Abstract
Behavioral signs of Autism Spectrum Disorder (ASD) are typically observable by the second year of life and a reliable diagnosis of ASD is possible by 2 to 3 years of age. Studying infants with familial risk for ASD allows for the investigation of early signs of ASD risk within the first year. Brain abnormalities such as hyper-connectivity within the first year may precede the overt signs of ASD that emerge later in life. In this preliminary study, we use functional near-infrared spectroscopy (fNIRS), an infant-friendly neuroimaging tool that is relatively robust against motion artifacts, to examine functional activation and connectivity during naturalistic social interactions in 9 high-risk (HR; older sibling with ASD) and 6 low-risk (LR; no family history of ASD) infants from 6 to 9 months of age. We obtained two 30-second baseline periods and a 5-minute social interaction period. HR infants showed reduced right and left-hemispheric activation compared to LR infants based on oxy (HbO2) and deoxy (HHb) signal trends. HR infants also had greater functional connectivity than LR infants during the pre- and post-social periods and showed a drop in connectivity during the social period. Our findings are consistent with previous work suggesting early differences in cortical activation associated with familial risk for ASD, and highlight the promise of fNIRS in evaluating potential markers of ASD risk during naturalistic social contexts.
Keywords: Infants, Autism Spectrum Disorder, Social Interaction, fNIRS, Cortical Activation, Functional Connectivity
1. Introduction
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental disorder that is characterized by deficits in social communication and the presence of restricted and repetitive behaviors and interests (American Psychiatric Association, 2013). The presence of other comorbidities in ASD such as sensory atypicalities and motor delays/impairments suggests it is indeed a whole brain disorder affecting multiple brain regions and functions (Bhat, Landa & Galloway, 2011; Sacrey, Bennett, & Zwaigenbaum, 2015; Elsabbagh & Johnson, 2016; van Etten et al., 2016). Some initial evidence suggests that atypical development in ASD is related to aberrations in whole brain connectivity that may begin prior to birth (Bauman & Kemper, 2005; Courchesne, Campbell & Solso, 2007). Prospective studies of infants with familial risk (i.e., younger siblings of children with ASD or high-risk [HR] siblings) have elucidated several behavioral signs of emerging ASD during the second year of life, including delays in language and gesture production as well as poor spontaneous initiation of and response to the joint attention bids of others (i.e., the ability to shift attention between objects and caregivers) (Sacrey et al., 2015; Zwaigenbaum, Bryson, & Garon, 2013; Zwaigenbaum et al., 2015). However, common consensus among researchers is that early behavioral signs within the first year of life do not reliably predict future ASD outcomes (Zwaigenbaum, Bryson, & Garon, 2013; Zwaigenbaum et al., 2015). It is possible that differences in brain development within the first year of life precede overt behavioral signs of ASD in the second year (Wolff et al., 2015). The discovery of these differences have the potential to contribute to our understanding of ASD as it first emerges and may facilitate earlier detection and improved access to early intervention. To this end, we conducted a preliminary study using functional near-infrared spectroscopy (fNIRS), a neuroimaging method that is well suited for infants (Lloyd-Fox, Blasi, & Elwell, 2010), to compare cortical activation and connectivity during naturalistic social interactions between HR and LR infants.
There is a growing literature examining differences in structural brain development associated with ASD during infancy and toddlerhood. The Infant Brain Imaging Study (IBIS) network has published a series of studies showing excessive cortical connectivity as early as 6 months of age in infants who later developed ASD (HR-ASD) compared to infants who did not develop ASD (HR-non ASD) (Wolff et al., 2012; 2015; Elison et al., 2013; Hazlett et al., 2017). Specifically, studies using structural MRI and diffused weighted imaging techniques have provided evidence of abnormal connectivity between 6 and 24 months of age in HR-ASD infants (Wolff et al., 2012; 2015). The thickness of the corpus callosum was greater in the 6-month-old HR-ASD infants compared to low-risk (LR) control infants and correlated with rates of repetitive behavior at two years of age within the HR-ASD group (Wolff et al., 2015). Hazlett et al (2017) reported early hyper-expansion of the cortical surface between 6 and 12 months and an increase in total brain volume between 12 and 24 months of age in HR-ASD infants. They also found strong correlations between these early patterns of brain development and infants’ autism severity at 24 months. Similarly, HR-ASD infants showed early abnormalities in visual orienting at 7 months that were not associated with radial diffusivity (i.e., a measure of white matter organization) of the corticospinal tract and the splenium of the corpus callosum (Elison et al., 2013) suggesting that disorganization in the microstructure of the corpus callosum of the HR-ASD infants may be linked to infants’ early gaze abnormalities; this was not the case in the LR infants. Taken together, findings from structural neuroimaging studies of HR infants suggest that differences in brain development are apparent within the first two years of life and are associated with some aspects of ASD symptomatology.
A step further would be to investigate functional activation (FA) and functional connectivity (FC) measures to describe brain activity patterns associated with resting states or during social stimuli presentation in infants with an elevated risk for ASD. The fMRI literature in this area is relatively limited given the challenges of fMRI with regard to data collection in awake infants. One fMRI study, however, examined brain responses to auditory social stimuli (i.e., human vocalization) in 4 to 7-month-old HR infants during natural sleep, finding that HR infants had less selective fronto-temporal activation in response to vocalizations compared to LR infants (Blasi et al., 2015). Given the limitations of using fMRI in infants, recent studies have turned to functional near-infrared spectroscopy (fNIRS), a novel neuroimaging tool that is relatively robust in the presence of motion artifacts, allowing for the investigation of brain activation in awake and interactive infants (Lloyd-Fox, Blasi, & Elwell, 2010; McDonald et al., 2019). Importantly, fNIRS is an ideal method to study functional brain development in the context of naturalistic social interactions that more closely approximate to infants’ daily experiences (Jones et al., 2015). Paradigms using more complex, naturalistic social stimuli may better elicit difficulties experienced by HR infants during real-world, caregiver interactions. Moreover, parent-child interactions could be used as assessments or as parent-mediated treatment strategies to address the behavioral impairments of HR infants (Bradshaw et al., 2015).
To date, a small number of fNIRS studies have investigated FC and FA in HR infants or young children with ASD (Keehn et al., 2013; Lloyd-Fox et al. 2013; Fox et al., 2013; Zhu et al., 2015). During a face perception task, HR infants showed a lack of difference in both right and left orbito-frontal activation between conditions of smile vs. neutral facial emotions compared to LR infants who showed increased smile-related activation in the same region (Fox et al., 2013). Lloyd-Fox et al. (2013) found reduced activation mainly in the right temporal cortex in HR infants compared to LR infants during presentation of visual and auditory social stimuli. Keehn et al. (2013) conducted a longitudinal comparison of HR and LR infants and found intra- and inter-hemispheric hyper-connectivity at 3 months and hypo-connectivity at 12 months of age during passive listening to linguistic stimuli. At later ages, school-age children with ASD showed no differences in left prefrontal cortex activation when observing videos of a person engaged in joint attention (JA) vs. non-JA behaviors (Zhu et al., 2015). However, they showed greater right prefrontal cortex activation during the non-JA condition compared to the JA condition. In contrast, age-matched, typically developing (TD) children showed greater right and left prefrontal cortex activation during the JA condition vs. the non-JA condition. In terms of connectivity, ASD children showed no differences in inter-hemispheric connectivity between the JA and non-JA conditions whereas TD children showed reduced inter-hemispheric connectivity and greater left-left prefrontal connectivity during the JA condition (vs. the non-JA condition) (Zhu et al., 2015). Overall, these studies confirm atypical findings of FA and FC in infants at high-risk for and children with ASD with differing patterns across ages, contexts, regions, and hemispheres. Additionally, studies of typical infant social development exemplify the utility of fNIRS in assessing infant brain activity during social interactions. For instance, 4- to 6-month-old infants had greater activation in the fronto-temporal cortices, specifically, the inferior frontal gyrus (IFG) and superior temporal sulcus (STS); to a combination of natural social inputs of infant-directed social gaze and infant-directed speech compared to either provided individually (Lloyd-Fox et al., 2015). In another study, TD infants had greater dorsomedial prefrontal cortex activation in response to direct social gaze compared to averted gaze during structured social interactions with a live social partner playing “peek-a-boo” (Urakawa et al., 2015). To date, fNIRS studies involving HR infants have not utilized naturalistic social stimuli; which are inherently more complex due to co-occurring social verbal and non-verbal (affective and gestural) input offered by caregivers.
In the present study, we addressed the aforementioned limitation by capitalizing on the strengths of the fNIRS approach to conduct a preliminary study comparing FC and FA in HR and LR infants during ecologically valid, naturalistic social interactions with a parent at 6 to 9 months of age. Based on previous literature, we hypothesized that HR infants would show reduced FA during periods of social interaction compared to LR infants (Lloyd-Fox et al., 2013; Fox et al., 2013) and greater FC within and between hemispheres compared to LR infants (Keehn et al., 2013).
2. Materials and Methods
2.1. Participants
Fifteen infants between 6 and 9 months of age were recruited from a larger, ongoing longitudinal study at the XXX involving infant siblings of children with ASD. These included 9 infant siblings of children with ASD (HR infants, 7 males, HR=7.0 ±1.5 months) and 6 infants without any known family history of ASD (LR infants, 1 male, LR= 7.5±1. months). The two groups in our small study sample were similar with regard to age (p>0.1) but not gender (p<0.01). Given the preliminary nature of the current study, we were unable to control for gender. Multiple studies suggest that affective engagement during social interactions do not differ between young male and female infants (Cossette et al., 1996; Forbes et al., 2004); hence, we do not expect the larger proportion of female infants in the LR group to be a confounding factor contributing to group differences. The high proportion of males in our HR group indicates a higher likelihood of ASD-related impairments in the future, including ASD symptoms and developmental delays (Christensen et al., 2016) and, hence, of greater relevance to the ASD population.
Participating families were recruited through onsite and local clinical services, local ASD advocacy groups, public birth announcements, and word of mouth. The older siblings of HR infants held a clinical diagnosis of ASD. Exclusion criteria for both groups included preterm birth (<36 weeks gestation), hearing or vision impairments, seizure disorders, or any other genetic disorder leading to ASD (e.g., Fragile X syndrome, Rett’s disorder). This study was carried out in accordance with the recommendations of the Human Investigations Committee of Yale University School of Medicine who also approved the study protocol. All parents signed a written informed consent approved by the IRB at the Yale University School of Medicine. They had to specifically agree to participate in the fNIRS component of the longitudinal study.
2.2. Experimental Paradigm
In the first year of life, infants learn to socialize in the context of face-to-face interactions with their caregivers (Feldman et al., 2015). The Social Interaction paradigm was designed to replicate these natural interactions in a laboratory setting (Figure 1). During the pre-social period (30 seconds), infants were offered a toy to play with and parents were given a form to complete, thus naturally reducing the amount of parent-child interaction. Parents were instructed to avoid interactions with their infants during the pre/post-social periods. During the social period (5 minutes or 300 seconds), parents were instructed to play with their infants as they normally would at home. No toys were made available during this period in order to encourage face-to-face interactions. During the post-social period (30 seconds), infants were offered the same toy and the parents returned to completing the questionnaires.
Figure 1:
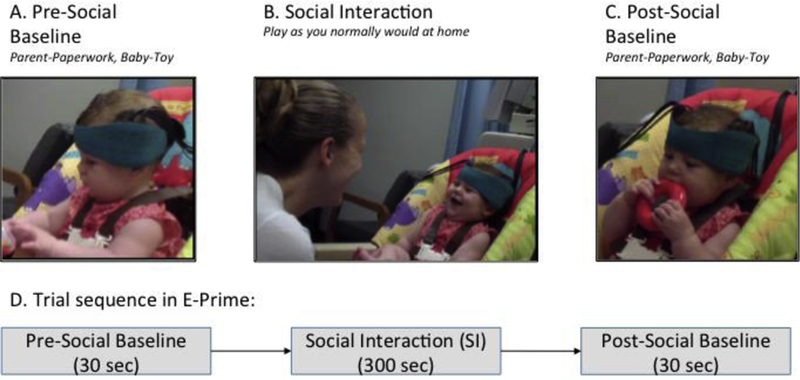
Experimental Paradigm.
2.3. fNIRS Data Collection
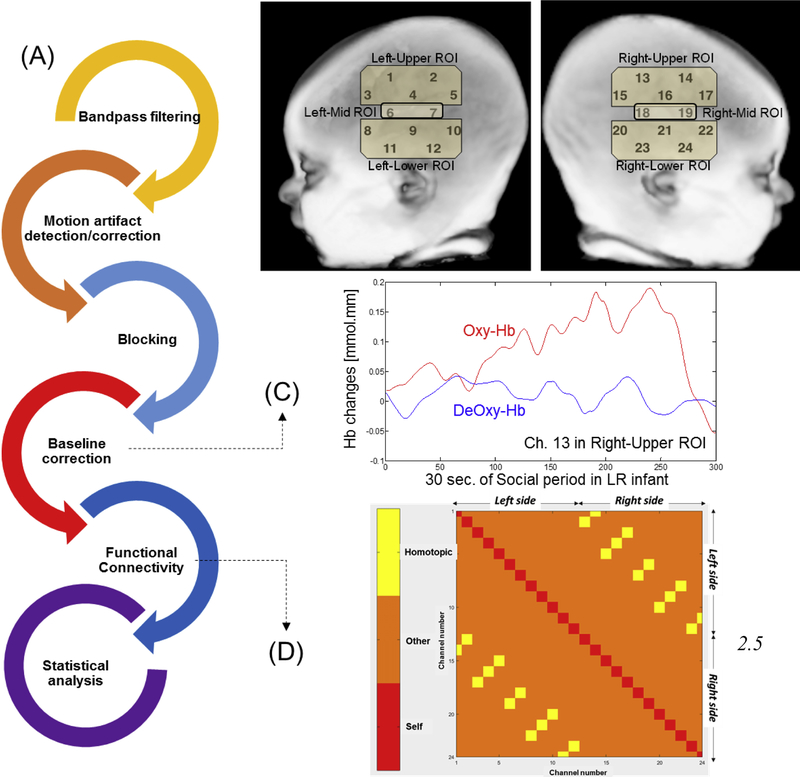
Changes in oxygenation were captured using the Hitachi ETG-4000 system (Hitachi Medical Systems, Tokyo, Japan; Sampling Rate: 10 Hz). Two 3×3 optode sets consisting of five infrared emitters and four receivers (i.e., 24 data channels) were centered above the infant’s ears. The middle column of the optode set aligned with the tragus of the ear and the lowermost row of the optode set aligned with the ear lobes. We hypothesized that some of the lower/center channels of the 3×3 probes were around the T3 level or just over temporal cortices, per the international 10–20 system (Klem, Luders, Jasper, & Elger, 1999; Jurczak, Tsuzuki, & Dan, 2007, Figure 2C shows the ROIs used). The remaining channels within each holder may have covered some portions of the frontal and parietal cortices. Spatial registration or the process of attributing fNIRS channel activation to specific cortical regions was not implemented in this study due to difficulties with infant head stabilization; which is consistent with other published fNIRS studies (Keehn et al., 2013; Fox et al., 2013). It is only in the last few years effective infant spatial registration methods are being developed (Lloyd-Fox et al., 2014; Emberson et al., 2017). However, these approaches are non-trivial and not yet widely implemented. Additionally, a clear dearth of available infant MRI reference databases as well as infant brain atlases limits our ability to register the bony landmarks obtained from infants onto an average infant brain. We hope to collaborate on developing new spatial registration methods with experts in the field which can be incorporated in our future work. Nevertheless, in this study we attempted to distinguish activation in the upper vs. mid, vs. lower ROIs for each hemisphere with the assumption that upper ROIs were closer to fronto-parietal cortices (inferior frontal and inferior parietal gyri), lower ROIs around the ears were closer to the superior temporal cortices and the mid ROIs were unclear and covering any of the three cortices (inferior frontal, inferior parietal, or superior temporal) (Figure 2B shows the ROIs used). Without spatial registration data we cannot affirmatively confirm the cortical regions in these ROIs, hence, we believe this was the next best approach we could have used.
Figure 2:
Data processing workflow (A), localizing activation to regions of interest (B), exemplar oxy and deoxy profiles in one channel (C) and types of connectivity measured (D).
Adjacent pairs of probes, 20 mm apart, act as emitters and receivers for two wavelengths of infrared light (695 and 830 nm). The infrared light from the emitter passes through the skin, skull, and underlying brain tissue and is detected by adjacent receivers. The majority of the light that is detected will have reached cortical areas approximately 10 mm below the midpoint of the two probes; therefore, each pair forms a measurement channel. The change in infrared light attenuation can be used to calculate the changes in concentrations of oxygenated (HbO2) and deoxygenated hemoglobin (HHb) chromophores per channel using the Modified Beer-Lambert Law. We expect neural activation within a region to increase the concentration of HbO2 and decrease that of HHb (Lloyd-Fox et al., 2010). These data are exported within an output file in the comma-separated values (.csv) format and later post-processed. E-Prime presentation software (version 2.0) from a Windows PC triggered the Hitachi fNIRS system via a serial port to mark the start and end of each baseline and the start and end of the social interaction periods, also stored in the .csv output.
2.4. Data Processing
We incorporated functions from open-source software such as Hitachi POTATo (Sutoko et al., 2016) and HOMER2 (Huppert, Diamond, Franceschini, & Boas, 2009) within our own custom MATLAB codes to analyze the .csv output from ETG-4000 (see data processing steps in Figure 2A). For activation analysis, signals from each channel were band-pass filtered between 0.01 and 0.5 Hz to remove lower or higher frequencies associated with body movements and other hemodynamic signals such as respiration, heart rate, skin blood flow, etc. For connectivity analysis, we used data within a narrower frequency range from 0.009 to 0.08 Hz to remove noise-based correlations arising from other noise such as Mayer waves and other hemodynamic signals. For motion artifact detection/correction, we used two functions. We used the “motion check” function from Hitachi POTATo to identify motion artifacts. In this MATLAB function, motion artifact was defined as a signal deviation of 2SD above the mean within two consecutive sampling points (Sutoko et al., 2016). The removal of motion artifacts was done using the wavelet motion artifact correction method as implemented in the HOMER2 software (Sato et al., 2006), which is one of the most robust and effective methods for motion artifact removal (Hu et al., 2015). To identify the motion artifacts/outliers, an “iqr” paramater of 0.5 was used following visual analysis of how various parameter values affect the motion artifacts within the data (values between 0.5 and 1.0). The wavelet motion correction algorithm computes the distribution of wavelet coefficients (Molavi & Dumont, 2012). The coefficients greater than iqr times the interquartile range of the data are typically associated with motion artifacts, and hence, they are set to zero to remove such artifacts. Higher values of the iqr parameter means fewer coefficients will be deleted and vice versa. Lastly, the algorithm involves applying the inverse discrete wavelet transform and signal reconstruction. For baseline correction, the linear trend for a 5-second duration directly preceding and following each period was calculated and subtracted from the values within a given period (pre-social, social, post-social) as implemented within Hitachi POTATo (Sutoko et al., 2016). Average HbO2 and an average HHb values were obtained for each period (i.e., one average value per period) and trends from both signals are reported in this paper. However, it is widely accepted that changes in HbO2 signals are significantly greater in magnitude compared to HHb data. While some suggest that HbO2 profiles have a greater signal-to-noise ratio compared to HHb (Sato et al., 2005); others have suggested that HHb signals are less susceptible to systemic physiological interference (Tachtsidis & Scholkmann, 2015). Typically, neural activation is associated with a consistent hemodynamic response often seen in children and adults involving an increase in HbO2 with a corresponding decrease or no change in HHb values (Gervain et al., 2011). However, young infants are known to have inconsistent hemodynamic responses given their developing brain and neurovasculature (Gervain et al., 2011). Therefore, we were expecting to see some inconsistencies between the trends for HbO2 and HHb profiles. Functional activation or FA was defined as the average increase in HbO2 or a decrease in HHb concentration for the entire period (pre-social, social, or post-social). We have also provided an exemplar figure showing one channel’s hemodynamic response after filtering, motion artifact removal, blocking, and baseline correction (Figures 2C). Note that we have also compared data between equal durations of pre-social, social (1st 30 seconds only), and post-social periods and those findings are similar to the results presented below (later see supplementary materials, section A).
Functional Connectivity Analysis
Functional Connectivity or FC was measured using Pearson’s correlations between the 276 unique channel pairs to obtain a correlation matrix for each of the three periods (Zhu et al., 2015, Figure 2D). For each period, we computed a correlation matrix of 24 × 24 or 576 channel pairs (Figure 2D). There are 24 “self” correlations that were excluded i.e., correlation between channels 1–1, 2–2, etc. (see red squares in Figure 2D). Of the remaining 552 correlations, half the correlations are redundant (e.g., correlations between channels 1–2 and 2–1, 3–5 and 5–3, etc. are identical). Therefore, the number of meaningful/unique correlations is (576–24)/2 = 276. Higher correlation values indicate higher FC or greater neural synchronization. FC measures were averaged within each hemisphere or “intra-hemispheric” (left-left, right-right, 66 pairs each) and across the two hemispheres or “inter-hemispheric” (144 left-right pairs). We also report inter-hemispheric FC for the 12 homologous channel pairs termed “homotopic”. We only used positive correlations within our analysis given that negative correlations are considered less reliable and reproducible based on patterns reported in the fMRI resting connectivity literature (Uddin et al., 2010). Note that only ~15% of the connectivity data were negative correlations so majority of the data was included in this analysis. To confirm that this decision did not affect our results we have included both types of correlations within a different analysis reported under supplementary materials (section B).
2.6. Statistical Analyses
The lack of spatial registration data and an effort to retain statistical power motivated us to assess differences in hemispheric FA by averaging HbO2 data across the 12 channels for each hemisphere to obtain right and left FA measures. We also attempted to distinguish activation to upper/fronto-parietal vs. lower/temporal channels on each side. In terms of FC, intra-hemispheric FC between channels of the same hemisphere were averaged to obtain right and left hemispheric FC. Inter-hemispheric FC was calculated in two ways – by averaging FC across all left-right channels and by averaging FC values for homotopic channels alone. Based on our hypotheses, we conducted the following planned comparisons for FA and FC: a) within-group, condition-based differences were assessed using paired t-tests and b) between-group differences for each condition were assessed using independent t-tests. Due to the preliminary nature of our study, in an effort to avoid type II errors, we did not correct for multiple comparisons and instead set statistical significance at p < 0.05. We have also reported on statistical trends (ps between 0.05 and 0.1). This approach has been used in past fNIRS studies involving HR infants and small sample sizes (Lloyd-Fox et al., 2013; Keehn et al., 2013).
3. Results
3.1. Patterns of FA using HbO2 and HHb
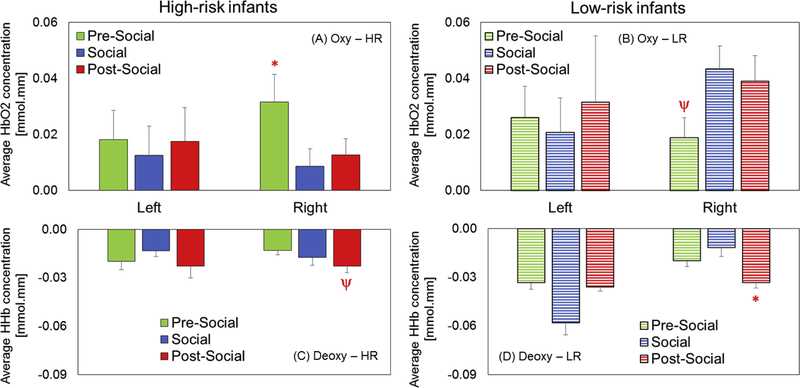
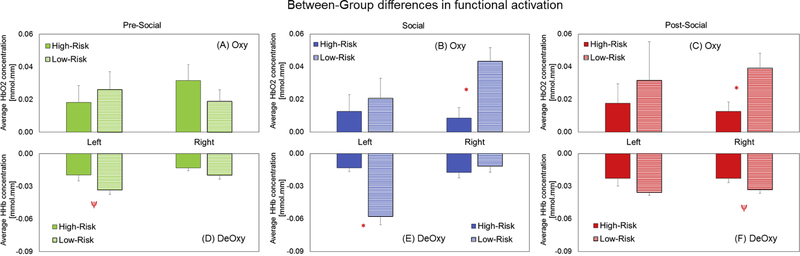
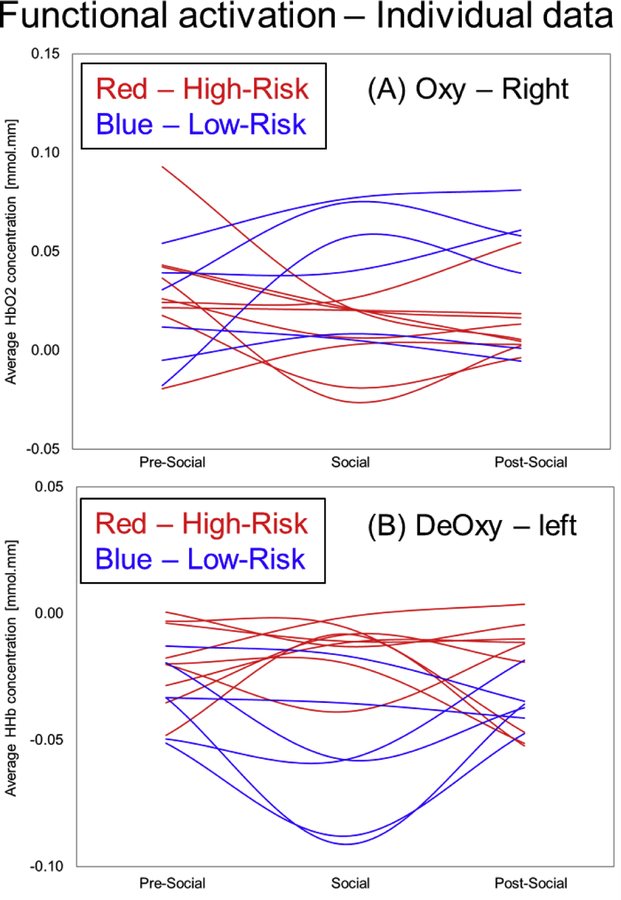
There were no significant within-group hemispheric differences in FA. In terms of condition-related, within-group differences in HbO2 signals, HR infants showed greater right hemispheric FA during the pre-social period compared to the social period (p=0.04, Figure 3A, Table 1). In contrast, LR infants showed a statistical trend for increased right hemispheric FA during the social and post-social periods compared to the pre-social period (ps = 0.09, Figure 3B, Table 1). In terms of context-related differences in HHb signals, the HR infants showed a trend for greater activation (i.e., a more negative value) in the post-social period compared to the pre-social period (p=0.08, Figure 3C, Table 1). Additionally, the LR infants had significantly greater activation in the post-social period compared to the pre-social period (p=0.01, Figure 3D, Table 1). Taken together, both groups showed condition-related differences in right hemispheric activation which was a pattern seen consistently across HbO2 or HHb trends. However, HR infants showed greater activation in the non-social periods (pre or post) compared to the social period. In contrast, the LR infants showed greater activation in the social and post-social periods compared to the pre-social period. In terms of between-group differences in HbO2 signals, HR infants showed reduced right hemispheric FA compared to LR infants during the social (p=0.004, Figure 4B, Table 1) and post-social periods (p=0.02, Figure 4C, Table 1). In terms of between-group differences in HHb signals, HR infants showed reduced left hemispheric FA compared to LR infants during the pre-social (p=0.05, Figure 4D, Table 1) and social periods (p=0.0002, Figure 4E, Table 1). Additionally, HR infants also showed similar trends for reduced right hemispheric FA compared to LR infants during the post-social period (p=0.06, Figure 4F, Table 1). Taken together, HR infants showed reduced FA in both hemispheres compared to LR infants consistent across HbO2 and HHb trends most prominently in the social and post-social periods (Figure 4B and 4C). In terms of individual data for both, HbO2 and HHb signals, 8 out of 9 HR infants and 4 out of 6 LR infants followed the group trends (Figure 5A and 5B). Interestingly, individual data trends were similar across the oxy and deoxy signals in that LR infants showed higher FA in the social and post-social periods compared to the pre-social period and this pattern was not seen in the majority of the HR infants. Next, we localized the significant group differences (*in Figure 4) by comparing FA between the upper (fronto-parietal), mid (fronto-parieto-temporal) vs. lower (temporal) channels (Figure 2B shows the chosen ROIs). In terms of right hemispheric group differences in HbO2 trends, we found that the lower activation in the HR infants compared to the LR infants during the social period was attributed to the right upper ROI (p=0.006) and the same trend during the post-social period was attributed to the right mid ROI (p=0.03). In terms of left hemispheric group differences in HHb trends, we found that lower activation in the HR infants compared to LR infants during the social period was attributed to both, the left upper and left lower ROIs (ps<0.01). Overall, the lower right/left-sided FA seen in HR infants during the social and post-social periods (compared to LR infants) was localized to bilateral fronto-parieto-temporal cortices based on HbO2 and HHb trends. Note that the spatial localization of statistical trends for group differences in FA are not elaborated further, but are listed in Table 1.
Figure 3:
Context-related differences in functional activation for HR infants (A) and LR infants (B). Red asterisk (*) indicates significant differences between conditions (p values < 0.05) and red psi (ψ) indicates a trend (p values from 0.05 to 0.1) distinguishing the marked (*/ψ) condition and the remaining ones.
Table 1:
A listing of p-values and direction of effects for relevant statistical comparisons of Oxy and Deoxy FA data
| Within-group differences | Between-group differences | ||
|---|---|---|---|
| HR infants | LR infants | Hemisphere/Conditions | |
| 1) R Oxy, Social < Pre-Social, p=0.04* | 1) R Oxy, Social & Post-Social > Pre-Social, ps=0.09| | 1) Oxy, R Social | - LR >HR, p=0.004*. ~Also seen in R upper ROI, p=0.006* |
| 2) R Deoxy, Post-Social > Pre-social, p=0.08| | 2) R Deoxy, Post-Social > Pre-Social, p=0.01* | 2) Oxy, R Post-Social | - LR >HR, p=0.02*. ~ Also seen in R mid ROI, p=0.03* |
| 3) Deoxy, L Pre-Social | - LR >HR, p=0.05| .~Seen in R upper and mid ROIs, ps=0.1| | ||
| 4) Deoxy, L Social | - LR >HR, p=0.0002*.~Also seen in L upper (p=0.002*) and lower (p=0.01*) ROIs | ||
| 5) Deoxy, R Post-Social | - LR >HR, p=0.07|.~Similar trend in R lower RO1, p=0.06| | ||
= p < 0.05
= 0.05–0.1; HR=High-risk, LR=Low-risk.
~Note that when present, we are also reporting consistent trends in the upper and lower ROIs of the right &/or left hemispheres.
R=right, L=left
Figure 4:
Group differences in functional activation (left, right) across periods – A) Pre-social, B) Social, and C) Post-social. Red asterisks (*) indicate significant group differences (p values < 0.05) and red psi (ψ) indicates a trend (p values from 0.05 to 0.1). Note that only significant group differences (*) were further explored in terms of localizing activation to certain ROIs.
Figure 5:
Individual differences in functional activation between HR and LR infants.
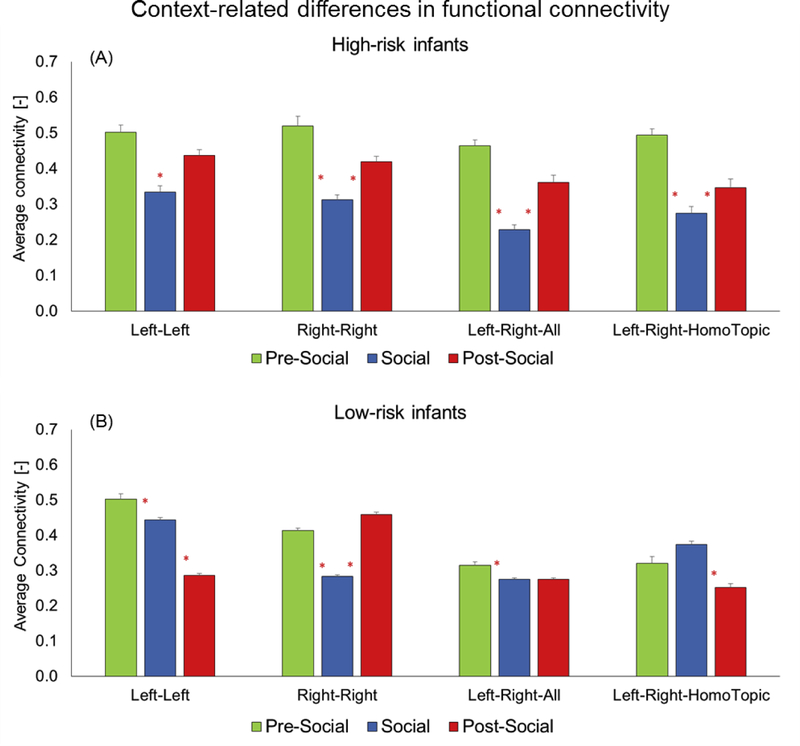
3.2. Patterns of FC
In terms of within-group, context-related differences in FC, HR infants showed a unique U-shaped pattern of connectivity with greater intra-hemispheric (left-left, right-right, Figure 6A, ps=0.04–0.0001, Table 2) and inter-hemispheric connectivity in the pre and post-social periods compared to the social period (left-right, all and homotopic channels, Figure 6A, ps=0.005–0.0001 for social vs. pre-social and social vs. post-social periods, also see Table 2). In contrast, the LR infants had more variable patterns of FC for both intra-hemispheric and inter-hemispheric connectivity (Figure 6B). Specifically, they showed varied patterns of FC in the social period compared to the pre-social or post-social periods including patterns where social periods had reduced, greater or the same levels of connectivity as the pre/post social periods depending on the type of FC being measured (ps=0.03 – 0.0001).
Figure 6:
Context-related differences in functional connectivity for HR infants (A) and LR infants (B). Red asterisks are placed between-conditions being compared to highlight significant differences.
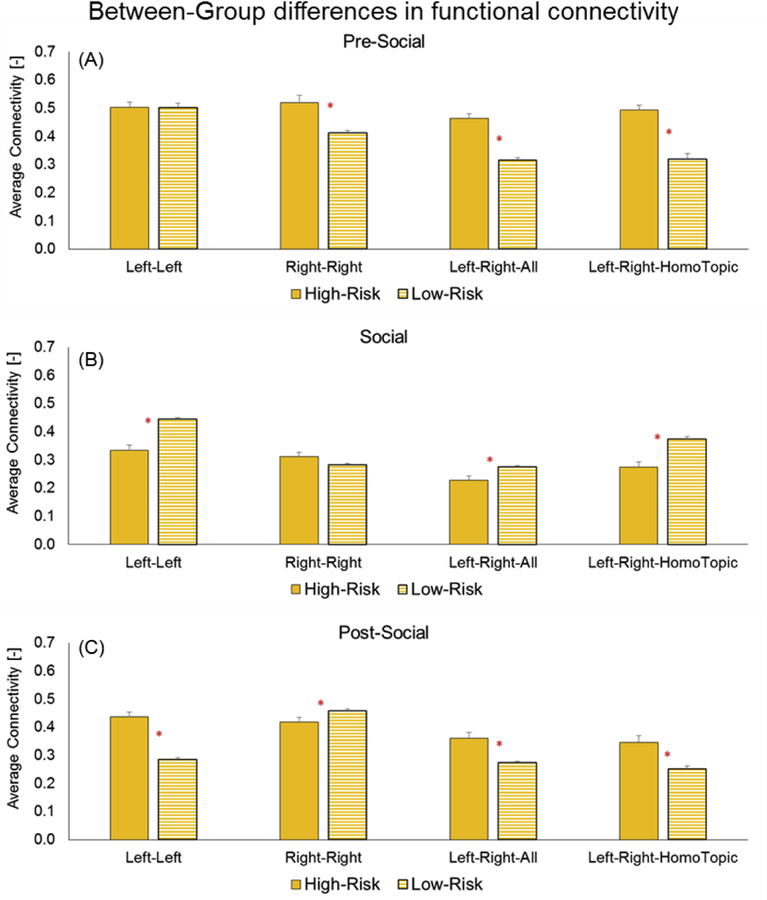
In terms of between-group differences, during the pre-social period, HR infants had greater FC compared to LR infants for right-right intra-hemispheric, left-right all, left-right homotopic, inter-hemispheric FC (ps=0.001 – 0.0001, Figure 7A). In contrast, during the social period, the LR infants showed greater FC compared to HR infants for left-left, intra-hemispheric, left-right all, and left-right homotopic, inter-hemispheric FC (Figure 7B). During the post-social period, once again, HR infants had greater FC compared to LR infants for left-left, intra-hemispheric, left-right all, and left-right homotopic, inter-hemispheric FC (ps=0.0001, Figure 7C). Interestingly, in the post-social period, LR infants had greater FC in right-right, intra-hemispheric FC compared to the HR infants.
Table 2:
A listing of p-values for relevant statistical comparisons for FC data
| Groups | Left-left | Right-Right | Left-Right-All | Left-Right Homotopic |
|---|---|---|---|---|
| Within-Group Comparisons | ||||
| LR infants | Pre-Social > Social, p=0.02* | Pre-Social> Social, p=0.0003* | Pre-Social> Social, p=0.04* | No differences |
| Social>Post-Social, p<0.0001** | Social<Post-Social, p<0.0001** | No difference | Social>Post-Social, p=0.001* | |
| Pre-Social> Post-Social, p=0.0005* | Pre-Social < Post-Social, p=0.02* | Pre-Social> Post-Social, p=0.06| | No differences | |
| HR Infants | Pre-Social> Social, p<0.0001** | Pre-Social> Social, p<0.0001** | Pre-Social> Social, p<0.0001** | Pre-Social> Social, p<0.0001** |
| Social< Post-Social, p=0.0002* | Social< Post-Social, p<0.0001* | Social< Post-Social, p<0.0001* | Social< Post-Social, p=0.0006* | |
| Pre-Social> Post-Social, p=0.05* | Pre-Social> Post-Social, p=0.01* | Pre-Social> Post-Social, p=0.0002* | Pre-Social> Post-Social, p<0.0001** | |
| Between-Group Differences | ||||
| Condition | Left-left | Right-Right | Left-Right-All | Left-Right Homotopic |
| Pre-social | No differences | HR>LR, p=0.003* | HR>LR, p<0.0001** | HR>LR p<0.0001** |
| Social | LR>HR, p=0.0002* | No differences | LR>HR, p=0.01* | LR>HR p=0.0004* |
| Post-social | HR>LR p<0.0001** | HR<LR p=0.04* | HR>LR p=0.002* | HR>LR p=0.006* |
p between 0.05 and 0.1
p between 0.0001 and 0.05
= p<0.0001; HR=High-risk, LR=Low-risk
During the social period, intra-hemispheric FC (left-left or right-right) of LR infants was lower compared to the pre/post-social periods, a pattern similar to that of the HR infants. However, in terms of inter-hemispheric connectivity (left-right - all channels and homotopic channels) LR infants had similar or greater FC than the pre/post-social periods; this pattern was not seen in the HR infants.
4. Discussion
The current study compared cortical activation and connectivity patterns between infants with and without familial risk for ASD during the first year of life, prior to the development of overt behavioral signs of ASD. We developed a novel paradigm that examined infant cortical responses (i.e., FA and FC) during naturalistic social interactions when HR infants may be more likely to show differences given the complex nature of naturalistic social play. We used fNIRS technology, an infant friendly neuroimaging tool, to compare FC and FA between young HR and LR infants. Compared to LR infants, HR infants had reduced right-hemispheric FA during the social and post-social periods based on HbO2 trends as well as reduced left-hemispheric FA during the social period based on HHb trends. In terms of ROI analysis, we localized the group differences in FA to the right upper and mid ROIs as well as left upper and lower ROIs suggesting reduced FA in the HR infants over bilateral fronto-parieto-temporal cortices. During the pre and post-social periods, consistent with past studies, young infants with familial risk for ASD showed greater intra-hemispheric and inter-hemispheric FC (i.e., hyper-connectivity) compared to LR infants. In contrast, during the social period, FC in the HR infants was lower compared to LR infants, especially in terms of left-left intra-hemispheric and inter-hemispheric connectivity. Our findings provide support for differential patterns of cortical responses during social interactions versus spontaneous object play and evidence of hyper-connectivity in infants with high-risk for ASD during the first year of life.
4.1. Context-related and group differences in functional activation in HR infants
Based on analysis of HbO2 signals, during the social and post-social baseline periods, HR infants had reduced right-sided FA compared to their own pre-social activation as well as compared to LR infants. An opposite trend was noted in the LR infants who had greater right-sided FA during the social and post-social periods compared to their own pre-social activation and compared to HR infants. Based on HHb signal analysis, there were no clear within-group differences for HR infants. However, HR infants had lower left-sided FA during the social period and the post-social period compared to their own pre-social activation and compared to LR infants. In terms of localization, HR infants had reduced activation in the right fronto-parieto-temporal cortices (based on multiple HbO2 and one HHb trends) as well as left fronto-parieto-temporal cortices (based on HHb trends only) during the social and post-social periods. The lower levels of FA in the right and left fronto-parieto-temporal cortices of HR infants during the social and post-social periods suggest that HR infants may process social information differently than LR infants. Findings from past studies in HR infants (Lloyd-Fox et al., 2013; Fox et al., 2013) as well as typically developing (TD) infants are consistent with our results (Urakawa et al., 2015, Lloyd-Fox et al., 2011; 2016). HR infants showed diminished fNIRS activation in the right STS region in response to social visual and auditory stimuli (vs. non-social stimuli) in comparison to LR infants (Lloyd-Fox et al., 2013). Similarly, children with ASD showed greater right prefrontal activation while observing non-JA behaviors compared to JA/social behaviors (Zhu et al., 2015). Additionally, children with ASD did not alter their left prefrontal activation between JA/social versus non-JA/non-social conditions. In contrast, TD children showed greater activation during the social/JA condition versus the non-social/non-JA condition in both right and left prefrontal ROIs (Zhu et al., 2015). In contrast, three studies in TD infants report preferential activation in the right/both hemispheres, in different cortical ROIs, namely, STS, IFG, orbitofrontal, and prefrontal cortices during structured tasks involving dynamic social stimuli or adult-child interactions (Lloyd-Fox et al., 2011, 2016; Urakawa et al., 2015). First, five-month-old TD infants responded with more activation in the right vs. the left hemisphere (i.e., IFG and STS) while watching human movements involving hands and/or eyes (Lloyd-Fox et al., 2011). Second, Gambian TD Infants between 4 to 24 months of age showed consistent activation in response to visual social vs. non-social stimuli in the right STS region (Lloyd-Fox et al., 2016). Third, during a structured social interaction involving a peek-a-boo game, TD infants showed greater dorsomedial prefrontal cortex activation in response to direct social gaze compared to averted gaze; which was not found to be hemisphere specific (Urakawa et al., 2015).
Our findings of both right and left hemispheric atypicalities in activation are also consistent with previous structural and functional MRI studies reporting both right and left hemisphere abnormalities in adults/children with ASD compared to those without ASD (Cauda et al., 2014; Subburaju et al., 2017; Richter et al., 2017). Using a large fMRI database of over 1000 adults with ASD, Subburaju et al. (2017) revealed more abnormalities in right hemispheric activation (i.e., right STS and right inferior parietal lobule [IPL]) compared to adults without ASD. In contrast, Richter et al. (2015) compared surface cortical thickness of pre-adolescent children with and without ASD and found reduced thickness in the left frontal, temporal, parietal, and occipital cortices in children with ASD compared to TD children. Similarly, sleeping 1-year-old children at-risk for ASD who listened to natural language vs. auditory stimuli had lower activation in the left superior temporal cortices during natural speech (i.e., reading of bedtime stories) compared to control children with typical language outcomes (Eyler et al., 2012); with no group differences when hearing simple auditory stimuli. It is suggested that right hemisphere encodes prosodic aspects of speech such as “motherese” and pragmatic aspects of communication such as pauses and intonations (Ozonoff & Miller, 1996) whereas left hemisphere (Broca’s and Wernicke’s areas) is important for processing of semantics and sentence structure and hence, both would play important roles when processing relatively complex, natural speech (Herringshaw et al., 2016). In short, both fronto-parietal and superior temporal cortices in both hemispheres play an important role in processing of social, non-verbal and verbal components of naturalistic interactions with caregivers. It is possible that young infants at risk for ASD do not adequately process mother’s social and verbal inputs or do not appropriately respond to her inputs through the use of gestures, speech, and gaze. Taken together, our findings suggest that future studies must validate our result of reduced right &/or left-hemispheric, frontoparieto-temporal activation during natural social interactions as a potential neurobiomarker of ASD risk or social delay.
FA patterns observed in the social period continued to occur in the post-social period in both groups (i.e., greater FA in LR compared to HR infant in both periods). Given the intense nature of the infant-caregiver interactions during the social period, it is possible that cortical activation associated with the social period did not return to baseline entirely during the post-social period given the gradual changes in the fNIRS hemodynamic response. It is also possible that while parents were disengaged from their infants and busy completing paperwork, infants were still seeking to socially connect with them (through glances or smiles) which may have led to similarity in trends between the social and post-social periods in terms of activation. To confirm whether infants initiated social interactions, we coded the number of social overtures (e.g., looks to parent) by the infants during the pre- and post-social periods. Both groups showed no differences in self-initiated gaze between the pre-social and post-social periods. We also confirmed that all caregivers followed instructions during the post-social period and did not attempt to interact with their infant. Hence, the similarities in activation between the social and post-social periods is most likely the result of a prolonged hemodynamic response continuing in the post-social period.
4.2. Increased functional connectivity in HR infants during spontaneous play
During the pre-social and post-social periods, HR infants had greater intra-hemispheric and inter-hemispheric FC compared to LR infants. These findings are consistent with previous work suggestive of a pattern of cortical hyper-connectivity in white matter as well as brain overgrowth in the first year of life (Courchesne, Campbell, Solso, 2011). At this time, we cannot confirm how many HR infants in our study eventually developed autism-related delays/diagnoses. It is estimated that about 20% of HR infants develop ASD (Ozonoff et al., 2011) and another 20% evidence developmental delays or subclinical ASD symptoms known as the broader autism phenotype (Ozonoff et al., 2014), although this rate may be a bit higher in our sample given the high proportion of boys (Christensen et al., 2016). Our findings of hyper-connectivity in the HR infants suggest that the pattern of brain overgrowth may extend to the broader autism phenotype. This is consistent with the latest IBIS study that reported cortical hyper-expansion in the first year of life followed by overall brain overgrowth in the second year of life in HR infants who developed ASD by two years of age (Hazlett et al, 2017); a similar neurodevelopmental pattern was present to a lesser degree in infants with familial risk who did not meet criteria for ASD at two years of age. Likewise, recent DTI study findings of increased intra-hemispheric connectivity in the frontal cortices and increased inter-hemispheric connectivity in the splenium of the corpus callosum in 6-month-old HR infants who later developed ASD compared to LR infants (Wolff et al., 2012; 2015) further support our finding of increased FC during the first year of life in HR infants. Keehn et al. (2013) similarly found that HR infants had increased intra-hemispheric and inter-hemispheric connectivity during an fNIRS-based auditory, linguistic processing task at 3 months (but not at 6 and 9 months) along with patterns of hypo-connectivity emerging by 12 months.
4.3. Context-related differences in FC in HR infants
Ours is the first study to examine fNIRS-based FC in HR infants in the context of a naturalistic social interaction. HR infants showed a context-dependent, U-shaped pattern of FC with greater connectivity during the pre-social and post-social periods as compared to the social period, for both intra- and inter-hemispheric connectivity. The LR infants showed more variable patterns of FC and for multiple forms of connectivity showed an inverted-U with greater connectivity during the social period compared to the pre- and post-social periods. The differing patterns of FC between the two groups revealed different patterns of social engagement and underlying neural processing. During the quiet play periods, HR infants perhaps exhibited reduced self-initiated, social engagement along with persistent object play; most likely associated with increased FC. Furthermore, during the social period, FC was lower in the HR infants perhaps associated with greater social engagement with caregivers compared to their independent play periods. In contrast, LR infants perhaps displayed spontaneous social engagement (i.e.; caregiver-directed vocalizations/social gaze) during the non-social periods as well as higher-level or more complex social interactions during the social period leading to more variable patterns of connectivity.
While there are no studies evaluating “task-based” FC in young infants, fNIRS-based FC studies in children with ASD report atypical FC in school-age children with ASD characterized by a lack of difference in connectivity between a social task involving JA and a non-JA task (Zhu et al., 2015). This pattern differed in the TD children who showed clear differences in FC patterns during the JA task compared to a non-JA task (Zhu et al., 2015). Our findings are also consistent with past behavioral studies reporting subtle behavioral differences between HR and LR infants during dyadic and triadic interactions with caregivers (Bhat, Galloway, & Landa, 2010; Srinivasan & Bhat, 2016; Rozga et. al., 2011; Yirmiya et al., 2006). During dyadic social interactions of the still face paradigm, two separate studies did not reveal significant differences in social gaze or affect in 6-month-old HR infants who later developed ASD (vs. the LR infants) (Yirmiya et al., 2006; Rozga et al., 2011). Similarly, during triadic contexts of social-object play, when caregivers overtly facilitated their infants’ to play with a cause and effect toy, both HR and LR infants showed no differences in social gaze (Bhat et al., 2010). It was only during the independent play periods (i.e., when caregivers reduced their social engagement) HR infants increased their object-related gaze compared to LR infants (Bhat et al., 2010). Studies examining free object exploration suggest that HR infants under one year of age may be engaging in repetitive play, excessive visual exploration, and undergo few changes in body posture in turn affecting their social interaction abilities (Ozonoff et al., 2008; Kaur, Srinivasan, & Bhat, 2015; Nickel et al., 2013; van Etten et al., 2016). A combination of the aforementioned early risk behaviors may have contributed to the U-shaped pattern of FC in the HR infants. The more variable and inverted-U pattern of FC in the LR infants may point to their more variable and complex forms of social engagement throughout the task. Overall, a concurrent assessment of changes in fNIRS-based FC across different contexts of parent-child interaction could potentially enhance the sensitivity of the subtle early behavioral markers of ASD.
4.4. Clinical Implications
The methodology and findings from the current study may have translational relevance for ASD clinicians and clinical researchers. We uniquely measured infant brain responses in the context of naturalistic social interactions with a caregiver; a paradigm that more closely approximates the complex social world infants must navigate on a daily basis. Our paradigm also allowed us to assess brain function during periods of spontaneous or independent play (pre- and post-social periods) that are critical for observing early behavioral markers such as persistent object play, repetitive limb and body movements, reduced exploration, as well as lack of self-initiated, social engagement. fNIRS systems are also becoming increasingly portable and cost effective, making these systems potentially useful in clinical contexts. A combination of behavioral and biomedical testing may have the potential to enhance the reliability of early detection of ASD by combining behavioral markers as well as cortical connectivity/activation related neurobiomarkers. fNIRS-related variables could also serve as objective predictive or outcome measures in clinical trials of caregiver-mediated early interventions. Finally, our finding that the observed hyper-connectivity “normalized” (similar levels to LR infants) during the social interactive periods of play provides additional support to the utility of early interventions that enhance positive parent-infant interactions to promote optimal development in HR infants (Green et al., 2015; 2017).
4.5. Study Limitations and Future Directions
This study was our first proof-of-concept study to assess cortical activation and develop new methodology to study naturalistic infant-caregiver interactions. Nevertheless, this study has several limitations that must be acknowledged. First, ours is a preliminary study with a small sample size and we were unable to perform corrections for multiple comparisons. Second, we were unable to conduct spatial registration in our infant sample. Difficulty in registering bony landmarks on infants’ heads (due to their inability to stay still) as well as lack of widely available infant brain atlases for registering bony landmarks onto an average infant brain limited our ability to implement spatial registration in this study. However, based on the size and placement of the fNIRS probes we may have covered portions of bilateral STS, IPL, and IFG regions. Due to the lack of spatial registration data and the small sample, we limited our analyses to left versus right hemisphere FA or intra and inter-hemispheric FC. However, our additional ROI analyses suggest that group differences were localized to right upper/mid ROIs as well as left upper and lower ROIs, potentially, bilateral fronto-parieto-temporal cortices which is also consistent with past studies. Third, we do not have a language or ASD outcome for the at-risk infants; hence, our findings are not ASD-specific but reflect possible delays associated with a familial risk for ASD. Fourth, our groups were uneven with regard to gender, but our at-risk sample is more biased towards males who are at a greater autism risk. A common limitation of fNIRS is that light waves can only penetrate through a depth of approximately 20 mm and pick up changes in cortical oxygenation; so we are unable to report subcortical activation patterns. Lastly, we only had one social and two non-social periods to reduce potential distress experienced by infants resulting from repeated disengagement with their caregiver. Future studies should include larger, gender-matched samples using more robust experimental designs with repeated receding/reintroducing of social interactions as well as synchronized video and fNIRS data collections to confirm important brain-behavior relations as well as individual differences across dyads and to understand whether early brain responses predict later language outcomes/ASD symptomatology.
Conclusions
In this study, we compared the FC and FA patterns of infant siblings of children with ASD (HR infants) compared to a group of infants with no family history of ASD (LR infants) using a novel, ecologically valid context of parent-child interactions. Consistent with past studies in HR infants and adults with ASD, we found reduced right and left-sided FA in the HR infants compared to the LR infants during the social and post-social periods. Consistent with the limited number of studies on FC in HR infants, we found intra- and inter-hemispheric hyper-connectivity in the HR infants during the quiet/independent play periods and a reduction in FC in the HR infants during the social period. In contrast, LR infants showed more variable and at times an opposite pattern of FC. Future studies must replicate our current results using larger sample sizes and long-term follow-up. Nevertheless, this preliminary study offers a promising methodology that future studies could utilize in the context of longitudinal analyses of brain-behavior relations using fNIRS as well as clinical trials of very early interventions for HR infants.
Supplementary Material
Figure 7:
Group differences in functional connectivity for three periods: A) Pre-social, B) Social, and C) Post-social. X-axis lists the different types of FC - intra-hemispheric (left-left, right-right) and inter-hemispheric (for all channels and for homotopic channels only). Red asterisk (*) indicates significant group differences (p-values ranging from 0.001 to 0.0001).
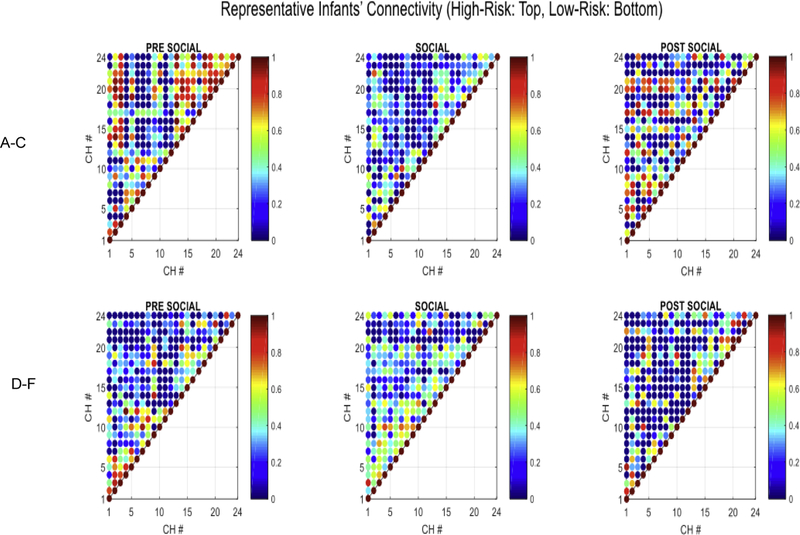
Individual data confirm that all the infants within a group followed the connectivity group trends for their own group suggesting that our group trends are robust (Figure 8). We are also providing a visual depiction of the entire correlation matrix for one exemplar HR and LR infant for each period (Figure 9A–9F). Note the change in connectivity in the HR infant – greater connectivity in pre-social and post-social period and lower functional connectivity (FC) for the social period indicating a drop in connectivity during the social period (Figures 9A–9C). In contrast, the LR infant showed opposite connectivity profile with a slight increase in overall connectivity in the social period compared to the pre- and post-social period (Figures 9D–9F).
Figure 8:
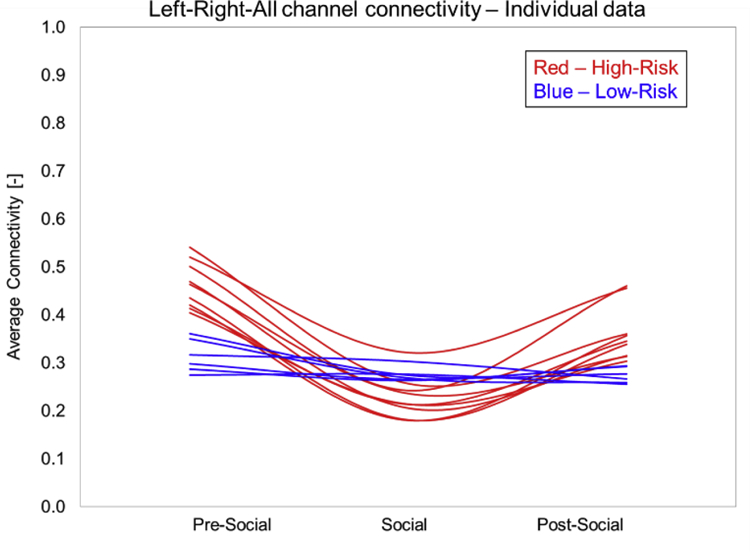
Individual data on functional connectivity in HR and LR infants.
Figure 9.
shows a visual depiction of correlation matrices obtained for the pre-social (A, D), social (B, E) and post-social (C, F) for one HR infant (A–C) and one LR infant (D–F). Each point represents a specific channel correlation and values range from 0 = blue to 1 = red and shades in between. The HR infant had higher FC in the pre- and post-social periods compared to the social period. The LR infant had higher FC in the social period compared to the pre- and post-social periods.
Highlights.
Cortical hyper-connectivity in the first year precedes overt signs of ASD seen in the second year.
fNIRS examined functional activation (FA) and connectivity (FC) in high- and low-risk infants.
HR infants showed reduced right and left hemispheric FA in the social period and greater FC than LR infants in the non-social periods.
Early differences in cortical FA and FC may be associated with ASD risk.
Acknowledgments and Funding Sources
This research was conducted at the Yale Child Study Center. We thank Cara Keifer, Courtney Paisley, and Hannah Friedman who helped with participant scheduling and data collection, as well as the families who volunteered their time to participate in the study. This research was supported by Autism Speaks (R1115). A. Bhat’s work in this project was supported by the following awards: a) Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54- GM104941 (Site PI: Binder-Macleod) for a CTR-Pilot (Project PI), CTR-SHORE (Project PI), and CTR-OrBITS (Co-I), b) Dana foundation’s clinical neuroscience award as well as an NIH S10 grant (1S10OD021534-01, PI: Anjana Bhat). N. McDonald’s work on this project was supported by a National Institute for Mental Health fellowship (F32MH108283-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors confirm no conflicts of interest. These data have not been published elsewhere.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (Fifth Ed.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Bauman ML, & Kemper TL (2005). Neuroanatomic observations of the brain in autism: A review and future directions. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 23(2–3), 183–187. doi:S0736-5748(04)00136-4. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, & Landa RJ (2010). Social and non-social visual attention patterns and associative learning in infants at risk for autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51(9), 989–997. 10.1111/j.1469-7610.2010.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Landa RJ, & Galloway JC (2011). Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical therapy, 91(7), 1116–1230. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, & Landa RJ (2012). Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior & Development, 35(4), 838–846. 10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A, Lloyd-Fox S, Sethna V, Brammer MJ, Mercure E, Murray L, …Johnson M (2015) Atypical processing of voice sounds in infants at risk for autism spectrum disorder. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior 71: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J, Steiner A, Gengoux G, & Koegel L (2015) Feasibility and effectiveness of very early intervention for infants at-risk for autism spectrum disorder: A systematic review. Journal of Autism and Developmental Disorders 45(3): 778–794. [DOI] [PubMed] [Google Scholar]
- Cauda F, Costa T, Palermo S, D’Agata F, Diano M, Bianco F, …Keller R (2014). Concordance of white matter and gray matter abnormalities in autism spectrum disorders: A voxel-based meta-analysis study. Human Brain Mapping, 35(5), 2073–2098. 10.1002/hbm.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Braun KV, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ 2016;65(No. SS-3)(No. SS-3):1–23. 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossette L, Pomerleau A, Malcuit G, & Kaczorowski J (1996). Emotional expression of female and male infants in a social and a nonsocial context, Sex Roles, 35(11):693–709. [Google Scholar]
- Courchesne E, Campbell K, & Solso S (2011). Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Research, 1380, 138–145. 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, …Piven J – IBIS Network. (2013). White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. The American Journal of Psychiatry, 170(8), 899–908. 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, & Johnson M (2016) Autism and the social brain: The first year puzzle. Biological Psychiatry, 80(2):94–9. 10.1016/j.biopsych.2016.02.019. [DOI] [PubMed] [Google Scholar]
- Emberson L, Cannon G, Palmeri H, Richards J, Aslin R (2017) Using fNIRS to examine occipital and temporal responses to stimulus repetition in young infants: Evidence of selective frontal cortex involvement. Developmental Cognitive Neuroscience 23: 26–38. 10.1016/j.dcn.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Pierce K, Courchesne E (2012) A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain 135, 949–960. 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R (2015). Mutual influences between child emotion regulation and parent-child reciprocity support development across the first 10 years of life: Implications for developmental psychopathology. Development and Psychopathology, 27(4 Pt 1), 1007–1023. 10.1017/S0954579415000656. [DOI] [PubMed] [Google Scholar]
- Forbes E, Cohn J, Allen N, & Lewinsohn P (2004) Infant Affect during Parent-Infant Interaction at 3 and 6 Months: Differences Between Mothers and Fathers and Influence of Parent History of Depression. Infancy 5(1): 61–84. 10.1207/s15327078in0501_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Wagner JB, Shrock CL, Tager-Flusberg H, Nelson CA (2013) Neural processing of facial identity and emotion in infants at high-risk for autism spectrum disorders. Frontiers in Human Neuroscience 7:89 10.3389/fnhum.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y, Ajichi Y, & Okada E (2003) Monte Carlo prediction of near-infrared light propagation in realistic adult and neonatal head models. Applied Optics 42, 2881–2887. [DOI] [PubMed] [Google Scholar]
- Gervain J, Mehler J, Werker J, Nelson C, Csibra G, Lloyd-Fox S, …Aslin R (2011) Near-infrared spectroscopy: a report from the McDonnell infant methodology consortium. Developmental Cognitive Neuroscience 1(1): 22–46. 10.1016/j.dcn.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Charman T, Pickles A, Wan M, Elsabbagh M, Slonims V, …Johnson M, the BASIS team (2015) Parent-mediated intervention versus no intervention for infants at high risk of autism: a parallel, single-blind, randomised trial. Lancet Psychiatry 2(2): 133–140. 10.1016/S2215-0366(14)00091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Pickles A, Pasco G, Bedford R, Wan M, Elsabbagh M, …Johnson M (2017) Randomised trial of a parent-mediated intervention for infants at high risk for autism: longitudinal outcomes to age 3 years. British Autism Study of Infant Siblings (BASIS) Team. Journal of Child Psychology & Psychiatry 10.1111/jcpp.12728. [DOI] [PMC free article] [PubMed]
- Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, …Piven J (2011). Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Archives of General Psychiatry, 68(5), 467–476. 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringshaw A,J, Ammons CJ, DeRamus TP, Kana RK (2016) Hemispheric differences in language processing in Autism Spectrum Disorders: A meta-analysis of neuroimaging studies. Autism Research 9, 1046–1057. 10.1002/aur.1599. [DOI] [PubMed] [Google Scholar]
- Hu XS, Arredondo MM, Gomba M, Confer N, DaSilva AF, Johnson TD, …Kovelman I (2015). Comparison of motion correction techniques applied to functional near-infrared spectroscopy data from children. Journal of Biomedical Optics, 20(12), 126003 10.1117/1.JBO.20.12.126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, & Boas DA (2009). HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Applied Optics, 48(10), D280–98. doi:177567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Gliga T, Bedford R, Charman T, & Johnson M (2014) Developmental pathways to autism: a review of prospective studies of infants at risk. Neuroscience & Biobehavioral Reviews 39:1–33. 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Venema K, Lowy R, Earl R, & Webb SJ (2015) Developmental changes in infant brain activity during naturalistic social experiences. Developmental Psychobiology 57: 842–853. 10.1002/dev.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurczak V, Tsuzuki D, & Dan I (2007). 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. NeuroImage, 34(4), 1600–1611. 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kaur M, Srinivasan SM, & Bhat AN (2015). Atypical object exploration in infants at-risk for autism during the first year of lifer. Frontiers in Psychology, 6, 798 10.3389/fpsyg.2015.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Wagner JB, Tager-Flusberg H, & Nelson CA (2013). Functional connectivity in the first year of life in infants at-risk for autism: A preliminary near-infrared spectroscopy study. Frontiers in Human Neuroscience, 7, 444 10.3389/fnhum.2013.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem GH, Luders HO, Jasper HH, & Elger C (1999). The ten-twenty electrode system of the international federation. The international federation of clinical neurophysiology. Electroencephalography and Clinical Neurophysiology Supplement, 52, 3–6. [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, & Elwell CE (2010). Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neuroscience and Biobehavioral Reviews, 34(3), 269–284. 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Everdell N, Elwell CE, & Johnson MH (2011). Selective cortical mapping of biological motion processing in young infants. Journal of Cognitive Neuroscience, 23(9), 2521–2532. 10.1162/jocn.2010.21598. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE, Charman T, Murphy D, & Johnson MH (2013). Reduced neural sensitivity to social stimuli in infants at risk for autism. Proceedings in Biological Sciences, 280(1758), 20123026 10.1098/rspb.2012.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Richards J, Blasi A, Murphy D, Elwell C, Johnson M (2014) Coregistering functional near-infrared spectroscopy with underlying cortical areas in infants. Neurophotonics 1(2): 1–16. 10.1117/1.NPh.1.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Begus K, Halliday D, Pirazzoli L, Blasi A, Papademetriou M, …Elwell CE (2016). Cortical specialisation to social stimuli from the first days to the second year of life: A rural Gambian cohort. Developmental Cognitive Neuroscience 10.1016/j.dcn.2016.11.005. [DOI] [PMC free article] [PubMed]
- McDonald NM, Perdue KL, Eilbott JE, Loyal J, Shic F, & Pelphrey KA (2019). Infant brain responses to social sounds: a longitudinal functional near-infrared spectroscopy study. Developmental Cognitive Neuroscience 20 March, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molavi B & Dumont GA (2012) Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiological Measures 33(2):259–70. 10.1088/0967-3334/33/2/259. [DOI] [PubMed] [Google Scholar]
- Nickel LR, Thatcher AR, Keller F, Wozniak RH, & Iverson JM (2013). Posture development in infants at heightened vs. low risk for autism spectrum disorders. Infancy: The Official Journal of the International Society on Infant Studies, 18(5), 639–661. 10.1111/infa.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, & Miller JN (1996). An exploration of right-hemisphere contributions to the pragmatic impairments of autism. Brain and Language, 52(3), 411–434. doi:s0093-934X(96)90022-X. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, & Rogers SJ (2008). Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism, 12(5), 457–472. 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, …Stone WL (2011). Recurrence risk for Autism Spectrum Disorders: A baby siblings research consortium study. Pediatrics, 128(3), e488–e495. 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, …Steinfeld M (2014). The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry, 53 (4), 398–407. 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Henze R, Vomstein K, Stieltjes B, Parzer P, Haffner J, Brandeis D, Poustka L (2015) Reduced cortical thickness and its association with social reactivity in children with autism spectrum disorder. Psychiatry Research: Neuroimaging 234, 15–24. 10.1016/j.pscychresns.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young G, Rogers S, & Ozonoff S (2011) Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother–infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders 41 (3): 287–301. 10.1037/t54175-000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacrey LA, Bennett JA, & Zwaigenbaum L (2015). Early infant development and intervention for autism spectrum disorder. Journal of Child Neurology, 30(14), 1921–1929. 10.1177/0883073815601500. [DOI] [PubMed] [Google Scholar]
- Sato H, Fuchino Y, Kiguchi M, Katura T, Maki A, Yoro T, & Koizumi H (2005). Intersubject variability of near-infrared spectroscopy signals during sensorimotor cortex activation. Journal of Biomedical Optics, 10(4), 44001 10.1117/1.1960907. [DOI] [PubMed] [Google Scholar]
- Sato H, Tanaka N, Uchida M, Hirabayashi Y, Kanai M, Ashida T, …Maki A (2006). Wavelet analysis for detecting body-movement artifacts in optical topography signals. NeuroImage, 33(2), 580–587. doi:S1053-8119(06)00684-7. [DOI] [PubMed] [Google Scholar]
- Srinivasan SM, & Bhat AN (2016). Differences in object sharing between infants at risk for autism and typically developing infants from 9 to 15 months of age. Infant Behavior & Development, 42, 128–141. 10.1016/j.infbeh.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subburaju V, Belathur S, Sundaram S, & Narasimhan S (2017). Identifying differences in brain activities and an accurate detection of autism spectrum disorder using resting state functional-magnetic resonance imaging: A spatial filtering approach. Medical Image Analysis, 35, 375–389. 10.1016/j.media.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Sutoko S, Sato H, Maki A, Kiguchi M, Hirabayashi Y, Atsumori H, …Katura T (2016). Tutorial on platform for optical topography analysis tools. Neurophotonics, 3(1), 010801 10.1117/1.NPh.3.1.010801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L, Supekar K, and Menon V (2010). Typical and atypical development of functional human brain networks: insights from resting-state fMRI. Frontiers in Systems Neuroscience, 4, 21 10.3389/fnsys.2010.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa S, Takamoto K, Ishikawa A, Ono T, & Nishijo H (2015). Selective medial prefrontal cortex responses during live mutual gaze interactions in human infants: An fNIRS study. Brain Topography, 28(5), 691–701. 10.1007/s10548-014-0414-2. [DOI] [PubMed] [Google Scholar]
- Van Etten H, Kaur M, Srinivasan S, Cohen S, Bhat A, Dobkins K (2017) Increased Prevalence of Unusual Sensory Behaviors in Infants at Risk for, and Teens with, Autism Spectrum Disorder. Journal of Autism and Developmental Disorders August 2 10.1007/s10803-017-3227-9. [Epub ahead of print]. [DOI] [PubMed]
- Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, …Piven J – IBIS Network. (2015). Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain: A Journal of Neurology, 138(Pt 7), 2046–2058. 10.1093/brain/awv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, …Piven J – IBIS Network. (2012). Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. The American Journal of Psychiatry, 169(6), 589–600. 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pillowsky T, Feldman R, Baron-Cohen S, & Sigman M (2006). The development of siblings of children with autism at 4 and 14 months: social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry, 47(5), 511–523. [DOI] [PubMed] [Google Scholar]
- Zhu H, Li J, Fan Y, Li X, Huang D, He S (2015) Atypical prefrontal cortical responses to joint/non-joint attention in children with autism spectrum disorder (ASD): A functional near-infrared spectroscopy study. Biomedical Optics Express 6(3): 1–12. 10.1364/BOE.6.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bauman ML, Choueiri R, Fein D, Kasari C, Pierce K, …Wetherby A (2015). Early identification and interventions for autism spectrum disorder: Executive summary. Pediatrics, 136 Suppl 1, S1–9. 10.1542/peds.2014-3667B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, & Garon N (2013). Early identification of autism spectrum disorders. Behavioural Brain Research, 251, 133–146. 10.1016/j.bbr.2013.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.