Abstract
Objective:
To investigate whether rates of self-reported Woman’s Condom (WC) clinical failure and semen exposure from a functionality study are comparable to results from a contraceptive efficacy substudy.
Study Design:
We structured our comparative analysis to assess whether functionality studies might credibly supplant contraceptive efficacy studies when evaluating new female condom products. Couples not at risk of pregnancy in the functionality breakage/slippage/invagination/penile misdirection) study and women in the contraceptive efficacy study completed condom self-reports and collected pre and postcoital vaginal samples for up to four uses of the WC. Both studies used nearly identical self-report questions and the same self-sampling procedures and laboratory for prostatic specific antigen (PSA), a well-studied semen biomarker. We compared condom failure and semen exposure proportions using generalized estimating equations methods accounting for within-couple correlation.
Results:
Ninety-five (95) efficacy substudy participants used 334 WC and 408 functionality participants used 1572 WC. Based on self-report, 19.2% WC (64 condoms) clinically failed in the efficacy substudy compared to 12.3% WC (194 condoms) in the functionality study (p-value=0.030). Of the 207 WC efficacy uses with evaluable post-coital PSA levels, 14.5% (30 uses) resulted in semen exposure compared to 14.2% (184 uses) of the 1293 evaluable WC functionality study uses.
Conclusions:
When evaluating the ability of an experimental condom to prevent semen exposure, the rate of clinical condom failure reported by participants risking pregnancy in an efficacy substudy was significantly higher than the rate reported by participants not risking pregnancy in a functionality study. The rate of semen exposure, assessed by an objective biomarker was nearly identical for the two studies.
Implications:
Our results suggest that an objective marker of semen exposure in functionality studies could provide a reasonable alternative to contraceptive efficacy studies in evaluating risk of unintended pregnancy and inferring protection from sexually transmitted infection than condom failure rates based on self-report.
Keywords: prostate-specific antigen (PSA), semen biomarkers, female internal condom, contraceptive efficacy, condom clinical failure, sexually transmitted infection
1.0. Introduction
Despite the public health importance of developing a variety of female condom products to counter both unintended pregnancy and sexually transmitted infection (STI) transmission, only four female condom products are widely available in multiple markets around the world, having received WHO/UNFPA prequalification and the European Union’s CE marking [1–2]. Only one of these products, the FC2, is FDA approved and available for sale in the U.S. While FDA approval of a new male condom product is contingent on successful completion of a large functionality (breakage and slippage) study demonstrating non-inferiority compared to a predicate condom, a new female condom product requires a large scale contraceptive efficacy study prior to market approval [3]. The rationale for this expensive requirement, which impedes additional female condom products from becoming available in the U.S. [4], stems from the variations and complexities in female condom design that can lead to failure modes other than breakage and slippage.
Regulators have laid the groundwork for female condom functionality studies by defining additional failure modes which include invagination (condom pushed into the vagina) and misdirection (condom bypassed by penis) [5]. Concerns remain about how accurately participants can discern multiple failure modes and the degree to which these failure modes reflect actual risk of unintended pregnancy or STI transmission. The incorporation of quantifiable semen exposure measurement within functionality studies is a viable response to these concerns [6–10]. Prostate specific antigen (PSA), is a reliable biomarker of semen exposure [7–20]. Studies have demonstrated the utility of collecting pre- and postcoital vaginal samples which can be stored at room temperature and later assayed for PSA following well-established laboratory procedures [21–23]. Comparative studies have shown that semen exposure estimates are more consistent than self-collected failure reports across different study populations [18]. Furthermore, semen exposure estimates are more directly related to the risk of STI transmission than pregnancy rates [8].
Whether a study population not at risk of pregnancy in a functionality study (breakage/slippage/invagination/penile misdirection) would be representative of a study population at risk of pregnancy in a contraceptive efficacy study requires further investigation. Functionality studies would not be an acceptable substitution for efficacy studies if participants not at risk of pregnancy experienced lower condom failure rates and/or semen exposure rates than participants at risk of pregnancy.
Close collaboration between CONRAD and the National Institute of Child and Human Development (NICHD) on their respective studies of the Woman’s Condom (WC) allowed for the use of nearly identical report forms, and identical sample collection and laboratory testing procedures to estimate female condom failure rates and biomarker confirmed semen exposure estimates based on PSA results. Our objective was to determine whether the self-reported condom failure and semen exposure rates were comparable between a functionality study and a contraceptive efficacy substudy of the WC.
2.0. Materials and methods
2.1. Study design
We report on two studies which evaluated the WC. The CONRAD functionality study was a randomized, open-label, cross-over study of clinical condom failure and vaginal semen exposure which compared the WC with the FC2 condom. Participating couples completed a self-report of clinical failure and collected a precoital vaginal swab and postcoital swabs from the vagina and condom interior after each of four condom uses of both condom types. California Family Health Council (CFHC) conducted this study in 2010-2011.
The NICHD contraceptive efficacy study was a phase III multicenter, open-label, non-comparative trial of the WC. Seven of 11 study sites (two managed by CFHC), participated in a nested substudy in which participants completed a self-report of clinical failure and collected a precoital vaginal swab and postcoital swabs from the vagina and condom interior after the first four uses of the WC (study conducted in 2011-2012).
Participants in both studies recorded each study condom use on a condom self-report form on which they recorded the circumstances of condom use, how the condom performed, and whether they collected swabs (Supplement Sections 6 and 7). All participants provided written informed consent in conformance with 21 CFR Part 50.25 [24]. Institutional Review Boards associated with the respective study sites approved the study.
2.2. Study population
Participants in both the functionality and efficacy studies were sexually active, between the ages of 18 and 45, in monogamous relationships of at least 3 months, and reported themselves at low STI risk. Couples in the functionality study were required to use effective back-up contraception such as a hormonal method, IUD or sterilization to prevent pregnancy. Women in the efficacy study had no known factors impairing their fertility and depended solely on the WC to prevent pregnancy.
2.3. Study products
The WC (Shanghai Dahua Medical Apparatus Corp., Ltd, Shanghai, China) consists of a 0.03-mm-thick pliable plastic pouch that easily conforms to the shape of the vagina. It is 22.7 +/− 0.25 cm (9.0 +/− 0.1 inches) long and has a flexible soft outer ring designed to hug the external genitalia. Foam shapes on the outside of the pouch cling lightly to vaginal walls to stabilize the device. The insertion capsule made from dissolvable polyvinyl alcohol (PVA) is similar to the PVA used in C-Film (Apothecus Pharmaceutical Corporation, New York, NY). Since the WC is not pre-lubricated, participants were instructed to use only study-provided a water based personal lubricant which has been shown not to interfere with the PSA assay used in this study [25] (Supplement Section 1).
2.4. Swab collection
Participants in both the functionality study and efficacy substudy collected vaginal and condom samples using Falcon Swubes® (Becton Dickinson and Company) [26]. Participants collected the precoital vaginal swabs immediately before any genital sexual contact and the postcoital vaginal and condom swabs immediately after removal of the WC (Supplement Sections 2 and 3).
2.5. Laboratory procedures
The same technician at the Center for Disease Control (CDC) followed identical laboratory procedures for both studies [22, 27–28] (Supplement Section 4).
2.6. Statistical analysis
The objective of this analysis is to compare the rates of self-reported clinical condom failure and lab-confirmed semen exposure among WC users in the functionality study with comparable rates among WC users in the efficacy substudy. For functionality study results to supplant efficacy study results, it is critical that condom failure and semen exposure rates from a not-at-risk functionality study population not be lower than the rates obtained from an at-risk efficacy study population. Results included in our analysis met the criteria described in the Figure 1 [13, 8, 29] (Supplement Section 5).
Figure 1.
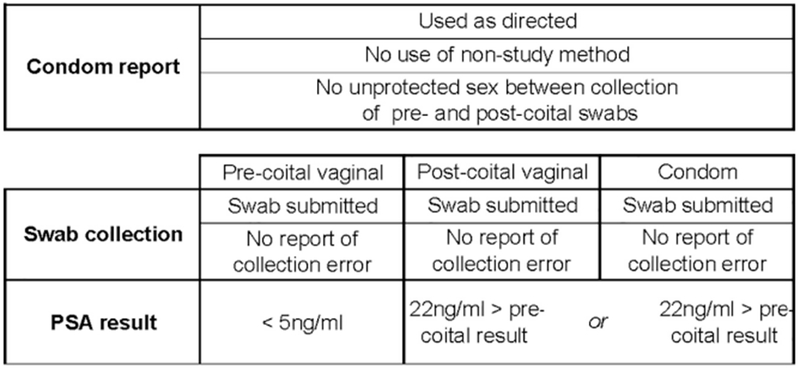
We calculated the sensitivity and specificity of self-reported condom failure in the functionality study and efficacy substudy using PSA as the reference standard since PSA is a highly sensitive marker of semen exposure and self-reported condom failure does not directly measure semen exposure [16, 18] (Supplement Section 6).
The rates of self-reported condom failure (CFF) and semen exposure (SEF) in the functionality study were deemed “not appreciably lower” than the rates of self-reported condom failure (CFE) and semen exposure (SEE) in the efficacy substudy if the lower bounds of the 90% confidence interval for (CFF-CFE; SEF-SEE) were greater than −0.03 (−3%), the margin used in both the CONRAD functionality study and NICHD substudy. We used generalized estimating equations (GEE) methods to perform tests of noninferiority, accounting for within-couple correlation. Missing outcome data were assumed missing completely at random. The model used an identity link function and independence working correlation matrix. The output was a 90% confidence interval around the difference in the semen exposure rates from the two studies. For all other comparisons, we evaluated statistical significance as a p-value of < 0.05.
3.0. Results
All 490 couples in the functionality study and 154 efficacy substudy participants agreed to complete a condom self-report and collect samples in conjunction with four condom uses. Table 1 presents demographic characteristics for the 408 female functionality study participants and 95 efficacy substudy participants who completed at least one condom self-report. Table 2 provides a breakdown of condom self-reports and swab sets used in the analysis, as well as the reasons for the exclusion of results from the analysis. The percentage of uses excluded from the analysis was higher in the efficacy substudy than in the functionality study for all categories.
Table 1.
Demographic characteristics and sexual/contraceptive history of participants at enrollment in two studies of a female condom
| Functionality Study | Efficacy Substudy | |
|---|---|---|
| Characteristic | n (%) | n (%) |
| Total Participants | 408 | 95 |
| Social/demographic/physical | ||
| Female age (median)Female age (median) | 25 | 28 |
| Race/Ethnicity1 Race/Ethnicity1 | ||
| Asian/Pacific Islander | 34 (8.3) | 11 (11.6) |
| Black | 45 (11.0) | 40 (42.1) |
| White | 193 (47.3) | 42 (44.2) |
| Hispanic | 75 (18.4) | 21 (22.1) |
| Native American | 4 (1.0) | 4 (4.2) |
| Other/Multiple | 57 (14.0) | 10 (10.5) |
| Some college or above | 360 (88.2) | 75 (78.9) |
| Not married | 286 (70.1) | 66 (69.5) |
| Current smoker | 48 (11.8) | 19 (20.0) |
| Current alcohol drinker | 359 (88.0) | 48 (50.5) |
| BMI (lb*703/in2) (median)2BMI (lb*703/in2) (median)2 | 24 | 27 |
| Sexual activity/contraceptive use | ||
| Lifetime sexual partners (median) | 5 | 5 |
| Used hormonal method in past 6 months | NC3 | 21 (22.1)4 |
| Currently using hormonal method | 270 (66.2)5 | NC3 |
| Previous experience using female condom | 28 (6.9) | 18 (18.9) |
Multiple responses allowed in Efficacy Study
Calculated from height and weight measures
Not collected from study participants
Includes oral contraceptives, patch, implant, vaginal ring, emergency contraception
Includes oral contraceptives, injectables, and patch
Table 2.
Evaluable condom self-reports and swab sets in two studies of a female condom
| Functionality | Efficacy | |||
|---|---|---|---|---|
| Study | Substudy | |||
| n | % | n | % | |
| Total condom self-reports submitted | 1582 | 353 | ||
| Disqualifications based on self-report | ||||
| Used for anal intercourse | 1 | 0.1 | 1 | 0.3 |
| Used non-study lubricant | 4 | 0.3 | 11 | 3.1 |
| Not used for vaginal intercourse | 0 | 0.0 | 1 | 0.3 |
| Total evaluable condom self-reports | 1577 | 340 | ||
| Disqualifications based on swabs (multiple reasons possible) | ||||
| Precoital vaginal swab not collected or interpretable | 10 | 0.6 | 18 | 5.3 |
| Postcoital vaginal swab not collected or interpretable | 16 | 1.0 | 22 | 6.5 |
| Postcoital condom swab not collected or interpretable | 33 | 2.1 | 21 | 6.2 |
| Precoital vaginal sample ≥ 5ng/ml PSA1 | 56 | 3.6 | 34 | 10.0 |
| Unprotected intercourse between collection of pre- and postcoital swabs2 | 42 | 2.7 | 14 | 4.1 |
| Non-study method use between collection of pre- and postcoital swabs3 | NA | NA | 16 | 4.7 |
| Neither vaginal nor condom postcoital sample 22ng/ml > precoital sample4 | 162 | 10.3 | 53 | 15.6 |
| Total evaluable swab sets | 1293 | 207 | ||
Indicative of semen exposure from previous act of intercourse
Swabs not disqualifed if unprotected intercourse only occurred after a condom failure
Swabs not disqualified if non-study method use occurred after a condom failure
No possibility of semen expsosure due to insufficient amount of semen
3.1. Condom functionality measures
One hundred twenty-four (32%) couples in the functionality study and 25 (35%) participants in the efficacy substudy who contributed at least one evaluable swab set reported one or more female condom failure modes while using the WC. Table 3 presents the frequencies of these failure modes (penile misdirection, invagination, completely slipping out of the vagina while not clinging to the penis, clinical breakage) for both studies. While none of the individual failure modes were statistically different (p-value < 0.05) between the two studies, the total clinical failure was significantly higher in the efficacy substudy (19.2%) compared to the functionality study (12.3%) (p-value < 0.03), reflecting the cumulative effect of the higher incidence of failure reported by efficacy substudy participants for each of the failure modes compared to functionality study participants.
Table 3.
Self-reported condom failure events and semen exposure in two studies of a female condom, by failure mode
| Event Type | Functionality Study | Efficacy Substudy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Uses with Reported Events7 | % | Total Uses8 | 95% CI | Uses with Reported Events7 | % | Total Uses8 | 95% CI | P-Value | |
| Misdirection1 | 59 | 3.8 | 1570(2.7-4.9) | 19 | 5.7 | 333(2.7-8.7) | 0.23 | ||
| Invagination2 | 115 | 7.3 | 1570(5.6-9.0) | 37 | 11.0 | 336(6.3-15.7) | 0.15 | ||
| Complete slip out3 | 13 | 0.8 | 1577(0.4-1.3) | 7 | 2.1 | 340(0.6-2.5) | 0.18 | ||
| Clinical breakage4 | 40 | 2.5 | 1577(1.6-3.5) | 11 | 3.2 | 339(1.1-5.4) | 0.55 | ||
| Total clinical failure5 | 194 | 12.3 | 1572(10.3-14.4) | 64 | 19.2 | 334(13.3-25.0) | 0.03 | ||
| Semen exposure6 | 184 | 14.2 | 1293(11.7-16.7) | 30 | 14.5 | 207(8.4-20.6) | NS9 | ||
Penis was inserted between condom and the vagina
Open end of the condom was pushed completely into the vagina
Slipped completely out of vagina during intercourse while NOT clinging to the penis
Broke during vaginal intercourse or removal of the condom from the vagina
Includes penile misdirection, complete invagination, complete slip out while not clinging to penis or clinical break
Difference between pre-coital and post-coital vaginal PSA result ≥ 22ng/ml
Event occurred one or more times
Excludes uses where event occurrence is unknown due to missing data
Not statistically significant: p-value > 0.05
3.2. Semen exposure measures
The rates of semen exposure were nearly identical for the two studies (Table 3). Likewise, quantitative PSA results for the pre- and postcoital vaginal samples and the sample from the interior of the used condom were similar for both studies (Table 4).
Table 4.
PSA results in two studies of a female condom, by swab source
| Swab Source | n= | Functionality Study | n= | Efficacy Substudy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 ng/ml | 1-4 ng/ml | 5-22 ng/ml | 23-99 ng/ml | ≥100 ng/ml | <1 ng/ml | 1-4 ng/ml | 5-22 ng/ml | 23-99 ng/ml | ≥100 ng/ml | |||
| % | % | % | % | % | % | % | % | % | % | |||
| Pre-coital vaginal | 1293 | 98 | 2 | --- | --- | --- | 207 | 94 | 6 | --- | --- | --- |
| Post-coital vaginal | 1293 | 75 | 7 | 4 | 4 | 10 | 207 | 63 | 15 | 8 | 4 | 10 |
| Post-coital condom | 1287 | 2 | 1 | 1 | 2 | 95 | 207 | 1 | 0 | 0 | 4 | 94 |
In Table 5, we compare the rate of semen exposure following a reported condom failure or performance problem for the two studies. We also include the median PSA results for condom failure modes and performance problems collected in the functionality study but not the efficacy substudy. Clinical breakage resulted in the highest rate of semen exposure among the failure modes (76%) and the second highest median PSA result (5225 ng/ml). Penile misdirection had the second highest probability of semen exposure (32%) and the highest median PSA result (8175 ng/ml), followed by invagination (29%) with a lower PSA result (2648 ng/ml). Surprisingly, slipping out of the vagina while clinging to the penis, which is not defined as a failure mode, was much more likely to result in semen exposure (36%) than slipping out of the vagina while not clinging to the penis (6%), which is defined as a failure mode [5]. Slipping out of the vagina while clinging to the penis also resulted in a median PSA result of 4691 ng/ml, comparable to PSA results of clinical breakage (5225 ng/ml).
Table 5.
Self-reported condom failure events and corresponding percent (%) semen exposure in two studies of a female condom
| Functionality Study (n=1289) | Efficacy Substudy (n=205) | Total (n=1494) | |||||
|---|---|---|---|---|---|---|---|
| Self-Reported Events | Semen Exposure | Self-Reported Events | Semen Exposure | Self-Reported Events | Semen Exposure | ||
| n | % (+) ≥22ng/ml8 | Median (+) PSA (ng/ml)9 | n | % (+) ≥22ng/ml8 | n | % (+) ≥22ng/ml8 | |
| No clinical failures reported | 1135 | 9 | 232 | 171 | 13 | 1306 | 9 |
| Clinical breakage1 | 17 | 82 | 5225 | 4 | 50 | 21 | 76 |
| Misdirection2 | 15 | 40 | 8175 | 4 | 0 | 19 | 32 |
| Invagination3 | 54 | 31 | 2648 | 12 | 17 | 66 | 29 |
| Slipped out while not clinging to penis4 | 3 | 0 | --- | 3 | 0 | 6 | 0 |
| Slipped out while clinging to penis5 | 29 | 14 | 4691 | 7 | 29 | 36 | 17 |
| Slipped out during withdrawal6 | 85 | 4 | 67 | 13 | 8 | 98 | 4 |
| Total clinical failure7 | 154 | 53 | 3243 | 34 | 21 | 188 | 47 |
Broke during vaginal intercourse or withdrawal of the condom, no other functionality problem reported
Penis was inserted between condom and the vagina at least once, no other functionality problem reported
Open end of the condom was pushed completely into the vagina at least once, no other functionality problem reported
Slipped completely out of vagina while not clinging to the penis during intercourse at least once, no other functionality problem reported
Slipped completely out of vagina while clinging to the penis during intercourse at least once, no other functionality problem reported (not a failure mode)
Slipped completely out of vagina during withdrawal of the penis at least once, no other functionality problem reported (not a failure mode)
One or more of the following reported: clinical breakage, penile misdirection, complete invagination, or slipped completely out of vagina while clinging to the penis during intercourse; use not included in clinical failure estimate if information on any of the failure modes was missing
Difference between pre- and post-coital vaginal PSA result; percentage based on self-reported events within category
Serial dilutions on swabs >100ng/ml were not performed in the Efficacy substudy
3.3. Sensitivity and specificity of self-reported clinical failure using PSA level as the reference standard
Since the PSA is a highly sensitive marker of semen exposure and measures semen exposure directly, we used PSA as the reference standard for calculating the sensitivity and specificity of self-reported condom failure as an indicator of semen exposure. The sensitivity is the probability that a condom failure would be self-reported when the PSA level indicated semen exposure and the specificity is the probability that a condom failure was not self-reported (when the PSA level actually did not indicate semen exposure). Thus, the sensitivity and specificity estimates presented in Table 6 quantify the loss of information about semen exposure when relying on self-reports of condom failure.
Table 6.
Sensitivity and specificity of self-reported condom clinical failure using semen exposure as the reference standard in two studies of a female condom
| Functionality Study | Efficacy Substudy | ||
|---|---|---|---|
| A | Uses with self-reported clinical failure1 | 154 | 34 |
| B | Uses with semen exposure2 | 182 | 30 |
| C | Uses with both self-reported clinical failure and semen exposure | 81 | 7 |
| C/B | Sensitivity | 45% | 23% |
| 95% Confidence Interval | (36%-53%) | (7%-40%) | |
| D | Uses with no self reported clinical failure | 1135 | 171 |
| E | Uses with no semen exposure | 1107 | 175 |
| F | Uses without either self-reported clinical failure or semen exposure | 1034 | 148 |
| F/E | Specificity | 93% | 85% |
| 95% Confidence Interval | (92%-95%) | (77%-92%) |
One or more of the following was reported: condom breakage, penile misdirection, complete invagination, slipped completely out of vagina during intercourse while not clinging to the penis like a male condom
Either the condom swab PSA result or post-coital vaginal PSA result was 22ng/ml greater than the pre-coital vaginal PSA result
While the specificity of failure self-reports was high in both studies (93% functionality, 85% efficacy), the sensitivity was low, especially in the efficacy study (45% functionality, 23% efficacy). The sensitivity and specificity of condom self-reports were consistently higher in the functionality study than in the efficacy substudy.
3.4. Rates of self-reported clinical failure and semen exposure
The rate of clinical condom failure based on self-report was considerably lower in the functionality study (12.3%) than in the efficacy substudy (19.2%) (Table 7). The two studies’ semen exposure rates based on uses that met all evaluable criteria were nearly identical (14.2% functionality, 14.5% efficacy substudy). We did not have the statistical power to show that semen exposure in the functionality study was not appreciably lower than in the efficacy substudy.
Table 7.
Test of non-inferiority (alpha= 0.051) for the probabilities of semen exposure and self-reported clinical condom failure2
| Probability | Functionality Study | Efficacy Substudy | Estimate difference | Lower bound for two-sided | Reject Ho3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Uses | Events | % | Uses | Events | % | % | 90% CI | ||
| Clinical condom failure4 | 1572 | 194 | 12.3 | 334 | 64 | 19.2 | −6.8 | −12.0% | No |
| Semen exposure (evaluable uses)5 | 1293 | 184 | 14.2 | 207 | 30 | 14.5 | −0.3 | −5.78% | No |
| Semen exposure (all uses)6 | 1553 | 229 | 14.7 | 318 | 37 | 11.6 | 3.1 | −1.24% | Yes |
Based on the lower bond of the two-sided 90% Confidence Interval (CI)
SAS GENMOD procedure, controlling for within-couple correlation and self-reported ejaculation
The null hypothesis of inferiority is rejected if the lower bound of the two-sided 90% CI for delta > −3.00
Includes penile misdirection, invagination, complete slip out while not clinging to penis or clinical break
Difference between pre-coital and post-coital vaginal PSA result ≥ 22ng/ml
Difference between pre-coital and post-coital vaginal PSA result ≥ 22ng/ml; includes all uses with interpretable pre- and post-coital vaginal swabs
4.0. Discussion
We found that the rate of self-reported clinical condom failure was significantly higher (19.2%) in the efficacy substudy compared to the functionality study (12.3%) which may reflect the difference in the risk status of the participants by study. While participants in the efficacy study were at risk of pregnancy if the WC failed, we required participants in the functionality study to use an effective back-up method of birth control (hormonal, IUD, sterilization). This difference may have led to differences in how much care participants exercised in using condoms, as well as their attentiveness to functionality problems, acceptability of female condom use, and motivation to use the condom throughout any entire act of intercourse. These factors may have contributed to the shorter length of intercourse reported in the functionality study (13.2 minutes vs. 17.7 minutes for the efficacy substudy) and the increased propensity of functionality study participants to remove the condom before ending intercourse (8.7% functionality, 5.2% efficacy).
In contrast, the semen exposure rates for the two studies were almost identical (14.2% functionality, 14.5% efficacy). Previous studies have also found greater agreement between semen exposure rates than between self-reported condom failure rates. Functionality studies of the female condom which shared common protocols and procedures conducted in Brazil and Alabama, found very similar PSA postcoital levels (19% > 1ng/ml PSA Brazil, 17% > 1ng/ml PSA Alabama) but dissimilar self-reported clinical failure rates (5% Brazil, 29% Alabama) [18]. These findings strongly suggest that semen exposure might be a more consistent and objective measure of condom functionality than self-reported condom failure which relies on the ability of participants to interpret failure events during intercourse.
The low sensitivity of self-reported clinical failure (45% functionality study, 23% efficacy study) is explained by the poor correspondence between some of the clinical failure modes and semen exposure. For example, while 82% of the clinical breakages in the functionality study and 50% of the clinical breakages in the efficacy substudy resulted in semen exposure, none of the condoms that slipped completely out of the vagina while not clinging to penis resulted in semen exposure in either study. The sensitivity of reported condom failure in the efficacy substudy was further reduced because slipping out of the vagina while clinging to the penis, resulting in semen exposure on 17% of the 36 occasions in which it was reported in the efficacy substudy, is not defined as a standard female condom failure mode [5]. Ironically, slipping out of the vagina while not clinging to the penis, which is defined as a failure mode, did not result in any semen exposure, possibly reflecting the difficulties condom users encounter detecting failure modes during intercourse. Alternatively, these results may suggest that the set of functionality problems currently defined as standard condom failure modes for female condoms merit reevaluation based on semen exposure results.
The identical study procedures and nearly identical data collection instruments used by the CONRAD functionality study and the NICHD efficacy substudy allowed us to test the feasibility of condom evaluation strategies applied to different study designs and study populations. Participants in both studies submitted swabs for a very high percentage of their reported condom uses (98% functionality, 94% efficacy). The demographic diversity of the substudy population suggests that self-collection of swabs may be feasible in various study populations.
An inherent limitation to any study based on self-reported outcomes and self-collected swabs is that the investigator cannot verify the authenticity of the data. Although our analysis includes only condom uses in which at least one of the post-coital samples was >22ng PSA, there was no way to distinguish swabs that had been collected poorly or not at all. In a crossover functionality study randomized to order of condom use, deficient swabs evenly distributed between the condom types would mitigate this limitation.
Another notable limitation to this comparative analysis involves the limited number of evaluable samples from the efficacy substudy. First, only 156 of the 380 participants consented to the efficacy substudy sites. Second, only 95 of the 156 consented participants ended up contributing condom self-reports with their swab sets. A substantial number of the non-contributors (34 participants) never used or reported any WC use, an indicator that WC acceptability was an issue for some participants. Furthermore, compliance with swab collection instructions was lower in the efficacy substudy than in the functionality study leading to a lower proportion of evaluable WC uses in the efficacy substudy than in the functionality study (82% functionality, 61% efficacy substudy). This was due to a variety of factors including a lower rate of swab collection, a higher incidence of precoital vaginal samples with PSA ≥ 5ng/ml, more frequent unprotected intercourse between collection of pre- and postcoital swabs and use of non-study methods. Motivational factors may account for some of these differences; functionality study participants agreed to test mechanical properties of a limited number of study condoms over several weeks while efficacy study participants assented to using study condoms for months.
Although we lacked statistical power due to the small sample size of the efficacy substudy, our results suggest that while participants in a functionality study are much less likely to self-report condom failure than participants in an efficacy study, the rates of objectively measured semen exposure are very similar for both study populations. Our results support the premise that functionality studies that measure semen exposure using PSA as a biomarker can provide a reasonable alternative to contraceptive efficacy studies in evaluating the contraceptive effectiveness of female condoms. In addition, since it is not feasible to directly evaluate the prevention of the transmission of STIs in clinical trials, semen exposure estimates in functionality studies may provide a better inference of STI protection.
Supplementary Material
Acknowledgments
The authors thank Deborah Boerschig, Joann Cooper, Anne Davis, Anna Garcia, Kate Hodges, Jackie Seguin, and Elizabeth Steider for data collection; Carolyn Brown for PSA laboratory oversight, Teresa Brown for laboratory testing; Sam Bell, Sarah Godfrey, Robert Muraguri Gitonga, and Clint Dart for data management; Maggie Kilbourne-Brook and Matthew Reeves for technical and medical expertise; and Karen Peacock for her assistance with manuscript review and editing.
Funding
Eunice Kennedy Shriver National Institute of Child and Human Development (NICHD) (Protocol #CCN008) and CONRAD (Protocol # D09-108) under USAID (PPRD) Cooperative Agreement [GPO-A-00-08-00005-00] through PEPFAR supported this work. The US Centers for Disease Control and Prevention (CDC), Division of Reproductive Health, and the Division of Scientific Resources provided quantified prostate specific antigen testing for this project.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of NICHD, CDC, or CONRAD. Use of trade names is for identification only and does not imply endorsement by NICHD, CDC, or CONRAD.
Authors’ Disclosures: J.L.S. is an employee of CONRAD, a not for profit organization, and serves on safety committees for Medicines 360 and Femasys®. D.L.B. is an employee of the NIH and is principal investigator of a CRADA between NICHD and HRA Pharma. D.F.A. (within the past 3 years) has received research support from Actavis, Bayer Healthcare, Endoceutics, Glenmark, Merck, Radius Health, Shionogi, and TherapeuticsMD; and has served as a consultant to AbbVie, Actavis, Agile Therapeutics, Bayer Healthcare, Endoceutics, Exeltis, InnovaGyn, Merck, Pfizer, Radius Health, Sermonix, Shionogi, Teva Women’s Healthcare, and TherapeuticsMD. K.T.B. has consulted for AbbVie and Bayer Healthcare. J.T.J. has received payments for consulting and research support from Bayer Healthcare, Merck, Sebela, Abbvie, HRA Pharma, and the Population Council, consulting only from Cooper Surgical, and research support only from Estetra SPRL and Medicines360. These potential conflicts of interest have been reviewed and managed by OHSU. A.L.N. has received research support through Agile Therapeutics, ContraMed/Sebela, Estetra SPRL, Evofem Inc., FHI 360, Mathra Pharma, and Merck. She has also received honoraria or consultation fees from Avion, Bayer Healthcare, CooperSurgical, Merck, Agile, AMAG Pharma, ContraMed/Sebela, and Pharmanest. These potential conflicts of interest have been reviewed and managed by Essential Access Health. M.A.T. has received research support through Agile Therapeutics, Bayer Healthcare, and Medicines 360, all managed through the University of Cincinnati College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registrations: clinicaltrials.gov: (NICHD-CCN008); (CONRAD-108)
References
- [1].United Nations Population Fund. UNFPA Prequalified Female Condom Products (in alphabetical order), 2019. http://www.unfpaprocurement.org/documents/10157/37547/UNFPA+Female+Condom+Prequalification+List/05feba45-4893-474a-81d4-7b61e4f68ae7 (accessed 5 May 2019).
- [2].Forbes A. Promoting Global Access to Female Condoms. Our Bodies Ourselves, 2014. https://www.ourbodiesourselves.org/book-excerpts/health-article/promoting-global-access-to-female-condoms/ (accessed 5 May 2019) [Google Scholar]
- [3].US Food and Drug Administration Obstetrical and Gynecological Devices. Reclassification of single-use female condom, to be renamed single-use internal condom. Final Order Federal Register 2018; 83(188):48711–48713(2018-09-27). [PubMed] [Google Scholar]
- [4].Kosova E. The power of protection: why the FDA’s policy on female condoms matters. National Women’s Health Network, 2018. https://nwhn.org/power-protection-fdas-policy-female-condoms-matters/ (accessed 5 May 2019) [Google Scholar]
- [5].Beksinska M, Joanis C, Manning J, Smit J, Callahan M, Deperthes B, et al. Standardized definitions of failure modes for female condoms. Contraception 2007;75:251–5. [DOI] [PubMed] [Google Scholar]
- [6].Lawson ML, Macaluso M, Bloom A, Hortin G, Hammond KR, Blackwell R. Objective markers of condom failure. Sex Transm Dis 1998;25:427–32. [DOI] [PubMed] [Google Scholar]
- [7].Macaluso M, Lawson L, Akers R, Valappil T, Hammond K, Blackwell R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception 1999;59:195–201. [DOI] [PubMed] [Google Scholar]
- [8].Mauck CK, Doncel GF, Biomarkers of Semen Exposure Clinical Working Group. Biomarkers of semen in the vagina: applications in clinical trials of contraception and prevention of sexually transmitted pathogens including HIV. Contraception 2007; 75:407–419. [DOI] [PubMed] [Google Scholar]
- [9].Mauck CK, Weaver MA, Schwartz JL, Walsh T, Joanis C. Critical next steps for female condom research-Report from a workshop. Contraception 2009; 79:339–344. [DOI] [PubMed] [Google Scholar]
- [10].Snead MC, Black CM, Kourtis AP. The use of bilomarkers of semen exposure in sexual and reproductive health studies. J. Womens Health (Larchmt) 2014; 23(10):787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Walsh TL, Frezieres RG, Nelson AL, Wraxall BG, Clark VA. Evaluation of prostate-specific antigen as a quantifiable indicator of condom failure in clinical trials. Contraception 1999; 60:289–298. [DOI] [PubMed] [Google Scholar]
- [12].Lawson ML, Macaluso M, Duerr A, Hortin G, Hammond KR, Blackwell R, et al. Partner characteristics, intensity of the intercourse, and semen exposure during use of the female condom. Am J Epidemiol 2003;157:282–8. [DOI] [PubMed] [Google Scholar]
- [13].Macaluso M, Lawson ML, Hortin G, Duerr A, Hammond KR, Blackwell R, et al. Efficacy of the female condom as a barrier to semen during intercourse. Am J Epidemiol 2003;157:289–97. [DOI] [PubMed] [Google Scholar]
- [14].Walsh TL, Frezieres RG, Peacock K, Nelson AL, Clark VA, Bernstein L, Wraxall BGD. Use of prostate-specific antigen (PSA) to measure semen exposure resulting from male condom failures: implications for contraceptive efficacy and the prevention of sexually transmitted disease. Contraception 2003;67:139–150. [DOI] [PubMed] [Google Scholar]
- [15].Galvao LW, Oliveira LC, Jua D, Kim Dj, Marchi N, van Dam J, et al. Effectiveness of female and male condoms in preventing exposure to semen during vaginal intercourse: A randomized trial. Contraception 2005;71:130–6. [DOI] [PubMed] [Google Scholar]
- [16].Walsh T, Warner L, Macaluso M, Frezieres R, Snead M, Wraxall B. Prostate-specific antigen as a biomarker of condom failure: comparison of three laboratory assays and self-reported condom use problems in a randomized trial of female condom performance. Contraception 2012; 86(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gallo MF, Behets FM, Steiner MJ, Hobbs MM, Hoke TH, Van Damme K, et al. Prostate-specific antigen to ascertain reliability of self-reported coital exposure to semen. Sex Transm Dis 2006;33:476–9. [DOI] [PubMed] [Google Scholar]
- [18].Chen MP, Macaluso M, Blackwell R, Galvao L, Kulczycki A, Diaz J, et al. Self-reported mechanical problems during condom use and semen exposure. Comparison of two randomized trials in the United States of America and Brazil. Sex Transm Dis 2007;34:557–62. [DOI] [PubMed] [Google Scholar]
- [19].Jamshidi R, Penman-Aguilar A, Wiener J, Gallo MF, Zenilman JM, Melendez JH, et al. Detection of two biological markers of intercourse: prostate-specific antigen and Y-chromosomal DNA. Contraception 2013; 88(6);749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kulczyski A, Brill I, Snead MC, Macaluso M. Prostate-specific antigen concentration in vaginal fluid after exposure to semen. Contraception 2017; 96(5)336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carey RN, Frye RM, Cook JD, Koch TR, Harris EK. Between-lot/between-instrument variations of the abbott imx method for prostate-specific antigen. Clin Chem 1992;38:2341–3. [PubMed] [Google Scholar]
- [22].Kort SA, Martens F, Vanpoucke H, van Duijnhoven HL, Blankenstein MA, Kort SAR, et al. Comparison of 6 automated assays for total and free prostate-specific antigen with special reference to their reactivity toward the WHO 96/670 reference preparation. Clin Chem 2006. August;52(8): 1568–74. [DOI] [PubMed] [Google Scholar]
- [23].Bahamondes L, Diaz J, Marchi NM, Castro S, Villarroel M, Macaluso M, et al. Prostate-specific antigen in vaginal fluid after exposure to known amounts of semen and after condom use: Comparison of self-collected and nurse-collected samples. Hum Reprod 2008;23:2444–51. [DOI] [PubMed] [Google Scholar]
- [24].United States Food & Drug Administration. CFR – Code of Federal Regulations Title 21, Part 50.25, 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=50.25 (accessed 10 June 2019).
- [25].Snead MC, Melendez JH, Kourtis AP, Chaney DM, Brown TM, Black CM, Mauck CK, Schwartz JL, Zenilman JM, Jamieson DJ, Macaluso M, Doncel GF. Effect of lubricants and a vaginal spermicide gel on the detection of prostate specific antigen, a biomarker of semen exposure, using a quantitative (Abbott ARCHITECT) assay. Contraception 2014;89(2):134–8.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Becton Dickinson and Company. BD Falcon SWUBE – Single Swab Sterile (cited 2009 FNovember 9); Available from http://www.bd.com/ds/productcenter/220210.asp
- [27].Abbott Diagnostics. ARCHITECT i2000SR (cited 2009 November 9); Available from: http://www.abbottdiagnostics.com/products/instrumentsbyplatform/default.cfm?system=architect&suffix=i2000sr®=us
- [28].Slev PR, La’ulu SL, Roberts WL. Intermethod differences in results for total PSA, free PSA, and percentage of free PSA. Am J Clin Pathol 2008. June;129(6):952–8. [DOI] [PubMed] [Google Scholar]
- [29].Macaluso M, Blackwell R, Jamieson DJ, Kulczycki A, Chen MP, Akers R, et al. Efficacy of the male latex condom and of the female polyurethane condom as barriers to semen during intercourse: A randomized clinical trial. Am J Epidemiol 2007;166:88–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


