Abstract
Background:
Neurophysiological measures of brain function, such as magnetoencephalography (MEG), are widely used in clinical neurology and have strong relations with cognitive impairment and dementia but are still underdeveloped in multiple sclerosis (MS).
Objectives:
To demonstrate the value of clinically applicable MEG-measures in evaluating cognitive impairment in MS.
Methods:
In eyes-closed resting-state, MEG data of 83 MS patients and 34 healthy controls (HCs) peak frequencies and relative power of six canonical frequency bands for 78 cortical and 10 deep gray matter (DGM) areas were calculated. Linear regression models, correcting for age, gender, and education, assessed the relation between cognitive performance and MEG biomarkers.
Results:
Increased alpha1 and theta power was strongly associated with impaired cognition in patients, which differed between cognitively impaired (CI) patients and HCs in bilateral parietotemporal cortices. CI patients had a lower peak frequency than HCs. Oscillatory slowing was also widespread in the DGM, most pronounced in the thalamus.
Conclusion:
There is a clinically relevant slowing of neuronal activity in MS patients in parietotemporal cortical areas and the thalamus, strongly related to cognitive impairment. These measures hold promise for the application of resting-state MEG as a biomarker for cognitive disturbances in MS in a clinical setting.
Keywords: Multiple sclerosis, magnetoencephalography, cognition, oscillatory activity, power
Introduction
An estimated 50% of multiple sclerosis (MS) patients suffer from cognitive decline, thereby affecting quality of life.1 Although validated neuropsychological test batteries exist, such measurements can be influenced by factors such as mood disorders, fatigue, and underperformance. A reliable biomarker for cognitive functioning would therefore greatly enhance its objective evaluation.
Adequate cognitive functioning requires coordinated interaction between neurons within and across specialized brain areas.2 Cortical gray matter demyelination and thalamic atrophy are thought to impact cognitive functioning by disturbing such coordinated neuronal interaction,3,4 with thalamic atrophy, in particular, as a strong predictor of cognitive decline.5 Unfortunately, thalamic atrophy can be difficult to measure in clinical practice, making it less useful as a biomarker for cognitive performance.
Magnetoencephalography (MEG) directly measures neuronal activity, with a high temporal resolution. Quantitative resting-state MEG measurements are clinically comparable with electroencephalography (EEG) measurements; such measures have been used for almost a century in standard neurological care. The principal advantage of MEG over EEG is its higher spatial resolution and ability to measure function in deep gray matter (DGM) structures such as the thalamus.
Previous studies have established the validity and test–retest reliability of oscillatory phenotypes as markers of cognitive performance in MEG and EEG.6,7 The aim of this study was to further extend these studies by identifying quantitative MEG biomarkers for a new and expanded cohort of cognitively impaired (CI) MS patients compared to preserved patients. In addition, we also focus on the DGM structures, including the thalamus. Such measures could hold promise for the detection of cognitive impairment in a clinical setting.
Methods
Participants
Existing data of 83 MS patients and 34 healthy controls (HC) from the Amsterdam MS cohort were analyzed. These data sets were acquired as part of an ongoing clinical study at the MS Center Amsterdam, as described previously.8 Patients were diagnosed with clinically definitive MS according to the revised McDonald criteria9 and involved relapsing-remitting multiple sclerosis (RRMS, N = 59), secondary progressive multiple sclerosis (SPMS, N = 16), and primary progressive multiple sclerosis (PPMS, N = 8). All participants underwent clinical assessment consisting of history taking, neurological examination, blood tests, neuropsychological tests, structural magnetic resonance imaging (MRI), and MEG-recording. Educational level was determined using a Dutch classification system, ranking from 1 (did not finish primary education) to 7 (university degree), described previously.4 Disability was classified using the Expanded Disability Status Scale (EDSS).10
Data acquisition
Neuropsychological evaluation
Directly after the MEG sessions, neuropsychological tests were performed, consisting of the brief repeatable battery of neuropsychological (BRB-N) tests, expanded with the concept shifting test (CST), the Stroop test, and the memory comparison test (MCT). See Supplementary Information for a more detailed description of the tests. For each patient, a Z-score (corrected for age, gender, and educational level) was calculated for each test, based on the mean and standard deviations (SDs) of the complete HC group from the original cohort. Z-scores were averaged for each domain separately and subsequently averaged into an average cognition Z-score, as previously described.11 Patients with a score lower than −2 SD on at least two domains below the HC scores were considered “cognitively impaired,” while patients with a score below 1.5–2 SD below HCs on at least two domains, while not fulfilling the CI-criteria, were defined as “mildly cognitively impaired” (MCI). Patients scoring better than MCI were considered “cognitively preserved” (CP).
MRI recordings
All subjects were scanned on a 3 T whole-body magnetic resonance system (General Electric Signa-HDxt, Milwaukee, WI, USA), using an eight-channel phased-array head coil, according to a previously described protocol (see Supplementary Information).12 Normalized gray matter volumes (NGMV), white matter volumes (NWMV), and whole-brain volumes (NBV) were measured with SIENAX (FSL 5, FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl),13 after lesion filling. Thalamic volumes were measured using FIRST (part of FSL), corrected for head size with the V-scaling factor of SIENAX. All scans were inspected by an experienced rater (M.M.S.).
MEG-recordings and pre-processing
MEG data were recorded and pre-processed according to a standardized pipeline.14 Specific details can be found in the Supplementary Information, but summarized: broad-band eyes-closed resting-state data were projected to 78 cortical and 10 DGM regions of interest (ROIs) of the automated anatomical labeling (AAL) atlas using an atlas-based beamformer.15 Afterwards, the data were cleaned using the temporal extension of Signal Space Separation (tSSS) in MaxFilter software.16,17 For each subject, five non-overlapping, artifact-free epochs of 16,384 samples (13.1072 seconds) were selected after careful visual inspection and down-sampled by a factor of 4.
Time-series analyses
The time series were digitally filtered using a discrete fast Fourier transform to calculate the relative power for each of the six classical EEG/MEG frequency bands (delta (0.5–4 Hz), theta (4–8 Hz), alpha1 (8–10 Hz), alpha2 (10–13 Hz), beta (13–30 Hz), and gamma (13–48 Hz), henceforth referred to as “relative spectral power”), and peak frequency, in each cortical ROI (n = 78) and DGM ROI (n = 10), resulting in six sets of five epochs for all ROIs. Peak frequency and relative powers were estimated for each cortical and subcortical ROI separately. In addition, cortical relative power was computed as mean relative power over all 78 cortical ROIs and epochs. The same was done for peak frequency to get the average cortical peak frequency. DGM relative powers and peak frequency were estimated as means over 10 DGM ROIs (Table 1).
Table 1.
Overview of quantitative MEG measurements; analyses that were performed in this study.
| MEG analyses |
|---|
| Cognition groups (HC, CI, CP, MCI) |
| Whole-brain measurements (average of 78 cortical ROIs) |
| peak frequency |
| relative powers (for six frequency bands) |
| Regional measurements (for each ROI, N = 78 cortical and 10 subcortical ROIs) |
| relative powers (for six frequency bands) |
| Correlation whole-brain measurements (average of N = 78 cortical ROIs) and specific cognitive domains (N = 8 cognitive domains) |
CP: cognitively preserved; MCI: mild cognitively impaired; CI: cognitively impaired; HC: healthy controls; ROI: region of interest.
Overview of quantitative MEG measurements; analyses that were performed in this study.
Statistical analysis
Baseline characteristics
Statistical analyses were performed in IBM SPSS Statistics 22.0 or MATLAB, version 7.14.0.739 (MathWorks, Natick (MA), USA). Normality was checked by visual inspection and the Kolmogorov–Smirnov test. Univariate and multivariate linear models were used (or, in the absence of normality, non-parametric testing) to identify group differences, using age, gender, and educational level as covariates.
If significant whole-brain (i.e. cortical and subcortical) relative spectral power differences were found within specific frequency bands, regional relative power values were compared between groups using a Mann–Whitney test. In order to determine which frequency bands correlated most strongly with overall cognition and each cognitive domain, a linear regression model was constructed, correcting for age, gender, and education, to obtain coefficients for the correlation between average cognition Z-scores and each of the MEG variables (i.e. whole-brain relative spectral power in a frequency band or peak frequency). A p-value of <0.05 was considered statistically significant with correction for multiple comparisons using the false discovery rate (FDR), correcting for six frequency bands plus peak frequency times eight cognitive domains.18 Furthermore, differences in whole-brain relative spectral powers and peak frequencies between MS subtypes (regardless of cognition scores) were explored using a logistic regression model correcting for age, gender, and education.
Results
Baseline characteristics
Characteristics of patients and HCs are summarized in Table 2. A total of 37 MS patients were classified as CP, 18 as MCI, and 28 as CI; and 59 had RRMS, 8 had PPMS, and 16 had SPMS. There were no significant differences in age, sex, education, symptom duration, or disease duration between the study groups. Not surprisingly, there were significant differences in average cognition Z-scores between the CI group and all other groups (p < 0.001) and between the MCI group and all other groups (p < 0.001) but not between the CP and the HC group. There was a significantly higher EDSS in the CI group compared to the MCI and CP groups.
Table 2.
Baseline characteristics.
| CP (N = 37) | MCI (N = 18) | CI (N = 28) | HC (N = 34) | |
|---|---|---|---|---|
| Age (years) | 51.9 ± 9.9 | 54.6 ± 8.6 | 53.9 ± 9.9 | 50.7 ± 9.8 |
| Sex (female, n (%)) | 27 (73) | 11 (61%) | 17 (61) | 21 (62) |
| Educational level (1–7) | 4.0 (IQR = 3) | 4.0 (IQR = 2.3) | 4.0 (IQR = 3) | 6 (IQR = 2.3) |
| EDSS (0–10)a | 3.0 (IQR = 2.3) | 3.25 (IQR = 2.3) | 4.5 (IQR = 2.5) | – |
| Average cognitionb (Z-score) | −0.24 ± 0.43 | −1.1 ± 0.36 | −1.8 ± 0.58 | −0.09 ± 0.48 |
| Symptom duration (years) | 17.1 ± 6.0 | 19.8 ± 8.3 | 19.7 ± 7.2 | − |
| Diagnosis duration (years) | 13.4 ± 4.1 | 14.7 ± 6.9 | 16.9 ± 7.0 | − |
| NWMV (l) | 0.65 ± 0.037 | 0.67 ± 0.036 | 0.65 ± 0.034 | 0.69 ± 0.024 |
| NCGMV (l)b | 0.74 ± 0.044 | 0.74 ± 0.037 | 0.71 ± 0.051 | 0.76 ± 0.034 |
| NDGMV (l)b | 0.057 ± 0.0056 | 0.055 ± 0.0052 | 0.051 ± 0.0071 | 0.062 ± 0.0029 |
| Thalamic volume (L)b | 0.018 ± 0.0019 | 0.018 ± 0.0019 | 0.016 ± 0.0031 | 0.020 ± 0.0011 |
| White matter lesion load (mL) | 17,414 ± 15,477 | 19,110 ± 15,221 | 31,214 ± 24535 | − |
CP: cognitively preserved; MCI: mild cognitively impaired; CI: cognitively impaired; HC: healthy controls; EDSS: Expanded Disability Status Scale; NWMV: normalized white matter volume; NCGMV: normalized cortical gray matter volume; NDGMV: normalized deep gray matter volume.
Depicted are mean values ± SD or median with the interquartile range (IQR) where appropriate.
Significant difference between the CI versus the CP (p-value = 0.037) and the CI versus the MCI (p-value = 0.04) groups.
Significant group differences exist, see described in text.
NWMVs were similar across all groups. For the NGMV, the CI group had a lower volume than the CP group (p = 0.015) and HCs (p < 0.001), but not than the MCI group. The CP group had a lower NGMV than HCs (p = 0.012) but also not than the MCI group. The MCI group had a lower NGMV than HCs (p = 0.036). There was only a significant difference between white matter lesion loads between the CI and CP groups (p = 0.043).
As mentioned before, thalamic atrophy is strongly associated with cognitive decline;5 we therefore also looked at group differences in thalamic volume. The CI group had a lower thalamic volume than the CP (p = 0.04) and HC group (p < 0.001), but not than the MCI group. The CP group had a lower thalamic volume than the HC group (p < 0.001), but not than the MCI group. Thalamic volume was lower in the MCI group than HCs (p < 0.01). See Table 2 for a full list of descriptives.
Quantitative MEG analysis
Global cortical power differences between groups
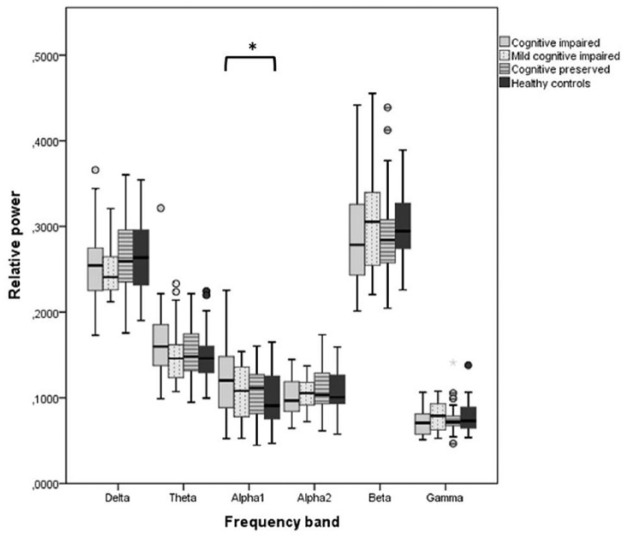
There was a diffuse slowing of oscillatory activity in CI patients compared to HCs, where only the CI group had significantly lower peak frequency than HCs (9.2 versus 9.7 Hz, p = 0.019). There were no differences in peak frequency between the other groups (Figure 1). When looking at the individual frequency bands, CI patients had a significantly higher whole-brain relative spectral alpha1 power than HCs (mean alpha1 power CI = 0.121, HC = 0.099; p = 0.029, Figure 2). Notably, a similar pattern was seen in the theta band without reaching statistical significance (mean theta power CI = 0.165, HC = 0.150; p = 0.090). No significant whole-brain differences between groups were found for the other frequency bands.
Figure 1.
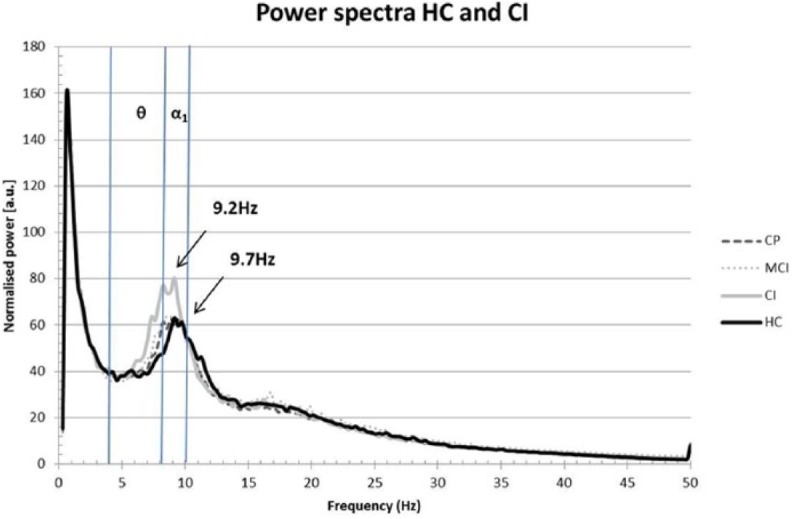
Power spectra for the different cognition groups versus healthy controls.
CI patients had a significantly lower peak frequency (9.2 Hz, p = 0.019) than the HC group (9.7 Hz). The CP and MCI groups are added for reference and were not significantly different from any of the groups. The blue lines represent the thresholds for the significant frequency bands (the theta band ranges from 4–8 Hz and alpha1 band from 8–10 Hz).
Figure 2.
Whole-brain cortical relative power in six frequency bands.
Whole-brain relative powers averaged over 78 cortical ROIs for each of the cognitive groups for each of the six different frequency bands.
*Significantly higher relative alpha1 power in the CI group than the HC group after correction for age, gender, education, and multiple comparisons (p = 0.029).
Regional cortical power differences between groups
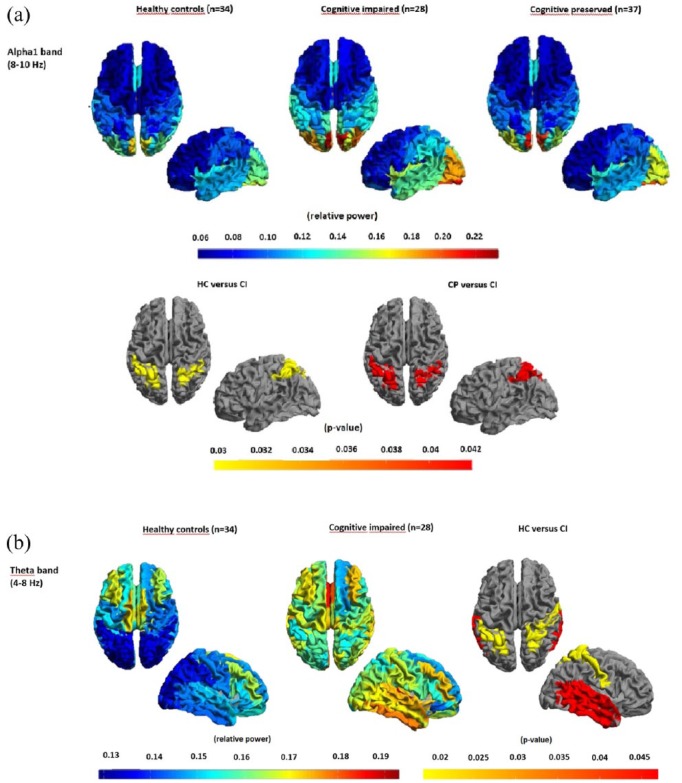
CI patients showed significantly increased cortical relative spectral theta and alpha1 power in bilateral superior and inferior parietal gyri, compared to HCs and HCs and CP patients, respectively (Figure 3). Bilateral superior, medial, and inferior temporal gyri showed increased theta power only in the CI group versus HCs. No additional significant differences were seen in any other cortical region or band.
Figure 3.
Regional relative power and significant group differences.
Regional relative power and significant group differences displayed as color-coded maps on a parcellated template mesh: (a) alpha1 band (first and second row) and (b) theta band (third row). Note the significantly higher relative alpha1 power in the CI group versus HC, and versus CI. Note that for the theta band, the anterior–posterior gradient in the HC group is absent in the CI group and that the relative power for the CI group is significantly higher for several regions in the parietal and temporal cortices.
Global and regional DGM differences between groups
The CI group had significantly higher average DGM relative spectral theta power as well as a lower alpha2 power than CP patients. A trend was seen for increased relative spectral alpha1 power (p = 0.09), which did not survive FDR-correction. In addition, group differences in alpha2 and theta power between CI patients and HCs were present in nearly all subcortical areas but with the most pronounced effect in both thalami (Tables 3 and 4). No differences were found within the other frequency bands or between the other groups.
Table 3.
Average relative power values of the deep gray matter for each cognition group.
| CP (N = 37), mean ± SD | MCI (N = 18), mean ± SD | CI (N = 28), mean ± SD | HC (N = 34), mean ± SD | |
|---|---|---|---|---|
| Peak frequency | 8.150 (0.912) | 8.192 (0.7160 | 8.021 (1.049) | 8.122 (1.034) |
| Delta power | 0.268 (0.061) | 0.255 (0.039) | 0.253 (0.053) | 0.258 (0.042) |
| Theta power | 0.167 (0.033) | 0.166 (0.040) | 0.184 (0.057) b | 0.162 (0.032) |
| Alpha1 power | 0.108 (0.031) | 0.110 (0.040) | 0.112 (0.036) | 0.100 (0.025) |
| Alpha2 power | 0.103 (0.024) | 0.098 (0.014) | 0.093 (0.016) a | 0.109 (0.022) |
| Beta power | 0.288 (0.062) | 0.303 (0.067) | 0.293 (0.074) | 0.304 (0.039) |
| Gamma power | 0.066 (0.018) | 0.068 (0.019) | 0.064 (0.018) | 0.067 (0.019) |
CP: cognitively preserved; MCI: mild cognitively impaired; CI: cognitively impaired; HC: healthy controls.
Average peak frequency and power values for each of the six frequency bands, averaged over 10 deep gray matter ROIs. Depicted are mean values (SD).
Significant difference between the CI and HC group (p = 0.031).
Significant difference between the CI versus the HC (p = 0.004) and a trend between the CI versus the CP (p = 0.09) groups. All other differences were not statistically significant.
Table 4.
Differences in relative power in deep gray matter structures between CI and HC groups.
| ROI (AAL) | CI group | HC group | Difference | Corrected p-value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | CI vs HC | |||
| Alpha2 power | Thalamus (90) | 0.101 | 0.021 | 0.122 | 0.034 | 0.021 | 0.01 |
| Thalamus (89) | 0.102 | 0.025 | 0.122 | 0.032 | 0.020 | 0.018 | |
| Pallidum R (88) | 0.092 | 0.016 | 0.111 | 0.023 | 0.019 | 0.003 | |
| Pallidum L (87) | 0.095 | 0.021 | 0.113 | 0.025 | 0.018 | 0.006 | |
| Amygdala R (82) | 0.096 | 0.023 | 0.111 | 0.022 | 0.015 | 0.014 | |
| Putamen R (86) | 0.090 | 0.016 | 0.104 | 0.022 | 0.014 | 0.014 | |
| Putamen L (85) | 0.092 | 0.018 | 0.106 | 0.024 | 0.014 | 0.018 | |
| Amygdala L (81) | 0.098 | 0.022 | 0.112 | 0.026 | 0.014 | 0.023 | |
| Caudate L (83) | 0.084 | 0.016 | 0.094 | 0.024 | 0.010 | 0.053 | |
| Caudate R (84) | 0.083 | 0.016 | 0.091 | 0.019 | 0.008 | 0.091 | |
| ROI (AAL) | Mean | SD | Mean | SD | Difference | Corrected p-value | |
| Alpha1 power | Caudate R (84) | 0.100 | 0.035 | 0.084 | 0.021 | –0.016 | 0.017 |
| Thalamus (90) | 0.126 | 0.051 | 0.111 | 0.041 | −0.015 | NS | |
| Amygdala L (81) | 0.124 | 0.042 | 0.110 | 0.029 | −0.014 | NS | |
| Thalamus (89) | 0.125 | 0.042 | 0.112 | 0.039 | −0.013 | NS | |
| Caudate L (83) | 0.099 | 0.035 | 0.086 | 0.024 | −0.013 | 0.094 | |
| Pallidum R (88) | 0.112 | 0.041 | 0.099 | 0.026 | −0.013 | 0.107 | |
| Putamen R (86) | 0.105 | 0.038 | 0.093 | 0.026 | −0.012 | 0.098 | |
| Pallidum L (87) | 0.114 | 0.038 | 0.102 | 0.025 | −0.012 | 0.1 | |
| Putamen L (85) | 0.103 | 0.030 | 0.094 | 0.022 | −0.009 | NS | |
| Amygdala R (82) | 0.115 | 0.044 | 0.112 | 0.034 | −0.003 | NS | |
| ROI (AAL) | Mean | SD | Mean | SD | Difference | Corrected p-value | |
| Theta power | Thalamus (90) | 0.183 | 0.055 | 0.156 | 0.035 | –0.027 | 0.014 |
| Pallidum R (88) | 0.188 | 0.062 | 0.161 | 0.032 | –0.027 | 0.031 | |
| Pallidum L (87) | 0.190 | 0.066 | 0.166 | 0.034 | –0.024 | 0.027 | |
| Thalamus (89) | 0.182 | 0.060 | 0.159 | 0.034 | –0.023 | 0.034 | |
| Putamen R (86) | 0.181 | 0.059 | 0.159 | 0.033 | –0.022 | 0.05 | |
| Amygdala L (81) | 0.187 | 0.056 | 0.166 | 0.036 | −0.021 | 0.053 | |
| Amygdala R (82) | 0.184 | 0.054 | 0.164 | 0.034 | −0.020 | 0.062 | |
| Caudate R (84) | 0.184 | 0.060 | 0.164 | 0.033 | −0.020 | 0.069 | |
| Putamen L (85) | 0.182 | 0.066 | 0.163 | 0.033 | −0.019 | 0.053 | |
| Caudate L (83) | 0.180 | 0.058 | 0.167 | 0.039 | −0.013 | NS | |
CI: cognitively impaired; HC: healthy controls; ROI: region of interest; SD: standard deviation; R: right; L: left; NS: not significant.
Differences in relative powers in 10 deep gray matter structures between CI and HC groups, ranked by effect size and, in case of equal effect size, significance. Depicted are the mean relative power values for each ROI in the alpha2, alpha1, and theta band for the CI and HC group, since group differences were only significant in these power bands. Analyses were corrected for age, gender, and multiple comparisons over regions (correcting for 10 regions times six frequency bands plus peak frequency). Significant regions are printed in bold and italics. Depicted are both significant p-values as well as p-values < 0.1 to show the trend across the majority of deep gray matter structures.
Individual cognitive domains and global cortical relative power
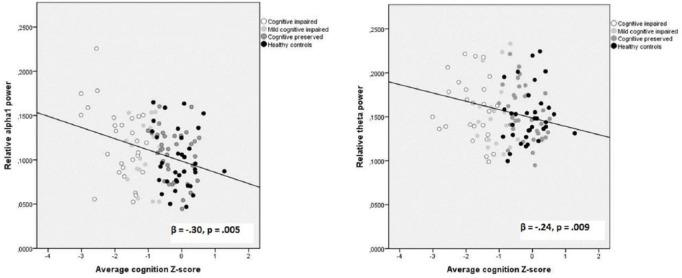
To determine whether specific cognitive domains were associated with frequency and power differences, we looked at the individual continuous cognition scores in a post hoc analysis. Increased whole-brain relative spectral alpha1 power was associated with impaired overall cognitive performance (standardized β = –0.30, t(116) = –3.50, p = 0.005, Figure 4) but specifically with attention (β = –0.41, t(116) = –3.61, p < 0.001), working memory (β = –0.39, t(116) = –4.25, p < 0.001), and verbal memory (β = –0.26, t(116) = –3.80, p = 0.020). Increased whole-brain relative spectral theta power was also associated with worse overall cognitive performance (β = –0.24, t(116) = –2.04, p = 0.009, Figure 4), as well as verbal memory (β = –0.33, t(116) = –3.45, p = 0.003). These correlations were not driven by the observed group differences alone, since a correlation analysis for the patient group only (see Supplementary Information) confirmed the alpha1 band result (standardized β = –0.27, p = 0.018), although the correlation within the theta band was reduced to a trend (standardized β = –0.15, p = 0.18).
Figure 4.
Correlation between global relative alpha1 and theta power (averaged over 78 cortical ROIs) and average cognition Z-scores.
β = standardized coefficient, corrected for age, gender, and education.
No significant correlation was found between cognition and whole-brain relative spectral power in the other frequency bands. A non-significant trend was found between relative power in the theta, alpha1, and alpha2 bands and information processing speed (see Supplementary Information).
Disease severity and MRI parameters
In a preliminary post hoc analysis, a significant correlation was found between SPMS subtype and higher relative spectral alpha1 (β = –0.228, p = 0.022) and theta power (β = –0.253, p = 0.010). Moreover, PPMS patients had higher alpha1 (0.126 and 0.107) and theta power (0.176 vs 0.157) than RRMS patients, although the sample size of the PPMS group was small (N = 8), and this difference was not statistically significant. Higher theta power was associated with higher lesion load and lower brain volume (Table 5). In addition, both alpha1 and gamma power were associated with lower DGM volumes. Delta power was associated with both cortical and DGM atrophy. No other significant associations between power values and MRI parameters existed.
Table 5.
Association between relative power values and MRI parameters.
| White matter lesion load | NWMV | NCGMV | NDGMV | Thalamic volume | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | p-value | Beta | p-value | Beta | p-value | Beta | p-value | Beta | p-value | |
| Peak frequency | 0.002 | NS | −0.020 | NS | −0.058 | NS | −0.018 | NS | −0.08 | NS |
| Delta power | −0.128 | NS | 0.134 | NS | 0.240 | 0.020 | 0.229 | 0.018 | 0.314 | <0.001 |
| Theta power | 0.268 | 0.018 | –0.255 | 0.011 | –0.289 | 0.005 | –0.324 | <0.001 | –0.357 | <0.001 |
| Alpha1 power | 0.148 | NS | −0.200 | NS | −0.123 | NS | –0.277 | 0.005 | –0.311 | <0.001 |
| Alpha2 power | 0.022 | NS | 0.027 | NS | −0.038 | NS | 0.044 | NS | 0.001 | NS |
| Beta power | −0.116 | NS | 0.124 | NS | 0.036 | NS | 0.107 | NS | 0.090 | NS |
| Gamma power | −0.230 | NS | 0.194 | NS | 0.205 | NS | 0.282 | 0.004 | 0.321 | <0.001 |
NWMV: normalized white matter volume, NCGMV; normalized cortical gray matter volume, NDGMV; normalized deep gray matter volume.
Relative power values were calculated as an average over 78 cortical ROIs. Depicted are standardized beta’s and corresponding p-values after correction for multiple comparisons. Analyses are corrected for age and gender. Significance level: p < 0.05.
Discussion
The aim of this study was to demonstrate the value of clinically applicable quantitative MEG measurements in evaluating cognitive impairment in MS. We found that cognitive impairment in MS is associated with global slowing of the posterior dominant rhythm, resulting, due to the use of relative power bands, in an increase in relative spectral theta and alpha1 (and reduced alpha2) power across the brain. Regionally, a specific involvement of parietotemporal regions and the DGM structures was seen. In terms of specific cognitive domains, this increase in alpha1 power correlated strongly with a reduced overall cognition but specifically with attention, working memory and verbal memory. In addition, we have shown that this neuronal slowing also exists within the DGM structures in general and the thalamus specifically.
Up to now, evidence for disease-specific and clinically applicable quantitative EEG/MEG alterations in MS remains sparse. Our current findings are in line with two branches of research. First, we would like to highlight two previous studies that have performed pioneering work regarding brain oscillatory activity in relation to cognitive functioning in MS. Van der Meer et al.6 previously reported a lower peak frequency in another cohort of early MS patients (n = 21), coinciding with a higher alpha1 power and a lower alpha2 power in the complete MS patient group than HCs. Increased relative power in the alpha 1 band also correlated with reduced overall cognition and reduced information processing speed. Similar observations were made in other studies, with EEG showing a higher theta power in MS patients in general (with a similar, non-significant pattern in CI patients)19 and an increased theta power over the temporal regions, which was related to disability.20 In addition, Keune et al.7 replicated the association reported by Van der Meer et al. between increased relative alpha1 activity and reduced Symbol Digit Modalities Test (SDMT) performance (a measure of information processing speed) in an EEG setting (n = 25), providing strong evidence that alpha power might serve as a marker of cognitive performance in MS. We have now confirmed these findings in a more heterogeneous, as well as larger, cohort of MS patients and have specifically shown that these effects are mainly present in CI patients.
Both Van der Meer et al. and Keune et al. reported a negative correlation between global relative power in the alpha1 band and information processing speed. Although our analysis showed a trend toward reduced information processing speed in relation to higher relative spectral theta and alpha1 power (see Supplementary Information) and mimicked the results from the previous studies, they did not reach statistical significance.
Second, an extensive branch of MS research has focused on evaluating cognitive performance by means of neuropsychological examination. Depending on the disease phase and type, 43%–70% of MS patients exhibit various aspects of cognitive dysfunction, with PPMS patients being the most severely affected.21 Previous research has shown that cognitive decline in MS is mostly domain specific; overt dementia is seldom seen. The domains most frequently affected are information processing speed, attention, working memory, verbal and visuospatial memory, and executive functions.21,22 Our findings showed a negative correlation between alpha1 power and attention, working memory, and verbal memory, as well as between theta power and verbal memory. This overlap between domain-specific cognitive impairment and the correlations with global oscillatory slowing may provide new insight into the underpinnings of the underlying pathophysiology, as discussed in the following paragraph.
We have, for the first time, demonstrated that the thalamus is involved in the global oscillatory slowing within the alpha band in MS and that there is an association with thalamic atrophy. The thalamus is a key region involved in the generation of alpha oscillations.23 As a “relay organ,” the thalamus serves as a gateway to the cerebral cortex and is involved in a wide range of neurological functions including motor, sensory, integrative, and higher cortical functions such as memory, emotion, consciousness, awareness, and attention.24 Reduction in thalamic volume has repeatedly been shown to strongly affect cognitive performance.5,25 Although traditionally viewed as a “passive” station, recent studies have identified the thalamus as a critical hub region involved in actively integrating diverse cognitive processes, as well as maintaining the modular structure of cortical functional networks.26 This property could potentially allow the thalamus to send and access information across diverse cortical functional networks, thus sub-serving multiple cognitive functions. Coincidentally, these networks have been shown to be altered in MS patients with cognitive complaints.5
Finally, we would like to highlight that slowing of resting-state oscillatory activity has been found in several other (primary neurodegenerative) diseases, such as Parkinson’s disease27 and Alzheimer’s disease28 in both EEG and MEG studies. Although less pronounced, the oscillatory slowing in MS shows a comparable pattern. It remains to be seen whether disease-specific mechanisms play a role, or whether such slowing is present as a final common pathway of neurodegenerative processes and focal pathology.
There are some potential limitations that need to be considered. A limitation of MEG is often thought to be its lower spatial resolution for deeper (subcortical) regions.29,30 The spatial resolution of beamformer-reconstructed images of neuronal activity ranges between sub-millimeter and several centimeters across the brain,31 meaning that there is a possibility of reduced spatial resolution for the thalamus, which is approximately 3-cm long. However, recent simulation and experimental studies have demonstrated the beamformers’ ability to accurately project sensor-space data to deeper brain regions.29,32–34 Another potential limitation is the absence of data on potential confounders, such as fatigue or the prevalence of depression, which are known to be related to impaired cognition. However, depression and anxiety have previously been evaluated in the larger cohort from which this sample was derived and were found to have no effect on cognitive outcome in both patients and controls. To minimize the potential effect of slowing of oscillatory activity by drowsiness, the epochs from the original set were assessed by an independent experienced assessor (MF), who checked for artifacts and effects of drowsiness and was, at the time of epoch selection, blind to the group assignment. Finally, the data were analyzed using canonical frequency bands, which simplified comparison with previous studies. Future studies could explore the use of approaches to parameterize the spectra35 and examine whether such approaches are sensitive to differences between patient groups.
To conclude, an important step forward has been made in identifying objective measures that can potentially discriminate between cognitive impairment and cognitive preservation in MS. Additional work needs to be done to assess sensitivity and specificity in a larger patient group as well as to evaluate the prospective value of such measures. Future studies also need to further elucidate the relationship between cortical and thalamic oscillatory slowing and further unravel the complex interplay between different pathological processes that ultimately determine the disease course and burden in MS.
Supplemental Material
Supplemental material, Supplementary_Figure_4 for Resting-state MEG measurement of functional activation as a biomarker for cognitive decline in MS by Deborah N Schoonhoven, Matteo Fraschini, Prejaas Tewarie, Bernard MJ Uitdehaag, Anand JC Eijlers, Jeroen JG Geurts, Arjan Hillebrand, Menno M Schoonheim, Cornelis J Stam and Eva MM Strijbis in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, Supplementary_Information_methods for Resting-state MEG measurement of functional activation as a biomarker for cognitive decline in MS by Deborah N Schoonhoven, Matteo Fraschini, Prejaas Tewarie, Bernard MJ Uitdehaag, Anand JC Eijlers, Jeroen JG Geurts, Arjan Hillebrand, Menno M Schoonheim, Cornelis J Stam and Eva MM Strijbis in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, Supplementary_Information_SDMT_FDR for Resting-state MEG measurement of functional activation as a biomarker for cognitive decline in MS by Deborah N Schoonhoven, Matteo Fraschini, Prejaas Tewarie, Bernard MJ Uitdehaag, Anand JC Eijlers, Jeroen JG Geurts, Arjan Hillebrand, Menno M Schoonheim, Cornelis J Stam and Eva MM Strijbis in Multiple Sclerosis Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that J.J.G.G. is an Editor in Europe for Multiple Sclerosis Journal. J.J.G.G. was not in any way involved in the handling of this paper.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: MM Schoonheim  https://orcid.org/0000-0002-2504-6959
https://orcid.org/0000-0002-2504-6959
Contributor Information
Deborah N Schoonhoven, Departments of Neurology and Clinical Neurophysiology, Magnetoencephalography Center Amsterdam UMC, location VUmc, Amsterdam, The Netherlands.
Matteo Fraschini, Departments of Neurology and Clinical Neurophysiology, Magnetoencephalography Center Amsterdam UMC, location VUmc, Amsterdam, The Netherlands/Department of Electrical and Electronic Engineering, University of Cagliari, Cagliari, Italy.
Prejaas Tewarie, Departments of Neurology and Clinical Neurophysiology, Magnetoencephalography Center Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands.
Bernard MJ Uitdehaag, Department of Neurology, Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands.
Anand JC Eijlers, Department of Anatomy and Neurosciences, Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands.
Jeroen JG Geurts, Department of Anatomy and Neurosciences, Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands.
Arjan Hillebrand, Department of Clini cal Neurophysiology, Magnetoencephalography Center Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands.
Menno M Schoonheim, Department of Anatomy and Neurosciences, Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands.
Cornelis J Stam, Department of Clinical Neurophysiology, Magnetoencephalography Center Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands.
Eva MM Strijbis, Departments of Neurology and Clinical Neurophysiology, Magnetoencephalography Center Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands.
References
- 1. Calabrese P. Neuropsychology of multiple sclerosis: An overview. J Neurol 2006; 253(Suppl. 1): I10–I15. [DOI] [PubMed] [Google Scholar]
- 2. Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron 2006; 52(1): 155–168. [DOI] [PubMed] [Google Scholar]
- 3. Inglese M, Oesingmann N, Casaccia P, et al. Progressive multiple sclerosis and gray matter pathology: An MRI perspective. Mt Sinai J Med 2011; 78(2): 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tewarie P, Schoonheim MM, Stam CJ, et al. Cognitive and clinical dysfunction, altered MEG resting-state networks and thalamic atrophy in multiple sclerosis. PLoS ONE 2013; 8(7): e69318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schoonheim MM, Hulst HE, Brandt RB, et al. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 2015; 84(8): 776–783. [DOI] [PubMed] [Google Scholar]
- 6. Van der Meer ML, Tewarie P, Schoonheim MM, et al. Cognition in MS correlates with resting-state oscillatory brain activity: An explorative MEG source-space study. Neuroimage Clin 2013; 2: 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keune PM, Hansen S, Weber E, et al. Exploring resting-state EEG brain oscillatory activity in relation to cognitive functioning in multiple sclerosis. Clin Neurophysiol 2017; 128(9): 1746–1754. [DOI] [PubMed] [Google Scholar]
- 8. Tewarie P, Steenwijk MD, Tijms BM, et al. Disruption of structural and functional networks in long-standing multiple sclerosis. Hum Brain Mapp 2014; 35(12): 5946–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 11. Amato MP, Portaccio E, Goretti B, et al. The Rao’s brief repeatable battery and stroop test: Normative values with age, education and gender corrections in an Italian population. Mult Scler 2006; 12(6): 787–793. [DOI] [PubMed] [Google Scholar]
- 12. Eijlers AJC, Meijer KA, van Geest Q, et al. Determinants of cognitive impairment in patients with multiple sclerosis with and without atrophy. Radiology 2018; 288: 544–551. [DOI] [PubMed] [Google Scholar]
- 13. Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002; 17(1): 479–489. [DOI] [PubMed] [Google Scholar]
- 14. Boon LI, Hillebrand A, Dubbelink KTEO Stam, et al. Changes in resting-state directed connectivity in cortico-subcortical networks correlate with cognitive function in Parkinson’s disease. Clin Neurophysiol 2017; 128(7): 1319–1326. [DOI] [PubMed] [Google Scholar]
- 15. Hillebrand A, Barnes GR, Bosboom JL, et al. Frequency-dependent functional connectivity within resting-state networks: An atlas-based MEG beamformer solution. Neuroimage 2012; 59(4): 3909–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taulu S, Hari R. Removal of magnetoencepha-lographic artifacts with temporal signal-space separation: Demonstration with single-trial auditory-evoked responses. Hum Brain Mapp 2009; 30(5): 1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taulu S, Simola J, Kajola M. MEG recordings of DC fields using the signal space separation method (SSS). Neurol Clin Neurophysiol 2004; 2004: 35. [PubMed] [Google Scholar]
- 18. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc B: Met 1995; 57(1): 289–300. [Google Scholar]
- 19. Leocani L, Locatelli T, Martinelli V, et al. Electroencephalographic coherence analysis in multiple sclerosis: Correlation with clinical, neuropsychological, and MRI findings. J Neurol Neurosurg Psychiatry 2000; 69(2): 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colon E, Hommes OR, de Weerd JP. Relation between EEG and disability scores in multiple sclerosis. Clin Neurol Neurosurg 1981; 83(3): 163–168. [DOI] [PubMed] [Google Scholar]
- 21. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008; 7(12): 1139–1151. [DOI] [PubMed] [Google Scholar]
- 22. Amato MP, Portaccio E, Goretti B, et al. Cognitive impairment in early stages of multiple sclerosis. Neurol Sci 2010; 31: 211–214. [DOI] [PubMed] [Google Scholar]
- 23. Roux F, Wibral M, Singer W, et al. The phase of thalamic alpha activity modulates cortical gamma-band activity: Evidence from resting-state MEG recordings. J Neurosci 2013; 33(45): 17827–17835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Minagar A, Barnett MH, Benedict RH, et al. The thalamus and multiple sclerosis: Modern views on pathologic, imaging, and clinical aspects. Neurology 2013; 80(2): 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Batista S, Zivadinov R, Hoogs M, et al. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 2012; 259(1): 139–146. [DOI] [PubMed] [Google Scholar]
- 26. Hwang K, Bertolero MA, Liu WB, et al. The human thalamus is an integrative hub for functional brain networks. J Neurosci 2017; 37(23): 5594–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bosboom JL, Stoffers D, Stam CJ, et al. Resting state oscillatory brain dynamics in Parkinson’s disease: An MEG study. Clin Neurophysiol 2006; 117(11): 2521–2531. [DOI] [PubMed] [Google Scholar]
- 28. Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol 2004; 115(7): 1490–1505. [DOI] [PubMed] [Google Scholar]
- 29. Attal Y, Schwartz D. Assessment of subcortical source localization using deep brain activity imaging model with minimum norm operators: A MEG study. PLoS ONE 2013; 8(3): e59856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hillebrand A, Barnes GR. A quantitative assessment of the sensitivity of whole-head MEG to activity in the adult human cortex. Neuroimage 2002; 16(3 Pt 1): 638–650. [DOI] [PubMed] [Google Scholar]
- 31. Hillebrand A, Barnes GR. Beamformer analysis of MEG data. Int Rev Neurobiol 2005; 68: 149–171. [DOI] [PubMed] [Google Scholar]
- 32. Quraan MA, Moses SN, Hung Y, et al. Detection and localization of hippocampal activity using beamformers with MEG: A detailed investigation using simulations and empirical data. Hum Brain Mapp 2011; 32(5): 812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mills T, Lalancette M, Moses SN, et al. Techniques for detection and localization of weak hippocampal and medial frontal sources using beamformers in MEG. Brain Topogr 2012; 25(3): 248–263. [DOI] [PubMed] [Google Scholar]
- 34. Engels MMA, Hillebrand A, van der Flier WM, et al. Slowing of hippocampal activity correlates with cognitive decline in early onset Alzheimer’s disease. An MEG study with virtual electrodes. Front Hum Neurosci 2016; 10: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haller M, Donoghue T, Peterson E, et al. Parameterizing neural power spectra. bioRxiv. Epub ahead of print 11 April 2018. DOI: 10.1101/299859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure_4 for Resting-state MEG measurement of functional activation as a biomarker for cognitive decline in MS by Deborah N Schoonhoven, Matteo Fraschini, Prejaas Tewarie, Bernard MJ Uitdehaag, Anand JC Eijlers, Jeroen JG Geurts, Arjan Hillebrand, Menno M Schoonheim, Cornelis J Stam and Eva MM Strijbis in Multiple Sclerosis Journal
Supplemental material, Supplementary_Information_methods for Resting-state MEG measurement of functional activation as a biomarker for cognitive decline in MS by Deborah N Schoonhoven, Matteo Fraschini, Prejaas Tewarie, Bernard MJ Uitdehaag, Anand JC Eijlers, Jeroen JG Geurts, Arjan Hillebrand, Menno M Schoonheim, Cornelis J Stam and Eva MM Strijbis in Multiple Sclerosis Journal
Supplemental material, Supplementary_Information_SDMT_FDR for Resting-state MEG measurement of functional activation as a biomarker for cognitive decline in MS by Deborah N Schoonhoven, Matteo Fraschini, Prejaas Tewarie, Bernard MJ Uitdehaag, Anand JC Eijlers, Jeroen JG Geurts, Arjan Hillebrand, Menno M Schoonheim, Cornelis J Stam and Eva MM Strijbis in Multiple Sclerosis Journal