Abstract
Objective:
Emerging evidence suggests that injury to the anterior cruciate ligament (ACL) typically initiates biological changes that contribute to the development of osteoarthritis (OA). The molecular biomarkers or mediators of these biological events remain unknown. The goal of this exploratory study was to identify novel synovial fluid biomarkers associated with early biological changes following ACL injury distinct from findings in end-stage OA.
Methods:
Synovial fluid was aspirated from patients with acute (≤30 days) and subacute (31–90 days) ACL tears and from patients with advanced OA and probed via tandem-mass-spectrometry for biomarkers to distinguish OA from ACL injury. Periostin (POSTN) was identified as a potential candidate. Further analyses of POSTN were performed in synovial fluid, OA cartilage, torn ACL remnants, and culture media by Western blot, PCR, immunostaining and ELISA.
Results:
Synovial fluid analysis revealed that POSTN exhibited higher expression in subacute ACL injury than OA. POSTN expression was relatively low in cartilage/chondrocytes suggesting it is produced by other intra-articular tissues. Conversely, high and time-dependent expression of POSTN in ACL tear remnants and isolated cells was consistent with the synovial fluid results.
Conclusions:
Elevated POSTN may provide a synovial fluid biomarker of subacute ACL injury setting separate from OA. Increased expression of POSTN in ACL suggests that the injured ACL may play a pivotal role in POSTN production, which is sensitive to time-from-injury. Previous studies have shown potential catabolic effects of POSTN, raising the possibility that POSTN contributes to the initiation of joint degeneration and may offer a window-of-opportunity to intervene in the early stages of post-traumatic OA.
Keywords: osteoarthritis, ACL cells, articular cartilage, chondrocytes, time-from-injury
INTRODUCTION
The lifetime risk of developing osteoarthritis (OA) is one in two, and it is the third leading cause of years lived with a disability [1, 2]. OA is a multifactorial disease and its pathophysiological mechanisms are complex and poorly understood, but it is now believed that the molecular changes to joint tissues begin much earlier than the clinical symptoms become apparent [3]. A prerequisite for developing disease modifying OA drugs is a reliable way for studying early pathogenic mechanisms [4]. While detection of early manifestations of primary OA is not currently possible due to complex nature of the disease, post-traumatic OA offers the opportunity to study early molecular changes following trauma as time and nature of initial trauma are generally known [5].
Anterior cruciate ligament (ACL) tears are a leading cause of joint trauma, and ACL-deficient knees have altered biomechanics that likely lead to cartilage damage [6] and eventually post-traumatic OA [7]. ACL reconstruction does not prevent OA progression [8, 9], suggesting that the high risk of OA after ACL tear is likely due to factors other than joint instability [7, 10, 11]. Unfortunately, the biological underpinning of how ACL injury leads to OA remains an unanswered question. It is believed that paracrine factors released in the joint following joint injury – potentially from the ACL itself – could exert a biological effect on the joint which might contribute to the development of OA [12]. However, given the long lag between ACL injury and development of OA, the biological response of ACL tears appears to vary with time-from-injury [13, 14]. For instance, we have reported that expression of periostin (POSTN) in ACL tear remnants, which is elevated following ACL injury changes with the chronicity of human ACL tear [15].
POSTN is a secreted matricellular protein that has been shown to be expressed in a variety of connective tissues such as periodontal ligament [16, 17], bone and periosteum [18], tendons [19] and skin [20], and is thought to influence collagen fibrillogenesis[17, 21]. While reduced POSTN levels result in defective connective tissues [22], higher levels of POSTN have been implicated in human and rodent osteoarthritic cartilage [12, 23]. In addition, higher levels of POSTN have been shown to correlate with severity of OA [24, 25]. Certain studies have shown that POSTN is involved in cartilage matrix degradation via MMP-13 [12, 23] and MMP-9 [26]. However, the levels of POSTN in knees after ACL injury has not been compared to levels in knees with OA.
The goal of this exploratory study was to identify novel synovial fluid biomarker candidates associated with early biological changes following ACL injury distinct from findings in end-stage OA. While it is thought that ACL tears initiate biological changes in the joint that contribute to the development of OA, the molecular mediators of these biological changes are not fully understood. We hypothesize that ACL tears result in a significant and predictable pattern of POSTN expression in the knee, which may be a critical mediator of biological changes in the knee after this injury. By cataloguing the proteins present in the synovial fluid of ACL tear and OA patients, we hope to characterize candidates for further study as potential targets for early therapeutic intervention.
METHODS
Study design
First, we compared the synovial fluid proteomics data from 3 groups: (i) acute (≤30 days from injury) ACL tears; (ii) subacute (31–90 days from injury) ACL tears; and (iii) knee OA patients. POSTN was identified as a candidate marker. The findings were validated by multiplex bead assay and Western blot. Next, we measured and compared the expression of POSTN between acute and subacute ACL tears using synovial fluid (multiplex bead assay), ACL tear remnants (real-time PCR) and cells (real-time PCR, Western blot) and cell culture media (ELISA). Finally, we measured and compared the expression of POSTN between ACL tear remnants/cells and articular cartilage/chondrocytes using real-time PCR, Western blot, ELISA, and immunostaining.
Study patients
The study protocol was approved by the Institutional Review Board, and the surgical specimens were handled in accordance with the current ethical standards. All patients (N=124) gave written and signed informed consent before inclusion in the study. Patients’ characteristics are presented in Table-1 and inclusion and exclusion criteria are detailed in Supplementary Text.
Table 1:
Characteristics of study patients
| Tissue | Condition | N | Age (years) | BMI (kg/m2) | Sex | Time from injury (day) Mean [95% CI] | Material | Experiment | Data in |
|---|---|---|---|---|---|---|---|---|---|
| Synovial fluid | Acute (≤ 30 days) | 3 | 35.3 | 22.8 | 3F, 0M | 4.0 [1.74 to 6.26] | Synovial fluid | Proteomics, Western blot | Fig. 1A–C, F, 2A, Tables 2, 5 |
| Synovial fluid | Subacute (31–90 days) | 3 | 47.0 | 24.1 | 1F, 2M | 45.0 [17.6 to 72.4] | Synovial fluid | Proteomics, Western blot | Fig. 1A–B, D, F, 2A, Tables 3, 5 |
| Synovial fluid | OA | 3 | 59.7 | 30.4 | 1F, 2M | - | Synovial fluid | Proteomics, Western blot | Fig. 1A–D, F, Tables 2, 3 |
| Synovial fluid | Acute (≤ 30 days) | 9 | 32.9 | 25.8 | 6F, 3M | 6.0 [3.5 to 8.5] | Synovial fluid | Multiplex ELISA | Fig. 1E, 2B, Table 4 |
| Synovial fluid | Subacute (31–90 days) | 4 | 40.3 | 24.2 | 3F, 1M | 43.7 [28.8 to 58.6] | Synovial fluid | Multiplex ELISA | Fig. 1E, 2B, Table 4 |
| Synovial fluid | OA | 16 | 55.8 | 31.0 | 7F, 12M | - | Synovial fluid | Multiplex ELISA | Fig. 1E, 2B, Table 4 |
| ACL | Acute (≤ 30 days) | 8 | 17.9 | 25.0 | 6F, 2M | 21.8 [18.6 to 25.0] | ACL remnant | Real-time PCR | Fig. 2C, 3D |
| ACL | Subacute (31–90 days) | 7 | 26.4 | 24.2 | 3F, 4M | 53.1 [38.7 to 67.5] | ACL remnant | Real-time PCR | Fig. 2C, 3D |
| ACL | 3–6 months | 4 | 36.5 | 27.6 | 1F, 3M | 206.5 [63.0 to 351.0] | ACL remnant | Real-time PCR | Fig. 2C |
| ACL | > 6 months | 3 | 32.0 | 29.6 | 0F, 3M | 1550.3 [470.0 to 2630.0] | ACL remnant | Real-time PCR | Fig. 2C |
| ACL | Acute (≤ 30 days) | 2 | 38.5 | 20.8 | 1F, 1M | 21.0 [15.1 to 26.9] | Progenitors | Western blot | Fig. 2D |
| ACL | Subacute (31–90 days) | 2 | 29.0 | 22.0 | 2F, 0M | 70.5 [46.0 to 95.0] | Progenitors | Western blot | Fig. 2D |
| ACL | Acute (≤ 30 days) | 3 | 17.7 | 24.2 | 2F, 1M | 13.7 [8.35 to 19.0] | Progenitors | ELISA | Fig. 2E |
| ACL | Subacute (31–90 days) | 3 | 47.0 | 24.1 | 3F, 0M | 49.3 [38.5 to 60.1] | Progenitors | ELISA | Fig. 2E |
| ACL | ACL-T | 6 | 29.8 | 26.8 | 2F, 4M | 75.7 [25.9 to 126.0] | Progenitors | Real-time PCR | Fig. 3A |
| ACL | ACL-T | 6 | 24.3 | 26.2 | 3F, 3M | 29.3 [18.5 to 40.11 | Fibroblasts | Real-time PCR | Fig. 3A |
| Cartilage | OA | 9 | 64.1 | 30.9 | 6F, 3M | - | Chondrocytes | Real-time PCR | Fig. 3A |
| ACL | ACL-T | 2 | 27.0 | 27.8 | 1F, 1M | 35.0 [−10.0 to 80.0] | Progenitors | Western blot | Fig. 3B |
| ACL | ACL-T | 2 | 33.5 | 22.7 | 2F, 0M | 34.0 [4.6 to 63.4] | Fibroblasts | Western blot | Fig. 3B |
| Cartilage | OA | 2 | 63.5 | 33.9 | 1F, 1M | - | Chondrocytes | Western blot | Fig. 3B |
| ACL | ACL-T | 6 | 32.3 | 24.1 | 5F, 1M | 31.5 [14.9 to 48.1] | Progenitors | ELISA | Fig. 3C |
| ACL | ACL-T | 3 | 31.0 | 26.4 | 2F, 1M | 36.0 [16.1 to 55.9] | Fibroblasts | ELISA | Fig. 3C |
| Cartilage | OA | 3 | 64.3 | 33.3 | 1F, 2M | - | Chondrocytes | ELISA | Fig. 3C |
| Cartilage | OA | 8 | 60.9 | 36.9 | 4F, 4M | - | Cartilage | Real-time PCR | Fig. 3D |
| ACL | ACL-T | 3 | 38.0 | 39.3 | 2F, 1M | 34.7 [22.3 to 47.1] | Progenitors | Immunofluorescence | Fig. 3E |
| ACL | ACL-T | 3 | 27.3 | 24.5 | 2F, 1M | 41.3 [32.2 to 50.4] | Fibroblasts | Immunofluorescence | FIG. 3E |
| Cartilage | OA | 4 | 64.8 | 28.6 | 2F, 2M | - | Chondrocytes | Immunofluorescence | Fig. 3E |
ACL = anterior cruciate ligament; ACL-T = ACL tear; BMI = body mass index; OA = osteoarthritis; M = male; F = female; CI = confidence interval PCR = polymerase chain reaction; ELISA = enzyme linked immunosorbent assay
Synovial fluid collection and processing
Synovial fluid was aseptically aspirated with an 18-gauge needle entering the joint from a superolateral approach with the knee in full-extension. There was no gross evidence for iatrogenic contamination of the synovial fluid samples at the time of aspiration. Since all of the sample fluids were collected in the clinic from patients with effusions that warranted aspiration due to their volume, lavage was not necessary. The collecting syringe was capped and transferred to the lab within 30–60 minutes of collection. Synovial fluid was centrifuged at 3,000×g for 20 minutes at 4°C to remove cells and particulate material and clear supernatant was stored in cryovials at −80°C until assayed [27]. None of the synovial samples were so viscous that they became a pellet after centrifuge.
nano-LC-MS/MS analysis
Synovial fluid was depleted of 14 highly abundant proteins [28, 29]. Fractionation, protein digestion, peptide preparation, and isobaric labeling were carried out using standard methods prior to nano-LC-MS/MS analysis. Data processing and analysis were performed using an R environment. The detail of the methods and analysis are given in Supplementary Text. Proteomic data have been deposited in the PRoteomics IDEntifications (PRIDE) PreoteomXchange and can be accessed by identifier PXD013043 (https://massive.ucsd.edu/ProteoSAFe/static/massive.jsp).
Multiplex assay
Synovial fluid was thawed, centrifuged at 15,000×g for 10 minutes at 4°C and diluted (1:8). Quantification of POSTN (and other proteins on the panel namely DKK-1, GDF-15, NSE, OPG, Osteonectin [SPARC], TRA5, TWEAK and YLKL-40) was performed using Milliplex MAP Luminex Assay Kit (Cat. #HCMBMAG-22K) by following the supplied protocol. The assay plate was read on Luminex® 200™ with xPONENT® software. Milliplex Analyst 5.1.0.0 was used as a curve fitting program. A 5-parameter logistic curve-fit and a linear polation logistic curve-fit were used to calculate the concentration in ng/mL from the standards.
Cartilage sampling and cell isolation
Cartilage was collected from knees of patients undergoing total knee arthroplasty. Chondrocytes were isolated via enzymatic digestion [12] as detailed in Supplementary Text.
ACL sampling and cell preparation
ACL remnants were removed from patients undergoing ACL reconstruction surgery. ACL progenitor cells that have been shown to express stem cell markers [30, 31] were isolated as described before [32]. Moreover, we also isolated ACL fibroblasts as described previously [31] with slight modifications. Detailed procedures are described in Supplementary Text.
Collection of cell culture supernatant
Chondrocytes, ACL progenitor cells, and ACL fibroblasts were cultured in individual wells of 12-well plates at a density of 2×105 cells/well. After 4 days, the culture media was collected to measure the levels of POSTN.
RNA isolation and first strand cDNA synthesis
Total RNA was prepared from tissues and cells using TRIzol solution and RNeasy Mini kit (Qiagen) according to supplied protocol [12]. RNA quality and concentration were checked by measuring the ratio of optical densities at 260 and 280 nm using Nanodrop 2000. A total of 250 ng RNA was treated with DNase-I (Invitrogen) to remove traces of genomic DNA. High-Capacity cDNA Reverse Transcription Kit (Thermo-Fisher-Scientific) and random primers were used to convert RNA to first strand of cDNA.
Real-time PCR
Real-time PCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems) as before [33]. The primer for POSTN included: POSTN (forward) 5’-TCTGCACTGCCAAGACTGAG-3’ and (reverse) 5’-TGGTCTTGCCATTCCTGGAC-3’. Peptidylprolyl isomerase A (PPIA) was used as housekeeping gene: (forward) 5’-CAACGCAGCGCTATTCTGAC and (reverse) 5’-CCAAGTTGTCCCAAGCCTCA-3’. Detailed method is provided in Supplementary Text.
Enzyme-linked immunosorbent assay
The levels of POSTN were measured in the cell culture supernatant using a human POSTN ELISA Kit (Cat. # EHPOSTN, Thermo-Fisher-Scientific) as detailed in Supplementary Text.
Immunostaining and confocal microscopy
POSTN localization was assessed by immunofluorescence using chondrocytes, ACL progenitors, or ACL fibroblasts as detailed in Supplementary Text.
Western blot
Western blot was used for detection of POSTN protein in synovial fluid samples as well as lysates prepared from cultured cells. A detailed description is provided in Supplementary Text.
Statistical analyses
All data were analyzed by non-parametric ANOVA (Kruskal-Wallis) test with Dunn’s multiple testing or non-parametric Mann-Whitney U test. Results are presented as mean ± standard error of the mean unless indicated otherwise. Results with a P<0.05 were considered statistically significant.
RESULTS
Higher levels of POSTN in the synovial fluid of subacute ACL tears than OA patients
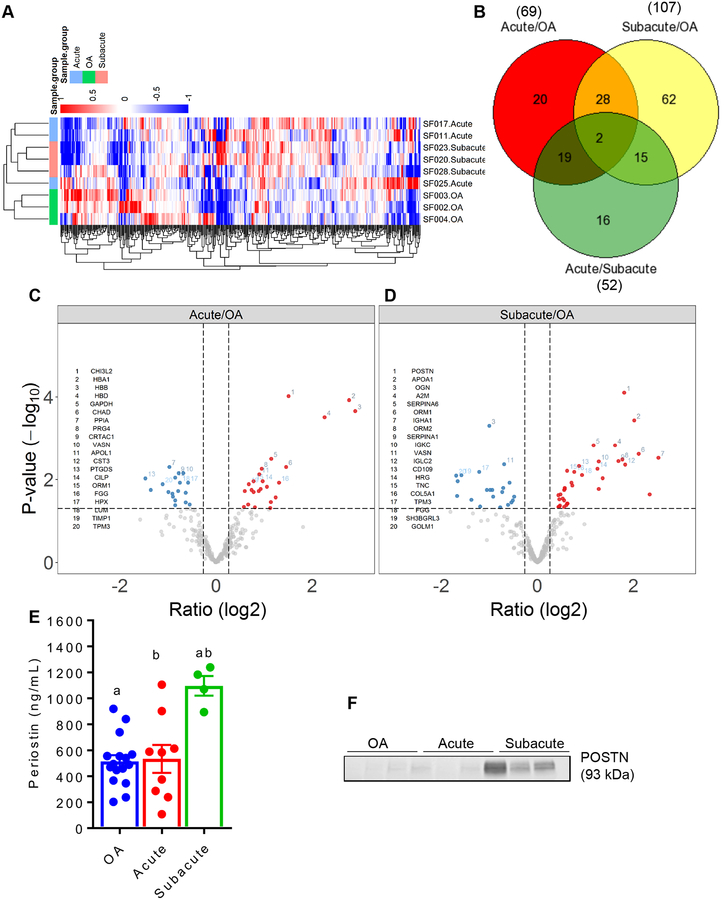
Tandem mass spectrometry on synovial fluid samples from acute and subacute ACL tear patients as well as OA patients quantified 491 proteins (Supplementary Table-1) from 5001 unique peptide sequences (Supplementary Table 2) in the synovial fluid. To assess the levels of POSTN, we compared the ACL tear (acute and subacute) groups with the OA group. Interestingly, MDS analysis revealed a discrete separation of sample from three groups (acute and subacute tear groups without OA and knees with OA) based on differentially expressed proteins. Heatmaps further confirmed distinct segregation of samples (Fig-1A). We observed that 69 proteins were significantly differentially expressed in the synovial fluid from acute tears and OA comparison. Among these, 38 were higher in acute tears and 31 were lower in this group (Table-2; Supplementary Table-3). While 20 proteins exclusively differed between acute tears and OA, 28 proteins overlapped with subacute tears and OA comparison, 19 proteins overlapped with acute and subacute tears comparison and 2 proteins were common to all three comparisons (Fig-1B). Our data demonstrated that POSTN was not significantly differentially expressed in the synovial fluid from patients with acute tears compared to the OA group (Fig-1C).
Figure 1.
A. Unsupervised clustering of the protein abundances relative to the reference standard generated from all samples analyzed using synovial fluid proteomic: acute (N = 3) and subacute (N = 3) tear groups and OA (N = 3) group. B. Venn diagram for the number of proteins that differed between any two groups and the number of proteins that were common to any two or all three groups. C-D. Volcano plot showing differentially expressed proteins between acute tear and OA groups (C) and between subacute tear and OA groups (D). The cutoffs for the horizontal dotted lines are at a P value of 0.05 and the cutoff for the vertical dotted lines are at an absolute fold change of 1.2. Blue dots represent proteins that are low in acute/OA comparison or subacute/OA comparison, while red dots show proteins higher in these comparisons. E. Multiplex assays performed on synovial fluid from acute (N = 9) and subacute (N = 4) tear groups and OA (N = 16) group confirmed significantly higher concentration of POSTN protein in the subacute tear group compared to both acute tear and OA groups. Kruskal-Wallis with Dunn’s multiple comparison test. Each bar graph is presented as mean ± standard error of the mean. Similar lowercase letters (a, b) indicate statistical significance across groups at P < 0.05. F. Western blot analysis of POSTN protein in the synovial fluid of selected patients further showed that the subacute tear group had strong bands corresponding to high POSTN protein compared to both acute tear and OA groups.
Table 2:
Proteins differentially expressed in acute and OA groups
| Symbol | Name | Fold change | P value |
|---|---|---|---|
| Proteins elevated in acute group | |||
| HBB | hemoglobin subunit beta | 7.45 | <0.001 |
| HBA1 | hemoglobin subunit alpha 1 | 6.83 | <0.001 |
| SIPA1L3 | signal induced proliferation associated 1 like 3 | 6.35 | <0.001 |
| HBD | hemoglobin subunit delta | 4.79 | <0.001 |
| SAA1 | serum amyloid A1 | 4.70 | 0.013 |
| THBS1 | thrombospondin 1 | 3.41 | 0.001 |
| CTSL | cathepsin L | 3.22 | 0.006 |
| CHI3L2 | chitinase 3 like 2 | 2.84 | <0.001 |
| CHAD | chondroadherin | 2.76 | 0.005 |
| SELENBP1 | selenium binding protein 1 | 2.49 | 0.013 |
| Proteins repressed in acute group | |||
| APOL3 | apolipoprotein L3 | −5.12 | 0.010 |
| PEBP4 | phosphatidylethanolamine binding protein 4 | −2.98 | 0.002 |
| PTGDS | prostaglandin D2 synthase | −2.77 | 0.009 |
| PIGR | polymeric immunoglobulin receptor | −2.70 | 0.010 |
| B2M | beta-2-microglobulin | −2.56 | 0.018 |
| TPM3 | tropomyosin 3 | −2.16 | 0.013 |
| SH3BGRL3 | SH3 domain binding glutamate rich protein like 3 | −1.98 | 0.020 |
| PPIA | peptidylprolyl isomerase A | −1.97 | 0.005 |
| IGFBP6 | insulin like growth factor binding protein 6 | −1.89 | 0.022 |
| TBC1D1 | TBC1 domain family member 1 | −1.89 | 0.032 |
Comparison between subacute tears and OA revealed that 107 proteins were differentially expressed between the two groups. Among these, 49 were higher in subacute tears and 58 were lower in this group (Table-3; Supplementary Table-4). While 62 proteins were exclusive to the comparison of subacute tears vs. OA, 15 proteins overlapped with acute and subacute comparison, 28 proteins overlapped between acute tears and OA comparison (Fig-1B). The expression of POSTN was ~3.5 fold higher in subacute tears than in OA (P<0.0001) (Fig-1D).
Table 3:
Proteins differentially expressed in subacute and OA groups
| Symbol | Name | Fold change | P value |
|---|---|---|---|
| Proteins elevated in subacute group | |||
| IGHA1 | immunoglobulin heavy constant alpha 1 | 5.75 | 0.003 |
| NES | nestin | 5.31 | <0.001 |
| ALB | albumin | 5.06 | 0.023 |
| ORM1 | orosomucoid 1 | 4.32 | 0.002 |
| APOA1 | apolipoprotein A1 | 4.05 | <0.001 |
| IGLC2 | immunoglobulin lambda constant 2 | 3.54 | 0.004 |
| POSTN | periostin | 3.51 | <0.001 |
| ORM2 | orosomucoid 2 | 3.41 | 0.003 |
| SERPINA1 | serpin family A member 1 | 3.23 | 0.004 |
| A2M | alpha-2-macroglobulin | 3.08 | 0.002 |
| Proteins repressed in subacute group | |||
| APOL3 | apolipoprotein L3 | −8.54 | 0.013 |
| NKPD1 | NTPase, KAP family P-loop domain containing 1 | −6.72 | 0.018 |
| COL2A1 | collagen type II alpha 1 chain | −4.43 | 0.006 |
| HNRNPA2B1 | heterogeneous nuclear ribonucleoprotein A2/B1 | −4.26 | 0.025 |
| GSS | glutathione synthetase | −3.81 | 0.007 |
| FKBP5 | FK506 binding protein 5 | −3.67 | 0.005 |
| YWHAB | 14-3-3 protein beta/alpha | −3.37 | 0.015 |
| GOLM1 | golgi membrane protein 1 | −3.23 | 0.008 |
| VIM | vimentin | −3.18 | 0.025 |
| DEFA1 | defensin alpha 1 | −3.15 | 0.011 |
Consistent with the proteomics results, POSTN was found to be significantly (P=0.001) different between subacute (1096.02 ± 76.13 ng/mL) and OA (488.17 ± 55.70 ng/mL) groups based on multiplex kit analysis (Fig-1E). Interestingly, none of the other 7 proteins, except for osteonectin i.e. SPARC, were either significantly differentially expressed by proteomics or by multiplex assay between acute ACL tears and OA (Table-4). High concentration of POSTN in the subacute phase of injury was further confirmed by Western blot analysis using synovial fluid samples from acute, subacute and OA groups (Fig-1F).
Table 4:
Proteins differentially expressed in acute, subacute and OA groups as determined by multiplex assay
| Symbol | Name | Group | N | Mean | S.E.M | 95% Confidence Interval for Mean | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Acute vs Subacute | Acute vs OA | Subacute vs OA | ||||||
| GDF15 | Growth Differentiation Factor 15 | Acute | 9 | 0.77 | 0.15 | 0.43 | 1.10 | 0.721 | 0.902 | 0.867 |
| Subacute | 4 | 0.54 | 0.21 | −0.11 | 1.10 | |||||
| OA | 16 | 0.68 | 0.13 | 0.40 | 0.96 | |||||
| DKK1 | Dickkopf-related protein 1 | Acute | 9 | 0.36 | 0.05 | 0.24 | 0.48 | 0.800 | 0.695 | 0.409 |
| Subacute | 4 | 0.27 | 0.08 | 0.03 | 0.51 | |||||
| OA | 16 | 0.44 | 0.07 | 0.29 | 0.59 | |||||
| NSE | Neuron-specific enolase | Acute | 9 | 31.59 | 6.18 | 17.33 | 45.84 | 0.812 | 0.990 | 0.841 |
| Subacute | 4 | 23.56 | 5.69 | 5.44 | 41.68 | |||||
| OA | 16 | 30.37 | 6.14 | 17.28 | 43.45 | |||||
| SPARK | Osteonectin | Acute | 9 | 3448.58 | 528.82 | 2229.13 | 4668.04 | 0.055 | <0.001 | 0.296 |
| Subacute | 4 | 2000.10 | 211.37 | 1327.41 | 2672.71 | |||||
| OA | 16 | 1159.78 | 138.84. | 863.84 | 1455.71 | |||||
| POSTN | Periostin | Acute | 9 | 533.43 | 107.21 | 286.21 | 780.66 | 0.003 | 0.902 | 0.001 |
| Subacute | 4 | 1096.02 | 76.13 | 853.76 | 1338.28 | |||||
| OA | 16 | 488.17 | 55.70 | 369.44 | 606.89 | |||||
| TRAP5 | tartrate-resistant acid phosphatase type 5 | Acute | 9 | 107.61 | 37.13 | 22.00 | 193.23 | 0.398 | 0.768 | 0.646 |
| Subacute | 4 | 19.23 | 6.72 | −2.14 | 40.60 | |||||
| OA | 16 | 75.27 | 30.51 | 10.23 | 140.31 | |||||
| OPG | Osteoprotegerin | Acute | 9 | 21.87 | 9.66 | 9.59 | 54.15 | 0.262 | 0.646 | 0.550 |
| Subacute | 4 | 6.02 | 2.59 | −2.22 | 14.26 | |||||
| OA | 16 | 21.83 | 7.04 | 6.82 | 36.84 | |||||
| YKL49 (CHI3L1) | Chitinase 3 Like 1 | Acute | 9 | 4270.52 | 915.03 | 2160.46 | 6380.58 | 0.928 | 0.950 | 0.808 |
| Subacute | 4 | 3599.47 | 1610.40 | −1524.59 | 8723.53 | |||||
| OA | 16 | 4655.36 | 779.16 | 2994.62 | 6316.11 | |||||
| TWEAK | tumor necrosis factor-like weak inducer of apoptosis | Acute | 9 | 0.88 | 0.17 | 0.49 | 1.27 | 0.285 | 0.123 | 0.988 |
| Subacute | 4 | 0.51 | 0.17 | −0.04 | 1.06 | |||||
| OA | 16 | 0.54 | 0.08 | 0.37 | 0.72 | |||||
Temporal changes in synovial fluid POSTN in ACL injury
We examined the temporal changes in POSTN in synovial fluid samples from ACL tear patients. The multidimensional scaling (MDS) analysis showed a distinct separation of sample from two groups (acute and subacute) based on differentially expressed proteins. Unsupervised hierarchical clustering also showed segregation of samples (Fig-1A). In total, 52 proteins were significantly different between acute and subacute tears. Among these, 21 proteins were more highly expressed and 31 had less expression in subacute tears compared to acute tears (Table-5; Supplementary Table-5). Sixteen proteins were exclusive to acute/subacute comparison while others also overlapped with subacute tears and OA comparison (15 proteins) and with acute tears and OA comparison (19 proteins) (Fig-1B).
Table 5:
Proteins differentially expressed in acute and subacute groups
| Symbol | Name | Fold change | P value |
|---|---|---|---|
| Proteins elevated in subacute group | |||
| NES | nestin | 7.01 | <0.001 |
| ALB | albumin | 5.38 | 0.016 |
| POSTN | periostin | 4.08 | 0.000 |
| IGHA1 | immunoglobulin heavy constant alpha 1 | 3.89 | 0.009 |
| HP | haptoglobin | 3.37 | 0.042 |
| SERPINA1 | serpin family A member 1 | 2.78 | 0.005 |
| IGLC2 | immunoglobulin lambda constant 2 | 2.66 | 0.011 |
| ORM1 | orosomucoid 1 | 2.51 | 0.023 |
| MMP3 | matrix metallopeptidase 3 | 2.07 | 0.004 |
| IGKC | immunoglobulin kappa constant | 1.98 | 0.012 |
| Proteins repressed in subacute group | |||
| SIPA1L3 | signal induced proliferation associated 1 like 3 | −6.02 | 0.008 |
| HBB | hemoglobin subunit beta | −5.13 | 0.014 |
| ALPL | alkaline phosphatase, liver/bone/kidney | −4.69 | 0.022 |
| HBA1 | hemoglobin subunit alpha 1 | −4.22 | 0.010 |
| HBD | hemoglobin subunit delta | −3.56 | 0.018 |
| H3F3A | H3 histone family member 3A | −2.80 | 0.004 |
| PZP | PZP, alpha-2-macroglobulin like | −2.72 | 0.019 |
| PSMA3 | proteasome subunit alpha 3 | −2.49 | 0.013 |
| YWHAB | 14-3-3 protein beta/alpha | −2.46 | 0.044 |
| CFHR2 | complement factor H related 2 | −2.30 | 0.027 |
As shown in Fig-2A, the data demonstrated that POSTN was among the most highly significantly differentially expressed protein in the synovial fluid of patients in the subacute ACL tear group compared to the acute ACL tear group. The expression of POSTN was ~4 fold higher in subacute group than the acute group (P<0.001). We also found that POSTN was strongly positively correlated with the time-from-injury based on proteomics data (r=0.94, P=0.006). As stated above, we validated the expression of POSTN protein using a multiplex kit that included POSTN and seven other proteins in a larger cohort of patients. Interestingly, only POSTN was found to be significantly (P=0.003) different between acute (533.43 ±107.21 ng/mL) and subacute (1096.02 ± 76.13 ng/mL) groups. Finally, we observed that POSTN was strongly positively correlated with the time-from-injury based on multiplex (r=0.78, P=0.002) data (Fig-2B).
Figure 2:
A. Volcano plot showing differentially expressed proteins between acute and subacute tear groups based on synovial fluid proteomic analysis. The cutoffs for the horizontal dotted lines are at a P value of 0.05 and the cutoff for the vertical dotted lines are at absolute fold change of 1.2. Blue dots represent proteins that are low in acute/subacute comparison, while red dots show proteins higher in this comparisons. B. Pearson’s correlation between POSTN protein in the synovial fluid and time from ACL tear showed significantly positive correlations based on multiplex data. C. mRNA expression of POSTN in ACL tear remnants (ACL tissues) across four time-from-injury time points showed that subacute tears (31–90 days; N = 7) have significantly higher expression of POSTN than acute tears (<30 days; N = 8) and chronic tears (> 6 months N = 8). ACL tears 3–6 months old (N = 6) showed higher levels of POSTN compared with acute and chronic groups. Kruskal-Wallis with Dunn’s multiple comparison test. Each bar graph is presented as mean ± standard error of the mean. Similar lowercase letters (a, b, c, d) indicate statistical significance across groups at P < 0.05. D. Western blot analysis of POSTN protein for acute and subacute ACL tear groups using total protein extracted from ACL progenitor cells by RIPA buffer showed strong bands for the subacute tear group corresponding to high POSTN protein compared to acute tear group. E. Assessment of secretary POSTN protein from ACL progenitor cells revealed that after 4 days of culture there was significantly higher levels of POSTN in the cell culture media from cells collected from subacute ACL remnants (N = 3) compared to those collected from acute ACL tear remnants (N = 3). Mann-Whitney U test. Each bar graph is presented as mean ± standard error of the mean. Similar lowercase letter (a) indicates statistical significance across groups at P < 0.05.
Temporal changes in POSTN in ACL tear remnants
Next, we assessed the changes in POSTN from ACL tear remnants over time from injury at the gene and protein level. Initial assessment of the natural history of gene expression from the time of ACL injury clearly suggests distinct levels of POSTN in the first 3 months after injury and more than 12 months from injury. Expression of POSTN was low for ≤30 days after ACL injury. Expression increased significantly 31–90 days from injury and remained at a similar high level of expression 3–6 months following injury. The expression started to decline 6 months after ACL injury (Fig-2C). Since ligament repair is unlikely to be a feasible option more than a year from injury, we focused on two time intervals, acute (≤30 days after injury), and subacute (30–90 days after injury) for all subsequent studies. Follow up analysis demonstrated low levels of POSTN ≥ 30 days from injury (acute) and the highest levels of POSTN 31–90 days after injury (subacute). POSTN mRNA was significantly (P=0.017) higher (1.8 fold) in the subacute group than the acute group (Fig-2C). This finding was further confirmed by Western blot analysis of proteins prepared from ACL progenitor cells from acute and subacute groups detecting a 93 kDa protein band corresponding to POSTN protein in the subacute phase of injury (Fig-2D). Acute samples yielded only weak bands. To further explore if subacute ACL progenitor cells produce higher levels of POSTN protein, we measured the concentration of POSTN released in the culture media over 4 days using ELISA. As depicted in Fig-2E, subacute ACL progenitor cells generated significantly more POSTN (P=0.003) compared to acute ACL progenitor cells (101.10±3.77 ng/mL vs. 64.55±4.07 ng/mL).
Comparative expression of POSTN in ACL tear remnants and articular cartilage
Finally, we compared expression of POSTN between ACL tear remnants and articular cartilage. We acknowledge that there is no direct physiological or pathophysiological link between the 2 tissues but comparing the levels of POSTN is informative as to the role of this molecule across the knee as a whole.
Real-time PCR and Western blot results showed that ACL progenitor cells had significantly higher expression of POSTN mRNA (Fig-3A) and protein (Fig-3B) compared to chondrocytes. In addition, culture media collected 4 days after culture showed higher levels of POSTN protein in ACL cells (both progenitors and fibroblasts) than chondrocytes (Fig-3C). ACL tissues had higher expression of POSTN mRNA than cartilage (Fig-3D). Finally, we confirmed that ACL progenitor cells and fibroblasts also have significantly higher staining for POSTN protein than chondrocytes (Fig-3E).
Figure 3:
A. mRNA expression of POSTN in ACL progenitor cells (N = 6), chondrocytes (N = 9) and ACL fibroblasts (N = 4) showed that ACL progenitor cells had significantly higher expression of POSTN mRNA than chondrocytes. Kruskal-Wallis with Dunn’s multiple comparison test. Each bar graph is presented as mean ± standard error of the mean. Similar lowercase letter (a) indicates statistical significance across groups at P < 0.05. B. Western blot analysis of POSTN protein extracted from ACL progenitor cells, chondrocytes and ACL fibroblasts showed that ACL progenitor cells showed strong bands corresponding to high POSTN protein compared to chondrocytes. The intensity of POSTN bands was in between progenitor cells and chondrocytes for ACL fibroblasts. C. POSTN ELISA performed on cell culture media collected after 4 days of culture from ACL progenitor cells (N = 3), ACL fibroblasts (N = 3) and chondrocytes (N = 3) showed that chondrocytes had significantly lower concentration of POSTN protein than both ACL progenitor cells and ACL fibroblasts. Kruskal-Wallis with Dunn’s multiple comparison test. Each bar graph is presented as mean ± standard error of the mean. Similar lowercase letters (a, b) indicate statistical significance across groups at P < 0.05. D. mRNA expression of POSTN was measured in ACL tear remnants and cartilage tissues demonstrating that both acute (N = 6) and subacute (N = 5) ACL tear remnants had significantly higher POSTN mRNA than cartilage (N = 8). Kruskal-Wallis with Dunn’s multiple comparison test. Each bar graph is presented as mean ± standard error of the mean. Similar lowercase letters (a, b) indicate statistical significance across groups at P < 0.05. E. Immunofluorescent staining of ACL progenitor (N = 3) cells, ACL fibroblasts (N = 3) and chondrocytes (N = 3) further showed that POSTN protein (green) was higher in ACL progenitors and fibroblasts than chondrocytes. Phalloidin (red-orange) was used to stain F-actin while DAPI (blue) staining showed nucleus. Scale bar = 50 μm
DISCUSSION
As expected, this study identified differential expression of a number of proteins in the synovial fluid of knees following ACL tear compared to knees with end-stage radiographic OA identifying biological differences between early changes following ACL tears and those with end-stage OA. POSTN stood out as a strong candidate biomarker because previous studies have demonstrated that mRNA expression of POSTN in ACL remnants is very sensitive to time-from-injury [15], torn ACL remnants co-cultured with chondrocytes exert paracrine effects on chondrocytes function potentially via POSTN [12], and POSTN has been shown to be highly expressed in osteoarthritic cartilage [12, 23, 34]. However, there is no information on the source of POSTN in the knee and no comparison of POSTN levels in ACL injured knees with OA knees has been made.
This study demonstrated that the levels of POSTN were higher in the ACL injured knees especially in the subacute phase of injury (31–90 days) compared to knees with end-stage radiographic OA. This is the first comparison of POSTN levels between ACL tear and OA knees. A number of other studies have reported that OA synovial fluid and serum had significantly higher concentrations of POSTN compared to that of rheumatoid arthritis (RA) patients and healthy controls, and that these higher levels are positively correlated with the severity and the risk of progression of knee OA [24, 25, 35]. one contrasting study reported higher levels of POSTN in the synovial fluid of RA patients than in OA patients [36]. Cartilage from human and mouse OA knees has been shown to have higher expression of POSTN than normal cartilage [12, 23]. This higher expression of POSTN was confined mainly to chondrocytes in the periphery of erosive lesions [34] and has been associated with higher expression of MMPs in chondrocytes [12, 23] and synoviocytes [26]. There are some studies that report significant up-regulation of the POSTN gene in subchondral bone during OA [37–39]. However, none of these studies compared POSTN expression in OA patients with ACL tear patients. Given the fact that ACL tear increases the risk of developing OA [8, 9] and that the biologic underpinnings of this elevated risk are unknown, our findings suggest that POSTN accumulation in the joint following ACL injury may contribute to the initiation of degenerative changes in the cartilage. If so, it may provide a therapeutic target for blocking the progress of OA at a very early stage following ACL injury, before cartilage degradation occurs.
Another interesting finding was that the expression of POSTN from subacute ACL tears was significantly higher than from acute ACL tears. Moreover, mRNA and protein level expression of POSTN was also significantly higher in ACL cells, tissue and culture media from the subacute injury group. These findings suggest that POSTN could serve as a marker of chronicity following ACL injury. These findings are consistent with our prior finding that ACL tear remnants express higher levels of POSTN mRNA, particularly in within 3 months after ACL tear [15]. In the current study, differences in POSTN expression can be detected within 3 months of injury, which may identify a window of opportunity to intervene and delay or prevent post-traumatic OA following ACL tears. our data showed relatively low levels of POSTN in the first month after injury, which increase within 3 months of injury, peak at 3–6 months following injury and then declined significantly. A similar expression pattern of POSTN mRNA has been shown in the medial compartment of the knee in a murine model of post-traumatic OA initiated by meniscus and ACL tears [23]. It is possible that this finding suggests a biological benefit from early surgical intervention following ACL injury, as stabilization within the first month of an ACL tear could reduce intra-articular accumulation of POSTN. If this accumulation contributes to the development of OA, early treatment of the tear could be chondroprotective.
The complete source of POSTN in the joint is not known although it is thought that many tissues in the joint express and produce POSTN such as bone [18], cartilage [12, 23, 34], ACL [12, 15], meniscus [40] and synovium [36]. However, to date, no quantitative comparison of POSTN expression between various joint tissues has been reported. Comparing cartilage and ACL, we found elevated expression of POSTN in ACL tissues and cells suggesting that ACL tissue and cells are a more significant source of POSTN than cartilage and chondrocytes. Another study reported that POSTN was more highly up-regulated in ACL than in patellar tendon [41], consistent with our finding that the ACL is perhaps a major source of POSTN mRNA and protein in the knee.
This study has some limitations. First, while it is important to point out that not all patients with an ACL tear go on to develop OA, comparing the biologic response of the knee to ACL injury with the biology of OA is relevant because at least some subset of ACL tear patients go on to develop OA. This information is a first step towards understanding the initial effect of the ACL tear on the knee, as it is unknown exactly when and how knees diverge in their response to this common injury such that some develop OA and some do not. The study only included patients with isolated ACL tears without concomitant meniscus tears to minimize the confounding effect of these injuries. Second, we did not analyze the synovial fluid or tissues from healthy joints for practical and ethical reasons. While the focus of the current study was on POSTN, the available data from other proteins offers the opportunity to study other candidate proteins in ACL injury and OA. Third, while we were not able to study the other proteins that showed significant differences between acute and subacute ACL tears or between OA and ACL tear groups, several other potential candidates emerged from proteomic analysis that deserve further investigation. For instance, nestin, which is an intermediate filament protein and a marker of neural progenitors [42], was elevated in the subacute group compared to acute ACL tears and OA patients, suggesting the subacute tear group may have an increased number of nestin-positive cells. The combination of elevated nestin and POSTN expression suggests a more substantial repair response. Another example is the expression of SPARC (osteonectin) protein. In this study SPARC was not significantly different between any groups in proteomic analysis, was found to be significantly higher (~3-fold) in the acute group compared to the OA group by multiplex assay. SPARC is a secreted extracellular matrix protein that has been shown to be associated with ligament morphogenesis, modulation of tissue remodeling and extracellular matrix interaction [43]. SPARC expression also increased following ACL injury, suggesting that it may also play a role in the response of the knee to this trauma [44]. Another limitation is the lack of controls from non-injured knees. While this is an area that deserves further study, the significant differences between acute ACL tears, subacute ACL tears and OA represent novel information about the post-traumatic response of the knee to injury irrespective of if and how this differs from the uninjured controls. Lastly, the sample sizes are small. No a priori power calculation was undertaken, and so the study should be considered exploratory. However, the consistency of results across different methods of analysis in 124 discrete patients should give some confidence that they could be replicated. other factors such as clearance rate across the synovial membrane could influence levels of POSTN over time and were not assessed in the current study. one area that deserves further evaluation is determining if there are sex based differences in the pattern of POSTN expression following ACL injury. While we did not find any evidence for a difference based on sex in our current analysis that was not the focus of the investigation and more study is needed.
It remains unclear what POSTN tells us as a biomarker and what tissues contribute to POSTN in the knee. At this stage, we can only speculate about the role of POSTN based on the available literature. As outlined above, POSTN is a secreted matricellular protein that influences collagen fibrillogenesis and its reduced levels result in defective connective tissues. It appears that it is an important biomarker for synthesis of matrix components in fibrous tissues such as ACL. However, in articular cartilage, expression of POSTN is low in the normal state and is increased in OA. This suggests that higher levels in the cartilage are not desirable as it may lead to MMP-13 expression that in turn results in cartilage degeneration and OA. Thus, POSTN likely plays a dual role in the knee, depending on which tissue and under what conditions. Future mechanistic studies are necessary to expand our understanding of this complex, likely multifaceted, role. Based on our recent findings [32] and current observations, the injured ACL could be a major sources of POSTN in the knee. However, screening of all knee joint tissues is necessary to make a final determination.
In conclusion, elevated expression of POSTN in knees with ACL tears compared to end-stage OA suggests it is a distinct synovial fluid biomarker following this common injury. Further investigation is needed to determine the implications of this elevated POSTN expression for the knee and whether it has any predictive value for sequelae such as post-traumatic OA. The primary effect of ACL injury may be to induce the expression of POSTN from the injured ligament itself. In comparison with ACL tear remnants, cartilage appears to have low levels of POSTN. The increased expression of POSTN in the injured ACL suggests the injured ACL plays a pivotal role in POSTN production, which is sensitive to time-from-injury. The implications of POSTN accumulation in the knee over time are unknown. Elevated levels of POSTN after ACL injury may serve as a marker of the repair/healing response, implying a benefit for the joint. Conversely, sustained overexpression of POSTN may contribute to the initiation of joint degeneration. In the latter case POSTN may be a predictor of and/°r a possible target for early intervention to prevent post-traumatic OA.
Further investigation is needed to confirm these preliminary findings and better understand the cause and consequence of POSTN overproduction in knees after ACL injury, particularly to explore if it plays a critical role in the pathogenesis of OA.
Supplementary Material
Acknowledgements
The expert technical assistance of Petra Erdmann-Gilmore, Yiling Mi, Jim Malone and Rose Connors is gratefully acknowledged. The mass spectrometric experiments were performed at the Washington University Proteomics Shared Resource (WU-PSR), The WU-PSR is supported in part by the WU Institute of Clinical and Translational Sciences (NCATS UL1 TR000448), the Mass Spectrometry Research Resource (NIGMS P41 GM103422) and the Siteman Cancer Center Support Grant (NCI P30 CA091842).
Role of the funding source
This study was supported by orthopedics Research and Education (OREF) Career Development Award No. 18–002 (Drs. Brophy and Rai) and through NIH grant (AG46927, AG15768, Dr. Guilak) from National Institute on Aging (NIA). Dr. Rai was supported through the National Institutes of Health (NIH) Pathway to Independence Award (R00-AR064837) from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIAMS, NIA or the NIH and OREF. We also acknowledge the services by P30-AR074992 (Musculoskeletal Research Center) and P30-AR073752 (Rheumatic Disease Research Resource-based Center)." before "The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIAMS, NIA or the NIH and OREF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors have no competing interest to declare.
Financial conflict of interest
All authors have nothing to disclose.
REFERENCES
- 1.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum 2008; 59: 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT, et al. The burden of disease and injury in the United States 1996. Popul Health Metr 2006; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, Hodgson R. Identifying and treating preclinical and early osteoarthritis. Rheum Dis Clin North Am 2014; 40: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer WC, Hendricks KJ, Wang J. Pathogenetic mechanisms of posttraumatic osteoarthritis: opportunities for early intervention. Int J Clin Exp Med 2011; 4: 285–298. [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res 2011; 29: 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson F, Billinghurst RC, Pidoux I, Reiner A, Langworthy M, McDermott M, et al. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis Cartilage 2006; 14: 114–119. [DOI] [PubMed] [Google Scholar]
- 7.Roos H, Adalberth T, Dahlberg L, Lohmander LS. osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage 1995; 3: 261–267. [DOI] [PubMed] [Google Scholar]
- 8.Nordenvall R, Bahmanyar S, Adami J, Mattila VM, Fellander-Tsai L. Cruciate ligament reconstruction and risk of knee osteoarthritis: the association between cruciate ligament injury and post-traumatic osteoarthritis. a population based nationwide study in Sweden, 1987–2009. PLoS one 2014; 9: e104681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 2007; 35: 1756–1769. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Chen C, Chen S. Posttraumatic knee osteoarthritis following anterior cruciate ligament injury: Potential biochemical mediators of degenerative alteration and specific biochemical markers. Biomed Rep 2015; 3: 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lattermann C, Conley CE, Johnson DL, Reinke EK, Huston LJ, Huebner JL, et al. Select Biomarkers on the Day of Anterior Cruciate Ligament Reconstruction Predict Poor Patient-Reported outcomes at 2-Year Follow-Up: A Pilot Study. Biomed Res Int 2018; 2018: 9387809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinzei N, Brophy RH, Duan X, Cai L, Nunley RM, Sandell LJ, et al. Molecular influence of anterior cruciate ligament tear remnants on chondrocytes: a biologic connection between injury and osteoarthritis. Osteoarthritis Cartilage 2018; 26: 588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohly NE, Murray IR, Keating JF. Revision anterior cruciate ligament reconstruction: timing of surgery and the incidence of meniscal tears and degenerative change. J Bone Joint Surg Br 2007; 89: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 14.Amano K, Huebner JL, Stabler TV, Tanaka M, McCulloch CE, Lobach I, et al. Synovial Fluid Profile at the Time of Anterior Cruciate Ligament Reconstruction and Its Association With Cartilage Matrix Composition 3 Years After Surgery. Am J Sports Med 2018: 363546517749834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brophy RH, Tycksen ED, Sandell LJ, Rai MF. Changes in Transcriptome-Wide Gene Expression of Anterior Cruciate Ligament Tears Based on Time From Injury. Am J Sports Med 2016; 44: 2064–2075. [DOI] [PubMed] [Google Scholar]
- 16.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 1999; 14: 1239–1249. [DOI] [PubMed] [Google Scholar]
- 17.Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci 2008; 1123: 30–40. [DOI] [PubMed] [Google Scholar]
- 18.Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H, et al. Expression and function of periostin-isoforms in bone. J Cell Biochem 2004; 92: 1044–1061. [DOI] [PubMed] [Google Scholar]
- 19.Yoshiba N, Yoshiba K, Hosoya A, Saito M, Yokoi T, Okiji T, et al. Association of TIMP-2 with extracellular matrix exposed to mechanical stress and its co-distribution with periostin during mouse mandible development. Cell Tissue Res 2007; 330: 133–145. [DOI] [PubMed] [Google Scholar]
- 20.Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A, et al. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci U S A 2007; 104: 14472–14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 2007; 101: 695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet N, Gineyts E, Ammann P, Conway SJ, Garnero P, Ferrari S. Periostin deficiency increases bone damage and impairs injury response to fatigue loading in adult mice. PLoS one 2013; 8: e78347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attur M, Yang Q, Shimada K, Tachida Y, Nagase H, Mignatti P, et al. Elevated expression of periostin in human osteoarthritic cartilage and its potential role in matrix degradation via matrix metalloproteinase-13. FASEB J 2015; 29: 4107–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lourido L, Calamia V, Mateos J, Fernandez-Puente P, Fernandez-Tajes J, Blanco FJ, et al. Quantitative proteomic profiling of human articular cartilage degradation in osteoarthritis. J Proteome Res 2014; 13: 6096–6106. [DOI] [PubMed] [Google Scholar]
- 25.Honsawek S, Wilairatana V, Udomsinprasert W, Sinlapavilawan P, Jirathanathornnukul N. Association of plasma and synovial fluid periostin with radiographic knee osteoarthritis: Cross-sectional study. Joint Bone Spine 2015; 82: 352–355. [DOI] [PubMed] [Google Scholar]
- 26.Tajika Y, Moue T, Ishikawa S, Asano K, Okumo T, Takagi H, et al. Influence of Periostin on Synoviocytes in Knee Osteoarthritis. In Vivo 2017; 31: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watt FE, Paterson E, Freidin A, Kenny M, Judge A, Saklatvala J, et al. Acute Molecular Changes in Synovial Fluid Following Human Knee Injury: Association With Early Clinical outcomes. Arthritis Rheumatol 2016; 68: 2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritter SY, Subbaiah R, Bebek G, Crish J, Scanzello CR, Krastins B, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum 2013; 65: 981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharjee M, Balakrishnan L, Renuse S, Advani J, Goel R, Sathe G, et al. Synovial fluid proteome in rheumatoid arthritis. Clin Proteomics 2016; 13: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KJ, Clegg PD, Comerford EJ, Canty-Laird EG. Ligament-Derived Stem Cells: Identification, Characterisation, and Therapeutic Application. Stem Cells Int 2017; 2017: 1919845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinert AF, Kunz M, Prager P, Barthel T, Jakob F, Noth U, et al. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng Part A 2011; 17: 1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai L, Brophy RH, Tycksen ED, Duan X, Nunley RM, Rai MF. Distinct expression pattern of periostin splice variants in chondrocytes and ligament progenitor cells. FASEB J 2019; 33: 8386–8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J Bone Joint Surg Am 2012; 94: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chijimatsu R, Kunugiza Y, Taniyama Y, Nakamura N, Tomita T, Yoshikawa H. Expression and pathological effects of periostin in human osteoarthritis cartilage. BMC Musculoskelet Disord 2015; 16: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rousseau JC, Sornay-Rendu E, Bertholon C, Garnero P, Chapurlat R. Serum periostin is associated with prevalent knee osteoarthritis and disease incidence/progression in women: the OFELY study. Osteoarthritis Cartilage 2015; 23: 1736–1742. [DOI] [PubMed] [Google Scholar]
- 36.Brown JM, Mantoku A, Sabokbar A, oppermann U, Hassan AB, Kudo A, et al. Periostin expression in neoplastic and non-neoplastic diseases of bone and joint. Clin Sarcoma Res 2018; 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou CH, Wu CC, Song IW, Chuang HP, Lu LS, Chang JH, et al. Genome-wide expression profiles of subchondral bone in osteoarthritis. Arthritis Res Ther 2013; 15: R190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou CH, Lee CH, Lu LS, Song IW, Chuang HP, Kuo SY, et al. Direct assessment of articular cartilage and underlying subchondral bone reveals a progressive gene expression change in human osteoarthritic knees. Osteoarthritis Cartilage 2013; 21: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang R, Fang H, Chen Y, Shen J, Lu H, Zeng C, et al. Gene expression analyses of subchondral bone in early experimental osteoarthritis by microarray. PLoS one 2012; 7: e32356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brophy RH, Zhang B, Cai L, Wright RW, Sandell LJ, Rai MF. Transcriptome comparison of meniscus from patients with and without osteoarthritis. Osteoarthritis Cartilage 2018; 26: 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Little D, Thompson JW, Dubois LG, Ruch DS, Moseley MA, Guilak F. Proteomic differences between male and female anterior cruciate ligament and patellar tendon. PLoS one 2014; 9: e96526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dealy CN, Scranton V, Cheng HC. Roles of transforming growth factor-alpha and epidermal growth factor in chick limb development. Dev Biol 1998; 202: 43–55. [DOI] [PubMed] [Google Scholar]
- 43.Tremble PM, Lane TF, Sage EH, Werb Z. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol 1993; 121: 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clements DN, Carter SD, Innes JF, Ollier WE, Day PJ. Gene expression profiling of normal and ruptured canine anterior cruciate ligaments. Osteoarthritis Cartilage 2008; 16: 195–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.