Abstract
Tissue injury and inflammation markedly alter touch perception, making normally innocuous sensations become intensely painful. Although this sensory distortion, known as tactile allodynia, is one of the most common types of pain, the mechanism by which gentle mechanical stimulation becomes unpleasant remains enigmatic. The stretch-gated ion channel PIEZO2 has been shown to mediate light touch, vibration detection, and proprioception. However, the role of this ion channel in nociception and pain has not been resolved. Here, we examined the importance of Piezo2 in the cellular representation of mechanosensation using in vivo imaging in mice. Piezo2-knockout neurons were completely insensitive to gentle dynamic touch but still responded robustly to noxious pinch. During inflammation and after injury, Piezo2 remained essential for detection of gentle mechanical stimuli. We hypothesized that loss of PIEZO2 might eliminate tactile allodynia in humans. Our results show that individuals with loss-of-function mutations in PIEZO2 completely failed to develop sensitization and painful reactions to touch after skin inflammation. These findings provide insight into the basis for tactile allodynia, identify the PIEZO2 mechanoreceptor as an essential mediator of touch under inflammatory conditions, and suggest that this ion channel might be targeted for treating tactile allodynia.
INTRODUCTION
Touch and proprioception (the sense of body position) are used in almost every aspect of daily life. Recent studies have demonstrated that a single ion channel, PIEZO2 (1), is responsible for detecting and transducing the subtle changes in force that characterize these sensory modalities in humans (2), mice, and flies (3, 4). Ordinarily, gentle touch is important for fine discrimination of objects and is innocuous or pleasant for humans. However, after injury, even a soft caress may elicit sharp pain. The mechanisms mediating this transformation of sensory input, called mechanical allodynia, are not understood. Current views have implicated two different mechanisms: increased sensitization of peripheral nociceptors and alterations of pain-related central processing (5–7). Here, we investigated the role of PIEZO2 in mechanical allodynia. Recently, we and others found that Piezo2 is very broadly expressed in somatosensory neurons including nociceptors in mice (8, 9). However, a consistent view for the function of this protein in pain is lacking (3, 10, 11) partly because complete loss of Piezo2 function in mice is lethal at birth (12).
Previous studies examining the molecular and cellular basis for tactile allodynia have often relied on in vitro assays in cultured neurons (13) or electrophysiological recordings both in vivo (14) or ex vivo preparations (15). However, no consensus view for the underlying mechanisms has emerged. We reasoned that in vivo functional imaging would provide a powerful way to study how injury and inflammation alter the detection of touch by exposing the global representation of sensory stimuli at a single-cell and single-spike resolution (16). Here, we used in vivo calcium imaging of the trigeminal ganglion to investigate the function of Piezo2 in mechanical nociception and tactile allodynia in mice.
Behavioral studies using genetically modified mice have proven extremely useful for interrogating how specific molecules and circuits enable sensation. Knockout mice were instrumental in defining Piezo2 as the receptor responsible for touch detection (3) and proprioception (17). However, pain is a subjective experience making translation from mice to humans more challenging. Thus, our recent identification of a small group of human participants with inactivating mutations in PIEZO2 provides an opportunity to directly evaluate whether this receptor is involved in pain sensation. Our results from quantitative sensory evaluation of human participants and the in vivo imaging of mice suggest a minor role for PIEZO2 in mechanical nociception but demonstrate its critical importance in tactile allodynia.
RESULTS
Viral recombination provides a strategy for generating and marking Piezo2-knockout neurons in vivo
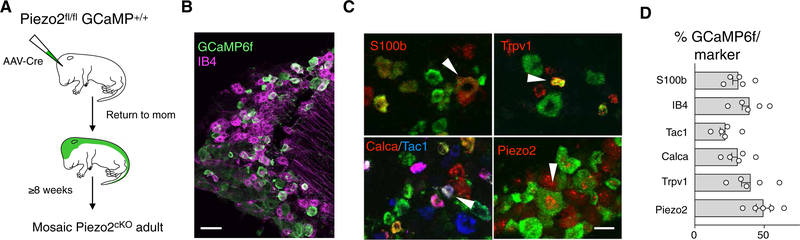
Global knockout of Piezo2 function in mice is lethal perinatally (12). Therefore, we developed a strategy to knock out Piezo2 in a large but mosaic subset of sensory neurons. This approach targets a broad range of neuronal classes and concomitantly marks the knockout cells (Fig. 1A). We generated mice carrying a Cre-dependent marker (tdTomato or GCaMP6f) crossed into a conditional Piezo2-knockout background. Viral delivery of Cre recombinase in newborn (up to P2) mice was then used to knock out Piezo2 function and activate marker expression (Fig. 1,B and C). Using this strategy, we were able to mark Piezo2-knockout neurons and showed that they belong to a broad range of neural classes (Fig. 1,B to D).
Fig. 1. A viral strategy efficiently generates viable Piezo2 mosaic knockouts.
(A) Cartoon depicting our strategy for simultaneously knocking out Piezo2 and marking neurons: A viral vector encoding Cre recombinase was introduced to new born (P0-P2) Piezo2cKO (Piezo2fl/fl/GCaMP+/+) mice; controls were GCaMP6f+/+. Mice were tested at > 8 weeks. (B and C) Representative confocal images of dorsal root ganglia from Piezo2cKO. (B) Green fluorescent protein antibody staining to reveal the GCaMP6f-positive cells (green) and the isolectin IB4 (magenta) to mark putative mechanonociceptors. (C) Representative confocal images of multilabel in situ hybridization; arrowheads highlight multilabeled cells. (D) Quantification of marker positive cells expressing GCaMP6f (N = 5 mice). Scale bars, 50 μm (B) and 25 μm (C).
To test for Piezo2 loss of function in the labeled cells, we carried out in vitro recordings (fig. S1, A and B) from representative populations of acutely cultured somatosensory neurons (fig. S1,C and D). GCaMP6f-positive neurons from control mice regularly exhibited mechanically activated currents, whereas labeled neurons from Piezo2 conditional knockout (Piezo2cKO) mice never responded in this assay (fig. S1, A and B), fully validating our strategy.
After injecting Piezo2cKO, we often observed behavioral pheno-types consistent with the importance of Piezo2 in proprioception (2,13) and touch (3,14–16), including ataxia, elevated touch withdrawal reflexes, and an absence of responses to dynamic touch stimulation of hind paw (brushing; fig. S2). As expected, post hoc analysis demonstrated that the extent of deficits was correlated with the efficiency of Cre recombination (fig. S2, C and D).
Piezo2 is required for neural responses to gentle but not noxious mechanical stimuli
We recently optimized calcium imaging of the trigeminal ganglion as a means to record activity from hundreds of sensory neurons responding to a range of sensory stimuli (16). This functional imaging platform relies on the genetic expression of the calcium reporter GCaMP6f (18) and is sensitive enough to measure single spikes in somatosensory neurons (16). Therefore, we hypothesized that the importance of Piezo2 in nociception and mechanical pain could be directly determined by in vivo imaging of sensory responses in control and Piezo2cKO backgrounds.
We first performed functional imaging of control Cre-dependent GCaMP6f mice (Ai95) injected with AAV-Cre to record neuronal responses to gentle dynamic brushing and pinch (Fig. 2A and fig. S3). As expected, given that both C and A fibers respond to these types of stimuli, activity was recorded from neurons with a wide range of diameters [Fig. 2A (inset) and fig. S1C]. We could also record robust responses to heat and vibration (fig. S4); the latter is a stimulus known to selectively activate Aβ fibers consistent with the large diameter of vibration responsive cells (fig. S4,B and C).
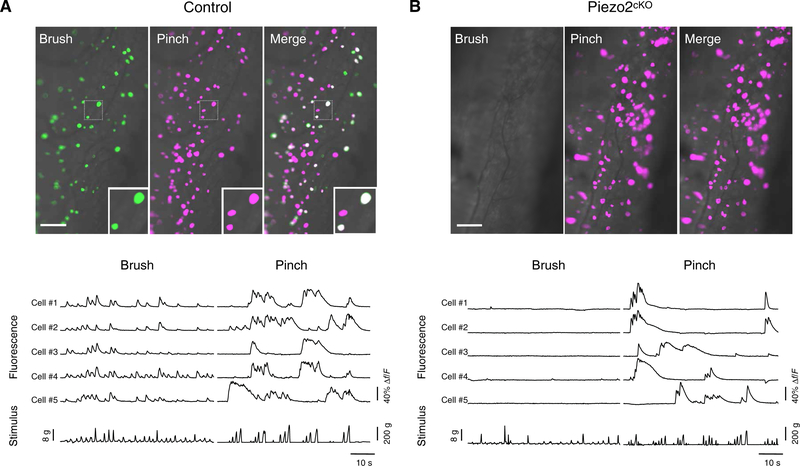
Fig. 2. Functional imaging reveals the role of Piezo2 in detecting mechanical stimuli in living mice.
In vivo functional imaging of the trigeminal ganglion neurons. Top: Typical imaging fields (grayscale) with responding neurons pseudocolored (green, brush; magenta, pinch) to highlight the magnitude of fluorescence changes. (A) Control mice; magnified boxed region: three responding cells, two activated by both stimuli with soma sizes of C-LTMRs (C-fiber low threshold mechanoreceptors) (small diameter) and fast-conducting touch neurons (large diameter), and one neuron that only responded to pinching (small diameter). Bottom: Examples of changes in fluorescence (Δf/F) for five cells that responded to both brushing and pinch. Stimuli were applied with a cotton swab (brush) or forceps (pinch) with integrated load cells (see fig. S3) that recorded the applied stimulus intensity (stimulus). (B) Piezo2cKO mice; as no neurons responded to brushing, bottom panel shows examples of changes in fluorescence (Δf/F) for five pinch-sensitive cells. Scale bars, 100 μm.
Given its role in detection of gentle mechanical stimuli, we predicted that labeled neurons in Piezo2cKO should lack responses to these stimuli while maintaining response to temperature. As expected, heat responses were observed (fig. S4A) in the mutant mice. By contrast, knockout neurons failed to detect either dynamic brush (Fig. 2B) or vibration (fig. S4,B and C), substantiating Piezo2 as the primary receptor for touch.
Piezo2 is broadly expressed in nociceptors (8, 9), and single-fiber recordings showed that it plays a role in firing dynamics of Aδ and C fibers in response to high-threshold mechanical stimuli (19). However, our imaging approach showed that the global representation of high-threshold mechanical stimulation in Piezo2cKO neurons resembled that of control animals (Fig. 1,E and F). Together, these results help explain why human participants with PIEZ02 loss-of-function variants (PIEZ02LOF) still report intense stimuli as unpleasant or painful with normal thresholds (2) but raise the possibility that PIEZ02 influences the quality of noxious mechanical sensation.
Piezo2 remains essential for gentle touch after inflammation
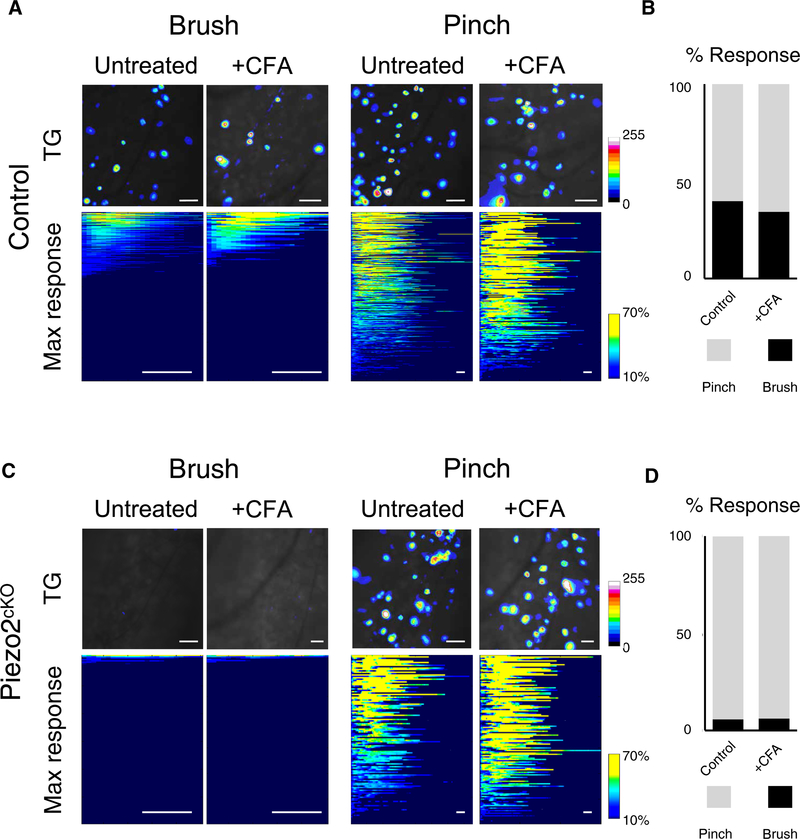
To test the role of Piezo2 in touch-evoked pain, we first used in vivo functional imaging to determine whether Piezo2cKO neurons that normally are unresponsive to gentle mechanical stimuli become sensitized after inflammation. Complete Freund’s adjuvant (CFA), a standard for activating immune responses, has been shown to produce robust long-lasting tactile allodynia and thermal sensitization in mice (20). As expected, CFA administration induced recruitment of immune cells (fig. S5A) and pronounced sensitization of trigeminal responses to heat (37° and 41°C; fig. S5, B and C). However, mechanical responses of control and CFA-treated mice appeared far less affected (Fig. 3A). To verify that mechanical responses after CFA closely resemble those of controls, we quantified the ratio of neurons responding to brush versus pinch (Fig. 3B) and the magnitude of cellular responses to pinch (fig. S5D). In Piezo2cKO mice, pinch responses also remained robust and after CFA treatment (Fig. 3,C and D) and were of similar magnitude to uninflamed controls (fig. S5D). In marked contrast, Piezo2cKO neurons never responded to brushing even after CFA (Fig. 3,C and D). Piezo2cKO neurons also remained insensitive to other gentle mechanical stimuli including air puff and vibration during inflammation (fig. S6). Thus, our results demonstrate that Piezo2 is required for detection of gentle touch both under normal conditions and after CFA-induced inflammation in mice.
Fig. 3. Piezo2 is essential for neural responses to gentle touch during chronic inflammation.
(A) Representative imaging fields from control mice with responding neurons pseudocolored to reflect fluorescence changes (SD projections; top) illustrate the numbers of neurons responding under normal (untreated) and inflamed (CFA) conditions. In the course of an experiment, multiple applications of each stimulus type were recorded, and the maximum-evoked response for every responding cell was identified, each of these is shown as a line in the heat maps (bottom); n = 364 untreated neurons and n = 174 CFA-treated neurons. TG, trigeminal ganglion. (B) Quantification of responses; P = 0.2537, Fisher’s exact test. (C) Representative fields and (D) quantitation of responses of Piezo2cKO neurons; the representation of pinch responses was not significantly altered by CFA treatment (P > 0.9999; N = 3 mice per group). Scale bars, 50 μm (top) and 1 s (bottom).
Gentle touch responses are also Piezo2-dependent in other pain models
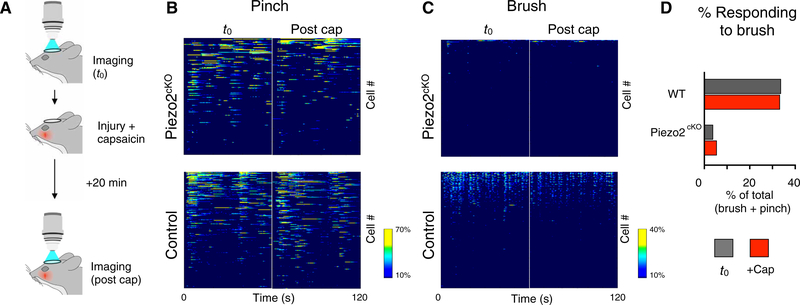
Next, we asked whether these findings generalize to an acute neuro-inflammatory challenge as well as to a completely different model of tactile allodynia involving nerve injury. Capsaicin, the pungent component of chili peppers, activates peptidergic nociceptors, causing local neurogenic inflammation, tactile allodynia, and sensitization (20). Because capsaicin is fast-acting, this model provides the opportunity to image the ganglion during development of inflammation (Fig. 4A). Moreover, because capsaicin-induced sensitization is relatively transient, it is also used as a test in human participants (21). In control neurons, capsaicin treatment had only minor effects on the global representation of pinching and brushing [Fig. 4,B and C (bottom); quantified in Fig 4D]. Nonetheless, about half of the neurons showed more than 50% change in Δf/F in brush responses between imaging sessions, but similar variability was also observed in the absence of capsaicin treatment (fig. S7). Therefore, capsaicin-induced neuroinflammation failed to sensitize Piezo2cKO neurons to brushing [Figs. 4B (top) and 3D].
Fig. 4. Piezo2 is required for gentle touch responses before and after neurogenic inflammation by capsaicin.
(A) Schematic representation of the experimental strategy. (B and C) Heatmaps representing Δf/F for mechanically responsive cells in Piezo2cKO mice (top; n = 176 neurons, N = 3 mice) and controls (bottom; n = 231 neurons, N = 3 mice), before (t0) and after inflammation (post cap). Each line represents the full stimulation session for a given cell; cell responses were sorted by response maxima for the two imaging sessions (see fig. S7 for analysis of response stability and controls). (B) Pinch responses. (C) Brush responses. (D) Quantitation of the proportion of mechanosensitive neurons responding to brush before and after neurogenic inflammation; capsaicin treatment had no effect on the percentage of mechanosensitive neurons responding to brush in either control (P > 0.9999) or Piezo2cKO neurons (P = 0.6212; Fisher’s exact test). WT, wild type
Spared nerve injury reliably induces neuropathic pain in animal models and has been used previously to sensitize trigeminal responses to mechanical stimulation. As predicted, from our results with inflammatory models, Piezo2cKO neurons failed to detect brush even 1 week after nerve injury (fig. S6B). Together, these data from three models of tactile allodynia reveal that Piezo2 remains the essential receptor for gentle touch detection even after treatments that make these types of stimuli painful.
Human with inactivating mutations of PIEZO2 detect mechanical pain
We recently identified two individuals with PIEZO2LOF who displayed selective touch deficits (including a failure to detect vibration) and difficulty producing coordinated movements (2). The phenotype that we reported for these two individuals helped us diagnose three additional participants carrying three recessive inactivating variants in PIEZO2 in compound heterozygosity (fig. S9). Sensory evaluations demonstrated that these participants, along with a fourth individual previously reported to lack PIEZO2 but not quantitatively tested (22), required orders of magnitude more force to detect von Frey filaments applied to palm (Fig. 5A) and forearm (fig. S10A) than volunteer controls. The four PIEZO2LOF individuals were also unable to use touch to perform a two-point discrimination task (fig. S10B) or distinguish between horizontal and vertical ridges on a touch cube (fig. S10C). Only one could detect vibration (fig. S10D), but this individual was hyposensitive compared to controls.
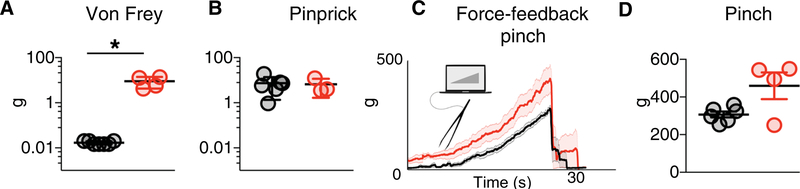
Fig. 5. PIEZO2 is required for the detection of gentle but not noxious mechanical stimuli in human participants.
Quantitative sensory evaluation of age- and gender-matched volunteers (black circles) and PIEZO2LOF individuals (red circles). (A) Detection thresholds for von Frey filaments applied to glabrous skin (palm). Mean thresholds for PIEZO2LOF individuals (N = 4) were significantly different from controls (N = 7; *P = 0.0061, Mann-Whitney). (B) Pinprick pain thresholds were no different between groups (P = 0.7619, Mann-Whitney); for controls (N = 7), pinprick pain required higher force than von Frey detection (P = 0.0006, Mann-Whitney), but for PIEZO2LOF individuals (N = 3), there was no difference between these thresholds (P = 0.2286, Mann-Whitney). (C and D) Pinch responses were tested using custom forceps and an integrated feedback system until the individual reported feeling pain. (C) Illustrative force traces for a control (black line) and PIEZO2LOF (red line; median ± SD, N = 3 trials). (D) Quantitation of threshold for controls (N = 7) and PIEZO2LOF individuals (N = 4); difference between groups is not significant (P = 0.2571, Mann-Whitney).
Given the uncertainty surrounding the role of PIEZO2 in pain, we also evaluated the sensitivity to mechanical pain in PIEZO2LOF individuals. Each of the four PIEZO2LOF individuals had normal mechanical pain responses; pressure (fig. S10E) and pinprick pain thresholds closely matched those of controls (Fig. 5B and fig. S10F). We note that von Frey detection and pinprick pain thresholds (normally orders of magnitude apart) were very similar for the PIEZO2LOF individuals. This finding is consistent with our previous study (2) and implies that PIEZ02 function is not required for detection of noxious mechanical stimuli in humans.
A reduced pinch sensitivity has been observed in mice lacking Piezo2 (19). To quantitatively test the role of PIEZ02 in this type of pain in humans, we recorded the threshold force needed to elicit discomfort in PIEZO2lOf individuals and control volunteers (Fig. 5C). Our results showed no significant difference between control and PIEZO2lOf individuals (Fig. 5D and fig. S10G) and support the conclusion that PIEZ02 plays at most a minor role in detecting noxious mechanical stimuli in humans.
PIEZO2 is required for tactile allodynia in humans
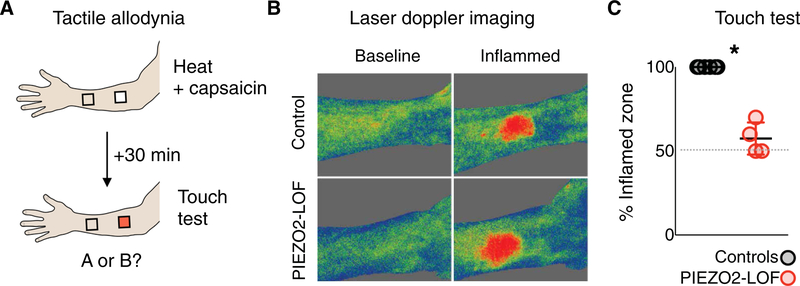
Our results, demonstrating Piezo2 as essential for light touch detection after injury in mice, predict a similar role for this channel in humans and raise the exciting possibility that inhibiting its function would effectively block tactile allodynia. Tactile allodynia has been evaluated experimentally using heat in combination with capsaicin cream to induce short-term but intense inflammation in humans (21, 23). Application of heat and capsaicin to the forearm causes a burning sensation and irritation through activation of the transient receptor potential vanilloid 1 (TRPVl)-expressing neurons (24). As expected, PIEZO2LOF individuals and controls reported discomfort and developed pronounced local inflammation (Fig. 6,A and B). Standard evaluation of allodynia involves mapping the primary and secondary areas of inflammation using a cotton swab or fine brush (23). However, the four PIEZO2LOF individuals were completely unable to detect this type of stimulation. Therefore, to quantitatively evaluate their deficits, we designed a two-alternative forced-choice assay where the participant simply needed to report the site where swabbing felt more unpleasant or painful (Fig. 6A). As expected, controls unfailingly reported light touch surrounding the inflamed area as unpleasant and painful compared to the same stimulus applied to a neighboring but uninflamed area of the skin. In the forced-choice assay, the four PIEZO2LOF individuals performed at chance (Fig. 6C). In combination, our data from mice and humans demonstrate that PIEZO2 is required for detecting several different low-threshold mechanical stimuli and remains essential under conditions that result in touch-evoked pain.
Fig. 6. PIEZO2 mediates touch-evoked pain in human participants.
(A) Model for testing touch allodynia after neurogenic inflammation based on application of topical capsaicin; as illustrated, capsaicin cream was applied to a 3 cm by 3 cm square of the forearm for 30 min. The cream was removed, and inflammation was confirmed using laser doppler imaging, illustrated in (B). Participants were then asked to report whether touching the treated area or a nearby unaffected spot was more painful or intense. (C) Quantitation of forced choice testing; the dotted line represents chance. PIEZO2LOF and control individuals had significantly different responses (*P = 0.0048, Mann-Whitney).
DISCUSSION
Our data, using in vivo imaging in mice and quantitative sensory testing in humans, provide a consistent view for the role of PIEZO2 in pain sensation. PIEZO2 function is dispensable for detection of noxious mechanical stimuli like pinprick, pinch, or pressure both at baseline (no injury) and during inflammation. On the other hand, all gentle mechanical stimuli tested including brush, air puff, and vibration are completely dependent on this mechanosensory channel even after nerve injury or during chronic inflammation. Human participants with PIEZO2LOF variants were completely unable to sense gentle stimuli applied to inflamed skin. In contrast, all control volunteers found these normally pleasant stimuli intensely painful. One direct implication of our findings is that blocking PIEZO2 function in normal individuals would be expected to prevent tactile allodynia and associated pain without affecting protective nociceptive responses.
The ability to directly translate findings related to mechanosensory detection in mice to human pain sensation highlights the value of studying rare congenital disorders in human participants. In the case of PIEZO2, identifying individuals with loss-of-function mutations allowed us to investigate the role of this ion channel in pain. Human participants are able to carry out complex tasks, describe their experience, and provide subjective information that would be difficult or impossible to obtain using animals. Several innovative measures of pain response in mice have been proposed (25,26), but ultimately, they all rely on investigator interpretation. In contrast, human participants reliably report when a stimulus becomes painful. Here, all four PIEZO2LOF individuals reported noxious mechanical stimuli as painful but were completely insensitive to gentle touch even under conditions where control volunteers experienced pain from this type of stimulus. These data are fully consistent with the results from in vivo functional imaging in mice, where high-threshold mechanical stimuli can be detected in the absence of Piezo2, but gentle mechanical stimuli require this channel even after injury or inflammation.
The PIEZO2LOF syndrome affects only very few individuals, and therefore, one limitation of our work is the small sample size. Although our data might hint that PIEZO2 affects pinch pain threshold, in line with behavioral studies in mice (19), interindividual variation means that we would need to study a far larger cohort than we have currently identified to be certain of this effect. Nonetheless, for touch and vibration detection, the effect size and consistency between individuals indicated the critical importance of PIEZO2 for sensation of gentle mechanical stimuli both under normal and pathological conditions.
How does injury induce touch-evoked pain? One view is that nociceptors may be directly detecting light touch, but equally, it may be that they are responding to mediators released by other cells (27–29). Regardless, activity of nociceptors has been proposed to be an important driver of tactile allodynia (5). Other studies suggest that changes in central processing may have a major role in touch-induced pain (30–33). Our in vivo imaging data reveal unexpected stability in the global representation of mechanical stimuli at the periphery, including an essential role for Piezo2 in gentle touch detection after inflammation or injury. The most parsimonious explanation for our findings is that tactile allodynia is primarily a central process. However, at present, we cannot assign the identity of neurons driving allodynia because Piezo2 is broadly expressed across many classes of sensory neurons. Recently, low-threshold mechanoreceptors expressing the tyrosine kinase type B receptor (TrkB) have been implicated as important for tactile allodynia (6, 34); these neurons express high levels of Piezo2 (8). However, it is possible that other cell classes (9, 35) are also drivers of this type of pain. Future studies using a range of Cre-driver lines will help establish whether specific cell types change their response profiles after injury. Nonetheless, our study pinpoints PIEZO2 as the essential transduction channel for gentle touch and tactile allodynia.
The pronounced sensitization and painful nature of touch after injury is something that almost everyone knows; it is also one of the most important drivers of chronic pain. We suggest that PIEZO2 antagonists will be valuable tools for understanding mechanical allodynia and may provide a strategy for relieving a variety of chronic pain conditions. Given that PIEZO2 is crucial for a wide range of mechanosensory processes, including proprioception, the use of systemic drugs is unlikely to be a feasible option. However, topically applied PIEZO2 antagonists might have potential for relieving mechanical allodynia caused by damage to nerves, chronic inflammation, or long-term injury. These types of pain are often difficult to treat with current therapies.
MATERIALS AND METHODS
Study design
The objective of the study was to assess the role of Piezo2 in injury-related pain and establish it as a molecular target for future development of novel inflammatory pain drugs. To achieve this, we developed mouse model of Piezo2LOF to investigate its role in responses to mechanical stimuli. We tested neuronal responses to low- and high-threshold mechanical stimuli using custom-built equipment directly measuring force applied to mouse cheek. Responses were visualized using a recently developed platform for in vivo imaging of trigeminal ganglia. To investigate the involvement of Piezo2 in mechanically induced responses under condition known to produce tactile allodynia, we used a set of three established hypersensitivity models both chronic (injection of CFA and spared nerve injury) and acute (capsaicin treatment). To test translatability of our findings in mice, we recruited a cohort of Piezo2LOF human participants and performed tests of their sensory perception, reporting of pain and tactile allodynia after inflammation.
Experimental animals
All experiments using animals followed National Institutes of Health (NIH) guidelines and were approved by the National Institute of Neurological Disorders and Stroke (NINDS) Animal Care and Use Committee. Rosa-LSL-GCaMP6f (Ai95) mice were purchased from the Jackson laboratory. Piezo2fl/fl mice were generated from embryonic stem cells received from the knockout mouse phenotyping (KOMP) facility with the help from the NINDS/National Institute of Mental Health Transgenic Core. Founder mice were crossed with C57BL/6 mice, and the offspring mated to obtain a homozygous breeding colony.
Viral injections and inflammatory models
Virus was injected into P0-P2 pups. The pups were briefly anesthetized on ice, and a Hamilton syringe was used to inject AAV9-Cre (1013 to 4 × 1013 virions/μl; Vigene, Rockville, USA). The virus was administered via three routes to increase chances of infection. For intracerebroventricular injection, a Hamilton (Hamilton Robotics, Reno, USA) syringe needle was inserted at a 45° angle and 3 mm deep in the space between the center of the eye and Bregma (about three-fifths of the distance, rostral-lateral of Bregma); 1.5 μl of virus was injected in each ventricle. Intravenous injection was performed by gently progressing the needle at sharp angle into the superficial temporal face vein; 1 ml of virus was injected. Standard intraperitoneal injection of 10-fold diluted virus (5 μl) was injected. After injections, pups were placed on a heating pad to recover before returning to the home cage.
To induce chronic cheek inflammation, CFA (Sigma-Aldrich, St. Louis, USA) was injected subcutaneously into the left cheek, and physiological and anatomical testing was performed 24 to 36 hours after injection. For spared nerve injury, animals were anesthesized, a small incision at the edge of the whisker pad was made, and subsequently, maxilliary branches of trigeminal nerve innervating the whisker pad were cut. For acute capsaicin (Sigma-Aldrich) injury model during in vivo imaging, two small longitudinal cuts (~1 mm) were made on a naired skin, and a 1% solution of capsaicin was applied to the region for 5 min after which the area was rinsed with saline.
Trigeminal ganglia in vivo functional imaging
Trigeminal imaging preparation was performed as described previously (16). Epifluorescence imaging of the ganglia was performed using an upright microscope (FVMPE-RS, Olympus) equipped with a 4×, 0.28-numerical aperture air objective. Illumination was provided with a 130-W halogen light source (U-HGLGPS, Olympus), using a standard green excitation/emission filter cube. Images were acquired using an ORCA-Flash 4.0 CMOS camera (Hamamatsu, Japan) at a frame rate of 10 Hz using MetaMorph (Molecular Devices). pClamp software was used to trigger the imaging acquisition and to generate TTL pulses for synchronizing other instruments (videography and force recording) through a Digidata 1550 (Molecular Devices).
Calcium imaging analysis
Acquired tiff stacks were cropped to remove out of focus areas. Stacks from multiple trials were concatenated and corrected for movement using standard ImageJ plugins (Linear Stack Alignment and StackReg tool). Regions of interest encompassing single cells were selected from active cells using the Cell Magic Wand Tool written for ImageJ. The resting florescence (f0) was calculated using the average of the five frames with lowest fluorescence within the entire stack. Neuropil subtraction was performed using custom-written MATLAB software described previously (16). The threshold for activation was set as Δf/F0 > 10%. To select the most effective bout of stimuli delivery across multiple trials, timing of the beginning and ending of every stimulus was established on the basis of videography and/or applied force recordings (see below). Most efficient pinching bout was selected for further analysis. Brushing was defined as stroking with a cotton swab with a force of <4 g (see fig. S3, A to C). All analyses were performed using custom-written MATLAB scripts. The total number of cells was calculated by counting cells that responded to any applied stimuli throughout the entire experiment.
Stimulation and videography
Videography was performed as described previously (16), using an infrared CMOS video camera (acA2000–165um, Basler) equipped with 0.6× telecentric lens (Edmund Optics). Images were captured at 20 Hz. Mechanical stimuli were delivered using custom-made devices using micro load cells (CZL639HD, Phidgets, Calgary, Canada) for measuring applied forces (fig. S3). Brush stimuli were delivered with custom-made aluminum cotton swab holder (fig. S3, A and B) in which the load cell was placed parallel to the plane of the cheek. This arrangement allowed for measurement of the applied force acting against the skin during the swab. Pinch stimuli were delivered using forceps in which one arm was custom-modified to include a load cell to allow the measurement of force acting against the skin (fig. S3, D and E). Load cells were connected to a bridge input device (Phidget-Bridge 4-Input, Phidgets) and a PC running Phidgets driver software and custom-written acquisition software (Python). For vibration delivery, a Solid Drive SD1SM (MS Audio, Plainview, Canada) sound transducer was mounted on a stand and fitted with a custom-made extension bar (diameter, ~5 mm) with a plastic screw cap (fig. S3F). The cap was placed in firm contact with the skin of the cheek after hair removal with a depilatory cream. The transducer was connected to a sound amplifier that received sine signals with frequency of 50 and 150 Hz. Temperature ramps were delivered as described previously (16), using recirculating water baths (Lauda-Brinkmann, Delran, USA) that could heat and cool a copper thermode touching the cheek after hair removal. Water flow from the baths was controlled using a set of valves that could be operated using TTL pulses.
Human participant enrollment and genetic testing
This study was approved by the Institutional Review Board of the NINDS and NIH. Written informed consent was obtained by a qualified medical investigator. Human participant enrollment and genetic testing were as described previously (2).
Quantitative sensory testing of human participants
Four patients and seven healthy age- and gender-matched controls were tested on a panel of sensory tests.
Von Frey touch detection threshold
The von Frey touch detection threshold was determined on the dorsum side of the left forearm and on the thenar eminence of the left palm using monofilaments with indentation forces ranging from 0.008 to 300 g (nylon fiber; Aesthesio, Bioseb, Pinellas Park, FL, USA). Stimulation forces were presented in the order of ascending, followed by descending intensities using the method of limits. The participants were asked to report “any sensation on the skin” after a verbal cue of stimulus onset. Participants were shown the von Frey monofilaments before testing but were deprived of visual input using a screen during the actual testing. The geometric mean of three detection threshold series determined the final touch detection threshold.
Von Frey pinprick pain threshold
The von Frey pinprick pain threshold was determined on the dorsum side of the left forearm and on the thenar eminence of the left palm using monofilaments with indentation forces ranging from 0.008 to 300 g (nylon fiber; Aesthesio, Bioseb, Pinellas Park, FL, USA). The pinprick pain threshold was determined immediately after the touch detection threshold in the same order of ascending intensities, followed by descending intensities, using the method of limits. After a verbal cue of stimulus onset, the participants were asked to report “any sensation on the skin with a sharp-pinprick character to it”. The geometric mean of three pinprick pain threshold series determined the final threshold.
Pinch pain threshold
The pinch pain threshold was determined for the hairy skin on the inner side of the left upper forearm and for the glabrous skin of the left ring finger. The skin on the arm was marked with two lines separated by 5 mm. The upper arm testing was repeated three times on skin locations separated by 3 cm. Marking on the skin on the finger was two lines separated by 10 mm. The finger testing was only done once per participant. Pinching forcep design was the same as for mouse model tests (see “Stimulation and videography” section). The participants were instructed to report any sensation of pain from the pinch, at which point the stimulus was stopped immediately. The force level at the stop point was recorded, and mean of all trials was used as the final threshold. Participants were shown the forceps before testing but were deprived of visual input during the actual testing.
Experimental tactile allodynia
The heat/capsaicin model of tactile allodynia is a safe and well-established model (23). Each participant’s baseline blood flow in the right forearm skin was captured using a laser speckle contrast imager (moorFLPI-2, Moor Instruments Ltd., Devon, United Kingdom). Baseline images were acquired at a frame rate of 25 images/min for 1 min before model application. After laser speckle contrast image acquisition, a 3 cm by 3 cm Peltier thermode (Medoc, TSA, Thermosensory Analyzer, Rimat Yishai, Israel) set at 45°C was applied to the skin in either the proximal or distal section of the dorsum side of the right forearm for 5 min. The area of the heat stimulation was marked. Immediately after the heat stimulation ended, a 1-mm-thick layer of 0.075% capsaicin cream was applied (Capzasin-HP) to the same 3 cm by 3 cm skin area and left on the skin for 30 min. After 30 min, the capsaicin cream was removed, and participants were asked for a verbal rating of ongoing pain in the treated skin zone using a numeric rating scale (0, no pain; 10, worst pain imaginable) to confirm model development. All participants developed a flare from the model, and changes quantified again using the laser speckle contrast imager (same as described above). The treated skin area was alternated between participants to be in either the proximal or distal area of the forearm. A control skin area (3 cm by 3 cm) was marked up 7 cm from the treated skin area within the forearm. Light skin stroking (stroking velocity, 3cm/s; stroking distance, 9 cm; i.e., around three sides of the marked square area; application force, about 0.3 N) was applied manually as paired stimuli consisting of one skin stroke in the intended allodynia zone and one in the corresponding area around the marked control zone using a cotton swab. Participants were deprived of visual input using a screen during the testing. After a verbal cue of stimulus onset, the participants were asked to report which of the paired stimuli was perceived as the most unpleasant or painful (two-alternative forced-choice assay). Ten paired stimuli were included in a randomized, counterbalanced order. The percentage of responses for each zone was compared between participants carrying loss-of-function mutations in PIEZO2 and control participants.
Statistical analysis
Data are presented as means ± SEM. For calcium imaging individual cells, data were collected from at least three independent in vivo preparations. Statistical difference between distributions of responding cells fraction was tested with χ2 or Fisher’s exact tests. For animal behavior test, differences were tested using Mann-Whitney. For comparison of psychophysical testing of Piezo2LOF human participant data, Mann-Whitney or Kruskal-Wallis tests were used where appropriate.
Supplementary Material
Fig. S1. In vitro responses of cultured neurons to touch demonstrate the efficiency of the mosaic knockout strategy.
Fig. S2. Mechanosensory phenotypes in adult PiezocKO mice.
Fig. S3. Custom-built devices for mechanical stimulation of the mouse cheek.
Fig. S4. Piezo2 is required for trigeminal responses to vibration but not heat.
Fig. S5. CFA induces chronic inflammation and sensitization to heat but not pinch.
Fig. S6. Responses to gentle mechanical stimuli require Piezo2 in the CFA inflammatory pain model.
Fig. S7. Controls for brush responses after capsaicin-induced inflammation.
Fig. S8. Responses to gentle mechanical stimuli require Piezo2 in the spared nerve injury neuropathic pain model.
Fig. S9. Four individuals with loss-of-function mutations in PIEZO2.
Fig. S10. Psychophysical evaluation of PIEZO2LOF individuals reveals diagnostic mechanosensory deficits.
Acknowledgments:
We are indebted to N. Ryba (NIDCR) for mentorship and advice throughout the study. We thank M. Hoon (NICDR) for advice and for generating and sharing the Piezo2fl/fl mice, C. Le Pichon (NICHD) for helpful suggestions, M. Nguyen for help with the trigeminal spared nerve surgeries, D. Ide and D. Yochelson (NIH section on instrumentation) for help designing and fabricating the custom-built instruments used in this study, and I Szczot for help with programming in Python. We also thank the following members of the clinical staff at the National Center for Complementary and Integrative Health (NCCIH) and the Bönnemann group for support in arranging the human studies: M. M. Lee, P. Yun, T. Ogata, K. Alter, C. Zampierei, C. Stanley, J. Matsubara, P. Mohassel, S. B. Neuhaus, Y. Hu, R. Kaur, T. Lehky, C. Hadigan, B. Solomon, A. Micheil Innes, J. K. Mah, C. M. Grosmann, C. Konersmann, N. Goyal, T. Mozaffar, G. Haliloglu, A. Munnich, C. Vuillerot, F. Munell, A. Nascimento, J. Colomer, A. Nickolls, L. MacLaren, C. Y. Alvarado, and M. Troncoso. We thank the GENIE project for the development of GCaMP6 and the Allen Institute for generating the Ai95 reporter strain.
Funding: This work was supported by the Intramural Research Program of the NIH, specifically the NCCIH (to A.T.C. and M.C.B.), the DDIR Innovation Award (to A.T.C.), and the NINDS (to C.G.B.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All the data are included in the main text or in the Supplementary Materials.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/10/462/eaat9892/DC1
Materials and Methods
REFERENCES AND NOTES
- 1.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A, Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, Hayes LH, Alter K, Zampieri C, Stanley C, Innes AM, Mah JK, Grosmann CM, Bradley N, Nguyen D, Foley AR, Le Pichon CE, Bönnemann CG, The role of PIEZO2 in human mechanosensation. N. Engl. J. Med 375, 1355–1364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranade SS, Woo S-H, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A, Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A, The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold MS, Gebhart GF, Nociceptor sensitization in pain pathogenesis. Nat. Med 16, 1248–1257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng C, Li L, Zhang M-D, Bengtsson Gonzales C., Parisien M, Belfer I, Usoskin D, Abdo H, Furlan A, Häring M, Lallemend F, Harkany T, Diatchenko L, Hökfelt T, Hjerling-Leffler J, Ernfors P, miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes. Science 356, 1168–1171 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Woolf CJ, Central sensitization: Implications for the diagnosis and treatment of pain. Pain 152, S2–S15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szczot M, Pogorzala LA, Solinski HJ, Young L, Yee P, Le Pichon CE, Chesler AT, Hoon MA, Cell-type specific splicing of Piezo2 regulates mechanotransduction. Cell Rep. 21, 2760–2771 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen MQ, Wu Y, Bonilla LS, von Buchholtz LJ, Ryba NJP, Diversity amongst trigeminal neurons revealed by high throughput single cell sequencing. PLOS ONE 12, e0185543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, Wood JN, A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun 4, 1682 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, Zhu Y, Cheng X, Hawke D, Mayor F Jr., Murga C, Heijnen CJ, Kavelaars A, Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc. Natl. Acad. Sci. U.S.A 113, 3036–3041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nonomura K, Woo S-H, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A, Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541, 176–181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weyer AD, O’Hara CL, Stucky CL, Amplified mechanically gated currents in distinct subsets of myelinated sensory neurons following in vivo inflammation of skin and muscle. J. Neurosci 35, 9456–9462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Hurwitz O, Shimada SG, Qu L, Fu K, Zhang P, Ma C, LaMotte RH, Chronic compression of the dorsal root ganglion enhances mechanically evoked pain behavior and the activity of cutaneous nociceptors in mice. PLOS ONE 10, e0137512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AK, O’Hara CL, Stucky CL, Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model. Mol. Pain 9, 61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghitani N, Barik A, Szczot M, Thompson JH, Li C, Le Pichon CE, Krashes MJ, Chesler AT, Specialized mechanosensory nociceptors mediating rapid responses to hair pull. Neuron 95, 944–954.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo S-H, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A, Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci 18, 1756–1762 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS, Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kühnemund J, Francisco AG, Keenan WT, Dubin AE, Lewin GR, Patapoutian A, The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med 10, aat9897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA, An overview of animal models of pain: Disease models and outcome measures. J. Pain 14, 1255–1269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen KL, Rowbotham MC, A new human experimental pain model: The heat/capsaicin sensitization model. Neuroreport 10, 1511–1516 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Haliloglu G, Becker K, Temucin C, Talim B, Küçükşahin N, Pergande M, Motameny S, Nürnberg P, Aydingoz U, Topaloglu H, Cirak S, Recessive PIEZO2 stop mutation causes distal arthrogryposis with distal muscle weakness, scoliosis and proprioception defects. J. Hum. Genet 62, 497–501 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Liljencrantz J, Björnsdotter M, Morrison I, Bergstrand S, Ceko M, Seminowicz DA, Cole J, Bushnell CM, Olausson H, Altered C-tactile processing in human dynamic tactile allodynia. Pain 154, 227–234 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D, The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, Mogil JS, Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 7, 447–449 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Browne LE, Latremoliere A, Lehnert BP, Grantham A, Ward C, Alexandre C, Costigan M, Michoud F, Roberson DP, Ginty DD, Woolf CJ, Time-resolved fast mammalian behavior reveals the complexity of protective pain responses. Cell Rep. 20, 89–98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, Geurts AM, Palygin O, Stucky CL, Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. eLife 7, e31684 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumbauer KM, DeBerry JJ, Adelman PC, Miller RH, Hachisuka J, Lee KH, Ross SE, Koerber HR, Davis BM, Albers KM, Keratinocytes can modulate and directly initiate nociceptive responses. eLife 4, e09674 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang Z, Sakamoto T, Tiwari V, Kim Y-S, Yang F, Dong X, Güler AD, Guan Y, Caterina MJ, Selective keratinocyte stimulation is sufficient to evoke nociception in mice. Pain 156, 656–665 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Liu S, Zhang Y-Q, Goulding M, Wang Y-Q, Ma Q, Timing mechanisms underlying gate control by feedforward inhibition. Neuron 99, 941–955.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wlaschin JJ, Gluski JM, Nguyen E, Silberberg H, Thompson JH, Chesler AT, Le Pichon CE, Dual leucine zipper kinase is required for mechanical allodynia and microgliosis after nerve injury. eLife 7, e33910 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI, Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci 19, 94–101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inquimbert P, Moll M, Latremoliere A, Tong C-K, Whang J, Sheehan GF, Smith BM, Korb E, Athié MCP, Babaniyi O, Ghasemlou N, Yanagawa Y, Allis CD, Hof PR, Scholz J, NMDA receptor activation underlies the loss of spinal dorsal horn neurons and the transition to persistent pain after peripheral nerve injury. Cell Rep. 23, 2678–2689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhandapani R, Arokiaraj CM, Taberner FJ, Pacifico P, Raja S, Nocchi L, Portulano C, Franciosa F, Maffei M, Hussain AF, de Castro Reis F, Reymond L, Perlas E, Garcovich S, Barth S, Johnsson K, Lechner SG, Heppenstall PA, Control of mechanical pain hypersensitivity in mice through ligand-targeted photoablation of TrkB-positive sensory neurons. Nat. Commun 9, 1640 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P, Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci 18, 145–153 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. In vitro responses of cultured neurons to touch demonstrate the efficiency of the mosaic knockout strategy.
Fig. S2. Mechanosensory phenotypes in adult PiezocKO mice.
Fig. S3. Custom-built devices for mechanical stimulation of the mouse cheek.
Fig. S4. Piezo2 is required for trigeminal responses to vibration but not heat.
Fig. S5. CFA induces chronic inflammation and sensitization to heat but not pinch.
Fig. S6. Responses to gentle mechanical stimuli require Piezo2 in the CFA inflammatory pain model.
Fig. S7. Controls for brush responses after capsaicin-induced inflammation.
Fig. S8. Responses to gentle mechanical stimuli require Piezo2 in the spared nerve injury neuropathic pain model.
Fig. S9. Four individuals with loss-of-function mutations in PIEZO2.
Fig. S10. Psychophysical evaluation of PIEZO2LOF individuals reveals diagnostic mechanosensory deficits.