Abstract
Background
Kawasaki disease is an acute, febrile vasculitis of childhood that affects medium-sized arteries, predominantly the coronary arteries. It is a multisystem disease; therefore, it may present with non-cardiac findings of disease.
Case presentation
Here, we report the case of 7-year-old Turkish girl who presented with symptoms of fever, chest pain, and vomiting, who was diagnosed as having Kawasaki disease. We also present a literature review on pulmonary involvement due to Kawasaki disease.
Conclusion
Pediatricians should consider the diagnosis of Kawasaki disease in the presence of pneumonia and pleural effusion that is nonresponsive to antibiotic therapy. This will prevent delay in diagnosis and the adverse consequences of the disease.
Keywords: Kawasaki disease, Pleural effusion, Pulmonary involvement
Background
Kawasaki disease (KD) is one of the most common vasculitis disorders of childhood [1]. Although it is a multisystem disease that mainly affects the coronary arteries, it can, rarely, present with unusual system involvement of the pulmonary system, gastrointestinal tract, central nervous system, and genitourinary system [1]. Here, we report the case of a patient with KD who presented with an unusual form of pleural effusion. We also present a literature review on the subject.
Case presentation
A 7-year-old Turkish girl presented to a local hospital with fever, chest pain, and vomiting. At hospital admission, she was febrile with a respiratory rate of 50 per minute. On physical examination, auscultation of her lungs revealed diminished breath sounds of the lower lobe of her left lung. An anteroposterior (AP) chest X-ray and chest ultrasonography showed a left lower lobar consolidation with minimal pleural effusion. She was hospitalized and sulbactam ampicillin (SAM), ceftriaxone, and clarithromycin were initiated. On the third day, her condition worsened with increasing pleural effusion (Fig. 1). Thoracentesis was performed. SAM and ceftriaxone treatments were discontinued and meropenem and vancomycin were started. A chest tube was inserted and 130 mL of pus was drained. Light’s criteria were positive for an exudative pleural effusion; a pleural fluid culture was sterile. After 4 days, the chest tube was removed. High fever persisted for 15 days despite broad spectrum antibiotics, and acute-phase reactants remained high; therefore, she was referred to our hospital for further evaluation.
Fig. 1.
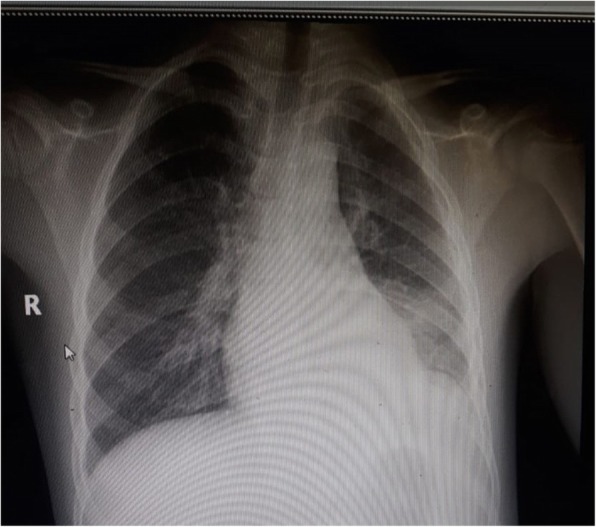
Chest X-ray of the patient showing left lower lobar consolidation with pleural effusion
She had a fever with a temperature of 38.1 °C, her respiratory rate was 48/minute, heart rate was 125/minute, blood pressure was 90/65 mm Hg, and oxygen saturation was 95%. A physical examination revealed non-purulent conjunctivitis in both eyes, perianal peeling, and periungual desquamation on her hand, fingers, and toes. All other findings in the physical examination were unremarkable. She had unilateral cervical lymphadenopathy and a rash on her extremities while in the other hospital. Her past medical history was unremarkable, as was her family history. Immunizations were up-to-date for her age.
On admission to our hospital, the laboratory findings were as follows: hemoglobin 10.2 g/dL, white blood cells 14,000/μL, and platelets 736,000/μL. C-reactive protein (CRP) was 4.26 mg/dL (normal, 0–0.8 mg/dL), the erythrocyte sedimentation rate (ESR) was 42 mm/hour (normal, 0–20 mm/hour), and the albumin, creatinine, aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transferase, blood urea nitrogen, calcium, sodium, chloride, and potassium levels were normal. Urine analysis was normal.
A chest X-ray was normal. Perivascular brightness and echogenicity of her right coronary artery was noted on transthoracic echocardiography (TTE). She was diagnosed as having KD based on the presence of fever, bilateral non-purulent conjunctivitis, cervical adenopathy, perianal peeling, periungual desquamation, elevated acute-phase reactants (ESR, CRP), thrombocytosis, and coronary artery involvement (CAI). Intravenous immunoglobulin (IVIG) (2 g/kg, infusion in 12 hours) and acetylsalicylic acid (60 mg/kg per day) were initiated. The fever resolved after IVIG infusion. At a 3-month follow-up visit, the acute-phase reactants and a TTE were normal. One year after the diagnosis, a TTE was normal and she was perfectly healthy.
Discussion and conclusion
The most important complication of KD is CAI, which leads to enlargement, aneurysm, ischemic heart disease, and sudden death [1]. The clinical course of KD is highly variable. There are no pathognomonic clinical or laboratory findings to help diagnose KD. The diagnosis of KD in this case was made using the criteria of the American Heart Association [1]. In the presence of at least 5 days of fever, if there are at least four of the five principal criteria (cervical adenopathy, bilateral non-purulent conjunctivitis, oropharyngeal mucosal changes, polymorphous rash, erythema of the palms or soles, and edema of the hands or feet) the patient is diagnosed as having KD [1].
KD may present with uncommon symptoms such as pneumonia, pleural effusion, diarrhea, vomiting, sterile pyuria, gallbladder hydrops, acute cholestatic hepatitis, arthritis, and aseptic meningitis [2–7]. Pulmonary system involvement of KD is very rare; KD can present as pneumonia, pulmonary nodules, bronchopneumonia, hydropneumothorax, and pleural effusion [6, 8, 9]. Singh et al. showed that 1.3% of patients had pulmonary involvement and pleural effusion was seen in 54.5% of these patients [6]. Ugi et al. reported the case of an adult patient who presented with pulmonary involvement, specifically bilateral massive pleural effusions [10]. Occasionally, pleural effusion may be associated with bacterial agents such as Mycoplasma pneumoniae and Streptococcus [11, 12]. Pulmonary symptoms are mostly initially treated with antibiotics. However, if fever and accompanying signs ensue, the diagnosis of KD should be considered. Patients with pulmonary involvement may be more likely to have CAI due to delays in diagnosing KD and administration of IVIG [12–17].
We performed a review of the literature using PubMed and the search terms: Kawasaki disease AND pulmonary involvement; OR Kawasaki disease AND pulmonary presentation; OR Kawasaki disease AND pleural effusion. The searches were limited to the English language and pediatric patients. Case series and single case reports involving pediatric patients with KD with pulmonary involvement were included. Inconsistencies were resolved through discussion with the author SO, who also reviewed the literature. The authors EAA and OA searched the literature and manually screened titles and abstracts for relevance. Inconsistencies were resolved through discussion with the author SO.
Figure 2 lists the schematic analyses of the systematic literature review. At first, 25 related articles were found, but nine articles were excluded because of duplication, non-English language, and adult age, which left 16 articles [6, 8, 11–24]. The characteristics of these patients are summarized in Tables 1, 2, and 3. Finally, 20 patients with pleural effusions due to KD were identified [6, 11–18, 20, 24]. Of the 20 reviewed patients, TTE results were available in nine patients and seven had CAI [6, 12–17, 24]. Eleven patients presented with respiratory symptoms such as cough, dyspnea, and tachypnea [6, 12–15, 20]. Only four patients [14, 17, 18, 24] had complete KD, 10 patients [6, 13, 15, 16, 20] had incomplete KD, and six patients’ [11, 12] presentations were not available. Although a definite infectious agent could be shown for two patients [18, 24], all of the patients received antibiotics except one [14]. Two patients [6, 17] received a second dose of IVIG, and five patients received a second dose of IVIG and corticosteroid treatment for KD [13–16, 18].
Fig. 2.
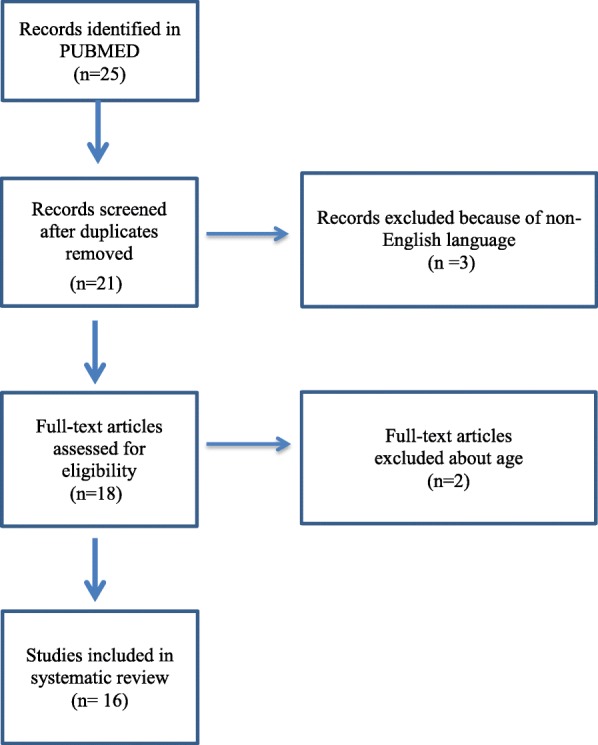
Study flowchart
Table 1.
Clinical symptoms and laboratory parameters of patients who had pulmonary involvement associated with Kawasaki disease
| Authors, year, reference number | Rash | Oral changes | Extremity changes | Red eyes | Adenitis | Other clinical symptoms | Hb (g/dL) | WBC (/mm3) | Plt (/mm3) | CRP (mg/dL) | ESR (mm/hour) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Singh et al., 2018, [6] | 5a | 1 a | 8a | 1 a | 0 a |
Perianal desquamation, 3 a; irritability, 1 a |
NA | 25,009b | 886,545b | 14.05c | 53.75 d |
| Alhammadi and Hendaus, 2013, [12] | NA | NA | NA | NA | NA | NA | NA | 24,000 | 600,000 | 10 | 65 |
| Lee et al., 2011, [11] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lee et al., 2010, [14] | Yes | Yes | Yes | Yes | Yes | Perianal desquamation | NA | 5500 | 178,000 | 2.8 | 21 |
| Falcini et al., 2009, [15] | No | No | No | Yes | No | Irritability | 9.5 | 21,800 | 710,000 | 27.8 | 99 |
| Elizabeth et al., 2007, [16] | No | Yes | Yes | No | Yes | Irritability | 10.2 | 12,800 | 550,000 | 7.7 | 86 |
| Yavuz et al., 2007, [17] | Yes | Yes | Yes | Yes | No | Non-pigmented keratic precipitates in both of the patient’s eyes, sterile pyuria | 10.4 | 32,800 | 734,000 | 21.8 | 90 |
| Sittiwangkul and Pongprot, 2004, [13] | Yes | No | Yes | Yes | No | Irritability | NA | 21,200 | 231,000 | 4.77 | 66 |
| de Magalhães et al., 2012, [21] | Yes | Yes | Yes | Yes | No | Induration at the BCG site, perianal desquamation | 6.5 | 25,000 | 905,000 | 34 | 120 |
| Hamada et al., 2005, [18] | Yes | Yes | Yes | No | Yes | Hepatomegaly | NA | 17,800 | NA | 13.9 | NA |
| D'Souza et al., 2006, [20] | No | No | Yes | No | Yes | Sterile pyuria | 9.8 | 56,800 | 690,000 | NA | 138 |
| de Maddi et al., 2009, [22] | |||||||||||
| Case 1 | No | Yes | No | Yes | Yes | No | 8.7 | 11,200 | 561,000 | 10.4 | 70 |
| Case 2 | No | No | No | No | No | No | 9 | 26,960 | 142,000 | 40.8 | 107 |
| Case 3 | Yes | Yes | No | Yes | No | Irritability, sterile pyuria | 11 | 18,500 | 1,087,000 | 2.95 | 50 |
| Freeman et al., 2003, [23] | |||||||||||
| Case 1 | Yes | Yes | No | Yes | No | Irritability | NA | NA | 1,120,000 | NA | NA |
| Case 2 | No | Yes | No | Yes | Yes | Torticollis | NA | NA | 1,102,000 | NA | 114 |
| Case 3 | Yes | No | No | No | Yes | Anorexia | NA | NA | 450,000 | 10.5 | NA |
| Kobayashi et al., 2006, [24] | |||||||||||
| Case 1 | Yes | Yes | No | Yes | Yes | 9.6 | 13,000 | 321,000 | 12.6 | 102 | |
| Case 2 | Yes | Yes | Yes | Yes | No | Induration at the BCG site | 11.6 | 18,800 | 314,000 | 7.6 | NA |
| Vaidya et al., 2017, [8] | Yes | Yes | Yes | No | No | 8.9 | 15,600 | 567,000 | 5.6 | 40 | |
| Akagi et al., 2017, [19] | |||||||||||
| Case 1 | Yes | Yes | Yes | No | No | NA | NA | NA | 4.26 | NA | |
| Case 2 | Yes | Yes | No | No | No | NA | NA | NA | 4.32 | NA | |
aNumber of the patients who had the symptom
b,c,dValues are expressed as mean for 11, 8, and 10 patients, respectively
BCG Bacille Calmette–Guérin, CRP C-reactive protein, ESR erythrocyte sedimentation rate, Hb hemoglobin, NA not available, Plt platelet, WBC white blood cell
Table 2.
Demographic parameters and clinical presentations of patients who had pulmonary involvement associated with Kawasaki disease
| Authors, year, reference number | Patients (n) | Sex | Age at onset of disease (months) | Initial symptoms | Fever duration (days) | Chest X-ray findings |
|---|---|---|---|---|---|---|
| Singh et al., 2018, [6] | 11 |
F, 6 a; M, 5 a |
30 b | Fever, cough, tachypnea | 14.1 b |
Consolidation,11 a; pleural effusion,6 a; empyema, 3 a; pneumothorax, 2 a |
| Alhammadi and Hendaus, 2013, [12] | 1 | F | 36 | Fever, cough, sore throat | 18 | Consolidation, pleural effusion |
| Lee et al., 2011, [11] | 54 | NA | NA | NA | NA |
Reticulonodular,17 a; opacification, 34 a; consolidation, 12 a; pleural effusion, 5 a diffuse interstitial, 5 a; atelectasis, 2 a; |
| Lee et al., 2010, [14] | 1 | M | 22 | Fever, cough, rhinorrhea | 6 | Infiltration, pleural effusion |
| Falcini et al., 2009, [15] | 1 | F | 30 | Fever, cough, | 12 | Pleural effusion |
| Elizabeth et al., 2007, [16] | 1 | F | 36 | Fever, gum bleeding | 21 | Pleural effusion |
| Yavuz et al., 2007, [17] | 1 | M | 11 | Fever, pharyngeal erythema, dyspnea | > 5 | Pleural effusion |
| Sittiwangkul and Pongprot, 2004, [13] | 1 | F | 11 | Fever, jaundice, diarrhea, dyspnea | 15 | Pleural effusion |
| de Magalhães et al., 2012, [21] | 1 | F | 3 | Fever | 10 | Infiltration |
| Hamada et al., 2005, [18] | 1 | F | 60 | Fever, abdominal pain, knee joint pain | 15 | Pleural effusion |
| D'Souza et al., 2006, [20] | 1 | M | 5 | Fever, diarrhea, dyspnea | 7 | Pleural effusion |
| Case 1 | 1 | M | 8 | Fever, sore throat | 8 | Consolidation |
| Case 2 | 1 | F | 11 | Febrile seizure, sore throat, cough | 14 | Consolidation |
| Case 3 | 1 | F | 23 | Fever, cough | 10 | Consolidation |
| Freeman et al., 2003, [23] | ||||||
| Case 1 | 1 | M | 4 | Fever, cough, rash | 21 | NA |
| Case 2 | 1 | M | 6 | Fever | 60 |
Normal; thorax CT, pulmonary nodule |
| Case 3 | 1 | NA | 5 | Fever cough, rash | 4 | Infiltration, multiple pulmonary nodules |
| Kobayashi et al., 2006, [24] | ||||||
| Case 1 | 1 | F | 24 | Fever, cracked lips, rash | 5 | Infiltration, pleural effusion |
| Case 2 | 1 | F | 24 | Fever, cough, nasal discharge | 4 | Atelectasis |
| Vaidya et al., 2017, [8] | 1 | F | 3 | Fever, rash, dyspnea | 32 | Hydropneumothorax, consolidation, pneumatoceles |
| Akagi et al., 2017, [19] | ||||||
| Case 1 | 1 | F | 4 | Fever, erythema of the lips, rash | NA |
NA; thorax MRI, bilateral multiple pulmonary nodules |
| Case 2 | 1 | F | 5 | Fever, erythema of the lips, rash | 9 |
Infiltration; thorax CT, bilateral pulmonary nodules |
aNumber of the patients who had noted findings
bValues are expressed as mean for 11 patients
CT computed tomography, F female, M male, MRI magnetic resonance imaging, NA not available
Table 3.
Treatment, coronary artery involvement, follow-up, and outcomes of patients who had pulmonary involvement associated with Kawasaki disease
| Authors, year, reference number | Infectious agent | Antibiotic treatment | CAI | Treatment | Follow-up and outcome |
|---|---|---|---|---|---|
| Singh et al., 2018, [6] | 2 a | 11 a | 3 a | 2 a, Second dose of IVIG | 9 a Normal, 2 a NA |
| Alhammadi and Hendaus, 2013, [12] | No | Yes | Yes | IVIG | Normal |
| Lee et al., 2011, [11] | NA | NA | NA | NA | NA |
| Lee et al., 2010, [14] | No | No | Yes | Second dose of IVIG and corticosteroid | Normal |
| Falcini et al., 2009, [15] | No | Yes | Yes | Second dose of IVIG and corticosteroid | Normal |
| Elizabeth et al., 2007, [16] | No | Yes | Yes | Second dose of IVIG and corticosteroid | Normal |
| Yavuz et al., 2007, [17] | No | Yes | Yes | Second dose of IVIG | Normal |
| Sittiwangkul and Pongprot, 2004, [13] | No | Yes | Yes | Second dose of IVIG and corticosteroid | Aneurysm persisted in 2 years |
| de Magalhães et al., 2012, [21] | No | Yes | Yes | Second dose of IVIG, corticosteroid, MTX, and ETN | Aneurysm decrease but persisted |
| Hamada et al., 2005, [18] | No | Yes | No | Second dose of IVIG and corticosteroid | Normal |
| D'Souza et al., 2006, [20] | No | Yes | No | IVIG | Normal |
| de Maddi et al., 2009, [22] | |||||
| Case 1 | No | Yes | No | IVIG | Normal |
| Case 2 | No | Yes | No | Not given IVIG | Normal |
| Case 3 | No | Yes | No | IVIG | Normal |
| Freeman et al., 2003, [23] | |||||
| Case 1 | No | Yes | Yes | IVIG | Death |
| Case 2 | NA | Yes | Yes | IVIG | Normal |
| Case 3 | No | Yes | Yes | IVIG | Normal |
| Kobayashi et al., 2006, [24] | |||||
| Case 1 | Yes | Yes | NA | IVIG | Normal |
| Case 2 | Yes | Yes | NA | IVIG | Normal |
| Vaidya et al., 2017, [8] | No | Yes | Yes | IVIG | NA |
| Akagi et al., 2017, [19] | |||||
| Case 1 | No | No | Yes | IVIG | Normal |
| Case 2 | No | Yes | Yes | IVIG | Normal |
aNumber of the patients
CAI coronary artery involvement, ETN etanercept, IVIG intravenous immunoglobulin, MTX methotrexate, NA not available
In this case, our patient initially had an exudative, noninfectious pleural effusion and no response to antibiotics. CAI was also noticed and IVIG was administered on the 15th day of fever. After IVIG treatment, our patient’s clinical and laboratory findings improved dramatically, and the fever and acute-phase reactants returned to normal. It remains unclear as to whether the KD was triggered by the infection of the pleural space or if the pulmonary finding was a feature of the inflammation of KD.
KD can affect various systems as well as the coronary arteries, and may present with an unusual clinical picture. The diagnosis of KD with atypical presentations may be difficult for pediatricians. Early diagnosis and treatment can prevent complications.
Acknowledgements
Not applicable.
Abbreviations
- AP
Anteroposterior
- CAI
Coronary artery involvement
- CRP
C-reactive protein
- ESR
Erythrocyte sedimentation rate
- IVIG
Intravenous immunoglobulin
- KD
Kawasaki disease
- SAM
Sulbactam ampicillin
- TTE
Transthoracic echocardiography
Authors’ contributions
EAA, OA, and SD drafted the initial manuscript. EAA and OA retrieved the pertinent literature. SD, YB, and SO contributed to the patient management. SO critically reviewed the manuscript. All authors have read and approved the final submitted manuscript.
Funding
No funding.
Availability of data and materials
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.
Ethics approval and consent to participate
No ethical committee approval is required for this case report.
Consent for publication
Written informed consent was obtained from the patient’s legal guardian(s) for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elif Arslanoglu Aydin, Email: arslanoglu0107@gmail.com.
Selcan Demir, Email: selcandemir@yahoo.com.
Orkun Aydin, Email: orkunaydin.89@gmail.com.
Yelda Bilginer, Email: yeldabilginer@yahoo.com.
Seza Ozen, Email: sezaozen@gmail.com.
References
- 1.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135(17):e927–ee99. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 2.Kaman A, Aydin-Teke T, Gayretli-Aydin ZG, Oz FN, Metin-Akcan O, Eris D, et al. Two cases of Kawasaki disease presented with acute febrile jaundice. Turk J Pediatr. 2017;59(1):84–86. doi: 10.24953/turkjped.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Du Z. A case of neonatal incomplete Kawasaki disease complicated with aseptic meningitis. Zhonghua Er Ke Za Zhi. 2014;52(1):63–64. [PubMed] [Google Scholar]
- 4.Sun Q, Zhang J, Yang Y. Gallbladder Hydrops Associated With Kawasaki Disease: A Case Report and Literature Review. Clin Pediatr (Phila) 2018;57(3):341–343. doi: 10.1177/0009922817696468. [DOI] [PubMed] [Google Scholar]
- 5.Saviour MJ, Hassan S. Kawasaki Disease Presenting with Bloody Diarrhea and Acute Renal Failure: First Case. Pediatr Rep. 2017;9(2):7163. doi: 10.4081/pr.2017.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Gupta A, Jindal AK, Gupta A, Suri D, Rawat A, et al. Pulmonary presentation of Kawasaki disease-A diagnostic challenge. Pediatr Pulmonol. 2018;53(1):103–107. doi: 10.1002/ppul.23885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilania RK, Bhattarai D, Singh S. Controversies in diagnosis and management of Kawasaki disease. World J Clin Pediatr. 2018;7(1):27–35. doi: 10.5409/wjcp.v7.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaidya PC, Narayanan K, Suri D, Rohit MK, Gupta A, Singh S, et al. Pulmonary presentation of Kawasaki disease: an unusual occurrence. Int J Rheum Dis. 2017;20(12):2227–2229. doi: 10.1111/1756-185X.12815. [DOI] [PubMed] [Google Scholar]
- 9.Leahy TR, Cohen E, Allen UD. Incomplete Kawasaki disease associated with complicated Streptococcus pyogenes pneumonia: A case report. Can J Infect Dis Med Microbiol. 2012;23(3):137–139. doi: 10.1155/2012/638357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ugi J, Lepper PM, Witschi M, Maier V, Geiser T, Ott SR. Nonresolving pneumonia and rash in an adult: pulmonary involvements in Kawasaki's disease. Eur Respir J. 2010;35(2):452–454. doi: 10.1183/09031936.00132309. [DOI] [PubMed] [Google Scholar]
- 11.Lee MN, Cha JH, Ahn HM, Yoo JH, Kim HS, Sohn S, et al. Mycoplasma pneumoniae infection in patients with Kawasaki disease. Korean J Pediatr. 2011;54(3):123–127. doi: 10.3345/kjp.2011.54.3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhammadi AH, Hendaus MA. Comorbidity of Kawasaki disease and group a streptococcal pleural effusion in a healthy child: a case report. Int J Gen Med. 2013;6:613–616. doi: 10.2147/IJGM.S49510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sittiwangkul R, Pongprot Y. Large pleural effusion: an unusual manifestation of Kawasaki disease. Clin Pediatr (Phila) 2004;43(4):389–391. doi: 10.1177/000992280404300412. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Jeon YH, Yang HJ, Pyun BY. Pleural effusion and disseminated intravascular coagulopathy: the rarely reported complications of kawasaki disease. Clin Pediatr (Phila) 2010;49(6):598–600. doi: 10.1177/0009922809356468. [DOI] [PubMed] [Google Scholar]
- 15.Falcini F, Vitale A, La Torre F, Conti G, Fede C, Calcagno G. Refractory pneumonia and high fever. Lancet. 2009;373(9677):1818. doi: 10.1016/S0140-6736(09)60694-2. [DOI] [PubMed] [Google Scholar]
- 16.Elizabeth KE, Ahamed MZ, Praveen KS. Atypical relapsing course of Kawasaki disease with hemorrhagic serous effusions and hepatic dysfunction. Indian Pediatr. 2007;44(10):785–787. [PubMed] [Google Scholar]
- 17.Yavuz T, Nisli K, Yilmaz C, Dindar A. Large pleural effusion necessitates chest tube drainage in a patient with Kawasaki disease. J Paediatr Child Health. 2007;43(3):191–192. doi: 10.1111/j.1440-1754.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamada H, Terai M, Honda T, Kohno Y. Marked pleural and pericardial effusion with elevated Vascular Endothelial Growth Factor production: an uncommon complication of Kawasaki disease. Pediatr Int. 2005;47(1):112–114. doi: 10.1111/j.1442-200x.2005.02015.x. [DOI] [PubMed] [Google Scholar]
- 19.Akagi K, Abe J, Tanaka K, Tomotaki S, Iki Y, Ueda K, et al. Kawasaki disease with pulmonary nodules and coronary artery involvement: a report of two cases and a review of the literature. Int J Rheum Dis. 2017;20(11):1862–1864. doi: 10.1111/1756-185X.12692. [DOI] [PubMed] [Google Scholar]
- 20.D'Souza S, Khubchandani RP, Shetty AK. Kawasaki disease presenting with hemorrhagic pleural effusion. J Trop Pediatr. 2006;52(4):299–301. doi: 10.1093/tropej/fmh076. [DOI] [PubMed] [Google Scholar]
- 21.de Magalhães CM, Alves NR, de Melo AV, Junior CA, Nomicronbrega YK, Gandolfi L, et al. Catastrophic Kawasaki disease unresponsive to IVIG in a 3-month-old infant: a diagnostic and therapeutic challenge. Pediatr Rheumatol Online J. 2012;10(1):28. doi: 10.1186/1546-0096-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Maddi F, Cinelli R, Rigante D, Mazzarella G, Siani P. Lung parenchymal consolidation as an uncommon presentation and cause of delayed diagnosis in atypical Kawasaki syndrome. Rheumatol Int. 2009;29(11):1373–1376. doi: 10.1007/s00296-008-0830-2. [DOI] [PubMed] [Google Scholar]
- 23.Freeman AF, Crawford SE, Finn LS, Lopez-Andreu JA, Ferrando-Monleon S, Perez-Tamarit D, et al. Inflammatory pulmonary nodules in Kawasaki disease. Pediatr Pulmonol. 2003;36(2):102–106. doi: 10.1002/ppul.10333. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y, Koike Y, Tokutomi T, Kuroki Y, Todoroki I. Case 2: fever, rash and pulmonary involvement. Diagnosis: Kawasaki disease. Acta Paediatr. 2006;95(9):1145–1148. doi: 10.1080/08035250600686953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.


