Abstract
Purpose
To test the hypothesis that the Burn time Per tumor Volume (BPV, min/ml), where burn time is the total time during which radiofrequency energy is being applied, is correlated with outcomes in the treatment of the hepatocellular carcinoma (HCC) using radiofrequency ablation (RFA) plus lyso-thermosensitive liposomal doxorubicin (LTLD).
Materials and Methods
The phase III HEAT study was a double-blind, randomized controlled phase III trial of RFA-only vs. RFA+LTLD in patients with 3–7 cm diameter hepatocellular carcinoma (HCC). The effect of BPV on the progression free survival (PFS) and overall survival (OS) was analyzed.
Results
BPV demonstrated differences between study groups that were statistically significant for OS (p=0.038, Hazard Ratio=0.85) but not for PFS (p=0.389, HR=1.059). In a separate analysis, treatment groups were independently analyzed to determine the effect of BPV within each individual group. Overall survival improves as BPV increases for RFA+LTLD patients (p=0.017, Hazard Ratio=0.836, CI=0.722–0.968). This same association was not observed in RFA-only patients (p=0.57, Hazard Ratio=0.99).
Conclusion
BPV may be a useful metric for this combination therapy for solitary HCC. The analysis suggested that the burn time for the tumor needs to be adjusted depending on the tumor volume. Because this is a post hoc study, the results are only suggestive and need to be confirmed with prospective studies.
INTRODUCTION
Radiofrequency ablation (RFA) has been widely used for unresectable hepatocellular carcinoma (HCC), but the application of RFA to tumors > 3 cm diameter has not been as successful due to technical limitations in creating a large enough treatment volume. This limitation results in incomplete treatment for larger tumors > 3cm (1–6). Recurrence at the treatment margin suggests untreated residual microscopic disease beyond the ablation zone.
Efforts to address tumor relapse due to under-treated margins have included alternative treatment methods, such as microwave ablation (7, 8), or combining RFA with an adjunctive therapy such as saline injection (9) or intravenous liposomal encapsulated doxorubicin. Lyso-thermosensitive liposomal doxorubicin (LTLD; ThermoDox; Celsion, Lawrenceville, NJ) was developed to release doxorubicin at temperatures above 40°C, which can potentially be used to deploy doxorubicin at the tumor margins during RFA. This synergistic combination of LTLD and RFA could possibly address RFA failure and disease recurrence on the tumor margins by depositing doxorubicin where it may be most beneficial. Safety and feasibility of systemic injection of LTLD and applying RFA (RFA+LTLD) have been shown in a phase I dose-escalation study for unresectable liver tumors (2).
More recently, a phase III HEAT study was completed that aimed to show efficacy of RFA+LTLD for liver tumors with long diameters between 3 and 7cm. The study found no significant difference between the outcomes of RFA-only and RFA+LTLD groups in terms of progression free survival (primary endpoint) and overall survival (secondary endpoint)(10). An initial post hoc sub-group analysis of 285 patients with a single tumor and dwell time (duration between needle-in and needle-out) longer than 45 minutes showed overall survival of RFA+LTLD patients improved compared with RFA-only patients (10). However, this dwell time included not only treatment burn time but also time for needle repositioning, which was not directly or mechanistically related to treatment efficiency. The primary mechanism for drug delivery with LTLD relies on rapid intravascular drug release from the liposomes while they are in circulation; this increases the intravascular drug concentration within the heated volume which facilitates drug delivery across vessel walls through Fickian diffusion (11). To take best advantage of this formulation, it is necessary to deliver thermal ablation while the drug is circulating. Pharmacokinetic modeling of this drug suggests that applying thermal ablation during the period of time in which drug concentration is highest would maximize tissue drug deposition, provided the thermal ablation were active for a prolonged and continuous duration before the serum drug levels declined (2).
In this study, it was hypothesized that the burn time (ablation duration, RFA energy “on”) together with tumor volume play an important role in the treatment of the HCC using RFA+LTLD. To test this hypothesis, HEAT study data were further analyzed retrospectively.
MATERIALS AND METHODS
Patient Population
In this post hoc study, data from the phase III HEAT study of LTLD were analyzed. The original study was double-blind and randomized controlled and compared RFA-only vs. RFA+LTLD patients with hepatocellular carcinoma (HCC) (10). Each participating site obtained approval by the institutional review board. The original trial included 701 patients single or multiple HCCs between 3 and 7 cm in diameter (10). Due to the nature of the LTLD pharmacokinetics and procedural variability (12), only data from patients with single tumors were analyzed in this study (RFA only n=210 vs. RFA+LTLD n=227 patients). The mean number (standard deviation) of overlapping ablations was 3.69 (3.45). A univariate and multivariate Cox proportional hazard model was used to investigate the effect of Burn time Per tumor Volume (BPV, min/ml), where burn time is the total time during which radiofrequency energy is being applied, and tumor diameter on the progression-free survival (PFS) and overall survival (OS). Volume measurements (ml) were determined by outlining the tumor area in each CT slice, multiplying by the slice thickness, and summing the values. Two radiologists from an independent contract research organization each measured tumor volumes. The average of the two values was used as the pre-treatment tumor volume.
Statistical analysis
To analyze the association between the BPV and overall survival of the two treatment groups, hazard ratios for different threshold values of BPV were calculated using RStudio (13). Also, Kaplan-Meier curves were plotted for two different BPV threshold values as examples.
A Cox proportional hazards model of R-studio was used on two separate outcomes: OS and PFS(13). Treatment groups (RFA only and RFA+LTLD), BPV, and burn time per tumor diameter were individually analyzed as input covariates using a simple Cox proportional hazard model. In addition, interaction of treatment group with the BPV and diameter were also tested in the complex multi covariate Cox proportional hazard model. To test Cox proportionality, Schoenfeld residuals were used to check non-zero slope, which indicates proportional hazard assumption is violated(14).
RESULTS
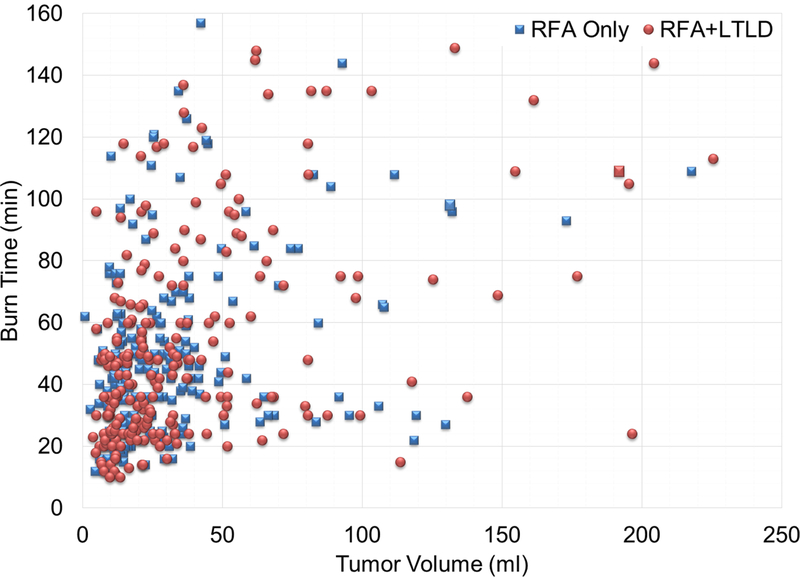
The volume measurements for the RFA only and RFA+LTLD groups (mean ± SD) were 35.3±40.0 ml vs. 35.5±38.5, respectively, with an average absolute measurement difference of 16.34%. Average burn times for the treatment groups were not significantly different: 52.01 vs. 49.71 min for RFA only and RFA+LTLD patients, respectively (two-sampled t-test h = 0, p = 0.3927, CI = [−8.30 3.27]). In addition, burn time was plotted against tumor volume and tumor largest diameter (Figures 1a and 1b). The data were highly scattered and were analyzed further for association. The results indicated a quadratic relationship between tumor volume and burn time, as evidenced by the p-value of the regression coefficients of tumor volume (p<0.0001) and tumor volume squared (p=0.0088). Similarly, burn time and tumor largest diameter were analyzed and the results indicated a linear relationship between tumor largest diameter and burn time, as evidenced by the p-value of the regression coefficient of largest tumor diameter (p<0.0001). The p-value for tumor largest diameter squared was equal to 0.1868.
Figure 1.
Burn Time vs. Tumor Volume and Tumor Largest Diameter. Aggregated data for burn time vs tumor volume (a) and tumor largest diameter (b) are highly scattered. There was a quadratic relationship between burn time and tumor volume and a linear relationship between burn time and tumor greatest diameter. Two sample sites showing burn time vs. tumor volume (c and e) and burn time vs. tumor diameter (d and f) with trend lines are shown. The first site (c and d) has an increasing burn time with both tumor volume and diameter. The relationship is linear for the diameter. In contrast, the second site has constant burn time without regard to increasing volume or diameter.
Each study site was examined individually to better understand their RFA treatment strategies and how operators were adjusting burn time depending on the tumor size. Two different sites’ results for burn time vs. tumor volume and burn time vs. tumor diameter are shown as examples. The first site shows a trend in which the burn time increased as the tumor volume or diameter increased (Figures 1c and d). In contrast, the second represents a site where burn time did not change as tumor diameter or volume increased (Figures 1e and f). Certain sites and certain operators demonstrated variability in terms of how and whether they adjusted the treatment times according to target tumor volumes. This practice pattern was measured and displayed using the graphical display, as a site-specific or operator-specific “practice pattern plot”.
In order to avoid the unintended effect of the dissociated tumor volume and burn time covariates, BPV (min/ml) was suggested as a new parameter to use in the statistical analysis. Average BPV for the treatment groups were not significantly different: 2.31 vs. 2.42 min/ml for RFA only and RFA+LTLD patients, respectively (two-sampled t-test h = 0, p = 0.55, CI = [−0.27 0.50]). Multiple covariate Cox survival analysis has been used to understand the interaction of different covariates (15) and the effects of these on survival. According to the Cox proportional hazard model, a secondary covariate may be a confounding or an effect modifier or neither. A confounding covariate (BPV/diameter) affects the outcome independent of the primary covariate (treatment groups). When BPV and diameter were included in the analysis, OS hazard ratio (HR) and p-value did not change significantly compared with crude treatment group analysis, which suggests that BPV and diameter were not confounding covariates (Table 1a, b, and d). Next, BPV and diameter were tested to determine whether they changed the effect of the treatment group. The interaction term for treatment group and BPV (TG*BPV) was significant (p=0.038) and hazard ratio (HR) was 0.85, which suggested that BPV was an effect modifier (Table 1c). The result suggested that an increase in BPV improved survival of the RFA+LTLD patients compared with RFA-only patients. The same analysis was also repeated for burn time per tumor longest diameter, but it was neither confounding nor an effect modifier (Table 1d and e). In addition, effects of the mentioned covariates were not significant when PFS was considered (results not shown). Burn time alone was also checked to determine whether it is a confounding or an effect modifier covariate, but results showed that burn time was neither (TG+Burn time: p=0.457, HR=0.897; TG*Burn time: p=0.164, HR=0.993). Schoenfield residuals were plotted to confirm that Cox proportional hazard assumption was valid in the analysis. There was a difference between the two treatment groups when the BPV was increased (Table 1). To further investigate the details of BPV effect, each group (RFA only and RFA + LTLD) was independently analyzed to determine the effect of BPV within each individual group (Table 2). Here, BPV was considered as the input covariate in the Cox survival analysis.
Table 1:
Cox analysis for (a) treatment group only (TG), and (b-e) including multiple covariates BPV (b-c) and burn time per longest diameter (d-e), together with treatment group using OS. To check whether the secondary covariates (BPV and BTPLD) were confounding variables, summation operation was used (b and d). Difference of P values and HRs was not statistically significant compared with crude model (a), which suggested that BPV and BTPLD were not confounding variables. (c) However, interaction term for BPV was significant, which means it is an effect modifier. (e) The same effect was not observed for BTPLD.
| Cox survival analysis of the covariates | ||||
|---|---|---|---|---|
| Covariates | Output | p-Val | Hazard Ratio | CI |
| (a) TG | TG | 0.445 | 0.895 | 0.674–1.189 |
| (b) TG+BPV | TG | 0.416 | 0.888 | 0.669–1.181 |
| BPV | 0.149 | 0.942 | 0.868–1.022 | |
| (c) TG*BPV | TG | 0.290 | 1.249 | 0.827–1.887 |
| BPV | 0.591 | 0.987 | 0.939–1.036 | |
| TG*BPV | 0.038 | 0.85 | 0.728–0.991 | |
| (d) TG+BPD | TG | 0.468 | 0.900 | 0.678–1.196 |
| BPV | 0.328 | 0.891 | 0.707–1.122 | |
| (e) TG*BPD | TG | 0.339 | 1.352 | 0.728–2.513 |
| BTPLD | 0.678 | 1.072 | 0.771–1.492 | |
| TG*BTPLD | 0.147 | 0.711 | 0.448–1.127 | |
TG: Treatment Group, BPV: Burn time Per tumor Volume, BPD: Burn time Per longest Diameter, n= 437, Events=191
Table 2:
Cox proportional hazard model to analyze the effect of BPV to each treatment group individually. The results of the PFS analysis of neither group were statistically significant. Response of the RFA only patients (n=210) to the increase of the BPV was also not statistically significant. However, one unit increase of BPV (1 min/ml) increased the survival chance of the RFA+LTLD patients (n=227) and the result was significant (p=0.017, HR=0.836).
| Effect of BPV on each individual treatment group | ||||||
|---|---|---|---|---|---|---|
| Group | OS/PFS | p-Val | Hazard Ratio | CI | Events (Deaths) | R2 |
| RFA Only | PFS | 0.133 | 0.925 | 0.835–1.024 | 133 | 0.02 |
| OS | 0.590 | 0.987 | 0.940–1.036 | 96 | 0.002 | |
| RFA + LTLD | PFS | 0.637 | 0.98 | 0.90–1.066 | 133 | 0.001 |
| OS | 0.017 | 0.836 | 0.722–0.968 | 95 | 0.033 | |
Cox univariate analysis was also performed by applying thresholds to the BPV and considering only the patients above the threshold value (Figure 2). As the threshold was increased, the hazard ratio became smaller, which was expected. Survival results with Kaplan Meier survival curves demonstrated significant difference with the addition of LTLD for the subset of patients analyzed who had single tumors and met specific thresholds of time per tumor volume. (Figure 3) For example, when only the patients with BPV larger than 2 min/ml were considered, HR was 0.7, which means 42.8% survival improvement for RFA+LTLD patients compared with RFA-only patients (Figure 3a). When the threshold was increased to 3.4 min/ml, HR was reduced to 0.5 and survival improvement became 100% (Figure 3b).
Figure 2.
Hazard ratio vs. lower threshold of burn time per tumor volume (BPV). As the patients with smaller values of BPV were excluded, the hazard ratio decreased, representing improved benefit for RFA+LTLD patients compared with RFA-only patients. When patients with less than 2 min/ml (or 3.4 min/ml) BPV were excluded, the hazard ratio became 0.7, which meant RFA+LTLD patients survival improved 42.8% compared with RFA only patients. With exclusion of patients with less than 3.4 min/ml BPV, the hazard ratio and survival improvement for RFA+LTLD patients became 0.5 and 100%, respectively, compared with RFA only patients.
Figure 3.
Kaplan-Meier plot for the patients treated with BPV greater than 2 min/ml (a) and 3.4 min/ml (b). Hazard ratio (HR) was 0.7 when the threshold was 2 min/ml reflecting a 42.8% survival improvement for RFA+LTLD patients compared with RFA-only patients (a). For BPV=3.4 min/ml, HR was reduced to 0.5 and survival improvement became 100% (b).
The percentage of the patient population that could be completely treated using a BPV of 2 min/ml, depending on the available burn time, is plotted (Figure 4). For example, if available burn time was 100 min, almost 80% of the tumors could have been treated using 2 min/ml BPV.
Figure 4.
Percentage of this patient population that could be treated with a BPV of 2 min/ml depending on the available burn time. As the available time for treatment increases, the percentage of patients who may be treated with the defined value of BPV increases. If BPV of 2 min/ml is used, approximately 80% of the patients can be treated in less than 100 min.
Tumor volume vs. longest diameter was plotted with fitted curve and the right side of the plot provides corresponding burn time to optimize the LTLD effect. A magnified version of the plot was also provided for the tumor longest diameter from 35 mm to 55 mm (Figure 5).
Figure 5.
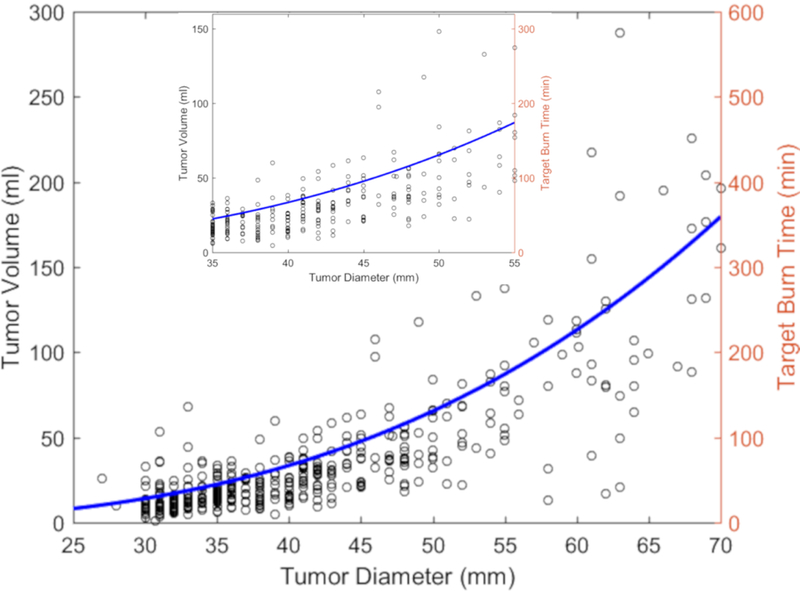
Tumor volume vs. tumor longest diameter vs. target burn time using burn time per volume of 2 min/ml as an example. The blue curve is the fit of the tumor volume vs. tumor longest diameter. Target burn time was calculated by multiplying the value of tumor volume in the fitted curve by the sample BPV value of 2 min/ml. The inset is a magnified detail for the diameters between 35 to 55 mm.
DISCUSSION
Although previous studies presented promising results for the RFA+LTLD combination (2), the results of the phase III HEAT study shows no statistically significant difference between the two treatment groups for the primary (PFS) and secondary (OS) endpoints (10, 16). On the other hand, the same study reported that RFA+LTLD patients had better OS compared with RFA-only patients when only a subgroup of patients, those treated longer than 45 minutes of dwell time, were considered (10, 16).
The referenced phase III HEAT study failed to meet the study endpoints when analyzed with conventional metrics. However, consideration of the mechanisms of action for this drug and device combination therapy supports analysis with a custom metric for critical evaluation of efficacy. In general, post hoc analyses may be inconclusive, unreliable, or convey a low level of data. However, this study may merit unique consideration and guide future studies, given the supportive preclinical mechanistic data for this drug plus device approach.
This work not only revealed a different perspective and implications of the HEAT trial, but also illustrated the importance of study design in drug plus device combination therapies in general, especially those that depend upon standardization of a variably subjective therapy such as thermal ablation. Although RFA is present in many algorithms for HCC management, the variability of the application or delivery of this therapy is frequently ignored or underrepresented. Specifically, there is a variability of practice patterns and an inherent lack of uniform technical guidance and instructions for use for RFA duration. Although an association between burn time and both tumor volume and tumor largest diameter was present, this link was not enforced or prospectively designed in practice, as evidenced by the widely scattered values (Figures 1 a and b). This degree of scatter and the demonstrated link between burn time and outcome highlights the importance of more tightly defined standardization of technique. This may be especially true for any operator-dependent procedure like RFA. Based upon the mechanism of drug-device combination action, this work suggests that appropriately extended RFA durations for larger volume tumors is required to increase efficacy of the LTLD+RFA, in terms of survival. Furthermore, BPV can be used to normalize and stratify the subjective variability among different operators and different sites, based upon practice patterns. This normalization may have broad implications for the field of combination drug plus device therapies.
In this study, only patients with a single tumor were considered due to the technical variability of the RFA+LTLD procedure in patients with multiple tumors, as well as the fact that the timing of the LTLD injection and RFA is critically important for maximizing the local drug release and optimizing the pharmacokinetics of LTLD combined with RFA (2, 12). The optimal combination of drug and device requires synchronization of the device use and the known temporal profile of the drug pharmacokinetics. Ablating multiple tumors in one session ensures that either none of the tumors gets optimum treatment or that only one tumor can be treated properly by RFA+LTLD, since the subsequent tumors will be exposed to lower levels of serum drug. Therefore, multiple tumor patients were excluded from the analysis.
Previous studies showed that the survival benefit for the LTLD patients in the phase III HEAT study was found only for a subgroup of patients who were treated with a longer than 45 min dwell time (10). Dwell time may not be the best measure for the completeness of treatment because it includes both burn time as well as time for electrode repositioning between ablation treatments, thus including time while the RFA system is off and being subject to wide variations in practice patterns and techniques. On the other hand, burn time per tumor volume may be a parameter directly related to completeness of the treatment, because RFA “on” duration is the major RFA variable adjusted by interventional radiologists according to the tumor size. BPV standardizes total burn time depending on the tumor volume and yields a normalized metric for comparison and verification of adequacy of technique.
The longest diameter is the usual standard for liver and other tumor size measurements as well as treatment planning. Burn time per longest diameter was found not to be a confounding or effect modifying covariate (Table 1). Although further investigations are crucial, tumor volume may be a better way to characterize the size of the tumor and determine the requisite burn time to ensure successful tumor coverage and overall successful local treatment. Therefore, BPV is the more consistent parameter to more accurately reflect the effects of ablation duration, especially for the RFA+LTLD patients.
The analysis showed that each unit (1 min/ml) increase of BPV increases RFA+LTLD patients’ survival rate by 17.6% compared with RFA only patients (Table 1c). This result was confirmed by separating the treatment groups, inputting BPV, and running a crude analysis (Table 2). The result showed that RFA+LTLD patients’ survival improved as BPV was increased. Every unit increase in BPV improved RFA+LTLD patients’ survival rate by 19.6% (1/0.836=1.196), which indicated that BPV may be critical for the RFA+LTLD patients, as the drug deposition increases with extended burn time as reflected by extended BPV. On the other hand, analysis using BPV demonstrated a survival benefit when comparing RFA only versus RFA+LTLD in solitary hepatocellular carcinomas between 3–7 cm. Such survival benefit was not statistically significant when PFS was considered, for unknown reasons.
The effect of BPV increase was not statistically significant in the RFA-only patients (p=0.590, HR=0.987). This result might explain why prolonged treatments may not have produced the same amount of cell killing in the periphery of the ablated zone, compared with RFA+LTLD, which defines whether the drug provides benefit or not. A general guideline for liver RFA duration does not exist because the technical specifications of the different RFA systems vary. RFA duration may change depending on tumor shape, vascularization, and size, as well as the RFA system and other operator factors. It is intriguing that specific centers and specific operators were clustered according to practice patterns or a practice “phenotype”. Such a tool (with future validation of metrics) might prove useful for training or screening out the outlying and underperforming or non-uniform operators or sites.
Extended duration of anesthesia required for treating large tumors may increase procedural risks, but this is true for RFA alone as well. A feasibility analysis showed that if the operators had 100 minutes of burn time, almost 80% of the tumor patients with single tumors in this study could have been treated longer than 2 min/ml (Figure 4).
Using the target burn time plots, one can determine how long RFA needs to be performed. For example, if an interventional radiologist aims to ablate a single tumor using 2 min/ml with a single electrode, ablation of a 45 mm diameter tumor (approximately 50 ml volume) would require 100 min (Figure 5). Feasibility of delivering the burn time is an important part of the treatment planning. To achieve 2 min/ml burn time, patients with a 70 mm tumor would need to be treated for nearly 360 minutes, which is not realistic (Figure 5). Use of multiple electrodes may address this limitation.
The main limitation of this study is that it was a post hoc analysis. Although the data were derived from a randomized, double-blind investigation that was both multi-institutional and international in scope, the reported measure was not included in the original analysis plan. RFA device, electrode and power variations were beyond the scope of this paper and were not included in the analyses. One additional limitation of this study is the variability introduced by the use of three different RFA vendors, which may introduce a risk of comparing non-comparable data. For this reason, the study was limited to the subset of solitary lesions which may improve standardization across the study. Also, the basic mechanisms of RFA in the range near 500 kilohertz rely upon the same mechanistic principles, regardless of the shape of the RFA probe, or the presence of water cooling. Thus, similar dependency upon thermal conductivity is present in all RFA systems, as well as the requisite minimal treatment time per volume tumor metric, which is the central hypothesis of this work. These results need to be confirmed in a prospective clinical trial which controls for these device and operational parameters. Any post hoc subpopulation or subgroup study may be subject to more risk for confounding variables (such as asymmetric Eastern Cooperative Oncology Group (ECOG) Performance Status or sample selection bias). However, a large randomized well-controlled trial with this as an endpoint defined a priori will be less prone to these biases.
Analysis using the parameter, burn time per tumor volume (BPV) demonstrated a survival benefit when comparing RFA+LTLD versus RFA only in solitary HCC between 3–7 cm in diameter. Device-drug combination studies need to be optimized and customized, depending on the interaction of the device and drug. The data suggest BPV may be used to filter survival curves for significant differences and may also identify different performing or potentially underperforming operators or sites. Such a metric may have implications for future studies of this device drug combination therapy. Defining rationale metrics based on mechanisms of action may play a role in standardization and normalization of human factors during clinical trial design.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH and the NIH Center for Interventional Oncology: Grant ZID# BC011242–9 & CL040015–9. NIH has a Cooperative Research and Development Agreement with Celsion Inc. We would like to thank Verma Walker, NIH Library Editing Service, for reviewing the manuscript.
Dr. Castro reports grants and non-financial support from Celsion Corp, during the conduct of the study.
Dr. Celik reports grants and non-financial support from Celsion Corp, during the conduct of the study.
Dr. Dewhirst reports other from Celsion Corp, outside the submitted work.
Dr. Karanian reports grants and non-financial support from Celsion Corp, during the conduct of the study;
Dr. Lencioni reports personal fees from Celsion Corporation, personal fees from BTG, personal fees from Guerbet, outside the submitted work;.
Dr. Leonard reports grants and non-financial support from Celsion Corp, during the conduct of the study;
Dr. Pritchard reports grants and non-financial support from Celsion Corp, during the conduct of the study;
Dr. Wakim has nothing to disclose.
Dr. Wood reports grants and non-financial support from Celsion Corp, during the conduct of the study; In addition, Dr. Wood has a patent Philips and NIH share licensed intellectual property issued, and multiple patents in the field owned by the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local Recurrence After Hepatic Radiofrequency Coagulation. Annals of Surgery 2005;242:158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood BJ, Poon RT, Locklin JK, et al. Phase I Study of Heat-Deployed Liposomal Doxorubicin during Radiofrequency Ablation for Hepatic Malignancies. Journal of vascular and interventional radiology : JVIR 2012;23:248–55 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency Thermal Ablation. American Journal of Roentgenology 2001;177:777–82. [DOI] [PubMed] [Google Scholar]
- 4.Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Annals of surgery 2007;246:559–65; discussion 65–67. [DOI] [PubMed] [Google Scholar]
- 5.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221:159–66. [DOI] [PubMed] [Google Scholar]
- 6.van Duijnhoven FH, Jansen MC, Junggeburt JMC, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Annals of surgical oncology 2006;13:651–8. [DOI] [PubMed] [Google Scholar]
- 7.Lu MD, Chen JW, Xie XY, et al. Hepatocellular carcinoma: US-guided percutaneous microwave coagulation therapy. Radiology 2001;221(1):167–72. [DOI] [PubMed] [Google Scholar]
- 8.Strickland AD, Clegg PJ, Cronin NJ, et al. Experimental study of large-volume microwave ablation in the liver. Br J Surg 2002;89(8):1003–7. [DOI] [PubMed] [Google Scholar]
- 9.Lin YC, Chen JH, Han KW, Shen WC. Ablation of liver tumor by injection of hypertonic saline. AJR Am J Roentgenol 2005;184(1):212–9. [DOI] [PubMed] [Google Scholar]
- 10.Tak WY, Lin SM, Wang Y, et al. Phase III HEAT Study Adding Lyso-Thermosensitive Liposomal Doxorubicin to Radiofrequency Ablation in Patients with Unresectable Hepatocellular Carcinoma Lesions. Clin Cancer Res 2018;24(1):73–83. [DOI] [PubMed] [Google Scholar]
- 11.Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer research 2012;72(21):5566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swenson CE, Haemmerich D, Maul DH, Knox B, Ehrhart N, Reed RA. Increased Duration of Heating Boosts Local Drug Deposition during Radiofrequency Ablation in Combination with Thermally Sensitive Liposomes (ThermoDox) in a Porcine Model. PLOS ONE 2015;10:e0139752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Team RC. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria, 2015. [Google Scholar]
- 14.UCLA Institute for Digital Research and Education. Applied Survival Analysis, Chapter 6 | R Textbook Examples 2017. [cited 2017 31 March 2017]; Available from: http://stats.idre.ucla.edu/r/examples/asa/r-applied-survival-analysis-ch-6/. [Google Scholar]
- 15.Hosmer D, Lemeshow S, May S. Applied Survival Analysis. Controlled Clinical Trials 2000;21:56–8. [Google Scholar]
- 16.Lencioni R, Cioni D. RFA plus lyso-thermosensitive liposomal doxorubicin: in search of the optimal approach to cure intermediate-size hepatocellular carcinoma. Hepatic Oncology 2016;3:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]