Abstract
PF-06647263, a novel antibody-drug conjugate consisting of an anti-EFNA4 antibody linked to a calicheamicin payload, has shown potent antitumor activity in human xenograft tumor models, including triple-negative breast cancer (TNBC). In the dose-escalation part 1 of this multicenter, open-label, phase I study (NCT02078752), successive cohorts of patients (n, 48) with advanced solid tumors and no available standard therapy received PF-06647263 every 3 weeks (Q3W) or every week (QW), following a modified toxicity probability interval (mTPI) method (initial dosing: 0.015 mg/kg Q3W). Primary objective in part 1 was to estimate the maximum tolerated dose (MTD) and select the recommended phase 2 dose (RP2D). In part 2 (dose-expansion cohort), 12 patients with pretreated, metastatic TNBC received PF-06647263 at the RP2D to further evaluate tumor response and overall safety. PF-06647263 QW administration (n, 23) was better tolerated than the Q3W regimen (n, 25) with only 1 DLT reported (thrombocytopenia). The most common AEs with the QW regimen (fatigue, nausea, vomiting, mucosal inflammation, thrombocytopenia, and diarrhea) were mostly mild to moderate in severity. The MTD was not estimated. PF-06647263 exposures increased in a dose-related manner across the doses evaluated. The RP2D was determined to be 0.015 mg/kg QW. Six (10%) patients achieved a confirmed partial response and 22 (36.7%) patients had stable disease. No correlations were observed between tumor responses and EFNA4 expression levels. Study findings showed manageable safety and favorable PK for PF-06647263 administered QW at the RP2D, with preliminary evidence of limited antitumor activity in patients with TNBC and ovarian cancer.
Keywords: EFNA4, PF-06647263, ADC, calicheamicin, solid tumors
Introduction
Ephrin receptors (Eph) are tyrosine kinase receptors that modulate signaling pathways involved in embryogenesis and adult tissue homeostasis. Overexpression of Eph receptors and ephrin ligands can lead to tumorigenesis and metastasis in various types of cancer.1–4 Expression levels of ephrin-A4 ligand (EFNA4) and other ephrin/Eph family members have been found elevated in tumor samples from patients with breast cancer, including triple-negative breast cancer (TNBC), ovarian, colorectal, and non-small-cell lung cancer, when compared to the corresponding normal tissues.5–12 In addition, EFNA4 is expressed in aggressive tumor cell populations, such as tumor-initiating cells, as identified by analysis of gene expression profiles.8 Although these findings have suggested ephrin ligands and their receptors as potential therapeutic targets, excessive toxicity may be associated with pan-ephrin inhibition. Conversely, the efficacy of selectively targeted inhibitors may be limited by the existence of functional redundancy in this complex receptor/ligand system. Thus, we have opted to target EFNA4 with a novel antibody-drug conjugate (ADC) which would exert antitumor activity by selective binding and intracellular delivery of a potent anticancer agent.8,12,13
PF-06647263 is an anti-EFNA4 ADC comprised of the humanized immunoglobulin G1 (IgG1) antibody huE22 and the AcButDMH-N-Ac-calicheamicin-γ1 linker-payload.8 Calicheamicin, hydrolytically released at the lower pH of the lysosomal compartment, generates double-strand DNA breaks and therefore can affect both rapidly proliferating and quiescent or slowly proliferating tumor cells. Preclinical studies have demonstrated that PF-06647263 binds specifically to EFNA4-expressing cells and subsequently induces DNA cleavage and apoptosis/cell death, consistent with the known mechanism of action of calicheamicin.14,15 Evaluation of PF-06647263 in patient-derived xenograft tumor models demonstrated potent antitumor activity against breast and ovarian cancers, with sustained tumor regression and a reduction in the frequency of tumor-initiating cells.8
In view of these promising preclinical findings and the urgent unmet need of more effective treatment strategies for patients with advanced TNBC and other solid tumors,16–19 we conducted this first-in-human, phase I study to investigate the safety profile, tolerability, pharmacokinetics (PK), and antitumor activity of PF-06647263 in patients with advanced solid malignancies, including an expansion cohort of patients with previously treated, metastatic TNBC.
Patients and Methods
Study design and treatment
This was a multicenter, open-label, multiple-dose, 2-part, non-randomized phase I study conducted in adult patients with advanced solid tumors unresponsive to available therapies or for whom there was no available standard therapy.
In the dose escalation/de-escalation part of the trial (part 1), successive cohorts of 2–4 patients were administered PF-06647263 IV (~60-min infusion on an outpatient basis) every 3 weeks (Q3W) or every week (QW), using a modified toxicity probability interval (mTPI) method.20 The initial dosing regimen of PF-06647263, 0.015 mg/kg administered once Q3W, was selected based on findings from preclinical safety investigations. Evaluation of a QW regimen was to be initiated when the first patient treated with the Q3W regimen experienced a dose-limiting toxicity (DLT) or treatment-related, grade 2 thrombocytopenia. The starting dose of the QW regimen should not have exceeded 1/3 of the highest Q3W dose evaluated. Once initiated, the 2 regimens were evaluated in parallel and independently. In part 2 of the study, patients with previously treated, meta-static TNBC were enrolled in a dose-expansion cohort and treated with PF-06647263 at the identified recommended phase II dose (RP2D).
Patients were to participate in the study for ~6 months in part 1 and up to ~18 months in part 2. Treatment was continued until the patient had clinical benefit or until disease progression, patient refusal, unacceptable toxicity, or study termination.
The primary objective of part 1 was to estimate the maximum tolerated dose (MTD) and select the RP2D based on DLTs occurring in the first treatment cycle; secondary objectives included overall safety; single- and multiple-dose PK of PF-06647263, total anti-EFNA4 antibody, and unconjugated payload; immunogenicity; and preliminary evidence of anti-tumor activity of PF-06647263 based on objective response rate (ORR).
The primary objective for the expansion cohort (part 2) was to confirm safety and tolerability, and assess antitumor activity of PF-06647263 based on ORR at the RP2D. Secondary objectives included further evaluation of overall safety; single- and multiple-dose PK of PF-06647263, total antibody, and unconjugated payload; and immunogenicity of PF-06647263. Analyses of potential correlations between EFNA4 expression levels and response were included as an exploratory study objective.
The study was approved by the institutional review board or independent ethics committee of the participating institutions and it followed the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent. The study was sponsored by Pfizer and registered at ClinicalTrials.gov (NCT02078752).
Patients
Patients were eligible for part 1 of the study if they had a histological or cytological diagnosis of advanced solid tumors resistant to standard therapy or for which no standard therapy was available. Patients were enrolled in the expansion cohort (part 2) if they had previously treated TNBC,21 and at least 1 measurable lesion by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. In addition, across all the study, patients had to be ≥18 years of age; have Eastern Cooperative Oncology Group performance score (ECOG PS) 0 or 1; and have adequate bone marrow, renal, and liver functions.
Patients were not eligible if they had known, unstable, symptomatic brain metastases requiring steroid therapy; had received major surgery, radiation therapy or systemic anticancer therapy within 4 weeks; or hormonal, biological or investigational agents within 2 weeks (or within 5 times the half-life of the agent) of starting study treatment; previous high-dose chemotherapy requiring stem cell rescue or bone marrow transplant; prior irradiation to >25% of the bone marrow; or >4 prior systemic chemotherapy-containing regimens (part 2 only). Patients were also excluded if they had experienced prior significant allergic reactions to recombinant human or murine proteins; had evidence or history of veno-occlusive disease (VOD) or sinusoidal obstruction syndrome (SOS); had clinically significant cardiac or chronic liver disease; or had an active and clinically significant bacterial, fungal, or viral infection.
Study assessments
Safety.
Safety evaluations included physical examinations, vital signs, laboratory test results, 12-lead electrocardiograms, and monitoring of adverse events (AEs). AEs were characterized by type, incidence, seriousness, and relationship to study drug and graded for severity according to National Cancer Institute (NCI) common terminology criteria for adverse events (CTCAE) version 4.03. AEs were collected for 28 days after the last treatment administration or until all drug-related toxicities had resolved.
Any of the following AEs occurring in the first treatment cycle and considered not related to disease progression was classified as a DLT. A) hematologic AEs: grade 4 neutropenia lasting >7 days, febrile neutropenia, grade ≥ 3 neutropenia with infection, any grade thrombocytopenia associated with clinically significant or life-threatening bleeding, grade 4 thrombocytopenia ≥72 h or platelets ≤10,000/mm3 regardless of duration; b) nonhematologic: a bilirubin increase ≥2 × upper limit of normal (ULN) not related to disease progression or other known cause; all other, maximally treated, grade ≥ 3 AEs (e.g., nausea, vomiting, diarrhea); or a delay >2 weeks in receiving the next scheduled cycle due to persisting toxicities not attributable to disease progression.
Pharmacokinetics and immunogenicity.
Blood samples were collected at protocol-specified time points for the measurement of PF-06647263 (conjugated payload), PF-06523432 (total anti-EFNA4 antibody), and CL-184538 (unconjugated payload). Serum concentrations of each compound were quantified using validated bioanalytical assays. The lower limit of quantification (LLOQ) was 0.100 ng/mL for the hybrid liquid chromatography-tandem mass spectrometry (LC-MS/ MS) PF-06647263 assay, 10.0 ng/mL for the electrochemiluminescent assay (ECLA) for PF-06523432, and 0.050 ng/mL for the LC-MS/MS unconjugated payload assay. PK parameters for PF-06647263 (ADC) and PF-06523432 (total antibody) were calculated for each patient and each treatment using noncompartmental analysis of concentration-time data.
Blood samples were collected at baseline and at protocol-defined time points during treatment for the analysis of serum antidrug antibodies (ADAs) using an ECLA. Samples positive for ADAs were also analyzed for neutralizing antibodies (Nabs) using a competitive enzyme-linked immunosorbent assay (ELISA).
Antitumor activity.
Tumor assessments were performed at baseline and every 6 weeks until disease progression, death, or permanent discontinuation of study treatment, by computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis. Assessments included brain scans for patients with known/suspected brain metastases and bone scans or X-rays for patients with known/suspected bone metastases. Objective tumor responses were determined using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 and considered confirmed if they persisted on repeat imaging ≥4 weeks after initial documentation of response.
Biomarker analysis.
Available archival (formalin-fixed, paraffin-embedded tumor samples) or de novo tumor tissue samples were analyzed retrospectively for EFNA4 expression using a NanoString® assay, in a Clinical Laboratory Improvement Amendments (CLIA) certified laboratory at Covance Genomics Laboratory (Redmond, WA).
Sample size and statistical analyses
A maximum of ~70 patients were expected to be enrolled in part 1 of the study. The total number of patients enrolled depended upon tolerability of PF-06647263 and the number of dose levels required to identify the MTD in the Q3W and QW dosing regimens. Dose escalation in part 1 was to be stopped if the maximum sample size had been reached, at least 9 patients had been treated at a dose predicted to be the MTD, or all doses explored appeared to be overly toxic and the MTD could not be determined. Approximately 24 patients were to be enrolled in the expansion cohort in part 2.
A mTPI method targeting a DLT rate of 25% with an equivalence interval of 20–30% was used to estimate the MTD.20 Patients were enrolled in cohorts of 2–4, starting at 0.015 mg/kg. Subsequent dose levels included a maximum 100% escalation until the dose was ≥0.060 mg/kg, a patient experienced a DLT, or a patient developed treatment-related grade 2 thrombocytopenia. Dose escalation in subsequent cohorts followed a modified Fibonacci scheme with maximum dose increases of 67% in the first cohort of the scheme, 50% in the following cohort, and then 33% in the rest of the cohorts. Intra-patient dose escalation was not permitted. If the DLT rate exceeded 33% in the patients treated at the RP2D in part 2, enrollment was to be interrupted to determine whether the MTD and/or RP2D needed to be reassessed.
Results
Patients
A total of 60 patients were enrolled in the study and treated with PF-06647263: 48 patients in the dose-escalation groups (part 1) and 12 patients in the expansion cohort (part 2) (Table 1 and Supporting Information Table S1). In part 1, 25 patients received PF-06647263 Q3W at doses ranging from 0.015 mg/kg to 0.134 mg/kg and 23 patients received PF-06647263 QW at doses ranging from 0.01 mg/kg to 0.02 mg/kg (Table 2). In the expansion cohort, 12 patients received PF-06647263 at the RP2D of 0.015 mg/kg QW.
Table 1.
Patient demographics and baseline characteristics
| PF-06647263 |
PF-06647263 |
|
|---|---|---|
| Dose Escalation N = 48 |
Expansion Cohort 0.015 mg/kg QW N = 12 |
|
| Female: Male, n (%) | 42 (87.5): 6 (12.5) | 12 (100): 0 |
| Mean age, yrs (range) | 59.9 (35–82) | 55.8 (27–73) |
| ≥65 yrs, n (%) | 15 (31.3) | 3 (25.0) |
| Race, n (%) | ||
| White | 38 (79.2) | 10 (83.3) |
| Black | 6 (12.5) | 0 |
| Asian | 1 (2.1) | 2 (16.7) |
| Other | 2 (4.2) | 0 |
| Unspecified | 1 (2.1) | 0 |
| ECOG PS, n (%) | ||
| 0 | 16 (33.3) | 4 (33.3) |
| 1 | 32 (66.7) | 8 (66.7) |
| Primary tumor diagnosis, n (%) | ||
| Ovarian cancer | 16 (33.3) | 0 |
| TNBC cancer | 8 (16.7) | 12 (100) |
| Breast cancer (non TNBC) | 4 (8.3) | 0 |
| Malignant peritoneal neoplasm | 2 (4.2) | 0 |
| Other1 | 18 (37.5) | 0 |
| Prior systemic anticancer therapy2 | ||
| Yes, n (%) | 48 (100) | 12 (100) |
| 1 | 3 (6.3) | 0 |
| 2 | 10 (20.8) | 1 (8.3) |
| 3 | 10 (20.8) | 2 (16.7) |
| 25 (52.1) | 9 (75.0) | |
One patient each had adenocarcinoma of the cervix, transitional-cell carcinoma of the bladder, cancer of the appendix, carcinoma of the cervix, colon cancer, endometrial cancer, invasive ductal breast carcinoma, pancreatic carcinoma, malignant melanoma, medullary thyroid cancer, papillary serous endometrial carcinoma, rectal cancer, renal cancer, sarcoma, squamous-cell lung carcinoma, uterine cancer, malignant neoplasm of the lung, and malignant neoplasm of unknown origin.
Among the 20 patients with TNBC (part 1 and 2), all had received ≥1 line of prior taxane therapy in the adjuvant/neoadjuvant and/or metastatic setting and 80% had received prior anthracycline therapy, mostly in the adjuvant/neoadjuvant setting. Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; TNBC, triple-negative breast cancer.
Table 2.
Dose-limiting toxicities by schedule and dose level
| PF-06647263 Dose level mg/kg |
Treated patients n |
Patients with DLTs n |
DLTs |
|---|---|---|---|
| Q3W schedule | |||
| 0.015 | 2 | 0 | |
| 0.03 | 3 | 0 | |
| 0.05 | 3 | 0 | |
| 0.075 | 3 | 0 | |
| 0.1 | 3 | 0 | |
| 0.134 | 2 | 2 | - Grade 2 epistaxis and grade 3 increased AST - Grade 3 stomatitis |
| 0.1 | 3 | 2 | - Grade 3 thrombocytopenia - Grade 3 thrombocytopenia |
| 0.05 | 6 | 2 | - Grade 4 thrombocytopenia - Grade 4 thrombocytopenia |
| QW schedule | |||
| 0.01 | 3 | 0 | |
| 0.02 | 7 | 1 | - Grade 2 and 3 thrombocytopenia |
| 0.015 | 13 | 1 | - Death of unknown cause |
Abbreviations: AST, aspartate aminotransferase; DLT, dose-limiting toxicity; Q3W, every 3 weeks; QW, every week.
In part 1, the majority of patients were women (87.5%), with a mean age of 59.9 (range, 35–82) years and ECOG PS 1 (66.7%) (Table 1). Sixteen (33.3%) patients had a primary diagnosis of ovarian cancer, 8 (16.7%) of TNBC, 4 (8.3%) of non-TNBC breast cancer, and 2 (4.2%) of malignant peritoneal neoplasm. The other 18 (37.5%) patients had each a different tumor type as listed in Table 1 (footnote). All patients in the part 2 expansion cohort were women with TNBC cancer, with a mean age of 55.8 (range 27–73) years; 8 (66.7%) patients in this group had ECOG PS 1. In both part 1 and 2, the majority of patients had received ≥3 lines of prior systemic anticancer therapy (72.9% in part 1 and 91.7% in the TNBC expansion cohort). All patients with TNBC (part 1 and 2) had received ≥1 line of prior taxane therapy in the adjuvant, neoadjuvant, and/or metastatic setting.
Following a protocol amendment leading to an adjustment in patient sample size, patients with ovarian cancer were not enrolled in the expansion cohort as initially planned. The study was closed to enrollment by the sponsor in Nov 2016, for reasons not related to safety.
DLT and safety
In dose escalation, none of the patients receiving PF-06647263 Q3W up to 0.1 mg/kg experienced DLT (Table 2). Two patients in the 0.134 mg/kg Q3W group developed DLTs: grade 2 epistaxis and grade 3 increased aspartate aminotransferase (n =1) and grade 3 stomatitis (n = 1). The Q3W doses of 0.1 and 0.05 mg/kg were then re-evaluated in dose de-escalation; 2 patients experienced grade 3 thrombocytopenia at 0.1 mg/kg Q3W and 2 patients experienced grade 4 thrombocytopenia at 0.05 mg/kg Q3W. Once-weekly treatment with PF-06647263 was initiated at 0.01 mg/kg with no DLT observed in 3 patients. One of the 7 patients treated at 0.02 mg/kg QW experienced DLT (grade 2 and grade 3 thrombocytopenia) and 1 patient in the 0.015 mg/kg QW group died of unknown cause. None of the other 12 patients treated with PF-06647263 0.015 mg/kg QW experienced DLT. The MTD was not determined for either the Q3W or QW treatment regimen.
All 60 treated patients were evaluable for safety. All patients experienced at least 1 treatment-emergent all-causality AE and 53 (88.3%) had a treatment-related AE of any grade. Thirty-two (53.3%) patients developed an all-causality grade 3 or 4 AE across all treatment groups (Supporting Information Table S2). The main cause of death in both part 1 and part 2 (including follow-up >28 days after last treatment dose) was disease under study (4 of 5 deaths in part 1 and 3 of 4 deaths in part 2). In both other cases, the cause of death was unknown.
In patients treated with PF-06647263 Q3W (n = 25), the most frequently reported treatment-related AEs were fatigue (64.0%), nausea (64.0%), decreased appetite (48.0%), thrombocytopenia (44.0%), dysgeusia (36.0%), diarrhea (28.0%), mucosal inflammation (28.0%), skin hyperpigmentation (28.0%), stomatitis (28.0%), vomiting (28.0%), and rash (24.0%) (Table 3). Eight (32.0%) patients experienced grade 3 AEs and 4 (16.0%) patients experienced grade 4 AEs, including 5 (20%) patients with grade 3 (8%) or grade 4 (12%) thrombocytopenia. Two (8%) patients had a treatment-related serious AE (SAE) of grade 3 hyperbilirubinemia (0.134 mg/kg Q3W and 0.075 mg/kg Q3W) and 1 (4.0%) patient of grade 3 vomiting (0.134 mg/kg Q3W) (Supporting Information Table S3). None of the patients in the Q3W groups died of a treatment-related AE.
Table 3.
Treatment-related adverse events reported in >3 patients treated with PF-06647263 Q3W1
| Adverse event n (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total N = 25 |
|---|---|---|---|---|---|
| Any AE | 1 (4.0) | 11 (44.0) | 8 (32.0) | 4 (16.0)2 | 24 (96.0) |
| Nausea | 12 (48.0) | 3 (12.0) | 1 (4.0) | 0 (0.0) | 16 (64.0) |
| Fatigue | 4 (16.0) | 11 (44.0) | 1 (4.0) | 0 (0.0) | 16 (64.0) |
| Decreased appetite | 5 (20.0) | 7 (28.0) | 0 (0.0) | 0 (0.0) | 12 (48.0) |
| Thrombocytopenia | 2 (8.0) | 4 (16.0) | 2 (8.0) | 3 (12.0) | 11 (44.0) |
| Dysgeusia | 7 (28.0) | 2 (8.0) | 0 (0.0) | 0 (0.0) | 9 (36.0) |
| Diarrhea | 6 (24.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) | 7 (28.0) |
| Mucosal inflammation | 1 (4.0) | 4 (16.0) | 2 (8.0) | 0 (0.0) | 7 (28.0) |
| Skin hyperpigmentation | 6 (24.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 7 (28.0) |
| Stomatitis | 3 (12.0) | 2 (8.0) | 2 (8.0) | 0 (0.0) | 7 (28.0) |
| Vomiting | 4 (16.0) | 2 (8.0) | 1 (4.0) | 0 (0.0) | 7 (28.0) |
| Rash | 5 (20.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 6 (24.0) |
| Abdominal pain | 1 (4.0) | 4 (16.0) | 0 (0.0) | 0 (0.0) | 5 (20.0) |
| Dry mouth | 4 (16.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 5 (20.0) |
| Nail disorder | 5 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (20.0) |
| Anemia | 1 (4.0) | 2 (8.0) | 1 (4.0) | 0 (0.0) | 4 (16.0) |
| Oral candidiasis | 2 (8.0) | 2 (8.0) | 0 (0.0) | 0 (0.0) | 4 (16.0) |
No grade 5 AEs were reported.
One patient experienced grade 4 neutropenia. AE: adverse event; Q3W: every 3 weeks.
Of the 2 patients with a treatment-related SAE of grade 3 hyperbilirubinemia, 1 patient (a 35-year-old woman with metastatic colorectal cancer) presented with right upper quadrant pain, lower extremity edema, hyperbilirubinemia, and thrombocytopenia −2 months after discontinuing the study (due to disease progression in the lung) and from the last treatment dose (1 dose at 0.134 mg/kg Q3W and 1 dose at 0.1 mg/kg Q3W). A liver biopsy showed pericellular and periportal fibrosis consistent with chronic hepatic vein outflow obstruction, leading to a diagnosis of grade 1 hepatic VOD. The patient had received multiple prior chemotherapy regimens and targeted agents, and had previously undergone extended right liver lobectomy, hepaticojejunostomy, chole-cystectomy, and extrahepatic bile duct resection. The second patient (a 65-year-old woman with primary peritoneal malignancy and response to treatment) experienced grade 3 hyperbilirubinemia ~6 weeks after the last treatment dose (2 cycles at 0.075 mg/kg Q3W and 5 cycles at 0.05 mg/kg Q3W). A liver biopsy showed portal vein pressure venous gradient consistent with portal hypertension and nodular regenerative hyperplasia (idiopathic noncirrhotic portal hypertension). The patient had received multiple prior chemotherapy regimens and had previously undergone cholecystectomy and debulking surgery among other surgical procedures.
In addition, among patients treated Q3W, 1 (4%) patient with endometrial cancer (0.05 mg/kg group) experienced an AE of grade 2 hyperbilirubinemia, still present at the end of the study, with no evidence of congestive hepatic disease (VOD/SOS) at liver biopsy.
The most frequently reported treatment-related AEs in patients treated with the PF-06647263 QW regimens (n = 35) were fatigue (51.4%), nausea (51.4%), vomiting (40.0%), mucosal inflammation (37.1%), thrombocytopenia (31.4%), diarrhea (28.6%), decreased appetite (25.7%), and dysgeusia (22.9%) (Table 4). The majority of these AEs were mild to moderate in severity. One patient with ovarian cancer, receiving PF-06647263 0.015 mg/kg QW, experienced a treatment-related AE of grade 1 (2.9%) and grade 2 (2.9%) hyperbilirubinemia not resolved at study discontinuation due to disease progression. Six (17.1%) and 1 (2.9%) patients developed treatment-related grade 3 and grade 4 AEs, respectively. Grade 3 thrombocytopenia was observed in 2 (5.7%) patients (0.015 mg/kg QW and 0.02 mg/kg QW). The reported grade 4 AE consisted of grade 4 gastritis (0.015 mg/kg QW). One patient had a treatment-related SAE of grade 3 nausea (0.015 mg/kg QW) (Supporting Information Table S3). One patient (a 44-year-old woman with an 8.4-year history of stage IV invasive ductal breast carcinoma, treated at 0.015 mg/kg QW) died of unknown reason 1 week after receiving the first treatment dose. The patient had received multiple lines of prior systemic, anticancer treatment and radiation therapy to the right breast, axilla, and lower outer quadrant. The patient presented to the clinic on day 8 of cycle 1 with grade 1 hypotension, grade 3 dehydration, and renal failure (glomerular filtration rate 35 ml/min). Physical examination demonstrated a necrotic mass invading the left chest wall with foul smelling drainage. The patient expired before blood cultures could be performed. The primary cause of death (potential sepsis from unclear source or pulmonary embolism) in the absence of an autopsy remains unknown and a role of the study drug cannot be excluded.
Table 4.
Treatment-related adverse events reported in >3 patients treated with PF-06647263 QW
| Adverse event n (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total N = 35 |
|---|---|---|---|---|---|---|
| Any AE | 8 (22.9) | 13 (37.1) | 6 (17.1) | 1 (2.9)1 | 1 (2.9) | 29 (82.9) |
| Fatigue | 10 (28.6) | 6 (17.1) | 2 (5.7) | 0 (0.0) | 0 (0.0) | 18 (51.4) |
| Nausea | 10 (28.6) | 7 (20.0) | 1 (2.9) | 0 (0.0) | 0 (0.0) | 18 (51.4) |
| Vomiting | 11 (31.4) | 3 (8.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 14 (40.0) |
| Mucosal inflammation | 10 (28.6) | 2 (5.7) | 1 (2.9) | 0 (0.0) | 0 (0.0) | 13 (37.1) |
| Thrombocytopenia | 6 (17.1) | 3 (8.6) | 2 (5.7) | 0 (0.0) | 0 (0.0) | 11 (31.4) |
| Diarrhea | 8 (22.9) | 2 (5.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (28.6) |
| Decreased appetite | 5 (14.3) | 4 (11.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (25.7) |
| Dysgeusia | 5 (14.3) | 3 (8.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (22.9) |
| Alopecia | 5 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (14.3) |
| Asthenia | 2 (5.7) | 2 (5.7) | 1 (2.9) | 0 (0.0) | 0 (0.0) | 5 (14.3) |
| Dry skin | 4 (11.4) | 1 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (14.3) |
| Anemia | 2 (5.7) | 2 (5.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (11.4) |
| Nail disorder | 4 (11.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (11.4) |
| Pruritus | 4 (11.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (11.4) |
| Pyrexia | 4 (11.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (11.4) |
| Skin discoloration | 3 (8.6) | 1 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (11.4) |
One patient experienced grade 4 gastritis.
Abbreviations: AE, adverse event; QW, every week.
The most frequent reason for permanent treatment discontinuation was disease progression, reported in 28 (46.7%) patients overall. Twelve patients discontinued treatment due to treatment-related AEs: 10 (20.8%) patients in dose-escalation (part 1) and 2 (16.7%) patients in the expansion cohort (part 2) (Supporting Information Table S1). Among these, 6 (17.1%) of 35 patients treated with PF-06647263 0.015 mg/kg QW discontinued due to treatment-related AEs: 4 (17.4%) patients in part 1 (thrombocytopenia, asthenia, and fatigue; all grade 2) and 2 (16.7%) patients in the expansion cohort (grade 2 thrombocytopenia). Treatment was temporarily discontinued due to treatment-related AEs in 18 (37.5%) patients in part 1 and 3 (25.0%) patients in part 2; 10 (20.8%) patients and 1 (8.3%) patient, respectively, had dose reductions; and 8 (16.7%) patients and 1 (8.3%) patient, respectively, had temporary discontinuations plus dose reductions due to treatment-related AEs (Supporting Information Table S2). Median duration of treatment ranged from 0.14 to 36.14 weeks in the dose-escalation groups (part 1) and from 2.14 to 20.29 weeks in the expansion cohort with a median duration of 5.1 weeks (part 2).
Pharmacokinetics and immunogenicity
Serum concentrations of PF-06647263 (measured as conjugated payload) and PF-06523432 (total anti-EFNA4 antibody) generally increased in a dose-related manner over the dose range tested for both the Q3W and QW regimens (Supporting Information Fig. S1, Tables S4, and S5). PF-06647263 exhibited a bi-exponential decline over time with estimated terminal half-lives (t1/2) ranging between 2.6 to 6.9 days for both single and multiple dosing. PF-06523432 (total antibody) t/ values ranged between 1.9 to 14.8 days for both single and multiple dosing. Serum concentrations of unconjugated payload were very low (peak concentrations <0.3 ng/mL at all dose levels) and declined below the LLOQ (0.05 ng/mL) within a few days after dosing.
In the absence of an estimated MTD with either the Q3W or QW regimen, the tolerable regimens expanded in part 1 and 2 were 0.05 mg/kg Q3W and 0.015 mg/kg QW, respectively. At these dose levels, the maximum serum concentration (Cmax) of PF-06647263 was reduced in the QW regimen (12.0 ng/mL in part 1 [n = 13]; 16.9 ng/mL in part 2 [n = 12]) compared to the Q3W regimen (58.3 ng/mL [n = 9]). In part 1, total exposure of PF-06647263 across the first 3-week dosing cycle was similar between the 0.015 mg/kg QW (mean area under the plasma concentration-time curve from time 0 to 504 h after dosing [AUC504] = 2,777 ng·h/mL [n = 13]) and the 0.05 mg/kg Q3W (mean AUC over the dosing interval [AUCτ] = 3,475 ng·h/mL [n = 9]) treatment groups, allowing selection of the 0.015 mg/kg QW regimen as the RP2D without compromising the total exposure. In part 2, total PF-06647263 exposure for the 0.015 mg/kg QW regimen (mean AUC504 = 3,414 ng·h/mL [n = 12]) was similar to that in part 1.
In part 1, 19 (39.6%) of the 48 patients tested positive for ADAs to PF-06647263 (ADC), 3 (6.3%) patients tested positive for ADAs to PF-06523432 (mAb) (evaluated n = 19/48), and 16 (33.3%) patients tested positive for ADAs to the unconjugated payload (evaluated n = 19/48). Sixteen patients tested positive (titer ≥1.3) for Nabs and 3 patients tested negative for Nabs. In the expansion cohort, 8 (66.7%) of the 12 patients tested positive for ADAs to PF-06647263, 1 (8.3%) patient for ADAs to PF-06523432 (evaluated n = 8/12), and 6 (50.0%) patients for ADAs to the unconjugated payload (evaluated n = 8/12). Eight patients tested positive for Nabs.
Of the 27 patients overall who tested positive for ADAs against PF-06647263, only 1 patient was considered to have treatment-emergent ADA, while the other patients were all positive for ADAs at baseline and without treatment-boosted titers. Positive ADA status did not appear to affect PF-06647263 and PF-06523432 PK, based on individual concentration-time profiles by treatment and ADA status.
Antitumor activity
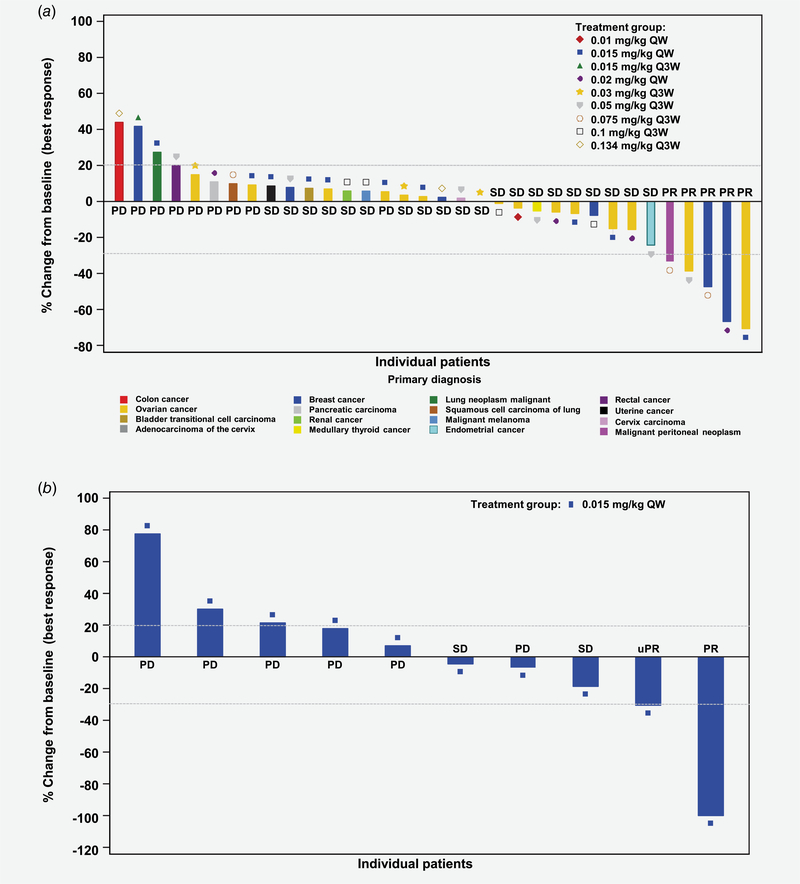
In part 1, PRs were observed as best overall response (BOR) in 5 patients treated with PF-06647263 0.015 mg/kg QW (ovarian cancer), 0.02 mg/kg QW (TNBC), 0.05 mg/kg Q3W (ovarian cancer), and 0.075 mg/kg Q3W (TNBC and malignant peritoneal neoplasm), for an ORR of 10.4% across all dose-escalation groups (Fig. 1). The ORR was 9.1% (95% CI 0.2–41.3) in the 0.015 mg/kg QW group (part 1, n = 11). No patient had a CR. Twenty (41.7%) patients had stable disease as BOR, which lasted ≥126 days in 8 (16.7%) patients (Table 5). In the TNBC expansion cohort (part 2), 1 patient achieved a PR and 1 patient had an unconfirmed PR (uPR) as BOR, for an ORR of 8.3% (95% exact CI, 0.2–38.5). Two (16.7%) patients had stable disease, which lasted ≥126 days in 1 (8.3%) patient. Duration of treatment and treatment responses are shown in Supporting Information Figure S2. Median duration of response was 18.9 (N.E.-N.E.) weeks with the Q3W regimen (n = 3) and 18.6 (13.0–24.1) weeks with the QW regimen (n = 3).
Figure 1.
Maximum percent change in tumor size for target lesions (a) in dose-escalation patients with solid tumors by dose level (n = 34), and (b) in dose-expansion patients with TNBC (n = 10). Only patients with both baseline and postbaseline tumor assessments were included, in this analysis. Dashed lines indicate a 30% decrease and a 20% increase from baseline in the sum of longest diameters for target lesions. PD: progressive disease; PR: partial response; Q3W: every 3 weeks; QW: every week; SD: stable disease; TNBC: triple-negative breast cancer; uPR: unconfirmed PR.
Table 5.
Best overall response by RECIST
| PF-06647263 |
PF-06647263 |
|
|---|---|---|
| Part 1 |
Part 2 |
|
| Best overall response, n (%) | Dose escalation N = 481 |
Expansion cohort 0.015 mg/kg QW N= 12 |
| CR | 0 | 0 |
| PR | 5 (10.4) | 1 (8.3) |
| uPR | 0 | 1 (8.3) |
| SD ≥126 days | 8 (16.7) | 1 (8.3) |
| SD <126 days | 12 (25.0) | 1 (8.3) |
| Disease progression | 8 (16.7) | 6 (50.0) |
| Symptomatic deterioration | 0 | 1 (8.3) |
| Early death | 2 (4.2) | 0 |
| Indeterminate | 6 (12.5) | 1 (8.3) |
| ORR (CR + PR) | 5 (10.4) | 1 (8.3) [95% exact CI 0.2–38.5] |
| CBR rate (CR + PR + uPR + SD ≥ 126 days) | 13 (27.1) | 3 (25.0) [95% exact CI 5.5–57.2] |
Seven (14.6%) patients had no measurable disease at baseline.
Abbreviations: CBR, clinical benefit response rate; CI, confidence interval; CR, complete response; ORR, objective response rate; PR, partial response; QW, weekly; SD, stable disease; uPR, unconfirmed PR.
Biomarker analysis
Although evaluated in a small number of patients in the TNBC expansion cohort, response to treatment with PF-06647263 did not appear to correlate with expression levels of EFNA4. Three patients with high EFNA4 levels in archived tumor samples and 1 patient with low EFNA4 levels in de novo tumor tissue had disease progression as BOR (Supporting Information Fig. S2). No information is available on EFNA4 tumor expression levels for the patients with PR, uPR, or stable disease due to operational difficulties in obtaining samples for analysis of EFNA4 expression.
Discussion
This is the first-in-human study of PF-06647263, a novel ADC consisting of the anti-EFNA4 mAb PF-06523432 conjugated to a calicheamicin payload. Treatment with PF-06647263 was administered in Q3W and QW regimens to patients with TNBC, ovarian cancer, and other advanced solid tumors, who had received multiple lines of prior systemic anticancer therapy.
The QW regimen appeared to have a more favorable safety profile than the Q3W regimen. DLTs were reported in 6 patients receiving PF-06647263 Q3W at dose levels ≥0.05 mg/kg, including grade 3 and 4 thrombocytopenia. With the QW regimen, only 1 patient experienced a DLT of grade 3–4 thrombocytopenia, at the 0.02 mg/kg dose level. Thrombocytopenia was not unexpected as acute thrombocytopenia and liver microvascular injury with platelet sequestration in liver sinusoids have been previously described in cynomolgus monkeys and in patients treated with other antibody-calicheamicin conjugates such as gemtuzumab ozogamicin and inotuzumab ozogamicin.22,23
Hyperbilirubinemia was reported as a treatment-related SAE in patients treated with the Q3W regimen, at the higher dose levels evaluated (n = 2, 0.075 and 0.134 mg/kg). One of these 2 patients with grade 3 SAEs of hyperbilirubinemia was diagnosed with grade 1 hepatic VOD and the other one with nodular regenerative hyperplasia and idiopathic noncirrhotic portal hypertension. Although diagnosis of VOD may be challenging, biopsies from both patients showed patterns that could be explained by postsinusoidal and sinusoidal damage. Multifocal, sinusoidal dilation or hepatocyte atrophy, associated with intervening nodular foci of hepatocyte hypertrophy/regeneration, had been previously observed at doses ≥0.1 mg/kg in safety studies of PF-06647263 conducted in cynomolgus monkeys (data not shown). Treatment with chemo-therapeutic agents (i.e., azathioprine, cyclophosphamide, oxaliplatin, and irinotecan) or ADCs may be associated with the development of VOD/SOS.24 In addition, vascular lesions consistent with hepatic VOD/SOS have been reported in patients with hematologic malignancies treated with other calicheamicin immunoconjugates, targeted to CD22 and CD33.25–28 The relative contribution to the development of these abnormalities by a potential ADC uptake in hepatic sinusoidal cells through man-nose receptors or on-target effects in normal tissues remains to be defined.24,25
The MTD was not determined for PF-06647263 in either the Q3W or QW regimen. Treatment at the RP2D of 0.015 mg/kg QW was generally well tolerated. The most common AEs with this regimen were mostly mild to moderate in severity including fatigue, nausea, vomiting, mucosal inflammation, thrombocytopenia, diarrhea, decreased appetite, and dysgeusia. EFNA4 expression in normal tissues such as skin, esophagus, and colon may contribute to the on-target toxicities observed in these organs.1,2 The development of gastrointestinal AEs in these patients may also reflect toxicities reported with other antibodies conjugated to a calicheamicin payload (a DNA synthesis inhibitor), such as nausea, vomiting, and diarrhea. Neutropenia, previously described with other calicheamicin-conjugated antibody therapies in patients with hematologic malignancies, did not contribute to DLT in this study and it was infrequently observed in this patient population treated with PF-06647263.25 The rate of permanent treatment discontinuation due to treatment-related AEs among patients receiving PF-06647263 at the RP2D in the TNBC expansion cohort was ~17%.
PF-06647263 (ADC) and PF-06523432 (anti-EFNA4 antibody) exposures, based on geometric mean AUCT and Cmax values, appeared to increase in a dose-related manner across the dose range evaluated, for both the QW and Q3W regimens, following single and multiple IV dosing of PF-06647263. PF-06647263 exposure in patients with TNBC dosed at 0.015 mg/kg QW in the expansion cohort was similar to that observed at this dose level in part 1 of the study.
ADAs detected in a proportion of patients were predominantly pre-existing (first observed at baseline) and specific to the calicheamicin payload. None of the patients was positive for treatment-boosted ADAs to PF-06647263 and the ADAs detected did not appear to affect the PK of PF-06647263. No clear relationship was noted between Nabs and tumor response: 4 (66.7%) of the 6 patients achieving a PR as BOR had positive Nabs.
Partial responses and stable disease ≥126 days were observed following treatment with PF-06647263 in patients with advanced TNBC or ovarian cancer and unknown EFNA4 tumor expression, in part 1 of the study. The RP2D for PF-06647263 was determined to be 0.015 mg/kg QW based on the favorable safety profile relative to 0.05 mg/kg Q3W and observed antitumor activity at comparable total cycle exposure. One PR and 1 uPR were achieved by patients with TNBC treated at the RP2D in the expansion cohort. In addition, 1 patient had stable disease for ≥126 days for an overall clinical benefit response rate (CR + PR + uPR + stable disease ≥126 days) of 25% in this group of patients with heavily pretreated TNBC. Although difficulties in obtaining sufficient numbers of tumor samples to conduct EFNA4 expression analysis prevented an unambiguous insight into potential correlations between EFNA4 expression levels and tumor response, response to treatment with PF-06647263 did not appear to correlate with EFNA4 expression levels in the small number of patients analyzed.
Other studies have investigated alternative approaches, aimed at the ephrin type-B receptor 4 (EphB4), by developing biologic or small-molecule inhibitors.29,30 Preclinical results reported with monomeric soluble EphB4 fused to human serum albumin (sEphB4-HSA) indicated antitumor activity in xenografts models of human mesothelioma, with induction of apoptosis in target cancer cells.29 Using an alternative effector strategy, EphB4 was one of the targets recognized by JI-101, an oral multikinase inhibitor that blocks vascular endothelial growth factor receptor type 2 (VEGFR-2), platelet-derived growth factor receptor β (PDGFR-β), and EphB4. Initial phase I findings with JI-101 combined with everolimus in patients with advanced ovarian cancer showed that combination treatment was well tolerated, but associated with limited antitumor activity in this patient population.30 In a recent study, the multitarget receptor tyrosine kinase inhibitor of VEGFR-2, TIE-2, and EphB4 QDAU5 was shown to mediate antiangio-genesis and anticancer activity in vitro and an in vivo MCF-7 breast cancer xenograft model.31
In conclusion, the anti-EFNA4 ADC PF-06647263 evaluated in this study has shown manageable safety and a favorable PK profile at the RP2D administered once weekly. Although preliminary evidence of antitumor activity was observed in patients with heavily pretreated TNBC and ovarian cancer, study enrollment was terminated due to the limited response to adequate exposure of PF-06647263 in patients with TNBC.
Supplementary Material
What’s new?
The ephrin-A4 (EFNA4) ligand is aberrantly expressed in solid tumors and aggressive tumor-initiating cells, making it a promising target for novel anticancer therapies. Of particular interest is the anti-EFNA4 antibody-drug conjugate, PF-06647263, which previously was shown to induce DNA damage and cell death in EFNA4-expressing xenograft models of breast and ovarian cancer. Here, PF-06647263 was evaluated for the first time in human patients with advanced malignancies. PF-06647263 was relatively safe for patients and had a favorable pharmacokinetic profile. While partial responses and stable disease were observed, patients with advanced triple-negative breast cancer and ovarian cancer showed limited benefit.
Acknowledgements
This study was sponsored by Pfizer. The authors thank the patients and their families/caregivers, and the investigators, research nurses, study coordinators, and operations staff who contributed to this study. Medical writing and editorial support was provided by S. Mariani, MD PhD of Engage Scientific Solutions and was funded by Pfizer.
Grant sponsor: Pfizer
I. Krop has disclosed research funding from Genentech/Roche and Pfizer; and consulting/advisory roles and honoraria from Context Therapeutics, Daiichi/Sankyo, Genentech/Roche, Macrogenics, and Taiho Oncology. H. A. Burris has disclosed employment, a leadership position, and stock/ownership interests of HCA Healthcare/Sarah Cannon Research Institute; institutional research funding from AbbVie, Agios, Astra Zeneca, Bayer, BioAtla, BioMed Valley Discoveries, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celldex, CicloMed, CytomX Therapeutics, Daiichi Sankyo, eFFECTOR Therapeutics, Gilead Sciences, GSK, Harpoon Therapeutics, H3 Biomedicine, Immunocore, Incyte, Janssen, Jiangsu Hengrui Medicine, Jounce Therapeutics, Lilly, Loxo, Macrogenics, MedImmune, Merck, Mersana, Millennium, Moderna Therapeutics, Neon Therapeutics, Novartis, OncoMed, Pfizer, Regeneron, Roche/Genentech, PTC Therapeutics, Revolution Medicines, Sanofi, Seattle Genetics, Tarveda Therapeutics, Takeda, Tesaro, TG Therapeutics, Valent Technologies, Verastem, and Vertex; consulting/advisory roles for Astra Zeneca, Bristol-Myers Squibb, Forma Therapeutics, Janssen, MedImmune, Mersana, Novartis, Roche/Genentech, and TG Therapeutics; and expert testimony for Novartis. E. Hamilton has disclosed consulting/advisory roles for Pfizer, Genentech/Roche, Flatiron Health, Lilly, Puma Biotechnology, Daiichi Sankyo, Mersana, Boehringer Ingelheim, Cascadian Therapeutics, and Eisai; institutional research funding from Astra Zeneca, Hutchinson MediPharma, OncoMed, MedImmune, Stem CentRx, Genentech/Roche, Curis, Verastem, Zymeworks, Syndax, Lycera, Rgenix, Novartis, Mersana, Millennium, TapImmune Inc, Cascadian Therapeutics, Lilly, BerGenBio, Medivation, Pfizer, Tesaro, Kadmon, Boehringer Ingelheim, Eisai, H3 Biomedicine, Radius Health, Acerta Pharma, Takeda, Macrogenics, Abbvie, Immunomedics, Fujifilm, eFFECTOR Therapeutics, Mallinckrodt, Merus, Nucana, TetraLogic Pharmaceuticals, PharmaMar, Regeneron, Leap Therapeutics, Taiho Pharmaceutical, EMD Serono, Daiichi Sankyo, ArQule, Syros Pharmaceuticals, Clovis Oncology, CytomX Therapeutics, InventisBio, Oncothyreon; and travel/accomodations expense reimbursement from Amgen, Astra Zeneca, Bayer, Bristol-Myers Squibb, Clovis Oncology, Eisai, EMD Serono, Lilly, Genentech/Roche, Genzyme, Helsinn Therapeutics, HERON, Lexicon, Lilly, Medivation, Merck, Novartis, Pfizer, Roche, Sysmex, Tesaro, Guardant Health, and Foundation Medicine. F. Braiteh has disclosed advisory board/consulting honoraria from Amgen, Astra Zeneca/ MedImmune, Boehringer Ingelheim, Clovis, Eisai, Lilly, Exelixis, Gilead, Incyte, Ipsen, Lexicon, Loxo Oncology, Merck, Pfizer, Roche/Genentech, Taiho, and Takeda; speaker honoraria from Amgen, Astra Zeneca/MedImmune, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Clovis, Lilly, Exelixis, Incyte, Ipsen, Loxo Oncology, Merck, Pfizer, Puma Biotechnology, Roche/Genentech, Taiho, and Takeda; and travel expense reimbursement from Amgen, Astra Zeneca/MedImmune, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Pfizer, Lilly, Exelixis, Incyte, Ipsen, Loxo Oncology, Merck, and Roche/Genentech. A. M. Weise has disclosed consulting and speaker honoraria from Array BioPharma. M. Abu-Khalaf has disclosed advisory board/consulting honoraria from Agedia, Astra Zeneca, Biothera, Immunomedics, Pfizer, and Puma Biotechnology. T. L. Werner has disclosed no relevant conflicts of interest. S. Pirie-Shepherd, C. J. Zopf, M. Lakshminarayanan, J. S. Holland, and R. Baffa were employees of Pfizer during the conduct of this study. J. S. Holland has disclosed holding stock from Pfizer. D. S. Hong has disclosed consulting/advisory roles for Alpha Insights, Axiom, Adaptimmune, Baxter, Bayer, Genentech, GLG, Group H, Guidepoint Global, Infinity, Janssen, Merrimack, Medscape, Numab, Pfizer, Seattle Genetics, Takeda, and Trieza Therapeutics; research funding from AbbVie, Adaptimmune, Amgen, Astra Zeneca, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Fate Therapeutics, Genentech, Genmab, Ignyta, Infinity, Kite, Kyowa, Lilly, LOXO, Merck, MedImmune, Mirati, MiRNA, Molecular Templates, Mologen, NCI-CTEP, Novartis, Pfizer, Seattle Genetics, and Takeda; travel/accomodations expense reimbursement from LOXO and MiRNA; and other ownership interests in Molecular Match (advisor), OncoResponse (founder), and Presagia Inc. (advisor).
Abbreviations:
- ADA
antidrug antibody
- ADC
antibody–drug conjugate
- AE
adverse event
- AUC504
area under the plasma concentration–time curve from time 0 to 504 h after dosing
- AUCτ
area under the plasma concentration–time curve over the dosing interval
- BOR
best overall response
- CBR
clinical benefit response rate
- CI
confidence interval
- Cmax
maximum serum concentration
- CR
complete response
- DLT
dose-limiting toxicity
- ECOG PS
Eastern Cooperative Oncology Group performance score
- EFNA4
ephrin-A4 ligand
- Eph
ephrin
- LC–MS/MS
liquid chromatography–tandem mass spectrometry
- LLOQ
lower limit of quantification
- MTD
maximum tolerated dose
- mTPI
modified toxicity probability interval
- N.E.
not estimable
- Nab
neutralizing antibody
- ORR
objective response rate
- PD
progressive disease
- PK
pharmacokinetics
- PR
partial response
- Q3W
every 3 weeks
- QW
every week
- RECIST
Response Evaluation Criteria in Solid Tumors
- RP2D
recommended phase II dose
- SAE
serious adverse event
- SD
stable disease
- SOS
sinusoidal obstruction syndrome
- TNBC
triple-negative breast cancer
- ULN
upper limit of normal
- uPR
unconfirmed partial response
- VOD
veno-occlusive disease
Footnotes
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Conflict of interest: This study was sponsored by Pfizer. I. Garrido-Laguna has disclosed no relevant conflicts of interest.
References
- 1.Hafner C, Schmitz G, Meyer S, et al. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem 2004;50:490–9. [DOI] [PubMed] [Google Scholar]
- 2.Perez White BE, Getsios S. Eph receptor and ephrin function in breast, gut, and skin epithelia. Cell Adh Migr 2014;8:327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev 2004;15:419–33. [DOI] [PubMed] [Google Scholar]
- 4.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signaling and beyond. Nat Rev Cancer 2010;10:165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Q, Suo Z, Risberg B, et al. Expression of Ephb2 and Ephb4 in breast carcinoma. Pathol Oncol Res 2004;10:26–33. [DOI] [PubMed] [Google Scholar]
- 6.Kumar SR, Singh J, Xia G, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol 2006;169:279–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brantley-Sieders DM, Jiang A, Sarma K, et al. Eph/ephrin profiling in human breast cancer reveals significant associations between expression level and clinical outcome. PLoS One 2011;6: e24426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damelin M, Bankovich A, Park A, et al. Anti-EFNA4 calicheamicin conjugates effectively target triple-negative breast and ovarian tumor-initiating cells to result in sustained tumor regressions. Clin Cancer Res 2015;21:4165–73. [DOI] [PubMed] [Google Scholar]
- 9.Kaenel P, Mosimann M, Andres AC. The multifaceted roles of Eph/ephrin signaling in breast cancer. Cell Adh Migr 2012;6:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar SR, Masood R, Spannuth WA, et al. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br J Cancer 2007;96:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne PD, Dasgupta S, Blayney JK, et al. EphA2 expression is a key driver of migration and invasion and a poor prognostic marker in colorectal cancer. Clin Cancer Res 2016;22:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov 2014;13:39–62. [DOI] [PubMed] [Google Scholar]
- 13.Sapra P, Hooper AT, O’Donnell CJ, et al. Investigational antibody drug conjugates for solid tumors. Expert Opin Investig Drugs 2011;20:1131–49. [DOI] [PubMed] [Google Scholar]
- 14.Nicolaou KC, Pitsinos EN, Theodorakis EA, et al. Synthetic calicheamicin mimics with novel initiation mechanisms: DNA cleavage, cytotoxicity and apoptosis. Chem Biol 1994;1:57–66. [DOI] [PubMed] [Google Scholar]
- 15.Prokop A, Wrasidlo W, Lode H, et al. Induction of apoptosis by enediyne antibiotic calicheamicin proceeds through a caspase-mediated mitochondrial amplification loop in an entirely Bax- dependent manner. Oncogene 2003;22:9107–20. [DOI] [PubMed] [Google Scholar]
- 16.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429–34. [DOI] [PubMed] [Google Scholar]
- 17.Lin NU, Vanderplas A, Hughes ME, et al. Clinico-pathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the national comprehensive cancer network. Cancer 2012;118:5463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders CK, Zagar TM, Carey LA. The management of early-stage and metastatic triple-negative breast cancer: a review. Hematol Oncol Clin North Am 2013;27:737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardoso F, Costa A, Senkus E, et al. ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol 2017;28:16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji Y, Wang S-J. Modified toxicity probability interval design: a safer and more reliable method than the 3 + 3 design for practical phase I trials. J Clin Oncol 2013;31:1785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 22.Guffroy M, Falahatpisheh H, Biddle K, et al. Liver microvascular injury and thrombocytopenia of antibody-calicheamicin conjugates in cynomolgus monkeys-mechanism and monitoring. Clin Cancer Res 2017;23:1760–70. [DOI] [PubMed] [Google Scholar]
- 23.Ricart AD. Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res 2011;17: 6417–27. [DOI] [PubMed] [Google Scholar]
- 24.Tewari P, Wallis W, Kebriaei P. Manifestations and management of veno-occlusive disease/sinusoidal obstruction syndrome in the era of contemporary therapies. Clin Adv Hematol Oncol 2017;15:130–9. [PubMed] [Google Scholar]
- 25.Donaghy H Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs 2016;8:659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumeister P, Eibl M, Zinke-Cerwenka W, et al. Hepatic veno-occlusive disease in two patients with relapsed acute myeloid leukemia treated with anti-CD33 calicheamicin (CMA-676) immuno- conjugate. Ann Hematol 2001;80:119–20. [DOI] [PubMed] [Google Scholar]
- 27.Cohen AD, Luger SM, Sickles C, et al. Gemtuzumab ozogamicin (Mylotarg) monotherapy for relapsed AML after hematopoietic stem cell transplant: efficacy and incidence of hepatic veno-occlusive disease. Bone Marrow Transplant 2002; 30:23–8. [DOI] [PubMed] [Google Scholar]
- 28.Kantarjian HM, DeAngelo DJ, Advani AS, et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INOVATE study. Lancet Haematol 2017;4:e387–98. [DOI] [PubMed] [Google Scholar]
- 29.Liu R, Ferguson BD, Zhou Y, et al. EphB4 as a therapeutic target in mesothelioma. BMC Cancer 2013;13:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werner TL, Wade ML, Agarwal N, et al. A pilot study of JI-101, an inhibitor of VEGFR-2, PDGFR-β, and EphB4 receptors, in combination with everolimus and as a single agent in an ovarian cancer expansion cohort. Invest New Drugs 2015;33: 1217–24. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Shan Y, Ji X, et al. Discovery and evaluation of triple inhibitors of VEGFR-2, TIE-2 and EphB4 as anti-angiogenic and anti-cancer agents. Oncotarget 2017;8: 104745–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.