Abstract
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE) that affects cervid species throughout North America. We evaluated gene expression in white‐tailed deer collected by Illinois Department of Natural Resource wildlife managers during annual population reduction (e.g., sharpshooting) and disease monitoring efforts throughout the CWD‐endemic area of northcentral Illinois. We conducted comparative transcriptomic analysis of liver and retropharyngeal lymph node tissue samples between CWD‐positive (n = 5) and CWD‐not detected (n = 5) deer. A total of 74,479 transcripts were assembled, and 51,661 (69.36%) transcripts were found to have matched proteins in NCBI‐NR and UniProt. Our analysis of functional categories showed 40,308 transcripts were assigned to at least one Gene Ontology term and 37,853 transcripts were involved in at least one pathway. We identified a total of 59 differentially expressed genes (DEGs) in CWD‐positive deer, of which 36 and 23 were associated with liver and retropharyngeal lymph node tissues, respectively. Functions of DEGs lend support to previous relationships between misfolded PrP and cellular membranes (e.g., STXBP5), and internal cellular components. We identified several genes that suggest a link between CWD and retroviruses and identified the gene ADIPOQ that acts as a tumor necrosis factor (TNF) antagonist. This gene may lead to reduced production of TNF and impact disease progression and clinical symptoms associated with CWD (i.e., wasting syndrome). Use of candidate genes identified in this study suggests the activation of endogenous processes in CWD‐positive deer, which in turn may enable earlier detection of the disease.
Keywords: chronic wasting disease, differential gene expression, Illinois, Odocoileus virginianus, RNA‐Seq, white‐tailed deer
We evaluated gene expression in white‐tailed deer collected by the Illinois Department of Natural Resources during annual population reduction and disease monitoring efforts throughout the CWD‐endemic area of northcentral Illinois. We identified 59 differentially expressed genes (DEGs) in CWD–infected deer LV (n = 36) and RPLN (n = 23) tissues, of which 28 genes were up‐regulated and 31 down‐regulated. Use of candidate genes identified in this study suggests the activation of endogenous processes in CWD‐positive deer, which in turn may enable earlier detection of the disease.
1. INTRODUCTION
Transmissible spongiform encephalopathies (TSEs) are fatal neurodegenerative prion diseases (proteinaceous infectious particle; Prusiner, 1982) that infect humans (Creutzfeldt‐Jakob disease, kuru; Brown, 2013), mink (transmissible mink encephalopathy; Hartsough & Burger, 1965), sheep (scrapie; Prusiner, 1989), cattle (bovine spongiform encephalopathy; Hope et al., 1988), and cervids (chronic wasting disease [CWD]; Williams & Young, 1980). Prion diseases are caused by conversion of naturally occurring protease‐sensitive, cellular prion protein (PrPc) into a conformationally altered isoform (PrPSc) of prions (Griffith, 1967; Prusiner, 1982). These abnormal prions, or amyloids, accumulate in the central nervous system and peripheral lymphoid tissues (Caughey, Race, & Chesebro, 1988; Kimberlin & Walker, 1982) of a host, which are detergent‐insoluble and partially proteinase K‐resistant (Pan et al., 1993; Prusiner, 1989). Post‐translational alterations from PrP to PrPSc have been implicated as the causative factor leading to infection (Prusiner, 1989).
Chronic wasting disease (CWD) is of considerable interest and concern to wildlife managers throughout North America (Williams, Miller, Kreeger, Kahn, & Thorne, 2002). Due to the potential risk of transmission, CWD poses a possible threat to domestic species such as cattle (Basu et al., 2012) and swine (Moore et al., 2017), and cannot be ruled out as a potential risk to human health (Waddell et al., 2018). Risk of cross‐species transmission is increased by the ability of prions to affect captive and free‐ranging animals (Williams et al., 2002) as captive wildlife are more likely to come in contact with domestic species. Prevalence rates as high as 50% in free‐ranging herds and 90% in captive herds have been documented (Haley & Hoover, 2015), though prions are difficult to diagnose in live cervids (Cheng et al., 2016) due to the logistical challenges of collecting diagnostic samples. Tonsil, lymphoid, and third eyelid tissues may be biopsied to confirm CWD infection in live animals; however, these tissue biopsies would require sedation or anesthesia in accordance with Institutional Animal Care and Use Committee (IACUC) protocols, and are impractical in free‐ranging herds (Haley & Richt, 2017). Clinical signs may help in visual diagnoses of positive animals, but are only apparent in final stages of disease progression (i.e., months to years after initial infection; Gilch et al., 2011; Williams, 2005), and may be the result of other chronic disease processes.
Little is known about prion transmission in native hosts (Saunders, Bartelt‐Hunt, & Bartz, 2012). Some research suggests underlying mechanisms of PrPSc formation may be dependent on the type of prion disease (i.e., infectious, sporadic, genetic; Harris, 1999). Chronic wasting disease is transmitted primarily through direct contact between positive and susceptible animals via oral and mucosal membranes (Safar et al., 2008). Contact with prions in the environment via horizontal transmission (Saunders et al., 2012) and mother‐to‐offspring vertical transmission (Nalls et al., 2013; Selariu et al., 2015) contribute to the rate of disease spread. Furthermore, prions are stable enough to withstand environmental changes such as ultraviolet radiation, freeze–thaw cycles, and bacterial and fungal enzymes (Gilch et al., 2011) and persist for at least 1 year (Kuznetsova, Cullingham, McKenzie, & Aiken, 2018; Wyckoff et al., 2016) in soil in the absence of CWD‐positive deer (Johnson et al., 2006).
A single prior study identified gene expression changes in CWD‐positive Rocky Mountain elk (Cervus elaphus) using microarray analysis and predetermined transcripts (Basu et al., 2012). This study provided evidence for the involvement of genes assigned to functional groups associated with biological regulation, metabolic process, and cellular process. Moreover, Basu et al. (2012) identified novel genes and numerous pathways that contributed to infection, including calcium signaling, apoptosis and cell death, immune cell trafficking, and inflammatory response. However, confinement to known transcripts is a disadvantage of traditional microarray studies. Studies addressing hypothesis‐driven questions related to the role of specific genes in facilitating or reducing disease infection in white‐tailed deer (Odocoileus virginianus; hereafter deer) are difficult to conduct given the limited availability of annotated deer genomes and transcripts currently available in the literature. Furthermore, researchers have evaluated the potential for CWD resistance in relation to sequence polymorphisms (Brandt et al., 2015; Kelly et al., 2008) and potential genetic risk factors (Matsumoto, Samuel, Bollinger, Pybus, & Coltman, 2013), but have not previously examined differentially expressed genes in CWD‐infected and noninfected deer.
Next‐generation sequencing (NGS) allows for discovery of novel transcripts in a more rapid and comprehensive method than other current technologies available (Mardis, 2008) at comparatively low costs (Metzker, 2010). Discovery of novel genes using NGS does not require a priori knowledge of genes that may be present, thus mitigating ascertainment bias. Thus, a need exists for NGS application (Basu et al., 2012) and discovery of novel transcripts to further gene expression studies in all TSE‐impacted species. At the initiation of this research, transcriptome‐level gene expression evaluation in free‐ranging deer using ribonucleic acid (RNA)‐sequencing technology had not previously been conducted.
Regardless of protein polymorphisms, liver tissues from CWD‐positive and clinically affected deer are CWD immunohistochemistry (IHC)‐negative, while retropharyngeal lymph nodes are CWD IHC‐positive (Otero et al., 2019). Therefore, we sought to identify differentially expressed genes in liver, which produces proteins involved in the innate immune response (Gao, Jeong, & Tian, 2008), and retropharyngeal lymph nodes, which are sites of prion accumulation (Williams, 2005), from CWD IHC‐positive and CWD IHC‐not detected (hereafter CWD‐positive and CWD‐ND, respectively), free‐ranging deer. Our study may contribute to an increased understanding of molecular mechanisms involved in the pathology and replication of CWD in cervid species. To our knowledge, this is the first study evaluating gene expression in CWD using NGS to identify novel transcripts.
2. MATERIALS AND METHODS
2.1. Tissue extraction
From January to March 2015, liver, obex, and retropharyngeal lymph node samples were collected from 380 free‐ranging adult (>1.5 years old) deer (Severinghaus, 1949) euthanized by Illinois Department of Natural Resources wildlife managers during annual population reduction and disease management efforts throughout the CWD‐endemic area of northcentral Illinois (Manjerovic, Green, Mateus‐Pinilla, & Novakofski, 2014; Mateus‐Pinilla, Weng, Ruiz, Shelton, & Novakofski, 2013). Following euthanasia, deer were transported to central processing locations within 6 hr of death, at which time tissues were rinsed using double‐distilled water (ddH2O) and any blood removed prior to collection. At the time of necropsy, liver and retropharyngeal lymph node biopsy tissue samples were extracted using 6‐mm Miltex surgical biopsy punches (Ref. Num. 33–36). For each animal, liver tissue samples were collected at the approximate center of the right anterior section of the right lobe. We randomly selected the right or left retropharyngeal lymph node for sampling, and collected tissue from the approximate center of the node. Biopsy punches were placed into 1.5‐ml centrifuge tubes and stored in 1.5 ml RNAlater (Thermo Fisher Scientific, Cat. No. AM7020) per the manufacturer's recommendations (Qiagen, Inc.). Tissue samples were refrigerated for 24 hr at 2°C after which they were placed in a freezer (−10°C) on site. Each week, we transported biopsy samples on dry ice to Western Illinois University, at which time they were stored at −20°C until transported to the Core Genomics Laboratory at University of Illinois Chicago for sequencing.
Wildlife managers from the Illinois Department of Natural Resources submitted CWD diagnostic samples (i.e., retropharyngeal lymph nodes, obex) to the Animal Disease Laboratory in Galesburg, Illinois, USA, for disease testing via immunohistochemistry (IHC). We used test results to select CWD‐positive and CWD‐ND deer from our biopsy sample collection. We conducted RNA integrity analysis to determine suitable samples for sequencing. We paired CWD‐positive suitable samples with randomly selected CWD‐ND individuals of similar locations and age classes (i.e., adults). None of 380 deer sampled showed clinical signs; thus, we assumed that if they were CWD‐positive, they were in similar stages of disease progression (Williams, 2005). The health status of sampled free‐ranging deer was unknown as they had not been tested for other diseases (e.g., epizootic hemorrhagic disease, tuberculosis). From the 380 deer sampled, we selected 10 adult deer for this study. Inclusion criteria were based on integrity of RNA samples, age, sex, and having CWD test results from both obex and retropharyngeal lymph nodes. We used tissue biopsy samples from 10 (5 CWD‐positive [2 males and 3 females; treatment group] and 5 CWD‐ND [3 males and 2 females; control group]) adult free‐ranging deer for RNA‐Seq analyses. All five CWD‐positive deer were IHC‐positive in obex and retropharyngeal lymph nodes.
2.2. RNA extraction and sequencing
Using a Qiagen RNeasy Mini Kit (Cat. No. 74104), we extracted RNA from each sample according to the manufacturer's instructions. We examined RNA integrity and quantity using a NanoDrop 1000 and a 2200 TapeStation system using RNA ScreenTape (Agilent, Cat. No. 5067‐5576). A total of 5 μg RNA with an RNA integrity number (RIN) > 7 was used for RNA‐Seq library construction. Additionally, a complementary deoxyribonucleic acid (cDNA) library was prepared with the TruSeq Stranded mRNA LT Sample Prep Kit—Set A (Illumina, Cat. No. RS‐122‐2101) and amplified using polymerase chain reaction (PCR), specifically the Illumina HiSeq 2500 Sequencing System with a HiSeq SBS sequencing kit (Illumina Inc.). Resulting paired‐end reads were 100 base pairs in length and sequenced on one lane from each end for 101 cycles. We generated and demultiplexed FASTQ data files with the bcl2fastq v1.8.4 Illumina Conversion Software (Illumina Inc.). We used RNA‐Seq to analyze liver and retropharyngeal lymph node tissue samples from CWD‐positive and CWD‐ND deer. Low integrity RNA samples (i.e., RIN < 7.0) were excluded from RNA‐Seq analyses.
2.3. De novo assembly
Raw sequencing reads were trimmed by Trimmomatic software (Bolger, Lohse, & Usadel, 2014) to remove low‐quality sequencing reads before assembly. Reads with an average quality score below 15 in a 4 base pair sliding window, and reads with quality below 5 at the beginning and end were filtered. After trimming, cleaned reads were used for the reference transcriptome assembly based on Trinity version 2.06 with paired‐end mode (Grabherr et al., 2011). Transcripts from liver and retropharyngeal lymph node tissues were separately assembled de novo. To obtain a comprehensive reference transcriptome, the two assemblies were merged and redundant transcripts were filtered by CD‐HIT software with default parameters (Li & Godzik, 2006). To filter out misassembled transcripts and transcripts with low expression, raw sequenced reads were mapped to assembled reference transcriptomes using Bowtie 1.0.0 (Langmead, Trapnell, Pop, & Salzberg, 2009). Then, transcript abundance, fragments per kilobase per transcript per million mapped reads (FPKM), values were calculated using RNA‐Seq by expectation maximization (RSEM) software (Li & Dewey, 2011), and transcripts with FPKM < 1 were filtered out (Li & Godzik, 2006). Filtered transcripts were used as the deer reference transcriptome for downstream analysis (Grabherr et al., 2011).
2.4. Transcriptome annotation
Assembled transcriptomes were annotated using BLASTX against NCBI‐NR and UniProt protein databases, with a cutoff E‐value of <1e−6. We imported BLASTX results into BLAST2GO software (Conesa et al., 2005), and Gene Ontology (GO) terms, Enzyme Commission numbers, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were annotated by BLAST2GO software. Protein‐coding DNA sequence region was predicted using TransDecoder implemented in Trinity software (Haas et al., 2013). Sequences with a corresponding protein length greater than 100 were retained for further analysis.
2.5. Differential gene expression analysis
Clean reads generated from liver and retropharyngeal lymph node tissues from CWD‐positive and CWD‐ND groups were mapped back to our assembled reference transcriptome separately, and fragments per kilobase of exon model per million fragments mapped values were calculated by RSEM software for each individual deer (Li & Dewey, 2011). The resulting data matrix that contained FPKM expression values for liver and retropharyngeal lymph node tissues of each individual was generated by “rsem‐generate‐data‐matrix” script. This data matrix with FPKM values was imported into edgeR 2.14 (Robinson, McCarthy, & Smyth, 2010) to create a pairwise comparison between CWD‐positive and CWD‐ND deer, and identify differentially expressed genes (DEGs) with fold change > 22 (log fold change = log 2 [CWD‐positive FPKM/CWD‐ND FPKM]) and a p‐value < .001 for false discovery rate. We presented expressed genes with a false discovery rate ≤ 0.001. Differential expression analyses were conducted between liver samples from CWD‐positive versus CWD‐ND deer, and between retropharyngeal lymph node samples from CWD‐positive versus CWD‐ND deer. We defined an up‐regulated gene as a gene that was differentially expressed in CWD‐positive deer as compared to a CWD‐ND deer, and a down‐regulated gene as a gene that was differentially expressed in CWD‐ND deer as compared to a CWD‐positive deer based on log fold change and false discovery rate results. Genes were analyzed using Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes. Gene Ontology enrichment analysis of the DEGs detected was conducted by DAVID function annotation tool with Fisher's exact test p‐value ≤ .05 (Huang, Sherman, & Lempicki, 2008), to classify DEGs that were molecularly validated based on cellular components, biological processes, and molecular functions.
2.6. Gene validation
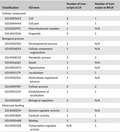
Real‐time quantitative PCR (qRT‐PCR) was conducted to validate the DEGs identified by RNA‐Seq. Two assays were designed for each region using PrimerQuest Tool from Integrated DNA Technologies, Inc. A total of 40 assays were used, with one assay repeated twice. Additionally, Flex Six BioMark chip (Fluidigm, Inc.) and Eva Green RT‐PCR (Bio‐Rad Laboratories, Inc.) assays were used. Samples were treated with DNase I (Zymo DNase I set, E1010) followed by column purification (i.e., Qiagen RNeasy Micro, Qiagen Cat. ID 74004). Samples were analyzed using a 2200 TapeStation (Thermo Scientific, Cat. No. 4368814) to verify removal of gDNA. Conversion of RNA to cDNA was accomplished using 1 μg of total RNA per reaction and a High Capacity cDNA Reverse Transcription Kit from Applied Biosystems. Complementary DNA was target‐specific preamplified according to a gene expression preamp protocol (Fluidigm, Inc.). We used 12 amplification cycles in the thermal cycling step. Final products were diluted fivefold, and each sample was analyzed in three technical replicates and five biological replicates (i.e., 5 CWD‐positive and 5 CWD‐ND). BioMark reactions were set up as per Fluidigm's quick reference protocol (Fluidigm, Inc.). We performed qRT‐PCR cycling and signal acquisition on the BioMark System and analyzed data using Fluidigm qRT‐PCR analysis software (Spurgeon, Jones, & Ramakrishnan, 2008). We further classified DEGs as passing validation when all above parameters were met and amplification plots showed a clear exponential phase and saturation plateau and no residual primer dimers. We classified DEGs as failing validation when the previously mentioned parameters were not met, which led to no or aberrant amplification plots and melt curves. We classified DEGs as interpret with caution for a variety of reasons (e.g., remaining primer dimers, differing temperature peaks, or failed primers; Table 3) per the protocol of the Core Genomics Lab at the University of Chicago to aid in downstream biological interpretation and prioritization.
Table 3.
Top 40 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in the white‐tailed deer de novo assembly transcriptome
| Pathway | Sequences in pathway | Number of enzymes |
|---|---|---|
| Purine metabolism | 843 | 55 |
| Biosynthesis of antibiotics | 841 | 126 |
| Pyrimidine metabolism | 343 | 33 |
| Glycerophospholipid metabolism | 255 | 31 |
| Phosphatidylinositol signaling system | 250 | 22 |
| Lysine degradation | 244 | 19 |
| Aminoacyl‐tRNA biosynthesis | 230 | 24 |
| Glycolysis/gluconeogenesis | 228 | 26 |
| Glutathione metabolism | 199 | 19 |
| Drug metabolism—cytochrome P450 | 194 | 7 |
| Fatty acid degradation | 186 | 15 |
| Tryptophan metabolism | 183 | 21 |
| Glycerolipid metabolism | 180 | 16 |
| Metabolism of xenobiotics by cytochrome P450 | 178 | 8 |
| Oxidative phosphorylation | 178 | 7 |
| Pyruvate metabolism | 174 | 20 |
| Amino sugar and nucleotide sugar metabolism | 174 | 35 |
| Glycine, serine, and threonine metabolism | 170 | 31 |
| Carbon fixation pathways in prokaryotes | 163 | 20 |
| Inositol phosphate metabolism | 162 | 21 |
| T‐cell receptor signaling pathway | 162 | 2 |
| Thiamine metabolism | 161 | 4 |
| Valine, leucine, and isoleucine degradation | 159 | 24 |
| Nicotinate and nicotinamide metabolism | 157 | 16 |
| Citrate cycle (TCA cycle) | 156 | 17 |
| Arachidonic acid metabolism | 142 | 17 |
| Sphingolipid metabolism | 142 | 20 |
| Cysteine and methionine metabolism | 139 | 25 |
| Pentose phosphate pathway | 125 | 17 |
| Drug metabolism—other enzymes | 122 | 17 |
| Methane metabolism | 120 | 15 |
| Steroid hormone biosynthesis | 117 | 16 |
| Butanoate metabolism | 111 | 15 |
| Arginine and proline metabolism | 108 | 23 |
| Retinol metabolism | 105 | 10 |
| Alanine, aspartate, and glutamate metabolism | 103 | 24 |
| Starch and sucrose metabolism | 102 | 18 |
| Propanoate metabolism | 101 | 16 |
| Biosynthesis of unsaturated fatty acids | 100 | 8 |
3. RESULTS
We generated 488,145,350 (243,310,654 from liver tissue and 244,834,696 from retropharyngeal lymph node tissue) clean pair‐end reads through RNA‐sequencing. Mean assembled transcripts ranged in size from 400 base pairs to >5,000 base pairs. After removal of transcripts with low expression and redundancy, we retained 74,479 transcripts as a reference transcriptome (number of N50 transcripts = 14,877, N50 length = 3,204 base pairs, mean length = 2,108 base pairs). In addition, 51,647 transcripts were assigned genes in NCBI‐NR and 47,292 transcripts were assigned genes in UniProt; 51,661 (69.36%) were assigned to a known gene. Ovis aries, Bos taurus, Bubalus bubalis, Bos mutus, and Capra hircus (Table 1) were the top species associated with BLAST hits against the NCBI‐NR database. These species accounted for 49.08% (20,980) of the BLAST hits, whereas the “others” category accounted for 8,696 BLAST hits. The “others” category accounted for all other organisms (>290 species) annotated beyond the top 29 species (Table 1). There were 7,899 genes shared between liver and retropharyngeal lymph node tissues.
Table 1.
Species distribution of top BLAST results of de novo‐assembled white‐tailed deer transcripts against NCBI‐NR database
| Species | BLAST top‐hits |
|---|---|
| Ovis aries | 8,904 |
| Othersa | 8,696 |
| Bos taurus | 7,361 |
| Bubalus bubalis | 5,837 |
| Bos mutus | 4,831 |
| Capra hircus | 2,951 |
| Pantholops hodgsonii | 2,771 |
| Bison bison | 2,535 |
| Homo sapiens | 1,150 |
| Sus scrofa | 582 |
| Mus musculus | 513 |
| Balaenoptera acutorostrata | 508 |
| Camelus ferus | 442 |
| Physeter catodon | 398 |
| Orcinus orca | 349 |
| Myotis brandtii | 291 |
| Equus przewalskii | 278 |
| Equus caballus | 277 |
| Pteropus alecto | 276 |
| Lipotes vexillifer | 275 |
| Tursiops truncatus | 272 |
| Cricetulus griseus | 271 |
| Synthetic construct | 252 |
| Tupaia chinensis | 251 |
| Rattus norvegicus | 250 |
| Ursus maritimus | 239 |
| Ailuropoda melanoleuca | 237 |
| Chlorocebus sabaeus | 222 |
| Canis lupus | 221 |
| Macaca mulatta | 207 |
The “others” category accounts for all other organisms (>290) annotated beyond the top 29 species summarized in the table.
We assigned 40,308 transcripts at least one GO term, and 37,853 transcripts were assigned to at least one pathway; we limited reporting of GO terms to those with ≥1,000 assigned transcripts. Gene Ontology analysis identified transcripts successfully mapped to 16 GO biological processes (GO level 2; Table 2). Most (59.0%) transcripts were related to cellular process, metabolic process, single‐organism process, or biological regulation. Additionally, transcripts were mapped to six cellular components and seven molecular functions (GO level 2). Most (60.8%) transcripts assigned to a cellular process were related to cells and organelles. Similarly, most (80.9%) transcripts mapped to molecular functions were related to binding and catalytic activity. Top Kyoto Encyclopedia of Genes and Genomes pathways include purine metabolism, biosynthesis of antibiotics, pyrimidine metabolism, glycerophospholipid metabolism, and phosphatidylinositol signaling system (Table 3).
Table 2.
Number of Gene Ontology (GO) analysis identified white‐tailed deer transcripts with a cutoff E‐value of <1e−6 and corresponding protein length greater than 100, successfully mapped to cellular component, biological process, and molecular function classifications
| GO number | Classification | No. of transcriptsa |
|---|---|---|
| Cellular component | ||
| GO:0005623 | Cell | 27,177 |
| GO:0043226 | Organelle | 21,631 |
| GO:0016020 | Membrane | 11,980 |
| GO:0032991 | Macromolecular complex | 9,618 |
| GO:0031974 | Membrane‐enclosed lumen | 5,757 |
| GO:0005576 | Extracellular region | 2,021 |
| Biological process | ||
| GO:0009987 | Cellular process | 27,280 |
| GO:0008152 | Metabolic process | 24,872 |
| GO:0044699 | Single‐organism process | 22,464 |
| GO:0065007 | Biological regulation | 16,474 |
| GO:0050896 | Response to stimulus | 11,215 |
| GO:0071840 | Cellular component organization or biogenesis | 8,501 |
| GO:0051179 | Localization | 8,496 |
| GO:0023052 | Signaling | 7,363 |
| GO:0032501 | Multicellular organismal process | 7,242 |
| GO:0032502 | Developmental process | 6,538 |
| GO:0002376 | Immune system process | 3,473 |
| GO:0051704 | Multi‐organism process | 2,741 |
| GO:0040011 | Locomotion | 1,799 |
| GO:0022610 | Biological adhesion | 1,762 |
| GO:0022414 | Reproductive process | 1,165 |
| GO:0040007 | Growth | 1,157 |
| Molecular function | ||
| GO:0005488 | Binding | 24,330 |
| GO:0003824 | Catalytic activity | 16,073 |
| GO:0098772 | Molecular function regulator | 1,982 |
| GO:0005215 | Transporter activity | 1,914 |
| GO:0060089 | Molecular transducer activity | 1,804 |
| GO:0001071 | Nucleic acid binding transcription factor activity | 1,391 |
| GO:0005198 | Structural molecule activity | 1,138 |
Reporting of GO terms was limited to those with ≥1,000 assigned transcripts.
We identified 59 genes as differentially expressed in CWD‐positive (as compared to CWD‐ND) deer liver and retropharyngeal tissues (Table 4). Among these, 36 were found in liver tissue (16 up‐regulated, 20 down‐regulated) and 23 (12 up‐regulated, 11 down‐regulated) in retropharyngeal lymph node tissue; 29 genes have a known function when compared to UniProt and NCBI databases. Of 59 genes, 33 passed validation, 14 failed, and 12 should be interpreted with caution (Table 4). Function of genes that passed validation includes sodium channel proteins, endogenous retrovirus proteins, and cell death activators. Differentially expressed genes associated with liver and retropharyngeal lymph node tissues included top functions assigned by Gene Ontology associated with cellular membranes, binding, apoptosis, metabolic processes, cellular processes, and catalytic activity (Table 5). Furthermore, we identified several DEGs (i.e., ERVK13‐1, ERVK‐24) which were up‐regulated in the disease state and assigned a Gene Ontology cellular component of plasma membrane.
Table 4.
Differentially expressed genes in chronic wasting disease‐positive liver (LV) and retropharyngeal lymph node (RPLN) tissues from white‐tailed deer collected in the chronic wasting disease‐endemic area of northern Illinois during annual population reduction, winter 2015
| Differentially expressed gene identificationa | logFCb | FDR | CWD‐ positive FPKMc | CWD‐ ND FPKMd | Annotation |
|---|---|---|---|---|---|
| Up‐regulated in CWD‐positive LV | |||||
| L_TR43469|c2_g3_i5e | 3.39 | 6.12E−06 | 6.05 | 0.57 | Endogenous retrovirus group k member 25 env poly; ERVK13‐1 |
| L_TR63450|c1_g1_i1f | 5.28 | 1.50E−05 | 6.29 | 0.16 | Uncharacterized protein loc105607204 isoform x1 |
| L_TR45335|c0_g1_i1g | 2.97 | 2.34E−05 | 3.9 | 0.49 | Unknown |
| L_TR29095|c7_g3_i1g | 3.24 | 2.67E−05 | 4.24 | 0.44 | Sodium channel protein type 11 subunit partial |
| L_TR56520|c6_g2_i1g | 4.64 | 0.001065 | 2.15 | 0.08 | Unknown |
| L_TR56520|c6_g3_i2g | 5.79 | 0.002325 | 1.15 | 0.02 | Syntaxin‐binding protein 5; STXBP5 |
| L_TR41343|c0_g1_i2g | 4.28 | 0.004595 | 3.61 | 0.18 | Uncharacterized protein loc102402433 isoform x1 |
| L_TR49285|c0_g1_i1e | 5.16 | 0.00801 | 2.73 | 0.07 | Unknown |
| L_TR77350|c0_g1_i1e | 3.98 | 0.00963 | 4.46 | 0.27 | Gag protein, ERVK‐24 |
| L_TR47646|c1_g1_i1f | 2.32 | 0.028849 | 4.67 | 0.92 | Unknown |
| L_TR56520|c6_g1_i1g | 4.24 | 0.030398 | 1.14 | 0.06 | Syntaxin‐binding protein 5 |
| L_TR53215|c2_g3_i1e | 7.08 | 0.038974 | 1.71 | 0 | Dual metabolic roles of gluconeogenesis and glyoxylate detoxification; AGXT |
| L_TR41343|c0_g1_i3f | 3.77 | 0.040655 | 2.86 | 0.2 | Unknown |
| L_TR79592|c7_g2_i1g | 3.36 | 0.04327 | 1.8 | 0.17 | Interleukin‐17 receptor a isoform x2 |
| L_TR27390|c2_g1_i1g | 11.61 | 0.046946 | 3.22 | 0 | Tumor necrosis factor antagonist; ADIPOQ |
| L_TR41343|c0_g1_i1f | 5.01 | 0.046946 | 4.06 | 0.12 | Unknown |
| Down‐regulated in CWD‐positive LV | |||||
| L_TR47259|c0_g1_i1f | −9.76 | 1.50E−05 | 0.03 | 34.22 | Uncharacterized protein loc105607204 isoform x1 |
| L_TR10266|c1_g2_i3e | −3.83 | 1.50E−05 | 1.52 | 21.59 | Acyl‐binding protein; DBI |
| L_TR8752|c0_g3_i2g | −2.05 | 0.00088 | 28.5 | 117.84 | Zinc ion binding; Rsp29 |
| L_TR28955|c0_g1_i1g | −8.13 | 0.001462 | 0.02 | 10.09 | Unknown |
| L_TR30917|c0_g1_i1 | −3.87 | 0.002742 | 0.07 | 1.09 | Upf0545 protein c22orf39 homolog isoform x1 |
| L_TR28955|c0_g2_i1g | −6.93 | 0.00801 | 0.05 | 8.13 | Ubiquinone biosynthesis protein coq4 mitochondrial isoform x5 |
| L_TR29354|c0_g1_i1g | −3.05 | 0.00801 | 0.51 | 4.21 | Positive regulation of tumor necrosis factor; CCL3 |
| L_TR5337|c0_g1_i1e | −4.73 | 0.00801 | 0.12 | 3.34 | Unknown |
| L_TR60746|c4_g1_i1g | −4.48 | 0.00801 | 0.05 | 1.3 | Uncharacterized protein partial |
| L_TR74636|c14_g8_i1e | −2.02 | 0.00801 | 11.5 | 46.47 | Craniofacial development protein 2; TMCO5B |
| L_TR26826|c4_g1_i1g | −2.95 | 0.009568 | 0.38 | 3.06 | Endonuclease–reverse transcriptase |
| L_TR22918|c2_g1_i1e | −3.99 | 0.015372 | 1.48 | 23.38 | Myomegalin isoform x14 |
| L_TR43779|c0_g1_i2f | −7.74 | 0.015372 | 0 | 3.7 | Unknown |
| L_TR62456|c0_g1_i1g | −2.65 | 0.015945 | 1.74 | 13.38 | Unknown |
| L_TR69615|c0_g1_i1g | −3.65 | 0.019822 | 2.1 | 25.99 | Unknown |
| L_TR3476|c0_g1_i1g | −2.61 | 0.023558 | 1.05 | 6.36 | Unknown |
| L_TR46171|c0_g2_i2g | −2.84 | 0.023558 | 0.51 | 3.59 | Unknown |
| L_TR15492|c1_g1_i1f | −4.05 | 0.027461 | 0.13 | 2.14 | Endonuclease–reverse transcriptase, POL; P11369 |
| L_TR26463|c1_g1_i1g | −4 | 0.028849 | 0.93 | 15.15 | Unknown |
| L_TR23471|c1_g1_i3g | −2.45 | 0.028849 | 0.42 | 2.31 | Cell death activator cide‐3 isoform x2; CIDEC |
| Up‐regulated in CWD‐positive RPLN | |||||
| N_TR92656|c3_g7_i1f | 3.15 | 9.60E−05 | 3.82 | 0.4 | Unknown |
| N_TR181264|c0_g1_i1g | 5.42 | 0.000109 | 4.8 | 0.1 | Low‐quality protein: saoe class i histocompatibility a alpha chain‐like |
| N_TR87145|c0_g1_i4g | 7.75 | 0.000242 | 2.38 | 0 | Unknown |
| N_TR101034|c0_g4_i11f | 2.03 | 0.002067 | 4.01 | 0.99 | Unknown |
| N_TR113697|c0_g2_i1g | 2.5 | 0.00294 | 3.31 | 0.59 | Low‐quality protein: endogenous retrovirus group k member 25, POL; ERVK‐25 |
| N_TR160975|c0_g1_i1g | 2.02 | 0.003354 | 5.01 | 1.24 | Collagen VI acts as a cell‐binding protein; COL6A5 |
| N_TR122168|c0_g4_i1e | 2.25 | 0.012216 | 5.26 | 1.08 | Acts as coactivator in regulation of translation initiation of poly(A)‐mRNA; PAIP1 |
| N_TR108513|c1_g2_i1g | 2.69 | 0.016226 | 3.93 | 0.58 | Unknown |
| N_TR100013|c5_g1_i1g | 2.31 | 0.018102 | 2.91 | 0.58 | Unknown |
| N_TR113697|c0_g5_i1g | 2.45 | 0.022443 | 5 | 0.91 | Uncharacterized protein loc104969954 isoform x1 |
| N_TR158607|c0_g2_i2g | 3.42 | 0.02443 | 2.2 | 0.19 | Unknown |
| N_TR164441|c0_g1_i1g | 2.46 | 0.035534 | 6.64 | 1.19 | Unknown |
| Down‐regulated in CWD‐positive RPLN | |||||
| N_TR151520|c0_g2_i1f | −2.13 | 4.98E−06 | 6.29 | 27.09 | Nascent polypeptide‐associated complex subunit alpha; NACA |
| N_TR149140|c6_g1_i2f | −8.29 | 1.23E−05 | 0 | 3.72 | Unknown |
| N_TR81328|c8_g2_i1g | −8.58 | 0.000142 | 0 | 4.69 | Unknown |
| N_TR81328|c8_g5_i1f | −7.3 | 0.000255 | 0 | 2.33 | Unknown |
| N_TR81328|c8_g1_i1e | −6.65 | 0.00079 | 0 | 1.69 | Unknown |
| N_TR84619|c1_g1_i3e | −2.03 | 0.002067 | 1.38 | 5.52 | Uridine‐cytidine kinase 1, UCK1 |
| N_TR93637|c1_g1_i1g | −2.32 | 0.002599 | 0.59 | 2.99 | Unknown |
| N_TR41415|c0_g1_i1f | −2.51 | 0.00294 | 0.26 | 1.48 | Unknown |
| N_TR43226|c0_g1_i1f | −3.67 | 0.005155 | 0.4 | 5.16 | Unknown |
| N_TR164448|c1_g2_i2e | −4.39 | 0.007536 | 0.14 | 2.94 | Unknown |
| N_TR97881|c0_g1_i1g | −5.73 | 0.026083 | 0.16 | 8.02 | May be involved in signal transduction as component of a multimeric receptor complex; Ms4a |
We defined an up‐regulated gene as a gene that was differentially expressed in CWD‐positive white‐tailed deer as compared to a CWD‐ND (i.e., not detected) white‐tailed deer, and a down‐regulated gene as a gene that was differentially expressed in CWD‐ND white‐tailed deer as compared to a CWD‐positive white‐tailed deer based on log fold change (FC) and false discovery rate (FDR) results.
DEGs are those with fold change (log FC) > 22 and a p‐value < .001 for false discovery rate (FDR).
FC = log 2 (CWD‐positive FPKM/CWD‐ND FPKM).
CWD‐positive FPKM = fragments per kilobase of exon model per million fragments mapped expression values for liver and retropharyngeal lymph node tissues for CWD‐positive white‐tailed deer.
CWD‐ND FPKM = fragments per kilobase of exon model per million fragments mapped expression values for liver and retropharyngeal lymph node tissues for white‐tailed deer in which CWD was not detected.
Genes that should be interpreted with caution.
Genes that failed validation.
Genes that passed validation.
Table 5.
Gene ontology (GO) classifications for differentially expressed genes (DEGs) in liver (LV) and retropharyngeal lymph node (RPLN) tissue of chronic wasting disease‐positive white‐tailed deer collected in the chronic wasting disease‐endemic area of northern Illinois during annual population reduction, winter 2015
| Classification | GO term | Number of transcripts in LV | Number of transcripts in RPLN |
|---|---|---|---|
| Cellular component | |||
| GO:0005623 | Cell | 3 | 1 |
| GO:0044464 | Cell part | 3 | 1 |
| GO:0032991 | Macromolecular complex | 1 | N/A |
| GO:0043226 | Organelle | 3 | 1 |
| Biological process | |||
| GO:0032502 | Developmental process | 2 | N/A |
| GO:0016043 | Cellular component organization | 1 | N/A |
| GO:0008152 | Metabolic process | 3 | 2 |
| GO:0016265 | Death | 1 | N/A |
| GO:0043473 | Pigmentation | 2 | N/A |
| GO:0051179 | Localization | 1 | 1 |
| GO:0032501 | Multicellular organismal process | 1 | N/A |
| GO:0009987 | Cellular process | 3 | 2 |
| GO:0051234 | Establishment of localization | 1 | 1 |
| GO:0065007 | Biological regulation | 2 | N/A |
| Molecular binding | |||
| GO:0030234 | Enzyme regulator activity | 1 | N/A |
| GO:0003824 | Catalytic activity | 1 | 1 |
| GO:0005488 | Binding | 3 | 2 |
| GO:0030528 | Transcription regulator activity | N/A | 1 |
4. DISCUSSION
4.1. PrP misfolding on plasma membranes potentially linked to CWD
Differentially expressed genes assigned to Gene Ontology cellular component plasma membrane may suggest a change occurring in the plasma membrane of CWD animals, in agreement with previous research by Ersdal, Goodsir, Simmons, McGovern, and Jeffrey (2009). Naturally occurring PrPC is attached to the outer surface of the plasma membrane (Peters et al., 2003) and has been shown to be expressed during infection (Linden et al., 2008). Naturally occurring PrP has multiple binding partners involved in cytoskeletal processes (e.g., maintenance, cell growth; Zafar et al., 2011), and its function has been linked to copper homeostasis, oxidative stress, cell survival differentiation, cell signaling, and cell proliferation. Additionally, it has been associated with synaptic function, maintenance, or structure and a regulatory role at central and peripheral synapses (Westergard, Christensen, & Harris, 2007). Functions of PrP and DEG syntaxin‐binding protein 5 (STXBP5) are overlapped. Like PrP, STXBP5 may regulate the ability of presynaptic vesicles to fuse and dock with presynaptic membranes (Bennett, Calakos, & Scheller, 1992) by inhibiting formation of SNARE complexes (i.e., SNAp REceptor; complexes that involve syntaxin, SNAP‐25, and synaptobrevin), which play a role in the release of neurotransmitters (Asuni, Cunningham, Vigneswaran, Perry, & O'Connor, 2008). Furthermore, an interaction critical to the successful conversion of PrP to PrPSc may take place on the plasma membrane (Caughey, Raymond, Ernst, & Race, 1991). Blocking the specific site of conversion may potentially prevent the misfolding of PrP into PrPSc.
4.2. Potential link between CWD and retroviruses
All vertebrate genomes studied include integrated RNA viral sequences known as endogenous retroviruses (ERVs), which were previously considered “junk DNA” (Lee, Jeong, Choi, & Kim, 2013). Several ERV genes, including ERVK13‐1 and ERVK‐24, ERVK‐25, and P11369 (Table 3), were identified in this study, suggesting a potential link between CWD and retroviruses. In addition, at least two DEGs are associated with ERV Gag, the protein responsible for synthesis of structural proteins necessary for the viral core. Proteins Pol and Env, which encode for reverse transcriptase, and proteins of the viral envelope, respectively, also were differentially expressed in CWD‐positive deer (Coffin, Hughes, & Varmus, 1997). Combined with Pro, a virion protease, Gag, Env, and Pol create the backbone of replicating retroviruses (Petropoulos, 1997). Although Pro is not specifically listed in our DEGs, one of the unknown genes may be a form of the protein. Endogenous retroviruses may be activated in prion diseases (Lee et al., 2013), and previous studies suggest retroviruses can serve as cofactors involved in prion diseases (Leblanc et al., 2006) potentially altering endocytic pathways (Ashok & Hegde, 2006). Leblanc et al. (2006) suggested retroviruses may increase prion infectivity by acting as transport vectors in the spread of infective prions throughout an individual. Retroviral Gag was suggested to enhance the release of prion proteins in cellular culture when expressed (Leblanc et al., 2006) as was further demonstrated by Bian et al. (2010) using CWD prions. Additionally, PrP has been suggested to influence retroviral activity as it may act as an antiretroviral, specifically in the spleen after immune stimulation (Lötscher et al., 2007). Prions and retroviral cells may be localized in the same cellular compartments, thus acting as cofactors in infection (Leblanc et al., 2006).
4.3. Association between CWD and immune‐related genes
Several differentially expressed genes identified in our study (i.e., ADIPOQ, CCL3; Table 3) are related to tumor necrosis factor (TNF), a cytokine that produces an immune response to help prevent the spread of infection. It induces fever and apoptotic cell death, and inhibits viral replication. Chronic exposure to TNF can lead to shock‐like symptoms including a wasting syndrome (Chu, 2013). It also is important to maintaining follicular dendritic cell networks (Sallusto & Lanzavecchia, 1994). Kitamoto, Muramoto, Mohri, Doh‐Ura, and Tateishi (1991) suggested follicular dendritic cells were important to the replication of prions in lymphoid tissues as early PrpSc accumulates on these cells. Specifically, ADIPOQ, a TNF antagonist (Masaki et al., 2004), is up‐regulated in liver tissues of positive deer. A monokine, CCL3, is down‐regulated in liver tissues of positive deer and is responsible for positive regulation of TNF production (Ramos et al., 2005). Such regulation of TNF suggests CWD‐positive deer may have down‐regulated TNF production at the time of sampling. However, IHC results were positive in retropharyngeal lymph node and obex, suggesting that CWD‐positive deer were not in the early stages of infection. The presence of TNF genes associated with liver tissue and their absence within retropharyngeal lymph node tissue are to be expected, as the liver is a part of the innate immune system (Gao et al., 2008).
In this study, IHC determined whether a deer was CWD‐positive or CWD‐ND; a CWD‐positive deer was assumed to be far enough (i.e., not recently infected) in disease progression to exhibit an accumulation of prions in the RPLN tissue and obex. Although speculative, it is possible that prior to the IHC detectable stage of CWD infection, an initial increase in TNF production occurs in response to initial infection, which overtime becomes detrimental to deer whose response is to down‐regulate TNF as identified in this study. There was no recorded evidence of declining physical condition in CWD‐positive deer, which may be associated with long incubation periods (2–4 years) and absence of clinical symptoms during early stages of prion infection (Williams, 2005). Nevertheless, during later stages of infection chronic exposure to low concentrations of TNF (Wajant, Pfizenmaier, & Scheurich, 2003) may contribute to the wasting syndrome, depression, and cachexia associated with CWD (Chu, 2013). Additionally, an up‐regulated DEG in liver tissue of positive deer associated with interleukin‐17 (IL‐17; Table 3), is responsible for communication between cells, specifically as an inflammatory response in positive individuals (Huang, Zhang, & He, 2015). Furthermore, other studies have shown interleukin genes to be implicated in prion disease pathogenesis and the innate immune system (Bradford & Mabbott, 2012). Moreover, Meling, Skovgaard, Bårdsen, Heegaard, and Ulvund (2018) reported transcriptional innate immune responses in liver tissues of TSE‐positive animals. Although our study only used 5 CWD‐positive and 5 CWD‐negative deer, further investigation of these genes across known stages of disease progression in a larger sample of infected individuals may lead to a better understanding of the immune response to CWD (or other TSEs) or the identification of additional genes.
4.4. Use of candidate genes for early detection
Logistics of collecting and preserving high‐quality tissue samples and transporting samples to laboratory settings for storage make field‐based RNA studies difficult. However, these types of studies are important in eliminating confounding factors (i.e., exposure to artificially high concentrations of prions, inheritance of partial CWD resistance‐conferring PrP polymorphism at greater frequency than in natural settings) induced by captive breeding and evaluating transmission in a natural setting. Animals in captive facilities are exposed to higher concentrations of CWD prions over less space than their free‐ranging counterparts. An increase in the number of CWD‐positive animals in a smaller area may lead to higher infection rates in captive individuals and exposure to higher infectious doses of prions than their free‐ranging conspecifics (Miller & Wild, 2004). A difference in prion concentration may affect gene expression and time of detection, thereby highlighting the importance of examining CWD gene expression in free‐ranging naturally infected individuals. Consequently, a CWD‐positive animal may not exhibit prion concentrations high enough for detection using traditional methods if tested too early in disease progression (Haley, Mathiason, Zabel, Telling, & Hoover, 2009). However, use of gene chips and in situ hybridization may enable researchers to select specific candidate genes as indicators of disease status (Lein, Zhao, & Gage, 2004). Gene expression analyses allow for the detection of genetic responses to stimuli before they are phenotypically visible (Klaper & Thomas, 2004), and use of DEGs identified in this study as candidate genes suggests the activation of endogenous process in CWD‐infected deer that may advance the pathological process.
4.5. Validation of unknown DEGs and potential functions
Genes that passed validation and were unannotated are candidates for further study. These genes could have implications for the transmission or replication of infectious prion proteins. Even genes that did not pass validation or that should be interpreted with caution may benefit from testing with additional primer sets. Any potential role of the DEGs discussed in this study should be examined in normal prion proteins. Normal PrP function is ambiguous, and DEGs identified in this study may further the understanding of PrP. While many normal prion protein functions have been described, underlying pathogenesis of TSEs is not well understood as amyloid deposits can be found in outwardly healthy individuals (Diack et al., 2016). It also remains unclear whether conversion of PrPC to PrPSc leads to a gain of function in PrPSc‐positive individuals (Collins, Lawson, & Masters, 2004) or a loss of function (Samaia & Brentani, 1998). Additionally, DEGs associated with retroviruses warrant further investigation as they may be involved in CWD endocytic cell pathways related to CWD. Future studies should build upon CWD‐associated DEGs identified in this study by examining DEGs in other tissues (i.e., brain stem, blood, rectoanal mucosa‐associated lymph tissue) used in routine disease surveillance (Williams, 2005). Further genetic analyses at a transcriptome level could lead to a greater understanding of naturally occurring prion protein functions and thus aid in the understanding of disease‐causing prion infection and formation mechanisms.
5. CONCLUSIONS
Chronic wasting disease has been widely studied; however, many of the underlying mechanisms that influence transmission and disease spread in infected deer are not well understood. Our research highlights several areas for further investigation. Similar to Ersdal et al. (2009), our research suggests a change occurs in the plasma membrane of CWD‐positive deer. Although not explicitly evaluated in this study, this could be due to coinfections with retrovirus or activation of endogenous retrovirus. Furthermore, as Gag, Env, and Pol proteins are differentially expressed, this suggests a link between endogenous retroviruses and CWD as previously presented by Leblanc et al. (2006). Additionally, further investigation of DEGs collected from a wider range of CWD tissues (i.e., obex, blood, tonsils, spleen) may provide greater insight into the mechanisms of disease progression. Investigation of DEGs we have presented may allow for the monitoring of specific genes and their expression, suggesting the activation of endogenous process in CWD‐infected deer.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
CNJ and PAS conceived and designed the research; EKT‐L and PAS collected data; EKT‐L, GL, and JW contributed reagents/materials and conducted analyses; EKT‐L, GL, JTL, JW, PRZ, NEM‐P, and CNJ wrote and substantially edited the manuscript.
ACKNOWLEDGMENTS
This research was supported by Federal Aid in Wildlife Restoration, Project W‐178‐R, administered through the Illinois Department of Natural Resources and the Department of Graduate Studies at Western Illinois University. Sample analysis was conducted at the Core Genomics Lab at the University of Illinois at Chicago. The Illinois Department of Natural Resources aided in collection of tissue samples. We thank A. Brandt, S. Dubay, E. Schauber, and three anonymous reviewers for providing helpful comments on earlier drafts of our manuscript. We thank Illinois hunters for their willingness to participate in the management of CWD by donating tissue samples during the surveillance efforts at hunter harvest, and the cooperating landowners who allowed targeted reduction of deer on their property in CWD‐infected areas.
Trone‐Launer EK, Wang J, Lu G, et al. Differential gene expression in chronic wasting disease‐positive white‐tailed deer (Odocoileus virginianus). Ecol Evol. 2019;9:12600–12612. 10.1002/ece3.5724
Contributor Information
Emma K. Trone‐Launer, Email: Emma.Trone@illinois.gov.
Christopher N. Jacques, Email: cn-jacques@wiu.edu.
DATA AVAILABILITY STATEMENT
RNA‐Seq data are available at the NCBI SRA SRP158695.
REFERENCES
- Ashok, A. , & Hegde, R. S. (2006). Prions and retroviruses: An endosomal rendezvous? EMBO Reports, 7(7), 685–687. 10.1038/sj.embor.7400749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni, A. A. , Cunningham, C. , Vigneswaran, P. , Perry, V. H. , & O'Connor, V. (2008). Unaltered SNARE complex formation in an in vivo model of prion disease. Brain Research, 1233, 1–7. 10.1016/j.brainres.2008.07.083 [DOI] [PubMed] [Google Scholar]
- Basu, U. , Almeida, L. M. , Dudas, S. , Graham, C. E. , Czub, S. , Moore, S. S. , & Guan, L. L. (2012). Gene expression alterations in Rocky Mountain elk infected with chronic wasting disease. Prion, 6(3), 282–301. 10.4161/pri.19915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M. K. , Calakos, N. , & Scheller, R. H. (1992). Syntaxin: A synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science, 257(5067), 255–259. 10.1126/science.1321498 [DOI] [PubMed] [Google Scholar]
- Bian, J. , Napier, D. , Khaychuck, V. , Angers, R. , Graham, C. , & Telling, G. (2010). Cell‐based quantification of chronic wasting disease prions. Journal of Virology, 84(16), 8322–8326. 10.1128/JVI.00633-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger, A. M. , Lohse, M. , & Usadel, B. (2014). Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30(15), 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, B. M. , & Mabbott, N. A. (2012). Prion disease and the innate immune system. Viruses, 4(12), 3389–3419. 10.3390/v4123389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, A. L. , Kelly, A. C. , Green, M. L. , Shelton, P. , Novakofski, J. , & Mateus‐Pinilla, N. E. (2015). Prion protein gene sequence and chronic wasting disease susceptibility in white‐tailed deer (Odocoileus virginianus). Prion, 9(6), 449–462. 10.1080/19336896.2015.1115179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, P. (2013). Transmissible spongiform encephalopathy: From its beginnings to Daniel Carlton Gajdusek In Zou W.‐Q., & Gambetti P. (Eds.), Prions and diseases (pp. 1–19). New York, NY: Springer. [Google Scholar]
- Caughey, B. , Race, R. E. , & Chesebro, B. (1988). Detection of prion protein mRNA in normal and scrapie‐infected tissues and cell lines. Journal of General Virology, 69(Pt 3), 711–716. 10.1099/0022-1317-69-3-711 [DOI] [PubMed] [Google Scholar]
- Caughey, B. , Raymond, G. J. , Ernst, D. , & Race, R. E. (1991). N‐terminal truncation of the scrapie‐associated form of PrP by lysosomal protease(s): Implications regarding the site of conversion of PrP to the protease‐resistant state. Journal of Virology, 65(12), 6597–6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y. C. , Hannaoui, S. , John, T. R. , Dudas, S. , Czub, S. , & Gilch, S. (2016). Early and non‐invasive detection of chronic wasting disease prions in elk feces by real‐time quaking induced conversion. PLoS ONE, 11(11), e0166187 10.1371/journal.pone.0166187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, W.‐M. (2013). Tumor necrosis factor. Cancer Letters, 328(2), 222–225. 10.1016/j.canlet.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin, J. M. , Hughes, S. H. , & Varmus, H. E. (1997). The place of retroviruses in biology. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK19382/ [Google Scholar]
- Collins, S. J. , Lawson, V. A. , & Masters, C. L. (2004). Transmissible spongiform encephalopathies. The Lancet, 363(9402), 51–61. [DOI] [PubMed] [Google Scholar]
- Conesa, A. , Götz, S. , García‐Gómez, J. M. , Terol, J. , Talón, M. , & Robles, M. (2005). Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics, 21(18), 3674–3676. 10.1093/bioinformatics/bti610 [DOI] [PubMed] [Google Scholar]
- Diack, A. B. , Alibhai, J. D. , Barron, R. , Bradford, B. , Piccardo, P. , & Manson, J. C. (2016). Insights into mechanisms of chronic neurodegeneration. International Journal of Molecular Sciences, 17(1), 82 10.3390/ijms17010082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersdal, C. , Goodsir, C. M. , Simmons, M. M. , McGovern, G. , & Jeffrey, M. (2009). Abnormal prion protein is associated with changes of plasma membranes and endocytosis in bovine spongiform encephalopathy (BSE)‐affected cattle brains. Neuropathology and Applied Neurobiology, 35(3), 259–271. 10.1111/j.1365-2990.2008.00988.x [DOI] [PubMed] [Google Scholar]
- Gao, B. , Jeong, W. , & Tian, Z. (2008). Liver: An organ with predominant innate immunity. Hepatology, 47(2), 729–736. 10.1002/hep.22034 [DOI] [PubMed] [Google Scholar]
- Gilch, S. , Chitoor, N. , Taguchi, Y. , Stuart, M. , Jewell, J. E. , & Schätzl, H. M. (2011). Chronic wasting disease In Tatzelt J. (Ed.), Prion proteins (pp. 51–77). Berlin, Heidelberg, Germany: Springer; Retrieved from http://link.springer.com/chapter/10.1007/128_2011_159 [DOI] [PubMed] [Google Scholar]
- Grabherr, M. G. , Haas, B. J. , Yassour, M. , Levin, J. Z. , Thompson, D. A. , Amit, I. , … Regev, A. (2011). Full‐length transcriptome assembly from RNA‐Seq data without a reference genome. Nature Biotechnology, 29(7), 644–652. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith, J. S. (1967). Nature of the scrapie agent: Self‐replication and scrapie. Nature, 215(5105), 1043–1044. 10.1038/2151043a0 [DOI] [PubMed] [Google Scholar]
- Haas, B. J. , Papanicolaou, A. , Yassour, M. , Grabherr, M. , Blood, P. D. , Bowden, J. , … Regev, A. (2013). De novo transcript sequence reconstruction from RNA‐seq using the Trinity platform for reference generation and analysis. Nature Protocols, 8, 1494 10.1038/nprot.2013.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley, N. J. , & Hoover, E. A. (2015). Chronic Wasting disease of cervids: Current knowledge and future perspectives. Annual Review of Animal BioSciences, 3(1), 305–325. 10.1146/annurev-animal-022114-111001 [DOI] [PubMed] [Google Scholar]
- Haley, N. J. , Mathiason, C. K. , Zabel, M. D. , Telling, G. C. , & Hoover, E. A. (2009). Detection of sub‐clinical CWD infection in conventional test‐negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS ONE, 4(11), e7990 10.1371/journal.pone.0007990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley, N. J. , & Richt, J. A. (2017). Evolution of diagnostic tests for chronic wasting disease, a naturally occurring prion disease of cervids. Pathogens, 6(35), 53–74. 10.3390/pathogens6030035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, D. A. (1999). Cellular biology of prion diseases. Clinical Microbiology Reviews, 12(3), 429–444. 10.1128/CMR.12.3.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsough, G. R. , & Burger, D. (1965). Encephalopathy of mink: I. Epizootiologic and clinical observations. Journal of Infectious Diseases, 115(4), 387–392. [DOI] [PubMed] [Google Scholar]
- Hope, J. , Reekie, L. J. , Hunter, N. , Multhaup, G. , Beyreuther, K. , White, H. , … Wells, G. A. (1988). Fibrils from brains of cows with new cattle disease contain scrapie‐associated protein. Nature, 336(6197), 390–392. 10.1038/336390a0 [DOI] [PubMed] [Google Scholar]
- Huang, D. W. , Sherman, B. T. , & Lempicki, R. A. (2008). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols, 4(1), 44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Huang, X.‐D. , Zhang, H. , & He, M.‐X. (2015). Comparative and evolutionary analysis of the Interleukin 17 gene family in invertebrates. PLoS ONE, 10(7), e0132802 10.1371/journal.pone.0132802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, C. J. , Phillips, K. E. , Schramm, P. T. , McKenzie, D. , Aiken, J. M. , & Pedersen, J. A. (2006). Prions adhere to soil minerals and remain infectious. PLoS Pathogens, 2(4), e32 10.1371/journal.ppat.0020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, A. C. , Mateus‐Pinilla, N. E. , Diffendorfer, J. , Jewell, E. , Ruiz, M. O. , Killefer, J. , … Novakofski, J. (2008). Prion sequence polymorphisms and chronic wasting disease resistance in Illinois white‐tailed deer (Odocoileus virginianus). Prion, 2(1), 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin, R. H. , & Walker, C. A. (1982). Pathogenesis of mouse scrapie: Patterns of agent replication in different parts of the CNS following intraperitoneal infection. Journal of the Royal Society of Medicine, 75(8), 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto, T. , Muramoto, T. , Mohri, S. , Doh‐Ura, K. , & Tateishi, J. (1991). Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt‐Jakob disease. Journal of Virology, 65(11), 6292–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaper, R. , & Thomas, M. A. (2004). At the crossroads of genomics and ecology: The promise of a canary on a chip. BioScience, 54(5), 403–412. 10.1641/0006-3568(2004)054[0403:ATCOGA]2.0.CO;2 [DOI] [Google Scholar]
- Kuznetsova, A. , Cullingham, C. , McKenzie, D. , & Aiken, J. M. (2018). Soil humic acids degrade CWD prions and reduce infectivity. PLoS Pathogens, 14(11), e1007414 10.1371/journal.ppat.1007414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead, B. , Trapnell, C. , Pop, M. , & Salzberg, S. L. (2009). Ultrafast and memory‐efficient alignment of short DNA sequences to the human genome. Genome Biology, 10(3), R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc, P. , Alais, S. , Porto‐Carreiro, I. , Lehmann, S. , Grassi, J. , Raposo, G. , & Darlix, J. L. (2006). Retrovirus infection strongly enhances scrapie infectivity release in cell culture. The EMBO Journal, 25(12), 2674–2685. 10.1038/sj.emboj.7601162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.‐J. , Jeong, B.‐H. , Choi, E.‐K. , & Kim, Y.‐S. (2013). Involvement of endogenous retroviruses in prion diseases. Pathogens, 2(3), 533–543. 10.3390/pathogens2030533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein, E. S. , Zhao, X. , & Gage, F. H. (2004). Defining a molecular atlas of the hippocampus using DNA microarrays and high‐throughput in situ hybridization. Journal of Neuroscience, 24(15), 3879–3889. 10.1523/JNEUROSCI.4710-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B. , & Dewey, C. N. (2011). RSEM: Accurate transcript quantification from RNA‐Seq data with or without a reference genome. BMC Bioinformatics, 12(1), 323 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , & Godzik, A. (2006). Cd‐hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics, 22(13), 1658–1659. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- Linden, R. , Martins, V. R. , Prado, M. A. , Cammarota, M. , Izquierdo, I. , & Brentani, R. R. (2008). Physiology of the prion protein. Physiological Reviews, 88(2), 673–728. 10.1152/physrev.00007.2007 [DOI] [PubMed] [Google Scholar]
- Lötscher, M. , Recher, M. , Lang, K. S. , Navarini, A. , Hunziker, L. , Santimaria, R. , … Zinkernagel, R. M. (2007). Induced prion protein controls immune‐activated retroviruses in the mouse spleen. PLoS ONE, 2(11), e1158 10.1371/journal.pone.0001158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjerovic, M. B. , Green, M. L. , Mateus‐Pinilla, N. , & Novakofski, J. (2014). The importance of localized culling in stabilizing chronic wasting disease prevalence in white‐tailed deer populations. Preventive Veterinary Medicine, 113(1), 139–145. 10.1016/j.prevetmed.2013.09.011 [DOI] [PubMed] [Google Scholar]
- Mardis, E. R. (2008). The impact of next‐generation sequencing technology on genetics. Trends in Genetics, 24(3), 133–141. 10.1016/j.tig.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Masaki, T. , Chiba, S. , Tatsukawa, H. , Yasuda, T. , Noguchi, H. , Seike, M. , & Yoshimatsu, H. (2004). Adiponectin protects LPS‐induced liver injury through modulation of TNF‐α in KK‐Ay obese mice. Hepatology, 40(1), 177–184. 10.1002/hep.20282 [DOI] [PubMed] [Google Scholar]
- Mateus‐Pinilla, N. , Weng, H. , Ruiz, M. O. , Shelton, P. , & Novakofski, J. (2013). Evaluation of a wild white‐tailed deer population management program for controlling chronic wasting disease in Illinois, 2003–2008. Preventive Veterinary Medicine, 110(3–4), 541–548. 10.1016/j.prevetmed.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Matsumoto, T. , Samuel, M. D. , Bollinger, T. , Pybus, M. , & Coltman, D. W. (2013). Association mapping of genetic risk factors for chronic wasting disease in wild deer. Evolutionary Applications, 6(2), 340–352. 10.1111/eva.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meling, S. , Skovgaard, K. , Bårdsen, K. , Heegaard, P. M. H. , & Ulvund, M. J. (2018). Expression of selected genes isolated from whole blood, liver, and obex in lambs with experimental classical scrapie and healthy controls, showing a systemic innate immune response at the clinical end‐stage. BMC Veterinary Research, 14(1), 281 10.1186/s12917-018-1607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzker, M. L. (2010). Sequencing technologies — the next generation. Nature Reviews Genetics, 11(1), 31–46. 10.1038/nrg2626 [DOI] [PubMed] [Google Scholar]
- Miller, M. W. , & Wild, M. A. (2004). Epidemiology of chronic wasting disease in captive white‐tailed and mule deer. Journal of Wildlife Diseases, 40(2), 320–327. 10.7589/0090-3558-40.2.320 [DOI] [PubMed] [Google Scholar]
- Moore, S. J. , Greenlee, M. H. W. , Kondru, N. , Manne, S. , Smith, J. D. , Kunkle, R. A. , … Greenlee, J. J. (2017). Experimental transmission of the chronic wasting disease agent to swine after oral or intracranial inoculation. Journal of Virology, 91, e00926-17 10.1128/JVI.00926-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls, A. V. , McNulty, E. , Powers, J. , Seelig, D. M. , Hoover, C. , Haley, N. J. , … Mathiason, C. K. (2013). Mother to offspring transmission of chronic wasting disease in Reeves' Muntjac deer. PLoS ONE, 8(8), e71844 10.1371/journal.pone.0071844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero, A. , Duque Velásquez, C. , Johnson, C. , Herbst, A. , Bolea, R. , Badiola, J. J. , … McKenzie, D. (2019). Prion protein polymorphisms associated with reduced CWD susceptibility limit peripheral PrPCWD deposition in orally infected white‐tailed deer. BMC Veterinary Research, 15, 50 10.1186/s12917-019-1794-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, K. M. , Baldwin, M. , Nguyen, J. , Gasset, M. , Serban, A. , Groth, D. , … Cohen, F. E. (1993). Conversion of alpha‐helices into beta‐sheets features in the formation of the scrapie prion proteins. Proceedings of the National Academy of Sciences of the United States of America, 90(23), 10962–10966. 10.1073/pnas.90.23.10962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, P. J. , Mironov, A. , Peretz, D. , van Donselaar, E. , Leclerc, E. , Erpel, S. , … Prusiner, S. B. (2003). Trafficking of prion proteins through a caveolae‐mediated endosomal pathway. Journal of Cell Biology, 162(4), 703–717. 10.1083/jcb.200304140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos, C. (1997). Retroviral taxonomy, protein structures, sequences, and genetic maps. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK19417/ [Google Scholar]
- Powers, J. G. , Hoover, E. A. , Zabel, M. M. , Fullaway, S. , Nalls, A. , Mathiason, C. K. , … Mayfield, A. (2015). In utero transmission and tissue distribution of chronic wasting disease‐associated prions in free‐ranging Rocky Mountain elk. Journal of General Virology, 96(11), 3444–3455. 10.1099/jgv.0.000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner, S. B. (1982). Novel proteinaceous infectious particles cause scrapie. Science, 216(4542), 136–144. [DOI] [PubMed] [Google Scholar]
- Prusiner, S. B. (1989). Scrapie prions. Annual Reviews in Microbiology, 43(1), 345–374. 10.1146/annurev.mi.43.100189.002021 [DOI] [PubMed] [Google Scholar]
- Ramos, C. D. L. , Canetti, C. , Souto, J. T. , Silva, J. S. , Hogaboam, C. M. , Ferreira, S. H. , & Cunha, F. Q. (2005). MIP‐1α[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF‐α and LTB4. Journal of Leukocyte Biology, 78(1), 167–177. 10.1189/jlb.0404237 [DOI] [PubMed] [Google Scholar]
- Robinson, M. D. , McCarthy, D. J. , & Smyth, G. K. (2010). edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 26(1), 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar, J. G. , Lessard, P. , Tamgüney, G. , Freyman, Y. , Deering, C. , Letessier, F. , … Prusiner, S. B. (2008). Transmission and detection of prions in feces. Journal of Infectious Diseases, 198(1), 81–89. 10.1086/588193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto, F. , & Lanzavecchia, A. (1994). Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony‐stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. Journal of Experimental Medicine, 179(4), 1109–1118. 10.1084/jem.179.4.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaia, H. B. , & Brentani, R. R. (1998). Can loss‐of‐function prion‐related diseases exist? Molecular Psychiatry, 3(3), 196–197. 10.1038/sj.mp.4000378 [DOI] [PubMed] [Google Scholar]
- Saunders, S. E. , Bartelt‐Hunt, S. L. , & Bartz, J. C. (2012). Occurrence, transmission, and zoonotic potential of chronic wasting disease. Emerging Infectious Diseases, 18(3), 369–376. 10.3201/eid1803.110685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinghaus, C. W. (1949). Tooth development and wear as criteria of age in white‐tailed deer. Journal of Wildlife Management, 13(2), 195–216. 10.2307/3796089 [DOI] [Google Scholar]
- Spurgeon, S. L. , Jones, R. C. , & Ramakrishnan, R. (2008). High throughput gene expression measurement with real time PCR in a microfluidic dynamic array. PLoS ONE, 3(2), e1662 10.1371/journal.pone.0001662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell, L. , Greig, J. , Mascarenhas, M. , Otten, A. , Corrin, T. , & Hierlihy, K. (2018). Current evidence on the transmissibility of chronic wasting disease prions to humans‐A systematic review. Transboundary and Emerging Diseases, 65(1), 37–49. 10.1111/tbed.12612 [DOI] [PubMed] [Google Scholar]
- Wajant, H. , Pfizenmaier, K. , & Scheurich, P. (2003). Tumor necrosis factor signaling. Cell Death and Differentiation, 10, 45–65. 10.1038/sj.cdd.4401189 [DOI] [PubMed] [Google Scholar]
- Westergard, L. , Christensen, H. M. , & Harris, D. A. (2007). The cellular prion protein (PrPC): Its physiological function and role in disease. Biochimica Et Biophysica Acta (BBA) – Molecular Basis of Disease, 1772(6), 629–644. 10.1016/j.bbadis.2007.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, E. S. (2005). Chronic wasting disease. Veterinary Pathology Online, 42(5), 530–549. 10.1354/vp.42-5-530 [DOI] [PubMed] [Google Scholar]
- Williams, E. S. , Miller, M. W. , Kreeger, T. J. , Kahn, R. H. , & Thorne, E. T. (2002). Chronic wasting disease of deer and elk: A review with recommendations for management. Journal of Wildlife Management, 66(3), 551–563. 10.2307/3803123 [DOI] [Google Scholar]
- Williams, E. S. , & Young, S. (1980). Chronic wasting disease of captive mule deer: A spongiform encephalopathy. Journal of Wildlife Diseases, 16(1), 89–98. 10.7589/0090-3558-16.1.89 [DOI] [PubMed] [Google Scholar]
- Wyckoff, C. A. , Kane, S. , Lockwood, K. , Seligman, J. , Michel, B. , Hill, D. , … Zabel, M. (2016). Clay components in soil dictate environmental stability and bioavailability of cervid prions in mice. Frontiers in Microbiology, 7, 1885 10.3389/fmicb.2016.01885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar, S. , von Ahsen, N. , Oellerich, M. , Zerr, I. , Schulz‐Schaeffer, W. J. , Armstrong, V. W. , & Asif, A. R. (2011). Proteomics approach to identify the interacting partners of cellular prion protein and characterization of Rab7a interaction in neuronal cells. Journal of Proteome Research, 10(7), 3123–3135. 10.1021/pr2001989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNA‐Seq data are available at the NCBI SRA SRP158695.


