Abstract
Background:
The proliferation of computerized neuropsychological assessment devices (CNADs) for screening and monitoring cognitive impairment is increasing exponentially. Previous reviews of computerized tests for multiple sclerosis (MS) were primarily qualitative and did not rigorously compare CNADs on psychometric properties.
Objective:
We aimed to systematically review the literature on the use of CNADs in MS and identify test batteries and single tests with good evidence for reliability and validity.
Method:
A search of four major online databases was conducted for publications related to computerized testing and MS. Test–retest reliability and validity coefficients and effect sizes were recorded for each CNAD test, along with administration characteristics.
Results:
We identified 11 batteries and 33 individual tests from 120 peer-reviewed articles meeting the inclusion criteria. CNADs with the strongest psychometric support include the CogState Brief Battery, Cognitive Drug Research Battery, NeuroTrax, CNS-Vital Signs, and computer-based administrations of the Symbol Digit Modalities Test.
Conclusion:
We identified several CNADs that are valid to screen for MS-related cognitive impairment, or to supplement full, conventional neuropsychological assessment. The necessity of testing with a technician, and in a controlled clinic/laboratory environment, remains uncertain.
Keywords: Multiple sclerosis, computerized tests, cognition, systematic review, reliability, validity
Introduction
Roughly 50%–60% of patients with multiple sclerosis (MS) suffer from cognitive impairment (CI).1–3 Currently, widespread screening for MS-related CI is limited by the considerable time and staff training required to administer traditional neuropsychological (NP) tests. Computerized neuropsychological assessment devices (CNADs) require fewer staff resources and less time to administer and score and may represent a viable alternative. This perspective hinges on both CNADs and conventional NP tests meeting adequate psychometric standards prior to clinical implementation.4 Furthermore, confounding factors that may influence test validity should be accounted for, such as the degree of test automation and technician involvement/supervision. Previous reviews concluded that, despite some promising results, the scope of impairment targeted by CNADs is limited, motor confounders are not accounted for, and psychometric data are lacking.5,6 While informative, the reviews did not cover psychometric properties in depth and omitted several individual tests not part of a larger battery.
Our objective was to assess the status of psychometric research on CNADs in MS and identify the most promising approaches for future research and clinical application. We endeavored to examine systematically the literature focusing specifically on test–retest reliability (consistency over repeated trials), ecological or predictive validity (relationships with real-world outcomes and general clinical measures), discriminant/known groups validity, and concurrent validity (correlations with established NP tests). Our overall aim was to help MS researchers and clinicians make informed decisions to apply select measures in MS research and clinical care.
Methods
Search protocol and inclusion
As per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,7 we utilized PubMed, MEDLINE, PsycINFO, and Embase databases. The search terms and Boolean operators applied for each database were as follows: multiple sclerosis AND (neuropsychological test OR cognition OR cognitive function OR cognitive impairment OR memory OR processing speed OR executive function OR language OR attention OR visuospatial) AND (computer OR internet OR iPad OR tablet OR CNAD). Test names known to the authors were also employed: Automated Neuropsychological Assessment Metrics (ANAM), Central Nervous System-Vital Signs (CNSVS), Cognitive Drug Research (CDR) Battery, NeuroTrax, Cognitive Stability Index (CSI), Neurobehavioral Evaluation System (NES), Amsterdam Neuropsychological Test (ANT), Cambridge Neuropsychological Test Automated Battery (CANTAB), CogState Brief Battery (CBB), Cognivue, Cognistat, NeuroCog BAC App, CogniFit, BrainCheck, Standardized Touchscreen Assessment of Cognition (STAC), and the National Institutes of Health (NIH) Toolbox.
Inclusion criteria for the selected articles were as follows: (a) included an MS and healthy control (HC) sample, (b) included at least one measure with computerized stimuli and computerized response collection, (c) published in a peer-reviewed journal after 1990, and (d) included sufficient data to evaluate at minimum one of the following:
|
1. Test/retest reliability: Consistency of scores across multiple administrations using (a) Pearson (r) or intraclass correlation (ICC), (b) index of stability comparing scores between two or more administrations, or (c) if otherwise qualifying test development literature shows test–retest reliability in an HC sample. 2. Discriminant or known groups validity: MS and HC compared by (a) statistical test for difference between means, (b) means and standard deviations provided for both groups, (c) sensitivity/specificity values, or (d) area under the curve (AUC). |
|
3. Ecological validity: Association between test and relevant patient outcomes via (a) correlation with or prediction of employment status or (b) correlation with Expanded Disability Status Scale (EDSS)8 or Multiple Sclerosis Functional Composite (MSFC).9
4. Concurrent validity: Comparing CNAD and conventional NP tests via (a) correlations, (b) partial correlations accounting for demographic variables, (c) impairment detection rates, or (d) regression analyses with conventional test(s) predicting CNADs or vice versa. |
The only exclusion criterion was a failure to meet one or more of the inclusion criteria. To obtain additional information regarding test characteristics (e.g. administration mode, hardware, etc.), information from CNAD websites was reviewed or provided by the test owner upon inquiry by the authors.
Effect size calculation
We calculated effect sizes (Cohen’s d) comparing MS and HCs using available data or by converting another reported metric (e.g. Hedge’s g). The average discriminant effect size for CNADs was calculated for each cognitive domain.
Domain identification
Battery subtests and individual tests were classified as measuring specific cognitive domains based largely on author descriptions, and tests used in multiple studies with varying domain attribution (e.g. N-Back—both processing speed and working memory) were classified as measuring multiple domains. Simple reaction time tests were classified as measuring cognitive processing speed.
Results
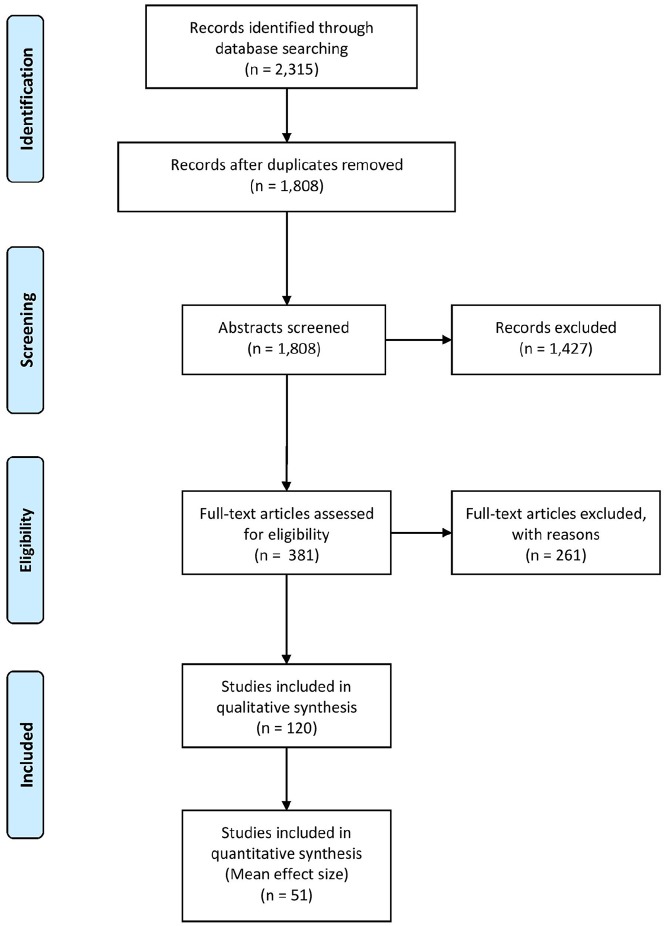
The PRISMA search found 120 articles (Figure 1), covering 11 batteries (i.e. multiple distinct cognitive functions) and 33 individual tests. CNADs with multiple indices within a single domain were classified as individual tests. For example, the Test of Attentional Performance (TAP) has multiple subtests, but each measures attention, so it was not classified as a “battery.” Certain subtests were not included in the review, as they were never used in any study with an MS sample (e.g. several subtests of the CANTAB, ANAM, and TAP were not included as they were not used in a published MS study).
Figure 1.
PRISMA systematic review flow diagram.
CNAD administration characteristics
Tables 1 and 2 reveal that most CNADs are Personal Computer (PC) based but several are dually compatible with either PC or iPad®/tablet administration, as claimed by the authors, but only the CBB empirically established the equivalence of PC and iPad administrations.55 Most batteries were presented as requiring a technician for administration, as in NeuroTrax, CSI, NES2, CANTAB, ANAM, ANT, and CNSVS. The influence of technician supervision was investigated in only a few studies, results supporting the idea that human-supervised administration yields equivalent data to self-administration,40,56,57 although this conclusion is deemed preliminary and likely to vary by test. Of the 11 CNAD batteries, 9 included a large normative database from which scores could be standardized. Few individual tests not part of a battery had large normative databases for standardized scores, but most were administered in at least one study that included HCs.
Table 1.
Test administration characteristics.
| Battery | Administration | Time to administer | Alternate forms | Number of MS-related publications | Normative sample | |
|---|---|---|---|---|---|---|
| Mode | Technician | |||||
| Cognitive Drug Research (CDR) Battery10 | PC | Self or Tech | 15–20 minutes | Yes, at least 30 for dementia battery11 | 1 | Several |
| NeuroTrax12 | PC | Tech | 45 minutes for full battery or shorter depending subtests selected | Yes, 3 equivalent | 23 | N = 1569, age range: 8–93, 58% female |
| Cognitive Stability Index (CSI)13 | PC | Tech | 25–35 minutes | Yes, 5 to 6 depending on subtest | 1 | N = 284, age range: 18–87, 53% female |
| Cambridge Neuropsychological Test Automated Battery (CANTAB)14 | iPad | Tech | Depends on tests selected | Yes, but equivalence not yet established | 8 | Several |
| Neurobehavioral Evaluation System 2 (NES2)15 | PC | Tech | 20+ minutes | Unknown | 1 | N = 67, mean age = 56.5, 57.6% femalea |
| Automated Neuropsychological Assessment Metrics (ANAM)16 | PC | In MS, performance is better on some subtests in at-home, webcam-guided session vs live technician guidance | 20–25 minutes | Yes, multiple alternate forms for each test | 6 | N = 107,671, age range: 17–65, 90% male |
| Amsterdam Neuropsychological Test (ANT)17 | PC | Tech | Depends on tests selected | Yes, 3 | 3 | Various normative samples depending on the subtest; ranges N = 14 to N = 330 |
| Cognivue18 | PC | Self | 10 minutes | No | 1 | N = 401, age range: 55–95 |
| CogniFit (CAB Battery)19 | iPad/PC | Self | 30–40 minutes | Unknown | 1 | N = 861, mean age = 65.7 |
| CogState Brief Battery (CBB)20 | iPad/PC | Self | 20 minutes | Yes, stimuli can be randomized | 5 | N > 2500, age range: 18–99 |
| Central Nervous System-Vital Signs (CNSVS)21 | iPad/PC | Tech | Depends on tests selected | Except for memory tests, randomization allows for unlimited alternate forms | 3 | N > 1600, age range: 8–90 |
Reported sample details are those currently available for each test.
Healthy sample from a research study, not currently used for standardization of scores.
Table 2.
Test administration characteristics.
| Test | Administration | Time to Administer | Alternate Forms | Number of MS-related Publications | Normative Sample | |
|---|---|---|---|---|---|---|
| Mode | Technician | |||||
| Computerized Test of Information Processing (CTIP)22 | PC | Self | 15 minutes | No, but no practice effects observed | 8 | N = 386, age range: 15–74 |
| Computerized Stroop Test23 | PC | Self | Varies, depending on modification of the task | Stimuli can be customized | 8 | Severala |
| Computerized Symbol Digit Modalities Test (C-SDMT)24 | PC | Tech | Varies, ~90 seconds | Yes, randomizes key pairings | 7 | Severala |
| fMRI-SDMT25 | fMRI | Tech | 5 minutes | Unknown | 2 | 1. N = 18, mean age = 41.14, 44% femalea
2. N = 16, mean age = 19.1, 69% femalea |
| Auto-SDMT26 | PC | Self | Varies depending on participant | Fixed and variable key pairings | 1 | N = 32, mean age = 43.13, 72% femalea |
| Computerized Incidental Learning Test (c-ILT)27 | PC | Tech | Unknown | Yes, 2 | 1 | N = 38, mean age = 54.3, 66% femalea |
| Computerized Speed Cognitive Test (CSCT)28 | PC | Tech | 90 seconds | Yes, randomizes key pairings | 4 | N = 415, age range: 18 to >65 |
| Paced Visual Serial Addition Test (PVSAT)29 | PC | Tech | 2–3 minutes | Yes, 2 | 5 | Severala |
| Visual Threshold Serial Addition Task (VT-SAT)30 | PC | Tech | 3 minutes (depending on subject response to first trial) | Unknown | 4 | N = 32, mean age = 44a |
| N-Back (0-Back, 1-Back, and 2-Back)31 | PC | Self | Varies depending on modification of the task | Stimuli can be customized | 17 | Severala |
| Janculjak Tests32 | PC | Tech | Unknown | No | 1 | N = 42, mean age = 37, 55% femalea |
| Computerized Paired Associate Learning (CPAL) Test23 | PC | Tech | Unknown | Unknown | 1 | N = 40, mean age = 44.9, 62.5% femalea |
| Sternberg Memory Scanning Task33 | PC | Self | Varies, can be modified | Yes, can customize stimuli | 2 | Several |
| Computerized Salthouse Operational Working Memory Test/Keeping Track Task (KTT)34 | PC | Tech | Varies depending on participant response speed | Yes, can customize | 4 | 1. N = 35, mean age = 38, 74% femalea
2. N = 36, mean age = 43.62a |
| Attention Network Test35 | PC | Self | 30 minutes | Yes, can customize stimuli | 7 | Severala |
| fMRI Tower of London Test36 | fMRI | Tech | Unknown | Unknown | 1 | N = 18, mean age = 36.6, 33% femalea |
| Denney Tests37 | PC | Unknown | 60–75 minutes | Unknown | 1 | N = 40, mean age = 40.3, 90% femalea |
| Kujala Tests38 | PC | Tech | 60–120 minutes | No | 1 | N = 35, mean age = 43.5, 51% femalea |
| Graded Conditional Discrimination Tasks (GCDT)39 | Visual Display Unit monitor with a sloping front panel with two push button switches | Unknown | 30 minutes | Unknown | 1 | N = 25, mean age = 44.1, 56% femalea |
| The Processing Speed Test (PST)40 | iPad | Self or Tech | 2 minutes | Yes, randomizes key pairings | 3 | 1. N = 116, mean age = 42.5, 53% femalea
2. N = 85, mean age = 43.1, 75% femalea 3. N = 24, mean age = 44, 88% femalea |
| SRT & CRT41 | PC | Self, with Tech present | Unknown, part of laboratory session lasting 10 minutes | No | 2 | N = 98, mean age = 46.4, 65.3% femalea |
| Foong Tests42 | PC | Unknown | Unknown | Unknown | 1 | N = 20, mean age = 40.55, 55% femalea |
| Conners’ CPT-II43,44 | PC | Self with Tech present | 7–8 minutes | Yes | 2 | CPT-II: 1920 CPT-III: 1400 |
| Computerized Digit Span45 | PC | Tech | Unknown | Unknown | 1 | N = 20, mean age = 38.7a |
| Amieva Computerized Tests46 | Touchscreen PC | Tech | Unknown | Unknown | 2 |
N = 43a
N = 44, mean age = 37.8, 68% femalea |
| Test of Attentional Performance (TAP)47 | PC | Self with Tech present | Unknown | Some subtests have randomized stimuli | 5 | Varies depending on subtest |
| Computerized Reading Span Task/Automated Ospan48 | PC | Self | Unknown, part of laboratory session lasting 1 hour | Yes, can customize stimuli | 4 | N = 5537, age range: 17–35 |
| NIH Toolbox (Cognitive Battery)49 | iPad | Tech | Depends on tests selected | Only for the Picture Sequence Memory Test | 1 | N = 1038, mean age = 49.1 |
| Touch Panel Tests50 | Large touch panel screen desktop | Unknown | Unknown | No | 1 | Two samples: 1. N = 40, mean age = 42.7, 68% femalea 2. N = 15, mean age = 54.6, 80% femalea |
| Urban DailyCog©51 | Virtual reality environment | Self with Tech present | 20 minutes | No | 1 | N = 22, mean age = 37.8 years, 72.7% femalea |
| Actual Reality Task52 | Virtual reality environment | Self with Tech present | 10–20 minutes | No | 3 |
N = 18, mean age = 44.1, 22.2% femalea
N = 32, mean age = 43.8, 72% femalea |
| The Memory and Attention Test (MAT)53 | PC | Self with Tech present | Unknown | Unknown | 2 | N = 84, mean age = 39.9, 65.5% femalea |
| Visual Search Task54 | PC (touchscreen) | Self with Tech present | 15–20 minutes | Performed on E-Prime, can program to change stimuli | 1 | N = 40, mean age = 36.3, 70% femalea |
fMRI: functional magnetic resonance imaging; SDMT: Symbol Digit Modalities Test; SRT: Simple Reaction Time; CRT: Cued Recall Test; CPT II: Continuous Performance Test II; CPT III: Continuous Performance Test III; NIH: National Institutes of Health.
Reported sample details are those currently available for each test.
Healthy sample from a research study, not currently used for standardization of scores.
Psychometric findings by cognitive domain
Table 3 lists CNADs by functional domain (standalone or part of a larger battery) and shows results for the four psychometric properties. Supplementary Tables A and B provide more details regarding samples, effect sizes, and validity coefficients from each study.
Table 3.
Psychometric characteristics of computerized tests.
| Functional domain | Battery/test name | Subtest | Comparator(s) | Test–retest reliability | Validity | ||
|---|---|---|---|---|---|---|---|
| Discriminant/known groups | Ecological | Concurrent | |||||
| Processing speed | C-SDMT | C-SDMT | SDMT | ✓ | ✓ | ✓ | ✓a |
| CSCT | CSCT | SDMT | ✓ | ✓ | ✓ | ✓a | |
| NeuroTrax | IPS | SDMT, DSST, PASAT | ✓ | ✓ | ✓ | ✓a | |
| CDR | Speed of Memory | SDMT, DSST, PASAT | ✓ | ✓ | ✓ | ✓a | |
| PST | PST | SDMT | ✓ | ✓ | ✓a | ||
| CNSVS | Processing Speed | SDMT, DSST, PASAT | Mixed | ✓ | ✓ | ✓a | |
| CTIP | SRT | SDMT, DSST, PASAT | Mixed | ✓ | ✓ | ✓a | |
| CTIP | CRT | SDMT, DSST, PASAT | Mixed | Mixed | ✓a | ||
| CTIP | SSRT | SDMT, DSST, PASAT | Mixed | ✓ | ✓a | ||
| CBB | Detection | SDMT, DSST, PASAT | Mixed | Mixed | ✓ | ✓a | |
| ANAM | Procedural RT | SDMT, DSST, PASAT | ✓ | ✓ | ✓ | ||
| NES2 | Symbol Digit | SDMT, DSST, PASAT | ✓ | ✓ | |||
| NES2 | Pattern Recognition | SDMT, DSST, PASAT | ✓ | ✓ | |||
| SRT & CRT | SRT | SDMT, DSST, PASAT | ✓ | ✓ | ✓ | ||
| CRT | SDMT, DSST, PASAT | ✓ | ✓ | ✓ | |||
| ANAM | SRT | SDMT, DSST, PASAT | ✓ | ✓ | |||
| Amsterdam Neuropsychological Test | Baseline Speed | Novel | ✓ | ✓ | |||
| Foong Tests | SRT | SDMT, DSST, PASAT | ✓ | ||||
| Foong Tests | Cued CRT | SDMT, DSST, PASAT | ✓ | ||||
| Foong Tests | Warned CRT | SDMT, DSST, PASAT | ✓ | ||||
| Foong Tests | Computerized Symbol Digit Substitution | SDMT, DSST, PASAT | ✓ | ||||
| Kujala Tests | Number Set | SDMT, DSST, PASAT | ✓ | ||||
| Kujala Tests | Letter Set | SDMT, DSST, PASAT | ✓ | ||||
| Kujala Tests | SRT | SDMT, DSST, PASAT | ✓ | ||||
| Kujala Tests | 2-Choice RT | SDMT, DSST, PASAT | ✓ | ||||
| Kujala Tests | 10-Choice RT | SDMT, DSST, PASAT | ✓ | ||||
| Kujala Tests | Statement Verification Test | SDMT, DSST, PASAT | ✓ | ||||
| Kujala Tests | Subtraction Test | SDMT, DSST, PASAT | ✓ | ||||
| c-ILT | c-ILT | SDMT, DSST, PASAT | ✓ | ||||
| GCDT | GCDT | SDMT, DSST, PASAT | ✓ | ||||
| Denney Tests | Picture Naming Test | SDMT, DSST, PASAT | ✓ | ||||
| Denney Tests | Rotated Figures Test | SDMT, DSST, PASAT | ✓ | ✓ | |||
| Janculjak Tests | Visual Reaction Task | SDMT, DSST, PASAT | ✓ | n.s. | |||
| CANTAB | RT | SDMT, DSST, PASAT | ✓ | n.s. | |||
| Denney Tests | Remote Associates Test | SDMT, DSST, PASAT | Mixed | ✓ | |||
| Auto-SDMT | Auto-SDMT | SDMT | ✓ | n.s. | ✓a | ||
| CSI | Number Sequencing | Letter Number Sequencing (WMS) | ✓a | ||||
| CSI | Animal Decoding | DSST | ✓a | ||||
| CSI | Symbol Scanning | Symbol Search (WAIS) | ✓a | ||||
| N-Back | 0-Back | SDMT, DSST, PASAT | Poor | Mixed | ✓a | ||
| CSI | Processing Speed Composite | SDMT, DSST, PASAT | ✓ | ✓a | |||
| ANAM | Coding Substitution Learning | SDMT or DSST | ✓ | n.s. | ✓a | ||
| fMRI-SDMT | fMRI-SDMT | SDMT | n.s. | ✓a | |||
| CNSVS | Reaction Time | SDMT, DSST, PASAT | Mixed | ✓ | |||
| Working memory | CANTAB | Spatial Working Memory | WMS Symbol Span | ✓ | ✓ | ✓ | |
| PVSAT | PVSAT-4 seconds | PASAT | Poor | ✓ | ✓a | ||
| PVSAT | PVSAT-3 seconds | PASAT | Mixed | n.s. | ✓a | ||
| PVSAT | PVSAT-2 seconds | PASAT | ✓ | ✓ | n.s. | ✓a | |
| NeuroTrax | Working Memory | PASAT | ✓ | ||||
| Automated Ospan | AOspan | PASAT, Digit Span, Ospan (non-automated) | ✓ | ✓ | ✓a | ||
| Sternberg Memory Scanning Task | Sternberg Memory Scanning Task | PASAT, Digit Span, Reading Span | ✓ | Mixed | n.s. | ||
| VT-SAT | 1-Back | PASAT | ✓ | ||||
| VT-SAT | 2-Back | PASAT | ✓ | ✓a | |||
| VT-SAT | 2-Back at 1-Back Threshold | PASAT | ✓ | ||||
| Computerized Digit Span | Computerized Digit Span | Digit Span | ✓ | ||||
| CBB | 1-Back | PASAT, WMS Spatial Span, Digit Span | Mixed | Mixed | ✓ | ✓a | |
| N-Back | Overall | PASAT, Digit Span, Reading Span | Mixed | ||||
| N-Back | 1-Back | PASAT, Digit Span, Reading Span | Poor | Mixed | ✓a | ||
| N-Back | 2-Back | PASAT, Digit Span, Reading Span | Poor | Mixed | ✓a | ||
| N-Back | 3-Back | PASAT, Digit Span, Reading Span | Mixed | Mixed | Mixed | ||
| CANTAB | Spatial Span | WMS Spatial Span | ✓ | Mixed | |||
| Computerized Salthouse Operational Working Memory Test/Keeping Track Task (KTT) | KTT | PASAT or Digit Span | ✓ | n.s. | ✓a | ||
| MAT | Verbal Working Memory | PASAT | ✓ | ||||
| MAT | Episodic Working Memory | WMS Logical Memory Immediate | ✓ | ||||
| MAT | Figural Working Memory | WMS Spatial Span | n.s. | ||||
| CDR | Quality of Working Memory | PASAT | Mixed | n.s. | ✓ | Mixed | |
| Episodic memory | NeuroTrax | Memory | CVLT2, BVMTR | ✓ | ✓ | ✓ | ✓a |
| CBB | Continuous PAL Task | BVMTR, 10/36 | ✓ | ✓ | ✓ | ✓ | |
| CNSVS | Composite Memory | CVLT2, BVMTR | Mixed | ✓ | ✓ | ✓a | |
| CBB | One Card Learning | BVMTR | Poor | ✓ | ✓ | ✓a | |
| ANAM | Coding Substitution Delayed Recall | WAIS-III DSST Recall | ✓ | ✓ | ✓a | ||
| CANTAB | PALT | BVMTR, 10/36 | ✓ | ✓ | n.s. | ||
| Cognivue | Memory | BVMTR, 10/36 | ✓ | ✓ | |||
| CANTAB | Delayed Matching To Sample | BVMTR learning | Poor | ✓ | ✓ | ||
| CANTAB | Spatial Recognition Memory | 10/36, WMS Designs | Mixed | ✓ | |||
| Touch Panel Tests | Flipping Cards Game | 10/36, WMS Designs | ✓ | ✓ | |||
| CPALT | Recall: Unrelated Items | WMS Verbal Paired Associates, CVLT2 | ✓ | ||||
| CPALT | Delayed Recall | WMS Verbal Paired Associates, CVLT2 | ✓ | ||||
| CSI | Memory Composite | 10/36, WMS Designs | ✓ | ✓ | |||
| CSI | Memory Cabinet 1 | 10/36, WMS Designs | ✓a | ||||
| CSI | Memory Cabinet 2 | 10/36, WMS Designs | ✓a | ||||
| MAT | Episodic Short-Term Memory | WMS Logical Memory I | ✓ | ✓ | |||
| MAT | Figural Short-Term Memory | BVMTR Learning | n.s. | ||||
| MAT | Verbal Short-Term Memory | CVLT2 Learning, RAVLT | n.s. | ||||
| CDR | Quality of Episodic Memory | CVLT2, RAVLT, BVMTR | Mixed | n.s. | ✓ | ✓ | |
| NES2 | Paired Associate Learning | WMS Verbal Paired Associates | n.s. | ||||
| CPALT | Recall: Related Items | WMS Verbal Paired Associates, CVLT2 | n.s. | ||||
| Attention | CDR | Power of Attention | Digit Span, BTA, TMT, CPT | ✓ | ✓ | ✓ | ✓ |
| CDR | Continuity of Attention | Digit Span, BTA, TMT, CPT | ✓ | ✓ | ✓ | ✓ | |
| NeuroTrax | Attention | Digit Span, BTA, TMT, CPT | ✓ | ✓ | ✓ | ✓a | |
| Amsterdam Neuropsychological Test | Focused Attention | Digit Span, BTA, TMT, CPT | ✓ | ✓ | |||
| Amsterdam Neuropsychological Test | Memory Search | Digit Span, BTA, TMT, CPT | ✓ | ✓ | |||
| CBB | Identification | Digit Span, BTA, TMT, CPT | Mixed | ✓ | ✓a | ||
| Conners’ CPT | CPT | Digit Span, BTA, TMT, CPT | ✓ | ✓ | |||
| NES2 | Continuous Performance | Digit Span, BTA, TMT, CPT | ✓ | ✓ | |||
| Janculjak Tests | Visual Attention Task | Digit Span, BTA, TMT, CPT | ✓ | ||||
| Janculjak Tests | Visual Reaction Task | Digit Span, BTA, TMT, CPT | ✓ | n.s. | |||
| MAT | Selective Attention | DKEFS CW, Stroop | ✓ | ||||
| CANTAB | Rapid visual Processing | CPT | ✓ | n.s. | |||
| CNSVS | Complex Attention | DKEFS CW, Stroop, DSST | ✓ | Mixed | |||
| Attention Network Test | Overall | Novel | ✓ | Mixed | ✓ | n.s. | |
| Attention Network Test | Alerting | CPT | Mixed | Mixed | n.s. | n.s. | |
| Attention Network Test | Orienting | Novel | Mixed | Mixed | n.s. | ||
| Attention Network Test | Conflict | Novel | ✓ | n.s. | n.s. | ||
| TAP | Alertness | CPT | Mixed | ||||
| TAP | Divided Attention | Novel | Mixed | ||||
| TAP | Go-No-Go | DKEFS CW, Stroop | n.s. | ||||
| CSI | Attention Composite | Digit Span, BTA, TMT, CPT | ✓ | ✓a | |||
| CNSVS | Simple Attention | Digit Span, BTA, TMT, CPT | Poor | ✓ | |||
| ANAM | Running Memory CPT Throughput | Digit Span, BTA, TMT, CPT | ✓ | ✓a | |||
| Executive function | NeuroTrax | Executive Function | DKEFS CW, Stroop | ✓ | ✓ | ✓ | ✓ |
| Computerized Stroop Test | Word | DKEFS CW, Stroop | ✓ | ✓ | ✓ | ||
| Computerized Stroop Test | Color | DKEFS CW, Stroop | ✓ | ✓ | ✓ | ||
| Computerized Stroop Test | Color-Word | DKEFS CW, Stroop | ✓ | ✓ | ✓ | ||
| CBB | Groton Maze Learning | NAB Mazes | ✓ | ✓ | ✓a | ||
| NIH Toolbox | Modified Flanker | DKEFS CW, Stroop | ✓ | ✓ | ✓a | ||
| Foong Tests | Computerized Stroop | DKEFS CW, Stroop | ✓ | ||||
| fMRI Tower of London Test | fMRI Tower of London Test | DKEFS Tower Test | ✓ | ||||
| Denney Tests | Tower of London | DKEFS Tower Test | Mixed | ||||
| Amieva Computerized Tests | Computerized Stroop | DKEFS CW, Stroop | ✓ | n.s. | |||
| CANTAB | Stockings of Cambridge | DKEFS Tower Test | Mixed | ||||
| CNSVS | Cognitive Flexibility | WCST, Stroop, NES Switching Attention | ✓ | ✓ | ✓a | ||
| CNSVS | Executive Function | WCST, Stroop, NES Switching Attention | ✓ | Mixed | ✓a | ||
| Amieva Computerized Tests | Go-No-Go | DKEFS CW, Stroop | n.s. | n.s. | |||
| Other/miscellaneous | NeuroTrax | Visual Spatial | JLO | ✓ | ✓ | ✓ | ✓a |
| CNSVS | Psychomotor Speed | NES Finger Tapping Test | ✓ | ✓ | ✓ | ✓ | |
| NeuroTrax | Global Cognitive Score | Novel | ✓ | ✓ | ✓ | ||
| NeuroTrax | Motor Skills | NES Finger Tapping Test | ✓ | ✓ | |||
| ANAM | Index of Cognitive Efficiency | Novel | ✓ | ✓ | |||
| Cognivue | Perception | Novel | ✓ | ✓ | |||
| Cognivue | Visuomotor | Novel | ✓ | ✓ | |||
| CANTAB | Gambling Task | Iowa Gambling Test | ✓ | ✓ | |||
| Visual Search Task | Visual Search Task | Novel | ✓ | n.s. | |||
| Actual Reality Task | Actual Reality Task | Novel | Mixed | ✓ | ✓ | ||
| Kujala Tests | Motor Programming Speed | Novel | ✓ | ||||
| CANTAB | Information Sampling Task | Iowa Gambling Test | ✓ | ||||
| CogniFit | Multiple | Multiple | |||||
| Urban DailyCog© and DSDT | Daily functioning and driving | Novel | n.s. | n.s. | |||
| CNSVS | Global Score | Novel | |||||
| Touch Panel Tests | Arranging Pictures Game | Novel | ✓ | ✓ | |||
| Touch Panel Tests | Finding Mistakes Game | Novel | n.s. | ✓ | |||
| Touch Panel Tests | Beating Devils game | Novel | n.s. | ||||
| NeuroTrax | Verbal Function | COWAT | Poor | n.s. | ✓ | ||
| NeuroTrax | Problem Solving | Raven’s Progressive Matrices | n.s. | ✓ | |||
SSRT: Semantic Search Reaction Time, WMS: Wechsler Memory Scales, WAIS: Wechsler Adult Intelligence Scale, CVLT: California Verbal Learning Test, PAL: Paired Associate Learning, WAIS III: Wechsler Adult Intelligence Scale–Third Edition, SRT: Simple Reaction Time, PALT: Paired Associate Learning Test, CPALT: Computerized Paired Associate Learning Test, RAVLT: Rey’s Auditory Verbal Learning Test, BTA: Brief Test of Attention, TMT: Trail Making Test, WCST: Wisconsin Card Sorting Test, JLO: Judgment of Line Orientation, DSDT: Driving Simulator Dual Task, COWAT: Controlled Oral Word Association Test, NAB: Neuropsychological Assessment Battery, DSST: Digit Symbol Substitution Test, CRT: Complex Reaction Time, c-ILT: Computerized Incidental Learning Test, AOspan: Automated Operation Span Task, and DKEFS CW: Delis Kaplan Executive Function System Color Word Interference Test C-SDMT: Computerized Symbol Digit Modalities Test; CSCT: Computerized Speed Cognitive Test; IPS: Information Processing Speed; PASAT: Paced Auditory Serial Addition Task; CDR: Cognitive Drug Research; PST: Processing Speed Test; CNSVS: Central Nervous System-Vital Signs; CTIP: Computerized Test of Information Processing; CBB: CogState Brief Battery; ANAM: Automated Neuropsychological Assessment Metrics; NES: Neurobehavioral Evaluation System; GCDT: Graded Conditional Discrimination Tasks; CANTAB: Cambridge Neuropsychological Test Automated Battery; CSI: Cognitive Stability Index; PVSAT: Paced Visual Serial Addition Test; VT-SAT: Visual Threshold Serial Addition Task; MAT: The Memory and Attention Test; BVMTR: Brief Visuospatial Memory Test-Revised; CPT: Continuous Performance Test; TAP: Test of Attentional Performance; NIH: National Institute of Health; n.s.: non-significant; Mixed: mixed results from one or more studies showing both significance and non-significance or both poor and adequate reliability; Poor: test–retest reliability below r = 0.60.
The symbol ‘✓’ indicates significant results for respective psychometric measure (p < 0.05). Blank boxes represent missing/uninvestigated for the respective psychometric measure.
Concurrent validity was with a comparator test of the same domain/cognitive function.
Cognitive processing speed
Of the 44 processing speed tests reviewed, all four psychometric properties were examined for the Computerized Symbol Digit Modalities Test (C-SDMT),24 Computerized Speed Cognitive Test (CSCT),28 and NeuroTrax Information Processing Speed (IPS) test.12
For test–retest reliability, the C-SDMT demonstrated the highest test–retest coefficient among processing speed tests in MS patients (ICC = 0.97) over a mean interval of 103 days.24 By comparison, the test–retest reliability for the Processing Speed Test (PST) was 0.88 in MS patients.40 Most every test evaluated in an MS sample showed acceptable cognitive processing speed reliability (see Table 3 and also Supplementary Tables A and B).
For discriminant validity (MS vs HCs), the average effect size for processing speed tests was d = 1.03 (SD = 0.42). The C-SDMT showed medium (d = 0.76)24 to large (d = 1.48)58 effect sizes comparable to the conventional SDMT.59 The CSCT had similar effect sizes comparing HCs with primary progressive MS (d = 1.8, p < 0.001) and relapsing remitting MS (d = 0.80, p < 0.01).60 Other tests of cognitive processing speed discriminated equally well, including the Computerized Test of Information Processing (CTIP; mean d = 0.82, SD = 0.37),61–65 the PST (d = 0.75, p < 0.001),40 and to a lesser extent the Auto-SDMT (d = 0.68, p < 0.01)26 and CNSVS Processing Speed subtest (d = 0.52, p = 0.046).66
For ecological validity, EDSS correlated with C-SDMT (r = 0.35),24 NeuroTrax IPS (r = 0.20),67 CNSVS Processing Speed (r = 0.31),66 CTIP (range: r = 0.39–0.52),62 and CBB Detection (r = 0.46).68 EDSS did not correlate with CANTAB Reaction Time69 and Auto-SDMT.26 Like the conventional SDMT,70,71 impairment on the CSCT significantly predicted unemployment among MS patients.28 No other CNAD cognitive processing speed test was studied in this manner.
For concurrent validity, computerized tests based on SDMT correlated with the conventional version: CSCT (r = 0.88),28 C-SDMT (r = 0.78),72 Auto-SDMT (r = 0.81),26 and PST (r = 0.80 and r = 0.75).40,73 SDMT also correlated with CTIP (range: r = 0.29–0.40),62 CBB Detection (r = 0.40),74 and CSI Processing Speed Composite (r = 0.58),13 while the following processing speed tests each correlated significantly with their non-SDMT comparators: CDR Speed of Memory,10 CSI Number Sequencing,75 CNSVS Processing Speed,21 and NeuroTrax IPS.12
Working memory
Of the 22 working memory CNAD tests, we found published results on three of the psychometric properties for CANTAB Spatial Working Memory and Paced Visual Serial Addition Test 2 (PVSAT-2).
For test–retest reliability, the PVSAT-2 (ICC = 0.75)41 had the best consistency in MS patients over a mean of 71.7 days.
Overall, working memory CNADs had moderate discriminant validity with an average effect size of d = 0.70 (SD = 0.30). The PVSAT showed smaller effects (range: d = 0.27–0.61)29,41 depending on the rate of stimulus presentation. Except for CANTAB Spatial Span (mean d = 1.11)76 and Automated Ospan Test (d = 0.90),77 other CNAD working memory tests generally demonstrated lower effect sizes. For example, the effects derived from the CDR Quality of Working Memory (d = 0.20)10 and some of the Memory and Attention (MAT) Working Memory subtests (range: d = 0.05–0.32)78 were not statistically significant.
For ecological validity, EDSS was significantly correlated with CDR Quality of Working Memory (r = 0.48),10 CANTAB Spatial Working Memory (r = 0.43),69 CBB One-Back (r = 0.44),68 and Sternberg Memory Scanning Task.32 However, the Sternberg task did not correlate significantly with EDSS in two other studies.79,80 EDSS correlations with PVSAT29 and the Computerized Salthouse Operational Working Memory Test were also non-significant.79 Ecological validity was unexplored for the remaining working memory tests (listed in Table 3).
In regards to construct validity, validity coefficients with either Paced Auditory Serial Addition Task (PASAT) or Digit Span were as follows: Computerized Salthouse Operational Working Memory Test (r = 0.51, r = 0.57),79 CBB One-Back (r = 0.41, r = 0.50),68 PVSAT (r = 0.74),29 and the Two-Back level (r = 0.59).81
Episodic memory
Of the 21 episodic memory tests, all four psychometric properties were established for NeuroTrax Memory and CNSVS Composite Memory.
CNSVS Verbal and Visual Memory demonstrated mixed results for test–retest reliability in HCs.82 ANAM Coding Substitution Delayed Recall had very high consistency (ICC = 0.88) over 30 days.83 The NeuroTrax Memory Composite84 also showed very good reliability with an r value of 0.84. There was a wide range of reliability coefficients for the CDR Quality of Episodic Memory in MS patients, from r = 0.50 to r = 0.82, depending on the test–retest interval.10
For discriminant/known groups validity, the mean effect size for episodic memory tests was d = 0.70 (SD = 0.30), and the largest effect size noted was for the CNSVS Composite Memory (d = 1.34).85
For ecological validity, EDSS correlated modestly but significantly with several CNAD memory indices, including CNSVS Composite Memory (r = 0.29),86 CBB Continuous Paired Associate Learning (CPAL) Task (r = 0.32),68 Touch Panel Tests Flipping Cards Game (r = 0.45),50 CDR Quality of Episodic Memory (r = 0.33),10 CANTAB Delayed Matching to Sample (r = 0.40),69 and MAT Episodic Short-Term Memory (r = 0.21).78 The CANTAB Paired Associates Learning Test showed non-significant correlations with EDSS69,87 and all other episodic memory tests were uninvestigated for this standard.
Four episodic memory tests showed good or excellent concurrent validity: CBB One Card Learning correlated with the Brief Visuospatial Memory Test—Revised (BVMTR)88 (r = 0.83),89 CNSVS Verbal Memory correlated with Rey Auditory Verbal Learning Test90 (r = 0.54, r = 0.52),21 NeuroTrax Memory Composite correlated with the Selective Reminding Test91 (range: r = 0.61–0.65),12 and CSI Memory Cabinet75 correlated with Family Pictures subtest from the Wechsler Memory Scales92 (r = 0.65, r = 0.61).75
Attention
Of the 23 attention tests reviewed, all four psychometric properties were explored for CDR Power and Continuity of Attention and NeuroTrax Attention.
The test–retest reliability was examined in most CNAD attention tests, but for the most part only in HCs, seldom in MS. The CDR Power of Attention subtest had the strongest test–retest consistency in an MS sample (range: r = 0.86–0.94).10
For discriminating MS patients and HCs, the average effect size for attention was d = 0.81 (SD = 0.27) with the CANTAB Rapid Visual Processing showing the largest effect size (d = 1.12).69
Ecological validity data were scant as correlation with EDSS or other validators was tested in only a few CNADs. For CDR, Power of Attention correlated with EDSS at r = 0.62 and the correlation with Continuity of Attention was r = 0.43.10 Other EDSS correlations were as follows: NeuroTrax Attention r = 0.2667 and Attention Network Test Overall score r = 0.48.93 EDSS did not correlate with CANTAB Rapid Visual Processing,69 Attention Network Test-Alerting,94 and the Amieva Go-No-Go Test.95
Concurrent validity data were similarly sparse. Broadening the construct to include tests such as PASAT, Digit Span, or Trail Making Test,96 we find that NeuroTrax Attention,12 CBB Identification,68,89 CSI Attention Composite,13 and ANAM Running Memory CPT (Continuous Performance Test) throughput97 correlate with each of their comparators.
Executive function
A total of 14 executive function CNAD tests were reviewed, with NeuroTrax Executive Function being the only one to be evaluated on all four psychometric properties.
NeuroTrax Executive Function showed good test–retest reliability over 3 weeks to several months (r = 0.80).84 The average discriminant effect size of executive function measures was d = 0.99 (SD = 0.40) with CNSVS cognitive flexibility showing the largest (d = 1.67).85
Ecological validity was demonstrated by significant EDSS correlations with NeuroTrax Executive Function (r = 0.28),67 the Computerized Stroop Test (range: r = 0.32–0.40),98 and CBB Groton Maze Learning (r = 0.32).68 EDSS did not correlate with the Amieva Computerized Stroop,95 while the remaining executive function measures did not examine ecological validity. The NeuroTrax Global Cognitive Score and Executive Function subtest were studied in relation to employment status, both of which discriminated working and non-working MS patients (p < 0.05).99
A few computerized executive function measures showed moderate associations with similar conventional tests. For instance, the CBB Groton Maze Learning correlated with a maze completion task (r = 0.56)100 and the CNSVS measures showed moderate associations with both the Stroop Test and a test of mental set shifting.21
Other domains
Briefly, there were several other tests which do not clearly fall within one of the above cognitive domains, most discriminating MS from HCs, but with limited reporting of reliability or ecological validity (Table 3 and Supplementary Tables A and B).
Discussion
In this review, we cast a high bar for designating CNADs as ready for use in MS. We apply the usual standards of psychometric reliability and validity59,101 and expect that CNAD authors and vendors will publish research focused on MS samples. We recognize that this may present an economic hardship as the CNAD market is highly competitive and vendors are marketing to the wider neurology community. Nevertheless, we maintain that these psychometric standards are important for optimal quality of care, and that research findings should be publicly available, as in conventional NP validation.102–104
We find that tests from the CDR, the CBB, NeuroTrax, and CNSVS show acceptable psychometrics. The CSCT, PST, and C-SDMT may be the best single tests for relatively quick screens in busy clinics unable to provide a full NP assessment or carry out longer screening procedures. These tests fulfill the four psychometric criteria (i.e. test–retest reliability, discriminant/known groups validity, ecological validity, and concurrent validity) and are sensitive to MS-related impairment. While the PST lacks a published normative database, we know by personal communication that these data will soon be submitted for peer review. The CSCT and the C-SDMT were used in large healthy samples for comparison. The tests could be made more clinically relevant if age-, sex-, and/or education-specific norms are published to facilitate interpretation of individual results.
There are also notable differences in administration and outcome measures between these versions of the SDMT. The PST uses a manual response on a touchscreen keyboard, while the CSCT, C-SDMT, and traditional SDMT use oral responses recorded by an examiner. Furthermore, the time limits for each of the tests are not identical, as the PST has a 120-second limit, while the CSCT and traditional SDMT both last 90 seconds. The C-SDMT has no time limit as its primary outcome is completion time. These differences prevent valid comparison of raw scores.
Our committee debated the use of the term computerized neuropsychological testing device (CNAD) as published by the American Academy of Clinical Neuropsychology.4 Likewise, the term paper-and-pencil testing fails to accurately characterize conventional tests. It makes some sense to draw a distinction between tests that present stimuli on a computer screen as opposed to a person speaking to a patient (as in reading a word list or asking a patient to orally list words conforming with a category) or showing them a visual stimulus (as in presenting the Rey figure or interlocking pentagons with the instruction to copy it). Yet there are many shades of gray. The PASAT stimuli are often presented via audio files played on a computer device. Shall we refer to the PASAT as a computerized test? Clearly, the terms computerized and automated refer to a spectrum of technology, ever growing in our effort to improve test accuracy and ease of access.
Relatedly, very few tests have published data on the importance of technician oversight. Supervision could influence performance on several levels. First, it may improve patient motivation to perform well. Second, a technician could provide guidance or clarify instructions for patients with relatively little computer experience or having difficulty understanding a given task. Third, a technician may assist with any technical issues that may arise during test-taking, including malfunctioning hardware or confusion over interacting with a software interface. Recent findings on the CBB and PST suggest that a technician is not necessary in an MS sample.40,56 However, future research considering other CNADs, particularly those containing more complex tasks, may yield different results, especially for at-home self-administrations. This latter application would necessitate a basic capacity to manage digital platforms such as iPads and the like, as the home-use technology continues to evolve.
For each battery and test, a distinction should be made between the frequency of use in MS research/clinical trials, the amount of available psychometric information, and the quality of available psychometric information. As shown in Tables 1 and 2, some CNADs were included in numerous publications with MS patients, but as evident in Supplementary Tables A and B, few of these publications aimed specifically to validate the test. In addition, while some tests may have data for reliability and/or validity, the reported coefficients and effect sizes may be low or non-significant. In selecting a CNAD for routine NP assessment or clinical trials in MS, the degree to which a test meets these psychometric categories must be considered.
That said, we acknowledge that some psychometric standards are more important than others. EDSS is not a crucial ecological validity standard as it is notoriously insensitive to CI and physical and mental MS symptoms are generally weakly correlated. Concurrent validity, a process of construct validation, is not as relevant as predicting quality of life and some CNADs assess novel domains/functions for which there are no existing conventional tests. As is evident in Table 3, several CNAD outcomes lack a good comparator and are simply missed by conventional tests. For example, conventional tests seldom measure reaction time, and when they do it is not to the millisecond per stimulus. Another example is the Information Sampling Task (IST) from CANTAB that requires visual/spatial processing and also decision-making based on perceived probabilities of gain. While not yet validated against an established measure, it may prove valuable within a very narrow sub-area of executive function. Furthermore, construct validation depends on an established metric for the cognitive domain studied and the issue has not been fully examined even for conventional NP tests in MS.
Yet novel CNADs should still possess adequate test–retest reliability and sensitivity before routine clinical application or inclusion in a clinical trial. An interesting issue for sensitivity is the technological limitation preventing CNAD memory tests from evaluating recall, as opposed to recognition memory, the former notably more sensitive in MS.105,106 Someday CNADs may employ voice recognition or other methods to record and score recall responses, but in this review all of the memory tests use a recognition format. On the other hand, CNADs offer metrics absent from person-administered testing. Many CNADs are automated, which allows for easy administration and often foregoes the need for trained professionals to give instructions, present stimuli, record responses, and score results. In this way, automation can avoid possible bias or error introduced by a psychometrician. Stimuli can also be more readily changed or randomized in CNADs, providing many alternate test forms that may reduce practice effects with repeat testing. Similarly, some CNADs are less subject to ceiling and floor effects because they have the ability to vary the difficulty or presentation of items based on examinee performance.
Computerized tests might particularly benefit the detection of MS-related CI through their improved sensitivity in measuring reaction time and response speed. Declines in cognitive processing speed are the hallmark CI seen in MS.107 Computerized tests may facilitate the identification of prodromal deficits by capturing minute differences in response time not identified by traditional tests. In contrast to a few raw score indices derived from a conventional SDMT protocol, most CNADs also generate measures of change in accuracy over the course of a task, change in reaction time, and measures of vigilance decrements. In sum, we opine that CNADs are quite good at measuring cognitive processing speed in MS, and their sensitivity and validity in other domains merit further investigation.
Finally, the practicality of CNADs and their cost-effectiveness are frequently cited as reasons to utilize this approach. All cognitive performance tests, be they person- or computer-administered, require certain physical or sensory capacities of patients (e.g. adequate manual dexterity and visual acuity). Conventional, person-administered tests, require skilled trained examiners, are time-consuming, and may be expensive in some cases. Setting aside the potential advantages of a technician (ensuring motivation to perform well, understanding of instructions), we would like to point out that the SDMT costs roughly US$2 per administration and in its oral-response format requires 5 minutes or less. While a human examiner is needed, there is no cost for computer devices, high-speed internet, or paying a vendor for the service (in our experience vendors typically charge US$20 per test). It is interesting that, in a recent investigation of the conventional and computer forms of SDMT,26 patients reported a preference for the self-administered computer version. If replicated, this perspective may add value to CNADs over conventional tests, much like the PASAT was largely abandoned due to its stressing patients.59,101 The newly published CMS (Centers for Medicare & Medicaid Services) guidelines for reimbursement add another layer to the discussion. The new CPT code for automated computerized testing reimburses less than US$5 in the United States. Reimbursement rises when a professional becomes involved in the testing process. Thus, the relative value of completely self-administered CNADs is not yet fully recognized by payers, at least in the United States.
Conclusion
Several computerized tests of cognition are available and applied in MS research. As they currently stand, most CNAD batteries and individual tests do not yet demonstrate adequate reliability and validity to supplant well-established conventional NP procedures such as MS Cognitive Endpoints battery (MS-COG), BICAMS (Brief International Cognitive Assessment for MS), or MACFIMS (Minimal Assessment of Cognitive Function in MS). However, some tests (e.g. certain subtests of the CDR, CBB, NeuroTrax, CNSVS, C-SDMT, PST, and CSCT) possess psychometric qualities that approach or maybe even exceed conventional, person-administered tests and can serve as useful screening tools or supplements to full assessments. Further investigations of these CNADs, especially as they relate to ecological measures and patient-relevant outcomes, are needed before widespread implementation with an MS population.
Supplemental Material
Supplemental material, MSJ879094_supplemental_table_a for Computerized neuropsychological assessment devices in multiple sclerosis: A systematic review by Curtis M Wojcik, Meghan Beier, Kathleen Costello, John DeLuca, Anthony Feinstein, Yael Goverover, Mark Gudesblatt, Michael Jaworski, Rosalind Kalb, Lori Kostich, Nicholas G LaRocca, Jonathan D Rodgers and Ralph HB Benedict in Multiple Sclerosis Journal
Supplemental Material
Supplemental material, MSJ879094_supplemental_table_b for Computerized neuropsychological assessment devices in multiple sclerosis: A systematic review by Curtis M Wojcik, Meghan Beier, Kathleen Costello, John DeLuca, Anthony Feinstein, Yael Goverover, Mark Gudesblatt, Michael Jaworski, Rosalind Kalb, Lori Kostich, Nicholas G LaRocca, Jonathan D Rodgers and Ralph HB Benedict in Multiple Sclerosis Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Curtis M Wojcik, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA.
Meghan Beier, Division of Rehabilitation Psychology and Neuropsychology, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Kathleen Costello, National Multiple Sclerosis Society, New York, NY, USA.
John DeLuca, Department of Physical Medicine and Rehabilitation and Department of Neurology, Rutgers New Jersey Medical School, Newark, NJ, USA.
Anthony Feinstein, Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
Yael Goverover, New York University, New York, NY, USA/South Shore Neurologic Associates, New York, NY, USA.
Mark Gudesblatt, South Shore Neurologic Associates Islip, NY.
Michael Jaworski, III, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA.
Rosalind Kalb, National Multiple Sclerosis Society, New York, NY, USA.
Lori Kostich, The Mandell MS Center, Mount Sinai Rehabilitation Hospital, Hartford, CT, USA.
Nicholas G LaRocca, National Multiple Sclerosis Society, New York, NY, USA.
Jonathan D Rodgers, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA/Jacobs Neurological Institute, Buffalo, NY, USA/Canisius College, Buffalo, NY, USA.
Ralph HB Benedict, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA/Jacobs Neurological Institute, Buffalo, NY, USA.
References
- 1. Rao SM, Leo GJ, Bernardin L, et al. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 1991; 41(5): 685–691. [DOI] [PubMed] [Google Scholar]
- 2. Rao SM, Grafman J, DiGiulio D, et al. Memory dysfunction in multiple sclerosis: Its relation to working memory, semantic encoding, and implicit learning. Neuropsychology 1993; 7: 364–374. [Google Scholar]
- 3. Benedict RH, Cookfair D, Gavett R, et al. Validity of the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS). J Int Neuropsychol Soc 2006; 12: 549–558. [DOI] [PubMed] [Google Scholar]
- 4. Bauer RM, Iverson GL, Cernich AN, et al. Computerized neuropsychological assessment devices: Joint position paper of the American Academy of Clinical Neuropsychology and the National Academy of Neuropsychology. Arch Clin Neuropsychol 2012; 27: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lapshin H, Oconnor P, Lanctt KL, et al. Computerized cognitive testing for patients with multiple sclerosis. Mult Scler Relat Disord 2012; 1: 196–201. [DOI] [PubMed] [Google Scholar]
- 6. Korakas N, Tsolaki M. Cognitive impairment in multiple sclerosis: A review of neuropsychological assessments. Cogn Behav Neurol 2016; 29(2): 55–67. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. Ann Int Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 8. Kurtzke JF. Rating neurologic impairment in multiple sclerosis—An Expanded Disability Status Scale (EDSS). Neurology 1983; 33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 9. Fischer JS, Rudick RA, Cutter GR, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): An integrated approach to MS clinical outcome assessment. Mult Scler 1999; 5(4): 244–250. [DOI] [PubMed] [Google Scholar]
- 10. Edgar C, Jongen PJ, Sanders E, et al. Cognitive performance in relapsing remitting multiple sclerosis: A longitudinal study in daily practice using a brief computerized cognitive battery. BMC Neurol 2011; 11: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Snyder PJ, Jackson CE, Petersen RC, et al. Assessment of cognition in mild cognitive impairment: A comparative study. Alzheimers Dement 2011; 7(3): 338–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Achiron A, Doniger GM, Harel Y, et al. Prolonged response times characterize cognitive performance in multiple sclerosis. Eur J Neurol 2007; 14(10): 1102–1108. [DOI] [PubMed] [Google Scholar]
- 13. Erlanger DM, Kaushik T, Broshek D, et al. Development and validation of a web-based screening tool for monitoring cognitive status. J Head Trauma Rehabil 2002; 17(5): 458–476. [DOI] [PubMed] [Google Scholar]
- 14. Lowe C, Rabbitt P. Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: Theoretical and practical issues. Cambridge Neuropsychological Test Automated Battery. Neuropsychologia 1998; 36(9): 915–923. [DOI] [PubMed] [Google Scholar]
- 15. Letz R, Green RC, Woodard JL. Development of a computer-based battery designed to screen adults for neuropsychological impairment. Neurotoxicol Teratol 1996; 18(4): 365–370. [DOI] [PubMed] [Google Scholar]
- 16. Ibarra S. Automated Neuropsychological Assessment Metrics. In: Kreutzer JS, DeLuca J, Caplan B. (eds) Encyclopedia of clinical neuropsychology. New York: Springer, 2011, pp. 325–327. [Google Scholar]
- 17. DeSonneville LM, Boringa JB, Reuling IE, et al. Information processing characteristics in subtypes of multiple sclerosis. Neuropsychologia 2002; 40(11): 1751–1765. [DOI] [PubMed] [Google Scholar]
- 18. Smith AD, 3rd, Duffy C, Goodman AD. Novel computer-based testing shows multi-domain cognitive dysfunction in patients with multiple sclerosis. Mult Scler J Exp Transl Clin 2018; 4(2). DOI: 10.1177/2055217318767458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shatil E, Metzer A, Horvitz O, et al. Home-based personalized cognitive training in MS patients: A study of adherence and cognitive performance. Neurorehabilitation 2010; 26(2): 143–153. [DOI] [PubMed] [Google Scholar]
- 20. Darby D, Maruff P, Collie A, et al. Mild cognitive impairment can be detected by multiple assessments in a single day. Neurology 2002; 59(7): 1042–1046. [DOI] [PubMed] [Google Scholar]
- 21. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol 2006; 21(7): 623–643. [DOI] [PubMed] [Google Scholar]
- 22. Barker-Collo SL. Quality of life in multiple sclerosis: Does information-processing speed have an independent effect. Arch Clin Neuropsychol 2006; 21(2): 167–174. [DOI] [PubMed] [Google Scholar]
- 23. Denney DR, Lynch SG, Parmenter BA, et al. Cognitive impairment in relapsing and primary progressive multiple sclerosis: Mostly a matter of speed. J Int Neuropsychol Soc 2004; 10(7): 948–956. [DOI] [PubMed] [Google Scholar]
- 24. Akbar N, Honarmand K, Kou N, et al. Validity of a computerized version of the Symbol Digit Modalities Test in multiple sclerosis. J Neurol 2011; 258(3): 373–379. [DOI] [PubMed] [Google Scholar]
- 25. Akbar N, Banwell B, Sled JG, et al. Brain activation patterns and cognitive processing speed in patients with pediatric-onset multiple sclerosis. J Clin Exp Neuropsychol 2016; 38(4): 393–403. [DOI] [PubMed] [Google Scholar]
- 26. Patel VP, Shen L, Rose J, et al. Taking the tester out of the SDMT: A proof of concept fully automated approach to assessing processing speed in people with MS. Mult Scler J 2018; 25: 1506–1513. [DOI] [PubMed] [Google Scholar]
- 27. Denney DR, Hughes AJ, Elliott JK, et al. Incidental learning during rapid information processing on the Symbol–Digit Modalities Test. Arch Clin Neuropsychol 2015; 30(4): 322–328. [DOI] [PubMed] [Google Scholar]
- 28. Ruet A, Deloire MS, Charre-Morin J, et al. A new computerised cognitive test for the detection of information processing speed impairment in multiple sclerosis. Mult Scler 2013; 19(12): 1665–1672. [DOI] [PubMed] [Google Scholar]
- 29. Nagels G, Geentjens L, Kos D, et al. Paced Visual Serial Addition Test in multiple sclerosis. Clin Neurol Neurosurg 2005; 107(3): 218–222. [DOI] [PubMed] [Google Scholar]
- 30. Lengenfelder J, Bryant D, Diamond BJ, et al. Processing speed interacts with working memory efficiency in multiple sclerosis. Arch Clin Neuropsychol 2006; 21(3): 229–238. [DOI] [PubMed] [Google Scholar]
- 31. Sweet LH, Rao SM, Primeau M, et al. Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp 2006; 27(1): 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Janculjak D, Mubrin Z, Brinar V, et al. Changes of attention and memory in a group of patients with multiple sclerosis. Clin Neurol Neurosurg 2002; 104(3): 221–227. [DOI] [PubMed] [Google Scholar]
- 33. Sternberg S. High-speed scanning in human memory. Science 1966; 153(3736): 652–654. [DOI] [PubMed] [Google Scholar]
- 34. Leavitt VM, Lengenfelder J, Moore NB, et al. The relative contributions of processing speed and cognitive load to working memory accuracy in multiple sclerosis. J Clin Exp Neuropsychol 2011; 33(5): 580–586. [DOI] [PubMed] [Google Scholar]
- 35. Fan J, McCandliss BD, Sommer T, et al. Testing the efficiency and independence of attentional networks. J Cogn Neurosci 2002; 14(3): 340–347. [DOI] [PubMed] [Google Scholar]
- 36. Lazeron RH, Rombouts SA, Machielsen WC, et al. Visualizing brain activation during planning: The Tower of London Test adapted for functional MR imaging. AJNR Am J Neuroradiol 2000; 21(8): 1407–1414. [PMC free article] [PubMed] [Google Scholar]
- 37. Denney DR, Gallagher KS, Lynch SG. Deficits in processing speed in patients with multiple sclerosis: Evidence from explicit and covert measures. Arch Clin Neuropsychol 2011; 26(2): 110–119. [DOI] [PubMed] [Google Scholar]
- 38. Kujala P, Portin R, Revonsuo A, et al. Automatic and controlled information processing in multiple sclerosis. Brain 1994; 117(Pt 5): 1115–1126. [DOI] [PubMed] [Google Scholar]
- 39. Macniven JAB, Davis C, Ho MY, et al. Stroop performance in multiple sclerosis: Information processing, selective attention, or executive functioning? J Int Neuropsychol Soc 2008; 14: 805–814. [DOI] [PubMed] [Google Scholar]
- 40. Rao SM, Losinski G, Mourany L, et al. Processing Speed Test: Validation of a self-administered, IPad®-based tool for screening cognitive dysfunction in a clinic setting. Mult Scler J 2017; 23: 1929–1937. [DOI] [PubMed] [Google Scholar]
- 41. Lapshin H, Lanctot KL, O’Connor P, et al. Assessing the validity of a computer-generated cognitive screening instrument for patients with multiple sclerosis. Mult Scler 2013; 19(14): 1905–1912. [DOI] [PubMed] [Google Scholar]
- 42. Foong J, Rozewicz L, Chong WK, et al. A comparison of neuropsychological deficits in primary and secondary progressive multiple sclerosis. J Neurol 2000; 247(2): 97–101. [DOI] [PubMed] [Google Scholar]
- 43. Conners CK, Sitarenios G. Conners’ Continuous Performance Test (CPT). In: Kreutzer JS, DeLuca J, Caplan B. (eds) Encyclopedia of clinical neuropsychology. New York: Springer, 2011, pp. 681–683. [Google Scholar]
- 44. Conners CK, Pitkanen J, Rzepa SR. Conners 3; Conners 2008. In: Kreutzer JS, DeLuca J, Caplan B. (eds) Encyclopedia of clinical neuropsychology. 3rd ed New York: Springer, 2011, pp. 675–678. [Google Scholar]
- 45. Sfagos C, Papageorgiou CC, Kosma KK, et al. Working memory deficits in multiple sclerosis: A controlled study with auditory P600 correlates. J Neurol Neurosurg Psychiatry 2003; 74(9): 1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amieva H, Lafont S, Auriacombe S, et al. Inhibitory breakdown and dementia of the Alzheimer type: A general phenomenon. J Clin Exp Neuropsychol 2002; 24(4): 503–516. [DOI] [PubMed] [Google Scholar]
- 47. Zimmermann P, Fimm B. A test battery for attentional performance. In: Leclercq M. (ed.) Applied neuropsychology of attention. London: Psychology Press, 2004, pp. 124–165. [Google Scholar]
- 48. Unsworth N, Heitz RP, Schrock JC, et al. An automated version of the operation span task. Behav Res Methods 2005; 37(3): 498–505. [DOI] [PubMed] [Google Scholar]
- 49. Zelazo PD, Anderson JE, Richler J, et al. NIH Toolbox Cognition Battery (CB): Validation of executive function measures in adults. J Int Neuropsychol Soc 2014; 20(6): 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kawahara Y, Ikeda M, Deguchi K, et al. Cognitive and affective assessments of multiple sclerosis (MS) and neuromyelitis optica (NMO) patients utilizing computerized touch panel-type screening tests. Intern Med 2014; 53(20): 2281–2290. [DOI] [PubMed] [Google Scholar]
- 51. Lamargue-Hamel D, Deloire M, Saubusse A, et al. Cognitive evaluation by tasks in a virtual reality environment in multiple sclerosis. J Neurol Sci 2015; 359(1–2): 94–99. [DOI] [PubMed] [Google Scholar]
- 52. Goverover Y, O’Brien AR, Moore NB, et al. Actual reality: A new approach to functional assessment in persons with multiple sclerosis. Arch Phys Med Rehabil 2010; 91: 252–260. [DOI] [PubMed] [Google Scholar]
- 53. Adler G, Bektas M, Feger M, et al. Computer-based assessment of memory and attention: Evaluation of the Memory and Attention Test (MAT). Psychiatr Prax 2012; 39(2): 79–83. [DOI] [PubMed] [Google Scholar]
- 54. Utz KS, Hankeln TMA, Jung L, et al. Visual search as a tool for a quick and reliable assessment of cognitive functions in patients with multiple sclerosis PLoS ONE 2013; 8: e81531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stricker NH, Lundt ES, Edwards KK, et al. Comparison of PC and iPad administrations of the CogState Brief Battery in the Mayo Clinic Study of Aging: Assessing cross-modality equivalence of computerized neuropsychological tests. Clin Neuropsychol 2018; 33: 1102–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cromer JA, Harel BT, Yu K, et al. Comparison of cognitive performance on the CogState Brief Battery when taken in-clinic, in-group, and unsupervised. Clin Neuropsychol 2015; 29(4): 542–558. [DOI] [PubMed] [Google Scholar]
- 57. Wojcik CM, Rao SM, Schembri AJ, et al. Necessity of technicians for computerized neuropsychological assessment devices in multiple sclerosis. Mult Scler 2018, https://www.ncbi.nlm.nih.gov/pubmed/30465463 [DOI] [PubMed]
- 58. Hughes AJ, Denney DR, Owens EM, et al. Procedural variations in the Stroop and the Symbol Digit Modalities Test: Impact on patients with multiple sclerosis. Arch Clin Neuropsychol 2013; 28(5): 452–462. [DOI] [PubMed] [Google Scholar]
- 59. Benedict RH, DeLuca J, Phillips G, et al. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 2017; 23(5): 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ruet A, Deloire M, Charre-Morin J, et al. Cognitive impairment differs between primary progressive and relapsing-remitting MS. Neurology 2013; 80(16): 1501–1508. [DOI] [PubMed] [Google Scholar]
- 61. Walker LA, Cheng A, Berard J, et al. Tests of information processing speed: What do people with multiple sclerosis think about them. Int J MS Care 2012; 14(2): 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hughes AJ, Denney DR, Lynch SG. Reaction time and rapid serial processing measures of information processing speed in multiple sclerosis: Complexity, compounding, and augmentation. J Int Neuropsychol Soc 2011; 17(6): 1113–1121. [DOI] [PubMed] [Google Scholar]
- 63. Tombaugh TN, Berrigan LI, Walker LAS, et al. The Computerized Test of Information Processing (CTIP) offers an alternative to the PASAT for assessing cognitive processing speed in individuals with multiple sclerosis. Cogn Behav Neurol 2010; 23(3): 192–198. [DOI] [PubMed] [Google Scholar]
- 64. Smith AM, Walker LAS, Freedman MS, et al. Activation patterns in multiple sclerosis on the Computerized Tests of Information Processing. J Neurol Sci 2012; 312(1–2): 131–137. [DOI] [PubMed] [Google Scholar]
- 65. Mazerolle EL, Wojtowicz MA, Omisade A, et al. Intra-individual variability in information processing speed reflects white matter microstructure in multiple sclerosis. Neuroimage Clin 2013; 2: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Papathanasiou A, Messinis L, Georgiou VL, et al. Cognitive impairment in relapsing remitting and secondary progressive multiple sclerosis patients: Efficacy of a computerized cognitive screening battery. ISRN Neurol 2014; 151379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Golan D, Doniger GM, Wissemann K, et al. The impact of subjective cognitive fatigue and depression on cognitive function in patients with multiple sclerosis. Mult Scler 2018; 24(2): 196–204. [DOI] [PubMed] [Google Scholar]
- 68. De Meijer L, Merlo D, Skibina O, et al. Monitoring cognitive change in multiple sclerosis using a computerized cognitive battery. Mult Scler J Exp Transl Clin 2018; 4(4), https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6293367/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cabeca HLS, Rocha LC, Sabba AF, et al. The subtleties of cognitive decline in multiple sclerosis: An exploratory study using hierarchical cluster analysis of CANTAB results. BMC Neurol 2018; 18(1): 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morrow SA, Drake A, Zivadinov R, et al. Predicting loss of employment over three years in multiple sclerosis: Clinically meaningful cognitive decline. Clin Neuropsychol 2010; 24(7): 1131–1145. [DOI] [PubMed] [Google Scholar]
- 71. Benedict RHB, Drake AS, Irwin LN, et al. Benchmarks of meaningful impairment on the MSFC and BICAMS. Mult Scler 2016; 22(14):1874–1882. [DOI] [PubMed] [Google Scholar]
- 72. Patel VP, Walker LAS, Feinstein A. Deconstructing the symbol digit modalities test in multiple sclerosis: The role of memory. Mult Scler Relat Disord 2017; 17: 184–189. DOI: 10.1016/j.msard.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 73. Rudick RA, Miller D, Bethoux F, et al. The Multiple Sclerosis Performance Test (MSPT): An iPad-based disability assessment tool. J Visual Exp 2014; 30: e51318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Charvet LE, Shaw M, Frontario A, et al. Cognitive impairment in pediatric-onset multiple sclerosis is detected by the Brief International Cognitive Assessment for Multiple Sclerosis and computerized cognitive testing. Mult Scler J 2017; 24: 512–519. [DOI] [PubMed] [Google Scholar]
- 75. Younes M, Hill J, Quinless J, et al. Internet-based cognitive testing in multiple sclerosis. Mult Scler 2007; 13: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 76. Foong J, Rozewicz L, Quaghebeur G, et al. Executive function in multiple sclerosis: The role of frontal lobe pathology. Brain 1997; 120(Pt 1): 15–26. [DOI] [PubMed] [Google Scholar]
- 77. Grech LB, Kiropoulos LA, Kirby KM, et al. Coping mediates and moderates the relationship between executive functions and psychological adjustment in multiple sclerosis. Neuropsychology 2016; 30(3): 361–376. [DOI] [PubMed] [Google Scholar]
- 78. Adler G, Lembach Y. Memory and selective attention in multiple sclerosis: Cross-sectional computer-based assessment in a large outpatient sample. Eur Arch Psychiatry Clin Neurosci 2015; 265(5): 439–443. [DOI] [PubMed] [Google Scholar]
- 79. Archibald CJ, Wei X, Scott JN, et al. Posterior fossa lesion volume and slowed information processing in multiple sclerosis. Brain 2004; 127(Pt 7): 1526–1534. [DOI] [PubMed] [Google Scholar]
- 80. Janculjak D, Mubrin Z, Brzovic Z, et al. Changes in short-term memory processes in patients with multiple sclerosis. Eur J Neurol 1999; 6: 663–668. [DOI] [PubMed] [Google Scholar]
- 81. Parmenter BA, Shucard JL, Benedict RH, et al. Working memory deficits in multiple sclerosis: Comparison between the n-back task and the Paced Auditory Serial Addition Test. J Int Neuropsychol Soc 2006; 12(5): 677–687. [DOI] [PubMed] [Google Scholar]
- 82. Littleton AC, Register-Mihalik JK, Guskiewicz KM. Test-retest reliability of a computerized concussion test: CNS Vital Signs. Sports Health 2015; 7(5): 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vincent AS, Roebuck-Spencer TM, Fuenzalida E, et al. Test-retest reliability and practice effects for the ANAM General Neuropsychological Screening Battery. Clin Neuropsychol 2018; 32(3): 479–494. [DOI] [PubMed] [Google Scholar]
- 84. Schweiger A, Doniger G, Dwolatzky T, et al. Reliability of a novel computerized neuropsychological battery for mild cognitive impairment. Acta Neuropsychologica 2003; 1: 407–413. [Google Scholar]
- 85. Papathanasiou A, Messinis L, Zampakis P, et al. Corpus callosum atrophy as a marker of clinically meaningful cognitive decline in secondary progressive multiple sclerosis. Impact on employment status. J Clin Neurosci 2017; 43: 170–175. [DOI] [PubMed] [Google Scholar]
- 86. Papathanasiou A, Messinis L, Georgiou V, et al. Cognitive impairment in multiple sclerosis patients: Validity of a computerized cognitive screening battery. Eur J Neurol 2014; 21: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cotter J, Vithanage N, Colville S, et al. Investigating domain-specific cognitive impairment among patients with multiple sclerosis using touchscreen cognitive testing in routine clinical care. Front Neurol 2018; 9: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Benedict RHB, Schretlen D, Groninger L, et al. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychol Assess 1996; 8: 145–153. [Google Scholar]
- 89. Maruff P, Thomas E, Cysique L, et al. Validity of the CogState Brief Battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol 2009; 24(2): 165–178. [DOI] [PubMed] [Google Scholar]
- 90. Rey A. L’examen Clinique en Psychologie. Paris: Press Universitaire de France, 1964. [Google Scholar]
- 91. Buschke F, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974; 24(11): 1019–1025. [DOI] [PubMed] [Google Scholar]
- 92. Wechsler D. Wechsler Memory Scale—Revised manual. New York: Psychological Corporation, 1987. [Google Scholar]
- 93. Ayache SS, Palm U, Chalah MA, et al. Orienting network dysfunction in progressive multiple sclerosis. J Neurol Sci 2015; 351(1–2): 206–207. [DOI] [PubMed] [Google Scholar]
- 94. Urbanek C, Weinges-Evers N, Bellmann-Strobl J, et al. Attention Network Test reveals alerting network dysfunction in multiple sclerosis. Mult Scler 2010; 16: 93–99. [DOI] [PubMed] [Google Scholar]
- 95. Deloire MSA, Salort E, Bonnet M, et al. Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 2005; 76(4): 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Motor Skills 1958; 8: 271–276. [Google Scholar]
- 97. Wilken JA, Kane R, Sullivan CL, et al. The utility of computerized neuropsychological assessment of cognitive dysfunction in patients with relapsing-remitting multiple sclerosis. Mult Scler 2003; 9(2): 119–127. [DOI] [PubMed] [Google Scholar]
- 98. Lynch SG, Dickerson KJ, Denney DR. Evaluating processing speed in multiple sclerosis: A comparison of two rapid serial processing measures. Clin Neuropsychol 2010; 24(6): 963–976. [DOI] [PubMed] [Google Scholar]
- 99. Gudesblatt M, Zarif M, Bumstead B, et al. Multiple sclerosis, cognitive profile and cognitive testing: Predictability of SDMT and computerized cognitive testing in differentiating employment versus unemployment in patients with multiple sclerosis (P5. 207). Neurology 2015; 84, https://n.neurology.org/content/84/14_Supplement/P5.207 [Google Scholar]
- 100. Pietrzak RH, Olver J, Norman T, et al. A comparison of the CogState Schizophrenia Battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol 2009; 31: 848–859. DOI: 10.1080/13803390802592458. [DOI] [PubMed] [Google Scholar]
- 101. LaRocca NG, Hudson LD, Rudick R, et al. The MSOAC approach to developing performance outcomes to measure and monitor multiple sclerosis disability. Mult Scler 2018; 24(11): 1469–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Erlanger DM, Kaushik T, Caruso LS, et al. Reliability of a cognitive endpoint for use in a multiple sclerosis pharmaceutical trial. J Neurol Sci 2014; 340(1–2): 123–129. [DOI] [PubMed] [Google Scholar]
- 103. Benedict RHB, Amato MP, Boringa J, et al. Brief International Cognitive Assessment for MS (BICAMS): International standards for validation. BMC Neurol 2012; 12: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Benedict RHB, Cookfair D, Gavett R, et al. Validity of the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS). J Int Neuropsychol Soc 2006; 12: 549–558. [DOI] [PubMed] [Google Scholar]
- 105. DeLuca J, Gaudino EA, Diamond BJ, et al. Acquisition and storage deficits in multiple sclerosis. J Clin Exp Neuropsychol 1998; 20(3): 376–390. [DOI] [PubMed] [Google Scholar]
- 106. Rao SM. On the nature of memory disturbance in multiple sclerosis. J Clin Exp Neuropsychol 1989; 11(5): 699–712. [DOI] [PubMed] [Google Scholar]
- 107. DeLuca J, Chelune GJ, Tulsky DS, et al. Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? J Clin Exp Neuropsychol 2004; 26: 550–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ879094_supplemental_table_a for Computerized neuropsychological assessment devices in multiple sclerosis: A systematic review by Curtis M Wojcik, Meghan Beier, Kathleen Costello, John DeLuca, Anthony Feinstein, Yael Goverover, Mark Gudesblatt, Michael Jaworski, Rosalind Kalb, Lori Kostich, Nicholas G LaRocca, Jonathan D Rodgers and Ralph HB Benedict in Multiple Sclerosis Journal
Supplemental material, MSJ879094_supplemental_table_b for Computerized neuropsychological assessment devices in multiple sclerosis: A systematic review by Curtis M Wojcik, Meghan Beier, Kathleen Costello, John DeLuca, Anthony Feinstein, Yael Goverover, Mark Gudesblatt, Michael Jaworski, Rosalind Kalb, Lori Kostich, Nicholas G LaRocca, Jonathan D Rodgers and Ralph HB Benedict in Multiple Sclerosis Journal