Abstract
Background:
Diabetes mellitus and concomitant dyslipidemia, being referred to as ‘diabetic dyslipidemia’, are the foremost detrimental factors documented to play a pivotal role in cardiovascular illness. Diabetic dyslipidemia is associated with insulin resistance, high plasma triglyceride levels, low HDL-cholesterol concentration and elevated small dense LDL-cholesterol particles. Maintaining an optimal glucose and lipid levels in patients afflicted with diabetic dyslipidemia could be a major task that might require a well-planned diet-management system and regular physical activity, or otherwise an intake of combined antidiabetic and antihyperlipidemic medications. Synchronized treatment which efficiently controls insulin resistance-associated diabetes mellitus and co-existing dyslipidemia could indeed be a fascinating therapeutic option in the management of diabetic dyslipidemia. Peroxisome proliferator-activated receptors α/γ (PPARα/γ) dual agonists are such kind of drugs which possess therapeutic potentials to treat diabetic dyslipidemia. Nevertheless, PPARα/γ dual agonists like muraglitazar, naveglitazar, tesaglitazar, ragaglitazar and aleglitazar have been reported to have undesirable adverse effects, and their developments have been halted at various stages. On the other hand, a recently introduced PPARα/γ dual agonist, saroglitazar is an emerging therapeutic agent of glitazar class approved in India for the management of diabetic dyslipidemia, and its treatment has been reported to be generally safe and well tolerated.
Conclusion:
Some additional and new compounds, at initial and preclinical stages, have been recently reported to possess PPARα/γ dual agonistic potentials with considerable therapeutic efficacy and reduced adverse profile. This review sheds light on the current status of various PPARα/γ dual agonists for the management of diabetic dyslipidemia.
Keywords: PPARα/γ dual agonists, Insulin resistance, Diabetic dyslipidemia, Adverse effects, Cardiovascular events, Oedema
1. INTRODUCTION
Dyslipidemia is a crucial menace for cardiovascular disease in patients afflicted with diabetes mellitus. The diabetic dyslipidemia is associated with high plasma triglycerides, reduced high-density lipoproteins (HDL), and elevated levels of small dense low-density lipoproteins (LDL) [1]. These changes could be caused by an increase in free fatty acid flux secondary to insulin resistance and be aggravated by elevation of inflammatory adipokines [1]. Principle consequence is required to prevent the incessant growing of morbidity and mortality associated with diabetes mellitus and hyperlipidemia across the world.
Peroxisome proliferator-activated receptors (PPARs) are documented as major regulators of lipid and glucose metabolism [2]. PPARs are the member of nuclear hormone receptor superfamily that act as ligand-dependent transcription factors and expressively regulate the lipid and glucose metabolism. PPARs act on the DNA response elements as heterodimers with the nuclear retinoic acid receptor to modulate the expression of a target gene. Three isoforms of PPARs have been recognized namely PPARα, PPARγ and PPARδ [3, 4]. Activation of PPARα decreases triglyceride levels, whereas activation of PPARγ causes insulin sensitization to enhance glucose metabolism (Fig. 1). Fibrate class of hypolipidemic drugs activates PPARα, while thiazolidinedione class of antidiabetic agents activates PPARγ [5-7]. Patients with diabetes mellitus are at higher risk of having the cardiovascular disease onset. In addition, the cardiovascular disease risk is further high in those diabetic patients afflicted with hyperlipidemia [8]. Therefore, diabetic dyslipidemia is a major concern and needs an optimal therapeutic strategy for consistent management. Concurrent activation of PPARα and PPARγ alongside targeting both lipid and glucose metabolism could be a beneficial therapeutic option for the management of diabetic dyslipidemia (Fig. 1). In view of this context, various PPARα/γ dual agonists such as muraglitazar, naveglitazar, tesaglitazar, ragaglitazar and aleglitazar were studied for their therapeutic potentials and safety profiles; however, the undesirable adverse effects of these drugs noted in preclinical and clinical studies (Table 1) raised a safety concern, and as a result, the development of these glitazars has been halted at various stages [9-13]. On the other hand, saroglitazar, a recently developed PPARα/γ dual agonist, is emerging as a potent therapeutic agent of glitazar class for the management of diabetic dyslipidemia [14]. The Phase III clinical trial results indicate that saroglitazar is devoid of conventional side effects caused by fibrates and pioglitazone [14]. This opens up a new way of hope for the development of novel PPARα/γ dual agonists for the management of diabetic dyslipidemia. The present review enlightens the current status of dual acting PPARα/γ agonists in the management of diabetic dyslipidemia.
Fig. (1).
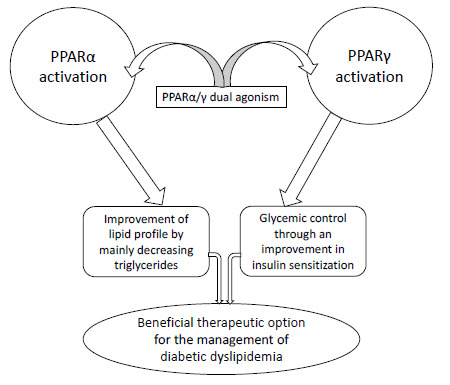
Therapeutic rationale of PPARα/γ dual agonism for the management of diabetic dyslipidemia.
Table 1. Key adverse effects pertaining to some PPARα/γ dual agonists in bench and clinical studies.
| PPARα/γ Dual Agonists | Adverse Effects |
|---|---|
| Muraglitazar | Oedema, congestive heart failure, and major adverse cardiovascular events like myocardial infarction, stroke and transient ischemic attack in diabetic patients [9, 18]. |
| Naveglitazar | Hypertrophic and proliferative effects on the urothelium in rats [19]. |
| Tesaglitazar | Elevation of serum creatinine, increase in body weight and peripheral oedema, and reduction in glomerular filtration rate in type 2 diabetic patients [21-23]. |
| Ragaglitazar | Carcinogenic effect in the rodent urinary bladder urothelium [24]. |
| Aleglitazar | It increases the risks of heart failure, renal dysfunction and gastrointestinal hemorrhage in diabetic patients [27]. In addition, aleglitazar increases the incidence of hypoglycemia and muscular events as compared to placebo [13]. |
2. UNDESIRABLE ADVERSE EFFECTS OF PPARΑ/Γ DUAL AGONISTS: PRECLINICAL AND CLINICAL EVIDENCES
The simultaneous activation of PPARα and PPARγ had been initially anticipated to provide improvements in glycemic control and dyslipidemia in type 2 diabetic patients, and therefore PPARα/γ dual agonists were evaluated preclinically and clinically for their therapeutic potentials. Muraglitazar is a non-thiazolidinedione, oxybenzylglycine PPARα/γ dual agonist [15]. Initially, Buse et al. [16] reported that 24 weeks muraglitazar treatment was effective in type 2 diabetic patients who were inadequately controlled with diet and exercise [16]. Subsequent studies, however, pointed out some serious adverse effects of this PPARα/γ dual agonist [9,17]. In diabetic patients, when compared with pioglitazone (PPARγ agonist), muraglitazar was reported to be associated with an excess incidence of the composite endpoint of death, congestive heart failure, and major adverse cardiovascular events like myocardial infarction, stroke and transient ischemic attack [9]. In addition, oedema-related event was noted to occur with muraglitazar in a dose-dependent incidence in patients with type 2 diabetes mellitus [18].
Another agent, naveglitazar, a gamma-dominant PPARα/γ dual agonist, was suggested to be associated with hypertrophic and proliferative effects on the urothelium in rats [19]. Of note, Fagerberg et al. [20] evaluated the effect of tesaglitazar, another dual acting PPARα/γ agonist, on lipid and glucose metabolism in patients having evidence of insulin resistance. Tesaglitazar, in this study, was noted to be well tolerated and it produced dose-dependent improvements in the lipid and glucose metabolism as well as in the insulin sensitivity [20]. In addition, this study suggested that tesaglitazar could have a potential to prevent vascular complications and delay the progression to diabetes in these patients [20]. However, in subsequent studies, tesaglitazar was noted to be associated with a greater increase in serum creatinine level than placebo in type 2 diabetic patients [21, 22]. Moreover, tesaglitazar treatment was shown to be associated with an increase in body weight and peripheral oedema in a dose-dependent manner in patients afflicted with type 2 diabetes mellitus [22]. Furthermore, it was shown that tesaglitazar-treated type 2 diabetic patients have had a reduction in glomerular filtration rate [23]. Another dual-acting PPARα/γ agonist, ragaglitazar, had a carcinogenic effect in the rodent urinary bladder urothelium [24]. In the urothelium of ragaglitazar-treated rats, hypertrophy was noted to be an early change that affected the whole bladder urothelial cell population [24].
Further intense research in the PPAR area has yielded a balanced dual-acting PPARα/γ agonist, aleglitazar [25]. This balanced PPARα/γ dual agonist had also been evaluated for its safety and therapeutic potentials. Aleglitazar was shown to produce dose-dependent improvements in fasting and postprandial glucose levels, insulin resistance as well as lipid parameters in type 2 diabetic patients [26]. Despite the reduction in glycated hemoglobin and improvement in serum HDL-cholesterol and triglyceride levels, aleglitazar did not significantly decrease the incidence of cardiovascular risk, rather aleglitazar was noted in the AleCardio randomized clinical trial to increase the risks of heart failure, renal dysfunction and gastrointestinal hemorrhage [27]. In addition, aleglitazar has been reported to increase the incidence of hypoglycemia and muscular events as compared to placebo [13]. Aleglitazar was reported to be associated with adverse events even within a short duration of exposure [13]. Based on these results, it was suggested that coupled with the previous failure, the dual agonists of PPAR α/γ might hold a little promise for cardiovascular therapeutics [13]. However, current research is still active in identifying the novel PPARα/γ dual agonists for the management of diabetic dyslipidemia.
3. SAROGLITAZAR: A NEWLY INTRODUCED PPARΑ/Γ DUAL AGONIST
Saroglitazar has predominant PPARα and moderate PPARγ agonistic activities [28]. Saroglitazar is the first approved agent in the Glitazar class for the management of diabetic dyslipidemia, and it has been approved in India [29]. Saroglitazar treatment was reported to be generally safe and well tolerated while no serious adverse events were reported, and it appeared to be an effective therapeutic option for improving hypertriglyceridemia in type 2 diabetic patients [30, 31]. An observational study evaluated, in Indian diabetic dyslipidemia patients, the safety and efficacy of saroglitazar [32]. In this study, saroglitazar was suggested to be a potential therapeutic option for type 2 diabetic patients with high triglyceride (TG) levels, not controlled by statins, for comprehensive lipid and glycemic control with acceptable safety profile [32]. A recent study reported that, in Indian patients with diabetic dyslipidemia, the add on therapy of saroglitazar with metformin significantly decreased TG, glycosylated haemoglobin, fasting plasma glucose and post prandial plasma glucose levels as compared to the add on therapy of fenofibrate with metformin [33]. The aforementioned studies indicate the therapeutic potentials of saroglitazar for the management of diabetic dyslipidemia.
4. AN UPDATE ON THE NEW PPARΑ/Γ DUAL AGONISTS BEING EXPLORED
Recent basic drug discovery studies aim to identify and develop potential PPARα/γ dual agonists devoid of unwanted adverse effects. The compound, LT175 has been reported to be a novel PPARα/γ ligand with potent insulin-sensitizing effects and reduced adipogenic properties [34]. Administration of LT175 to high-fat diet fed mice was reported to decrease body weight, adipocyte size and white adipose tissue mass. In addition, LT175 has been shown to significantly reduce the plasma glucose, non-esterified fatty acids, triglycerides and cholesterol [34]. Jeong et al. [35] have reported that via activation of PPARα/γ, a compound named, CG301269 improved the lipid and glucose metabolism. CG301269 was reported to enhance the fatty acid oxidation in vitro and to ameliorate the insulin resistance and hyperlipidemia in vivo. Moreover, CG301269 was noted to reduce inflammatory responses and fatty liver without body weight gain in db/db mice [35].
Interestingly, a recent study by Jung et al. [36] reported that amodiaquine, an antimalarial agent, has a potential to concurrently activate PPARα and γ. The authors showed that amodiaquine has a potential to improve insulin resistance and lipid metabolism in diabetic mice model [36]. Intriguingly, amodiaquine not only ameliorated insulin resistance, hyperlipidemia and fatty liver; but, also decreased the body weight gain in high-fat diet-induced obese and genetically modified obese/diabetic mice [36]. In a recent study, Ren et al. [37] have reported a novel PPARα/γ dual agonist, propane-2-sulfonic acid octadec-9-enyl-amide (N15), which was shown to ameliorate insulin resistance and gluconeogenesis in vivo and in vitro. The compound, N15 has exerted beneficial action over the glucose and lipid metabolism without triggering the weight gain and hepatotoxicity in mice [37]. The authors suggested that N15 could have the potential to be a prophylactic and therapeutic agent for the management of type 2 diabetes mellitus and associated metabolic disorders [37].
Continued research has identified a few more potential dual activators of PPARα and PPARγ. In this regard, Jung et al. [38] have reported antidiabetic effect of a compound, (E)-N-(4-(3-(5-bromo-4-hydroxy-2-methoxyphenyl)acryloyl) phenyl)-4-tert-butylbenzamide (SN158) via PPARα/γ dual activation in ob/ob mice. This study showed that SN158 markedly lowered the plasma glucose, TG and free fatty acid levels in ob/ob mice without severe weight gain and hepatomegaly, suggesting that SN158 could have a potential for the management of type 2 diabetes mellitus and associated metabolic disorders by alleviating glucose and lipid abnormalities [38]. A recent study evaluated the effect of Huangkui capsule (HKC), an extract from Abelmoschus manihot (L.) medic (a natural medicinal plant of China), in a diabetic nephropathic rat model [39]. In this study, HKC was noted to enhance the transcriptional activity of PPARα and PPARγ in cultured cells, liver and kidney of diabetic nephropathic rats, while it reduced the serum levels of TG and cholesterol and the fat in the liver of diabetic nephropathic rats [39]. Moreover, HKC was shown to reduce inflammatory genes expression in the kidney of diabetic nephropathic rats. This study showed that HKC improved lipid metabolic disorders by activating PPARα/γ, and it could ameliorate renal inflammation and glomerular injury in diabetic nephropathic rats [39].
Another study investigated the effect of 2-[4-(5-chlorobenzothiazothiazol-2-yl)phenoxy]-2-methyl-propionic acid (MHY908), a synthetic dual acting PPARα/γ agonist, in aged rats [40]. The aged rats administered with MHY908 showed a reduced levels of serum glucose and TG, and reduced liver TGs as well. In addition, MHY908 was shown to reduce endoplasmic reticulum stress and activate c-Jun N-terminal kinase in the liver of aged rats, which were suggested to consequently improve the insulin signaling [40]. Moreover, in the kidney of the aged rats, the anti-inflammatory potential of MHY908 was shown by its suppression of NF-κB activation via an inhibition of the Akt/IκB kinase signaling system. This preclinical study suggested that MHY908 could have a therapeutic potential against age-related inflammation and associated insulin resistance through activation of PPARα and PPARγ [40]. In another study, the effect of amorphastilbol on glucose and lipid metabolism was evaluated using in vitro and db/db mice models [41]. Amorphastilbol was noted to selectively stimulate the transcriptional activities of both PPARα and PPARγ, which were able to enhance fatty acid oxidation as well as glucose utilization [41]. Importantly, there were no significant adverse effects such as weight gain or hepatomegaly in amorphastilbol-treated animals [41]. The authors suggested that amorphastilbol could have a therapeutic potential against type 2 diabetes mellitus and associated metabolic disorders by enhancing glucose and lipid metabolism [41]. A recent study has reported a new thiazolidine compound, namely GQ-11, as a partial PPAR α/γ dual agonist having experimental antidiabetic effects [42]. Moreover, GQ-11 has been reported to improve the lipid profile and ameliorate the chronic inflammation associated with obesity in atherosclerosis-prone mice [42]. Taken in concert, the aforementioned literature have outlined the early stage potentials of newly identified dual activators of PPARα and PPARγ.
A recent study has identified some marine oxohexadecenoic acids such as (7E)-9-oxohexadec-7-enoic acid, and (10E)-9-oxohexadec-10-enoic acid (from the marine algae Chaetoceros karianus) having a potential to activate PPARα and PPARγ [43]. The authors, through the synthesis and biological evaluations, have denoted both compounds as semi-potent dual PPARα/γ agonists [43]. Of note, both compounds have been shown to induce anti-diabetic gene programs in adipocytes by upregulating insulin-sensitizing adipokines and repressing pro-inflammatory cytokines [43]. Developing such kind of natural products as dual acting PPARα/γ agonists might be associated with minimal adverse effects; however, further studies are needed to assess their therapeutic potentials and adverse profile. The key pharmacological actions and chemical structures of newly identified PPARα/γ dual agonists which are under preclinical stages have been provided in Table 2.
Table 2. The chemical structures of some novel PPARα/γ dual agonists which are under preclinical stages and their key pharmacological effects.
| PPARα/γ Dual Agonist | Chemical Structure | Key Effects/Advantages |
|---|---|---|
| LT175 | 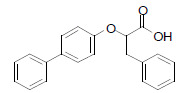 |
In mice fed a high-fat diet, the LT175 administration has decreased body weight, adipocyte size and the white adipose tissue mass, and it also significantly reduced the plasma glucose, insulin, non-esterified fatty acids, triglycerides and cholesterol [34]. |
| CG301269 | 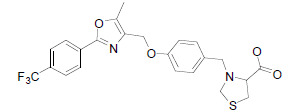 |
CG301269 has a potential to selectively stimulate the transcriptional activities of PPARα and PPARγ. Interestingly, CG301269 was noted to reduce the inflammatory responses and fatty liver without inducing the body weight gain in db/db mice. Moreover, CG301269 was noted to ameliorate insulin resistance and hyperlipidemia in vivo [35]. |
| Amodiaquine | 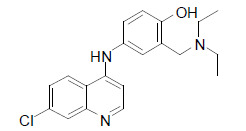 |
Amodiaquine has a potential to selectively activate PPARα/γ transcriptional activities. Intriguingly, in high fat diet-induced obese and genetically modified obese-diabetic mice, amodiaquine was noted to remarkably ameliorate insulin resistance, hyperlipidemia and fatty liver, and also to decrease the body weight gain [36]. |
| Propane-2-sulfonic acid octadec-9-enyl-amide (N15) | 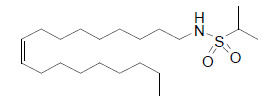 |
The compound, N15 has advantageous effects on glucose and lipid metabolism without triggering the weight gain in mice. Its glucose-lowering effect has been suggested to be associated with PPARγ-mediated upregulation of hepatic glucose consumption and downregulation of gluconeogenesis [37]. |
| SN158 | 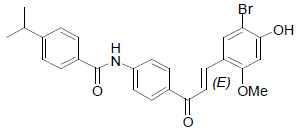 |
The compound, SN158 has been shown to interact with PPARα and PPARγ and to increase the transcriptional activities of both. Interestingly, SN158 has been noted to significantly lower the plasma glucose, triglycerides, and free fatty acids in ob/ob mice without having severe weight gain and hepatomegaly [38]. |
| MHY908 | 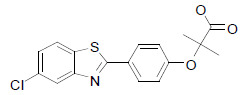 |
Supplementation with MHY908 showed reduced serum glucose and triglyceride levels, and also reduced liver triglyceride levels in aged rats [40]. |
| Amorphastilbol | 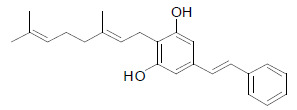 |
Amorphastilbol has been shown to selectively stimulate the transcriptional activities of PPARα and PPARγ. It improved glucose and lipid impairment in db/db mice. Moreover, there were no significant adverse effects like weight gain or hepatomegaly noted in amorphastilbol-treated animals [41]. |
Synchronized treatment which proficiently manages insulin resistance-and associated diabetes mellitus and co-existing hyperlipidemia is considered a promising therapeutic option for effective management of diabetic dyslipidemia. The novel PPARα/γ dual agonists currently being explored at preclinical stages might need to be evaluated clinically in order to determine their therapeutic efficacies and adverse profiles. An ideal dual acting PPARα/γ agonist is expected to efficiently manage type 2 diabetes mellitus and concurrent hyperlipidemic conditions without showing major adverse effects especially cardiovascular and renal events and metabolic abnormalities like weight gain.
CONCLUDING REMARKS
The diabetic prevalence is incessantly increasing across the world, while preventing the diabetes-associated high morbidity and mortality is of great clinical importance. Optimal glycemic control and effective lipid management, in order to avert cardiovascular complications, are the most important therapeutic goals in patients afflicted with chronic type 2 diabetes mellitus and dyslipidemia. Activating PPARs has long been an efficient target for developing antidiabetic therapy owing to their key role in insulin sensitization, glycemic management and lipid metabolism. Despite the undesirable adverse profile of PPARα/γ dual agonists like muraglitazar, naveglitazar, tesaglitazar, ragaglitazar and aleglitazar, the development of promising PPARα/γ dual agonists with potential therapeutic effects in diabetic dyslipidemic patients without any serious adverse effects is of continued interest. The early stage drug discovery studies have recently identified some novel PPARα/γ dual agonists such as LT175, CG301269, amodiaquine, propane-2-sulfonic acid octadec-9-enyl-amide (N15), SN158, MHY908, amorphastilbol, GQ-11 and some marine oxohexadecenoic acids; however, further studies are needed to precisely determine the therapeutic potentials and adverse profile of these newly identified compounds at clinical levels for the efficient management of diabetic dyslipidemia.
ACKNOWLEDGEMENTS
The authors are thankful to the Chairperson Smt. N. Sendamaraai, and the Director Mr. S. Ommsharravana of J.K.K. Nattraja Educational Institutions, Komarapalayam, Tamil Nadu, India for the inspiration and support.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Chehade J.M., Gladysz M., Mooradian A.D. Dyslipidemia in type 2 diabetes: prevalence, pathophysiology, and management. Drugs. 2013;73:327–339. doi: 10.1007/s40265-013-0023-5. [DOI] [PubMed] [Google Scholar]
- 2.Chigurupati S., Dhanaraj S.A., Balakumar P. A step ahead of PPARγ full agonists to PPARγ partial agonists: therapeutic perspectives in the management of diabetic insulin resistance. Eur. J. Pharmacol. 2015;755:50–57. doi: 10.1016/j.ejphar.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 3.Ferré P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53:S43–S50. doi: 10.2337/diabetes.53.2007.s43. [DOI] [PubMed] [Google Scholar]
- 4.Balakumar P., Jagadeesh G. PPAR ligands and cardiovascular disorders: Friend or foe. Curr. Mol. Pharmacol. 2012;5:219–223. doi: 10.2174/1874467211205020219. [DOI] [PubMed] [Google Scholar]
- 5.Balakumar P., Rose M., Ganti S.S., Krishan P., Singh M. PPAR dual agonists: Are they opening Pandora’s Box? Pharmacol. Res. 2007;56:91–98. doi: 10.1016/j.phrs.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Balakumar P., Rohilla A., Mahadevan N. Pleiotropic actions of fenofibrate on the heart. Pharmacol. Res. 2011;63:8–12. doi: 10.1016/j.phrs.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Balakumar P., Kathuria S. Submaximal PPARγ activation and endothelial dysfunction: New perspectives for the management of cardiovascular disorders. Br. J. Pharmacol. 2012;166:1981–1992. doi: 10.1111/j.1476-5381.2012.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balakumar P., Maung-U K., Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016;113:600–609. doi: 10.1016/j.phrs.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 9.Nissen S.E., Wolski K., Topol E.J. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- 10.Conlon D. Goodbye glitazars? Br. J. Diabetes Vasc. Dis. 2006;6:135–137. [Google Scholar]
- 11.Ratner R.E., Parikh S., Tou C. GALLANT 9 Study Group. Efficacy, safety and tolerability of tesaglitazar when added to the therapeutic regimen of poorly controlled insulin-treated patients with type 2 diabetes. Diab. Vasc. Dis. Res. 2007;4:214–221. doi: 10.3132/dvdr.2007.042. [DOI] [PubMed] [Google Scholar]
- 12.Tseng C.H., Tseng F.H. Peroxisome proliferator-activated receptor agonists and bladder cancer: Lessons from animal studies. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2012;30:368–402. doi: 10.1080/10590501.2012.735519. [DOI] [PubMed] [Google Scholar]
- 13.Erdmann E., Califf R., Gerstein H.C., Malmberg K., Ruilope L., Schwartz G.G., Wedel H., Volz D., Ditmarsch M., Svensson A., Bengus M. Effects of the dual peroxisome proliferator-activated receptor activator aleglitazar in patients with Type 2 Diabetes mellitus or prediabetes. Am. Heart J. 2015;170:117–122. doi: 10.1016/j.ahj.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Joshi S.R. Saroglitazar for the treatment of dyslipidemia in diabetic patients. Expert Opin. Pharmacother. 2015;16:597–606. doi: 10.1517/14656566.2015.1009894. [DOI] [PubMed] [Google Scholar]
- 15.Cox S.L. Muraglitazar: An agent for the treatment of type 2 diabetes and associated dyslipidemia. Drugs Today (Barc) 2005;41:579–587. doi: 10.1358/dot.2005.41.9.925347. [DOI] [PubMed] [Google Scholar]
- 16.Buse J.B., Rubin C.J., Frederich R., Viraswami-Appanna K., Lin K.C., Montoro R., Shockey G., Davidson J.A. Muraglitazar, a dual (alpha/gamma) PPAR activator: A randomized, double-blind, placebo-controlled, 24-week monotherapy trial in adult patients with type 2 diabetes. Clin. Ther. 2005;27:1181–1195. doi: 10.1016/j.clinthera.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Doggrell S.A. Muraglitazar: beneficial or detrimental in the treatment of Type 2 diabetes? Expert Opin. Pharmacother. 2006;7:1229–1233. doi: 10.1517/14656566.7.9.1229. [DOI] [PubMed] [Google Scholar]
- 18.Rubin C.J., Viraswami-Appanna K., Fiedorek F.T. Efficacy and safety of muraglitazar: A double-blind, 24-week, dose-ranging study in patients with type 2 diabetes. Diab. Vasc. Dis. Res. 2009;6:205–215. doi: 10.1177/1479164109336048. [DOI] [PubMed] [Google Scholar]
- 19.Long G.G., Reynolds V.L., Lopez-Martinez A., Ryan T.E., White S.L., Eldridge S.R. Urothelial carcinogenesis in the urinary bladder of rats treated with naveglitazar, a gamma-dominant PPAR alpha/gamma agonist: Lack of evidence for urolithiasis as an inciting event. Toxicol. Pathol. 2008;36:218–231. doi: 10.1177/0192623307311757. [DOI] [PubMed] [Google Scholar]
- 20.Fagerberg B., Edwards S., Halmos T., Lopatynski J., Schuster H., Stender S., Stoa-Birketvedt G., Tonstad S., Halldórsdóttir S., Gause-Nilsson I. Tesaglitazar, a novel dual peroxisome proliferator-activated receptor alpha/gamma agonist, dose-dependently improves the metabolic abnormalities associated with insulin resistance in a non-diabetic population. Diabetologia. 2005;48:1716–1725. doi: 10.1007/s00125-005-1846-8. [DOI] [PubMed] [Google Scholar]
- 21.Ratner R.E., Parikh S., Tou C. GALLANT 9 Study Group. Efficacy, safety and tolerability of tesaglitazar when added to the therapeutic regimen of poorly controlled insulin-treated patients with type 2 diabetes. Diab. Vasc. Dis. Res. 2007;4:214–221. doi: 10.3132/dvdr.2007.042. [DOI] [PubMed] [Google Scholar]
- 22.Bays H., McElhattan J., Bryzinski B.S. GALLANT 6 Study Group. A double-blind, randomised trial of tesaglitazar versus pioglitazone in patients with type 2 diabetes mellitus. Diab. Vasc. Dis. Res. 2007;4:181–193. doi: 10.3132/dvdr.2007.039. [DOI] [PubMed] [Google Scholar]
- 23.Hamrén B., Ohman K.P., Svensson M.K., Karlsson M.O. Pharmacokinetic-pharmacodynamic assessment of the interrelationships between tesaglitazar exposure and renal function in patients with type 2 diabetes mellitus. J. Clin. Pharmacol. 2012;52:1317–1327. doi: 10.1177/0091270011416937. [DOI] [PubMed] [Google Scholar]
- 24.Oleksiewicz M.B., Thorup I., Nielsen H.S., Andersen H.V., Hegelund A.C., Iversen L., Guldberg T.S., Brinck P.R., Sjogren I., Thinggaard U.K., Jørgensen L., Jensen M.B. Generalized cellular hypertrophy is induced by a dual-acting PPAR agonist in rat urinary bladder urothelium in vivo. Toxicol. Pathol. 2005;33:552–560. doi: 10.1080/01926230500214657. [DOI] [PubMed] [Google Scholar]
- 25.Bénardeau A., Benz J., Binggeli A., Blum D., Boehringer M., Grether U., Hilpert H., Kuhn B., Märki H.P., Meyer M., Püntener K., Raab S., Ruf A., Schlatter D., Mohr P. Aleglitazar, a new, potent, and balanced dual PPARalpha/gamma agonist for the treatment of type II diabetes. Bioorg. Med. Chem. Lett. 2009;19:2468–2473. doi: 10.1016/j.bmcl.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Sanwald-Ducray P., Liogier D’ardhuy X., Jamois C., Banken L. Pharmacokinetics, pharmacodynamics, and tolerability of aleglitazar in patients with type 2 diabetes: Results from a randomized, placebo-controlled clinical study. Clin. Pharmacol. Ther. 2010;88:197–203. doi: 10.1038/clpt.2009.259. [DOI] [PubMed] [Google Scholar]
- 27.Lincoff A.M., Tardif J.C., Schwartz G.G., Nicholls S.J., Rydén L., Neal B., Malmberg K., Wedel H., Buse J.B., Henry R.R., Weichert A., Cannata R., Svensson A., Volz D., Grobbee D.E. AleCardio Investigators. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: The AleCardio randomized clinical trial. JAMA. 2014;311:1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- 28.Jani R.H., Kansagra K., Jain M.R., Patel H. Pharmacokinetics, safety, and tolerability of saroglitazar (ZYH1), a predominantly PPARα agonist with moderate PPARγ agonist activity in healthy human subjects. Clin. Drug Investig. 2013;33:809–816. doi: 10.1007/s40261-013-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agrawal R. The first approved agent in the Glitazar’s Class: Saroglitazar. Curr. Drug Targets. 2014;15:151–155. doi: 10.2174/13894501113149990199. [DOI] [PubMed] [Google Scholar]
- 30.Pai V., Paneerselvam A., Mukhopadhyay S., Bhansali A., Kamath D., Shankar V., Gambhire D., Jani R.H., Joshi S., Patel P.A. Multicenter, Prospective, Randomized, Double-blind Study to Evaluate the Safety and Efficacy of Saroglitazar 2 and 4 mg Compared to Pioglitazone 45 mg in Diabetic Dyslipidemia (PRESS V). J. Diabetes Sci. Technol. 2014;8:132–141. doi: 10.1177/1932296813518680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jani R.H., Pai V., Jha P., Jariwala G., Mukhopadhyay S., Bhansali A., Joshi S. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI). Diabetes Technol. Ther. 2014;16:63–71. doi: 10.1089/dia.2013.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shetty S.R., Kumar S., Mathur R.P., Sharma K.H., Jaiswal A.D. Observational study to evaluate the safety and efficacy of saroglitazar in Indian diabetic dyslipidemia patients. Indian Heart J. 2015;67:23–26. doi: 10.1016/j.ihj.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh A., Sahana P.K., Das C., Mandal A., Sengupta N. Comparison of effectiveness and safety of add-on therapy of saroglitazar and fenofibrate with metformin in Indian patients with diabetic dyslipidaemia. J. Clin. Diagn. Res. 2016;10:FC01–FC04. doi: 10.7860/JCDR/2016/16908.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilardi F., Giudici M., Mitro N., Maschi O., Guerrini U., Rando G., Maggi A., Cermenati G., Laghezza A., Loiodice F., Pochetti G., Lavecchia A., Caruso D., De Fabiani E., Bamberg K., Crestani M. LT175 is a novel PPARα/γ ligand with potent insulin-sensitizing effects and reduced adipogenic properties. J. Biol. Chem. 2014;289:6908–6920. doi: 10.1074/jbc.M113.506394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong H.W., Lee J.W., Kim W.S., Choe S.S., Kim K.H., Park H.S., Shin H.J., Lee G.Y., Shin D., Lee H., Lee J.H., Choi E.B., Lee H.K., Chung H., Park S.B., Park K.S., Kim H.S., Ro S., Kim J.B. A newly identified CG301269 improves lipid and glucose metabolism without body weight gain through activation of peroxisome proliferator-activated receptor alpha and gamma. Diabetes. 2011;60:496–506. doi: 10.2337/db09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung H.Y., Kim B., Ryu H.G., Ji Y., Park S., Choi S.H., Lee D., Lee I.K., Kim M., Lee Y.J., Song W., Lee Y.H., Choi H.J., Hyun C.K., Holzapfel W.H., Kim K.T. Amodiaquine improves insulin resistance and lipid metabolism in diabetic model mice. Diabetes Obes. Metab. 2018;20:1688–1701. doi: 10.1111/dom.13284. [DOI] [PubMed] [Google Scholar]
- 37.Ren T., Yang W.S., Lin Y., Liu J.F., Li Y., Yang L.C., Zeng K.Y., Peng L., Liu Y.J., Ye Z.H., Luo X.M. Ke. Y.J.; Diao, Y.; Jin, X. A novel PPARα/γ agonist, propane-2-sulfonic acid octadec-9-enyl-amide, ameliorates insulin resistance and gluconeogenesis in vivo and vitro. Eur. J. Pharmacol. 2018;826:1–8. doi: 10.1016/j.ejphar.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Jung Y., Cao Y., Paudel S., Yoon G., Cheon S.H., Bae G.U., Jin L.T., Kim Y.K., Kim S.N. Antidiabetic effect of SN158 through PPARα/γ dual activation in ob/ob mice. Chem. Biol. Interact. 2017;268:24–30. doi: 10.1016/j.cbi.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Ge J., Miao J.J., Sun X.Y., Yu J.Y. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-α/γ and attenuating endoplasmic reticulum stress in rats. J. Ethnopharmacol. 2016;189:238–249. doi: 10.1016/j.jep.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 40.Park M.H., Kim D.H., Kim M.J., Lee E.K., An H.J., Jeong J.W., Kim H.R., Kim S.J., Yu B.P., Moon H.R., Chung H.Y. Effects of MHY908, a new synthetic PPARα/γ dual agonist, on inflammatory responses and insulin resistance in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:300–309. doi: 10.1093/gerona/glv043. [DOI] [PubMed] [Google Scholar]
- 41.Lee W., Ham J., Kwon H.C., Kim Y.K., Kim S.N. Anti-diabetic effect of amorphastilbol through PPARα/γ dual activation in db/db mice. Biochem. Biophys. Res. Commun. 2013;432:73–79. doi: 10.1016/j.bbrc.2013.01.083. [DOI] [PubMed] [Google Scholar]
- 42.Silva J.C., de Oliveira E.M., Turato W.M., Trossini G.H.G., Maltarollo V.G., Pitta M.G.R., Pitta I.R., de Las Heras B., Boscá L., Rudnicki M., Abdalla D.S.P. GQ-11: A new PPAR agonist improves obesity-induced metabolic alterations in LDLr-/- mice. Int. J. Obes. 2018;42:1062–1072. doi: 10.1038/s41366-018-0011-7. [DOI] [PubMed] [Google Scholar]
- 43.Sæther T., Paulsen S.M., Tungen J.E., Vik A., Aursnes M., Holen T., Hansen T.V., Nebb H.I. Synthesis and biological evaluations of marine oxohexadecenoic acids: PPARα/γ dual agonism and anti-diabetic target gene effects. Eur. J. Med. Chem. 2018;155:736–753. doi: 10.1016/j.ejmech.2018.06.034. [DOI] [PubMed] [Google Scholar]


