Abstract
Background
Trisectionectomy is a treatment option in extensive liver malignancy, including colorectal liver metastases (CRLM). However, the reported experience of this procedure is limited. Therefore, we present our experience with right hepatic trisectionectomy (RHT) for CRLM as an example and discuss the changing role of trisectionectomy in the context of modern treatment alternatives based on a literature review.
Methods
Between January 1993 and December 2014 all patients undergoing RHT at a single center in the UK for CRLM were included. Patient and tumor characteristics were reviewed and a multivariate analysis was done. Based on a literature review the role of trisectionectomy in the treatment of HPB malignancies was discussed.
Results
A total of 211 patients undergoing RHT were included. Overall perioperative morbidity was 40.3%. Overall 90‐day mortality was 7.6% but reduced to 2.8% over time. Multivariate analysis identified additional organ resection (P = .040) and blood transfusion (P = .028) as independent risk factors for morbidity. Multiple tumors, total hepatic vascular exclusion, and R1 resection were independent risk factors for significantly decreased disease‐free and disease‐specific survival. Further surgery for recurrence after RHT significantly prolonged survival compared with palliative chemotherapy only.
Conclusion
With the further development of surgical and multimodal treatment strategies in CRLM the indications for trisectionectomy are decreasing. Having being formerly associated with high rates of perioperative morbidity and mortality, this single‐center experience clearly shows that these concomitant risks decrease with experience, liberal use of portal vein embolization and improved patient selection. Trisectionectomy remains relevant in selected patients.
Keywords: colorectal liver metastases, liver resection, right hepatic trisectionectomy
Systematic review on the use of trisectionectomies in the treatment of HPB malignancies.

1. INTRODUCTION
Left hepatic trisectionectomy (LHT) was first described in detail by Starzl and colleagues as a left trisegmentectomy in 1982, and then as an extended left hepatectomy by Blumgart et al in 1993.1, 2 The designation LHT was adopted following the International Hepato‐Pancreato‐Biliary Association Brisbane 2000 consensus statement on the nomenclature of liver anatomy and resection. LHT is defined as excision of Couinaud liver segments 2, 3, 4, 5 and 8, with or without segment 1.3 Despite improvements in surgical techniques and perioperative patient management, only a few papers have reported outcomes of LHT in more than 10 patients.4, 5, 6, 7, 8 Morbidity and mortality after LHT is higher than for other hepatectomies, and this procedure is reserved for patients with a significant tumor burden and an otherwise dismal prognosis. The high morbidity rate is attributable mainly to the aggressive nature of the disease being treated, but may also be related to the extent of liver volume resected, estimated to be as high as 80 per cent.2 In 2005, the Leeds group reported long‐term outcomes of LHT in 70 consecutive patients.4 Morbidity rate was high, but the potential for cure supported an aggressive surgical resection policy where other treatment options had been exhausted. In 2016, the same group described changes in surgical practice over time, and analyzed the short‐ and long‐term outcomes of LHT for hepatobiliary malignancy, in order to identify factors associated with morbidity and mortality in the modern era.9
Right hepatic trisectionectomy (RHT) was first described by Lortat‐Jacob, Robert and Henry as right lobectomy in 1952.10 This operation has had a number of different names, but, until recently, it has been most commonly known as right trisegmentectomy. The designation RHT was adopted following the International Hepato‐Pancreato‐Biliary Association Brisbane 2000 consensus statement on the nomenclature of liver anatomy and resection.3, 11 This procedure requires excision of segments 4, 5, 6, 7 and 8 ± 1 and it also remains one of the most challenging major hepatectomies. Despite improvements in surgical technique and perioperative critical management, perioperative morbidity remains high and only a few hepatobiliary centers worldwide have reported their experience.12, 13
Modifications of LHT and RHT by in‐contiguity and non‐anatomical extension and repeat liver resection after LHT or RHT are also rarely reported.14, 15
The role of these technically demanding and extensive resections in contemporary hepatobiliary practice is established for primary liver cancers and for those tumors with no significant neoadjuvant strategies, but it is also changing as new treatments emerge. This is particularly true for patients with colorectal liver metastases (CRLM), and it is likely that this trend will be followed for other HPB malignancies as more effective preoperative strategies are developed. Emerging data for intrahepatic cholangiocarcinoma, for example, is encouraging.16, 17, 18, 19
For patients with CRLM, despite the lack of compelling data for most patients, there has been a paradigm shift in the oncological assessment of patients and the use of neoadjuvant and “downstaging” strategies before resection. This has been combined with a sensible development of surgical strategies aimed at parenchymal preservation, along with new developments in liver surgery such as multistage resection as a classical two‐stage approach (TSH) or associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). In the classical two‐stage approach, portal vein embolization (PVE) or portal vein ligation (PVL) is carried out to stimulate hypertrophy in the planned future liver remnant, along with resection of tumors from the planned future liver remnant (FLR). After an interval of 4‐8 weeks, with adequate hypertrophy of the FLR, the definitive resection is carried out.20 Besides PVL/PVE, the first step in ALPPS includes at least a 50% transection of liver parenchyma.21 By this modification, ALPPS seems to be able to accelerate liver growth of the FLR and to shorten the interstage interval.22, 23 A recent Scandinavian randomized controlled trial has shown the benefits of ALPPS in providing a higher resection rate compared to the classical two‐stage procedure, with comparable margins, complications and short‐term mortality.24
However, besides the evolvement of these promising strategies, there remains a place for up‐front major resection for many patients. In the light of this trend, we have reviewed in detail a 22‐year single‐center experience of RHT for CRLM and evaluated factors affecting morbidity and survival in order to provide a critical appraisal for the role of RHT for CRLM in order to add these data to our previous work on LHT.
2. METHODS
2.1. Study design
Patients undergoing RHT between January 1993 and December 2014 were identified from a prospectively maintained database at a single institution. Additional data from the database included radiological investigations and interventions, presence or absence of jaundice, extent of surgical resection, duration of operation, requirement for transfusion of blood or blood products, need for Pringle maneuver or total vascular exclusion, additional surgery (lymphadenectomy, extrahepatic bile duct excision with reconstruction, or vascular reconstruction), histopathological diagnosis, size and distribution of tumors, perioperative morbidity and mortality, and long‐term disease‐free and disease‐specific survival. This work has been reported in line with the PROCESS criteria.25
All patients undergoing liver resection were offered adjuvant chemotherapy according to guidelines unless they had received adjuvant therapy following their colonic resection within the past 12 months. However, detailed data on adjuvant chemotherapy after colorectal and hepatic surgery were not routinely collected in the database owing to the large number of patients presenting from a wide geographical area of referring hospitals using chemotherapy. In 12 patients, neoadjuvant chemotherapy was used as either a downsizing technique or as a “test of time approach.”
2.2. Preoperative evaluation
Preoperative radiological assessment in all patients included thoracic, abdominal and pelvic computed tomography (CT), and magnetic resonance imaging (MRI) of the liver. The investigations were reviewed in a multidisciplinary team meeting to discuss and define the extent of resection. In selected cases, positron emission tomography CT (PET‐CT) was used. From 2007, PVE was used when the future liver remnant was estimated to be <20% and was carried out 3 to 4 weeks before scheduled liver resection, but no formal volumetry studies have been done in our center.
2.3. Perioperative care
Techniques of RHT and extensions of RHT have been described previously.14, 26, 27 Intraoperative ultrasound was carried out in all patients to identify any additional lesions in segments 2 and 3, and their relation to the left portal structures and hepatic veins. All liver transections were carried out using a Cavitron ultrasonic surgical aspirator (CUSA). Pringle's maneuver was applied in selected patients to reduce blood loss and total hepatic vascular exclusion (TVE) (portal triad and hepatic vein or inferior vena cava [IVC] clamping) was used when necessary for tumors located at the hepatocaval confluence. Intraoperative allogeneic red blood cells (ARBC) and fresh frozen plasma were transfused at the discretion of the anesthesiologist. ARBC were also transfused postoperatively if the hemoglobin level fell to <8.0 g/dL in the absence of cardiac disease and <10.0 g/dL for patients with risk factors for cardiac disease according to our unit policy. No patients received autologous blood transfusion.
2.4. Morbidity and mortality
Details of complications were obtained from the database and, where necessary, from the patient notes and graded according to the validated Clavien‐Dindo classification system.28 Postoperative liver failure was defined according to the International Study Group of Liver Surgery.29 Postoperative mortality was defined by the occurrence of death within 90 days of surgery or at any time during postoperative hospital stay.
2.5. Histopathological evaluation
Pathological reports were reviewed to determine tumor histological grade, margin status, and histological abnormalities in the non‐tumor‐bearing liver (NTBL). A tumor‐free resection margin of less than 1 mm was classified as (R1), and 1 mm or more was classified as (R0).30 In relation to NTBL, liver steatosis was defined as diffuse accumulation of fat droplets affecting >5% of hepatocytes.31 Fibrosis was scored according to the Metavir score, and defined as the presence of portal fibrosis with/without septa, numerous septa, or cirrhosis.32 Sinusoidal injury was graded and defined as the presence of centrilobular involvement beyond one‐third of the lobular area.33 These findings in NTBL were defined as parenchymal liver damage in the present study.
2.6. Follow up
All patients were followed up regularly at the outpatient clinic at 1, 3, 6 and 12 months in the first year, 18 and 24 months in the second year, and yearly thereafter if the patient remained disease‐free. Follow up included clinical examination and assessment of tumor markers (carcinoembryonic antigen [CEA], cancer antigen [CA]19‐9). Surveillance imaging included CT scans of the chest, abdomen, and pelvis at 3, 6, 12, 18 and 24 months, annually to 5 years and again at 7 and 10 years. MRI and PET‐CT were carried out if recurrence was suspected in routine follow up.
2.7. Survival
Disease‐free survival (DFS) was defined as the time from operation to the first documented disease recurrence on imaging. Disease‐specific survival (DSS) was defined as the time from operation to the time of death as a result of recurrence or the most recent follow‐up time. Patients dying of other causes with no evidence of recurrence were censored. In this study of effect on long‐term disease and survival, patients with death within 90 days of operation were excluded.
2.8. Statistical analysis
Continuous variables were expressed as median and interquartile range. To consider changes over the study period, patients were divided into three periods based on time interval of treatment: time period 1, 1993‐2000; time period 2, 2001‐2007; time period 3, 2008‐2014. The Kruskal‐Wallis test was used for continuous variables and the Pearson chi‐squared test or Fisher's exact test, where appropriate, for categorical variables. Univariate analysis for postoperative complications was carried out using the Pearson chi‐squared test or Fisher's exact test where appropriate. Multivariate analysis was carried out by Cox regression (stepwise forward model) for variables shown to have a significant influence on postoperative morbidity, 90‐day mortality, and disease‐specific overall and disease‐free survival in the univariate analysis. Date of last follow up was February 2015. All statistical analyses were done using SPSS for Windows/MacTM version 20.0 (IBM), and statistical significance was taken at the 5% level.
3. RESULTS
3.1. Patient characteristics
Between January 1993 and December 2014, a total of 3946 liver resections were carried out at this single UK center. Of these, 399 (10%) patients underwent RHT, of whom 188 (47%) patients (hepatocellular carcinoma, n = 35 (18.5%); non CRLM, n = 31 (16.5%); hilar cholangiocarcinoma, n = 36 (19%); intrahepatic cholangiocarcinoma, n = 20 (11%); gallbladder cancer, n = 16 (8.5%); benign liver tumor, n = 15 (8%); other malignant liver tumor, n = 6 (3.2%); other benign bile duct disease, n = 5 (2.7%); RHT as part of auxiliary orthotopic liver transplantation, n = 24 (12.8%) were excluded.
A total of 211 patients were included: 126 (60%) male, 85 (40%) female with a median age of 62 years (range, 25‐85). All of the 211 patients included in this analysis underwent RHT for CRLM.
3.2. Tumor characteristics
In the cohort, 49 patients (23%) had solitary tumors and median size of the largest tumor was 50 (range 8‐410) mm. Neoadjuvant chemotherapy was given to 12 (5.6%) patients. In 13 (6%) patients, portal vein embolization was done before the actual surgery. Twenty‐five (12%) and 80 (38%) patients underwent concomitant segment (S)1 and/or S2/S3 metastectomy, respectively. Three (1.4%) patients underwent ex vivo resection. Thirty‐two (15%) patients required additional organ resection: in 19 (59%) patients, the diaphragm had to be resected, followed by large bowel n = 8 (25%) and others n = 5 (16%). In 72 (34%) of the patients, some form of parenchymal liver damage was noted: 57 (27%), six (3%) and 18 (9%) with steatosis, fibrosis and sinusoidal obstructive syndrome, respectively. Some patients had two or three duplicate types of liver damage.
3.3. Short‐ and long‐term outcomes, morbidity and mortality
Forty‐four patients (21%) received a blood transfusion, with a median ARBC transfusion of 4 units (range 1‐40). Median hospital stay was 10 days (range, 4‐139 days). Of the 211 patients who underwent RHT for CRLM, 85 (40.3%) had postoperative complications as described in Table 1. Thirty‐eight (18%) patients had more than two postoperative complications. Re‐laparotomy was carried out in 19 (9%) patients of the cohort, the main reason being intra‐abdominal bleeding n = 9 (47.4%). Four (21%) of the patients who underwent re‐laparotomy died in hospital.
Table 1.
Postoperative outcomes after right trisectionectomy for colorectal liver metastases
| Outcomes | n (%) |
|---|---|
| Overall morbidity | 85 (40.3) |
| Grade I | 10 (4.7) |
| Grade II | 21 (10.0) |
| Grade IIIa | 11 (5.2) |
| Grade IIIb | 13 (6.2) |
| Grade IVa | 7 (3.3) |
| Grade IVb | 8 (3.8) |
| Grade V (in‐hospital death) | 15 (7.1) |
| Median hospital stay, days (range)a | 10 (4‐139) |
| 90‐d mortality | 16 (7.6) |
| Morbidity detailsb | |
| Transient liver failure | 25 (11.8) |
| Wound infection | 12 (5.7) |
| Bile leak | 12 (5.7) |
| Sepsis | 11 (5.2) |
| Intra‐abdominal bleeding | 11 (5.2) |
| Renal failure | 9 (4.3) |
| Pneumonia | 8 (3.8) |
| Cardiac eventsc | 6 (2.8) |
| Gastrointestinal bleeding | 6 (2.8) |
| Intra‐abdominal fluid collection | 6 (2.8) |
| Wound dehiscence | 3 (1.4) |
| Intra‐abdominal abscess | 2 (0.9) |
| Bowel obstruction | 2 (0.9) |
| Pulmonary embolism | 2 (0.9) |
| Portal vein thrombosis | 2 (0.9) |
| Minor non‐specific complications | 9 (4.3) |
Excluding 15 patients who died in hospital.
38 patients had two or more complications.
Including myocardial infarction, congestive heart failure, and arrhythmia.
Of the whole cohort, 15 (7.1%) patients died in hospital. One other patient (0.5%) died within 90 days following surgery. Therefore, 16 patients (7.6%) died within 90 days. Among these 16 patients, main causes for mortality were as follows: seven (44%) patients died from multi‐organ failure; three (19%) patients died as a result of gastrointestinal bleeding; two (13%) due to acute myocardial infarction; one (6%) as a result of pneumonia or intra‐abdominal abscess (n = 1, 6%); massive abdominal bleeding (n = 1, 6%) in hospital; and unknown cause (n = 1, 6%) after discharge. The 211 included patients were further divided into three time periods where 70 patients were included in the first period, 70 patients in the second period and 71 patients in the third period, respectively. With increasing experience at our center we were able to decrease 90‐day mortality from 12.8% to 7.1% and 2.8% accordingly. These differences were not significant.
Univariate analysis for morbidity showed that other organ resection (P = .017) and ARBC transfusion (P = .012) were markers for poor outcome. Both variables were also found to be independent predictors for morbidity in multivariate analysis (odds ratio [OR] for other organ resection = 2.27; 95% confidence interval [CI], 1.04‐4.95; P = .040) and ARBC transfusion (OR = 2.16; 95% CI, 1.09‐4.30; P = .028). Notably, parenchymal liver damage in NTBL did not significantly impact morbidity, disease recurrence and survival (Table 2). Median follow up was 29.8 months (range, 0 to 255.7 months) in the entire cohort. DSS rates at 1, 3, 5, and 10 years after RHT were 89.7%, 55.7%, 33.7%, and 22.4%, respectively, with a median DSS of 39.7 months. One‐, 3‐, and 5‐year DFS were 58.7%, 26.6%, and 20.9%, respectively, with median DFS time of 13.3 months. Significant predictors of decreased DFS in univariate analysis were preoperative PVE (P = .030), preoperative chemotherapy (P = .029), multiple tumors (P < .001), caudate lobectomy (P = .034), additional metastectomy from segment 2 and/or 3 (P < .001), other organ resection (P = .015), TVE (P = .001), and R1 resection (P < .001). In multivariate analysis, multiple tumors (risk ratio [RR] = 1.99; 95% CI, 1.24‐3.20; P = .005), TVE (RR = 3.05; 95% CI, 1.42‐6.57; P = .004), and R1 resection (RR = 1.60; 95% CI, 1.11‐2.29; P = .012) were independent prognostic factors for DFS (Table 3). Likewise, for DSS, preoperative PVE (P = .009), preoperative chemotherapy (P = .043), multiple tumors (P < .001), combined caudate lobectomy (P = .042), partial resection of segment 2 and/or 3 (P = .023), TVE (P = .009), and R1 resection (P < .001) were found to be significant factors. In multivariate analysis, multiple tumors (RR = 2.95; 95% CI, 1.68‐5.20; P < .001), TVE (RR = 3.77; 95% CI, 1.73‐8.20; P = .001), and R1 resection (RR = 1.92; 95% CI, 1.31‐2.81; P < .001) were independent prognostic factors for DSS as with DFS (Table 4).
Table 2.
Univariate and multivariate analyses of variables affecting morbidity after right trisectionectomy
| Variables | Total | Morbidity (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| n = 211 | n = 85 (40.3) | Odds ratio (95% CI) | P‐value | Odds ratio (95% CI) | P‐value | |
| Preoperative variables | ||||||
| Gender | ||||||
| Female | 85 | 32 (37.6) | 1.20 (0.68‐2.11) | .521 | ||
| Male | 126 | 53 (42.1) | ||||
| Age | ||||||
| ≤70 y | 165 | 63 (38.2) | 1.48 (0.77‐2.87) | .238 | ||
| >70 y | 46 | 22 (47.8) | ||||
| Preoperative PVE | ||||||
| No PVE | 198 | 79 (39.9) | 1.29 (0.42‐3.99) | .656 | ||
| PVE | 13 | 6 (46.2) | ||||
| Preoperative chemotherapy | ||||||
| No | 122 | 49 (40.2) | 1.01 (0.58‐1.77) | .967 | ||
| Yes | 89 | 36 (40.4) | ||||
| Size of largest tumor | ||||||
| ≤100 mm | 170 | 73 (42.9) | 0.55 (0.26‐1.15) | .806 | ||
| >100 mm | 41 | 12 (29.3) | ||||
| No. of tumors | ||||||
| Solitary | 49 | 19 (38.8) | 1.09 (0.56‐2.09) | .806 | ||
| Multiple | 162 | 66 (40.7) | ||||
| Parenchymal liver damagea | ||||||
| No | 139 | 58 (41.7) | 0.84 (0.47‐1.50) | .553 | ||
| Yes | 72 | 27 (37.5) | ||||
| Intra‐ and postoperative variables | ||||||
| S1 resection | ||||||
| No | 186 | 71 (38.2) | 2.06 (0.89‐4.79) | .088 | ||
| Yes | 25 | 14 (56.0) | ||||
| S2/3 partial resection | ||||||
| No | 131 | 57 (43.5) | 0.70 (0.39‐1.24) | .221 | ||
| Yes | 80 | 28 (35.0) | ||||
| Other organ resection | ||||||
| No | 179 | 66 (36.9) | 2.50 (1.16‐5.39) | .017* | 2.27 (1.04‐4.95) | .040* |
| Yes | 32 | 19 (59.4) | ||||
| Vascular resectiona | ||||||
| No | 196 | 76 (38.8) | 2.37 (0.81‐6.92) | .106 | ||
| Yes | 15 | 9 (60.0) | ||||
| Pringle's maneuver | ||||||
| No | 74 | 30 (40.5) | 0.98 (0.55‐1.75) | .956 | ||
| Yes | 137 | 55 (40.1) | ||||
| Total hepatic vascular exclusion | ||||||
| No | 197 | 77 (39.1) | 2.08 (0.69‐6.22) | .183 | ||
| Yes | 14 | 8 (57.1) | ||||
| Allogenic red blood cell transfusion | ||||||
| No | 167 | 60 (35.9) | 2.35 (1.20‐4.61) | .012* | 2.16 (1.09‐4.30) | .028* |
| Yes | 44 | 25 (56.8) | ||||
| Fresh frozen plasma transfusion | ||||||
| No | 144 | 57 (39.6) | 1.10 (0.61‐1.98) | .761 | ||
| Yes | 67 | 28 (41.8) | ||||
PVE, portal vein embolization; S1, caudate lobe; S2/3, left lateral section.
Including steatosis, fibrosis, and sinusoidal obstruction syndrome.
Including resections of portal vein, hepatic artery, and inferior vena cava.
P < .05.
Table 3.
Univariate and multivariate analyses of variables predicting disease‐free survival after right trisectionectomy
| Variables | Total n = 195a | 3‐y DFS (%) | MST (months) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|---|
| P‐value | RR (95% CI) | P‐value | |||||
| Preoperative variables | |||||||
| Gender | |||||||
| Female | 80 | 25.1 | 13.0 | .561 | |||
| Male | 115 | 27.6 | 15.4 | ||||
| Age | |||||||
| ≤70 y | 157 | 26.1 | 13.1 | .348 | |||
| >70 y | 38 | 28.2 | 14.6 | ||||
| Preoperative PVE | |||||||
| No PVE | 182 | 28.0 | 13.9 | .030* | 1.22 (0.59‐2.51) | .589 | |
| PVE | 13 | 0.0 | 7.5 | ||||
| Preoperative chemotherapy | |||||||
| No | 111 | 31.4 | 18.5 | .029* | 1.23 (0.87‐1.74) | .246 | |
| Yes | 84 | 19.6 | 11.0 | ||||
| Neoadjuvant chemotherapy | |||||||
| No | 183 | 30.0 | 17.6 | .076 | |||
| Yes | 12 | 21.6 | 13.8 | ||||
| Size of largest tumor | |||||||
| ≤100 mm | 158 | 25.9 | 13.3 | .436 | |||
| >100 mm | 37 | 28.9 | 18.9 | ||||
| No. of tumors | |||||||
| Solitary | 42 | 50.5 | 37.0 | <.001* | 1.99 (1.24‐3.20) | .005* | |
| Multiple | 153 | 19.9 | 12.4 | ||||
| Type of liver metastasis | |||||||
| Synchronous | 107 | 23.0 | 13.3 | .436 | |||
| Metachronous | 88 | 30.3 | 13.1 | ||||
| Intra‐ and postoperative variables | |||||||
| S1 resection | |||||||
| No | 174 | 28.3 | 14.6 | .033* | 1.05 (0.59‐1.85) | .881 | |
| Yes | 21 | 11.0 | 7.4 | ||||
| S2/3 partial resection | |||||||
| No | 119 | 33.5 | 22.6 | <.001* | 1.40 (0.98‐1.99) | .065 | |
| Yes | 76 | 15.3 | 10.5 | ||||
| Extra bile duct resection | |||||||
| No | 185 | 26.3 | 13.3 | .569 | |||
| Yes | 10 | 30.0 | 9.9 | ||||
| Lymphadenectomy | |||||||
| No | 182 | 24.9 | 13.3 | .448 | |||
| Yes | 13 | 46.2 | 12.1 | ||||
| Other organ resection | |||||||
| No | 167 | 28.6 | 15.4 | .015* | 134 (0.84‐2.12) | .218 | |
| Yes | 28 | 13.6 | 6.9 | ||||
| Portal vein resection | |||||||
| No | 189 | 26.6 | 13.6 | .278 | |||
| Yes | 6 | 20.8 | 9.9 | ||||
| Hepatic artery resection | |||||||
| No | 193 | 26.2 | 13.3 | .855 | |||
| Yes | 2 | 0.5 | 9.9 | ||||
| IVC resection | |||||||
| No | 187 | 26.8 | 13.6 | .257 | |||
| Yes | 8 | 18.8 | 9.9 | ||||
| Pringle's maneuver | |||||||
| No | 66 | 29.3 | 16.7 | .401 | |||
| Yes | 129 | 25.1 | 13.1 | ||||
| Total hepatic vascular exclusion | |||||||
| No | 185 | 27.8 | 14.6 | .001* | 3.13 (1.46‐6.73) | .003* | |
| Yes | 10 | 0.0 | 6.3 | ||||
| Allogenic red blood cell transfusion | |||||||
| No | 159 | 26.5 | 14.7 | .575 | |||
| Yes | 36 | 26.3 | 12.4 | ||||
| Fresh frozen plasma transfusion | |||||||
| No | 134 | 23.7 | 13.0 | .376 | |||
| Yes | 61 | 31.8 | 14.5 | ||||
| Postoperative complications | |||||||
| No | 126 | 26.9 | 13.3 | .556 | |||
| Yes | 69 | 25.8 | 13.6 | ||||
| Histological variables | |||||||
| Tumor histological grade | |||||||
| Well differentiated | 17 | 25.5 | 21.6 | .970 | |||
| Moderate/poorly differentiated | 155 | 28.8 | 13.3 | ||||
| Margin status | |||||||
| R0 | 114 | 34.2 | 21.6 | <.001* | 1.60 (1.11‐2.29) | .012* | |
| R1 | 81 | 15.2 | 7.8 | ||||
| Hepatic parenchymal histology | |||||||
| Steatosis | |||||||
| No | 141 | 26.0 | 13.9 | .859 | |||
| Yes | 54 | 28.4 | 11.6 | ||||
| Fibrosis | |||||||
| No | 189 | 26.1 | 13.3 | .687 | |||
| Yes | 6 | 44.4 | 24.4 | ||||
| Sinusoidal obstruction syndrome | |||||||
| No | 177 | 27.4 | 14.5 | .224 | |||
| Yes | 18 | 18.1 | 7.8 | ||||
CI, confidence interval; DFS, disease‐free survival; IVC, inferior vena cava; MST, median survival time; PVE, portal vein embolization; RR, risk ratio; S1, caudate lobe; S2/3, left lateral section.
Excluding 16 patients who died within 90 d after operation.
P < .05.
Table 4.
Univariate and multivariate analyses of variables predicting disease‐specific survival after right trisectionectomy
| Variables | Total n = 195a | 5‐y DSS (%) | MST (months) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|---|
| P‐value | RR (95% CI) | P‐value | |||||
| Preoperative variables | |||||||
| Gender | |||||||
| Female | 80 | 37.5 | 36.6 | .999 | |||
| Male | 115 | 38.1 | 41.7 | ||||
| Age | |||||||
| ≤70 y | 157 | 39.0 | 39.9 | .380 | |||
| >70 y | 38 | 31.5 | 38.1 | ||||
| Preoperative PVE | |||||||
| No PVE | 182 | 39.1 | 40.2 | .009* | 1.74 (0.76‐3.97) | .190 | |
| PVE | 13 | 0.0 | 24.6 | ||||
| Preoperative chemotherapy | |||||||
| No | 111 | 44.0 | 43.9 | .043* | 1.20 (0.83‐1.74) | .338 | |
| Yes | 84 | 27.5 | 36.7 | ||||
| Neoadjuvant chemotherapy | |||||||
| No | 183 | 38.4 | 41.9 | .065 | |||
| Yes | 12 | 32.0 | 35.0 | ||||
| Size of largest tumor | |||||||
| ≤100 mm | 158 | 36.2 | 39.1 | .231 | |||
| >100 mm | 37 | 42.9 | 43.8 | ||||
| No. of tumor | |||||||
| Solitary | 42 | 64.6 | 204.2 | .001* | 2.95 (1.68‐5.20) | <.001* | |
| Multiple | 153 | 30.0 | 32.8 | ||||
| Type of liver metastasis | |||||||
| Synchronous | 107 | 36.7 | 39.9 | .678 | |||
| Metachronous | 88 | 38.9 | 39.1 | ||||
| Intra‐ and postoperative variables | |||||||
| S1 resection | |||||||
| No | 174 | 39.0 | 40.2 | .042* | 1.21 (0.64‐2.29) | .557 | |
| Yes | 21 | 28.1 | 26.4 | ||||
| S2/3 partial resection | |||||||
| No | 119 | 42.1 | 43.8 | .023* | 0.98 (0.67‐1.42) | .905 | |
| Yes | 76 | 30.8 | 31.8 | ||||
| Extra bile duct resection | |||||||
| No | 185 | 38.3 | 39.7 | .702 | |||
| Yes | 10 | 18.8 | 28.8 | ||||
| Lymphadenectomy | |||||||
| No | 182 | 35.9 | 39.1 | .222 | |||
| Yes | 13 | 66.7 | 79.8 | ||||
| Other organ resection | |||||||
| No | 167 | 38.0 | 39.7 | .344 | |||
| Yes | 28 | 38.8 | 48.6 | ||||
| Portal vein resection | |||||||
| No | 189 | 39.0 | 39.6 | .195 | |||
| Yes | 6 | 0.0 | 39.7 | ||||
| Hepatic artery resection | |||||||
| No | 193 | 37.6 | 39.6 | .456 | |||
| Yes | 2 | 50.0 | 48.6 | ||||
| IVC resection | |||||||
| No | 187 | 38.5 | 39.9 | .111 | |||
| Yes | 8 | 17.5 | 39.7 | ||||
| Pringle's maneuver | |||||||
| No | 66 | 36.6 | 37.8 | .717 | |||
| Yes | 129 | 38.1 | 40.0 | ||||
| Total hepatic vascular exclusion | |||||||
| No | 185 | 39.1 | 40.0 | .009* | 3.77 (1.73‐8.20) | .001* | |
| Yes | 10 | 11.3 | 16.7 | ||||
| Allogenic red blood cell transfusion | |||||||
| No | 159 | 37.2 | 39.7 | .581 | |||
| Yes | 36 | 39.5 | 38.5 | ||||
| Fresh frozen plasma transfusion | |||||||
| No | 134 | 36.0 | 37.8 | .651 | |||
| Yes | 61 | 41.1 | 42.7 | ||||
| Postoperative complications | |||||||
| No | 126 | 37.1 | 39.1 | .990 | |||
| Yes | 69 | 39.2 | 40.0 | ||||
| Histological variables | |||||||
| Tumor histological grade | |||||||
| Well differentiated | 17 | 37.6 | 39.7 | .635 | |||
| Moderate/poorly differentiated | 155 | 40.3 | 41.5 | ||||
| Margin status | |||||||
| R0 | 114 | 47.7 | 50.7 | <.001* | 1.92 (1.31‐2.81) | .001* | |
| R1 | 81 | 22.9 | 20.8 | ||||
| Hepatic parenchymal histology | |||||||
| Steatosis | |||||||
| No | 141 | 36 | 38.5 | .641 | |||
| Yes | 54 | 42.6 | 43.9 | ||||
| Fibrosis | |||||||
| No | 189 | 37.7 | 39.6 | .949 | |||
| Yes | 6 | 40 | 58.3 | ||||
| Sinusoidal obstruction syndrome | |||||||
| No | 177 | 38.6 | 40 | .339 | |||
| Yes | 18 | 27.6 | 36.7 | ||||
CI, confidence interval; DSS, disease‐specific survival; IVC, inferior vena cava; MST, median survival time; PVE, portal vein embolization; RR, risk ratio; S1, caudate lobe; S2/3, left lateral section.
Excluding 16 patients who died within 90 d after operation.
P < .05.
3.4. Changes in outcomes over time and redo‐surgery for recurrence
To evaluate the impact of the learning curve and changes over the study period, patients were divided into three operative experience periods: first (n = 70; 33.2%), second (n = 70; 33.2%), and third (n = 71; 33.6%) period as described in Table 5. Median DFS was 33.8, 26.8 and 25.5 months from the first to the third period, P = .774. Frequency of further surgery for recurrent disease after RT increased steadily over time. Of the 211 patients who underwent RHT, 152 patients (72.0%) had disease recurrence, of whom 38 (25.0%) were eligible for further surgery. Eleven patients (7.2%) underwent a second repeat surgery, six (3.9%) patients had a third surgery and one (0.7%) patient a fourth repeat surgery with curative intent. Median interval from RHT to further surgery was 13.3 months; there was a median of 38.8 months to the second, 43.6 to the third, and 129.6 to the fourth surgery. Mortality after further surgery was zero. One or more redo liver resections were carried out in 31 patients (20.4%). One or more pulmonary surgeries including radiofrequency ablation were done in seven patients (4.6%). Two patients (1.3%) underwent other surgery for recurrent lesions (para‐aortic lymph node extirpation and pancreaticoduodenectomy). Of the 152 patients with recurrence, 114 patients (75%) had palliative chemotherapy alone. Five‐and 10‐year DSS were 58.1% and 25.8%, respectively, with a median DSS time of 70.5 months for the patients who underwent further surgery for recurrence and 15.6% and 2.7%, respectively, with a median DSS time of 26.4 months for those who had palliative treatment only (P < .001) (Figure 1).
Table 5.
Changes in pre‐ and intraoperative management and postoperative outcomes according to the experience period
| Experience period, n (%) | P‐value | |||
|---|---|---|---|---|
| 1st period (n = 70) | 2nd period (n = 70) | 3rd period (n = 71) | ||
| Age, years (range) | 61 (36‐80) | 61.5 (42‐84) | 63 (25‐85) | .402 |
| Preoperative chemotherapy | 18 (25.7) | 23 (32.9) | 48 (67.6) | <.001* |
| Preoperative PVE | 0 (0) | 0 (0) | 13 (18.3) | <.001* |
| Parenchymal liver damage | 7 (10.0) | 16 (22.9) | 49 (69.0) | <.001* |
| Steatosis | 5 (7.1) | 14 (20.0) | 38 (53.5) | <.001* |
| Fibrosis | 2 (2.9) | 3 (4.3) | 1 (1.4) | .590 |
| Sinusoidal obstruction syndrome | 0 (0) | 0 (0) | 18 (25.4) | <.001* |
| S1 resection | 4 (5.7) | 7 (10.0) | 14 (19.7) | .031* |
| S2/3 partial resection | 19 (27.1) | 30 (42.9) | 31 (43.7) | .075 |
| Vascular resectiona | 5 (7.1) | 3 (4.3) | 7 (9.9) | .436 |
| Pringle's maneuver | 33 (47.1) | 43 (61.4) | 61 (85.9) | <.001* |
| Total hepatic vascular exclusion | 9 (12.9) | 3 (4.3) | 2 (2.8) | .023* |
| Allogenic red blood cell transfusion | 28 (40.0) | 12 (17.1) | 4 (5.6) | <.001* |
| Fresh frozen plasma transfusion | 47 (67.1) | 30 (42.9) | 8 (11.3) | <.001* |
| Morbidity | 28 (40.0) | 33 (47.1) | 24 (33.8) | .271 |
| Median hospital stay, days (range)b | 11 (6‐34) | 11 (4‐139) | 9 (5‐71) | .119 |
| 90‐d mortality | 9 (12.9) | 5 (7.1) | 2 (2.8) | .078 |
| Further surgery for recurrencec | 11 (21.6) | 12 (24.0) | 15 (29.4) | .645 |
PVE, portal vein embolization; S1, caudate lobe; S2/3, left lateral section.
Including resections of portal vein, hepatic artery, and inferior vena cava.
Excluding 15 patients who died in hospital.
51, 50, and 51 patients with recurrence in the 1st, 2nd, and 3rd periods, respectively.
P < .05.
Figure 1.
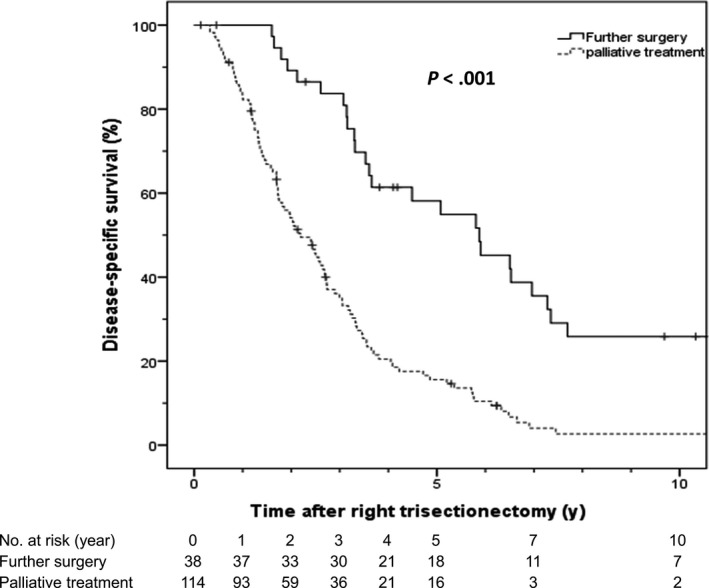
Disease‐specific survival following further surgery compared to palliative chemotherapy alone
4. DISCUSSION
Several centers worldwide have reported their experience with RHT or LHT and extensions of RHT for a variety of indications in HPB malignancies. However, the existing evidence on this topic is scarce and limited to small numbers. Only a few studies for RHT and LHT have reported their experience in larger cohorts.5, 7, 9, 13, 14, 26, 34, 35, 36 This limited evidence might reflect the changing role of these demanding and extensive resections in daily HPB practice, with decreasing numbers being carried out today. This must be at least in part due to promising results for newly implemented surgical strategies such as TSH and ALPPS, along with oncological advances with better understanding of tumor biology resulting in increasing success with neoadjuvant treatment.
Extended resections have been associated with high morbidity and mortality rates.37 In 1988, Iwatsuki and Starzl reported their experience showing a mortality rate of 6.3% following right trisectionectomies in their series.37
In our original report of 275 patients undergoing trisectionectomies including various HPB malignancies, postoperative morbidity was 41%.26 Thirty‐day and 90‐day mortality rates were 7% and 8%, respectively.26 In this up‐to‐date study, morbidity among all RHT trisectionectomies carried out was 40.3%, and 30‐day and 90‐day mortality rates were 7.1% and 7.6%, respectively, following this procedure. In a separate analysis assessing morbidity and mortality rates over time, we have been able to show a tendency for reduced morbidity and mortality. These results have been confirmed by other groups.36 In their series of RHT, Matsumoto et al showed a morbidity rate of 27% and a mortality rate of 0%.36 These results seem to be comparable with outcomes following modern approaches such as TSH or ALPPS.24
In extensive liver resections, the size of FLR may have a crucial impact on postoperative morbidity and mortality. In general, 3%‐5% of patients may develop liver failure following liver resection. In the present study, the incidence of transient liver failure was 12% (mainly grade A or B according to the International Study Group of Liver Surgery) and reflects the magnitude of surgery. Controversy exists about the amount of liver volume essential to prevent liver failure following these operations. After RHT, FLR is variable but approximately 15%‐30% of the total liver volume is preserved. Recent studies have shown that FLR of less than 25%‐30% is predictive of hepatic dysfunction.38 Therefore, the use of PVE before trisectionectomy has been advocated to decrease postoperative morbidity and mortality and make these operations safer.39 When assessing PVE in the preoperative setting of right trisectionectomies, several studies have shown the importance of embolizing segment 4 to achieve sufficient hypertrophy.40, 41 Indeed, this has become part of our routine for patients with CRLM after chemotherapy before RHT and, in recent years, we have not experienced any postoperative mortality.
In the present study, multivariate analysis identified additional organ resection at the time of RHT and perioperative ARBC transfusion as independent predictors of postoperative morbidity. In this series, concomitant organ resection was carried out in 32 patients (15.2%) of the cohort. The diaphragm or large bowel was resected most frequently. The inferior outcome following multi‐organ resection most likely reflects a poor and aggressive tumor biology as well as advanced tumor stage.
In other studies, intraoperative blood loss and concomitant blood transfusion were identified as independent risk factors influencing morbidity, mortality and DSS.9, 42, 43, 44 This measurement indirectly displays the extent and quality of the surgery itself. A median of 4 units 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 of red blood cells was transfused. Nearly 80% of patients did not require perioperative transfusion and there was a consistent decline in the need for transfusion over time. This may reflect improved surgical and anesthetic techniques as well as more conservative transfusion over time with higher thresholds for transfusion.
Our study further identified multiple tumors and R1 resections as independent predictors for recurrence and poor survival following liver resection for CRLM.45, 46, 47, 48, 49, 50 This corresponds to results of other studies.51, 52, 53 Sasaki et al clearly identified tumor size and number of CRLM as prognostic markers predicting outcome following resection of CRLM.54 Furthermore, our study identified TVE as an independent risk factor for poor survival. This might be due to advanced tumor stage when TVE was applied with invasion to the hepatocaval confluence or IVC. The radicality of surgery required, is associated with an increased potential for postoperative complications, this might be due to the fact that this type of surgery can potentially cause significant hemodynamic instability.55, 56, 57
Furthermore, in animal studies, TVE has been clearly linked to accelerated growth of hepatic micrometastases.50, 58 Indeed, nine out of 14 (64.3%) patients who required TVE had an R1 resection. In a separate analysis, a decrease of TVE over time was noted, again showing the increased experience with a higher caseload.
Repeat hepatic resection is technically challenging, with longer operative time than the initial surgery because of adhesions, altered anatomy, and fragile liver parenchyma as a result of chemotherapy, and it has rarely been reported following RHT.15, 59, 60, 61, 62, 63 However, in this series, repeat resection showed better 5‐ and 10‐year DSS of 58.1% and 25.8%, respectively, compared to DSS of 15.6% and 2.7% in patients undergoing palliative chemotherapy only. Furthermore, there was no postoperative mortality among patients who underwent repeat resections and none developed liver failure in the further postoperative course.
A limitation of the present study is the incomplete data on chemotherapy. As a result of this limitation, a detailed analysis of the role of chemotherapy in this treatment algorithm was not possible. Only a relatively small proportion of patients received neoadjuvant therapy for downsizing, but the proportion in whom downsizing strategies failed was not captured in the data set.
5. CONCLUSION
Left hepatic trisectionectomy and RHT are technically demanding liver resections with a high risk for perioperative morbidity and, in the past, also mortality. Our data show these risks are reducing with experience, better patient selection, and the more liberal use of PVE. LHT and RHT remain relevant for many situations but innovation in surgery and neoadjuvant treatments inevitably mean that the role of these challenging operations is decreasing.
DISCLOSURE
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Kron P, Kimura N, Farid S, Lodge JPA. Current role of trisectionectomy for hepatopancreatobiliary malignancies. Ann Gastroenterol Surg. 2019;3:606–619. 10.1002/ags3.12292
REFERENCES
- 1. Starzl TE, Iwatsuki S, Shaw BW Jr, et al. Left hepatic trisegmentectomy. Surg Gynecol Obstet. 1982;155(1):21–7. [PMC free article] [PubMed] [Google Scholar]
- 2. Blumgart LH, Baer HU, Czerniak A, Zimmermann A, Dennison AR. Extended left hepatectomy: technical aspects of an evolving procedure. Br J Surg. 1993;80(7):903–6. [DOI] [PubMed] [Google Scholar]
- 3. Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333–39. HPB (Oxford). 2002;4(2):99–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishio H, Hidalgo E, Hamady ZZ, et al. Left hepatic trisectionectomy for hepatobiliary malignancy: results and an appraisal of its current role. Ann Surg. 2005;242(2):267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lang H, Sotiropoulos GC, Brokalaki EI, et al. Left hepatic trisectionectomy for hepatobiliary malignancies. J Am Coll Surg. 2006;203(3):311–21. [DOI] [PubMed] [Google Scholar]
- 6. Natsume S, Ebata T, Yokoyama Y, et al. Clinical significance of left trisectionectomy for perihilar cholangiocarcinoma: an appraisal and comparison with left hepatectomy. Ann Surg. 2012;255(4):754–62. [DOI] [PubMed] [Google Scholar]
- 7. Esaki M, Shimada K, Nara S, et al. Left hepatic trisectionectomy for advanced perihilar cholangiocarcinoma. Br J Surg. 2013;100(6):801–7. [DOI] [PubMed] [Google Scholar]
- 8. Wicherts DA, de Haas RJ, Andreani P, et al. Short‐ and long‐term results of extended left hepatectomy for colorectal metastases. HPB (Oxford). 2011;13(8):536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farid SG, White A, Khan N, Toogood GJ, Prasad KR, Lodge JP. Clinical outcomes of left hepatic trisectionectomy for hepatobiliary malignancy. Br J Surg. 2016;103(3):249–56. [DOI] [PubMed] [Google Scholar]
- 10. Lortat‐Jacob JL, Robert HG, Henry C. Excision of the right lobe of the liver for a malignant secondary tumor. Arch Mal Appar Dig Mal Nutr. 1952;41(6):662–7. [PubMed] [Google Scholar]
- 11. Strasberg SM, Phillips C. Use and dissemination of the Brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257(3):377–82. [DOI] [PubMed] [Google Scholar]
- 12. Rui JA, Wang SB, Chen SG, Zhou L. Right trisectionectomy for primary liver cancer. World J Gastroenterol. 2003;9(4):706–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paik KY, Choi DW, Chung JC, Kang KT, Kim SB. Improved survival following right trisectionectomy with caudate lobectomy without operative mortality: surgical treatment for hilar cholangiocarcinoma. J Gastrointest Surg. 2008;12(7):1268–74. [DOI] [PubMed] [Google Scholar]
- 14. Lodge JP, Menon KV, Fenwick SW, Prasad KR, Toogood GJ. In‐contiguity and non‐anatomical extension of right hepatic trisectionectomy for liver metastases. Br J Surg. 2005;92(3):340–7. [DOI] [PubMed] [Google Scholar]
- 15. Ziff O, Rajput I, Adair R, Toogood GJ, Prasad KR, Lodge JP. Repeat liver resection after a hepatic or extended hepatic trisectionectomy for colorectal liver metastasis. HPB (Oxford). 2014;16(3):212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki T, Ebata T, Yokoyama Y, et al. Left trisectionectomy combined with resection of the right hepatic vein and inferior vena cava after right hepatic vein embolization for advanced intrahepatic cholangiocarcinoma. Surg Case Rep. 2019;5(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartsch F, Huber T, Lang H. Extended left hemihepatectomy with reconstruction of the right liver vein in a patient with an intrahepatic cholangiocellular carcinoma. Zentralbl Chir. 2016;141(6):604–6. [DOI] [PubMed] [Google Scholar]
- 18. Aoki Y, Suzuki T, Kato A, et al. A case of curative resection after downsizing chemotherapy in initially unresectable locally advanced intrahepatic cholangiocarcinoma. Gan To Kagaku Ryoho. 2014;41(12):1509–11. [PubMed] [Google Scholar]
- 19. Tran TB, Bal CK, Schaberg K, Longacre TA, Chatrath BS, Poultsides GA. Locally advanced intrahepatic cholangiocarcinoma: complete pathologic response to neoadjuvant chemotherapy followed by left hepatic trisectionectomy and caudate lobectomy. Dig Dis Sci. 2015;60(11):3226–9. [DOI] [PubMed] [Google Scholar]
- 20. Adam R, Miller R, Pitombo M, et al. Two‐stage hepatectomy approach for initially unresectable colorectal hepatic metastases. Surg Oncol Clin N Am. 2007;16(3):525–36. [DOI] [PubMed] [Google Scholar]
- 21. Linecker M, Kambakamba P, Reiner CS, et al. How much liver needs to be transected in ALPPS? A translational study investigating the concept of less invasiveness. Surgery. 2017;161(2):453–64. [DOI] [PubMed] [Google Scholar]
- 22. Langiewicz M, Schlegel A, Saponara E, et al. Hedgehog pathway mediates early acceleration of liver regeneration induced by a novel two‐staged hepatectomy in mice. J Hepatol. 2017;66(3):560–70. [DOI] [PubMed] [Google Scholar]
- 23. de Santibanes E, Clavien PA. Playing Play‐Doh to prevent postoperative liver failure: the "ALPPS" approach. Ann Surg. 2012;255(3):415–7. [DOI] [PubMed] [Google Scholar]
- 24. Sandstrom P, Rosok BI, Sparrelid E, et al. ALPPS improves resectability compared with conventional two‐stage hepatectomy in patients with advanced colorectal liver metastasis: results from a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg. 2018;267(5):833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agha RA, Borrelli MR, Farwana R, et al. The PROCESS 2018 statement: updating consensus Preferred Reporting Of CasE Series in Surgery (PROCESS) guidelines. Int J Surg. 2018;60:279–82. [DOI] [PubMed] [Google Scholar]
- 26. Halazun KJ, Al‐Mukhtar A, Aldouri A, et al. Right hepatic trisectionectomy for hepatobiliary diseases: results and an appraisal of its current role. Ann Surg. 2007;246(6):1065–74. [DOI] [PubMed] [Google Scholar]
- 27. Lodge JP, Dasgupta D, Prasad KR, et al. Emergency subtotal hepatectomy: a new concept for acetaminophen‐induced acute liver failure: temporary hepatic support by auxiliary orthotopic liver transplantation enables long‐term success. Ann Surg. 2008;247(2):238–49. [DOI] [PubMed] [Google Scholar]
- 28. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713–24. [DOI] [PubMed] [Google Scholar]
- 30. Hamady ZZ, Lodge JP, Welsh FK, et al. One‐millimeter cancer‐free margin is curative for colorectal liver metastases: a propensity score case‐match approach. Ann Surg. 2014;259(3):543–8. [DOI] [PubMed] [Google Scholar]
- 31. Hamady ZZ, Rees M, Welsh FK, et al. Fatty liver disease as a predictor of local recurrence following resection of colorectal liver metastases. Br J Surg. 2013;100(6):820–6. [DOI] [PubMed] [Google Scholar]
- 32. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–93. [DOI] [PubMed] [Google Scholar]
- 33. Rubbia‐Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin‐based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15(3):460–6. [DOI] [PubMed] [Google Scholar]
- 34. Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250(4):540–8. [DOI] [PubMed] [Google Scholar]
- 35. Neal CP, Mann CD, Pointen E, et al. Influence of hepatic parenchymal histology on outcome following right hepatic trisectionectomy. J Gastrointest Surg. 2012;16(11):2064–73. [DOI] [PubMed] [Google Scholar]
- 36. Matsumoto N, Ebata T, Yokoyama Y, et al. Role of anatomical right hepatic trisectionectomy for perihilar cholangiocarcinoma. Br J Surg. 2014;101(3):261–8. [DOI] [PubMed] [Google Scholar]
- 37. Iwatsuki S, Starzl TE. Personal experience with 411 hepatic resections. Ann Surg. 1988;208(4):421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127(5):512–9. [DOI] [PubMed] [Google Scholar]
- 39. Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long‐term follow‐up. Ann Surg. 2006;243(3):364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hammond CJ, Ali S, Haq H, et al. Segment 2/3 hypertrophy is greater when right portal vein embolisation is extended to segment 4 in patients with colorectal liver metastases: a retrospective cohort study. Cardiovasc Intervent Radiol. 2019;42(4):552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mise Y, Aloia TA, Conrad C, Huang SY, Wallace MJ, Vauthey JN. Volume regeneration of segments 2 and 3 after right portal vein embolization in patients undergoing two‐stage hepatectomy. J Gastrointest Surg. 2015;19(1):133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Boer MT, Molenaar IQ, Porte RJ. Impact of blood loss on outcome after liver resection. Dig Surg. 2007;24(4):259–64. [DOI] [PubMed] [Google Scholar]
- 43. Kooby DA, Stockman J, Ben‐Porat L, et al. Influence of transfusions on perioperative and long‐term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237(6):860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dionigi G, Boni L, Rovera F, et al. Effect of perioperative blood transfusion on clinical outcomes in hepatic surgery for cancer. World J Gastroenterol. 2009;15(32):3976–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10‐year experience. Ann Surg Oncol. 2006;13(5):668–76. [DOI] [PubMed] [Google Scholar]
- 46. Hamady ZZ, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JP. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1cm rule. Eur J Surg Oncol. 2006;32(5):557–63. [DOI] [PubMed] [Google Scholar]
- 47. Finch RJ, Malik HZ, Hamady ZZ, et al. Effect of type of resection on outcome of hepatic resection for colorectal metastases. Br J Surg. 2007;94(10):1242–8. [DOI] [PubMed] [Google Scholar]
- 48. Malik HZ, Hamady ZZ, Adair R, et al. Prognostic influence of multiple hepatic metastases from colorectal cancer. Eur J Surg Oncol. 2007;33(4):468–73. [DOI] [PubMed] [Google Scholar]
- 49. Vigano L, Capussotti L, Lapointe R, et al. Early, recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey‐based study of 6,025 patients. Ann Surg Oncol. 2014;21(4):1276–86. [DOI] [PubMed] [Google Scholar]
- 50. van der Bilt JD, Kranenburg O, Nijkamp MW, et al. Ischemia/reperfusion accelerates the outgrowth of hepatic micrometastases in a highly standardized murine model. Hepatology. 2005;42(1):165–75. [DOI] [PubMed] [Google Scholar]
- 51. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long‐term results. Ann Surg. 2000;231(4):487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189(3):291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sasaki K, Morioka D, Conci S, et al. The tumor burden score: a new, "Metro‐ticket" prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg. 2018;267(1):132–41. [DOI] [PubMed] [Google Scholar]
- 55. Hoekstra LT, van Trigt JD, Reiniers MJ, Busch OR, Gouma DJ, van Gulik TM. Vascular occlusion or not during liver resection: the continuing story. Dig Surg. 2012;29(1):35–42. [DOI] [PubMed] [Google Scholar]
- 56. Castro e Silva O, Mente ED, Sankarankutty AK, et al. Biochemical liver function after partial hepatic resection with or without partial hepatic vascular exclusion. Acta Cir Bras. 2011;26(Suppl 2):120–4. [DOI] [PubMed] [Google Scholar]
- 57. Tong Y, Yang JM, Lai EC, Lau WY, Wu MC. Complete hemihepatic vascular exclusion versus Pringle maneuver for liver resection: a comparative study. Hepatogastroenterology. 2011;58(109):1307–11. [DOI] [PubMed] [Google Scholar]
- 58. Nicoud IB, Jones CM, Pierce JM, et al. Warm hepatic ischemia‐reperfusion promotes growth of colorectal carcinoma micrometastases in mouse liver via matrix metalloproteinase‐9 induction. Cancer Res. 2007;67(6):2720–8. [DOI] [PubMed] [Google Scholar]
- 59. Elias D, Lasser P, Hoang JM, et al. Repeat hepatectomy for cancer. Br J Surg. 1993;80(12):1557–62. [DOI] [PubMed] [Google Scholar]
- 60. Nishio H, Hamady ZZ, Malik HZ, et al. Outcome following repeat liver resection for colorectal liver metastases. Eur J Surg Oncol. 2007;33(6):729–34. [DOI] [PubMed] [Google Scholar]
- 61. de Jong MC, Mayo SC, Pulitano C, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi‐institutional analysis. J Gastrointest Surg. 2009;13(12):2141–51. [DOI] [PubMed] [Google Scholar]
- 62. Thelen A, Jonas S, Benckert C, et al. Repeat liver resection for recurrent liver metastases from colorectal cancer. Eur J Surg Oncol. 2007;33(3):324–8. [DOI] [PubMed] [Google Scholar]
- 63. Yan TD, Sim J, Black D, Niu R, Morris DL. Systematic review on safety and efficacy of repeat hepatectomy for recurrent liver metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14(7):2069–77. [DOI] [PubMed] [Google Scholar]


