Abstract
Objective:
Analyzing ectocervical biopsy tissue from women before and after they initiated use of depot-medroxyprogesterone acetate (DMPA), we previously reported this progestin reduces levels of the cell-cell adhesion molecule (CCAM) desmoglein-1 and increases genital mucosal permeability. We likewise saw treating mice with 1.0 mg of DMPA reduced vaginal CCAM expression and increased genital pathogen susceptibility. Herein, we used dose-response studies to delimit DMPA doses and serum MPA levels in mice associated with impaired genital mucosal barrier function and enhanced susceptibility to low-dose herpes simplex virus type 2 (HSV-2) infection.
Study Design:
We compared genital CCAM expression, genital mucosal permeability, and susceptibility to genital inoculation with 103 plaque-forming units of HSV-2 among mice in estrus vs. after treatment with 0.01 mg, 0.1 mg, 0.3 mg, or 1.0 mg of DMPA.
Results:
Compared to mice in estrus, DMPA treatment in dose-dependent fashion significantly reduced desmoglein 1α (Dsg1a) and desmocollin-1 (Dsc1) gene expression, reduced DSG1 protein levels, and increased genital mucosal permeability to low molecular weight molecules. While no mice infected with HSV-2 in estrus died, we respectively saw 50% and 100% mortality in mice administered 0.1 mg or 0.3 mg of DMPA. At time of infection, mean serum MPA levels in mice administered the 0.1 mg or 0.3 mg doses were 3.8 nM and 13.0 nM respectively (values comparable to trough and peak MPA serum levels in women using DMPA).
Conclusions:
Mice with pharmacologically relevant serum MPA concentrations display significant changes in genital CCAM expression, genital mucosal barrier function, and HSV-2 susceptibility.
Keywords: DMPA, genital HSV-2 infection, genital mucosal barrier function
1. Introduction
It remains uncertain if use of the progestin-only injectable contraceptive depot-medroxyprogesterone acetate (DMPA) is a significant risk factor for HIV acquisition. While multiple observational studies found that DMPA use increases a woman’s susceptibility to HIV [1, 2], a recently completed randomized clinical trial (RCT) saw that women using DMPA were no more likely to acquire HIV than women using levonorgestrel (LNG) implants or copper intrauterine devices [3]. However, this RCT evaluated relative risk of HIV acquisition risk in women using 1 of these 3 contraceptives choices (i.e., it was not designed to compare HIV incidence rates to women using no form of contraception). It also had insufficient statistical power to detect any contraceptive-mediated increase in HIV acquisition lower than 30% [3]. These study design limitations are indicative of the types of obstacles that must be overcome to define the precise relationship between DMPA and HIV susceptibility and provides impetus for research that alternatively explores the effects of exogenous progestins on basic host defense mechanisms in the female genital tract. Our laboratory, among others, uses animal models to define immunomodulatory effects of progestins that have the potential to alter HIV susceptibility. To date, our studies uncovered DMPA treatment of mice reduces genital expression of the cell-cell adhesion molecules (CCAM) desmoglein-1α (Dsg1a) and desmocollin-1 (Dsc1) and weakens genital mucosal barrier function by increasing genital epithelial permeability [4]. We also reported treating mice with DMPA or levonorgestrel (LNG) increases susceptibility to genital infection with herpes simplex virus type 2 (HSV-2), Chlamydia trachomatis, and cell-associated HIV, whereas treatment with the potent corticosteroid methylprednisolone (MePRDL) does not increase genital pathogen susceptibility [4-6]. Because MPA binds both the progesterone receptor (PR) and glucocorticoid receptor (GR), but LNG and MePRDL more selectively bind the PR and GR, respectively [7], these findings implied progestin binding and activation of the PR initiates cellular processes that compromise an important host defense in the female genital tract.
Guided by our mouse model findings, we compared DSG1 levels and mucosal epithelial permeability in ectocervical biopsies from women before and 30 days after they began using Depo-Provera® (whose active component is MPA) or Mirena® (an intrauterine device releasing LNG). As reported in 2016 and 2017, these studies showed women initiating Depo-Provera® or Mirena® display changes in ectocervical DSG1 levels and genital mucosal barrier function that are analogous to effects seen in DMPA- and LNG-treated mice [4, 8]. This MPA-mediated reduction of genital DSG1 was independently confirmed in a follow-up study that interrogated ectocervical biopsy tissue from women before and 6 weeks after initiating use of Depo-Provera® [9]. Together, these findings showed that genital mucosal barrier function is comparably weakened in mice and humans by DMPA or LNG and identified mice as suitable models for exploring effects of exogenous progestins on this fundamentally important anti-virus defense mechanism. Herein, we sought to extend this work by conducting dose-response studies in mice to delimit DMPA doses that reduce genital Dsg1a and Dsc1 expression and to define serum MPA levels associated with increased susceptibility to low-dose genital HSV-2 infection.
2. Material and methods
All research was approved by the Stanford University Institutional Animal Care and Use Committee prior to study initiation. As indicated, 6- to 8-week old C57BL/6J female mice (The Jackson Laboratory, Bar Harbor ME) weighing approximately 20 g were subcutaneously (s.c.) injected with 100 μl of a PBS solution containing 0.01 mg, 0.1 mg, 0.3 mg, or 1.0 mg of DMPA (Pharmacia and Upjohn, New York NY). This dose range was selected based on animal modeling studies that specified it would generate serum MPA levels in mice that approach peak (Cmax) and trough (Ctrough) serum levels in women after s.c. or intramuscularly (i.m.) DMPA administration [10-12]. Where indicated, cells obtained from vaginal lavage were stained with crystal violet to identify mice that were in the estrus stage of the estrous cycle [13]. Using methods detailed elsewhere [4], mice in estrus or injected with indicated DMPA doses 5 days earlier (n = 5 per group) were euthanized and vaginal tissue excised to define Dsg1a and Dsc1 expression via quantitative real time PCR (qRT-PCR) (with relative expression quantified using pyruvate carboxylase as a housekeeping reference gene). Using methods already described [4], mice were also used to quantify vaginal DSG1 protein levels via immunofluorescence staining and confocal microscopy. As detailed before, mice in estrus and DMPA-treated mice were sedated to intravaginally administer 10 μl of a PBS solution containing 62.5 μg of dextran Texas-Red (MW = 70,000 Da) and 50 μg of Lucifer yellow CH, lithium salt (LY) (MW = 457 Da) (n = 4 per group). Mice were euthanized 45 minutes later, and the vaginal tissues formaldehyde-fixed, agarose-embedded, and DAPI-stained to assess fluorescent molecule penetration into the vaginal mucosa by confocal microscopy [4]. Depth of penetration of the low molecular weight (LMW) molecule LY was calculated as the fraction of full-thickness vaginal epithelium that contained this fluorescent molecule. In separate studies, peripheral blood was collected from mice in estrus or 5 days after indicated DMPA treatment (n = 4 per group) to quantify serum MPA levels by liquid chromatography–tandem triple quadrupole mass spectrometry (LC–MS/MS) and serum estradiol levels by ELISA (limit of detection 3 pg/mL) [14]. After blood collection, mice were genitally infected with 103 plaque-forming units (pfu) of HSV-2 and monitored daily for encephalopathic changes and death [15]. For statistical analyses, normal distribution was assessed by evaluation of the residuals. For multiple comparisons, we used one-way ANOVA with Dunnett’s post hoc test or Kruskal–Wallis test with Dunn's post hoc test (depending of data distribution). Kaplan-Meier survival curves and log-rank tests used to assess mortality rates caused by HSV-2 infection. All tests were performed using Prism 7 software (GraphPad, La Jolla CA).
3. Results
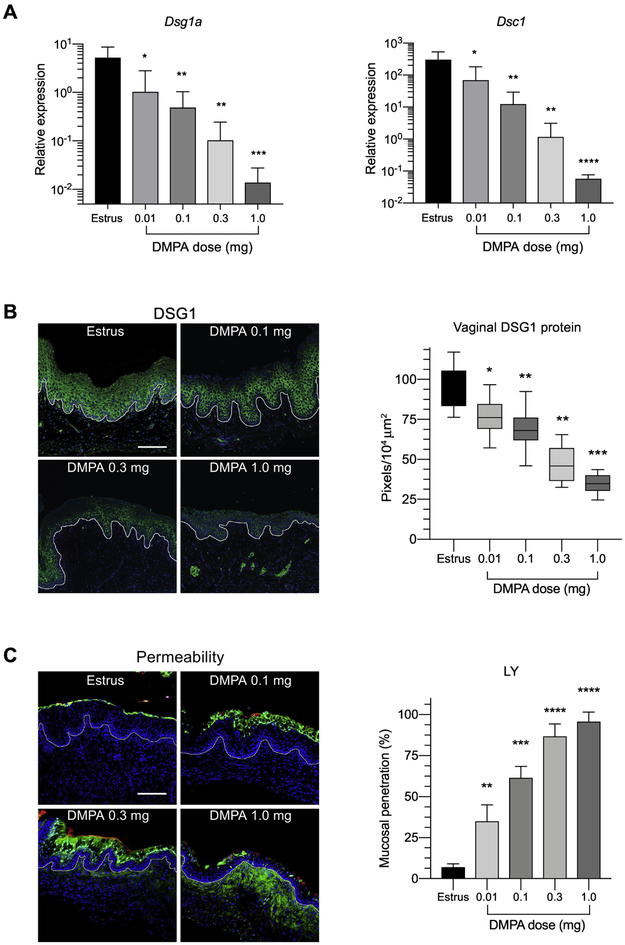
Prior work from our laboratory established that mice administered 1.0 mg of DMPA display reduced genital tract levels of the CCAM Dsg1a and Dsc1 and increased susceptibility to genital HSV-2 infection [4, 16]. To extend these findings, we began the current investigation by comparing vaginal expression of these same CCAM among mice in estrus vs. mice that were administered 1.0 mg of DMPA or one of 3 lower DMPA doses (i.e., 0.01 mg, 0.1 mg, or 0.3 mg) 5 days earlier. These studies showed that compared to mice in estrus, DMPA treatment induced significant, dose-dependent decreases in vaginal Dsg1a and Dsc1 gene expression (Fig. 1A) and DSG1 protein levels (Fig. 1B). Though the 1.0 mg dose most profoundly reduced Dsg1a and Dsc1 levels, the lowest DMPA dose examined (i.e., 0.01 mg) also significantly reduced CCAM expression of these CCAM (Fig. 1A & B). Congruent with its influence on CCAM expression, DMPA treatment also promoted significant, dose-dependent increase in the capacity of intravaginally administered LMW molecules to penetrate vaginal mucosal epithelium and submucosal tissue (Fig 1C). While these dose-response studies newly revealed DMPA doses < 1.0 mg decrease vaginal CCAM expression and significantly impair genital mucosal barrier function, we had yet to delineate the serum MPA concentrations produced by these doses or the effect of these lower MPA doses on susceptibility to genital HSV-2 infection.
Figure 1. DMPA decreases genital levels of CCAM and increases genital mucosal permeability in a dose-dependent fashion.
Mice in estrus and mice administered indicated DMPA doses 5 days previously were euthanized to process vaginal tissue for RNA isolation and immunofluorescence staining. A) Bar graphs show DMPA dose-dependent decrease in the relative expression of Dsg1a and Dsc1 mRNA. B) Images on left are representative of formaldehyde-fixed, paraffin-embedded vaginal tissue from mice in estrus and DMPA-treated mice that were stained with rabbit anti-DSG-1 and Alexa Fluor 488-conjugated anti-rabbit IgG (green) and counterstained with DAPI (blue) for confocal microscopy. Right-sided panel depicts the DMPA dose-dependent decrease in levels of DSG1 protein. C) Groups of mice treated as described in Figure 1B were anesthetized for intravaginal administration of a PBS solution containing Lucifer yellow (457 Da) (green) and 70 KDa dextran-Texas Red (red). Mice were euthanized after 45 minutes, and vaginal tissue processed and counterstained with DAPI (blue) for confocal microscopy. Images on left illustrate penetration of LY into vaginal mucosal epithelium. Right-sided panel shows the DMPA dose-dependent increase for penetration of this LMW fluorescent molecule into the vaginal epithelium. Data normality was defined by evaluation of the residuals. CCAM expression in DMPA treatment groups was compared to mice in estrus using one-way ANOVA and Dunnett’s post hoc tests. CCAM, cell-cell adhesion molecule; DAPI, 4,6-diamidino-2-phenylindole; DMPA, depot-medroxyprogesterone acetate; scale bar denotes 100 μm; Dsg1a, desmoglein-1α; Dsc1, desmocollin-1; and DSG1, desmoglein-1; *, **, ***, and **** denote p < 0.05, p < 0.01, p < 0.001, and p < 0.0001 respectively.
To define serum MPA levels associated with 0.01 mg, 0.1 mg, or 0.3 mg DMPA doses, MPA was measured in the peripheral blood of additional groups of identically DMPA-treated mice. This blood was collected 5 days after DMPA administration, and directly before mice in estrus and DMPA-treated mice were genitally inoculated with 103 pfu of HSV-2. These studies showed that mice treated with 0.01 mg, 0.1 mg, 0.3 mg, or 1.0 mg of DMPA had mean serum MPA concentrations of 1.4 nM (0.6 ng/mL), 3.8 nM (1.4 ng/mL), 13.0 nM (5.0 ng/mL), and 25.9 nM (10.0 ng/mL), respectively (Fig. 2A). We inspected these mice daily after HSV-2 infection, and observed no morbidity or mortality develop in untreated mice infected in estrus or mice that had been treated with 0.01mg of DMPA. However, infection caused 50% mortality in mice administered 0.1 mg of DMPA (i.e., mice with mean serum MPA levels = 3.8 nM) and 100% mortality in mice administered 0.3 mg of DMPA (i.e., mice with mean serum MPA = 13.0 nM) (Fig. 2B & 2C). Though HSV-2-induced mortality was significantly higher in mice administered 1.0 mg or 0.3 mg DMPA prior to infection compared to mice administered the 0.1 mg DMPA dose, serum levels of estradiol (i.e., endogenous estrogen) among these 3 DMPA-treated groups were indistinguishable from each other and lower than values seen in mice in proestrus (i.e., the estrus cycle stage with highest serum estradiol concentrations) (Fig. 2D).
Figure 2. DMPA-treated mice with MPA serum levels at the time of infection comparable to Ctrough serum concentrations in women were more susceptibility to genital HSV-2 infection.
Mice in estrus and mice treated with 0.01 mg, 0.1 mg, 0.3 mg, or 1.0 mg of DMPA 5 days earlier were genitally inoculated with 103 pfu of HSV-2. Immediately before infection, peripheral blood was collected to quantify serum concentrations of estradiol and MPA. A) Panel depicts positive linear correlation between DMPA dose and MPA serum levels (R2 = 0.9643). B) Survival curve data shows that HSV-2 was uniformly lethal in mice administered ≥ 0.3 mg DMPA, whereas 50% and 0% of mice that received 0.1 mg or 0.01 mg of DMPA respectively succumbed to infection. Between-group comparisons were performed using the long-rank test. C) Panel depicts relationship between serum MPA levels at infection and HSV-2-induced mortality rates. Red and blue dotted vertical lines denote typical values for Ctrough (red) and Cmax (blue) serum MPA levels in women using DMPA. D) Though mortality differed significantly in mice administered DMPA doses of 0.1 mg (50% mortality) vs. 0.3 mg or 1.0 mg (both 100% mortality), serum estradiol levels in these groups were comparable. Data normality was determined by evaluation of the residuals. Serum estradiol levels were compared by one-way ANOVA and Tukey’s post hoc tests (all DMPA doses examined decreased serum estradiol levels compared to mice in proestrus and diestrus. DMPA, depot-medroxyprogesterone acetate; HSV-2, herpes simplex virus type 2; pfu, plaque forming unit; * and ** denote p < 0.05 and p < 0.01 respectively.
4. Discussion
This study reveals that compared to mice in estrus there is significantly less expression of the CCAM Dsg1a and Dsc1 in vaginal tissue of DMPA-treated mice that have serum MPA levels roughly equivalent to Ctrough levels measured in women using DMPA for hormonal contraception. Also compared to mice in estrus, this study shows that mice with pharmacologically relevant serum MPA levels are significantly more susceptibility to genital HSV-2 infection. Strongly supporting that mouse susceptibility to a genital pathogen is DMPA dose dependent, we observed a 50% higher mortality rate in mice with MPA serum concentrations of 3.8 nM (1.4 ng/mL) vs. 13.0 nM (5.0 ng/mL) at the time of HSV-2 infection. On the other hand, these 2 groups of DMPA-treated mice (and mice administered 1.0 mg of DMPA) displayed similar serum concentrations of estradiol. Together, these findings indicate that serum MPA levels (rather than DMPA dose-dependent decreases in endogenous estrogen) are more predictive of mouse susceptibility to genital HSV-2 infection. Because prior reports established that large doses of the pure GR agonist MePRDL do not increase mouse susceptibility to genital HSV-2 infection and that testosterone treatment of castrated mice (i.e., stimulation of the androgen receptor) lowers the risk of HSV-induced encephalopathy [4, 18], current results further suggest that DMPA-mediated changes in susceptibility to genital HSV-2 infection are unrelated to MPA binding and activation of the glucocorticoid and androgen receptors.
As various clinical reports identified that women using Depo-Provera® (i.e., the 150 mg formulation of DMPA injected i.m. every 3 months) are more likely to acquire HIV than women using no hormonal contraception [1], it was recently postulated that risk of HIV transmission may be mitigated by s.c. administration of the 104 mg formulation [2]. While this outcome is possible, current mouse model findings do not support this conclusion. Specifically, while Ctrough serum MPA concentrations in women using the 104 mg or 150 mg formulations are comparable and about 2.6 nM (1.0 ng/mL) [11-12,17], we saw significantly reduced vaginal levels of DSG1 protein and enhanced HSV-2 susceptibility in mice with serum MPA concentration at time of infection roughly equivalent to Ctrough levels in women. Because greater HSV-2 susceptibility occurs in mice with serum MPA levels analogous to Ctrough levels in women, our findings also imply only large-scale longitudinal investigation will have sufficient statistical power to detect significant differences in HIV transmission efficiency between women using the 104 mg or 150 mg DMPA formulations. On the other hand, though current mouse model findings show MPA-mediated changes in genital CCAM expression and pathogen susceptibility are generated in mice with serum MPA concentrations comparable to Ctrough MPA concentrations in women, only clinical research will resolve if the compromise of genital mucosal barrier function seen in women initiating DMPA [4] increases their likelihood of acquiring genital infection. However, findings from the current study do suggest that executing clinical studies needed to illuminate links between exogenous progestins and genital infection will benefit from defining the connections between infection and genital levels of DSG1, a molecule emerged as a reliable biomarker of murine genital mucosal barrier function and susceptibility to genital HSV-2 infection.
Acknowledgements and funding:
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (under Award Number R01HD094634). The content is solely responsibility of the authors and does not necessarily represent official National Institutes of Health (NIH) views. Serum MPA levels were quantified by the Endocrine Technologies Core (ETC) (Oregon National Primate Research Center) (under Award Number P51OD011092) and serum estradiol levels were quantified by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (under Award Number P50HD28934).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Polis CB, Curtis KM, Hannaford PC, Phillips SJ, Chipato T, Kiarie JN, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS. 2016. November 13; 30(17):2665–2683. 10.1097/QAD.0000000000001228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Polis CB, Achilles SL, Hel Z, Hapgood JP. Is a lower-dose, subcutaneous contraceptive injectable containing depot medroxyprogesterone acetate likely to impact women's risk of HIV? Contraception. 2018. March; 97(3):191–197. 10.1016/j.contraception.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet. 2019. June 13 pii: S0140-6736(19)31288-7. 10.1016/S0140-6736(19)31288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Quispe Calla NE, Vicetti Miguel RD, Boyaka PN, Hall-Stoodley L, Kaur B, Trout W, et al. Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal Immunol. 2016. November; 9(6):1571–1583. 10.1038/mi.2016.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Quispe Calla NE, Vicetti Miguel RD, Glick ME, Kwiek JJ, Gabriel JM, Cherpes TL. Exogenous oestrogen inhibits genital transmission of cell-associated HIV-1 in DMPA-treated humanized mice. J Int AIDS Soc. 2018. January; 21(1). 10.1002/jia2.25063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Quispe Calla NE, Vicetti Miguel RD, Cherpes TL. Levonorgestrel and female genital tract immunity: time for a closer look. J Infect Dis. 2018. September 22; 218(9):1517–1518. 10.1093/infdis/jiy363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7.Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011. June; 76(7):636–52. 10.1016/j.steroids.2011.03.001 [DOI] [PubMed] [Google Scholar]
- [8].Quispe Calla NE, Vicetti Miguel RD, Trout W, Cherpes TL. HIV and hormonal contraception: bench and bedside. J Acquir Immune Defic Syndr. 2017. March 1; 74(3):e85–e86. 10.1097/QAI.0000000000001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zalenskaya IA, Chandra N, Yousefieh N, Fang X, Adedipe OE, et al. Use of contraceptive depot medroxyprogesterone acetate is associated with impaired cervicovaginal mucosal integrity. J Clin Invest. 2018. October 1; 128(10):4622–4638. 10.1172/JCI120583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016. March;7(2):27–31. 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shelton JD, Halpern V. Subcutaneous DMPA: a better lower dose approach. Contraception. 2014. May;89(5):341–3. 10.1016/j.contraception.2013.10.010 [DOI] [PubMed] [Google Scholar]
- [12].Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009. July;80(1):7–17. 10.1016/j.contraception.2009.02.005. [DOI] [PubMed] [Google Scholar]
- [13].McLean AC, Valenzuela N, Fai S, Bennett SA. Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation for Mouse Estrous Cycle Staging Identification. J Vis Exp. 2012. September 15;(67):e4389 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blue SW, Winchell AJ, Kaucher AV, Lieberman RA, Gilles C, et al. Simultaneous quantitation of multiple contraceptive hormones in human serum by LC-MS/MS. Contraception. 2018. April; 97(4):363–369. 10.1016/j.contraception.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cherpes TL, Harvey SA, Phillips JM, Vicetti Miguel RD, Melan MA, Quispe Calla NE, et al. Use of transcriptional profiling to delineate the initial response of mice to intravaginal herpes simplex virus type 2 infection. Viral Immunol. 2013. June;26(3):172–9. 10.1089/vim.2012.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vicetti Miguel RD, Hendricks RL, Aguirre AJ, Melan MA, Harvey SA, Terry-Allison T, et al. Dendritic cell activation and memory cell development are impaired among mice administered medroxyprogesterone acetate prior to mucosal herpes simplex virus type 1 infection. J Immunol. 2012. October 1;189(7):3449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mishell DR Jr. Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med. 1996. May; 41(5 Suppl):381–90. [PubMed] [Google Scholar]
- [18].Yirrell DL, Blyth WA, Hill TJ. The influence of androgens on paralysis in mice following intravenous inoculation of herpes simplex virus. J Gen Virol. 1987. September;68 (Pt 9):2461–4. [DOI] [PubMed] [Google Scholar]