Abstract
Researchers have reported associations between fetal sex and heart rate (FHR) and heart rate variability (FHRV) but rarely in the context of fetal behavioral sleep state. We examined differences in measures of fetal autonomic function by sex and sleep state. Fetal abdominal ECG monitoring technology was used to measure FHR and two measures of FHRV—standard deviation of FHR (SD) and beat-to-beat variability (RMSSD). FHR and movement patterns were also recorded with standard Doppler ultrasound monitor technology employed to code sleep states. Data were collected from 82 healthy fetuses ranging from 36–39 weeks gestation. A one-way MANOVA showed that FHR was significantly lower and SD was significantly higher for males than females. Independent samples t-tests found that these sex differences were only in the active sleep state. There were no significant differences in RMSSD by sex. Repeated measures MANOVA for a subset that exhibited more than one state (N=22) showed that SD was significantly different by state. RMSSD showed a marginally significant sleep state difference. In conclusion, fetal sex differences in HR and HRV may indicate more mature autonomic functioning in near-term males than females and fetal sleep state can influence abdominal fECG derived measures of FHR and FHRV.
Keywords: fetal heart rate, fetal heart rate variability, fetal sex differences, fetal behavioral sleep states
Introduction
Fetal heart rate (FHR) and heart rate variability (FHRV) provide indicators of brain development in the human fetus. Specifically, the gradual increase in FHRV and decrease in FHR as a function of gestational age, are indicative of a healthy developing fetus (Amorim-Costa, Costa-Santos, Ayres-de-Campos, & Bernardes, 2016; DiPietro et al., 2004; Lange, Van Leeuwen, Geue, Hatzmann, & Grönemeyer, 2005).
Traditionally females have been viewed as developmentally advanced at birth in comparison to males (Sontag & Richards, 1938) and the assumption was that this advantage precedes birth. Male fetuses have a higher risk of neonatal mortality (Eriksson, Kajantie, Osmond, Thornburg, & Barker, 2010) and are more negatively affected by exposure to environmental toxins during the prenatal period (DiPietro & Voegtline, 2017). Female fetuses display more advanced oral motor behaviors (Miller, Macedonia, & Sonies, 2006) and heart rate decelerations to speech sounds (Groome et al., 1999).
To better understand these sex differences, research has examined differences in FHR and FHRV by fetal sex. FHR does not differ between males and females in the first trimester (McKenna, Ventolini, Neiger, & Downing, 2006). However, evidence for sex differences in the third trimester is mixed. Ogueh & Steer (1998) did not find differences in FHR or FHRV between 79 male and female fetuses, but other research groups have found evidence for sex differences. Bernardes, Gonçalves, Ayres-de-Campos, & Rocha (2008) found that at 40 weeks a comparison between 23 male and 24 female fetuses showed that males have higher FHRV than females. In a larger study, DiPietro, Costigan, & Voegtline (2015) collected data from 740 fetuses and found that in the late term fetus, males show a more mature pattern of autonomic function than females. Specifically, late term female fetuses exhibited faster heart rates and lower heart rate variability than males. This was replicated by Bhide & Acharya (2018) with a sample of 9,259 fetuses.
Fetal state has a long history of inquiry (Nijhuis & ten Hof, 1999; Nijhuis, Prechtl, Martin, & Bots, 1982), and the body of work suggests that measures of fetal activity and autonomic function are state dependent. For example, FHRV has been found to progressively increase as a function of increased activity across 1F, 2F and 4F states; the fetus near term on average is in 1F (quiet sleep) 24% of the time, 2F (active sleep) 65% of the time and 4F (active awake) 11% of the time (Brändle et al., 2015). Fetal state organization may be a useful indicator of a healthy fetus, as researchers have found that fetuses who were impacted by intrauterine growth retardation had very few, if any incidences of 1F when compared to healthy fetuses (Arduini et al., 1989). In addition, Salisbury, Fallone, & Lester (2005) found state organization to be a significant predictor of babies who are at-risk at birth. One research study has examined differences in FHR and FHRV by sex in the context of state (Bernardes et al., 2008).
While patterns of accelerations and decelerations in fetal movement, heart rate and heart rate variability have been used to designate categories of fetal behavioral state, studies specifying these states in terms of quantified autonomic measures are rare. In addition, new technology has made possible the collection of true beat-to-beat tracings in the fetus (Huhn, et al., 2017), an improvement over data typically derived from less precise Doppler measurements (Reinhard, Hatzmann, & Schiermeier, 2008) but which, up to this point has been used primarily for fetal monitoring in clinical settings (Crawford, et al., 2018; Seliger et al., 2017).
This study seeks to extend previous findings assessing sex differences in fetal autonomic function via abdominal fECG in the late term fetus in the context of fetal behavioral sleep state.
Methods
Participants
The sample was comprised of a subset of pregnant women (N=89) recruited from Columbia University Medical Center as part of a larger study that analyzed fetal response to sound. These women met the following inclusion criteria: no gestational diabetes, hypertension, or other related medical conditions, did not smoke, consume alcohol, illicit or prescription drugs during pregnancy. Fifty-seven percent reported no caffeine consumption. Three women in this sample were excluded from the analysis due to having less than 70% heart rate data for the session; 3 subjects were excluded because fetal sex was not determined and 1 subject’s data was excluded as an outlier (more than 3 SDs above the mean in FHRV and mean FHR). The final sample consisted of 82 participants. Mothers’ mean age was 26 years (SD = 6.47). Ninety-two percent identified as Hispanic, 4% as African American, 1% as White, 1% as Asian, and 2% as Other. Fetal heart rate acquisition took place on average, 2.6 weeks prior to delivery (SD = 1.3). Mean gestational age (GA) at testing was 36.6 weeks (SD = 0.84), and ranged from 36–39 weeks. GA was recorded in the electronic medical record at the time of delivery and the mean was 39.31 weeks (SD = 1.07); however, 3 of the 82 fetuses did not have delivery information because they were delivered at a different hospital. The number of female and male fetuses was 38 and 44, respectively.
Procedure
Upon arrival, participants sat in a quiet room and were informed of the study purpose followed by signing the consent document. After answering demographic questions, 4 active electrodes were placed on the mother’s abdomen and connected to the transabdominal fECG Monica AN24 monitor (http://www.monicahealthcare.com/Monica_Healthcare/media/Monica/Products%20Sheets/Monica-AN24-IF24-Fetal-Monitor-Sales-Sheet.pdf, RRID:SCR_016455). The mother was then asked to sit comfortably in a left-oriented, semi-recumbent position. FHR and fetal movement (FM) were collected using a Toitu MT 516 fetal actocardiograph (http://en.toitu.co.jp/mt-516/, RRID:SCR_016438) via a Doppler transducer that was placed on the mother’s abdomen. Mothers were asked to rest with the lights off, wearing sound attenuating earplugs and headphones, while data were being collected during baseline and fetal sound presentation periods, which took approximately 50 minutes. Only baseline data are used for the current study. Studies were performed between 10 a.m. and 3 p.m.
Materials and Data Analysis
During data collection, the actocardiograph was connected to a computer that recorded and saved FHR and FM output at 20 samples per second using a custom-built Datacq analog to digital converter hardware device (http://www.medelex.com). These outputs were then processed in a customized Matlab program (http://www.mathworks.com/products/matlab/, RRID:SCR_001622) which cleaned and interpolated both signals during brief periods of signal artifact loss and made it possible to state code. Specifically, values less than 100bpm and more than 200bpm were considered outliers and were set to missing. Missing data were then replaced using linear interpolation, which was done by detecting heart rate change from one beat to the next. Any change of more than 4bpm was considered dropout and FHR was interpolated to the next value that was within 5bpm of the previous good value. FHR was then low-pass filtered at 3Hz to reduce remaining high frequency noise in the digitized FHR tracing. Finally, the Matlab program generated jpeg images used for fetal state coding (Figure 1). FHR values that were generated from the actocardiograph were used for state coding because they are smoothed and averaged of 2.6 seconds, allowing for more general patterns of fetal behavioral state to be identified. FHR values from the transabdominal fECGs are more frequently sampled (originally collected at 300–900Hz and then the interpolated intervals were smoothed at 4Hz) providing a more precise measurement of beat-to-beat intervals.
Figure 1.
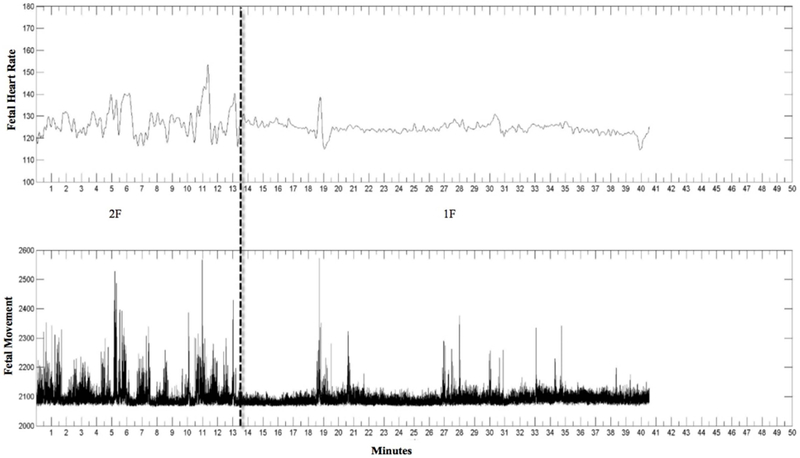
Jpeg image. The top row is FHR and the second row is fetal movement. Dashed line indicates state change. Here the first state is 2F (frequent movements and high HRV) and the second state is 1F (infrequent movements and low HRV).
Fetal states were determined with coding criteria developed by Nijhuis, et al. (1982; 1998). They identified four FHR states. In order to be defined as a state the interval must last at least 3 minutes. The variables that can be used to determine a state include fetal heart rate pattern (FHRP), fetal movement, and eye movement. However, in near term fetuses (>35 weeks GA) states can be identified by using just FHRP in the absence of fetal movement and eye movements (Pillai & James, 1990). Eye movements were not recorded in the present study thus, fetal state coding relied on FHRP and movement. Special efforts were made for state code training to assure inter-rater agreement. State coding criteria were as follows: State 1F is considered quiet sleep. It is a state of quiescence with a stable heart rate pattern and low heart rate variability (HRV). Fetal movement is infrequent, but in some instances there may be an isolated spike in FHR due to a startle detected in fetal movement. The FHR pattern of quiet sleep can vary across fetuses (Visser, Mulder, Stevens and Verweij, 1993). State 2F is considered active sleep. It is a state depicting variability in the heart rate pattern. Fetal movement is frequent and accompanied by accelerations in FHR pattern. State 3F is considered quiet awake. It is a state of quiescence with a stable heart rate pattern and low HRV. Startles are non-existent. State 4F is considered active awake. It is a state where the FHR pattern is highly variable with long-lasting accelerations fused with tachycardia. The process for state coding involved viewing FHR and FM in high resolution images. The tracings were segmented according to changes in FHRP and the respective FM, then a state was assigned using the above coding criteria. The entire length of the recording was state coded. Two coders independently coded 15% of the data for inter-rater reliability. The percentage of agreement was 83%, Cohen’s κ = 0.77. The remaining were coded by the first author.
Although state was coded for the entire length of the recording, only baseline periods were used for the current analyses. Baseline refers to epochs when no sound was played during data collection. The length of the baseline periods varied from fetus to fetus. The first baseline period lasted about 10–15 minutes, then the sound presentation was administered and lasted approximately another 10–15 minutes. After the sound stimulation ended, a second period of baseline was recorded which lasted about 10–20 minutes. Therefore, some recordings had only one state to be analyzed, while longer studies could have multiple states. In Figure 1, baseline periods for this example were from zero to 10 minutes and 20 to 40 minutes. These time periods were converted into seconds and synchronized with the FHR and FHRV data obtained from the fECG monitor.
The FHR data collected by the Monica fECG monitor were sampled at 4 samples per second and exported to text files. A customized program analyzed this data in 30 second intervals. The specific parameters extracted from each 30 second epoch were percentage of good data, number of R waves detected in each epoch, mean of the R-R intervals, standard deviation of the R-R intervals, and the root mean square of the successive differences between R-R intervals (RMSSD). Data that did not meet acceptable beat-to-beat change were excluded from the analysis. Specifically, it was excluded if the change from one R-R interval to the next exceeded ±10%. R-R intervals less than 300ms (greater than 200bpm) or greater than 667ms (less than 90bpm) were also excluded from the analysis. Of the acceptable data, only 30s epochs that had at least 70% of good data were included in the analysis. After applying these criteria, 36% of all possible 30s epochs were excluded from the analysis. There were a total of 110 instances of state that met our good data criteria during the baseline periods; 55 of these came from fetuses with good data in only 1 sleep state, 52 were from 26 fetuses which had good data in two sleep states, and 3 were from 1 fetus with three sleep states.
From the values collected by the fECG monitor, we calculated an estimate of heart rate (HR) with the following formula: ((60 ÷ mean R-R intervals) x 1000). These values were then averaged over periods of the same states to produce state-dependent values for FHR. Measures of FHRV were SD of mean R-R intervals and RMSSD, which were also averaged over periods of the same state per subject.
A one-way MANOVA analyzed the effects of fetal sex on the three dependent variables: FHR, SD, and RMSSD. An independent samples t-test was used to determine if there were differences in FHR between males and females when in 1F. A second t-test was used to determine if there were differences in FHR between males and females when in 2F, and a third test was used for testing differences in FHR between males and females when in 4F. Bonferroni adjustments to alpha were used to account for the multiple (3) tests. Parallel sets of analyses, with Bonferroni adjustments to alpha, were used to test for sex differences in SD and RMSSD in each of the three states. Lastly, a repeated measures MANOVA analyzed the effect of state (1F and 2F) as a within-subjects factor and fetal sex as a between-subjects factor on FHR, SD, and RMSSD. The latter was conducted on a smaller cohort (n=22) that had both states 1F and 2F and tested for effects of state and sex and their potential interaction.
Results
Fetal State Distribution
There were 110 epochs of fetal state, in which 22% were state 1F (9 females and 15 males), 63% state 2F (30 females and 39 males), and 15% state 4F (11 females and 6 males). There were no incidences of an indeterminate state for the periods of time used in the analysis. For repeated measures analyses 27% of the fetuses had data for both states 1F and 2F, 6% had data for 2F and 4F, and 1% had data for 1F, 2F, and 4F.
Gestational Age of Fetus
Pearson correlations did not show a significant relationship between GA and FHR, r = 0.07, p = 0.474; GA and SD, r = 0.08, p = 0.408; or GA and RMSSD, r = 0.03, p = 0.721. An independent samples t-test revealed that GA did not significantly differ by fetal sex, t(80) = −0.07, p = 0.947.
Effects of Fetal Sex and State
A one-way MANOVA compared differences in each dependent variable FHR, SD, and RMSSD by fetal sex. Results revealed a significant main effect for fetal sex, Wilks’ λ = 0.83, F(3, 78) = 5.34, p = 0.002, ηp2 = 0.17, power = 0.92.
Post-hoc univariate analyses revealed a significant main effect of fetal sex on FHR, F(1, 80) = 9.60, p = 0.003, ηp2 = 0.11, power = 0.87, where females had a higher mean FHR (M = 144.4bpm, SD = 8.4msec) than males (M = 138.7bpm, SD = 8.2msec). See Figure 2. There was also a significant main effect of fetal sex on SD, F(1, 80) = 7.40, p = 0.008, ηp2 = 0.09, power = 0.77, where males had a higher mean SD (M = 16.0, SD = ±3.2) than females (M = 14.0, SD = ±3.4). See Figure 2. There was no main effect of fetal sex on RMSSD, F(1, 80) = 2.67, p = 0.106, ηp2 = 0.03, power = 0.37, M = 9.6 (SD = 2.3) for males and M = 8.8 (SD = 2.1) for females.
Figure 2.
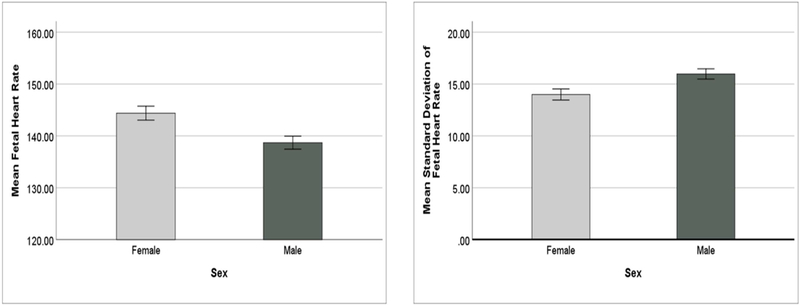
Main effect of Sex on FHR and SD (bars indicate +/− 1 SEM)
Because some fetuses experienced multiple behavioral sleep states, assumptions of independence necessary for statistical comparisons between states cannot be met. Therefore, independent samples t-tests were conducted to determine if there were significant differences by sex while in each behavioral sleep state for each dependent variable. Bonferroni correction was applied for the 3 comparisons (male vs. female in 1F, male vs. female in 2F, and male vs. female in 4F) made on each of the dependent variables (FHR, SD, and RMSSD). The altered p-value is 0.017 (αaltered = 0.05/3 = 0.017). There was a significant difference in FHR between males and females when in 2F, t(67) = 2.79, p = 0.007. Females had a significantly higher mean FHR (M = 142.11bpm, SD = 6.39msec) than males (M = 137.43bpm, SD = 7.29msec). However, there was no significant difference in FHR between males and females when in 1F, t(22) = 1.34, p = 0.194; or in 4F, t(15) = 0.46, p = 0.656. Results showed that there was a significant difference in SD between males and females when in 2F, t(67) = −3.32, p = 0.001. Males had a significantly higher mean SD (M = 15.86, SD = ±2.97) than females (M = 13.48, SD = ±2.92). However, there were no significant differences in SD between males and females when in 1F, t(22) = −1.78, p = 0.089; or in 4F, t(15) = −1.85, p = 0.085. In addition, there were no significant differences in RMSSD between males and females when in 1F, t(22) = −0.91, p = 0.372; 2F, t(67) = −1.33, p = 0.188; or 4F, t(15) = 0.160, p = 0.875. Figure 3 depicts the means for FHR and SD between males and females when in 1F, 2F, and 4F.
Figure 3.
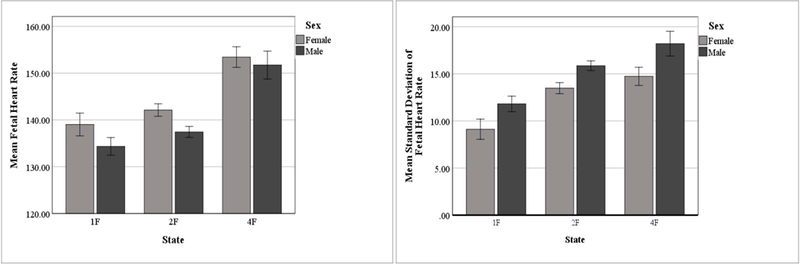
Effect of State and Sex on FHR and SD (bars indicate +/− 1 SEM)
Within-Subjects Analysis
Twenty-two subjects had occurrences of both states 1F and 2F. A repeated measures MANOVA analyzed the effect of state as a within-subjects factor on FHR, SD, and RMSSD. Fetal sex was included as a between-subjects factor. The results revealed a significant main effect of the within-subjects factor, state, Wilks’ λ = 0.44, F(3, 18) = 7.63, p = 0.002, ηp2 = 0.56, power = 0.96. The main effect of the between-subjects factor, fetal sex, and the interaction of state and fetal sex was not significant for this subset.
Univariate analyses for within-subjects revealed a significant main effect of state on SD, F(1, 20) = 24.85, p < 0.001, ηp2 = 0.55, power = 1.00, where the mean SD in 1F was 10.5 (SD = ±3.7), and 13.7 (SD = ±3.1) in 2F. There was a trend for a main effect for state on RMSSD, F(1, 20) = 3.15, p = 0.091, ηp2 = 0.14, power = 0.39, where the mean RMSSD in 1F was 8.8 (SD = ±3.2), and 9.4 (SD = ±2.7) in 2F. There was no significant main effect for state on FHR.
Although the multivariate main effect for fetal sex was not significant, univariate analyses showed that there was a main effect of fetal sex on SD, F(1, 20) = 7.49, p = 0.013, ηp2 = 0.27, power = 0.74, where the estimated marginal mean SD in females was 10.1 (SE = 0.9), and 13.3 (SE = 0.7) in males.
Discussion
Our results are consistent with research conducted by DiPietro et al. (2015) and Bhide & Acharya (2018), where near-term female fetuses show, on average, higher FHR and lower FHRV (SD of FHR) compared to male fetuses. When fetal behavioral sleep states were taken into account, the differences between males and females were observed in 2F. Specifically, when in 2F males had a significantly lower heart rate and higher heart rate variability than females. This pattern was not evident in states 1F or 4F. This follows a similar pattern to the findings of Bernardes et al. (2008) using spectral analysis of cardiotocogram derived FHR. They found that when in FHRP B (active sleep) males had significantly higher FHRV than females. They did not find differences in FHRP A (quiet sleep) and could not do a comparison between sexes in FHRP D (active awake) because none of the female fetuses exhibited FHRP D. Moreover, our results showed that there were sex differences in FHR when in 2F. This was not found in their study; however, they had a smaller sample size than we did in each state.
Our results did not show significant sex differences in RMSSD overall or when examined within each state. With regard to within-subjects analyses, marginal differences were found in RMSSD by state, with lower values for 1F than 2F. However, these findings follow a similar pattern of other research that used overnight recordings and found RMSSD to be lower in 1F when compared to 2F (Stone et al., 2017). Our results suggest that differences in RMSSD as a function of state should be examined as an individual characteristic. The within-subjects results also showed that when fetuses were in 1F the mean SD was significantly lower than when they were in 2F. The between-subjects analysis for the 22 fetuses showed that females had a lower mean SD than males, supporting the results from the entire sample.
FHR significantly decreases and FHRV significantly increases across GA (Amorim-Costa et al., 2016; DiPietro et al., 2004; Lange et al., 2005) indicating that higher FHRV and lower FHR is a more mature pattern. We did not find that GA was correlated with FHR and FHRV, perhaps due to low power and the small range for gestational age (36–39 weeks). Despite this, our results suggest that males exhibit the more mature pattern as they have lower FHR and higher FHRV than females (DiPietro et al., 2015). Similarly, FHRV was reported to be less complex for near-term male fetuses compared to female fetuses, indicating a more mature nervous system that functions more autonomously (Bernardes et al., 2008). The present findings are also convergent with reports showing that females had higher heart rates during labor than males (Dawes, Dawes, Moulden, & Redman, 1999).
Other researchers have found that females display what is interpreted as more mature neural development in studies of fetal learning (Hepper, Dornan, & Lynch, 2012; McCorry & Hepper, 2007). These findings show that female fetuses display a significant change in habituation to stimuli from 31 to 35 weeks, where a larger proportion habituate at 35 weeks than at 31 weeks, a trend not exhibited by male fetuses (McCorry & Hepper, 2007). Measures of habituation may be more closely associated with sensory development than autonomic functioning, however these discrepant findings might indicate that different nervous system functions may mature at different rates between sexes. Finally, some research suggests that female fetuses may show more mature patterns of cardiovascular development early in gestation, with males exhibiting accelerated autonomic maturity near term (Kim, Park, & Hoh, 2016).
Limitations
A limitation of this study is that the sample was predominantly Hispanic, and therefore may not generalize to other ethnicities. Also, we did not control for potential covariation of maternal and fetal heart rate. However, other research indicates that fetal and maternal heart rates are not correlated (Gavin, Kogutt, Fletcher, & Szymanski, 2018). Another potential limitation is lack of fetal weight and growth data; males tend to be heavier at birth than females (de Zegher, Devlieger, & Eeckels, 1999), and as fetuses develop, they show a more mature pattern of autonomic functioning. Therefore, the results regarding effects of sex may be in part moderated by physical growth, for which future research should control. Another limitation may be that mothers reported whether they consumed caffeine, but not if they had consumed caffeine immediately prior to the study, and caffeine intake by mothers is related to greater fetal heart rate reactivity (Buscicchio, Piemontese, Gentilucci, Ferretti, & Tranquilli, 2012).
Our results found that differences in FHR and SD between sexes were only significantly different in 2F, but not 1F or 4F. It may be that there were too few instances of these behavioral sleep states, or that 2F allows for these differences to be more apparent. Also, the within-subjects analysis only found that SD was significantly lower when fetuses were in 1F than when in 2F, and the differences in RMSSD were only marginally significant, however very few subjects entered into both 1F and 2F needed to investigate within subject differences. To address these concerns and to help clarify the present study’s findings, a larger sample size and longer duration of baseline data may be necessary.
Spectral analysis has been widely used to measure FHR and FHRV, but can be vulnerable to missing data (Peters, Vullings, Bergmans, Oei, & Wijn, 2008). We were unable to conduct a spectral analysis because 36% of our data was missing. If we had less missing data, and were able to apply spectral analyses, we would predict similar findings. Spectral analysis conducted in one study comparing FHR and FHRV between male and female fetuses found that female fetuses had lower FHRV than males prior to a cesarean (Bernardes et al., 2008). Moreover, as mentioned earlier, FHRV did differ by sex when in 2F which is consistent with Bernardes et al. (2008). However, in our larger sample we did find sex differences in FHR, but only when in state 2F.
Implications and Future Directions
The ability to detect specific, quantified differences in FHR and FHRV by state and by sex may help to assess the health of a fetus and understand outcomes later in life. For instance, disturbances in fetal behavioral sleep state regulation may foreshadow problems with sleep and mental health later on (Mulder & Visser, 2016). Thus, understanding differences in autonomic function by sex in the context of state in the late fetal period may help researchers and health professionals predict developmental outcomes and aid in the development of early interventions.
Acknowledgements:
The authors would like to thank Albany Perez for recruiting and helping with data collection, Dr. Joseph Isler, for building the Matlab customized program, Tracy Thai for building the fECG monitor customized program, Dr. Joel Yang, David Nugent, and Margaret Shair for helping with data cleaning and quality control, and MaryKate Carrillo for establishing inter-rater reliability with the first author for state coding. We would also like to convey our deepest gratitude to the mothers who participated in this research study. This research was funded by the National Institutes of Health [grant numbers 1P20MD002717, UL1TR000040, U01 HD55155, & U01 HD045935]. This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest:
The authors have no conflict of interest.
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Amorim-Costa C, Costa-Santos C, Ayres-de-Campos D, & Bernardes J (2016). Longitudinal evaluation of computerized cardiotocographic parameters throughout pregnancy in normal fetuses: A prospective cohort study. Acta Obstetricia et Gynecologica Scandinavica, 95(10), 1143–1152. 10.1111/aogs.12932 [DOI] [PubMed] [Google Scholar]
- Arduini D, Rizzo G, Caforio L, Boccolini MR, Romanini C, & Mancuso S (1989). Behavioural state transitions in healthy and growth retarded fetuses. Early Human Development, 19(3), 155–165. 10.1016/0378-3782(89)90076-5 [DOI] [PubMed] [Google Scholar]
- Bernardes J, Gonçalves H, Ayres-de-Campos D, & Rocha AP (2008). Linear and complex heart rate dynamics vary with sex in relation to fetal behavioural states. Early Human Development, 84(7), 433–439. 10.1016/j.earlhumdev.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Bhide A & Acharya G (2018). Sex differences in fetal heart rate and variability assessed by antenatal computerized cardiotocography. Acta Obstet Gynecol Scand, 9(12), 1486–1490. [DOI] [PubMed] [Google Scholar]
- Brändle J, Preissl H, Draganova R, Ortiz E, Kagan KO, Abele H, … Kiefer-Schmidt I (2015). Heart rate variability parameters and fetal movement complement fetal behavioral states detection via magnetography to monitor neurovegetative development. Frontiers in Human Neuroscience, 9(147), 1–8. 10.3389/fnhum.2015.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscicchio G, Piemontese M, Gentilucci L, Ferretti F, & Tranquilli AL (2012). The effects of maternal caffeine and chocolate intake on fetal heart rate. The Journal of Maternal-Fetal & Neonatal Medicine, 25(5), 528–530. doi: 10.3109/14767058.2011.636104 [DOI] [PubMed] [Google Scholar]
- Crawford A, Anyadi P, Stephens L, Thomas SL, Reid H, Higgins LE, Warrander LK, Johnstone ED, & Heazell AEP (2018). A mixed-methods evaluation of continuous electronic fetal monitoring for an extended period. Acta Obstet Gynecol Scand, 97(12), 1515–1523. [DOI] [PubMed] [Google Scholar]
- Dawes NW, Dawes GS, Moulden M, & Redman CWG (1999). Fetal heart rate patterns in term labor vary with sex, gestational age, epidural analgesia, and fetal weight. American Journal of Obstetrics and Gynecology, 180, 181–187. 10.1016/S0002-9378(99)70172-9 [DOI] [PubMed] [Google Scholar]
- de Zegher F, Devlieger H, & Eeckels R (1999). Fetal growth: Boys before girls. Hormone Research, 51(5), 258–259. DOI: 10.1159/000023382 [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Caulfield LE, Costigan KA, Merialdi M, Nguyen RHN, Zavaleta N, & Gurewitsch ED (2004). Fetal neurobehavioral development: A tale of two cities. Developmental Psychology, 40(3), 445–456. 10.1037/0012-1649.40.3.445 [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, & Voegtline KM (2015). Studies in fetal behavior: Revisited, renewed, and reimagined. Monographs of the Society for Research in Child Development, 80(3, Serial No. 318). 10.1111/mono.v80.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, & Voegtline KM (2017). The gestational foundation of sex differences in development and vulnerability. Neuroscience, 342, 4–20. 10.1016/j.neuroscience.2015.07.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Kajantie E, Osmond C, Thornburg K, & Barker DJP (2010). Boys live dangerously in the womb. American Journal of Human Biology, 22(3), 330–335. 10.1002/ajhb.20995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NR, Kogutt BK, Fletcher W, & Szymanski LM (2018). Fetal and maternal responses to yoga in the third trimester. The Journal of Maternal-Fetal & Neonatal Medicine, 1–5. doi: 10.1080/14767058.2018.1555815. [DOI] [PubMed] [Google Scholar]
- Groome LJ, Mooney DM, Holland SB, Smith LA, Atterbury JL, & Dykman RA (1999). Behavioral state affects heart rate response to low-intensity sound in human fetuses. Early Human Development, 54(1), 39–54. 10.1016/S0378-3782(98)00083-8 [DOI] [PubMed] [Google Scholar]
- Hepper PG, Dornan JC, & Lynch C (2012). Sex differences in fetal habituation. Developmental Science, 15(3), 373–383. 10.1111/j.1467-7687.2011.01132.x [DOI] [PubMed] [Google Scholar]
- Huhn EA, Muller MI, Meyer AH, Manegold-Brauer G, Holzgreve W, Hoesli I & Wilhelm FH (2017). Quality predictors of abdominal fetal electrocardiography recording in antenatal ambulatory and bedside settings. Fetal Diagnosis and Therapy, 41(4), 283–292. [DOI] [PubMed] [Google Scholar]
- Kim KN, Park YS & Hoh JK (2016). Sex-related differences in the development of fetal heart rate dynamics. Early Human Development, 93, 47–55. [DOI] [PubMed] [Google Scholar]
- Lange S, Van Leeuwen P, Geue D, Hatzmann W, & Grönemeyer D (2005). Influence of gestational age, heart rate, gender and time of day on fetal heart rate variability. Medical & Biological Engineering & Computing, 43(4), 481–486. 10.1007/BF02344729 [DOI] [PubMed] [Google Scholar]
- McCorry NK, & Hepper PG (2007). Fetal habituation performance: Gestational age and sex effects. British Journal of Developmental Psychology, 25(2), 277–292. 10.1348/026151006X120196 [DOI] [Google Scholar]
- McKenna DS, Ventolini G, Neiger R, & Downing C (2006). Gender-related differences fetal heart rate during first trimester. Fetal Diagnosis and Therapy, 21(1), 144–147. [DOI] [PubMed] [Google Scholar]
- Miller JL, Macedonia C, & Sonies BC (2006). Sex differences in prenatal oral-motor function and development. Developmental Medicine and Child Neurology, 48(6), 465–470. [DOI] [PubMed] [Google Scholar]
- Mulder EJH & Visser GHA (2016). Fetal behavior: Clinical and experimental research in the human. In Reissland N & Kisilevsky BS (Eds.), Fetal development: Research on brain and behavior, environmental influences, and emerging technologies (pp. 87–105). Switzerland: Springer International Publishing. [Google Scholar]
- Nijhuis IJM, & ten Hof J (1999). Development of fetal heart rate and behavior: indirect measures to assess the fetal nervous system. European Journal of Obstetrics & Gynecology and Reproductive Biology, 87(1), 1–2. 10.1016/S0301-2115(99)00143-8 [DOI] [PubMed] [Google Scholar]
- Nijhuis JG, Prechtl HF, Martin CB, & Bots RS (1982). Are there behavioural states in the human fetus? Early Human Development, 6(2), 177–195. [DOI] [PubMed] [Google Scholar]
- Nijhuis JG, Van De Pas M, & Jongsma HW (1998). State transitions in uncomplicated pregnancies after term. Early Human Development, 52(2), 125–132. 10.1016/S0378-3782(98)00016-4 [DOI] [PubMed] [Google Scholar]
- Ogueh O & Steer P (1998). Gender does not affect fetal heart rate variation. British journal of Obstetrics & Gynecology, 105, 1312–1314. [DOI] [PubMed] [Google Scholar]
- Peters C, Vullings R, Bergmans J, Oei G, & Wijn P (2008). The effect of artifact correction on spectral estimates of heart rate variability. Conf Proc IEEE Eng Med Biol Soc, 2669–2672. DOI: 10.1109/IEMBS.2008.4649751 [DOI] [PubMed] [Google Scholar]
- Pillai M, & James D (1990). The development of fetal heart rate patterns during normal pregnancy. Obstetrics & Gynecology, 76, 812–816. [DOI] [PubMed] [Google Scholar]
- Reinhard J, Hatzmann H & Schiermeier S (2008). Foetal electrocardiography (ECG) is an alternative to Doppler ultrasound cardiotocogram (CTG) for antenatal assessment of foetal well-being—preliminary results. Z Geburtshilfe Neonatol, 212(6), 226–9. [DOI] [PubMed] [Google Scholar]
- Salisbury AL, Fallone MD, & Lester B (2005). Neurobehavioral assessment from fetus to infant: The NICU Network Neurobehavioral Scale and the Fetal Neurobehavior Coding Scale. Mental Retardation and Developmental Disabilities Research Reviews, 11, 14–21. 10.1002/mrdd.20058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seliger G, Petroff D, Seeger S, Hoyer D, Tchirikov M & Schneider U (2017). Diurnal variations of short-term variation and the impact of multiple recordings on measurement accuracy. Journal of Perinatology, 37(3), 231–235. [DOI] [PubMed] [Google Scholar]
- Sontag LW, & Richards TW (1938). Studies in fetal behavior: I. Fetal heart rate as a behavioral indicator. Monographs of the Society for Research in Child Development, 3(4, Serial No. 17), 1–67. [Google Scholar]
- Stone PR, Burgess W, McIntyre J, Gunn AJ, Lear CA, Bennet L, … the Maternal Sleep In Pregnancy Research Group The University of Auckland. (2017). An investigation of fetal behavioural states during maternal sleep in healthy late gestation pregnancy: An observational study. Journal of Physiology, 595(24), 7441–7450. 10.1113/JP275084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser GHA, Mulder EJH, Stevens H, & Verweij R (1993). Heart rate variation during fetal behavioural states 1 and 2. Early Human Development, 34(1–2), 21–28. [DOI] [PubMed] [Google Scholar]


