Abstract
Microcin C (McC) is a peptide-nucleotide antibiotic that inhibits aspartyl-tRNA synthetase. Here, we show that McC is a strong inducer of persistence in Escherichia coli. Persistence induced by McC is mediated by (p)ppGpp and requires chromosomally encoded toxin-antitoxin modules. McC-producing cells have increased persistence levels due to a combined effect of McC imported from the cultured medium and intracellularly synthesized antibiotic. McC-producing cells also induce persistence in sensitive cells during co-cultivation, underscoring complex interactions in bacterial communities where an antagonistic compound produced by one community member can benefit other members by increasing their ability to withstand antibiotics.
Keywords: microcin C, peptide-nucleotide antibiotic, persistence, tRNA-synthetase, (p)ppGpp
Graphical Abstract
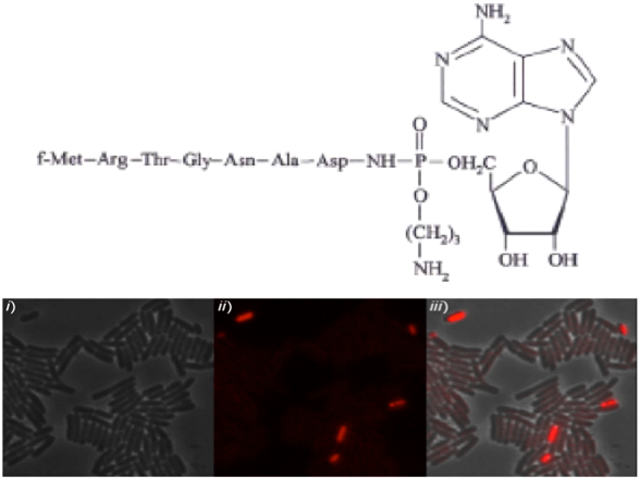
Microcin C is a peptide nucleotide antibiotic that inhibits protein synthesis. Cells that produce microcin C have increased levels or persistence, which prevents killing by other antibiotics. While inhibiting the growth of sensitive cells, microcin C also increases their level of persistence. In mixed producing-sensitive cells cultures, microcin C acts a “public good” compound benefiting the entire community.
INTRODUCTION
Since the pioneering 1944 publication by Bigger [1] it has been recognized that bacteria in growing cultures cannot be completely killed by antibiotic treatment. Cells that survive can be either genetically resistant to the drug or be in a state of persistence. Unlike the resistant mutants, cultures grown from persister cells are fully sensitive to the drug [2].
Persistence allows bacterial populations to protect themselves from various stresses, including many antibiotics or nutrient-deficient environments. It can be viewed as a risk-reducing strategy: at any given moment during favorable growth conditions a vast majority of cells in the population proliferate quickly but a small fraction of persister cells does not grow, providing a source of outgrowth in the case conditions abruptly change and the growing majority is eradicated [3, 4, 5]. Persistence is commonly observed in phylogenetically diverse bacteria, including major pathogens, and has contributed to the intractability of chronic and relapsing infections [6, 7].
One of the most studied agents that induces persistence is HipA, a toxin encoded by one of the many toxin-antitoxin (TA) systems of the Escherichia coli K-12 genome. HipA is a serine/threonine kinase that phosphorylates glutamyl-tRNA synthetase GltRS and inactivates it [8, 9]. Expression of hipA in the absence of cognate antitoxin arrests cell growth and strongly stimulates formation of persister cells [10]. Accumulation of uncharged tRNAGlu activates the synthesis of alarmone (p)ppGpp by RelA and SpoT, inhibiting rRNA gene transcription [8, 9]. Accordingly, the level of persistence induced by HipA is dramatically reduced in cells lacking (p)ppGpp synthetase RelA and SpoT [8, 9, 11,12].
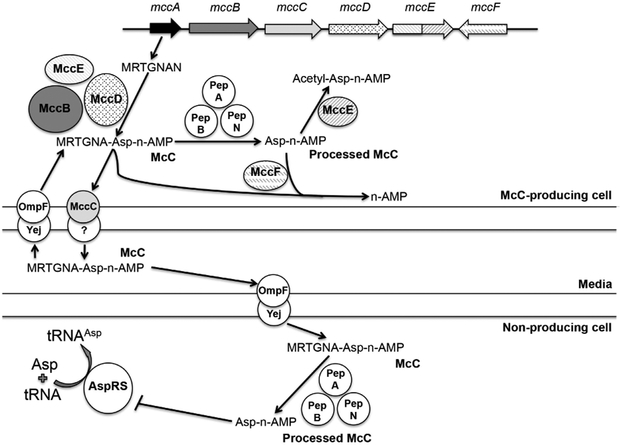
GltRS is one of the 20 aminoacyl-tRNA synthetases present in bacterial cells. Similarly to HipA, agents inhibiting the function of any one of these enzymes should stimulate persistence through the common RelA and SpoT-dependent (p)ppGpp synthesis pathway. However, this expectation has not been tested experimentally. Microcin C (McC) is a potent inhibitor of aspartyl-tRNA synthetase (AspRS) [13]. McC is produced by E. coli cells harboring a plasmid-borne gene cluster mccABCDE (Fig. 1) The mccA gene encodes a 7-aminoacid McC precursor. MccB adenylates the heptapeptide, while MccD and the N-terminal domain of MccE are required for phosphate modification with propylamine. MccC, MccE, and MccF jointly provide the producing cell with resistance to McC.
Figure 1. Biosynthesis and action of microcin C.
McC-producing cells contain the mccABCDE operon schematically presented at the top of the figure. The heptapeptide MRTGNAN encoded by the mccA gene is sequentially modified by the MccB, MccD, and N-terminal domain of MccE biosynthesis enzymes. The resulting mature peptide-nucleotide antibiotic McC can be i) processed intracellularly by aminopeptidases A, B, or N with a release of toxic processed McC, which is deactivated by the C-terminal domain of MccE or the MccF self-immunity enzyme or ii) exported outside of the producing cell by the MccC pump. Extracellular McC is taken up through the OmpF/Yej transporters. In sensitive cells it is processed by aminopeptidases and inhibits aspartyl-tRNA synthetase.
McC gets inside the inner membrane of sensitive E. coli cells through the YejABEF transporter [14]. The additional cellular function of the YejABEF transporter is unknown. No differences in cells with deletion of the individual genes from the yejABEF cluster and wild type cells were observed [14]. After uptake, the McC peptide is degraded by aminopeptidases [15], releasing processed McC, a non-hydrolysable aspartyl-adenylate that binds to and inhibits AspRS [13]. Here, we show that indeed McC induces (p)ppGpp synthesis and persistence in both sensitive and producer cells through the same mechanisms as those employed by HipA. Unlike HipA, which accumulates intracellularly, McC is produced into the cultured medium and then enters cells from the medium. We use this property to show that in co-cultivation experiments McC-producing cells induce persistence in McC-sensitive non-producing cells. The latter finding suggests that McC, and by extension other toxic Trojan-horse inhibitors, may function not just as inhibitors of bacterial growth but also serve as “public good” signals in bacterial communities by increasing resilience of all community members to various stresses, including multiple antibiotics.
RESULTS
Microcin C dramatically increases persistence
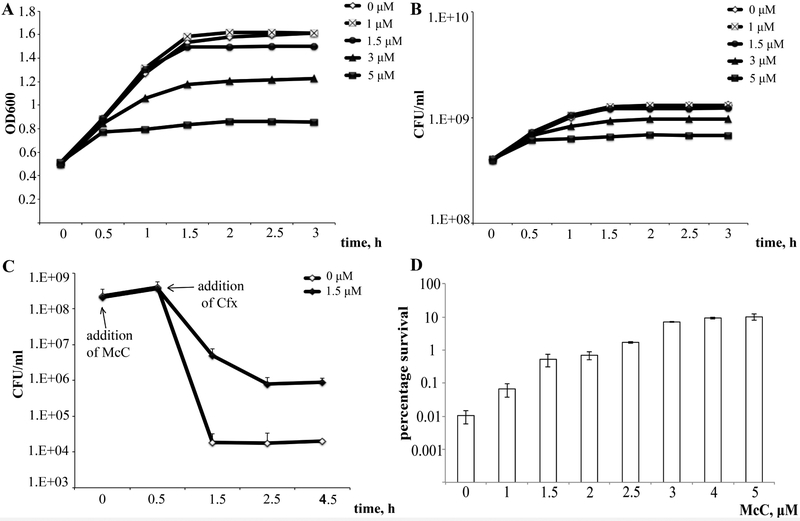
To determine whether McC is able to induce persistence, we treated growing cultures of McC-sensitive wild-type E. coli with various concentrations of McC. Bacterial growth was significantly inhibited by McC when present at concentrations of 5 μM or higher (Fig. 2A and B). Cells cultures that were supplemented with McC (and untreated control cultures) were next treated with lethal concentrations of ciprofloxacin (Cfx). At various times after the addition of Cfx, culture aliquots were removed, washed and plated on LB agar plates without antibiotics to determine the number of surviving colonies (CFUs). In the absence of McC the frequency of cells that survived the Cfx treatment was ~10−4 (Fig. 2C and D, 0.01% surviving cells). The addition of McC increased survival in a concentration-dependent manner. At the highest tested concentration of 5 μM, when McC strongly inhibited cell growth, 10% of cells in the culture survived the Cfx treatment. In the presence of 1.5 μM McC, when its effect on culture growth was hardly perceptible, up to 1% of cells survived the Cfx treatment, a 100-fold increase compared to control without McC. We therefore conclude that McC induces persister cell formation when added to growing E. coli cells culture.
Figure 2. Microcin C induces persistence in growing Escherichia coli cultures.
(A) Growth curves of MG1655 (wt) strain after McC treatment. An overnight culture was diluted 100-fold in LB and growth was allowed to continue at 37 °C. When OD600 reached 0.5 the indicated concentrations of McC were added to culture aliquots and further growth was monitored by following OD600 at indicated time points.
(B) As in A, but showing the number of colony forming units (CFUs) on LB agar plates at various time points after the McC addition.
(C) An example of killing curves obtained after ciprofloxacin (Cfx) treatment of MG1655 (wt) culture with or without McC (1.5 μM). Cultures were grown as in A. After 30 min incubation with McC, 1 mg/l Cfx was added. The incubation was continued and culture aliquots were removed at various time points followed by CFUs determination. Mean values and standard deviation obtained from three independent experiments are shown.
(D) For each killing curve obtained with or without McC, percentage of surviving cells (for details see Materials and Methods) after 4-hour incubation in the presence of Cfx (see panel C) was calculated. Error bars show standard deviations of mean values of at least 3 independent experiments.
McC induces the stringent response
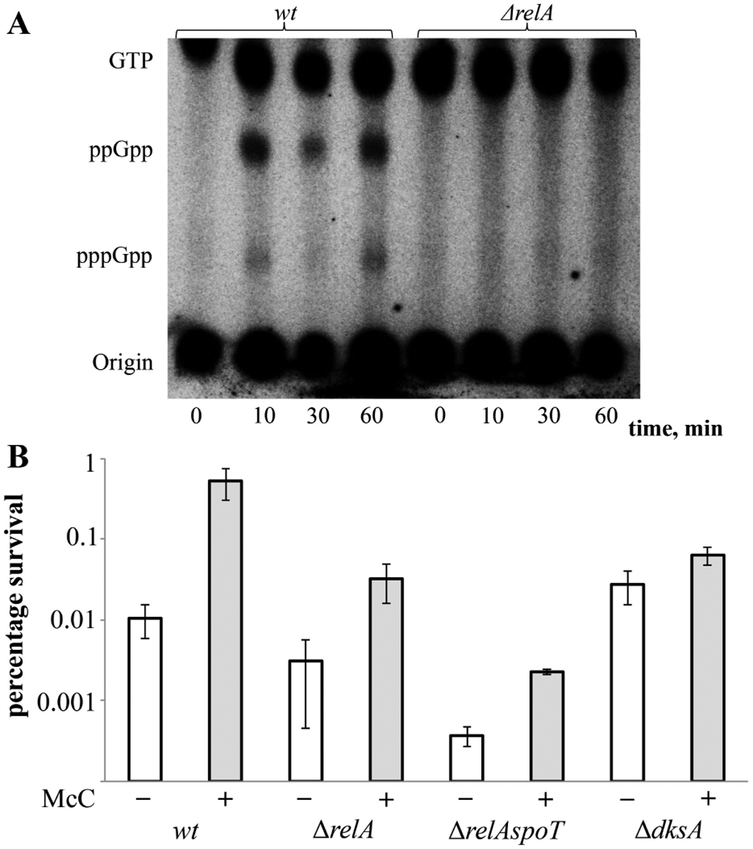
Since McC inhibits AspRS, it should cause accumulation of uncharged tRNAAsp, which in turn should cause production of (p)ppGpp. TLC analysis of cells treated with 3 3μM of McC indeed demonstrated strong accumulation of (p)ppGpp (Fig. 3A). The (p)ppGpp synthesis was dependent on the presence of ribosome-dependent (p)ppGpp synthetase RelA, as expected (Fig. 3A). To determine if McC induced persistence is mediated by (p)ppGpp accumulation, we repeated the persistence assay with mutant cells lacking the relA gene. Cells lacking both relA and spoT, were also tested. The ppGpp-mediated stringent response requires DksA, an RNA polymerase binding protein that inhibits transcription of ribosomal RNA genes [17]. Though DksA does not contribute to persistence induced by HipA, we also tested mutant cells lacking dksA in persistence assay along with (p)ppGpp synthetase-deficient cells. The results, shown in Fig. 3B, show that the fraction of cells surviving the Cfx treatment in the absence of McC decreased in the order wt > ΔrelA > ΔrelAspoT, as reported previously for HipA [8, 11, 12]. In the presence of McC, survival of relA and relAspoT mutants was increased 10-fold, whereas the survival of wild-type cells increased 100-fold at these conditions. Deletion of dksA had a smaller, but still a significant effect (Fig. 3B).” We conclude that McC-mediated persistence has a clear (p)ppGpp-dependent component, but other mechanisms also contribute.
Figure 3. McC treatment leads to (p)ppGpp production that contributes to persister formation.
(A) Accumulation of (p)ppGpp following McC addition. MG1655 and the ΔrelA isogenic deletion strain were grown exponentially in low phosphate MOPS minimal medium (see Materials and Methods). Samples were collected before and 10, 30 and 60 minutes after McC addition (3 μM) and separated by thin-layer chromatography (TLC). A representative autoradiograph of the thin-layer chromatography plates is shown.
(B) Exponentially growing cells of MG1655 and isogenic ΔrelA, ΔrelAspoT and ΔdksA deletion strains, untreated (white bars) or pretreated with 1.5 μM McC (grey bars), were exposed to 1 mg/l of ciprofloxacin (for details see Materials and Methods). Percentage of survival after 4 h of antibiotic treatment is shown (log scale). Error bars indicate the standard deviations of mean values obtained from at least 3 independent experiments, P < 0.05 (two-tailed Student’s t-test) for each pair of mean values obtained with and without McC.
McC induced persistence depends on TA modules, Lon and polyphosphate.
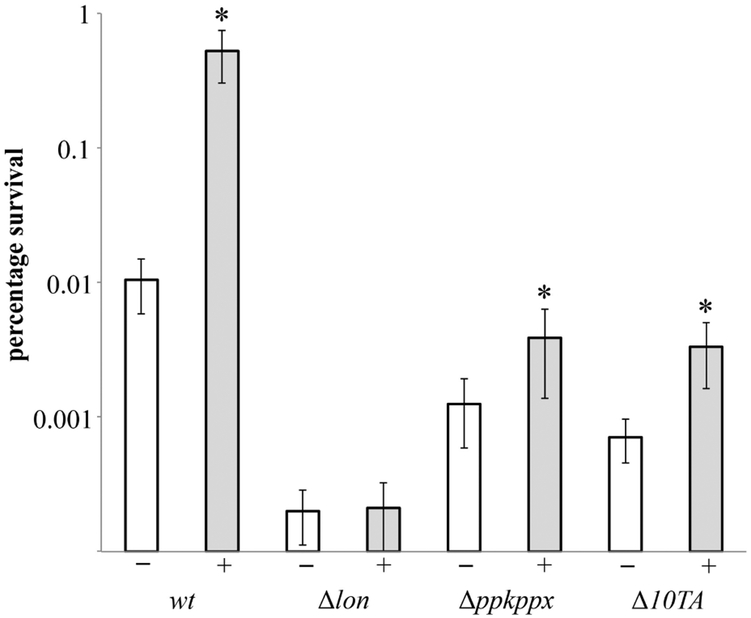
Previous analysis showed that the high (p)ppGpp level caused by HipA expression trans-activated the Toxin-Antitoxin(TA)-encoded mRNases which in turn triggered high persistence [8, 11, 12]. In addition, HipA-mediated trans-activation of mRNases depended hierarchically on (p)ppGpp, polyphosphate (Poly(P)) and Lon protease [8, 18]. That (p)ppGpp is an important component for McC-dependent persistence increase predicted that such increase should also depend on Poly(P), Lon and TAs. Consistently, when cultures of E. coli cells lacking all 10 Type II TA systems encoding mRNases were treated with McC, a more than 10-fold drop in percentage of surviving cells compared to the wild-type control was observed (Fig. 4). Moreover deletion of ppkppx, encoding poly(P) synthesis/degradation system had a similar effect. Finally deletion of lon abolished McC-dependent increase in persisters. Similar trends were observed with cells overproducing HipA [8, 18].
Figure 4. Persistence induction by McC depends on Lon protease, inorganic polyphosphate and toxin-antitoxin modules.
Exponentially growing cells of MG1655 and isogenic deletion strains Δlon, Δ(ppkppx) and Δ10TA, untreated (white bars) or pretreated with 1.5 μM McC (grey bars), were exposed to 1 mg/l of ciprofloxacin (for details see Materials and Methods). Percentage of survival after 4 h of antibiotic treatment is shown (log scale). Error bars show standard deviations of mean values of at least 3 independent experiments, * P < 0.05 for means obtained for samples with and without McC.
McC-producing cells have increased levels of persistence
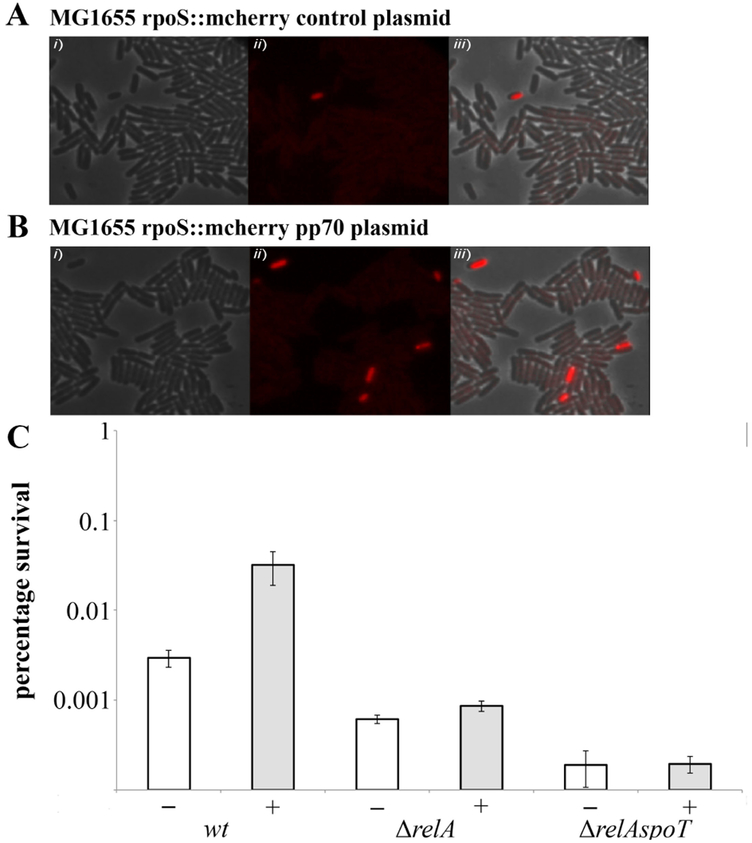
While McC-producing cultures continue to grow, they could be affected by the McC they produce in the medium and thus have an increased fraction of persister cells. To directly test this hypothesis and visualize persisters at the single cell level by fluorescence microscopy, we used the previously described MG1655 rpoS::mCherry E. coli strain. The rpoS::mCherry translational fusion (based on the activation of stationary-phase RNA polymerase σS factor) is a reliable proxy of the (p)ppGpp level and thus persistence of single cells [11]. MG1655 rpoS::mCherry cultures transformed with the pp70 plasmid, which carries the entire mcc-cluster under control of native promoters [19], or control empty vector were grown until late logarithmic phase, when McC production begins [19], and cells were examined microscopically. Statistical analysis of more than 120,000 cells indicated that in cells carrying pp70 the frequency of mCherry-positive cells was increased almost 30-fold compared to control cells (Figs. 5A and B and Fig. S1). In agreement with microscopic observations, we observed at least 10-times more colonies surviving the Cfx treatment in wild-type E. coli cultures transformed with pp70 than in control culture (Fig. 5C). Importantly cultures of relA and spoT mutants harboring pp70 produced McC normally but did not show increased levels of persisters compared to isogenic non-producing controls (Fig. 6C and Fig. S2). Thus, cells in McC producing cultures must have elevated levels of (p)ppGpp that lead to increased persisters levels.
Figure 5. McC-producing cells have increased frequency of (p)ppGpp “ON” cells and persister cells.
(A) Snap-shot of exponentially growing cells of MG1655 (top panel), and MG1655 harboring the pp70 plasmid (carries the entire McC cluster mccABCDEF under natural promoter) (bottom panel), carrying an rpoS::mcherry translational fusion. (i) Phase contrast, (ii) RpoS-mCherry fluorescence (iii) overlay of (i) and (ii). Statistical analysis of fluorescent cells is provided in Fig. S1. (Scale bar: 4 μm).
(B) Exponentially growing cells of MG1655 and isogenic deletion strains ΔrelA and ΔrelAspoT with (grey bars) the pp70 plasmid were exposed to 1 mg/l of ciprofloxacin (for details see Materials and Methods). Percentage of survival after 4 h of antibiotic treatment was compared to that of the control strains carrying the empty vector plasmid (white bars) (log scale). Error bars show standard deviation of mean values from at least 3 independent experiments, P < 0.05 for samples with and without McC.
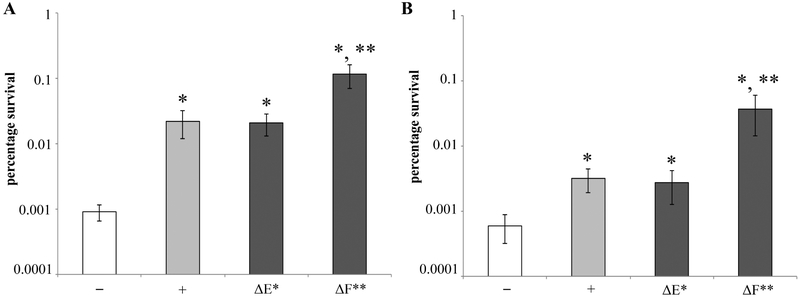
Figure 6. Persister levels in wild type and McC-resistant yejA mutant cells producing McC from complete plasmid-borne mcc operon or mcc operons with disrupted autoimmunity genes.
Exponentially growing cells of MG1655 (A) or the isogenic deletion strains ΔyejA (B) harboring either the pp70 or pp70ΔmccF (same as pp70 but lacking functional mccF, mutation S118A) or pp70ΔmccE (same as pp70 but mccE with inactivated acetyltransferase, mutations S553A and E572A) were exposed to 1 mg/l of ciprofloxacin (for details see Materials and Methods). Percentage of survival after 4 h of antibiotic treatment was compared to that of the control strains carrying the empty vector plasmid (white bars) (log scale). Error bars show standard deviations of mean values obtained from at least 3 independent experiments; * P < 0.05 compared to control cells not producing McC, ** P < 0.05 compared to cell harboring pp70.
To determine whether increased persistence in McC producers is due to endogenously produced or imported McC, persister levels were determined in cultures of McC-resistant yejA mutants (abolishing McC transport) transformed with pp70. Though the mutant cultures produced same amounts of McC (Fig. S3) as wild-type cultures, the frequency of persisters decreased by more than tenfold (Fig. 6). Thus, import of previously produced McC from the outside followed by processing clearly contributes to increased persistence levels of McC producing cultures. However, the percentage of yejA cells surviving the Cfx treatment in cultures transformed with pp70 was still ~10 times higher than in cultures transformed with control vector plasmid (Fig. 6). Thus, endogenous McC that accumulates in producing cells must also contribute to increased persistence levels.
The mcc operon encodes, in addition to the MccC export pump, two proteins that contribute to autoimmunity of producing cells. The C-terminal domain of MccE acetylates and inactivates processed McC [20]. MccF is a hydrolase that cleaves of the nucleotide moiety from either intact or processed McC [21]. Both proteins can lead to McC resistance when overexpressed, however, their contribution to auto-immunity of producing cells is not known. Cells that harbored pp70 derivatives encoding MccE with inactivated acetyltransferase or non-functional MccF were tested for persistence levels. Though both cells produced McC normally (Fig. S3), cells lacking the MccE acetyltransferase had the same levels of persisters as cells harboring parental pp70 (Fig. 6). In contrast, cells lacking functional MccF showed ~10-fold increase in persistence. It therefore follows that MccF is a more active contributor to McC detoxification in the producing cells. The same result was obtained in ΔyejA producers (Fig. 6), indicating that MccF, but not MccE detoxify endogenous McC.
McC producing cells induce persistence during co-culturing
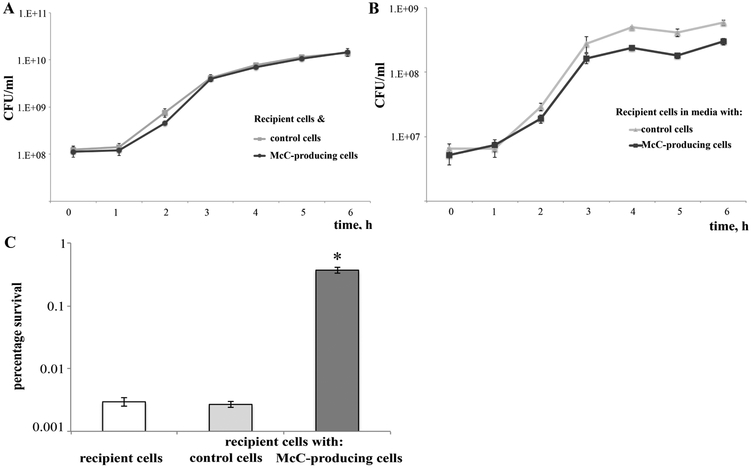
Since microcinogenic cells demonstrate increased persistence levels caused by a combined effect of internal and external McC, we tested whether McC produced in the culture can induce persistence in non-microcinogenic cells. To this end, we co-cultured cells transformed with pp70 (or control vector) with McC-sensitive “recipient” cells marked with a chromosomal tetracycline resistance gene. The co-culturing experiment was initiated by introducing equal amounts of both kinds of cells in fresh medium containing ampicillin to maintain the McC producing plasmid or control vector. The overall CFU numbers in co-cultures with pp70 or control vector cells were identical during the course of experiment (Fig. 7A). When the amounts of tetracycline-resistant non-microcinogenic cells were monitored, no difference between co-cultures with or without the pp70 plasmid was observed initially, however, when the cultures reached stationary phase, a ~5-fold lower amount of tetracycline-resistant cells in co-cultures with pp70-transformed cells was detected (Fig. 7B). Since inhibitory concentrations of McC were present when the co-culture reached stationary phase, we surmised that McC exported in the medium by the producing cells caused the decrease in tetracycline-resistant cells. Therefore, we determined the level of persistence in McC-sensitive recipient cells. As can be seen (Fig. 7C), recipient cells co-cultured with McC producers exhibited 100-fold higher persistence levels than same cells co-cultured with control non-producing cells or when grown in monoculture. We conclude that McC producers not just inhibit the growth of sensitive cells but also induce persistence.
Figure 7. McC-producing cells increase the persistence level of McC-non-producing cells during co-cultivation.
Growth curves of co-cultures of recipient tetracycline-resistant cells with McC-producing cells or non-producing control cells. (A) Total (recipient and McC-producing/non-producing (control)) cell counts. (B) Amounts of recipient cells grown with McC producing or non-producing control cultures (for details see Materials and Methods).
(C) Persister levels in recipient cells grown alone (white bar) or co-cultivated with McC-producing (dark grey bar) or non-producing control cells (grey bar). Error bars show standard deviations of mean values from at least 3 independent experiments; * P < 0.005 compared with control cells.
DISCUSSION
In this work we show that microcin C induces persistence in E. coli. Analogously to HipA, which inhibits GluRS, McC, an inhibitor of AspRS, causes production of (p)ppGpp, which activates the (p)ppGpp-dependent persistence formation pathway that is responsible for about 90% of the observed increase in persisters. Formation of the remaining, (p)ppGpp-independent persisters requires the toxin-antitoxin systems encoded in the E. coli genome.
While the levels of persistence caused by McC are dependent on its concentration, the increase obtained in the presence of concentrations that inhibit cell growth are the highest ever reported. Differences in persistence levels caused by agents inhibiting different aminoacyl-tRNA synthetases could be due to involvement of specific mechanisms activated by the inhibition of a particular aminoacyl-tRNA synthetase or may have to do with the frequency of use of a particular aminoacyl-tRNA or extent of (p)ppGpp synthesis activation by specific uncharged tRNAs.
The increase in persistence is observed at sub-inhibitory concentrations of McC. This finding led us to hypothesize that growing McC-producing cultures may also have increased persistence levels. The producing cells should maintain a steady-state level of internal McC determined by the balance between the synthesis rate and the export rate by the MccC pump and detoxification by MccE and MccF enzymes. When the concentration of McC in the medium is high, additional McC may be imported in the producing cells by the YejABEF transporter further increasing the concentration of intracellular McC. McC from the intracellular pool could be processed by proteases leading to accumulation of toxic processed McC and stimulating persistence. Indeed, we directly observed persister cells in McC producing cultures and demonstrated that import of earlier produced McC accounts for about 50% of the overall increase in persistence levels seen in producing cultures. The remaining increase must be due to processing of internally generated McC molecules that have not been exported. Thus, the autoimmunity enzymes MccE and MccF are not able to fully counter accumulation of processed McC in producing cells. Analysis of cells harboring mcc mutant plasmids shows that MccF is the primary contributor to detoxification of intracellular McC in the producing cells, since inactivation of this enzyme, but not of MccE, leads to additional increase in persistence levels.
The levels of McC produced by cells carrying the mcc cluster are sufficient to inhibit growth of neighboring sensitive cells, which is thought to be beneficial for microcinogenic cells at conditions when nutrient availability is limiting [22, 23]. Our results show, however, that the interaction between McC producers and non-microcinogenic sensitive cells may be more complex, since McC production also increases persistence in the recipient cells population. The observed increase is stronger than in the producing cells since recipient cells lack the McC export pump and dedicated detoxifying enzymes. Thus, at least from the point of view of persistence, McC producing cells seem to contribute to the “public good” of the community, ensuring their own survival and that of others. This property may contribute to the wide distribution on microcinogenicity in phylogenetically diverse bacteria.
MATERIALS AND METHODS
Growth media and biological materials
Cells were grown in LB or MOPS glucose medium supplemented with all amino acids at 37 °C with shaking. When required, the medium was supplemented with 10 μg/ml tetracycline or 50 μg/ml ampicillin. Bacterial strains and plasmids are listed in Table 1.
Table 1.
Bacterial Strains and Plasmids
| Strains/Plasmids | Genotype/Plasmids properties | Source/Reference |
|---|---|---|
| MG1655 | Wild-type E. coli K-12 | Laboratory collection |
| BL21 (DE3) | fhuA2 [lon] ompT gal (λ DE3) [dcm] ΔhsdS λ DE3 = λ sBamHIo ΔEcoRI-B int::(lacI::PlacUV5::T7 gene1) i21 Δnin5 | Laboratory collection |
| MGJ5987 (Δ10TA) | MG1655 ΔmazF *ΔchpB ΔrelBE Δ(dinJ-yafQ) Δ(yefM-yoeB) ΔhigBA Δ(prlF-yhaV) ΔyafNO ΔmqsRA ΔhicAB | Maisonneuve E et al. (2011) |
| MG1655 Δlon | MG1655 Δlon::tet | Winther KS and Gerdes K. (2009) |
| CF5802 (ΔppKppX) | MG1655 Δppk ppx::kan | Kuroda A and Kornberg A. (1997) |
| CF1693 (ΔrelAspoT) | MG1655 ΔrelA251::kan ΔspoT207::cat | Xiao H et al. (1991) |
| ΔrelA | MG1655 ΔrelA251::kan | P1 CF1698 × MG1655 |
| MG1655 ΔdksA | MG1655 ΔdksA::kan | Germain et al. (2015) |
| BW25113 ΔyejA | BW25113 ΔyejA::kan | Novikova M et al. (2007) |
| MG1655 ΔyejA | MG1655 ΔyejA | This work |
| HM21 | AT984 zde264::Tn10 dapA6 | Moyed HS and Bertrand KP. (1983) |
| MG1655 zde | MG1655 zde264::Tn10 dapA6 | P1 HM21 × MG1655 |
| MG1655 RpoS-Mcherry | MG1655 rpoS-mCherry::frt | Maisonneuve E et al. (2013) |
| pBad30 | Ara promoter, AmpR | Life Technologies |
| pp70 | pBad30-derived, McC cluster under natural promoter, AmpR | Zukher I et al. (2014) |
| pp70-F | pBad30-derived, McC cluster under natural promoter, mccFS118A, AmpR | Tikhonov A et al. (2010) |
| pp70-E | pBad30-derived, McC cluster under natural promoter, MccE S553A and E572A, AmpR | Novikova M et al. (2010) |
In the case of the mazEF locus, we only deleted the mazF gene.
Preparation and purification of McC
The E. coli K-12 strain BW25113 ΔyejA (Table 1) harboring McC-producing plasmid pp70 was grown for 18 h at 37 °C in M63 minimum medium containing 1% glycerol, 1 mg/l thiamine and 100 mg/l ampicillin. Cells were removed by centrifugation and the cultured medium was loaded onto Sep-Pak C8 cartridge (Waters). The cartridge was washed with water followed by a 0.1% aqueous trifluoroacetic acid wash, and bound material was eluted stepwise with 5, 10, and 20% acetonitrile in 0.1% trifluoroacetic acid. The 10% acetonitrile fraction was concentrated by lyophilization, dissolved in water, and subjected to reverse phase-HPLC (1 ml/min) on a ReproSil-Pur 300 ODS-3 column (5 μm, 250 × 4 mm) using a 0–20% linear gradient of acetonitrile in 0.1% trifluoroacetic acid. The total gradient volume was 50 ml. Pure McC eluted as a single peak; it was lyophilized, dissolved in water, and stored at −20 °C. The yield of chromatographically and mass spectrometrically pure McC ranged from 5 to 10 mg/l of cultured medium.
Bacterial growth experiments
Overnight cultures of MG1655 were diluted 100-fold in 50 ml of fresh LB medium and incubated for 2 h at 37 °C with shaking. 5-ml aliquots were transferred to 50 ml Falcon tubes and McC was added in different concentrations (0-5 μM). Cultures were allowed to continue growth and optical density at OD600 was monitored.
Persistence assay
Persistence was determined by measuring the number of colony forming units (CFUs)/ml upon exposure to 1 mg/l ciprofloxacin. Overnight cultures were diluted 100-fold in 10 ml of fresh LB medium and incubated for 2 h (otherwise indicated) at 37°C with shaking (typically reaching ~2×108 CFU/ml). Then aliquots of 5 ml were transferred to 28- by 114-mm Sarstedt conical polypropylene tubes and antibiotic was added. Tubes were placed in 45° inclination with shaking at 37°C for 4 hours. For determination of CFUs, 1 ml aliquots were removed, the cells harvested, resuspended in fresh medium, serially diluted and plated on solid LB medium. Persisters were calculated as the surviving fraction, by dividing the number of CFU/ml in the culture after 4h of incubation with the antibiotic by the number of CFU/ml in the culture before adding the antibiotic.
McC and persistence
To determine the number of persisters formed by cells experiencing pretreatment with McC, cells were grown in rich medium for 1.5 h (OD600 ~ 0.3) and various concentration of McC is added and cells were grown for an additional 30 min. Aliquots were then subjected to 1 mg/l ciprofloxacin antibiotic and persister cell formation determined as described above.
In vivo (p)ppGpp measurements
To determine the (p)ppGpp content formed by cells after McC treatment, overnight cultures were diluted 100-fold in 10 ml of MOPS glucose minimal medium supplemented with all amino acids as previously described [16] and incubated at 37 °C with shaking. At OD600~0.05, cells were labeled with H332PO4 (100 μCi/ml). After 2–3 generations (OD600~0.2–0.3), 3 μM McC were added. Samples were withdrawn 0, 10, 30 and 60 min after the addition of McC. Reactions were stopped by the addition of 10 μl of 2 M formic acid. Aliquots (10 μl) of each reaction were separated on PEI Cellulose TLC plates (GE Healthcare) at room temperature for 1 hour. 1.5 M KH2PO4 (pH 3.4) was used as the chromatographic solvent. The products were revealed by PhosphoImaging (GE Healthcare).
Microscopy
For phase contrast and fluorescence microscopy, cells were grown in LB to mid-exponential phase at 37 °C, centrifuged, resuspended in 10 times diluted LB in M9 medium, and mounted on pre-warmed microscope slides covered with a thin film of 1.2% agarose (in a 10 times dilute LB in M9 medium). Images were acquired with Zyla 5.5 CMOS camera attached to Nikon Eclipse Ti-E microscope. The images were acquired and analyzed with NIS-ELEMENTS.
Co-cultivation assay
Cells transformed with pp70 (or empty vector) and cells marked with a chromosomal tetracycline resistance gene the empty vector (i.e McC sensitive strains) were co-cultured. Overnight cultures were mixed in a 1:1 ration and diluted 200-fold in 10 ml of fresh LB medium and incubated for 6 h at 37 °C with shaking. At select time points OD600 and CFUs were measured on solid LB medium to enumerate the total CFU number of the co-culture or on LB medium with tetracycline to enumerate the number of CFUs from the McC-sensitive cells population. Persistence assay for co-cultivated cultures was slightly modified. Indeed before the addition of Cfx, the co-cultures were cultivated for 3 hours.
Supplementary Material
Acknowledgements.
This work was supported by NIAID R01 AI117210 grant to Satish A. Nair and KS, by Russian Science Foundation RSF 16-14-10356 to Svetlana Dubiley, the Danish National Research Foundation funded Centre of Excellence BASP (grant identifier DNRF120), a Novo Nordisk Foundation Laureate Research Grant and the ERC Advanced Investigator Grant PERSIST (294517).
References
- 1.Bigger JW. 1944. Treatment of staphylococcal infections with penicillin. Lancet ii: 497–500. [Google Scholar]
- 2.Moyed HS, Broderick SH. 1986. Molecular cloning and expression of hipA, a gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol 166:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kussell E, Kishony R, Balaban NQ, Leibler S. 2005. Bacterial persistence: A model of survival in changing environments. Genetics 169:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michiels JE, Van den Bergh B, Verstraeten N, Michiels J. 2016. Molecular mechanisms and clinical implications of bacterial persistence. Drug Resist Updat. 29:76–89. [DOI] [PubMed] [Google Scholar]
- 5.Harms A, Maisonneuve E, Gerdes K. 2016. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 354(6318). [DOI] [PubMed] [Google Scholar]
- 6.Lewis K 2010. Persister cells. Annu Rev Microbiol 64:357–72. [DOI] [PubMed] [Google Scholar]
- 7.Levin BR, Rozen DE. 2006. Non-inherited antibiotic resistance. Nat Rev Microbiol 4:556–62. [DOI] [PubMed] [Google Scholar]
- 8.Germain E, Castro-Roa D, Zenkin N, Gerdes K. 2013. Molecular mechanism of bacterial persistence by HipA. Mol Cell 52:248–54. [DOI] [PubMed] [Google Scholar]
- 9.Kaspy I, Rotem E, Weiss N, Ronin I, Balaban NQ, Glaser G. 2013. HipA-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat Commun 4 :3001. [DOI] [PubMed] [Google Scholar]
- 10.Korch SB, Hill TM. 2006. Ectopic overexpression of wild-type and mutant hipA genes in Escherichia coli: effects on macromolecular synthesis and persister formation. J. Bacteriol 188:3826–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maisonneuve E, Castro-Camargo M, Gerdes K. 2013. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell 154:1140–50. [DOI] [PubMed] [Google Scholar]
- 12.Ramisetty BC, Ghosh D, Chowdhury MR, Santhosh RS. 2016. What is the link between stringent response, endoribonuclease encoding Type II toxin-antitoxin systems and persistence? Front Microbiol. 7:1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metlitskaya A, Kazakov T, Kommer A, Pavlova O, Praetorius-Ibba M, Ibba M, Krasheninnikov I, Kolb V, Khmel I, Severinov K. 2006. Aspartyl-tRNA synthetase is the target of peptide nucleotide antibiotic microcin C. J Biol Chem 281:18033–18042. [DOI] [PubMed] [Google Scholar]
- 14.Novikova M, Metlitskaya A, Datsenko K, Kazakov T, Kazakov A, Wanner B, Severinov K. 2007. The Escherichia coli YejABEF transporter is required for the uptake of translation inhibitor microcin C. J. Bacteriol 189:8361–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazakov T, Vondenhoff GH, Datsenko KA, Novikova M, Metlitskaya A, Wanner BL, Severinov K. 2008. Escherichia coli peptidase A, B, or N can process translation inhibitor microcin C. J Bacteriol 190:2607–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cashel M 1994. Detection of (p)ppGpp accumulation patterns in Escherichia coli mutants. Methods in Molecular Genetics 3:341–356. [Google Scholar]
- 17.Doniselli N, Rodriguez-Aliaga P, Amidani D, Bardales JA, Bustamante C, Guerra DG, Rivetti C. 2015. New insights into the regulatory mechanisms of ppGpp and DksA on Escherichia coli RNA polymerase-promoter complex. Nucleic Acids Res 43:5249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germain E, Roghanian M, Gerdes K, Maisonneuve E. 2015. Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases. Proc Natl Acad Sci USA 112:5171–6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Zukher I, Novikova M, Tikhonov A, Nesterchuk MV, Osterman IA, Djordjevic M, Sergiev PV, Sharma CM, Severinov K. 2014. Ribosome-controlled transcription termination is essential for the production of antibiotic microcin C. Nucleic Acids Res 42:11891–11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novikova M, Kazakov T, Vondenhoff GH, Semenova E, Rozenski J, Metlytskaya A, Zukher I, Tikhonov A, Van Aerschot A, Severinov K. 2010. MccE provides resistance to protein synthesis inhibitor microcin C by acetylating the processed form of the antibiotic. J Biol Chem 285:12662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tikhonov A, Kazakov T, Semenova E, Serebryakova M, Vondenhoff G, Van Aerschot A, Reader JS, Govorun VM, Severinov K. 2010. The mechanism of microcin C resistance provided by the MccF peptidase. J Biol Chem 285:37944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Pastor JE, San Millan JL, Castilla MA, Moreno F. 1995. Structure and organization of plasmid genes required to produce the translation inhibitor microcin C7. J Bacteriol 177:7131–7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fomenko DE, Metlitskaya AZ, Peduzzi J, Goulard C, Katrukha GS, Gening LV, Rebuffat S, Khmel IA. 2003. Microcin C51 plasmid genes: possible source of horizontal gene transfer. Antimicrob Agents Chemother 47:2868–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.