Abstract
Background and Objective:
Warionia saharae Benth and Coss, is a medicinal plant used for its anti-diabetic properties in Morocco. This study was designed to examine the effect of the Flavonoid-Enriched Extract (FEE) obtained from Warionia saharae (W. saharae) on glucose and lipid metabolism in normal and streptozotocin (STZ)-induced diabetic rats.
Methods:
Acute (6 h) and sub-chronic (15 days) oral administration of FEE (10 mg/kg) was used to assess the glucose and lipid-lowering activity in normal and diabetic rats. Furthermore, glucose test tolerance, liver histopathological examination and in vitro antioxidant activity of FEE were carried out in this study.
Results:
Results indicated that FEE was able to exert antihyperglycemic activity. Additionally, FEE improved histopathological status of liver and pancreas in diabetic rats and possessed antioxidant activity.
Conclusion:
In conclusion, the present investigation revealed that FEE had potent antidiabetic effect in diabetic rats.
Keywords: Antioxidant, diabetes, flavonoid-enriched extract, histopathology, lipid profile, Warionia saharae
1. INTRODUCTION
Flavonoids are a large family of compounds that possess a common chemical structure and are synthesized by plants and presented broadly in nature [1-3]. Their extensive profitable bioactive proprieties including anti-viral, anti-bacterial, anti-inflammatory, cardioprotective, anti-diabetic, anti-cancer, anti-aging, anti-oxidant, have received great attention [3-5]. In fact, flavonoids are a class of the grand family of polyphenols. In this context, polyphenols may be useful nutraceuticals and supplementary treatments for various aspects of diabetes mellitus. Based on several in vitro and animal models and some clinical studies, polyphenols may play a role in many metabolic processes. They can regulate carbohydrate and lipid metabolism, reduce hyperglycemia, dyslipidemia and insulin resistance, improve adipose tissue metabolism, and alleviate oxidative stress [6-8]. The beneficial role of flavonoids in diabetes mellitus treatment is obvious because of outstanding ability in terms of decreased side reactions and complications. Numerous in vitro, in vivo and epidemiological studies support the hypoglycemic and hypolipidemic action of flavonoids [4, 9].
Warionia saharae Benth. & Coss. is a monotypic genus of Asteraceae, endemic to the northwestern edge of the African Sahara desert. The species Warionia saharae, locally named as “afessas”, is distributed in several localities on dry shale in northwestern Africa, in Morocco and Algeria [10]. In Morocco, this medicinal plant is traditionally used as a remedy against many health problems such as rheumatism, gastrointestinal disorders, migraine, kidney disorders and diabetes [11]. This species contains essential oil, polyphenols and flavonoids.
In this study, the flavonoid-enriched extract was obtained from leaves of Warionia saharae using organic solvent extraction (partitioning). Thereafter, this extract was utilized for assessing its hypoglycemic and hypolipidemic activities in streptozotocin-induced diabetic and normal rats and to evaluate the antioxidant activity.
2. MATERIAL AND METHODS
2.1. Plant Material
Specimens of Warionia saharae were collected from the Tafilalet region (semi-arid area) of Morocco in March-April 2015, and air-dried at 40°C. The plant was taxonomically identified and voucher specimen was deposited at the herbarium of the Faculty of Sciences and Techniques, Errachidia.
2.2. Preparation of the Flavonoid-Enriched Extract (FEE)
Plant material (leaves) was firstly dried at 40°C for one week, and then it was ground with an electric grinder. 20 g of the powder obtained was extracted in a Soxhlet apparatus during 12 hours, using 200 ml of 80% methanol as an organic solvent of extraction. Thereafter, the methanolic extract was filtered using a Millipore filter (Millipore 0.2 mm, St Quentin en Yvelines, France) to remove particulate matter. The filtrate obtained undergone successive fractionations, using a separatory funnel and this extract was concentrated by evaporation under reduced pressure using rotavapor apparatus at 50°C. The residue was dissolved in hot distilled water, filtered and left overnight in a refrigerator. In order to eliminate some organic waste (lipids, chlorophyll, etc.), this residue was defatted with petroleum ether (1:1 v/v), this process was repeated five times, the organic phase was discarded, while the aqueous one has been submitted to a second fractionation. Thereafter, the last aqueous phase was fractioned using chloroform (1:1 v/v), which is normally a less polar solvent, useful to extract aglycone flavonoids, this operation was repeated five times. Then, the organic phase (chloroform) obtained from the last fractionation has been assembled in a flask. The aqueous fraction has been submitted to a third fractionation using this time ethyl acetate (a high polar organic solvent) in order to extract flavonoid glycosides. Finally, the two fractions were mixed and submitted to an elimination of organic solvents using a rotatory vapor. Then, the extract obtained was lyophilized in a lyophilizator (LABCONCO, G.BOYER, Casablanca, Morocco) and stored in sealed flasks at a temperature of 4°C. Finally, Shinoda’s test [12] for detecting flavonoids was performed in this study. In addition, a test of evaluating the total flavonoids content of the final extract using FeCl3 method was performed for comparing the richness of total flavonoids in the two extracts.
2.3. Evaluation of the Anti-Free Radical Activity by Trapping the Free Radical (2,2-Diphenyl-1-picrylhyd-razyl: DPPH)
The scavenging activity of the DPPH radical was measured according to the protocol described by Louli et al. A solution of DPPH was prepared by solubilizing 4 mg of DPPH in 100 ml of methanol. The solution was then placed in the dark for 3 hours. In glass tubes, series of dilutions of the extract and the positive control butylhydroxytoluene (BHT) were prepared with a concentration of the stock solution of (5 mg / 10 ml) in order to obtain the following concentrations; 31, 25, 62.5, 125, 250 and 500 μg/ml. After this, 5 ml of the DPPH solution was added. After stirring, the tubes were placed in the dark at room temperature for 30 minutes. Concerning the negative control, it contains only the solution of DPPH and the methanol with an equal volume of 2.5 ml. The reading was carried out by measuring the absorbance at 515 nm using a UV / Vis spectrophotometer [13-15].
Antioxidant activity of FEE was estimated according to the following equation:
(I%) = [A control - A sample / A control]
where:
(I%): percentage of the anti-radical activity;
A control: Absorbance of the control;
A sample: Absorbance of the sample.
The result was expressed by mean (of three separated measures) ± SEM.
2.4. Ferric Reducing Antioxidant Power (FRAP) Assay
This method serves for determining the reduction of a ferric-tripyridyl-s-triazine complex (TPTZ) to its ferrous, thereby forming a violet-colored solution.
The sample (FEE) was taken in triplicate determination and distilled water was added to make an equal volume of solution in each test tube. Then, 900 µL of FRAP reagent was added, well vortexed and incubated at 37°C for 30 min. Finally, the absorbance of the chromophore developed has been read immediately after incubation at 593 nm using a UV / Vis spectrophotometer. The FRAP value is expressed as mmol Fe (II) equivalent/mg extract [14, 15]. The positive control used was the ascorbic acid.
The result was expressed by mean (of three separated measures) ± SEM.
2.5. Experimental Animals
Healthy albino adult male rats (Wistar strain) with a weight ranged between 120 and 200 g were kept in individual polyethylene cages and maintained in standard conditions and the animals were fed ad libitum.
2.6. Induction of Diabetes and Determination of Parameters
Diabetes was induced in the overnight fasted male albino Wistar rats by a single intraperitoneal injection (i.p.) of streptozotocin (65 mg/ kg body weight) dissolved in 0.1 M citrate buffer (pH = 4.5). Normal control rats received distilled water as a vehicle. After 3 days of STZ injection, blood samples were collected from the retro-orbital of the rat eyes and plasma glucose levels were determined. The animals used in this the study were confirmed as diabetics by the elevated plasma glucose levels over 300 mg/dl [14, 15].
Normal and diabetic rats were randomly assigned to three different groups containing six rats each. One control group received distilled water, a second treated group received the flavonoid-enriched extract of Warionia saharae (FEE) at a dose of 10 mg/kg (10 mg of lyophilized TE per kilogram of body weight) and the third group received a reference drug (glibenclamide at a dose of 5 mg/kg). The dose of 10 mg/kg was used according to a preliminary dose screening. For single oral administration, distilled water (control), glibencla-mide or the aqueous extract was administered and blood glucose levels were monitored during six hours. For repeated oral administration, rats were treated once daily for 15 days and blood glucose levels were followed during this period. The rats (n = 6 in each group) were treated orally. All experiments were performed in fasted rats. Blood samples from rats were collected from the retro-orbital sinus under ether anesthesia in order to determine their plasma lipid profile. Blood glucose levels were determined by the glucose oxidase method using a reflective glucometer (Contour™ TS) from Bayer Diabetes Care [14, 15].
Body weight of rats was measured at the start and the end of the study.
2.7. Histopathology
Pancreas and livers were collected and cut into small pieces, fixed in 10% natural buffered formaldehyde (formalin). Following fixation, specimens were dehydrated, embedded in paraffin, and then sectioned to 5 µm thicknesses using a semi-automated rotatory microtome. Sections were stained with hematoxylin and eosin. Finally, histopathological changes were examined under phase contrast microscope (Olympus B x 41, Japan) and images were captured at a magnification of 40× using a computer-assisted image analyzer (Nikon H600L, Nikon DS camera control Unit DS-U2, Version 4.4). The number of hepatocytes and Islets of Langerhans (IL), the diameter of the cores of hepatocytes and areas of IL were determined. For this reason, quantitative analyses were carried out using ImageJ software 1.50i (wayne Rasband National Institutes of Health, USA). Morphometric analysis was performed with ImageJ software 1.50i (wayne Rasband National Institutes of Health, USA) [14-16].
2.8. Plasma Lipids and Lipoproteins Profile in Diabetic and Normal Rats
The concentrations of two lipids (Total Cholesterol, Triglycerides) and a lipoprotein; High-Density Lipoprotein (HDL) were measured in plasma of STZ-diabetic and normal rats previously treated by FEE (10 mg/kg of body weight). Test Kits (SGM Italia) were used for their quantitative colorimetric determination [14-17].
For measurement of plasma cholesterol and triglycerides levels, the specific kits were used and in order to estimate the plasma HDL cholesterol levels, a precipitating reagent was employed (PEG 6000 precipitating method).
2.9. Statistical Analysis
Data were expressed as mean ± SEM. Statistical differences among the means studied were assessed by two-way ANOVA followed by Bonferroni multiple comparisons test. GraphPad Prism 7 software was used to carry out statistical analysis. Differences were considered to be significant when p < 0.05.
3. RESULTS
Flavonoid-Enriched Extract (FEE) was obtained by partitioning technique via solvent extraction from aerial parts of Warionia saharae. The richness of the extract on flavonoids was checked by the Shinoda test, where an intense pink coloration was detected in this extract at the end of this test, this coloration was more intense than that detected for the methanolic extract of W. saharae. Furthermore, the estimation of flavonoids content using the FeCl3 revealed that FEE contains 120.35 mg of an equivalent of quercetin per gram of extract (120.35 QE/g of the extract) against 21.07 mg of an equivalent of quercetin per gram of extract (21.07 QE/ g of the extract) for the methanolic extract. In the second time, FEE has been administrated orally to mal Albino Wistar rats for an acute investigation (6 hours) and a chronic study (15 days) in order to evaluate the lowering glucose activity of this extract in normal and STZ-induced diabetic rats.
3.1. Effect of FEE on Fasting Body Weight
Fig. (1) illustrates the effect of FEE on body weight. No change was observed concerning body weight values in both normal and diabetic rats after two weeks of treatment when compared to control animals.
Fig. (1).
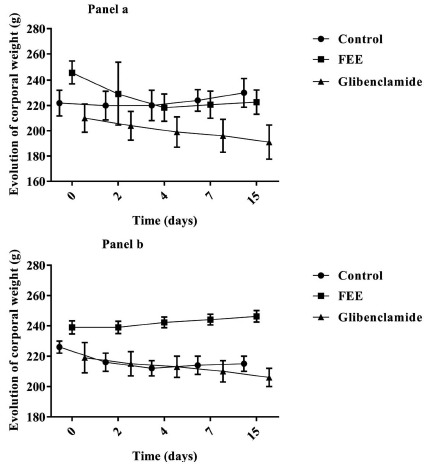
Effect of the flavonoids-enriched extract from Warionia saharae Benth. & Coss. (10 mg/kg body weight) on body weight in normal (panel a) and STZ-diabetic (panel b) rats. (All data are expressed as mean ± SEM (n=6). (*) p<0.05; (**) p<0.01; (***) p<0.001 vs. control.
3.2. Effect of FEE on Blood Glucose Levels
3.2.1. Single Oral Administration
Fig. (2) demonstrates the effect of the oral administration of FEE on blood glucose levels in normal and diabetic rats after 6 hours of treatment (acute test). Results showed that FEE did not affect glycemia in normal rats; while, the same extract was able to provoke an antihyperglycemic effect for STZ-induced diabetic rats after six hours of the single oral administration. FEE at 10 mg/kg significantly maintained diabetic blood glucose levels near the normal level after performing treatment for 4 and 6 hours, while a significant decrease (p < 0.0001) was mentioned at the end of the acute test.
Fig. (2).
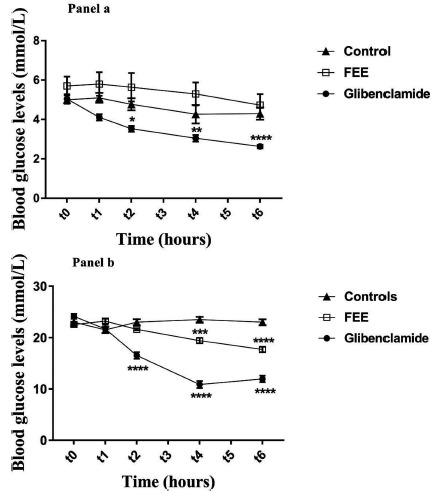
Plasma glucose levels over 6 h after single oral administration of the flavonoid-enriched extract from Warionia saharae Benth. & Coss. (10 mg/kg) in normal (Panel a) and diabetic rats (Panel b). Data are expressed as means ± S.E.M., n = 6 rats per group. *p<0.05; **p<0.01; ***p<0.001 and ****p<0.0001 when compared to baseline values.
3.2.2. Repeated Oral Administration
In normal rats, both FEE and glibenclamide significantly reduced fasting blood glucose levels (Fig. 3). FEE showed a significant reduction after 7 and 15 days of oral administration (p<0.01 and p<0.0001 respectively). In addition, glibenclamide showed a significant reduction from the 2nd day of treatment until the end of the chronic test (p<0.0001). These results suggest that hypoglycemic activity of glibenclamide was more pronounced than FEE. For diabetic rats, both FEE and glibenclamide manifested a significant reduction of blood glucose levels (p<0.0001). Glibenclamide (5 mg/kg) showed a significant reduction from the second day to the end of the chronic assay with the same level of reduction than FEE (p<0.0001).
Fig. (3).
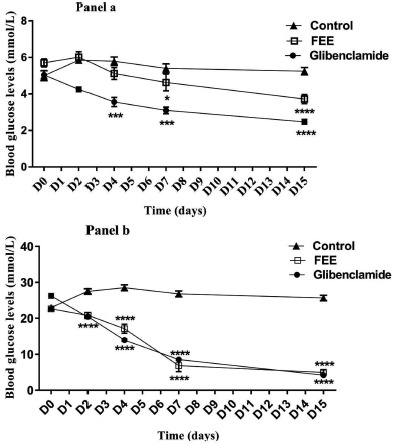
Plasma glucose levels over once daily repeated oral administration of the flavonoids-enriched extract from Warionia saharae Benth. & Coss. (10 mg/kg) for 10 days in normal (Panel a) and diabetic rats (Panel b). Data are expressed as means ± S.E.M., n= 6 rats per group. *p<0.05; **p<0.01; ***p<0.001 and ****p<0.0001 when compared to baseline values.
3.2.3. Effect of FEE on OGTT in Normal and STZ-induced Diabetic Rats During 120 min
The effect of FEE at a dose of 10 mg/kg body weight on glucose tolerance was estimated by measuring the blood glucose levels at different time points of 30, 60, 90 and 120 min after glucose challenge for both normal and STZ-diabetic rats. No significant changes were detected neither in normal nor in diabetic rats after oral administration of FEE for this dose (10 mg/kg of body weight) (Fig. 4, panels a and b). However, in glibenclamide-treated group, a significant reduction was observed in blood glucose levels after 60, 90 and 120 min (p<0.01, p<0.01 and p<0.001 respectively) in normal animals, while, no effect was observed in diabetic rats (Fig. 4, panel b).
Fig. (4).
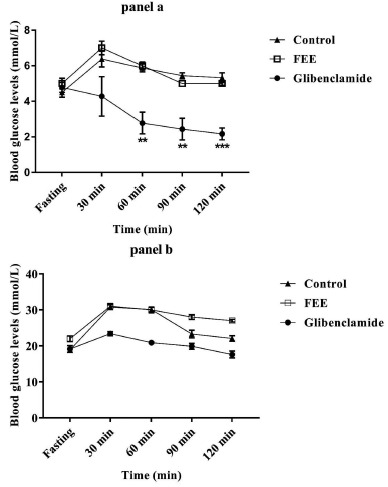
Effect of FEE (10 mg/kg) on oral glucose tolerance in normal rats (panel a), and STZ-diabetic rats (panel b). Values are means ± SEM; n=5. (**) p<0.01; (***) p<0.001 vs. control. The Oral Glucose Test Tolerance (OGTT) was carried out after 16 hours of fasting.
3.3. Histopathological Changes in the Liver and Morphometric Analysis
Fig. (5) displays the histopathological changes in the liver of diabetic rats 15 days after oral administration of FEE
Fig. (5).
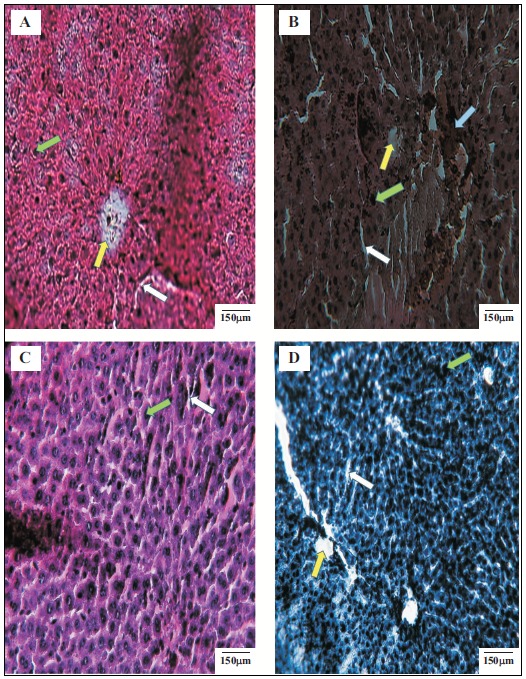
Histopathological examinations of the liver of different experimental groups at the end of the experiment. A: Normal control, B: Diabetic control, C: Diabetic treated with standard drug (glibenclamide), D: Diabetic treated with FEE of Warionia saharae. Green arrow indicates hepatocytes, withe arrow indicates sinusoids, the yellow one indicates central vein and the bleu one indicates injuries provoked by intoxication resulting from STZ. (The color version of the figure is available in the electronic copy of the article).
(10 mg/kg) or glibenclamide (5 mg/kg). Hepatic tissue of diabetic rat (Fig. 5B) showed distortion in the arrangement of cells around the central vein, enlargement and thickening of the walls of veins. Furthermore, development of fibrosis in the degenerated cells, when compared with this of control animals was detected (Fig. 5A). In STZ-induced diabetic rats treated with FEE (Fig. 5D) or glibenclamide (Fig. 5C), the liver architecture has been improved when compared to diabetic untreated rats (Fig. 5B). The hepatocytes became more organized, and mild sinusoid hyperemia was observed. The lack of central hemorrhagic necrosis and hepatocellular injuries has been also shown. Table 1 illustrates the morphometric analysis of hepatocytes in the normal rats, untreated-diabetic rats and rats treated with FEE or glibenclamide. It is well noticed that the diameter of the hepatic core of diabetic rats treated with FEE (27.90 ± 0.98) was significantly (p<0.001) larger when compared to the diabetic control rats (27.60 ± 2.11). In association to the number of hepatocytes counted in an area of 40000 µm2, findings of the present study revealed an increase in the number of hepatocytes in diabetic rats treated with FEE with 23 hepatocytes when compared to diabetic untreated rats (16 hepatocytes).
Table 1. Quantitative data of histopathological slides of liver illustrated in Fig.5, obtained from analysis with Image J software.
| - | Number of Hepatocytes | Diameter of the Cores (µm) |
|---|---|---|
| Normal control | 27 ± 1.00 | 25.29 ± 2.50 |
| STZ control | 16 ± 1.50 | 23.48 ± 0.48 |
| Glibenclamide STZ | 27 ± 2.30**** | 27.60 ± 2.11*** |
| FEE STZ | 23 ± 1.75*** | 27.90 ± 0.98*** |
3.4. Histopathological Changes of the Pancreas and Morphometric Analysis
Fig. (6) illustrates the histopathological changes in pancreas of rats treated with FEE (10 mg/kg) fifteen days after repeated oral administration of the flavonoid-enriched extract obtained from Warionia saharae. The structure of the pancreas of the control and diabetic rats is shown in Fig. (6). Pancreas of normal control animals presented normal islets, whereas diabetic rats exhibited atrophy of β-cells and vascular degenerative changes in the islets. A significant increase in the area of islets of Langerhans (2257.32 ± 512 µm2) was observed in diabetic rats treated with FEE when compared to the diabetic control rats (1725.36 ± 700 µm2). However, the number of islets of Langerhans in the area of 40000 μm2 was lesser in the group of diabetic animals treated by FEE (2 islets of Langerhans) (Table 2).
Fig. (6).
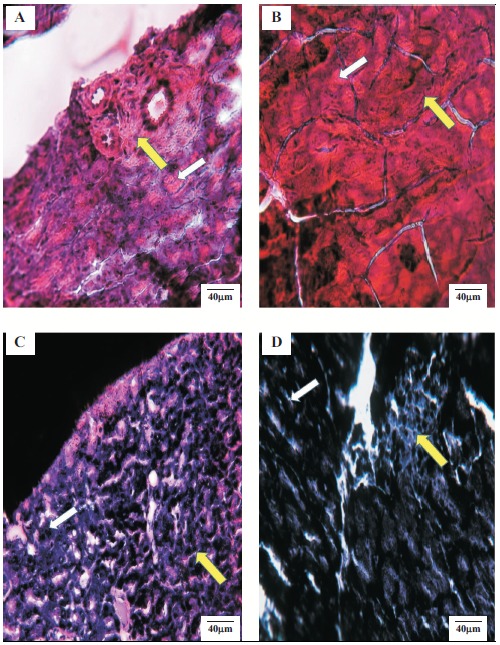
Histopathological examinations of the pancreas of different experimental groups at the end of the experiment. A: Normal control, B: Diabetic control, C: Diabetic treated with standard drug (glibenclamide), D: Diabetic treated with FEE of Warionia saharae. Yellow arrow indicates Islet of Langerhans and withe arrow indicates acini. (The color version of the figure is available in the electronic copy of the article).
Table 2. Quantitative data of histopathological slides of pancreas illustrated in Fig. 6, obtained from analysis with ImageJ software.
| - | Number of Ilets | Area of IL (µm2) |
|---|---|---|
| Normal control | 4 ± 0.07 | 2698.15 ± 346 |
| STZ control | 3 ± 0.08 | 1725.36 ± 700 |
| Glibenclamide STZ | 4 ± 0.05 | 3568.45 ± 981*** |
| FEE STZ | 2 ± 0.12 | 2257.32 ± 512** |
3.5. Effect on the Antioxidant Activity
The free radical scavenging activity (DPPH) of FEE was studied by its ability to reduce the DPPH. FEE exerted considerable free radical scavenging activity as indicated by the corresponding IC50 values (117.74 ± 1.02 µg/ml) in comparison to the synthetic antioxidant BHT (154.03 ± 1.98 µg/ml)
(Fig. 7) This finding shows the potential of FEE as an antioxidant agent.
Fig. (7).
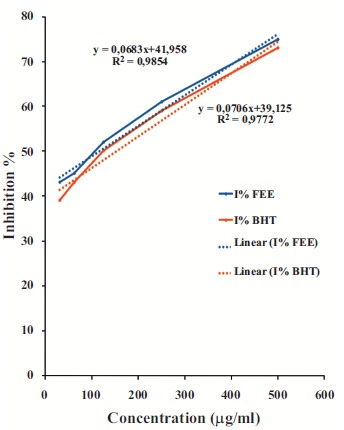
Percentage of inhibition of DPPH as a function of the concentrations of TE compared with butylhydroxytoluene (BHT).
3.6. Effects of FEE on Serum Lipid Profiles
The plasma cholesterol levels were significantly decreased (p<0.05) at the end of repeated oral administration of FEE in both normal and diabetic rats (Fig. 8). While no significant changes were noticed concerning triglycerides, HDL or LDL plasma concentrations for both normal and diabetic groups (Figs. 9-11). In addition, glibenclamide induced a significant reduction in plasma cholesterol levels in diabetic rats (p<0.001). This drug was also able to decrease plasma triglyceride levels in normal rats (p<0.05) after 15 days of treatment and increase HDL levels (p<0.05).
Fig. (8).
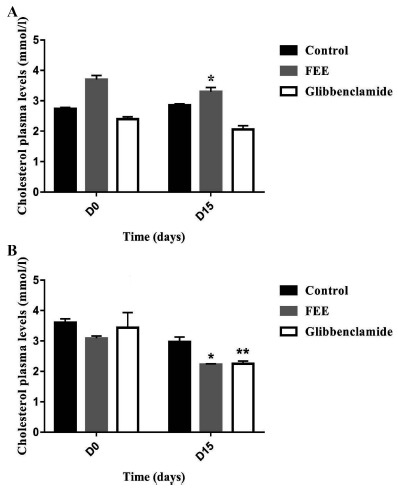
Cholesterol plasma levels over 15 days after repeated oral administration of FEE (10 mg/kg) in normal and STZ-induced diabetic rats. Data are expressed as means ± SEM., n = 6 rats per group. *p < 0.05, **p < 0.001, when compared to baseline values.
Fig. (9).
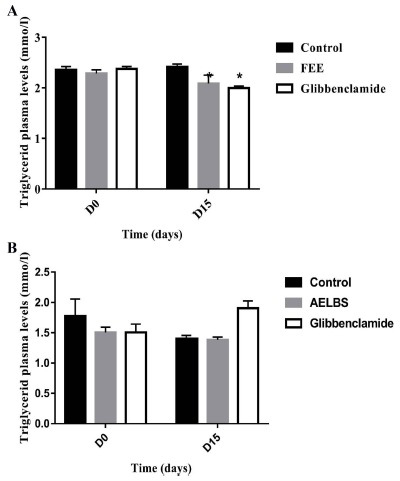
Triglyceride plasma levels over 15 days after repeated oral administration of FEE (10 mg/kg) in normal and STZ-induced diabetic rats. Data are expressed as means ± SEM., n = 6 rats per group. When compared to baseline values.
Fig. (11).
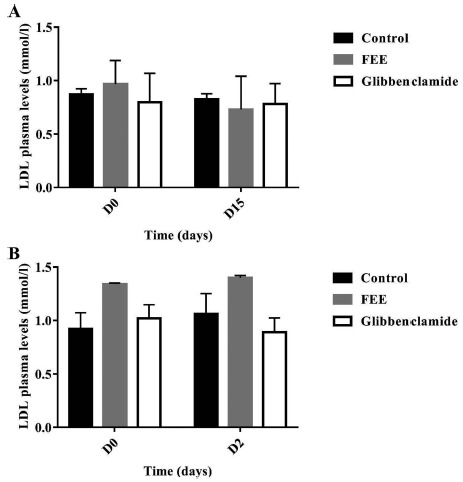
LDL plasma levels over 15 days after repeated oral administration of FEE (10 mg/kg) in normal and STZ-induced diabetic rats. Data are expressed as means ± SEM., n=6 rats per group. When compared to baseline values.
4. DISCUSSION
The purpose of the current study was to evaluate the effect of the flavonoid-enriched extract from W. saharae on the blood glucose levels and the lipid profile in normal and STZ-induced diabetic rats. Oral administration of FEE did not produce any change in body weight in normal and diabetic rats when treated for 15 days. In addition, single oral administration of FEE induced a decrease in blood glucose levels in STZ-induced diabetic rats. Results showed that FEE did not affect glycemia in normal rats; while, the same extract was able to provoke an antihyperglycemic effect on STZ-induced diabetic rats after both single and repeated oral administration of FEE. Evidence from a recent study demonstrated the potential antidiabetic effect of tannins extracted from the Warionia saharae [18]. This previous study has been performed in order to determine which fraction from the aqueous extract of aerial part of Warionia saharae is implicated in the antidiabetic effect. Consequently, the tannins were isolated from this aqueous extract and the antidiabetic effect was determined and demonstrated in normal and STZ rats at the dose of 10 mg/kg. Previously it was reported that the aqueous extract of Warionia saharae possesses a significant hypoglycemic effect when tested in normal and STZ-induced diabetic rats [19]. In this previous study, both single (6 h) and repeated (15 days) oral administration of the aerial part aqueous extract of Warionia saharae at a dose of 5 mg/kg have been demonstrated to possess hypoglycemic and antihyperglycemic activities in normal and STZ rats, respectively. The present study was performed in order to verify if the flavonoid-enriched fraction isolated from the aqueous Warionia saharae extract is also involved in the total antidiabetic effect of this plant.
To assess the ability of FEE in improving the body’s ability to utilize glucose, the oral glucose tolerance test OGTT was performed in normal and diabetic rats. Accordingly, no significant improvements were revealed from this test. In fact, the inability of FEE (at the dose of 10 mg/kg) to lower the blood glucose levels in oral glucose tolerance did not mean that rats treated with the extract did not have better glucose utilization capacity, however, it is possible that this dose is so insufficient to show amelioration of glucose utilization. These findings are in disagreement with those demonstrating that OGTT displayed a significant result concerning the effect of tannins obtained from W. saharae. This improvement was exclusively detected in diabetic rats where a significant decrease in blood glucose levels was alarming [18].
In diabetic untreated rats, histopathological changes in the liver showed characteristic degenerative damages which could be induced by streptozotocin. STZ is known as an agent causing damages in the liver and distorts its function [20]. The liver architecture in STZ diabetic rats treated with FEE (10 mg/Kg) (Fig. 5D) has been improved when compared to diabetic untreated rats (Fig. 5B). Findings of this histopathological study indicated that FEE markedly improved liver cellular population probably by overcoming the diabetic complications as described by similar studies [21]. This fact suggests the hepatoprotective effect of this extract. On the other hand, histopathological examinations of the pancreas revealed sever changes in untreated diabetic pancreas and morphologically presented degradation at the level of islets of Langerhans compared with control normal rats. These findings reveal that the antihyperglycemic effect of FEE may be partially associated with its favorable ability to improve histopathological proprieties of pancreas and liver.
Dyslipidemia is considered as the key component of metabolic syndrome which is characterized by increased CHL, LDL, TG and reduced level HDL [22]. The results elucidated significant differences due to treatments. The analysis of serum cholesterol levels indicated the favorable effect of FEE. However, no improvement was revealed concerning the serum levels of TG, HDL or LDL in both normal and diabetic groups. These results are in partial agreement with those reported for the tannins extracted from W. saharae which revealed the ability of this extract to reduce plasma cholesterol levels and to increase HDL-cholesterol concentrations [18].
Abnormal high levels of oxidative free radicals or Reactive Oxygen Species (ROS) is an important mediator of the pathogenic effects of high glucose and fatty acids [23]. Moreover, oxidative stress arises in parallel to the setting off of an imbalance between the body’s antioxidant defenses and the physiological redox status [24]. The results demonstrate clearly the antioxidant activity of FEE. Flavonoids display antioxidant activity by direct and indirect mechanisms; the former is mediated predominantly via direct scavenging of ROS, activation of antioxidant enzymes, metal chelating activity, inhibition of oxidases and more other processes [25].
Flavonoids improve insulin sensitivity, regulate insulin secretion, decrease serum glucose, and inhibit sorbitol accumulation in the lens of the eye and nerves [26, 27]. In the present study, FEE applies antihyperglycemic effect at least in part via its potent antioxidant ability, which contributes to improving pathological damages provoked by STZ in hepatic and pancreatic tissues.
CONCLUSION
The results showed that FEE could reduce glucose levels in normal and diabetic STZ-induced rats. Moreover FEE could improve cholesterol serum levels in both normal and diabetic rats. Furthermore, it possesses remarkable in vitro antioxidant activities. These data support the beneficial action of the flavonoid-enriched extract from Warionia saharae against diabetes mellitus.
Fig. (10).
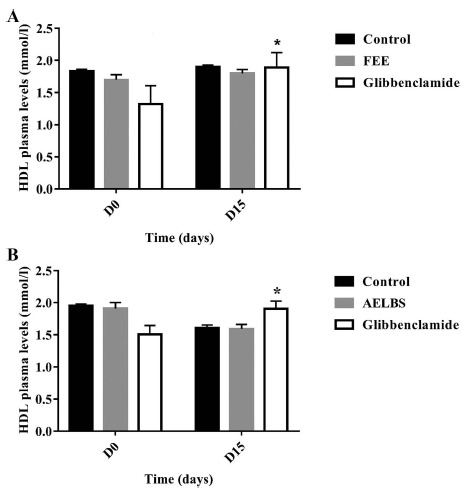
HDL plasma levels over 15 days after repeated oral administration of FEE (10 mg/kg) in normal and STZ-induced diabetic rats. Data are expressed as means ± SEM., n = 6 rats per group. *p<0.05, when compared to baseline values.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- DPPH
2,2-Diphenyl-1-Picrylhydrazyl
- FEE
Flavonoid-Enriched Extract
- HDL
High-Density Lipoprotein
- IL
Islets of Langerhans
- LDL
Low-Density Lipoprotein
- OGTT
Oral Glucose Tolerance Test
- ROS
Reactive Oxygen Species
- STZ
Streptozotocin
- TG
Triglycerides
- TPTZ
Ferric-Tripyridyl-s-Triazine complex
- W. saharae
Warionia saharae
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study on animal was approved by the local committee FSTE/2015. Faculty of Sciences and Techniques Errachidia, Morocco.
HUMAN AND ANIMAL RIGHTS
No human were used in this research. All animal research procedures were followed in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences, The National Academies Press, Washington, D.C.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This study was funded by CNRST (grant number PPR/2015/35).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ajebli M., Eddouks M. The promising role of plant tannins as bioactive antidiabetic agents. Curr. Med. Chem. 2018 doi: 10.2174/0929867325666180605124256. [DOI] [PubMed] [Google Scholar]
- 2.Testa R., Bonfigli A.R., Genovese S., De Nigris V., Ceriello A. The possible role of flavonoids in the prevention of diabetic complications. Nutrients. 2016;8:1–13. doi: 10.3390/nu8050310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krych J., Gebicka L. Catalase is inhibited by flavonoids. Int. J. Biol. Macromol. 2013;58:148–153. doi: 10.1016/j.ijbiomac.2013.03.070. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Huang H., Zhao X. Effects of flavonoids-rich Chinese bayberry (Myrica rubra Sieb. et Zucc.) pulp extracts on glucose consumption in human HepG2 cells. J. Funct. Foods. 2015;14:144–153. [Google Scholar]
- 5.Zhang J., Wu Y., Zhao X. Chemopreventive effect of flavonoids from Ougan (Citrus reticulata cv. Suavissima) fruit against cancer cell proliferation and migration. J. Funct. Foods. 2014;10:511–519. [Google Scholar]
- 6.Bahadoran Z., Mirmiran P., Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013;12:43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston K., Sharp P., Clifford M., Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005;579:1653–1657. doi: 10.1016/j.febslet.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 8.Jung U.J., Lee M.K., Jeong K.S., Choi M.S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepaticglucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004;134:2499–2503. doi: 10.1093/jn/134.10.2499. [DOI] [PubMed] [Google Scholar]
- 9.Yeon J.Y., Bae Y.J., Kim E.Y. Association between flavonoid intake and diabetes risk among the Koreans. Clin. Chim. Acta. 2015;439:225–230. doi: 10.1016/j.cca.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 10.Lewalle L. Warionia saharae Cosson. Al Biruniya. 1986;3:77–78. [Google Scholar]
- 11.Eddouks M., Ajebli M., Hebi M. Ethnopharmacological survey of medicinal plants used in Daraa-Tafilalet region (Province of Errachidia), Morocco. J. Ethnopharmacol. 2017;198:516–530. doi: 10.1016/j.jep.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Evans W.C. Trease and Evans Pharmacognosy. 14th ed. Singapore: Harcourt Brace and company, Asia Pvt. Ltd.; 1997. pp. 12–68. [Google Scholar]
- 13.Louli V., Ragoussis N., Magoulas K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour. Technol. 2004;92:201. doi: 10.1016/j.biortech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Hebi M., Farid O., Ajebli M., Eddouks M. Potent antihyperglycemic and hypoglycemic effect of Tamarix articulata Vahl. in normal and streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2017;87:230–239. doi: 10.1016/j.biopha.2016.12.111. [DOI] [PubMed] [Google Scholar]
- 15.Ajebli M., Eddouks M. Buxus sempervirens L improves streptozotocin-induced diabetes mellitus in rats. Cardiovasc. Hematol. Disord. Drug Targets. 2017;17:142–152. doi: 10.2174/1871529X17666170918140817. [DOI] [PubMed] [Google Scholar]
- 16.Ajebli M., Eddouks M. Pharmacological and phytochemical study of Mentha suaveolens Ehrh in normal and streptozotocin-induced diabetic rats. Nat. Prod. J. 2018;0327120434 doi: 10.2174/221031550866618. [DOI] [Google Scholar]
- 17.Ajebli M., Eddouks M. Buxus sempervirens L. improves lipid profile in diabetic rats. Cardiovasc. Hematol. Disord. Drug Targets. 2018;18:239–246. doi: 10.2174/1871529X18666180419100823. [DOI] [PubMed] [Google Scholar]
- 18.Ajebli M., El Ouady F., Eddouks M. Study of antihyperglycemic, antihyperlipidemic and antioxidant activities of tannins extracted from Warionia saharae Benth. & Coss. Endocr. Metab. Immune Disord. Drug Targets. 2018;1029160539 doi: 10.2174/187153031866618. [DOI] [PubMed] [Google Scholar]
- 19.Hebi M., Eddouks M. Glucose lowering activity of the aqueous extract of Warionia saharae in normal and diabetic rats. Cardiovasc. Hematol. Agents Med. Chem. 2018;16:66–72. doi: 10.2174/1871525716666180425125057. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh B.K., Maddirala D.R., Vinay K.K., Shaik S.F., Tiruvenkata K.E.G., Swapna S., Ramesh B., Rao C.A. Antihyperglycemic and antihyperlipidemic activities of methanol: Water (4:1) fraction isolated from aqueous extract of Syzygium alternifolium seeds in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2010;48:1078–1084. doi: 10.1016/j.fct.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Yuan M., Konstantopoulos N., Lee J. Reversal of obesity and diet induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 22.Imran A., Butt M.S., Arshad M.S., Arshad M.U., Saeed F., Sohaib M., Munir R. Exploring the potential of black tea based flavonoids against hyperlipidemia related disorders. Lipids Health Dis. 2018;17 doi: 10.1186/s12944-018-0688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Kregel K.C., Zhang H.J. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira J.F.S., Luthria D.L., Sasaki T., Heyerick A. Flavonoids from artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15:313570. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira M.A., Kottke T.E., Jordan C., O’Connor P.J., Pronk N.P., Carreon R. Preventing and managing cardiometabolic risk: The logic for intervention. Int. J. Environ. Res. Public Health. 2009;6:256884. doi: 10.3390/ijerph6102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceballos-Coronel M.L. Nutraceuticals in Diabetes Management. In: Yashwant P., editor. Handbook of nutraceuticals. Vol. 1. New York: Taylor and Francis Group, LLC; 2010. pp. 207–215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


