Abstract
The dermoskeleton of the earliest vertebrates is well known but their endoskeleton is thought to have been largely cartilaginous until the Late Silurian. We confirm that the dermal plates of Astraspis are three‐layered, with a superficial layer of enameloid and orthodentine, a middle layer of aspidin and a basal layer of lamellar acellular bone. This dermoskeleton is found in association with globular calcified cartilage, indicating the presence of a partially mineralized endoskeleton. In addition to the classical three‐layered organization, some dermal plates exhibit alignments of chondrocyte‐like lacunae, very similar to a pattern typical of chondroid metaplastic bone, previously unknown in early vertebrates. This discovery implies the presence of a proliferative cartilage, hitherto only known in Osteichthyans. This discovery indicates that a pattern similar to the first step of endochondral ossification was already present in the earliest vertebrates.
Keywords: dermal bone, histology, pteraspidomorph, skeleton
The dermoskeleton of the Ordovician vertebrate Astraspis possessed a newly described skeletal layer. This mineralized tissue exhibits similarities with proliferative cartilage, previously unknown in the early vertebrates. This discovery indicates that a pattern similar to the first step of endochondral ossification was already present in the earliest vertebrates.

Introduction
Early vertebrates possessed an external skeleton composed of bony plates (Janvier, 1996). Although their dermoskeleton is well known, their endoskeleton and its process of development remain a matter of debate (Smith & Hall, 1990; Janvier, 1996; Donoghue & Sansom, 2002; Donoghue et al. 2006).
The Harding Sandstone Formation was deposited during the Upper Ordovician and has yielded a rich fauna of early vertebrates (Allulee & Holland, 2005). Very few articulated specimens have been discovered but, instead, a huge quantity of bony fragments are available for study, especially on bone histology. Among the recorded taxa, the pteraspidomorphs Astraspis desiderata and Eriptychius americanus are the most numerous. Other taxa, such as thelodonts, chondrichthyans and the enigmatic Skiichtys have also been recorded (Sansom et al. 1996; Smith & Sansom, 1997). Some fragments of globular calcified cartilage attributed to Eriptychius strew the blocks of Harding sandstone (Denison, 1967).
The first study of the Harding Sandstone Formation vertebrates was published by Walcott (1892). It was followed by Vaillant (1902) and de Ricqlès (1995), who mainly focused on cellular bones, now attributed to Skiichtys. For many years they have been the subject of numerous studies (Bryant, 1936; Ørvig, 1958, 1989; Denison, 1967; Smith & Hall, 1990; Sansom et al. 1996, 1997; Smith & Sansom, 1997). The aim of this study is to describe for the first time new histological structures observed in the iconic taxon Astraspis.
Materials and methods
Some blocks of Harding sandstone were collected by Marcellin Boule and Albert Gaudry, Professors at the Muséum national d'Histoire naturelle (Paris, France) during a field trip organized by Charles Doolittle Walcott in the framework of the Fifth International Geological Congress in 1891 and are stored with the collection number MNHN.F.1891‐20. The blocks were then embedded in resin and thin‐sectioned. The sections were polished to a thickness of about 80 μm. Pictures were taken with a digital camera (Canon EOS 6D) adapted on a microscope Olympus CX31P with the Micro Tech Lab DSLRCFTC_Pro and TUST42C models.
Results
The dermoskeleton of Astraspis is a three‐layered skeleton: a superficial layer of odontodes (L1), a thick and trabecular middle layer (L2) of anosteocytic bone, termed aspidin (Keating et al. 2015) and a basal layer (L3) composed of thin anosteocytic lamellar bone, which in some plates embeds columns of ovoid spaces (Fig. 1).
Figure 1.

Histological section of a dermal plate of Astraspis, showing the different layers, labelled, with arrows indicating the chondroid layer. Scale bar: 1 mm.
Superficial layer (L1)
The tubercles are composed of a hypermineralized capping tissue and an acellular dentine layer underneath that constitutes the majority of the tubercles. The capping tissue is thick (about 50 μm), translucent and structureless, apart from numerous fine tubules extending throughout the whole layer (Fig. 2A). This is compatible with its interpretation as monocrystalline enameloid (Sansom et al. 1997). The main component of the tubercles is a layer composed of acellular dentine, penetrated by numerous, about 5‐μm‐wide tubules, which depart from the pulp cavity (Fig. 2B). In most cases, the pulp cavity is secondarily filled with dentine deposit (Fig. 2C) and is difficult, or even impossible, to observe. This is compatible with its interpretation as orthodentine (Sansom et al. 1997).
Figure 2.
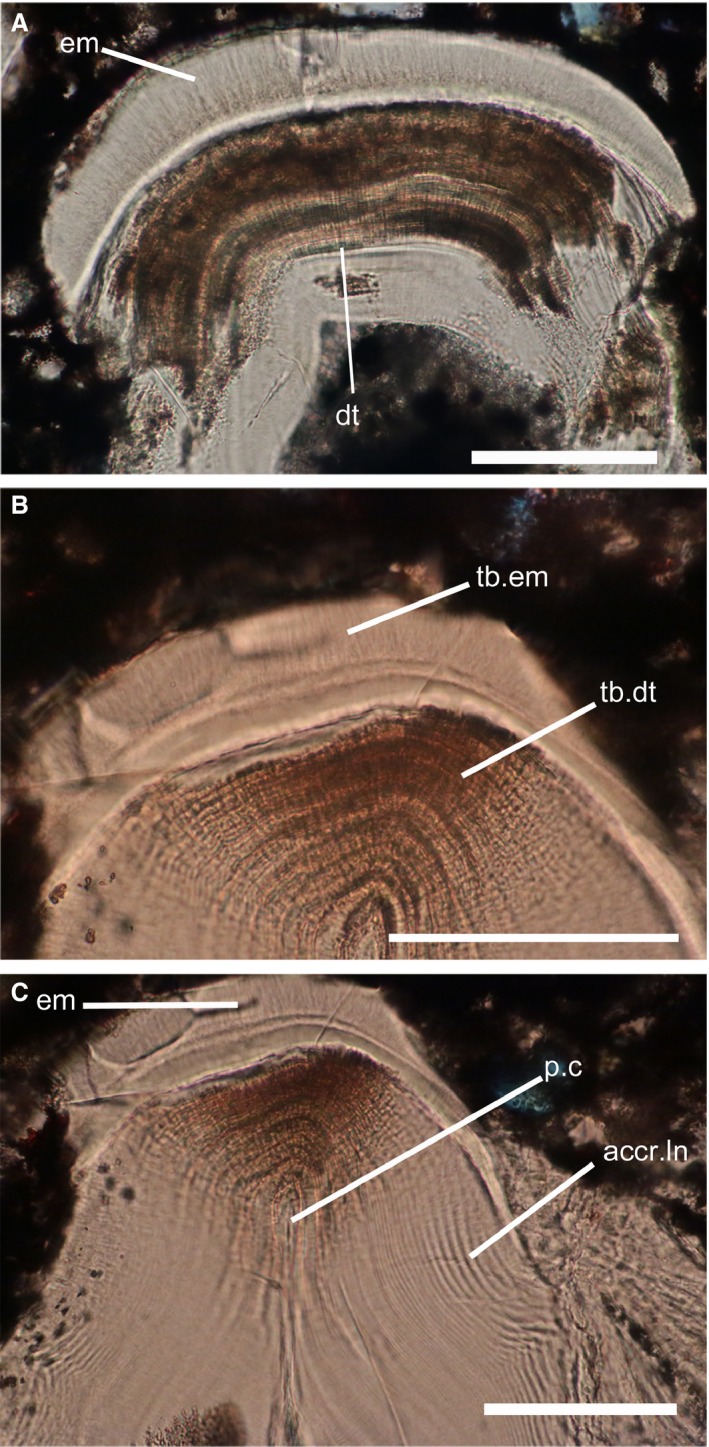
Histological sections through the superficial layer of Astraspis dermoskeleton. (A) Odontode showing the two layers composing the superficial layer. (B) Zoom on the enameloid layer. (C) Zoom on the dentine layer, showing the pulpar cavity. acc.ln, accretion lines; dt, dentine; em, enameloid; p.c, pulpar cavity; tb.dt, dentine tubule; tb.em, enameloid tubule. Scale bars: 100 μm.
Middle layer (L2)
This very thick layer, sometimes overlapping the basal layer, is composed of a centripetal deposit of bony tissue around vascular canals (Fig. 3A). In polarized light, these deposits form oval structures around canals, called aspidone (Sansom et al. 1997). These aspidones are separated by unlaminated collagen matrix (Fig. 3B). This layer is devoid of any cell spaces, and thus considered anosteocytic (Sansom et al. 1997) and is similar to the middle layer (L2) described by Keating et al. (2015, 2018) in the Heterostracan dermoskeleton, used to define aspidin (Gross, 1930).
Figure 3.

Histological sections through the middle layer of Astraspis. (A) Section showing the middle layer, with aspidone. (B) Section in polarized light, highlighting the aspidone present around the vascular space. (C) Zoom on an aspidone exhibiting small spaces interpreted as cell lacunae. aspd, aspidone; lac, lacunae; v. sp, vascular space. Scale bars: 100 μm.
Additionally, some plates of Astraspis exhibit, near the vascular spaces, small lacunae (3–5 μm) embedded between the accretion lines. They seem to be rounded or ovoid, flattened spaces. We also did not observe any ramification sprouting from these spaces. These lacunae are aligned radially around the vascular spaces (Fig. 3C), inside the aspidones.
Basal layer (L3)
This layer is composed of anosteocytic bone, with lamination of collagen bundle fibres more or less parallel to the base of the plates (Fig. 4A,B). They represent the different accretion phases of the layer. Cutting these incremental lines perpendicularly, fine laminations are visible in polarized light (Fig. 4B). In some dermal plates, other, coarser, perpendicular laminations, (about 5 μm of diameter) can be observed (Fig. 4) and are interpreted as Sharpey's fibre bundles.
Figure 4.

Histological sections through basal layers of Astraspis. (A) Section showing the basal layer of Astraspis, with white line indicating the limit with the middle layer. (B) Same section in polarized light, showing the structure of the layer. (C) Basal layer exhibiting small space, stacked in columns, interpreted as chondrocyte‐like cell lacunae. (D) Same section in polarized light with white arrows indicating chondrocyte‐like cell lacunae. acc.ln, accretion lines; aspd, aspidone; lac, lacunae. Scale bars: 100 μm (A,B); 50 μm (C,D).
Some specimens of Astraspis plates exhibit additional features, never before observed in this taxon.
Interestingly, the fine lamination present in the basal layer, and cutting across the deposit lines (Fig. 4C,D), embed small, flattened and ovoid spaces (about 9–10 μm) that are devoid of any ramifications. Unlike those observed in the middle layer, when varying the focus through the thickness of the thin section, we can observe that they really are flattened, ovoid structures. They are organized in linear stacks, forming columns of about a dozen spaces more or less parallel to each other (Fig. 5A). The laminations in this unusual and rare (observed in about 10 elements) layer, form wavy lines more or less parallel to the base of the layer. Additionally, the basal and middle layer are clearly separated by a conspicuous delimitation (Fig. 5B).
Figure 5.

Histological sections through the new basal layer of Astraspis, and comparison with metaplastic layer. (A) Zoom on chondrocyte‐like cell space area. (B) Zoom on the junction between the middle layer and the basal layer with dotted line indicating the limit. (C) Example of metaplastic bone resting on a proliferative cartilage (taken from Haines & Mohuiddin, 1968). acc.ln, accretion lines; aspd, aspidone; ch.lac, chondrocyte lacunae; lac, lacunae; ML, metaplastic layer; PC, proliferative cartilage. Scale bars: 50 μm (A,B); 100 μm (C).
This layer (L3) was also found in association with a fragment of calcified globular cartilage characterized by circular deposits forming Liesegang rings (Fig. 6A–C). Fragments of globular calcified cartilage can be observed throughout our sections (Denison, 1967) (Fig. 6D). Since this basal layer (L3) is only observed in Astraspis, we can assume that dermal elements with both this layer and the one of globular calcified cartilage came from this taxon as well.
Figure 6.

Histological sections through element of Astraspis. (A) Section showing the basal layer of Astraspis associated with global calcified cartilage. (B) Zoom showing the basal layer exhibiting the chondroid bone layer. (C) Zoom on the global calcified cartilage layer, showing Liesegang rings around cell spaces. (D) Example of globular calcified cartilage found in Harding sandstone. b.l, basal layer; gcc, globular calcified cartilage; lac, lacunae; L.r, Liesegang rings. Scale bars 100 μm.
Discussion
The endoskeleton of Astraspis
In the Harding Sandstone Formation, no dermal plates of Eriptychius (whose dermoskeleton is relatively similar to that of Astraspis) have been observed with this new basal layer, making it unique to Astraspis. Globular calcified cartilage fragments found in the Harding sandstone are often found isolated, without any bone attached, but one study recorded it associated with Eriptychius dermal plates in one partially articulated specimen (Denison, 1967).The association between this ‘basal’ layer and fragments of calcified globular cartilage can be either the consequence of an incidental taphonomic processes or a real anatomical connection between globular calcified cartilage and dermoskeletal plates of Astraspis. When observing the connection between the two layers (Fig. 6A), there is no evidence of a connection due to taphonomy. This is compatible with the calcified cartilage being part of the Astraspis skeleton.
This implies that both Astraspis and Eriptychius possessed an endoskeleton made of globular calcified cartilage. In Eriptychius, this endoskeleton is partially mineralized and its extension is still debated (Denison, 1967). A similar position for the cartilage in Astraspis can be proposed, suggesting that it possessed a partially mineralized endoskeleton as well. We have very little knowledge of the extension of the mineralized elements of the endoskeleton in Ordovician vertebrates. It is hypothetical to propose an extension or a location within the endoskeleton for these elements, but judging from those that are mineralized in the Galeaspida, which also possess a partially mineralized endoskeleton composed of globular calcified cartilage (Wang et al. 2005), Astraspis could have possessed a mineralized endoskeleton in the cephalic region.
Cell lacunae in Astraspis
We excluded the hypothesis of fungal ascospores, despite quite a similar columnar organization. The cells in ascospores are much less flattened and they do not appear in the fossil record before the Devonian (Taylor et al. 1999). The described Ordovician fungi are definitely not Ascomycetes and their morphology is totally different from what we observe here (Redecker et al. 2000).
It is surprising that such alignments of cell lacunae have never been observed before, despite the numerous histological studies on Astraspis (Bryant, 1936; Denison, 1967; Sansom et al. 1997). We also observed the historical thin sections of Vaillant, where none of these structures were present. This suggests that this tissue is rare along the body of Astraspis and that it is localized only in some particular parts of the head shield.
They are ovoid, flattened spaces. This is incompatible with collagen fibre bundles, which are not are not arranged in such a linear pattern, but is compatible with cell lacunae.
In the Harding formation, one taxon, Skiichthys, exhibits cellular hard tissues in its dermoskeleton. In this taxon, odontocytes and osteocytes are ovoid in shape, not flattened, and possess small ramifications that housed canaliculi (Fig. 7A,B), with a size about 3–5 μm. In Astraspis, the lacunae observed in the middle layer have roughly the same size, have no ramifications and resemble those observed in Ritchieichthys (Sansom et al. 2013).
Figure 7.

Histological section through dermal elements of Skiichthys. (A) Zoom showing the cellular dentine and enameloid layer. (B) Zoom showing the basal cellular layer. dt, dentine tubule; em, enameloid; od, odontocyte space; ost, osteocyte space. Scale bars: 100 μm.
However, the lacunae present in the basal layer of Astraspis are incompatible with osteocytes because of their ovoid, flattened shape and their absence of ramifications. The position of this layer, at the base of the dermoskeleton, is highly incompatible with odontoblast cell spaces, as they are characteristic of dentine, a superficial acellular tissue in Astraspis. When compared with chondrocyte cell lacunae, their size, 9–10 μm, is < 50% of those in extant vertebrates (Freitas, 1999), but it is close to the size of some chondrocyte cell spaces found in the mineralized tissue of several extinct vertebrates. In Euphanerops, the smallest chondrocytes are about 11–13 μm (Janvier & Arsenault, 2002) and in Paleospondylus, the small hypertrophied chondrocyte spaces are about 12–14 μm (Johanson et al. 2010). Moreover, chondrocytes are known to form similar columns of cells, called isogenic axial groups. Spaces in the basal layer of Astraspis are indeed compatible with chondrocyte cell spaces.
Although these plates of Astraspis possess a cellular bone layer, the majority of Astraspis plates remain with an anosteocytic basal layer.
This new layer described herein seems to exhibit the characteristics of both bone and cartilage (the presence of aligned chondrocyte‐like cell spaces). This is characteristic of chondroid bone (Witten et al. 2010). This new basal layer of Astraspis then represents the first evidence of chondroid bone in the fossil record (Smith & Hall, 1990). Moreover, this chondroid layer is similar to a type of metaplastic bone identified by Haines & Mohuiddin (1968). In their study, a metaplastic bone layer displays isogenic axial groups of chondrocyte cell lacunae embedded inside a collagenous (bony) matrix rather than in a cartilaginous matrix (Haines & Mohuiddin, 1968). This tissue presents columns of chondrocyte spaces embedded inside the matrix by lamination that cross‐cut the incremental growth lines of deposit (more or less parallel to the base of the layer). These lines take the form of wavy and/or rhythmic sinuous lines, showing the different growth phases. It is formed by the progressive mineralization front in the collagen matrix of proliferative cartilage, where chondrocytes are stacked as columns (Haines & Mohuiddin, 1968). It is clearly separated from the cancellar bone layer above by a line more or less parallel to the others (Haines & Mohuiddin, 1968). This is compatible with the tissue microstructure presented here, as it shows stacks of chondrocyte‐like cell lacunae arranged in columns, within a bone matrix, with wavy lines more or less parallel to its base (Fig. 5). We can observe a line, more or less parallel, that clearly separates the aspidin layer from the chondroid layer (Fig. 5B), similar to that seen in the metaplastic tissue (Fig. 5C). The microstructure of the chondroid bone layer in Astraspis is compatible with the type of metaplastic bone described by Haines & Mohuiddin (1968).
In extant vertebrates, metaplastic bone tissues can occur within the endoskeleton in areas of strong attachments, such as in tendons (Haines & Mohuiddin, 1968; Adams & Organ, 2005), or during the endochondral ossification of long bones (Haines & Mohuiddin, 1968). In the endoskeleton, this layer is situated underneath a layer of cancellar bone and overlies a layer of hyaline cartilage (Haines & Mohuiddin, 1968). In Astraspis, this chondroid layer is also situated beneath a layer of cancellar bone (Fig. 8) and some plates seem to exhibit a layer of globular calcified cartilage underneath this tissue (Fig. 6A). This is compatible with the location of the metaplastic bone layer within the skeletal structure, as globular calcified cartilage is considered as a type of hyaline cartilage, known in extant sharks (Adams & Organ, 2005).
Figure 8.

Schematic interpretation of the dermoskeleton of Astraspis. ext., external face; int., internal face; vc, vascular canals.
Its position is more ambiguous, as it seems to be at the base of the dermoskeleton, which would imply that cartilage was part of the dermoskeleton during the first phase of its growth. However, cartilage is not known in any vertebrate dermoskeleton (Donoghue et al. 2006) and is considered ‘unique’ to the endoskeleton; however, chondroid bone and chondrocyte‐like cells can occur during the development of dermal bones of archosaurians (Vickaryous & Hall, 2008).
The following processes of osteogenesis have been identified by Haines & Mohuiddin (1968). Metaplastic bone ossification first starts inside proliferative hyaline cartilage, where chondrocytes multiply and organize themselves into columns, termed isogenic axial groups (Hall, 2014). The tissue then undergoes metaplasia, where the cartilaginous matrix is progressively replaced by a bone matrix through the transfer of components by the bone layer that lies above it (Haines & Mohuiddin, 1968; Hall, 2014). In this tissue, lines can be observed inside the layer, representing different stages of bone growth (Haines & Mohuiddin, 1968). This implies an endoskeletal ossification for this kind of bone tissue. Concerning the chondroid bone layer of Astraspis, we have few clues that could help us to define its growth pattern. We see some wavy lines (Fig. 5B), which represent the different accretion phases, similar to the ones seen in metaplastic bone (Fig. 5C; Haines & Mohuiddin, 1968). In conclusion, structures characteristics of the growth pattern can still be observed, and are compatible with a metaplastic ossification. However, no non‐mineralized proliferative cartilage or any somewhat similar structure can be observed either in the layer or in the calcified cartilage layer that can be found beneath. As in the previous observations by Haines & Mohuiddin (1968), the process of metaplasia and mineralization may have occurred just after the proliferation phase.
Nevertheless, Astraspis ‘new’ basal layer is indeed chondroid bone and is compatible with the characteristics of metaplastic bone layer.
A similar pattern to endochondral ossification?
The presence of metaplastic bone in the internal parts of the dermoskeleton of Astraspis shows that some dermal plates were built not only by dermal ossification but also included an endoskeletal component. We can assume that the head region included a cartilaginous braincase overlain by and connected to the dermal cephalic shield. The presence of a metaplastic bone layer implies that during the growth of the dermo‐ and endoskeleton, a proliferative cartilage was present, at least around the area where the dermal plates exhibit this layer. This proliferative cartilage would have a structure similar to the one observed in the new layer described here, but with a non‐mineralized cartilage matrix (Hall, 2014). This structure is very similar to the first step in endochondral ossification (Francillon‐Vieillot et al. 1990), as metaplastic bone is associated with it (Haines & Mohuiddin, 1968). This does not mean that endochondral ossification occurred in Astraspis, as we found no evidence of the next steps of this ossification process: hypertrophic zone, calcification zone, destruction of the calcified cartilage and its replacement by bone (Francillon‐Vieillot et al. 1990). Interestingly, the second step of the endochondral ossification can be found in Paleospondylus (Johanson et al. 2010), but without the other steps being present. This is also different from the endoskeletal growth of Osteostracans, essentially composed of perichondral bone, or of Galeaspids and Eriptychius, which only show globular calcified cartilage as an endoskeletal mineralization, with no layer of proliferative cartilage either known or supposed to be present.
A main difference from the endoskeleton of Osteostracans is that the latter is made of perichondral bone or globular calcified cartilage (Janvier, 1996), whereas in Astraspis, it corresponds to a peri‐osseous calcified cartilage. In Galeaspids, the endoskeleton never shows any cell lacuna (Janvier, 1996; Min & Janvier, 1998; Wang et al. 2005).
Nevertheless, the histological structures involved in the first steps of endochondral ossification were already present in the Ordovician vertebrate Astraspis. It is difficult to assess whether this is a primitive feature among all vertebrates, or if the proliferative zone appeared twice, in Astraspis and in Osteichthyans (Fig. 9). In the case of a plesiomorphic condition, the absence of such a chondroid bone in all Paleozoic agnathans, except for Astraspis, may be due to a lack of endoskeletal calcification in most taxa. This implies that, although the presence of a proliferative cartilage is plesiomorphic, the mineralization of this cartilage would be homoplastic (Fig. 9). If we consider a homoplastic appearance of proliferative cartilage, we suggest that the vertebrate chondroblasts acquired very early the ability to grow by forming isogenous axial groups and that this ability ultimately favoured the appearance of the true endochondral ossification in Osteichthyans.
Figure 9.

Representation of the evolution of the dermoskeleton/endoskeleton interaction in early vertebrates (modified after Keating et al. 2018). In Astraspis, the mineralized endoskeleton and the proliferative cartilage appear simultaneously. In other vertebrates, the mineralized endoskeleton appears in the clade Galeaspida(Osteostraci(Gnathostomata)) and the proliferative cartilage appears with the true endochondral ossification in Osteichthyans.
Conclusion
Our study demonstrates the need to continue histological studies on already well‐known taxa and that the classical techniques of thin‐sectioning and optical microscopy are still essential in the understanding of ancient ossification processes. The modern analytical methods such as computed tomography scans, synchrotron light microtomography, geochemical approaches, etc., can obviously bring forth new data but are thus complementary and will not completely replace the old histological techniques. This study sheds some light on the skeleton of one of the earliest mineralized vertebrate, with the first evidence of chondroid bone in an Ordovician vertebrate. It confirms that the dermoskeleton of Astraspis is three‐layered, with a superficial layer of enameloid and orthodentine, a middle layer of aspidin and a basal layer of acellular bone. Some dermal plates exhibit a basal layer of chondroid metaplastic bone. This basal layer is associated with the mineralization of the endoskeleton into globular calcified cartilage, though only partially. This chondroid metaplastic bone tissue exhibits a proliferative cartilage layer, which resembles the first step in endochondral ossification. This suggests that the various steps of the endochondral ossification were established during early vertebrate evolution, but remained unmineralized.
Funding
This work was supported by the CR2P (MNHN, CNRS, Sorbonne Université).
Competing interests
Authors declare no competing interests.
Author contributions
Both authors A.L. and D.G. contributed equally to the study.
Data and materials availability
Thin sections are available for consultation upon request to the corresponding author.
Acknowledgements
We thank Séverin Morel (CR2P) for preparing the thin sections, Gaël Clément for access to collections and comments on the manuscript, François Meunier and Philippe Janvier for fruitful discussions. We are also indebted to Joseph Keating and an anonymous reviewer for the comments that greatly improved our manuscript.
References
- Adams JS, Organ CL (2005) Histologic determination of ontogenetic patterns and processes in hadrosaurian ossified tendons. J Vertebr Paleontol 25, 614–622. [Google Scholar]
- Allulee JL, Holland SM (2005) The sequence stratigraphic and environmental context of primitive vertebrates: Harding Sandstone, Upper Ordovician, Colorado, USA. Palaios 20, 518–533. [Google Scholar]
- Bryant WL (1936) A study of the oldest known vertebrates, Astraspis and Eriptychius. Proc Am Philos Soc 76, 409–427. [Google Scholar]
- Denison RH (1967) Ordovician Vertebrates From Western United States. Chicago: Field Museum of Natural History. [Google Scholar]
- Donoghue PCJ, Sansom IJ (2002) Origin and early evolution of vertebrate skeletonization. Microsc Res Tech 59, 352–372. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Sansom IJ, Downs JP (2006) Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. J Exp Zool B Mol Dev Evol 306B, 278–294. [DOI] [PubMed] [Google Scholar]
- Francillon‐Vieillot H, de Buffrénil V, Castanet J, et al. (1990) Microstructure and mineralization of vertebrate skeletal tissues In: Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. (ed. Carter JG.), pp. 471–548, New York: Van Nostrand Reinhold. [Google Scholar]
- Freitas RA (1999) Nanomedicine, Volume I: Basic Capabilities. Georgetown: Landes Bioscience. [Google Scholar]
- Gross W (1930) Die Fische des Mittleren Old Red Südlivlands. Palaeont Abh 18, 123–156. [Google Scholar]
- Haines RW, Mohuiddin A (1968) Metaplastic bone. J Anat 103, 527–538. [PMC free article] [PubMed] [Google Scholar]
- Hall BK (2014) Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. San Diego: Academic Press. [Google Scholar]
- Janvier P (1996) Early Vertebrates. Oxford: Oxford University Press. [Google Scholar]
- Janvier P, Arsenault M (2002) Calcification of early vertebrate cartilage: palaeobiology. Nature 417, 609. [DOI] [PubMed] [Google Scholar]
- Johanson Z, Kearsley A, den Blaauwen J, et al. (2010) No bones about it: an enigmatic Devonian fossil reveals a new skeletal framework—a potential role of loss of gene regulation. Semin Cell Dev Biol 21, 414–423. [DOI] [PubMed] [Google Scholar]
- Keating JN, Marquart CL, Donoghue PCJ (2015) Histology of the heterostracan dermal skeleton: insight into the origin of the vertebrate mineralised skeleton. J Morphol 276, 657–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating JN, Marquart CL, Marone F, et al. (2018) The nature of aspidin and the evolutionary origin of bone. Nat Ecol Evol 2, 1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Z, Janvier P (1998) The histological structure of the endoskeleton in galeaspids (Galeaspida, Vertebrata). J Vertebr Paleontol 18, 650–654. [Google Scholar]
- Ørvig T (1958) Pycnaspis splendens, new genus, new species, a new ostracoderm from the Upper Ordovician of North America. Proc US Natl Mus 108, 1–23. [Google Scholar]
- Ørvig T (1989) Histologic studies of ostracoderms, placoderms and fossil elasmobranchs. 6. Hard tissues of Ordovician vertebrates. Zool Scr 18, 427–446. [Google Scholar]
- Redecker D, Kodner R, Graham LE (2000) Glomalean fungi from the Ordovician. Science 289, 1920–1921. [DOI] [PubMed] [Google Scholar]
- de Ricqlès A (1995) Les vertébrés des grès de harding: Ce que Vaillant a pu observer. Geobios 28, 51–56. [Google Scholar]
- Sansom IJ, Smith MM, Smith MP (1996) Scales of thelodont and shark‐like fishes from the Ordovician of Colorado. Nature 379, 628–630. [Google Scholar]
- Sansom I, Smith MP, Smith MM, et al. (1997) Astraspis – the anatomy and histology of an Ordovician fish. Palaeontology 40, 625–643. [Google Scholar]
- Sansom IJ, Haines PW, Andreev P, et al. (2013) A new pteraspidomorph from the Nibil Formation (Katian, Late Ordovician) of the Canning Basin, Western Australia. J Vertebr Paleontol 33, 764–769. [Google Scholar]
- Smith MM, Hall BK (1990) Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev Camb Philos Soc 65, 277–373. [DOI] [PubMed] [Google Scholar]
- Smith MM, Sansom IJ (1997) Exoskeletal micro‐remains of an Ordovician fish from the Harding Sandstone of Colorado. Palaeontology 40, 645–658. [Google Scholar]
- Taylor TN, Hass H, Kerp H (1999) The oldest fossil ascomycetes. Nature 399, 648. [DOI] [PubMed] [Google Scholar]
- Vaillant L (1902) Sur la présence du tissu osseux chez certains Poissons des terrains paléozoïques de Canyon City (Colorado). Comptes rend Acad Sci 134, 1321–1322. [Google Scholar]
- Vickaryous MK, Hall BK (2008) Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J Morphol 269, 398–422. [DOI] [PubMed] [Google Scholar]
- Walcott CD (1892) Preliminary notes on the discovery of a vertebrate fauna in Silurian (Ordovician) strata. Geol Soc Am Bull 3, 153–172. [Google Scholar]
- Wang NZ, Donoghue PCJ, Smith MM, et al. (2005) Histology of the galeaspid dermoskeleton and endoskeleton, and the origin and early evolution of the vertebrate cranial endoskeleton. J Vertebr Paleontol 25, 745–756. [Google Scholar]
- Witten PE, Huysseune A, Hall BK (2010) A practical approach for the identification of the many cartilaginous tissues in teleost fish. J Appl Ichthyol 26, 257–262. [Google Scholar]


