Abstract
Background:
The CDC recommends PrEP for MSM at substantial risk of HIV acquisition, leaving clinicians unsure whether to prescribe PrEP to MSM who do not disclose HIV risk factors. In a real-world setting we followed a cohort of MSM using PrEP who during their clinical visits stated they were low-risk for HIV to assess the accuracy of their HIV risk perception.
Methods
A longitudinal cohort of MSM requesting PrEP despite reporting either 100% condom use or participation in oral sex only was followed over 13 months at a community clinic in San Francisco. Participants completed a sexual and substance use behavior questionnaire at baseline, followed by quarterly HIV/STI testing and condom use change questionnaires.
Results:
81 clients self-identified as low-risk for HIV (age range 22-71, 83% non-Hispanic, 17% Hispanic). The mean number of partners in the previous 12 months was 10. 80% of MSM who perceived themselves as low-risk for HIV reported at least one HIV-related risk behavior including, sex while intoxicated (38%), sex with a person of unknown HIV status (28%), injecting drugs (1%), consuming 5 or more alcoholic drinks at one sitting (40%), ecstasy (11%), and poppers (16%). Condomless sex increased to 12% at month 1, peaked at 22% at month 7, and then decreased to 6% at month 10 before increasing slightly to 8% at month 13. Rates of pharyngeal GC/CT varied from 7% at baseline to 11% at month 13, while rectal GC/CT decreased from 6% at baseline to 0% at month 13. The rate of syphilis was 1% both at baseline and at month 13, however, 11% and 15% of clients tested positive for syphilis at months 1 and 7 respectively.
Conclusions:
80% of participants who perceived themselves as low risk for HIV were actually high risk. Clients’ failure to report risk factors for HIV acquisition may lead to an increasingly unacceptable proportion of unidentified MSM at risk of HIV.
Introduction
The introduction of pre-exposure prophylaxis (PrEP) for HIV prevention in the form of Truvada® (emtricitabine + tenofovir dispoproxil fumarate) into clinical practice in the US in 2012 revolutionized HIV prevention among men who have sex with men (MSM) (Center For Disease Control and Prevention, 2014). PrEP is a once-daily pill that is simple to use, has low toxicity and, if adherence is maintained, is 92–99% effective in reducing HIV risk (van der Straten, Van Damme, Haberer, & Bangsberg, 2012). In order to assist with implementation the Centers for Disease Control and Prevention (CDC) issued guidelines for clinicians considering the use of PrEP in MSM, which include the following recommended indications: 1) Any anal sex without condoms (receptive or insertive) in past 6 months; 2) Any STI diagnosed or reported in past 6 months; 3) Is in an ongoing sexual relationship with an HIV-positive male partner; or 4) Intravenous drug use (Center For Disease Control and Prevention, 2014; Centers for Disease Control and Prevention, 2018). However, by attempting to define HIV risk, clinicians may be unintentionally creating barriers that prevent MSM from accessing PrEP (D. S. Krakower, Mayer, K H, 2015; Landovitz & Coates, 2014). Sexual health is a complex topic that involves psychological, social, cultural and historical factors (McKechnie, Bavinton, & Zablotska, 2012; World Health Organization, 2014) not typically discussed during a routine healthcare encounter, which may lead the client or clinician to fail to recognize risk factors for HIV (D. S. Krakower et al., 2015). Furthermore, multiple studies have shown that self-reporting sexual or substance use behaviors to a clinician are often unreliable (Collumbein, 2012) because social desirability may cause individuals not to disclose their “real” behaviors(Grimm, 2010). Overreliance on self-reporting may leave clinicians with little or no information on how to proceed when an MSM seeking PrEP does not disclose any qualifying risk factors.
The provision of PrEP to all MSM who request it is controversial due to concerns that this will increase sexual risk behavior and in turn reduce population-level prevention benefits, decrease cost effectiveness and increase STIs secondary to decreased condom use (Aghaizu et al., 2013; Arnold et al., 2017; Beymer et al., 2017; Calabrese et al., 2016). In order to assess the risks and benefits of providing PrEP to all MSM who request it, services at an urban, community-based PrEP program for gay, bi and transgender MSM were expanded to include MSM who stated they had 100% condom use or only participated in oral sex.
Purpose
The primary purpose of this study was to assess the prevalence of misclassification in HIV risk and evaluate changes in condom use and STI acquisition after initiation of PrEP over a 13-month period.
Sample and Setting
This study is part of a larger nurse-led longitudinal PrEP Health Program open to gay, bisexual and transgender men in the San Francisco Bay Area. Between November 2014 and May 2016, ~1,000 MSM were enrolled in the PrEP Health Program. To our knowledge this is the only clinic that included MSM that did not meet the CDC criteria for PrEP secondary to community demand. Clients were included in this analysis if they were age 18 and over; gay, bisexual or transgender; HIV negative; seeking PrEP; and self-identified as low risk for HIV acquisition by categorizing themselves as either 100% condom use or only engaging in oral sex. Client informed consent was obtained and the study was approved by the clinic site and the Institutional Review Board of the University of California, San Francisco (IRB Study #: 16-9026).
Study Procedures
At the baseline visit, conducted by a nurse practitioner, a history and physical was completed, blood tests for HIV, hepatitis B and C, renal function, and syphilis were performed, and samples for pharyngeal, urethral and anal gonorrhea and chlamydia were collected. If clients were HIV positive at this visit they were referred to an HIV clinic or their primary care provider. At baseline, as part of the clinic’s protocol, other HIV risk behaviors (including substance use, number of partners, sex with HIV positive or HIV status unknown partners) were reported by clients on a brief paper and pencil self-reported screening questionnaire. Follow-up visits began one month after initiation of PrEP and then continued quarterly for one year thereafter. Biological samples were collected at each follow-up visit to assess renal function, and to test for HIV, syphilis, gonorrhea and chlamydia. Condom use change was assessed by asking if condomless sex was more, less or the same compared to the last visit, and patients were also asked to give their reasons for remaining on or discontinuing PrEP. Each visit included HIV and STI prevention counseling based on a harm reduction model.
Methods
Each client was provided with an ID number in place of their name and medical record number and their visit history was logged in a database by the clinic’s research assistant. The database was checked the first author for missing data and updated on a monthly basis. The clients who stated at baseline that they used condoms 100% of the time or only participated in oral sex were identified as low risk in the database. The de-identified results from the low risk clients were entered into a statistical software package (Stata Statistical Software: Release 14, StataCorp, 2015) and descriptive statistics and bivariate analyses (chi–square and Fisher’s exact tests) were completed. Multilevel logistic regression analyses were performed to determine associations between condom use change, STIs risk behaviors and demographics reported by clients.
Results
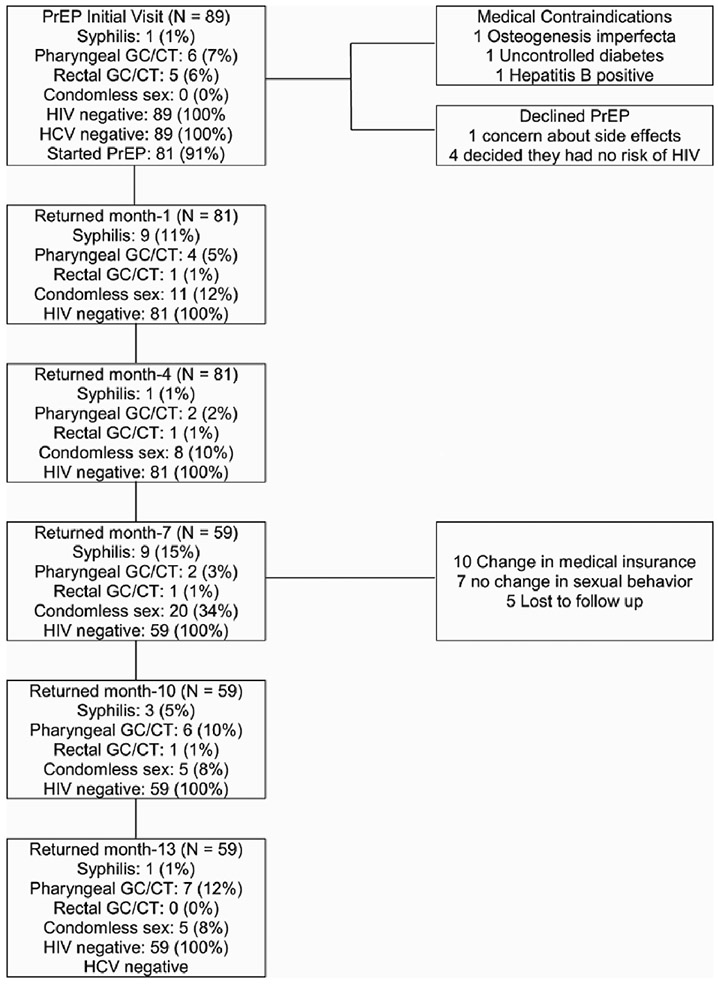
The overall rate of MSM self-identifying as low risk for HIV at the enrollment visit for PrEP was 8% (n = 89). Of the 89 clients self-identified as low risk, three clients were ineligible to start PrEP due to medical contraindications that included osteogenesis imperfecta, uncontrolled diabetes and undiagnosed hepatitis B. Five clients declined PrEP after completion of the education and counseling provided stating they had a better understanding of sexual risk of HIV and no longer felt they needed PrEP. Of the 89 clients who completed the intake visit for PrEP, 81 (91%) enrolled in the program and 59 (66%) remained on PrEP for 13 months.
The 81 clients who enrolled in the PrEP program had a mean age of 37 (range 22–71, SD 12), 83% identified as non-Hispanic (of whom 82% identified as white, 17% identified as Asian Pacific Islander, 5% identified as Middle Eastern and 3% identified as black) and 17% identified as Hispanic. The mean number of partners (at baseline) in the past 12 months was 10 (range 0–100, SD 17.5). None of the clients reported perceived stigma related to PrEP use.
Prevalence of Misperception of Risk at Baseline
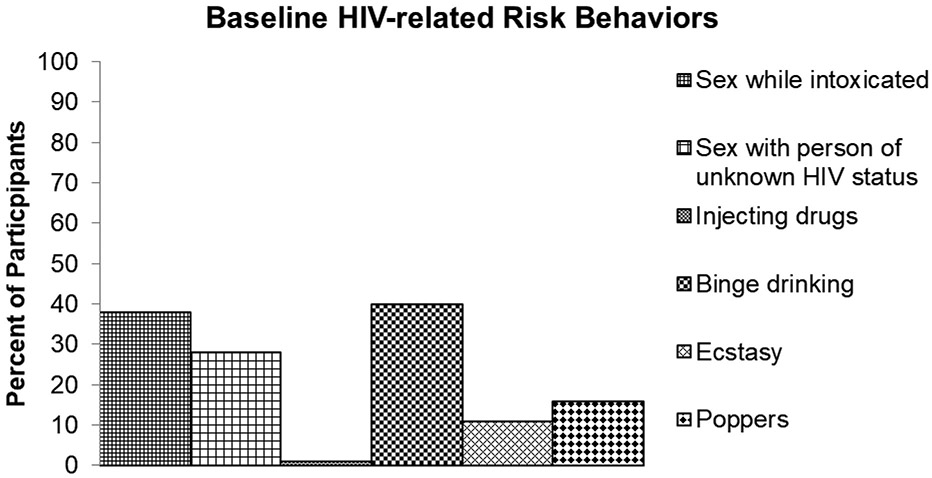
Although these clients reported at baseline that they perceived themselves to be low risk, a subsequent self-report questionnaire revealed certain risk behaviors that are known to be related to HIV acquisition. Overall, 80% of MSM who presented as low risk had reported at least one HIV-related risk behavior (i.e., high risk MSM misclassified as low risk). These behaviors included sex while intoxicated (n = 33, 38%), sex with a person of unknown HIV status (n = 25, 28%), injecting drugs (n = 1, 1%), and consuming 5 or more alcoholic drinks at one sitting (n = 36, 40%). Among other drug use, ecstasy (n = 10, 11%) and poppers (n = 14, 16%) were the most commonly used (see Figure 1).
Figure 1.
HIV related risk factors at baseline.
There was an association between number of partners and sex with a partner of unknown HIV status (Fisher’s exact p-value = 0.006). Being intoxicated during sex was also associated with an increase in the number of partners (Chi2: p = 0.048). There were no statistical differences in age. Multilevel logistic regression analysis did not reveal any associations with condom use or testing positive for an STI and age, race, ethnicity, substance use, partners HIV status, partner of unknown HIV status or number of partners.
Changes in Sexual Risk Behaviors
Clients were asked by the clinician if their condom use had increased, decreased or not changed since their last visit. We found that increase in condomless sex occurred at month 1 (n = 11, 12%) and peaked at month 7 (n = 20, 22%). Condomless sex decreased at month 10 (n = 5, 6%). At month 13, 52 of the 59 clients who remained on PrEP reported 100% condom use. The seven participants (8%) who reported not using condoms at month 13 all reported being in mutually monogamous relationships with a partner who was HIV positive (see Figure 2).
Figure 2.

Condomless Sex
Bacterial STI data laboratory results were accessed from the clinical charts and are summarized in Table 2. Before initiating PrEP all clients were HIV and HCV negative, while 14% of clients were positive for any bacterial STI (7% pharyngeal GC/CT, 6% rectal GC/CT and 1% syphilis). At month 1, 16% of clients were positive for any bacterial STI (4% pharyngeal GC/CT, 1% rectal GC/CT and 11% syphilis). At month 4, 4% of clients were positive for any bacterial STI (2% pharyngeal GC/CT, 1% rectal GC/CT and 1% syphilis). At month 7, 19% were positive for any bacterial STI (3% pharyngeal GC/CT, 1% rectal GC/CT and 15% syphilis). At month 13, 12% were positive for any bacterial STI (11% pharyngeal GC/CT and 1% syphilis). There were no new rectal, urethral GC/CT, HIV or HCV infections at month 13 (see Figure 3).
Table 2.
Sociodemographic and baseline risk behaviors (N = 89)
| Characteristics | Mean (min, max, SD) |
|---|---|
| Age in years | 37 (22, 71, 12) |
| Number of sex partners in last 12 months | 10 (0, 100, 17.5) |
| Characteristics | N (%) |
| Ethnicity | |
| Hispanic | 15 (17) |
| Non-Hispanic | 74 (83) |
| Race | |
| White | 78 (82) |
| Asian Pacific Islander | 15 (17) |
| Middle Eastern | 5 (6) |
| Black | 3 (3) |
| HIV-related risk behaviors | |
| Sex while intoxicated | 34 (38) |
| Sex with person of unknown HIV status | 25 (28) |
| Injecting drugs | 1 (1) |
| Alcohol binge | 36 (40) |
| Ecstasy | 10 (11) |
| Poppers | 14 (16) |
| Low risk category | |
| Oral sex only | 80 (90) |
| 100% condom use | 9 (10) |
| STI rates | |
| Pharyngeal GC/CT | 6 (7) |
| Rectal GC/CT | 5 (6) |
| Syphilis | 1 (1) |
Numbers may not add up to 100% as clients were allowed to check more than one category
Figure 3.
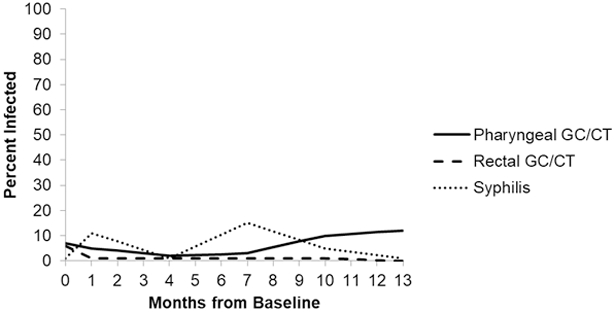
Bacterial STI Rates
Discussion
In this analysis of clinical records, at baseline, the majority of participants who perceived they were low risk for HIV acquisition were actually found to have behaviors linked to being high risk for HIV. These risks were not disclosed to a clinician but were noted in the self-reports. Non-disclosure of these risks during the clinical visit may be due to multiple reasons, including a client’s lack of understanding of risk, social desirability, discomfort in discussing risk behaviors with a health care provider, fear of being judged and a concern of how disclosing risk factors may affect their health insurance (Spector, Remien, & Tross, 2015; Underhill et al., 2015). This study affirms previous research findings that MSM may not perceive themselves to be at risk due to a misunderstanding of risk factors for HIV and STIs (Grov, Hirshfield, Remien, Humberstone, & Chiasson, 2013; Grov, Rendina, Ventuneac, & Parsons, 2013; Tobin, 2014; Yadav, 2014). Research shows that, for MSM who have lived with the threat of HIV, the fear and anxiety surrounding sex can trigger risk behaviors such as alcohol or substance use, which in turn can lead to multiple partners and incorrect use of condoms (Heidinger, 2015; Kahler, 2015; Smith, Herbst, Zhang, & Rose, 2015). If this behavior is a community norm, clients may not understand that they or their partners are involved in high risk behavior for HIV transmission (Chard, Metheny, & Stephenson, 2017). Risk misclassification may also be linked to lack of provider awareness of the need to discuss the effect alcohol and substance use may have on sexual practices (Smith, Pan, et al., 2015). The study findings do not support the perception among certain healthcare professionals and HIV advocacy groups that PrEP leads to increased risk behaviors and more STIs(D. S. Krakower et al., 2015; Venter, Allais, & Richter, 2014), as rectal GC/CT and syphilis decreased, and there were no new HIV, hepatitis C or urethral GC/CT infections. This may be attributed to the repetition of STI screening, treatment and counseling provided at the clinical encounters.
Condomless sex increased from 0% at baseline to a peak of 22% at month 7 but decreased to 8% at month 13, which is consistent with other PrEP studies (Grant et al., 2010; Liu et al., 2015; McCormack et al., 2015), which report first time condomless sex or decrease in condom use at ~6 months after initiating PrEP. This effect may be due to personal or partner desire to experience condomless sex, feelings of intimacy related to condomless sex, situational effects and social norms (Carlo Hojilla et al., 2016; Gamarel & Golub, 2015; Hoff et al., 2015). These results may indicate a period when more intensive counseling condom use would be beneficial and further research is needed to assess the impact this may have on STI infections. The return to 100% condom use at month 13 may be attributed to counseling at clinic quarterly visits or the acquisition of an STI at month 7. Another explanation of the return to baseline condom use may be that this group is truly risk adverse.
The study findings support the need for ongoing STI screening and education for MSM. Before initiating PrEP many of the MSM in this cohort reported they had not been previously tested for anal or pharyngeal GC/CT. Reasons cited were they had not reported risk factors, or they had only been previously offered urethral CT/GC testing. This is consistent with current issues in STI testing in primary care for MSM and builds on the body of research indicating clinician training for STI screening is crucial to decrease the current epidemic (Centers for Disease Control and Prevention, 2014; den Heijer et al., 2017). In this cohort, although there were no urethral GC/CT infections over 13 months, at baseline there were 6% rectal and 7% pharyngeal GC/CT infections. Therefore, had only urethral testing been completed, treatment for actual GC/CT infection would not have been initiated leading to further health complications and increased onward transmission. Albeit small, there was evidence of an increase in pharyngeal GC/CT infections from 7% at baseline to 11% at month 13. This reflects the current trend of increase in pharyngeal GC/CT in MSM (Cornelisse et al., 2018; Foschi et al., 2018; Park et al., 2012) and indicates the need for ongoing clinical education regarding STI screening for health care professionals. Additionally, prior to initiating PrEP, 60% of the cohort reported greater than 10 partners in the past six months. Although consistent condom use and oral sex are safer sexual practices to prevent HIV transmission they do not fully prevent other STI transmission (Centers for Disease Control and Prevention, 2015) indicating a need for counseling of clients on differences between HIV risk and STI risk.
Early detection and treatment of STIs benefit MSM at a community level by preventing onward transmission leading to a significant decline in STIs over the following decade(Katz, Dombrowski, Bell, Kerani, & Golden, 2016; Kelley et al., 2015). This study suggests that quarterly STI testing including pharyngeal, anal and urethral sites with prompt treatment and education are vital components in overcoming the current STI epidemic in MSM (Katz et al., 2016) and should remain an integral part of PrEP provision (Centers for Disease Control and Prevention, 2015; Volk et al., 2015).
This study offers insights into PrEP use in low risk MSM attending a gay-friendly clinic in San Francisco. The clients at the clinic are demographically comparable to the overall MSM population in California, but it is acknowledged that the data may not be generalizable to other settings. Additionally, an urban sexual health clinic that serves an MSM population may imply a sampling bias towards individuals wanting to initiate PrEP. Such individuals could be considered more likely to engage in sexual risk behaviors and thus there may be more STIs diagnosed than the general MSM population. Indeed, many of the participants initially came to the clinic for HIV testing or STI treatment and were unaware of PrEP until they met with an HIV counselor.
It is acknowledged that there is potential for improvement in the baseline questionnaire and adding the same questionnaire at 3 or 6 monthly intervals to assess change over time. Self-reporting of condom-use is known to be unreliable (Graham, 2012) and may have been subject to social desirability bias in this study. For example, although the criteria for low risk were 100% condom use or oral sex only, 5% of clients reported an increased use of condoms at month 1. The protocol could be revised to include questions regarding how and when condom use was initiated and what factors influenced decrease in condom use as these were not asked during the study. This would provide further insight into decision making practices and in turn inform the selection of interventions to help prevent STIs.
In this study, certain participants continued to self-identify as low risk for HIV despite evidence to contrary, such as when diagnosed with anal GC/CT and/or syphilis infections. This may have been secondary to social desirability bias during reporting, misunderstanding of the question or documentation errors in the chart. Generally, however, clients in this study appeared to speak frankly about sexual risk, as demonstrated by a willingness to discuss their feelings about PrEP with clinicians. This may have been influenced by repeat visits in a clinic supportive of a culture that allows organic and candid conversations to develop.
Conclusion
Given the discordance between perceived and actual HIV/STI risk, this study highlights the importance of clinicians providing PrEP and STI education for all MSM. It also validates previous research which indicates that low risk MSM do not accurately perceive their sexual risks (need ref). Clinicians should be prepared to discuss healthy sexuality and provide appropriate testing, treatment, and counseling including HIV/STI prevention methods in order to improve the overall health of all MSM. While PrEP can provide protection against acquisition of HIV, and thereby improve sexual pleasure, misconceptions regarding STI transmission and HIV risk remain. Utilizing strict criteria to decide who would benefit from PrEP during a clinical visit may unintentionally exclude MSM who either do not perceive themselves to be at risk for HIV or STIs or are not willing to discuss risk behavior. In order to prevent HIV/STI transmission, clinicians need to promote sexual health in all MSM through discussion and education regarding risk behaviors, PrEP, condom use, and HIV/STI transmission with quarterly testing to include extra genital screening. These services need to be in place so that advances in HIV prevention are not surpassed by challenges in the control and management of bacterial STIs.
CONSORT Diagram for Clinical Visits
Table 1.
Reasons Provided for Requesting and Continuing PrEP
| Baseline reasons for requesting PrEP (N=89) | N | (%) |
|---|---|---|
| Added protection | 89 | (100) |
| Extra layer of protection | 11 | (12) |
| Peace of mind | 5 | (6) |
| Added safety | 3 | (3) |
| Decrease anxiety | 3 | (3) |
| Another barrier to HIV | 1 | (1) |
| As many tools as possible to prevent HIV | 1 | (1) |
| As much protection as possible | 1 | (1) |
| Decrease fear of sex | 1 | (1) |
| Preventative measure against HIV | 1 | (1) |
| Reduce fear of HIV | 1 | (1) |
| Stay HIV free | 1 | (1) |
| To feel comfortable with sex | 1 | (1) |
| To feel safe | 1 | (1) |
| One-year reasons for continuing PrEP (N=59) | N | (%) |
| Added protection | 59 | (100) |
| Decreased fear of HIV | 59 | (100) |
| Peace of mind during sex | 13 | (22) |
| Feels safe from HIV | 8 | (14) |
| Feels secure during sex | 5 | (8) |
| Added protection against HIV | 4 | (7) |
| Decreased anxiety during sex | 4 | (7) |
| Feels able to relax during sex | 4 | (7) |
| Feels protected against HIV | 3 | (5) |
Table 3.
Bacterial STI rates
| Pharyngeal GC/CT |
Rectal GC/CT |
Syphilis | |
|---|---|---|---|
| Baseline | 7% | 6% | 1% |
| Month 1 | 4% | 1% | 11% |
| Month 4 | 2% | 1% | 1% |
| Month 7 | 3% | 1% | 15% |
| Month 13 | 11% | 0% | 1% |
References
- Aghaizu A, Mercey D, Copas A, Johnson AM, Hart G, & Nardone A (2013). Who would use PrEP? Factors associated with intention to use among MSM in London: a community survey. Sex Transm Infect, 89(3), 207–211. doi: 10.1136/sextrans-2012-050648 [DOI] [PubMed] [Google Scholar]
- Arnold T, Brinkley-Rubinstein L, Chan PA, Perez-Brumer A, Bologna ES, Beauchamps L, . . . Nunn A (2017). Social, structural, behavioral and clinical factors influencing retention in Pre-Exposure Prophylaxis (PrEP) care in Mississippi. PLoS One, 12(2), e0172354. doi: 10.1371/journal.pone.0172354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beymer MR, Weiss RE, Sugar CA, Bourque LB, Gee GC, Morisky DE, . . . Bolan RK (2017). Are Centers for Disease Control and Prevention Guidelines for Preexposure Prophylaxis Specific Enough? Formulation of a Personalized HIV Risk Score for Pre-Exposure Prophylaxis Initiation. Sex Transm Dis, 44(1), 48–56. doi: 10.1097/OLQ.0000000000000535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese SK, Underhill K, Earnshaw VA, Hansen NB, Kershaw TS, Magnus M, . . . Dovidio JF (2016). Framing HIV Pre-Exposure Prophylaxis (PrEP) for the General Public: How Inclusive Messaging May Prevent Prejudice from Diminishing Public Support. AIDS Behav. doi: 10.1007/s10461-016-1318-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo Hojilla J, Koester KA, Cohen SE, Buchbinder S, Ladzekpo D, Matheson T, & Liu AY (2016). Sexual Behavior, Risk Compensation, and HIV Prevention Strategies Among Participants in the San Francisco PrEP Demonstration Project: A Qualitative Analysis of Counseling Notes. AIDS Behav(1573–3254 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center For Disease Control and Prevention. (2014). Preexposure Prophylaxis for the Prevention of HIV infection in the United States -Clinical Providers’ Supplement Atlanta, Georgia: US Public Health Service [Google Scholar]
- Centers for Disease Control and Prevention. (2014). HIV Risk, Prevention, and Testing Behaviors— National HIV Behavioral Surveillance System: Men Who Have Sex With Men, 20 U.S. Cities, 2011. HIV Surveillance Special Report 8 Retrieved from http://www.cdc.gov/hiv/library/reports/surveillance/#special. [Google Scholar]
- Centers for Disease Control and Prevention. (2015). 2014 Sexually transmitted disease survaliance. Retrieved from http://www.cdc.gov/std/stats/
- Centers for Disease Control and Prevention. (2018). Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: a clinical practice guideline.: US Public Health Service; Retrieved from https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. [Google Scholar]
- Chard AN, Metheny N, & Stephenson R (2017). Perceptions of HIV Seriousness, Risk, and Threat Among Online Samples of HIV-Negative Men Who Have Sex With Men in Seven Countries. JMIR Public Health and Surveillance, 3(2), e37. doi: 10.2196/publichealth.7546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collumbein M, Busza Joanna., Cleland John., Campbell Oona. (2012). Social Science Methods for research on Sexual and Reproductive Health. Malta: WHO Press. [Google Scholar]
- Cornelisse VJ, Zhang L, Law M, Chen MY, Bradshaw CS, Bellhouse C, . . . Chow EPF (2018). Concordance of gonorrhoea of the rectum, pharynx and urethra in same-sex male partnerships attending a sexual health service in Melbourne, Australia. BMC Infect Dis, 18(1), 95. doi: 10.1186/s12879-018-3003-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer CDJ, Hoebe C, van Liere G, van Bergen J, Cals JWL, Stals FS, & Dukers-Muijrers N (2017). A comprehensive overview of urogenital, anorectal and oropharyngeal Neisseria gonorrhoeae testing and diagnoses among different STI care providers: a cross-sectional study. BMC Infect Dis, 17(1), 290. doi: 10.1186/s12879-017-2402-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foschi C, Gaspari V, Sgubbi P, Salvo M, D’Antuono A, & Marangoni A (2018). Sexually transmitted rectal infections in a cohort of ‘men having sex with men’. J Med Microbiol, 67(8), 1050–1057. doi: 10.1099/jmm.0.000781 [DOI] [PubMed] [Google Scholar]
- Gamarel KE, & Golub SA (2015). Intimacy motivations and pre-exposure prophylaxis (PrEP) adoption intentions among HIV-negative men who have sex with men (MSM) in romantic relationships. Ann Behav Med, 49(2), 177–186. doi: 10.1007/s12160-014-9646-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CA (2012). Condom use in the context of sex research: a commentary. Sex Health, 9(1), 103–108. doi: 10.1071/SH11103 [DOI] [PubMed] [Google Scholar]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, & Vargas L (2010). Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med, 363. doi: 10.1056/NEJMoa1011205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm P (2010). Social Desirability Bias In Wiley International Encyclopedia of Marketing: John Wiley & Sons, Ltd. [Google Scholar]
- Grov C, Hirshfield S, Remien RH, Humberstone M, & Chiasson MA (2013). Exploring the venue’s role in risky sexual behavior among gay and bisexual men: an event-level analysis from a national online survey in the U.S. Arch Sex Behav, 42(2), 291–302. doi: 10.1007/s10508-011-9854-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grov C, Rendina HJ, Ventuneac A, & Parsons JT (2013). HIV risk in group sexual encounters: an event-level analysis from a national online survey of MSM in the U.S. J Sex Med, 10(9), 2285–2294. doi: 10.1111/jsm.12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger B, Gorgens K, Morgenstern J. . (2015). The effects of sexual sensation seeking and alcohol use on risky sexual behavior among men who have sex with men. AIDS Behav, 19(3), 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff CC, Chakravarty D, Bircher AE, Campbell CK, Grisham K, Neilands TB, . . . Dworkin S (2015). Attitudes Towards PrEP and Anticipated Condom Use Among Concordant HIV-Negative and HIV-Discordant Male Couples. AIDS Patient Care STDS, 29(7), 408–417. doi: 10.1089/apc.2014.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler C, Wray TB, Pantalone DW, Kruis RD, Mastroleo NR, Monti PM, et al. (2015). Daily associations between alcohol use and unprotected anal sex among heavy drinking HIV-positive men who have sex with men. . AIDS Behav, 19(3), 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DA, Dombrowski JC, Bell TR, Kerani RP, & Golden MR (2016). HIV Incidence Among Men Who Have Sex With Men After Diagnosis With Sexually Transmitted Infections. Sex Transm Dis, 43(4), 249–254. doi: 10.1097/OLQ.0000000000000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CF, Vaughan AS, Luisi N, Sanchez TH, Salazar LF, & Frew PM (2015). The effect of high rates of bacterial sexually transmitted infections on HIV incidence in a cohort of black and white men who have sex with men in Atlanta, Georgia. AIDS Res Hum Retroviruses, 31. doi: 10.1089/aid.2015.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakower DS, Mayer KH (2015). Pre-Exposure Prophylaxis to Prevent HIV Infection: Current Status, Future Opportunities and Challenges. Drugs. doi: 10.1007/s40265-015-0355-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakower DS, Oldenburg CE, Mitty JA, Wilson IB, Kurth A, Maloney KM, . . . Mayer KH (2015). Knowledge, Beliefs and Practices Regarding Antiretroviral Medications for HIV Prevention: Results from a Survey of Healthcare Providers in New England. PLoS One, 10(7), e0132398. doi: 10.1371/journal.pone.0132398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landovitz RJ, & Coates TJ (2014). Moving HIV PrEP from research into practice. The Lancet Infectious Diseases, 14(9), 781–783. doi: 10.1016/S1473-3099(14)70747-9 [DOI] [PubMed] [Google Scholar]
- Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, . . . Kolber MA (2015). Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med, 1–11. doi: 10.1001/jamainternmed.2015.4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, & Gilson R (2015). Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKechnie ML, Bavinton BR, & Zablotska IB (2012). Understanding of Norms Regarding Sexual Practices Among Gay Men: Literature Review. AIDS Behav, 17(4), 1245–1254. doi: 10.1007/s10461-012-0309-8 [DOI] [PubMed] [Google Scholar]
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, & Bernstein KT (2012). Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men--San Francisco, 2010. Sex Transm Dis, 39(6), 482–484. doi: 10.1097/OLQ.0b013e3182495e2f [DOI] [PubMed] [Google Scholar]
- Smith DK, Herbst JH, Zhang X, & Rose CE (2015). Condom Effectiveness for HIV Prevention by Consistency of Use Among Men Who Have Sex With Men in the United States. J Acquir Immune Defic Syndr, 68(3), 337–344. doi: 10.1097/qai.0000000000000461 [DOI] [PubMed] [Google Scholar]
- Smith DK, Pan Y, Rose CE, Pals SL, Mehta SH, Kirk GD, & Herbst JH (2015). A Brief Screening Tool to Assess the Risk of Contracting HIV Infection Among Active Injection Drug Users. J Addict Med, 9(3), 226–232. doi: 10.1097/ADM.0000000000000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AY, Remien RH, & Tross S (2015). PrEP in substance abuse treatment: a qualitative study of treatment provider perspectives. Subst Abuse Treat Prev Policy, 10(1), 1. doi: 10.1186/1747-597x-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin K, Davey-Rothwell M, Yang C, Siconolfi D, Latkin C. . (2014). An examination of associations between social norms and risky alcohol use among African American men who have sex with men. . DrugAlcohol Depend., 134(1), 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill K, Morrow KM, Colleran C, Holcomb R, Calabrese SK, Operario D, . . . Mayer KH (2015). A Qualitative Study of Medical Mistrust, Perceived Discrimination, and Risk Behavior Disclosure to Clinicians by U.S. Male Sex Workers and Other Men Who Have Sex with Men: Implications for Biomedical HIV Prevention. J Urban Health. doi: 10.1007/s11524-015-9961-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Straten A, Van Damme L, Haberer JE, & Bangsberg DR (2012). Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS, 26(7), F13–F19. doi: 10.1097/QAD.0b013e3283522272 [DOI] [PubMed] [Google Scholar]
- Venter F, Allais L, & Richter M (2014). Exposure Ethics: Does Hiv Pre-Exposure Prophylaxis Raise Ethical Problems for the Health Care Provider and Policy Maker? Bioethics, 28(6), 269–274. doi: 10.1111/bioe.12021 [DOI] [PubMed] [Google Scholar]
- Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, & Follansbee S (2015). No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis, 61. doi: 10.1093/cid/civ778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2014). PrEP for MSM - systematic review – to inform the WHO Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva World Health Organization; [PubMed] [Google Scholar]
- Yadav D, Chakrapani V, Goswami P, Ramanathan S, Ramakrishnan L, George B, et al. ,. (2014). Association between alcohol use and HIV-related sexual risk behaviors among men who have sex with men (MSM): findings from a multi-site bio-behavioral survey in India. AIDS Behav., 18(7), 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]