = 0.28), indicating that although age was significant, most of the variation in the data was not explained by age (Fig 2). Further subgroup linear regression analysis of the DVC VAD and VLD for those 0 to 7 years of age and 8 to 18 years of age showed that age was a significant predictor in children 0 to 7 years of age for DVC VAD and VLD (P < 0.001; R2 = 0.41 and R2 = 0.43, respectively); although age was also a significant predictor of DVC VLD in children 8 to 18 years of age, it did not explain most of the variation in this linear model (P = 0.023; R2 = 0.11; Fig S1). Axial length (in patients 9weeks–5 years of age) significantly predicted vessel density and was a moderate fit for VAD (n = 23; P = 0.002; R2 = 0.38, linear regression) and VLD (n = 23; P < 0.001; R2 = 0.47, linear regression). The DVC VAD and VLD were not significantly different between races or genders.
The mean focus setting used was 0.584±2.85 (n = 89; median, 1.20; range, −6.54 to 6.96). Assessments of whether the focus setting and OCTA quality significantly affected the FAZ area and vessel density (VAD and VLD) measurements were unreliable. The regression model relationships between the variables were poor. Thus, most of the variation was not the result of the focus setting and OCTA quality.
In the subset of children with both right and left eyes imaged (n = 40 patients), there was no difference between the 2 eyes for the FAZ area and vessel length densities (P = 0.30, paired t test). The mean FAZ area difference between the 2 eyes was 0.01±0.01 mm2. The mean differences for the vessel densities were as follows: SVC VAD, 0.02±0.30%; SVC VLD, 0.11±0.36 mm−1; DVC VAD, 0.05±0.27%; and DVC VLD, 0.07±0.20 mm−1. The right and left eyes showed remarkable similarity for the FAZ area (ICC, 0.97; 95% confidence interval, 0.94–0.98). Agreement in vessel densities was moderate to good when comparing the VLD of the right and left eyes for the SVC and DVC (ICC, 0.72 and 0.82, respectively) and moderate for VAD comparisons in the SVC and DVC (ICC, 0.69 and 0.38, respectively).
Discussion
We successfully imaged and analyzed depth-resolved perifoveal microvasculature in healthy full-term infants and children and assessed the relationships between the FAZ area and vessel density with the following patient characteristics: age, gender, race or ethnicity, AL, and central subfield thickness. This study utilizes OCTA to quantify and analyze the normal perifoveal vasculature in a large cohort of healthy infants and young children (PubMed search on June 13, 2019, for optical coherence tomography angiography AND [pediatric OR infant OR children]). In our cohort of pediatric patients, we found that FAZ area differed between white and black children, whereas vessel density, particularly in the SVC, varied with patient age.
The racial differences in FAZ area between white and black pediatric patients are consistent with the racial and ethnic differences in foveal pit morphologic features; the larger FAZs seen in black patients could be explained by the deeper and broader foveal pit morphologic features.36 Furthermore, we observed that foveal thickness showed a significant inverse relationship with FAZ area: the thinner the CSFT, the larger the FAZ, a finding also noted previously by Falavarjani et al.20 A previous study by Borrelli et al18 in 5- to 17-year-olds that included only 1 black patient did not find an association between race and FAZ area. In terms of vessel density, different races and ethnicities did not show significant differences in the DVC, but the results for the SVC were mixed: the VLD was significantly different, whereas the VAD difference was not significant (P = 0.07). Such differences caused by different methods of vessel density analysis are important to note when determining the significance of various patient characteristics on perifoveal vasculature.
Our study contributes to the existing literature by providing information regarding OCTA measurements for FAZ and vessel density from infancy through the adolescent years. There seems to be a quadratic relationship between age and vessel density: VAD and VLD increased with age until approximately 10 to 15 years of age, after which the vessel density started to decrease. Interestingly, the peak of vascular density coincided with the age at which cone packing in the human fovea is complete, marking the attainment of foveal maturity.25 Our study was limited to pediatric patients, and thus we were unable to determine whether vessel density continues to decrease throughout young adulthood or stabilizes. A previous study by Iafe et al15 that analyzed the VLD of patients 9 to 88 years of age showed decreasing mean VLD between the fifth and sixth decades of life, and the decrease became significant between 60 and 70 years of age. Another study by Leng et al19 that studied patients 8 to 87 years of age with a mean age of 42.8±19.9 years found decreasing vessel density with increasing age. Together, our study findings suggest that perifoveal vasculature continues to grow starting in infancy until approximately the time of foveal maturity, at which point the vascular density stabilizes and then decreases later in adulthood.
We did not observe an effect of age on FAZ area in infants and children, even after including race in the multivariate analysis. This finding contrasts with that of Borrelli et al,18 who showed that increased age correlated with larger FAZs. Also, although we did not find any differences between genders for FAZ area or vessel density in our study, other studies have reported larger FAZs in females11 and increased superficial capillary plexus density in males.18 The different racial and ethnic composition of our study population when compared with that of others may explain some of the different results among studies; however, multivariate analysis of our data did not show a significant relationship of age and gender as a function of race. Other explanations to be considered include differences in study patient characteristics (whether patients in other studies were born full term23), statistical analysis (whether only 1 eye from each patient was used for independent data), vascular layer methodologies used by different authors to generate the en face OCTA images,49 and whether variations in AL were accounted for when calculating FAZ area on the OCTA images.13,50 Furthermore, differences in sample size, especially with regard to the number of infants and young children, may play a role in statistical differences. Our study assessing 89 eyes of healthy patients ranging from 9 weeks to 17 years of age contributes new information to the existing knowledge of quantitative OCTA measurements. Many factors such as race and ethnicity, age, and AL need to be considered when one evaluates whether a patient’s FAZ and vessel density are within so-called normal healthy ranges.
The cross-sectional design of our study limits our ability to draw conclusions regarding the effect of age on perifoveal microvasculature. Further follow-up of patients is necessary to assess the longitudinal changes in FAZ and vessel density as patients age. The imaging locations and methods also present different challenges. In the operating room, to minimize the additional time under anesthesia to acquire research images, we imaged only one rather than both eyes, thus precluding the assessment of interocular symmetry in these infants. In the clinic, to minimize impediment on clinic flow, AL was not measured, thus limiting our AL measurements in children older than 6 years. Although the same Spectralis OCTA software and camera head were used to image patients in both the operating room and the clinic, the patients were supine and had been administered supplemental oxygen and anesthetic agents for imaging in the operating room, and patients were upright in the clinic; this may have affected quantitative measures such as of VAD.
Based on the interocular symmetry demonstrated in this study for FAZ and vessel density, OCTA can be particularly useful in assessing eyes with unilateral ocular disease. Because the agreement in interocular vessel densities was better when using VLD instead of VAD, VLD may be a preferable measure of vessel density to the VAD. Furthermore, as discussed by Iafe et al,15 measurement of VLD is more important to assess pathologic features, because the finer vessels are often affected first and are the site of oxygen exchange and tissue perfusion. Thus, it is possible that VLD, rather than VAD, may prove more sensitive in early or mild disease states.
In conclusion, we used OCTA to measure the FAZ and vessel densities in normal maculae of healthy pediatric patients across ages from birth to 17 years. These data may provide a basis to assess foveal development and pathophysiologic features. These baseline measurements of healthy eyes in patients as young as 9 weeks not only provide a reference with which to compare pathologic features in infants and children but also, in the future, may help the clinician to detect, diagnose, or monitor disease.
Supplementary Material
Financial Disclosure(s):
The author(s) have made the following disclosure(s): S.T.H.: Financial support e Heidelberg Engineering (Heidelberg, Germany)
M.A.M.: Consultant – Castle Biosciences (Friendswood, TX)
G.J.J.: Consultant and Financial support – Heidelberg Engineering (Heidelberg, Germany)
C.A.T.: Financial support – Heidelberg Engineering (Heidelberg, Germany); Royalties – Alcon (Fort Worth, TX); Patent – ophthalmic OCT and handheld OCT angiography
L.V.: Consultant – Alcon (Fort Worth, TX), Janssen Pharmaceutical (Raritan, NJ), Roche/Genentech (Basel, Switzerland), DORC (Zuidland, The Netherlands), Second Sight, Inc (Sylmar, CA), Alimera Sciences (Alpharetta, GA), Bausch & Lomb (Rochester, NY), Guidepoint; Financial support – Alcon, Roche/Genentech, DORC, Second Sight, Inc, Heidelberg Engineering (Heidelberg, Germgsany)
Supported by Research to Prevent Blindness, Inc, New York, New York (unrestricted grant to Duke Eye Center; Career Development Award [X.C.]); Knights Templar Eye Foundation (L.V., M.A.E.); the National Institutes of Health, Bethesda, Maryland (grant nos.: P30EY005722, R01 EY25009 [C.A.T.]); National Eye Institute, National Institutes of Health, Bethesda, Maryland (grant no.: K23EY028227 [X.C.]); International Association of Government Officials Fund (L.V., S.T.H.); and Lions Duke Pediatric Eye Research Endowment (R.J.H.); these sponsors or organizations had no role in the design or conduct of this research. Research equipment (Spectralis HRA±OCT with Flex and OCT-A module) was provided by Heidelberg Engineering (Heidelberg, Germany), which participated in approval of the manuscript.
Abbreviations and Acronyms:
- AL
axial length
- CSFT
central subfield thickness
- DVC
deep vascular complex
- FAZ
foveal avascular zone
- ICC
intraclass correlation coefficient
- OCTA
OCT angiography
- SVC
superficial vascular complex
- VAD
vessel area density
- VLD
vessel length density
Footnotes
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at Duke University Health System approved the study. All research complied with the Health Insurance Portability and Accountability (HIPAA) Act of 1996 and adhered to the tenets of the Declaration of Helsinki. All participants’ parents or guardians provided informed consent.
No animal subjects were included in this study.
Presented in part at: American Academy of Ophthalmology Annual Meeting, October, 2018, Chicago, Illinois (recipient of the award for best original paper in the session for pediatric ophthalmology).
References
- 1.Jia Y, Bailey ST, Hwang TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A. 2015;112(18):E2395–E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashani AH, Chen C-L, Gahm JK, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57(8):3907–3913. [DOI] [PubMed] [Google Scholar]
- 4.Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160(1): 35–44. e31. [DOI] [PubMed] [Google Scholar]
- 5.Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015;35(11):2353–2363. [DOI] [PubMed] [Google Scholar]
- 6.Niestrata-Ortiz M, Fichna P, Stankiewicz W, Stopa M. Enlargement of the foveal avascular zone detected by optical coherence tomography angiography in diabetic children without diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2019;257(4):689–697. [DOI] [PubMed] [Google Scholar]
- 7.Li T, Jia Y, Wang S, et al. Retinal microvascular abnormalities in children with type 1 diabetes mellitus without visual impairment or diabetic retinopathy. Invest Ophthalmol Vis Sci. 2019;60(4):990–998. [DOI] [PubMed] [Google Scholar]
- 8.Hsu ST, Finn AP, Chen X, et al. Macular microvascular findings in familial exudative vitreoretinopathy on optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2019;50(5):322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpineto P, Mastropasqua R, Marchini G, et al. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol. 2016;100(5):671–676. [DOI] [PubMed] [Google Scholar]
- 10.Huang D, Jia Y, Rispoli M, et al. OCT angiography of time course of choroidal neovascularization in response to anti-angiogenic treatment. Retina. 2015;35(11):2260–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghassemi F, Mirshahi R, Bazvand F, et al. The quantitative measurements of foveal avascular zone using optical coherence tomography angiography in normal volunteers. J Curr Ophthalmol. 2017;29(4):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Jiang C, Wang X, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci. 2015;56(5):3212–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linderman R, Salmon AE, Strampe M, et al. Assessing the accuracy of foveal avascular zone measurements using optical coherence tomography angiography: segmentation and scaling. Transl Vis Sci Technol. 2017;6(3):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo J, She X, Liu X, Sun X. Repeatability and reproducibility of foveal avascular zone area measurements using AngioPlex spectral domain optical coherence tomography angiography in healthy subjects. Ophthalmologica. 2017;237(1):21–28. [DOI] [PubMed] [Google Scholar]
- 15.Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal capillary density and foveal avascular zone area are age-dependent: quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(13):5780–5787. [DOI] [PubMed] [Google Scholar]
- 16.Campbell JP, Zhang M, Hwang TS, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Chan S, Yang JY, et al. Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol. 2016;168:95–109. [DOI] [PubMed] [Google Scholar]
- 18.Borrelli E, Lonngi M, Balasubramanian S, et al. Macular microvascular networks in healthy pediatric subjects. Retina. 2019;39(6):1216–1224. [DOI] [PubMed] [Google Scholar]
- 19.Leng Y, Tam EK, Falavarjani KG, Tsui I. Effect of age and myopia on retinal microvasculature. Ophthalmic Surg Lasers Imaging Retina. 2018;49(12):925–931. [DOI] [PubMed] [Google Scholar]
- 20.Falavarjani KG, Shenazandi H, Naseri D, et al. Foveal avascular zone and vessel density in healthy subjects: an optical coherence tomography angiography study. J Ophthalmic Vis Res. 2018;13(3):260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coscas F, Sellam A, Glacet-Bernard A, et al. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9): Oct211–Oct223. [DOI] [PubMed] [Google Scholar]
- 22.Falavarjani KG, Sarraf D, Tsui I. Optical coherence tomography angiography of the macula in adults with a history of preterm birth. Ophthalmic Surg Lasers Imaging Retina. 2018;49(2):122–125. [DOI] [PubMed] [Google Scholar]
- 23.Falavarjani KG, Iafe NA, Velez FG, et al. Optical coherence tomography angiography of the fovea in children born preterm. Retina. 2017;37(12):2289–2294. [DOI] [PubMed] [Google Scholar]
- 24.Vajzovic L, Hendrickson AE, O’Connell RV, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012;154(5):779–789.e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol.154(5):767–778.e762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vajzovic L, Rothman AL, Tran-Viet D, et al. Delay in retinal photoreceptor development in very preterm compared to term infants. Invest Ophthalmol Vis Sci. 2015;56(2):908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provis JM, Hendrickson AE. The foveal avascular region of developing human retina. Arch Ophthalmol. 2008;126(4): 507–511. [DOI] [PubMed] [Google Scholar]
- 28.Provis JM, Diaz CM, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol. 1998;54(5):549–581. [DOI] [PubMed] [Google Scholar]
- 29.Maldonado RS, O’Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118(12):2315–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H, Purohit R, Sheth V, et al. Retinal development in infants and young children with achromatopsia. Ophthalmology. 2015;122(10):2145–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu ST, Chen X, Ngo HT, et al. Imaging infant retinal vasculature with OCT angiography. Ophthalmol Retina. 2019;3(1):95–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu S, Chen X, House RJ, et al. Visualizing macular micro-vasculature anomalies in 2 infants with treated retinopathy of prematurity. JAMA Ophthalmol. 2018;136(12):1422–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.House RJ, Hsu ST, Thomas AS, et al. Vascular findings in a small retinoblastoma tumor using OCT angiography. Ophthalmol Retina. 2019;3(2):194–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Viehland C, Carrasco-Zevallos OM, et al. Microscope-integrated optical coherence tomography angiography in the operating room in young children with retinal vascular disease. JAMA Ophthalmol. 2017;135(5):483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Huang X, Meng X, et al. In vivo assessment of macula in eyes of healthy children 8 to 16 years old using optical coherence tomography angiography. Sci Rep. 2017;7(1):8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner-Schuman M, Dubis AM, Nordgren RN, et al. Race-and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011;52(1): 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilat AV, Proudlock FA, Mohammad S, Gottlob I. Normal macular structure measured with optical coherence tomography across ethnicity. Br J Ophthalmol. 2014;98(7): 941–945. [DOI] [PubMed] [Google Scholar]
- 38.Doguizi S, Yilmazoglu M, Kiziltoprak H, et al. Quantitative analysis of retinal microcirculation in children with hyperopic anisometropic amblyopia: an optical coherence tomography angiography study. J AAPOS. 2019. May 18 pii: S1091–8531(19)30117-X. 10.1016/j.jaapos.2019.01.017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 39.Durbin MK, An L, Shemonski ND, et al. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135(4):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaur S, Singh SR, Sukhija J, Dogra MR. Comparison of quantitative measurement of foveal avascular zone and macular vessel density in eyes of children with amblyopia and healthy controls: an optical coherence tomography angiography study. J AAPOS. 2018;22(2):164–165. [DOI] [PubMed] [Google Scholar]
- 41.Matsushita I, Nagata T, Hayashi T, et al. Foveal hypoplasia in patients with stickler syndrome. Ophthalmology. 2017;124(6): 896–902. [DOI] [PubMed] [Google Scholar]
- 42.Kim AY, Rodger DC, Shahidzadeh A, et al. Quantifying retinal microvascular changes in uveitis using spectral-domain optical coherence tomography angiography. Am J Ophthalmol. 2016;171:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanga PE, Romano F, Chwiejczak K, et al. Swept-source optical coherence tomography angiography assessment of fellow eyes in Coats disease. Retina. 2019;39(3):608–613. [DOI] [PubMed] [Google Scholar]
- 44.Bayoumi NH. Fellow eye in unilateral primary congenital glaucoma. J Curr Glaucoma Pract. 2017;11(1):28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudnicka AR, Burk RO, Edgar DF, Fitzke FW. Magnification characteristics of fundus imaging systems. Ophthalmology. 1998;105(12):2186–2192. [DOI] [PubMed] [Google Scholar]
- 46.Frangi AF, Niessen WJ, Vincken KL, Viergever MA. Multi-scale Vessel Enhancement Filtering. Berlin, Heidelberg: Springer; 1998. [Google Scholar]
- 47.Li C, Xu C, Gui C, Fox MD. Distance regularized level set evolution and its application to image segmentation. Trans Img Proc. 2010;19(12):3243–3254. [DOI] [PubMed] [Google Scholar]
- 48.Schindelin J, Arganda-Carreras I, Frise E, et al. Fijidan open source platform for biological image analysis. Nat Methods. 2012;9(7):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spaide RF, Curcio CA. Evaluation of segmentation of the superficial and deep vascular layers of the retina by optical coherence tomography angiography instruments in normal eyes. JAMA Ophthalmol. 2017;135(3):259–262. [DOI] [PubMed] [Google Scholar]
- 50.Sampson DM, Gong P, An D, et al. Axial length variation impacts on superficial retinal vessel density and foveal avascular zone area measurements using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58(7):3065–3072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.