Abstract
The pharyngeal arches are a prominent and significant feature of vertebrate embryos. These are visible as a series of bulges on the lateral surface of the embryonic head. In humans, and other amniotes, there are five pharyngeal arches numbered 1, 2, 3, 4 and 6; note the missing ‘5’. This is the standard scheme for the numbering of these structures, and it is a feature of modern anatomy textbooks. In this article, we discuss the rationale behind this odd numbering, and consider its origins. One reason given is that there is a transient 5th arch that is never fully realized, while another is that this numbering reflects considerations from comparative anatomy. We show here, however, that neither of these reasons has substance. There is no evidence from embryology for a ‘5th’ arch, and the comparative argument does not hold as it does not apply across the vertebrates. We conclude that there is no justification for this strange numbering. We suggest that the pharyngeal arches should simply be numbered 1, 2, 3, 4 and 5 as this would be in keeping with the embryology and with the general numbering of the pharyngeal arches across the vertebrates.
Keywords: amniotes, human embryology, pharyngeal arches, pharyngeal pouches, tetrapod evolution
The numbering of the pharyngeal arches in humans, and other amniotes, should simply be from anterior to posterior, 1, 2, 3, 4 and 5.
Introduction
A key feature of the mid‐embryonic stage of human development is the presence of a series of bulges on the lateral surface of the head, the pharyngeal arches. These structures are of great developmental and evolutionary significance. It is within this segmental series of pharyngeal arches that the anatomy of the oro‐pharyngeal apparatus is believed to be organised. Furthermore, the presence of the pharyngeal arches is a defining feature of the phylotypic stage of vertebrate development; the most conserved stage, both in terms of morphology and gene expression, and lends this stage its name, pharyngula.
The pharyngeal arches are paired structures, which are conjoined at their ventral midline, and which consist of epithelia with a mesenchymal filling. The external surface of each arch is ectodermal and the internal endodermal, and these surround neural crest cells and a more centrally located mesodermal population. Between the arches, ectoderm and endoderm contact each other resulting in depressions that can be seen in the external surface between the arches, the pharyngeal clefts, and these overlie internal depressions, the pharyngeal pouches. The points of contact between the clefts and pouches come to define the limits of each of the arches.
At one level, the pharyngeal arches seem to constitute an iterated series with each generating the same basic set of components – each arch receives distinct sensorimotor innervation and forms skeletal elements, connective tissue, muscle and arteries. Fate mapping studies, in several vertebrates, have suggested that each of the different embryonic populations that constitute the arches generate these derivatives. Thus, it is the ectoderm that forms the skin and sensory neuronal populations, while the endoderm forms the inner surface of the pharynx, including the taste buds and the specialised glands that form here, such as the thyroid, parathyroid and thymus (D'Amico‐Martel & Noden, 1983; Couly & Le Douarin, 1990; Harlow & Barlow, 2007). The neural crest generates the skeletal and connective tissue components, and the mesoderm the musculature and endothelial cells of the arch arteries (Noden, 1983a,1983b).
As development progresses, the pharyngeal arches become remodelled. Once the full complement of arches has been realised, the second pharyngeal arch expands caudally covering the more posterior arches. The leading edge of the expanding second arch then fuses with the underlying tissue, and thus the more posterior arches are internalised. Initially, there exists a cavity between the inner surface of the expanded second arch and the outer surfaces of the posterior arches, termed the cervical sinus of His, but this subsequently collapses (Richardson et al. 2012). Thus, with time, the segmental organisation of the posterior pharyngeal region is lost.
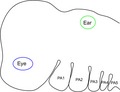
There are five pairs of pharyngeal arches in humans, and other amniotes, and these are numbered, from anterior to posterior, 1, 2, 3, 4 and 6 (Fig. 1). The 1st, most anterior, arch will form the jaws and the muscles of mastication, as well as the incus and malleus. This arch is innervated by the trigeminal nerve. The 2nd arch gives rise to portions of the hyoid, the muscles of facial expression and the stapes, and is innervated by the facial nerve. The 3rd arch will generate other distinct portions of the hyoid, form the stylopharyngeus muscle and is innervated by the glossopharyngeal nerve. The last two arches, the 4th and 6th, are thought to produce the laryngeal cartilages and muscles, and are innervated by branches of the vagus nerve.
Figure 1.
Longitudinal high‐resolution episcopic microscopy (HREM) section through the pharyngeal region of a Carnegie stage 15 (Cs15) human embryo from the DMDD website (https://dmdd.org.uk/) is shown. At this stage of development, the full complement of pharyngeal arches has formed and these are numbered from anterior to posterior – PA1, PA2, PA3, PA4 and PA6. Scale bar: 0.25 mm.
This somewhat odd numbering of the pharyngeal arches is a standard feature of most anatomy textbooks (Carlson, 2009; Sadler et al. 2012; Schoenwolf, 2014; Standring, 2015) and research papers. In general, two reasons are given for this numbering. One that is often cited is that it has been suggested that a fifth pharyngeal pouch/arch forms during development, but this is transient and regresses. The other reason is that this numbering reflects evolutionary considerations. The ancestral state for tetrapods is to have six pharyngeal arches (Shone et al. 2016), numbered 1, 2, 3, 4, 5 and 6, and this is the situation seen in anurans and urodeles. However, in amniotes the number of arches was reduced to five but the terminal arch is still labelled the ‘6th’ to imply homology with the last most posterior 6th arch of amphibia. Here, we show that neither of these arguments are valid, and that the numbering of the pharyngeal arches should be revised and simplified such that they are from anterior to posterior: 1, 2, 3, 4 and 5.
The presence of a transient ‘5th’ pouch/arch?
The idea that there are five pharyngeal pouches and six arches has been around for a long time and, for example, can be found in the 1918 edition of Gray's Anatomy (Lewis, 1918). In this text, it is stated on page 65 that “In the lateral walls of the anterior part of the fore‐gut five pharyngeal pouches appear”. Then on page 66 that “In all, six arches make their appearance, but of these only the first four are visible externally” and that “In each arch a cartilaginous bar, consisting of right and left halves, is developed, and with each of these there is one of the primitive aortic arches”. Finally, and of relevance here, it is written that “The ventral portions of the cartilages of the fourth and fifth arches unite to form the thyroid cartilage; from the cartilages of the sixth arch the cricoid and arytenoid cartilages and the cartilages of the trachea are developed”. Modern textbooks generally reflect this view, albeit with an emphasis on the transient nature of the ‘5th’ arch and, in general, they do not ascribe derivatives to that structure.
However, earlier textbooks stated that there are four pharyngeal pouches and five corresponding arches. In “Anatomie Menschlicher Embryonen III – zur Geschichte der Organe” (Anatomy of Human Embryos III – on the Ontogenesis of the Organs; 1885) by Wilhelm His, it is written on page 28 “Wenn einmal vier Bogenpaare unterscheid‐bar sind, so bilden diese, im Frontalschnitt gesehen, zwei nach abwärts convergirende Reihen. Die vierten Bogen stehen sich näher als die dritten und diese näher als die zweiten, wogegen der zweite Bogen unter dem ersten kaum zurücksteht. […..] Dazu kommt, dass die Bogen später auch hinsichtlich ihrer Mächtigkeit differiren, indem der vierte schwächer ist als der dritte, dieser schwächer als der zweite. Unterhalb des vierten Bogens aber besteht nur eine unvollkommene Correspondenz zwischen äusserer und innerer Furche, jene bildet einen nur niedrigen Einschnitt, diese dagegen eine relativ grosse blind auslaufende Bucht, welche jederseits neben dem Kehlkopfeingang liegt” (His, 1885) – which translates as “Once four arches are distinguishable, they form, in a coronal plane of section, two downwards converging rows. The fourth arches are closer together than the third ones and those are closer together than the second ones whereas the second arch does not significantly stand back under the first one [……] Furthermore, the arches differ in volume at later stages with the fourth arch being less substantial than the third one and that one smaller than the second one. Beneath the fourth arch there is only an incomplete correlation between the outer and inner pouch, the outer one forming a shallow groove whereas the inner one is forming a relatively broad bay on either side of the larynx”. Similarly, in “Human Embryology” (1897) by Charles Sedgewick Minot, it is stated on page 263, “In all birds and mammals there are four pairs of gill pouches”; and on page 265, “As there are four gill‐clefts it follows that there are five columns. These columns are known as the branchial arches, also as the gill or visceral arches” (Minot, 1897).
So, what is correct? Is there a transient fifth pouch and sixth arch or only four pouches and five arches? Can a modern perspective help resolve this issue? Importantly, our knowledge of the embryology of the pharyngeal arches has advanced greatly over the last two decades and, perhaps, the most significant development has been the understanding that it is the formation of the pharyngeal pouches that is key. Pharyngeal arch development is initiated with outpocketing of the pharyngeal endoderm at points along the anteroposterior axis to form the pharyngeal pouches (Veitch et al. 1999). These structures then grow to contact the overlying ectoderm, which invaginates to meet them, generating the pharyngeal clefts. It is the apposition between the pouches and clefts that define the limits of the arches, and neural crest cells and mesoderm subsequently migrate into these preformed units. Significantly, in mutants in which the pharyngeal pouches do not form, pharyngeal development overall fails (Piotrowski & Nusslein‐Volhard, 2000).
The formation of the pharyngeal pouches can be followed both morphologically and via the expression of genes that are expressed in these structures, such as PAX1 and PAX9, and studies in the two major models for understanding amniote development, chick and mouse, have shown that four pharyngeal pouches form (Okubo et al. 2011; Poopalasundaram et al. 2019). There have, of course, been fewer studies of pharyngeal pouch formation in human embryos, but again morphology and analysis of PAX9 expression demonstrate the formation of four pouches (Farley et al. 2013; Poopalasundaram et al. 2019).
Thus, there are three features that are associated with the pharyngeal pouches: 1 – they form during the segmental phase of pharyngeal development; 2 – they contact the overlying ectoderm; 3 – they express definitive molecular markers such as PAX1/9, TBX1, etc. (Poopalasundaram et al. 2019). We can conclude that in amniotes, including humans, there are five arches and four pouches, and their relationship is as follows: pouch 1 lies between arches 1 and 2; pouch 2 between arches 2 and 3; pouch 3 between arches 3 and 4; and pouch 4 between arches 4 and the terminal arch. There have, however, been other studies that have suggested the presence of additional pharyngeal pouches in human, yet none of the structures presented in these meet the criteria for being a pharyngeal pouch and are best viewed as being later forming undulations, or pocketings, of the endoderm ( see Grevellec & Tucker, 2010 for examples).
It was also suggested in Gray's Anatomy in 1918 that all the arches, including the ‘5th’, form a cartilage element and an arch artery, which would suggest that the development of arch ‘5’ has significance for later anatomy (Lewis, 1918). However, more recent studies suggest that this is not the case. We analysed chondrogenesis and myogenesis during the development of the pharyngeal arches in chick and mouse, and found that these processes occur within arches 1, 2 and 3, but not within the posterior arches 4 and 6 during their period of existence; the pharyngeal arches are remodelled before the initiation of either of these differentiation processes in these segments (Poopalasundaram et al. 2019). Thus, one cannot specifically ascribe the formation of muscle and cartilage primordia to arches 4 and 6 in these species. A similar conclusion has also been drawn from a recent study of human embryos (de Bakker et al. 2018). In this, a histological analysis was performed, and three‐dimensional models were prepared that demonstrate the formation of cartilage elements in arches 1, 2 and 3, but not in arches 4 or 6.
During normal development in amniotes, a pharyngeal arch artery forms within each of the arches, and thus 5 bilaterally paired vessels form, again numbered 1, 2, 3, 4 and 6. However, there have been suggestions that an additional ‘5th’ arch artery is seen in some rare cases, both in humans and other amniotes, and that this may indicate the possible existence of a transient 5th arch. One study described the presence of a collateral channel between the 4th and 6th arch arteries in a minority of mouse embryos and in one out of eight human embryos (Bamforth et al. 2013). However, it is questionable as to whether this can be considered a ‘5th’ arch artery. These vessels form at late stages, after the onset of the remodelling of the pharyngeal arches, they are either found unilaterally or bilaterally, and they are only sometimes seen dorsoventrally as an independent element. Thus, while the identification of these anomalies is undoubtedly important in explaining the aetiology of some congenital cardiovascular malformations, they are of no significance with respect to the terminology one uses to describe normal anatomy – one would never base normal anatomical terminology on infrequent anomalous states.
Evolutionary considerations and support for a ‘6th’ arch in amniotes
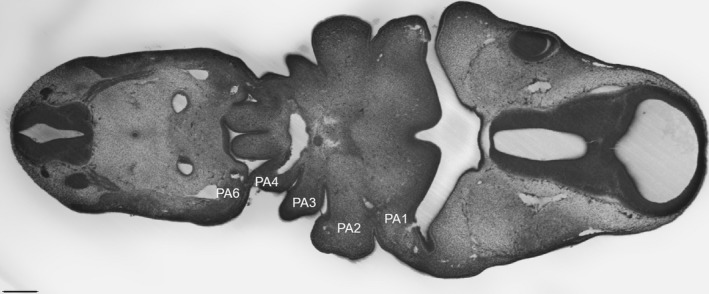
The other reason given for the curious numbering of the arches in amniotes is that this derives from comparative anatomy. With the evolution of the tetrapods a progressive reduction in the number of pharyngeal segments took place; amphibia: urodeles and anurans, have six, while amniotes: reptiles and mammals, have five. Thus, the labelling of the last arch in amniotes as the ‘6th’ was used to indicate its homology with the most caudal arch of amphibia. However, this argument suffers from a lack of overall consideration of the number of pharyngeal arches within the vertebrates.
Importantly, different groups of vertebrates have quite different numbers of pharyngeal arches. In extant vertebrates, the greatest numbers of pharyngeal segments are seen in the cyclostomes, with hagfish having up to 15 and lampreys nine (Romer, 1971; Takio et al. 2007; Oisi et al. 2015). However, most gnathostomes; chondrichthyans, actinopterygians and some basal sarcopterygians, have seven arches, while amphibia have six and amniotes have five (Kardong, 2012). In all these groups, the first arch forms the jaw, and the second the hyoid, while the more posterior arches in anamniotes will form the branchial apparatus, and in amniotes these segments are believed to give rise to components of the larynx.
Our recent studies have highlighted homologous features of the posterior limit of the pharyngeal arches across the vertebrates (Shone et al. 2016). It has been shown that the most posterior pouch is marked by expression of HOX1. Thus, in lampreys, it is the 8th pouch that expresses LjHox1w (Takio et al. 2007), in the catshark the 6th pouch expresses HoxB1, and in amniotes, chick and mouse, it is the 4th pouch that expresses HoxB1 (Shone et al. 2016). Additionally, in all vertebrates, the posterior margin of the pharynx is skirted by the hypoglossal (XIIth) nerve and the fourth pouch is the site of origin of the ultimobranchial body (Kardong, 2012; Shone et al. 2016). So, the most posterior arch of amniotes is homologous with the most posterior arch of amphibia, the 6th, but it is also homologous with the most posterior arch in the catshark, the 7th, and with the most posterior arch of the lamprey, the 9th. Thus, if one is considering that the numbering of the arches in amniotes should take account of comparative anatomy, then one could easily label the last arch the 9th to reflect homology with the lamprey, or the 7th to reflect homology with the basal gnathostomes. There is no reason to number the terminal arch in amniotes the 6th. Rather, and in keeping with the numbering of the arches across the vertebrates, it would be simpler to number the pharyngeal arches in amniotes, 1 to 5 (Fig. 2).
Figure 2.
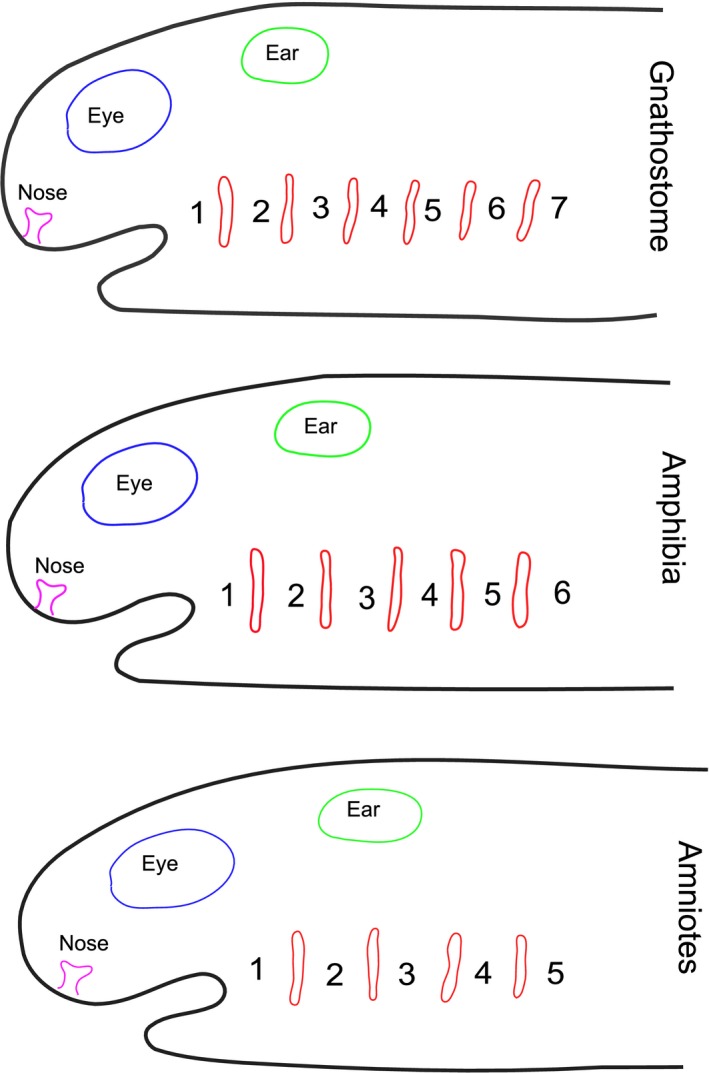
The number of pharyngeal arches varies across the vertebrates. The ancestral condition for jawed vertebrates (gnathostomes) is to have seven pharyngeal arches, numbered from anterior to posterior as 1 to 7. In the sarcopterygian lineage, there has been a trend towards a reduction in the number of pharyngeal arches. Thus, amphibia have six arches while amniotes have five. The position of the pharyngeal pouches is shown in red.
Conclusions
There is no evidence from amniote embryology for the existence of a transient ‘5th’ arch/pouch. Similarly, there is no good reason for the last arch of amniotes to be labelled the ‘6th’ to reflect considerations of comparative anatomy. Rather, the pharyngeal arches in amniotes, including humans, should simply be labelled from anterior to posterior as 1, 2, 3, 4 and 5 (Fig. 3). This is in keeping with the embryology and the general mode of numbering of the arches across the different vertebrate clades.
Figure 3.
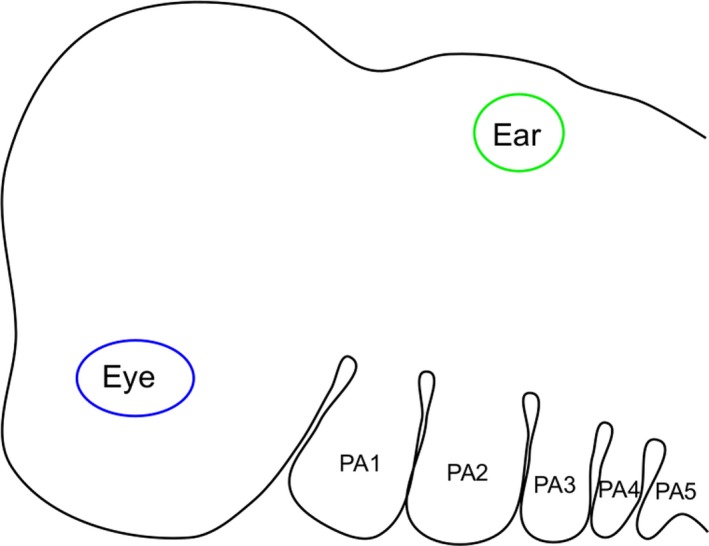
The numbering of the pharyngeal arches in humans, and other amniotes, should simply be from anterior to posterior, 1, 2, 3, 4 and 5.
Acknowledgements
The HREM data from the human embryos are from Deciphering the Mechanisms of Developmental Disorders website (https://dmdd.org.uk/), a programme funded by the Wellcome Trust with support from the Francis Crick Institute, is licensed under a Creative Commons Attribution licence. The authors thank Dr Tim Mohun for his assistance with the HREM data.
References
- de Bakker BS, de Bakker HM, Soerdjbalie‐Maikoe V, et al. (2018) The development of the human hyoid‐larynx complex revisited. Laryngoscope 128, 1829–1834. [DOI] [PubMed] [Google Scholar]
- Bamforth SD, Chaudhry B, Bennett M, et al. (2013) Clarification of the identity of the mammalian fifth pharyngeal arch artery. Clin Anat 26, 173–182. [DOI] [PubMed] [Google Scholar]
- Carlson BM (2009) Human Embryology and Developmental Biology. Philadelphia: Mosby/Elsevier. [Google Scholar]
- Couly G, Le Douarin NM (1990) Head morphogenesis in embryonic avian chimeras: evidence for a segmental pattern in the ectoderm corresponding to the neuromeres. Development 108, 543–558. [DOI] [PubMed] [Google Scholar]
- D'Amico‐Martel A, Noden D (1983) Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat 166, 445–468. [DOI] [PubMed] [Google Scholar]
- Farley AM, Morris LX, Vroegindeweij E, et al. (2013) Dynamics of thymus organogenesis and colonization in early human development. Development 140, 2015–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevellec A, Tucker AS (2010) The pharyngeal pouches and clefts: development, evolution, structure and derivatives. Semin Cell Dev Biol 21, 325–332. [DOI] [PubMed] [Google Scholar]
- Harlow D, Barlow L (2007) Embryonic origin of gustatory cranial sensory neurons. Dev Biol 310, 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- His W (1885) Anatomie Menschlicher Embryonen – III zur Geschichte der Organe. Leipzig: Verlag Von F. C.W.Vogel. [Google Scholar]
- Kardong KV (2012) Vertebrates: Comparative Anatomy, Function, Evolution. New York: McGraw‐Hill. [Google Scholar]
- Lewis WH (1918) Anatomy of the Human Body, by Henry Gray. 20th edn, thoroughly revised and re‐edited by Warren H. Lewis. Philadelphia: Lea & Febiger. [Google Scholar]
- Minot CS (1897) Human Embryology. London: MacMillan. [Google Scholar]
- Noden DM (1983a) The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat 168, 257–276. [DOI] [PubMed] [Google Scholar]
- Noden DM (1983b) The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol 96, 144–165. [DOI] [PubMed] [Google Scholar]
- Oisi Y, Fujimoto S, Ota KG, et al. (2015) On the peculiar morphology and development of the hypoglossal, glossopharyngeal and vagus nerves and hypobranchial muscles in the hagfish. Zoological Lett 1, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Kawamura A, Takahashi J, et al. (2011) Ripply3, a Tbx1 repressor, is required for development of the pharyngeal apparatus and its derivatives in mice. Development 138, 339–348. [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Nusslein‐Volhard C (2000) The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev Biol 225, 339–356. [DOI] [PubMed] [Google Scholar]
- Poopalasundaram S, Richardson J, Scott A, et al. (2019) Diminution of pharyngeal segmentation and the evolution of the amniotes. Zoological Lett 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J, Shono T, Okabe M, et al. (2012) The presence of an embryonic opercular flap in amniotes. Proc Biol Sci 279, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer A (1971) The Vertebrate Body‐shorter version. Philadelphia: W. B. Saunders. [Google Scholar]
- Sadler TW, Leland J, Sadler‐Redmond SL, et al. (2012) Langman's Medical Embryology. Philadelphia: Wolters Kluwer. [Google Scholar]
- Schoenwolf G (2014) Larsen's Human Embryology. London: Churchill Livingstone. [Google Scholar]
- Shone V, Oulion S, Casane D, et al. (2016) Mode of reduction in the number of pharyngeal segments within the sarcopterygians. Zoological Lett 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring S (2015) Gray's Anatomy: The Anatomical Basis of Clinical Practice, 41st edn, p. 1562. New York: Elsevier. [Google Scholar]
- Takio Y, Kuraku S, Murakami Y, et al. (2007) Hox gene expression patterns in Lethenteron japonicum embryos – insights into the evolution of the vertebrate Hox code. Dev Biol 308, 606–620. [DOI] [PubMed] [Google Scholar]
- Veitch E, Begbie J, Schilling TF, et al. (1999) Pharyngeal arch patterning in the absence of neural crest. Curr Biol 9, 1481–1484. [DOI] [PubMed] [Google Scholar]


