Abstract
Aldehyde dehydrogenase (ALDH) is one of the two enzymes primarily involved in alcohol metabolism. Several variants exist of the gene that produces ALDH. One of these gene variants, which generates a nonfunctional enzyme, is present in Asians but not in Caucasians and African-Americans. People with two copies of the defective gene respond to alcohol consumption with intense flushing and other unpleasant reactions, such as nausea. Consequently, these people consume very little alcohol and are at a much lower risk for alcoholism than people with functional ALDH genes. People with one copy of the defective gene also flush after ingesting alcohol and are at relatively lower risk for alcoholism than people with fully functional genes. In addition, these people have more intense, but not necessarily less pleasant, reactions to alcohol as assessed by both physiological and psychological measures. People with the defective gene variant also respond to alcohol consumption with characteristic changes in brain activity.
Keywords: alcohol flush reaction, Asian, aldehyde dehydrogenase, isoenzyme, gene, genotype, risk factors, brain, AOD use, AOD dependence, ethanol metabolism, racial differences
Alcoholism1 is a complex disorder arising from both biological (i.e., genetic) and sociocultural (i.e., environmental) factors. During the past two decades, studies in humans assessing individual differences in the response to alcohol have increased our understanding of how genetic traits may contribute to a person’s predisposition to alcoholism (Schuckit 1994). Some studies have aimed at identifying factors that might increase the risk for the disorder, whereas others have addressed protective factors. Most studies have focused on people who statistically are at high risk for alcoholism, such as children of alcoholic fathers, although some research has examined people with a statistically lower risk, such as those of Asian heritage. This article presents evidence that both alcohol use and alcoholism among Asians are genetically influenced, reviews studies examining individual differences in the response to alcohol that are associated with the genetic vulnerability for alcoholism, and provides a theoretical perspective of the results.
Alcohol-Induced Flushing Among Asians
People of Asian descent consistently experience lower levels of alcoholism and higher rates of abstinence than other ethnic groups (Helzer et al. 1990; Klatsky et al. 1983). Researchers have offered two explanations for these observations (reviewed in Sue and Nakamura 1984). An “environmental” hypothesis suggests that cultural values among Asians emphasize drinking in moderation. Conversely, the “genetic” theory proposes that Asians experience a different physiological reaction to alcohol. Bridging this dichotomy, Sue and Nakamura (1984) have attributed the low rates of alcohol consumption among Asians to an interaction between cultural and physiological factors.
One physiological factor that may protect Asians from heavy drinking is the alcohol-induced flushing reaction that about one-half of Chinese, Japanese, Koreans, and other Asians experience after drinking a moderate amount of alcohol (Chan 1986). This reaction, although documented in Chinese poetry as early as the first century B.C., first was reported in the scientific literature in 1972 (Wolff 1972). The flushing reaction is characterized by a rapidly increased blood flow to the skin of the face, neck, and chest; other symptoms may include increased heart rate (i.e., tachycardia), decreased blood pressure (i.e., hypotension), headache, nausea, and vomiting (Chan 1986). The manifestations of the flushing response vary widely: Some people intensely experience the full range of symptoms, whereas others have significantly milder reactions after consuming the same alcohol dose.
The Genetic Basis for the Flushing Reaction
The alcohol-induced flushing response is associated with the process by which the body metabolizes alcohol. Two enzymes are primarily involved in alcohol metabolism—alcohol dehydrogenase (ADH), which converts alcohol to acetaldehyde, and aldehyde dehydrogenase (ALDH), which converts acetaldehyde to acetate (figure 1). Researchers hypothesize that an elevated level of acetaldehyde, a highly reactive and potentially toxic by-product, in the blood and tissue causes the flushing reaction (Harada et al. 1981). Two mechanisms could contribute to increased acetaldehyde levels: a higher-than-normal acetaldehyde production by ADH or a slower-than-normal acetaldehyde breakdown by ALDH.
Figure 1.
The pathway of alcohol metabolism. Once in the liver, alcohol is converted into acetaldehyde and the acetaldehyde is converted into acetate. The enzyme alcohol dehydrogenase (ADH) catalyzes the first half of alcohol metabolism, and the enzyme aldehyde dehydrogenase (ALDH) catalyzes the second half. NAD+ is a coenzyme that plays an accessory role in enzyme catalysis.
Different Forms of ADH and ALDH
Both ADH and ALDH exist in different forms (i.e., isoenzymes) in the body. Iso-enzymes are groups of enzymes that perform the same chemical reaction but have a slightly different amino acid composition and different kinetic properties.2 ALDH, for example, has four isoenzymes: three that primarily exist in the cell’s cytoplasm and one—called ALDH2—that is located in the cell’s energy-producing structures, the mitochondria. ALDH2 is responsible for most of the acetaldehyde breakdown in the cell. Thus, alcohol-induced flushing and other symptoms related to alcohol sensitivity have been attributed primarily to an ALDH2 deficiency (Harada et al. 1981).
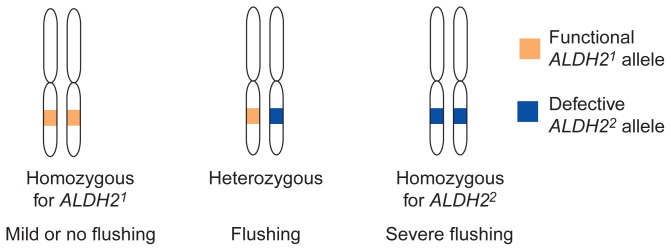
Each ALDH molecule consists of four parts, or subunits, all of which are produced (i.e., encoded) by the same gene. The gene encoding the ALDH2 isoenzyme is called ALDH2. This gene exists in two variants, or alleles. One allele, ALDH21, encodes a functional enzyme subunit; the other allele, ALDH22, contains a small change in the gene and thus encodes a defective subunit that causes ALDH deficiency. Because every person inherits two copies of each gene—one from the mother and one from the father—three different combinations of ALDH2 alleles (i.e., three different ALDH2 genotypes) are possible (figure 2): A person can have two ALDH21 alleles (ALDH21/21 genotype), one ALDH21 allele and one ALDH22 allele (ALDH21/22 genotype), or two ALDH22 alleles (ALDH22/22 genotype). A person with two identical alleles (e.g., ALDH21/21 genotype) is called homozygous for that allele; a person with two different alleles is called heterozygous.
Figure 2.
The three possible genotypes* for the ALDH2 gene and their associated phenotypes. Each person has a pair of chromosomes carrying the ALDH2 gene, one inherited from the mother and one from the father. The two chromosomes can carry either the same allele (homozygous) or two different alleles (heterozygous).
*For a definition of this term and others used in this figure, see central glossary, pp. 182–183.
The ALDH2 genotype determines how well the ALDH2 enzyme functions: An enzyme containing any defective subunits encoded by the ALDH22 allele is less active than an enzyme containing only functional subunits encoded by the ALDH21 allele. Furthermore, an enzyme consisting only of defective subunits (i.e., in a person homozygous for ALDH22) will be less active than an enzyme consisting of both functional and defective subunits (i.e., in a heterozygous person). As a result of the reduced enzyme activity in people with at least one ALDH22 allele, the conversion of acetaldehyde to acetate is slowed, creating excess levels of blood acetaldehyde after alcohol consumption.
Racial Differences in the Frequency of ALDH2 Alleles
Researchers can determine a person’s ALDH2 genotype using DNA extracted from a small blood sample. Such molecular analyses have found that the distribution of ALDH2 alleles varies among different ethnic groups (Goedde et al. 1992): Nearly all people of Caucasian and African-American descent are homozygous for the functional ALDH21 allele. Among Asians, however, only about 50 percent are homozygous for ALDH21, 30 to 40 percent are heterozygous, and 5 to 10 percent are homozygous for the defective ALDH22 allele.
As described previously, these genotypes among Asians predict their response to alcohol. People who have at least one ALDH22 allele experience elevated acetaldehyde levels as well as a readily ob-servable facial flush following alcohol ingestion. Asians homozygous for ALDH21, however, generally have no, or only a mild, flushing response.3
ALDH2 Genotype and Flushing as a Protective Factor for Alcoholism
The physiological characteristics of flushing are similar to the reactions to alcohol in people taking the medication disulfiram (i.e., Antabuse™), which is used to deter alcoholics from drinking. Molecular studies found that disulfiram mimics ALDH2 deficiency by inhibiting the enzyme and thus causing increased levels of acetaldehyde after alcohol consumption (Harada et al. 1982). The similarity between the effects of disulfiram and the flushing reaction suggests that the aversive effects of acetaldehyde may discourage drinking or excessive drinking in Asians and thus serve as a protective factor against alcoholism.
Several studies demonstrating significantly lower levels of alcohol use and alcoholism in Asians with ALDH22 alleles support this idea. For example, people homozygous for the ALDH22 allele drink very little alcohol (Takeshita et al. 1994) and are not found among alcoholics (Thomasson et al. 1991; Chao et al. 1994). Asians who are heterozygous drink significantly less and are much less likely to be alcoholic than Asians homozygous for the functional ALDH21 allele4 (Chao et al. 1994; Takeshita et al. 1994; Thomasson et al. 1991). The heterozygous genotype does not provide full protection from alcoholism, however, because 12 to 19 percent of Chinese and Japanese alcoholics have one ALDH22 allele (Chao et al. 1994; Thomasson et al. 1991).
Thus, a growing body of evidence suggests that possession of at least one ALDH22 allele reduces a person’s alcohol use and predisposition to alcoholism. The findings demonstrate that a genetic factor can influence, but not necessarily predetermine, the development of alcoholism. Each person’s predisposition to alcoholism probably depends on both genetic and environmental factors. Studies of alcoholics who are heterozygous for the ALDH2 gene may allow researchers to learn more about the interactions among genes or between genes and environmental factors that affect the development of alcoholism.
Physiological and Psychological Consequences of ALDH Genotype
Recent studies have investigated in more detail the physiological response to alcohol in Asians having different ALDH2 genotypes (Wall et al. 1992, 1993, 1994; Wall and Ehlers 1995). In these studies, the subjects were male, American-born university students of Chinese, Japanese, and Korean descent whose ALDH2 genotypes were determined from blood samples. Each subject was tested on two occasions after receiving either a placebo or 0.75 milliliters of alcohol per kilogram of body weight (mL/kg, equal to two to three alcoholic drinks). All subjects reacted comparably to the placebo beverage and had similar blood alcohol concentrations (BAC’s) after consuming the alcoholic beverage. Alcohol, however, induced very different physiological reactions in subjects, corresponding to the three different genotypes described earlier. For example, two subjects who were homozygous for the defective ALDH22 allele became ill after drink-ing the alcoholic beverage, experiencing nausea, vomiting, extreme tachycardia, and hypotension. These observations confirm the assumption that people who are homozygous for ALDH22 are most likely to experience severe physiological reactions; their intolerance of alcohol may help to explain why no one with this genotype has been found to be alcoholic.
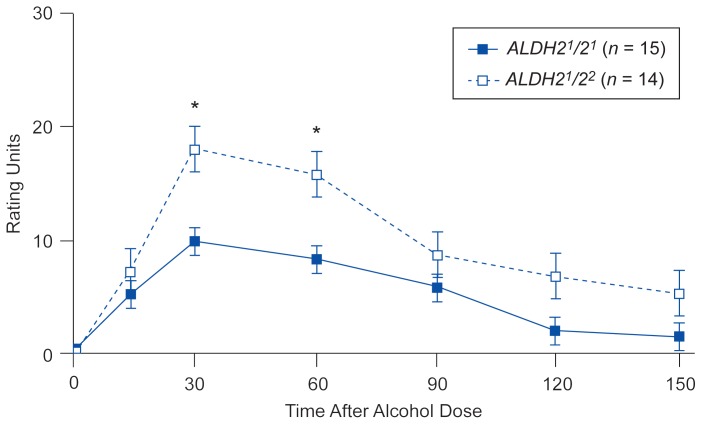
The ALDH2 genotype appears to affect not only physiological reactions but also more subjective responses to alcohol. In the experiment described above, the subjects were asked to assess the effects the alcoholic beverage had on them. The men who were heterozygous for ALDH2 reported more intense, but not necessarily more un-pleasant, reactions to alcohol than the men with two functional ALDH21 alleles, even though they had similar BAC’s and similar recent drinking patterns. For example, heterozygous subjects reported both significantly stronger “effects of alcohol” than subjects homozygous for ALDH21 (figure 3) and more intense attributes of intoxication, such as feeling “high,” “great overall,” and “drunk.” The heterozygous subjects also tended to more strongly experience the negative effects of intoxication, such as feeling “uncomfortable,” “nauseated,” and “terrible overall,” but statistically these results were not significantly different from the responses of the subjects homozygous for ALDH21.
Figure 3.
Subjective self-assessment of the “effects of alcohol” by 15 men homozygous** for ALDH21 (solid line) and 14 men with the ALDH21/ALDH22 genotype (dotted line) at different times after a single dose of alcohol (0.75 ml/kg body weight). The intensity of reported alcohol effects is presented in arbitrary rating units. Error bars indicate standard error of the mean.
*= significant difference between the groups (p < 0.05).
**For further explanation, see figure 2.
These findings contradict the hypothesis that people who are heterozygous for ALDH2 drink less because they experience only aversive alcohol effects. In fact, the results suggest that heterozygous people who regularly consume alcohol may have a more intense positive response to alcohol than those homozygous for ALDH21. Thus, one mechanism that may contribute to the decreased rate of alcohol use and alcoholism among Asians with at least one ALDH22 allele could be heightened sensitivity to relatively low doses of alcohol (Wall et al. 1992). A person with this heightened sensitivity would need to drink less to experience the same response to alcohol as a person with lower sensitivity. Consequently, a person carrying the ALDH22 allele may avoid high intake of alcoholic beverages and thus be less likely to develop alcoholism.
If the ALDH2 genotype affects not only people’s physiological responses but also their subjective feelings of intoxication, possession of an ALDH22 allele also may influence a person’s vulnerability to alcohol at the level of brain functioning. The following sections describe research aimed at understanding how different combinations of ALDH2 alleles may affect the brain’s response to alcohol.
Measuring the Brain’s Response to Alcohol
Electrophysiological techniques provide noninvasive ways to assess various elements of brain function. One of the most common methods, the electroencephalogram (EEG), is performed by attaching electrodes to a person’s scalp and recording the spontaneous electrical brain activity, known as brain waves. Brain waves can be divided into several different groups—slow alpha, fast alpha, beta, delta, and theta—according to their frequency5 (see table 1). Changes in brain waves (e.g., their amplitude6) correlate with changes in the level of consciousness or different psychological states. EEG’s allow researchers to characterize and quantify the brain’s spontaneous electrical activity and provide information about brain responses to external sensory stimuli. These responses also are called event-related potentials (ERP’s).
Table 1.
Typical Effects of Different Alcohol Doses on the Activities of Different EEG Frequency Bands1
| Frequency Band | Alcohol Dose | ||
|---|---|---|---|
|
|
|
||
| Low (< 0.5 g/kg) (less than 2–3 drinks) | Moderate (0.5–1.0 g/kg) (3–6 drinks) | High (> 1.0 g/kg) (more than 3–6 drinks) | |
|
|
|
|
|
| delta (< 4 Hz) | |||
| theta (4.0–7.5 Hz) | increased | increased | |
| slow alpha (7.5–9.0 Hz) | decreased | increased | |
| fast alpha (9.0–12.0 Hz) | decreased | decreased | |
| beta (> 12.0 Hz) | increased | ||
The brain waves measured with an electroencephalogram (EEG) are grouped into several “bands,” each containing waves with a certain frequency range. The frequency indicates how often a wave recurs in a certain time and is measured in hertz (Hz). 1 Hz = 1 cycle per second.
SOURCES: Summarized from Begleiter and Platz 1972; Ehlers et al. 1989; and Kalant and Woo 1981.
To measure ERP’s, the subjects are exposed to stimuli (e.g., sounds or lights) while their EEG is being recorded. When an uncommon stimulus occurs (e.g., a red light in a sequence of green and yellow lights), the brain produces a characteristic pattern of brain waves. One ERP wave that has been studied frequently in alcohol research is called P300 because it can be detected about 300 milliseconds after the uncommon stimulus. Although scientists believe that the P300 wave reflects specific neurocognitive functions (e.g., the brain’s evaluation of the stimulus and the process of selecting a response), they do not know the physiological basis for these functions.
Twin and family studies indicate that many EEG parameters are largely genetically determined (van Beijsterveldt and Boomsma 1994). A smaller number of studies also suggest moderate to high heritability for ERP’s (van Beijsterveldt and Boomsma 1994). Both EEG parameters and ERP’s are sensitive to the effects of acute and chronic alcohol use (Porjesz and Begleiter 1985).
Alcohol’s acute effects on EEG patterns have been studied since the 1930’s. In general, low doses of alcohol that result in mild intoxication and behavioral activation (less than 0.5 gram of alcohol per kilogram of body weight [g/kg], corresponding to fewer than two to three drinks) reduce the amplitudes of slow- and fast-alpha waves and increase the amplitudes of the beta waves (table 1). Moderate alcohol doses (0.5 to 1.0 g/kg) usually result in increased slow-alpha and theta waves and decreased fast-alpha waves. Larger doses of alcohol (more than 1.0 g/kg), which are associated with sedation and drowsiness, produce increases in the amplitude of the theta waves (Begleiter and Platz 1972; Ehlers et al. 1989; Kalant and Woo 1981). The magnitude of these alcohol effects, however, varies significantly among people.
The genetic vulnerability for alcoholism, as inferred from a family history of the disorder, partly determines a person’s brain response to alcohol. For example, children of alcoholics, who are at elevated risk for developing alcoholism, have a different EEG response to alcohol from control subjects without alcoholic relatives (Cohen et al. 1993; Ehlers and Schuckit 1991). Sons of alcoholic fathers in particular may have a less intense EEG response to alcohol, possibly reflecting an innate lower level of sensitivity to alcohol (Cohen et al. 1993; Ehlers and Schuckit 1991).
Not only does alcohol consumption affect the spontaneous brain activity reflected by the EEG patterns, it also alters brain responses to external stimuli (i.e., the ERP’s). Acute alcohol administration reduces the amplitude of P300 and/or increases the time between the stimulus and the appearance of P300 (i.e., the latency), suggesting reduced efficiency in brain processing (Porjesz and Begleiter 1985). The intensity of alcohol’s effect on P300 varies according to the dose ingested (Rohrbaugh et al. 1987). A person’s genetic vulnerability for alcoholism also appears to affect the P300 response. For example, alcohol-induced P300 changes were smaller in men at high risk for developing alcoholism (i.e., men having an alcoholic father) than in men at lower risk (Elmasian et al. 1982; Schuckit et al. 1988).
ALDH2 Genotype and the Electrophysiological Response to Alcohol
Because electrophysiological responses appear to be correlated with a person’s genetic predisposition to alcoholism, researchers have used EEG patterns and ERP’s to evaluate alcohol’s effects on brain functioning in college students of Asian origin with different ALDH2 genotypes (Wall et al. 1993; Wall and Ehlers 1995). The subjects received either a placebo or a moderate dose of 0.75 mL/kg alcohol before their brain waves were recorded. Subjects homozygous for the functional ALDH21 allele showed a typical EEG response, including increased theta and slow-alpha and decreased fast-alpha activity.
In the heterozygous subjects with one ALDH22 allele, however, the slow-alpha activity decreased significantly compared with that of the subjects homozygous for ALDH21. This pattern of alcohol-related EEG changes in the heterozygous subjects is consistent with the increased activation that may be caused by elevated acetaldehyde levels (Wall et al. 1993) and differs from the pattern seen in Caucasian sons of alcoholics (Ehlers and Schuckit 1991). These findings suggest that EEG responses may help to measure the effects of acute intoxication on brain functioning and genetically influenced reactions to alcohol.
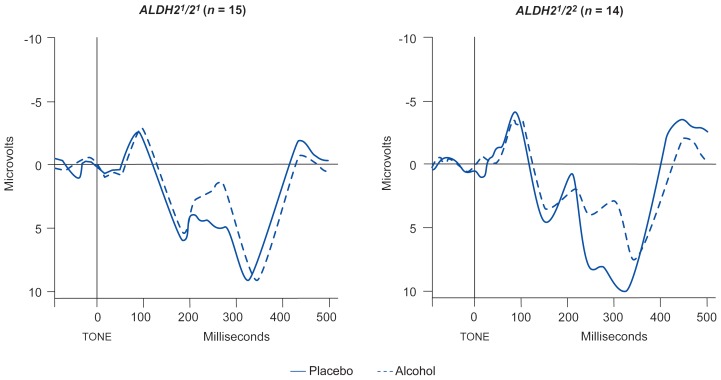
The response to alcohol in the same subjects also was assessed using ERP’s (Wall and Ehlers 1995). The study found that compared with a placebo, alcohol consumption in all subjects significantly decreased the amplitude and increased the latency of the P300 wave. In the heterozygous subjects, however, alcohol’s effects on P300 were significantly greater than in the subjects homozygous for ALDH21, although all subjects had equivalent BAC’s (figure 4). These data indicate that people heterozygous for ALDH2 experience a more intense response to alcohol and that their brain functioning may be more affected by alcohol than that of people homozygous for the ALDH21 allele.
Figure 4.
Mean event-related potentials in 15 subjects homozygous* for ALDH21 and 14 heterozygous* subjects after drinking a placebo beverage (solid line) or a beverage containing 0.75 ml/kg alcohol (dotted line). The subjects had to press a button when they heard a rarely presented tone. In both groups, alcohol delayed the appearance of the P300 wave (the segment occurring between 300 and 450 msec) and decreased P300 amplitude, but the effects were stronger in the heterozygous subjects than in the homozygous subjects.
*For further explanation, see figure 2.
SOURCE: Adapted from Wall and Ehlers 1995.
Theoretical and Research Implications
One model of genetic influences in alcoholism suggests that a person’s genetically determined reaction to alcohol may affect the likelihood of both drinking alcohol and developing alcoholism (Schuckit 1994). This theory predicts that compared with the general population, people at higher risk for developing alcoholism (e.g., children of alcoholic fathers) may respond less intensely to alcohol, as assessed by measures such as subjective feelings of intoxication and electrophysiological parameters. Conversely, people at lower risk for alcoholism (e.g., members of ethnic groups with a low prevalence of the disorder) would experience a heightened response to alcohol.
Studies reviewed in this article that examined the acute responses to alcohol in Asian men support this model. The men fell into three groups with respect to their genetic risk for developing alcoholism: extremely low risk (homozygous for the defective ALDH22 allele), moderately low risk (heterozygous for ALDH2), and relatively high risk (homozygous for the functional ALDH21 allele). The same groups were found to be highly sensitive, intermediately sensitive, and minimally sensitive to the effects of alcohol.
Although genetic factors may contribute to a person’s risk for or protection from alcoholism, their interactions with environmental factors determine their overall vulnerability. For example, as described here, the ALDH2 genotype helps determine the level of alcohol consumption among Asians. The proposed mechanism, however, by which this gene influences the development of alcoholism (i.e., by evoking a more intense response to alcohol in brain functioning) can only manifest itself after alcohol use and may even require a certain level of alcohol consumption. In contrast, environmental factors, such as cultural attitudes that emphasize abstinence or moderate alcohol consumption, can protect against the development of alcoholism in the absence of alcohol use, when genetic factors, such as the ALDH2 gene, likely have no influence.
No other gene has been identified that influences the level of alcohol consumption and risk for alcoholism to the same extent as ALDH2. Therefore, further studies of Asians with known ALDH2 genotypes would provide a unique opportunity to improve our understanding of how the interactions between genetic and environmental factors determine a person’s risk for alcoholism. Researchers have demonstrated, for example, that alcohol use among Asian-Americans varies with the number of generations their families have lived in the United States and their level of acculturation7 (Chu et al. unpublished master’s thesis 1978; Sue et al. 1979). Also, substantial differences in alcohol consumption patterns and the prevalence of alcoholism exist among different ethnic groups (e.g., Chinese, Japanese, and Koreans) (Helzer et al. 1990; Kitano and Chi 1989). These observations and their relationship to differences in the genotypic distribution of ALDH2 alleles among Asian groups warrant further investigation.
Future studies also should assess the interactions between different genes (e.g., between ALDH2 and ADH genotypes) in shaping a person’s drinking behavior. Finally, genetic factors, such as ALDH2 and their functions, have been largely unstudied in Asian women, and it is thus unclear to what extent biological or cultural differences explain gender differences in alcohol use and alcoholism prevalence among Asians. Consequently, more studies that include Asian women are needed.
Analyses of the relationships between alcohol-related genes, such as ALDH2, and behavior can provide insight into the determinants of alcohol-use patterns of people at various levels of genetic risk for alcoholism. These studies will contribute substantially to our knowledge of the causes of alcoholism and may help to improve alcoholism prevention and treatment. A better understanding of how the interactions of other biological and cultural factors with the ALDH2 genotype help determine the risk of Asians for alcoholism also may have important implications for evaluating the predisposition to alcoholism of other, non-Asian populations.
Footnotes
The terms “alcoholism” and “alcoholic” as used in this article are summary terms for the diagnoses of alcohol abuse and alcohol dependence.
For a definition of this and other technical terms used in this article, see central glossary, pp. 182–183.
The basis for this mild flushing response, which also has been reported in some other ethnic groups (e.g., Caucasians and Native Americans), is not well understood but may relate to other environmental or genetic factors, such as mutations in ADH genes.
Some researchers have proposed that genetic variations in alleles of ADH also could increase the rate of acetaldehyde formation, deterring heavy drinking (Takeshita et al. 1994) and contributing to decreased risk in Asians for developing alcoholism (Thomasson et al. 1991; Chao et al. 1994).
The frequency describes how often the wave goes through a complete cycle during a given time period. The frequency usually is measured in hertz (Hz); 1 Hz = 1 cycle per second.
The amplitude describes the height of a wave, from its lowest to its highest point. For electrical waves, the amplitude usually is measured in millivolts.
Acculturation refers to the degree to which immigrants adopt the values, attitudes, and behavior of the culture they enter.
This work has been supported by National Institute on Alcohol Abuse and Alcoholism grants AA00098, AA00155, and AA06420 as well as General Clinical Research Center grant RR00833.
REFERENCES
- Begleiter H, Platz A. The effects of alcohol on the central nervous system in humans. In: Kissin R, Begleiter H, editors. The Biology of Alcoholism: Volume 2. Physiology and Behavior. New York: Plenum Press; 1972. pp. 308–316. [Google Scholar]
- Chan AWK. Racial differences in alcohol sensitivity. Alcohol and Alcoholism. 1986;21:93–104. [PubMed] [Google Scholar]
- Chao Y-C, Liou S-R, Chung Y-Y, Tang H-S, Hsu C-T, Li T-K, Yin S-J. Polymorphism of alcohol and aldehyde dehydrogenase genes and alcoholic cirrhosis in Chinese patients. Hepatology. 1994;19:360–366. [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H. The effects of ethanol on EEG activity in males at risk for alcoholism. Electroencephalography and Clinical Neurophysiology. 1993;86:368–376. doi: 10.1016/0013-4694(93)90132-f. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. Evaluation of EEG alpha activity in sons of alcoholics. Neuropsychopharmacology. 1991;4:199–205. [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Schuckit MA. EEG spectral characteristics following ethanol administration in young men. Electroencephalography and Clinical Neurophysiology. 1989;73:179–187. doi: 10.1016/0013-4694(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Elmasian R, Neville H, Woods D, Schuckit M, Bloom F. Event-related brain potentials are different in individuals at high and low risk for developing alcoholism. Proceedings of the National Academy of Sciences USA. 1982;79:7900–7903. doi: 10.1073/pnas.79.24.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedde HW, Agarwal DP, Fritze G, Meier-Tackmann D, Singh S, Beckmann G, Bhatia K, Chen LZ, Fang B, Lisker R, Paik YK, Rothhammer F, Saha N, Segal B, Srivastava LM, Czeizel A. Distribution of ADH2 and ALDH2 genotypes in different populations. Human Genetics. 1992;88:334–346. doi: 10.1007/BF00197271. [DOI] [PubMed] [Google Scholar]
- Harada S, Agarwal DP, Goedde HW. Aldehyde dehydrogenase deficiency as cause of facial flushing reaction to alcohol in Japanese. Lancet. 1981;ii(8253):982. doi: 10.1016/s0140-6736(81)91172-7. [DOI] [PubMed] [Google Scholar]
- Harada S, Agarwal DP, Goedde HW. Mechanism of alcohol sensitivity and disulfiram-ethanol reaction. Substance and Alcohol Actions/Misuse. 1982;3:107–115. [PubMed] [Google Scholar]
- Helzer JE, Canino GJ, Yeh E-K, Bland RC, Lee CK, Hwu H-G, Newman S. Alcoholism: North America and Asia. Archives of General Psychiatry. 1990;47:313–319. doi: 10.1001/archpsyc.1990.01810160013002. [DOI] [PubMed] [Google Scholar]
- Kalant H, Woo N. Electrophysiological effects of ethanol on the nervous system. Pharmacology and Therapeutics. 1981;14:431–457. doi: 10.1016/0163-7258(81)90037-1. [DOI] [PubMed] [Google Scholar]
- Kitano HHL, Chi I. Asian Americans and alcohol: The Chinese, Japanese, Koreans, and Filipinos in Los Angeles. In: Spiegler DL, Tate DA, Aitken SS, Christian CM, editors. Alcohol Use Among U.S. Ethnic Minorities. Bethesda, MD: the Institute; 1989. pp. 373–382. (National Institute on Alcohol Abuse and Alcoholism Research Monograph No. 18. DHHS Pub. No. (ADM)89–1435). [Google Scholar]
- Klatsky AL, Sieglaub AB, Landy C, Friedman GD. Racial patterns of alcoholic beverage use. Alcoholism: Clinical and Experimental Research. 1983;7:372–377. doi: 10.1111/j.1530-0277.1983.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human brain electrophysiology and alcoholism. In: Tarter RE, Van Thiel DH, editors. Alcohol and the Brain. New York: Plenum Press; 1985. pp. 139–182. [Google Scholar]
- Rohrbaugh JW, Stapleton JM, Parasuraman R, Zubovic E, Frowein HW, Varner JL, Eckardt MJ, Linnoila M. Dose-related effects of ethanol on a visual sustained attention task and event-related potentials. Alcohol. 1987;4:293–300. doi: 10.1016/0741-8329(87)90026-7. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. A clinical model of genetic influences in alcohol dependence. Journal of Studies on Alcohol. 1994;55:5–17. doi: 10.15288/jsa.1994.55.5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO, Croot K, Finn P, Polich J. P300 latency after ethanol ingestion in sons of alcoholics and controls. Biological Psychiatry. 1988;24:310–315. doi: 10.1016/0006-3223(88)90199-0. [DOI] [PubMed] [Google Scholar]
- Sue S, Nakamura C. An integrative model of physiological and social/psychological factors in alcohol consumption among Chinese and Japanese. Journal of Drug Issues. 1984;14:349–364. [Google Scholar]
- Sue S, Zane N, Ito J. Alcohol drinking patterns among Asian and Caucasian Americans. Journal of Cross-Cultural Psychology. 1979;10:41–56. [Google Scholar]
- Takeshita T, Morimoto K, Mao XQ, Hashimoto T, Furuyama J-I. Characterization of the three genotypes of low Km aldehyde dehydrogenase in a Japanese population. Human Genetics. 1994;94:217–223. doi: 10.1007/BF00208273. [DOI] [PubMed] [Google Scholar]
- Thomasson HR, Edenberg HJ, Crabb DW, Mai X-L, Jerome RE, Li T-K, Wang S-P, Lin Y-T, Lu R-B, Yin S-J. Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men. American Journal of Human Genetics. 1991;48:667–681. [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): A review. Human Genetics. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- Wall TL, Thomasson HR, Schuckit MA, Ehlers CL. Subjective feelings of alcohol intoxication in Asians with genetic variations of ALDH2 alleles. Alcoholism: Clinical and Experimental Research. 1992;16:991–995. doi: 10.1111/j.1530-0277.1992.tb01907.x. [DOI] [PubMed] [Google Scholar]
- Wall TL, Ehlers CL. Acute effects of alcohol on P300 in Asians with different ALDH2 genotypes. Alcoholism: Clinical and Experimental Research. 1995;19:617–622. doi: 10.1111/j.1530-0277.1995.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Wall TL, Gallen CC, Ehlers CL. Effects of alcohol on the EEG in Asian men with genetic variations of ALDH2. Biological Psychiatry. 1993;34:91–99. doi: 10.1016/0006-3223(93)90261-b. [DOI] [PubMed] [Google Scholar]
- Wall TL, Nemeroff CB, Ritchie J, Ehlers CL. Cortisol responses following placebo and alcohol in Asians with different ALDH2 genotypes. Journal of Studies on Alcohol. 1994;55:207–213. doi: 10.15288/jsa.1994.55.207. [DOI] [PubMed] [Google Scholar]
- Wolff P. Ethnic differences in alcohol sensitivity. Science. 1972;175:449–450. doi: 10.1126/science.175.4020.449. [DOI] [PubMed] [Google Scholar]