Abstract
Living well with severe asthma can be challenging. People with severe asthma can be refractory to treatment, can experience poor symptom control and are at a heightened risk of death. Patients experience symptoms of shortness of breath, chest tightness, cough and wheeze. These symptoms influence many aspects of an individual's life, resulting in emotional, financial, functional and medication-related burdens that negatively impact quality of life. Quality of life is known to be influenced by individual levels of satisfaction that stem from real-life treatment experiences. This experience is portrayed through the lens of the patient, which is commonly referred to as the patient perspective.
The patient perspective is only one element of the patient experience. It influences health status, which, in severe asthma, is commonly assessed using validated health-related quality of life measures. A positive patient perspective may be achieved with implementation of management strategies tailored to individual needs. Management strategies developed in partnership between the patient, the severe asthma multidisciplinary team and the general practitioner may minimise disease-related impairment, allowing patients to live well with severe asthma.
Key points
Despite advances in treatment over the past decade, the experience of living with severe asthma has not significantly improved, with high levels of burden influencing the patient perspective.
The impact of severe disease is not only restricted to asthma symptoms and acute attacks. It causes significant emotional, financial, functional and medication-related burdens, leading to impaired health-related quality of life.
Clinical outcomes should not be stand-alone measures in severe asthma. Nonclinical measures should also be considered when evaluating health-related quality of life.
Disease burden may be minimised and quality of life improved via self-management strategies, including education sessions, written asthma action plans, symptom monitoring, breathing exercises, physical activity and psychotherapeutic interventions.
Educational aims
To demonstrate the importance of the patient perspective in severe asthma.
To identify the significant levels of disease burden associated with severe asthma.
To discuss quality of life in severe asthma.
To outline strategies that increase well-being in severe asthma.
Short abstract
Understanding the patient experience of the burden of severe asthma is key in enhancing patient–clinician relationships, and this may lessen the impact of severe asthma http://bit.ly/2wdm3tD
Introduction
Severe asthma is a high-burden illness that is known for its heterogeneity represented by varying types of airway inflammation, variable response to treatment and multiple comorbidities and risk-factors.
Severe asthma has been defined as asthma that requires treatment with guideline-suggested medications for asthma classified as “steps 4–5” by the Global Initiative for Asthma (high-dose inhaled corticosteroids and long-acting β2-agonists or leukotriene modifier/theophylline for the previous year, or systemic corticosteroids for ≥50% of the previous year) to prevent it from becoming “uncontrolled”, or that remains “uncontrolled” despite this therapy [1]. It should be noted that this definition excludes “difficult-to-treat” asthma, which improves considerably once an appropriate diagnosis and/or treatment of confounders occurs [1].
The reported prevalence of severe asthma varies. Research undertaken in the Netherlands reported that 3.6% of the Dutch adult population with asthma have true severe refractory disease [2], whereas severe asthma prevalence in Denmark has been reported at 8.1%; the latter estimate was derived using data from a nationwide prescription database [3].
Severe asthma is associated with frequent life-threatening acute attacks and a burdensome symptom profile. Consequently, living well with severe asthma can be a significant challenge for many patients as they experience poor symptom control and are at a heightened risk of death [1, 2]. Additionally, and importantly, people with severe asthma are also burdened by multiple comorbidities and risk factors, and these traits are of high priority to patients: for example, the risk factors of physical inactivity [4] and obesity, and the comorbidities of anxiety and depression [5]. The impacts of these embody not only asthma symptoms and acute attacks but also cause significant personal, physical, emotional, financial, functional and medication-related burdens that result in impaired health-related quality of life (HRQoL) [6].
Given the heterogeneous and widespread impact of severe asthma on physical, emotional and social well-being, disease burden cannot simply be measured by mortality and morbidity outcomes. Incorporating the patient's perspective can increase the likelihood of behaviour change, for example improved adherence to medications and physical activity, which may be beneficial to symptom control [7]. Shared decision-making allows asthma patients to prioritise the problems that they consider most significant and leads to improved outcomes [7]. In this article, we highlight the burden of severe asthma from the patient's perspective, discuss the concept of HRQoL among severe asthma patients and highlight strategies that can lead to improved overall well-being.
A hallmark of severe disease: health-related quality of life
Quality of life (QoL) is defined as an individual's satisfaction or happiness with life or well-being that is subjective, multidimensional and dynamic, compromising six domains: physical, psychological, level of independence, social relationships, environment, and spirituality/religion and/or personal beliefs [8]. However, with QoL so neatly and formally defined without the mention of health, researchers and academics developed and introduced the concept of HRQoL [8]. HRQoL is described as the part of a person's overall QoL determined primarily by their health status [8]. It is defined as “the functional effects of an illness and its consequent therapy upon a patient, as perceived by the patient” [9]. In severe asthma, HRQoL is an outcome measure used to characterise both individuals and populations as well as evaluate therapeutic interventions [10]. Importantly, HRQoL in severe asthma is frequently impaired and may be one of the hallmarks of severe disease.
The patient perspective
In severe asthma, patients endure the consequences of living with a chronic illness and require multifaceted treatment regimens that commonly include add-on administration of oral corticosteroids (OCSs). OCSs have significant side-effects that further increase the burden of disease experienced by patients [11]. In addition to complex treatment regimens, multiple pulmonary and extrapulmonary comorbidities coexist in patients with severe asthma. These comorbidities require their own complicated management plans, adding to disease burden [12, 13]. Coexisting comorbidities are problematic for patients because they are frequently undiagnosed, complicate severe asthma management and/or mimic the symptoms of severe disease [14]. Furthermore, comorbidities such as anxiety and depression not only worsen asthma outcomes but also negatively impact the patient's experience of living well with asthma [6, 14]. It is this arduous experience of living with severe asthma that influences and shapes the patient's perspective. Common comorbidities associated with severe asthma are listed in table 1.
Table 1.
Common comorbidities associated with severe asthma
| Pulmonary and upper airways | Extrapulmonary |
| Allergic and nonallergic rhinitis | Obesity |
| Chronic rhinosinusitis | Anxiety and depression |
| Dysfunctional breathing | Gastro-oesophageal reflux disease |
| Vocal cord dysfunction | Osteoporosis |
| Chronic obstructive pulmonary disease | Cardiovascular disease |
| Bronchiectasis | Metabolic disease |
| Obstructive sleep apnoea |
Reproduced and modified from [15] with permission.
The patient experience is considered to be the sum of the patient's perspective, satisfaction, engagement, participation and preferences [16]. In severe asthma, as with many chronic diseases, the patient experience may vary from individual to individual. Even though two patients may have been diagnosed with treatment-refractory disease and receive similar treatment, different levels of satisfaction and burden may be reported. This highlights the importance of understanding the experience of individual patients.
In clinical practice and in research, we often use “hard” outcome measures to evaluate severe asthma and the response to treatment; these include validated symptom frequency and severity scores, lung function and the frequency and severity of acute attacks. However, as demonstrated in the qualitative literature examining the experience of living with severe asthma [4, 17], factors including the ability to participate in physical activity, manage breathlessness, maintain relationships and maintain a healthy weight, are perhaps of greater importance to the patient. This being so, clinical outcomes (lung function, symptoms, acute attacks) should not stand alone in our assessment and measurement of severe asthma. Understanding the factors that define the patient experience should form part of the assessment. This may be done in a conversational way within the clinic setting or through the use of fit-for-purpose patient-reported outcome measures such as the Severe Asthma Questionnaire [5]. Furthermore, it is important to understand how the patient perspective can be negatively influenced by unmet needs. Past severe asthma research has largely focused on areas such as treatment, symptom control and exacerbation reduction. Recently, there has been an increase in research focused on the patient perspective and experience of living with severe asthma [4, 17–19]. These studies use the patients' own words and stories to shine a light on the day-to-day burdens, limitations and struggles of severe disease.
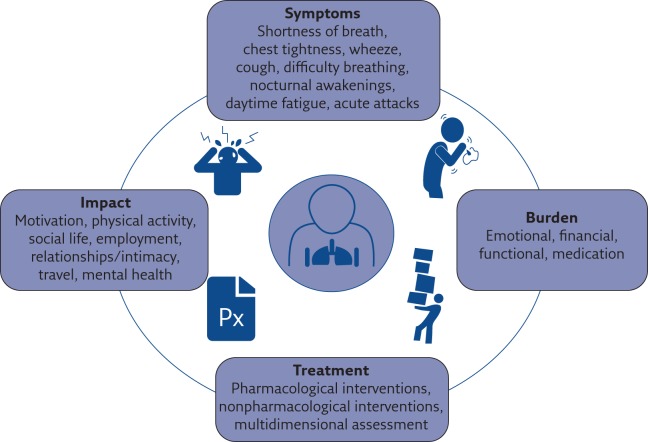
Patients diagnosed with severe asthma continually describe the negative impact/burden of living with severe treatment-refractory asthma, classified into specific domains (figure 1) [6, 20]. To gain deeper understanding of the adult patient's perspective of living with severe disease, an Australian study involving semi-structured qualitative interviews was conducted [4]. From the data collected in this study, four themes emerged: “The body as a hindrance”, “Burden of disease”, “Alone with asthma” and “Striving to adapt” [4]. Further to this, a systematic synthesis examining the daily challenges of people living with severe asthma by Eassey et al. [17] found that severe asthma was “disempowering”, a “threat to identity and life roles” and people were “striving to achieve a greater level of personal control over their condition”. Direct quotes from the study by Foster et al. [4] emphasise the immensity of the burden and the profound impact that patients commonly experience. For example, Stephanie (female, 38 years) quotes: “[It's] crap [sic] … [asthma] affects every hour of every day of every week of every month of every year of my life” [4]. Larry (male, 54 years) states: “You're a slave to this regimented ordeal you've got to go through every day” [4]. Brenda (female, 53 years) said: “It's frustrating, upsetting. There's things I really wanted to do and I haven't been able to do … It does make you depressed, because people don't see the sickness that has happened over my life” [4]. Severe asthma patients share similar stories that can be viewed on the Severe Asthma Toolkit [15].
Figure 1.
Symptoms, impacts, areas of burden and treatment options for people living with severe asthma.
Depression and guilt: emotional burden
Poor psychological health, including isolation, is common among those with severe asthma [21]. People with depression and/or anxiety coexisting with severe asthma engage in considerably more healthcare use, and have more adverse health outcomes and increased risk of acute attacks compared to people with severe asthma without depression and/or anxiety [22]. Furthermore, people with severe asthma seek treatment for mental health concerns significantly less often than the general population (6.5% versus 46%) [23]. “Feeling alone” is a known risk factor that contributes to the development of depressive disorders [21], with 25% of severe asthma patients currently reporting symptoms of depression [24]. This impact is illustrated in the study conducted by Foster et al. [4] with Rhonda, a 54-year-old female saying: “I have struggled with [depression] personally when I have been so, so sick, and I probably struggle with it every day now that I live alone. It's harder” [4]. Further to feeling alone, guilt is another component of the emotional burden experienced by severe asthma patients. Guilt is demonstrated to affect personal relationships. This was reported by the authors of a qualitative study that aimed to explore the impact of severe asthma on intimacy [18]. A 47-year-old female described her experience, saying: “I actually said to my husband … I wouldn't blame you for going, because I wouldn't want to be with me” [18]. Guilt of this nature may stem from patients feeling as though they cannot adequately fulfil the everyday roles that partners play in relationships [18]. With this in mind, important everyday roles that severe asthma patients play extend beyond personal relationships and into the workplace.
Individuals and healthcare services: financial burden
Financial hardship, including decreased earning capacity triggered by absenteeism, was experienced in 27% of patients with severe asthma in the Australasian Severe Asthma Web-based Database (SAWD) [25]. This hampers the ability to purchase prescribed medications and/or over-the-counter medications. Career choice and career longevity are further affected. In a qualitative study by Hyland et al. [19], a 44-year-old female described the impact this had on her life: “I lost my job, I was medically discharged because I was in and out of hospital.” While the financial burden is recognisably significant from a patient perspective, the impact on the healthcare system is also great, with >50% of asthma healthcare resources expended on patients with severe disease [26]. Urgent and unplanned visits to doctors and emergency departments, with subsequent hospital admissions, are costly to healthcare systems. In Australia, a portion of what is known as direct asthma cost is largely attributed to over 38 000 hospitalisations [27]. In the USA alone, acute attacks or a worsening of asthma symptoms resulted in 1.6 million emergency department visits in a single year [1].
Daily activities and physical activity: functional burden
Functional limitation is a major feature present in 70–100% of severe asthma patients [28]. Functional limitation is characterised by a decline in the ability to complete daily activities and was reported in 85% of severe asthma patients enrolled in the SAWD [24]. In this study, loss of productivity at work was experienced by 73% of patients with severe disease due to “feeling impaired” [24]. The study by Foster et al. [4] presented the impact of functional burden from the patient perspective, with Larry, a 54-year-old male, stating: “There comes a point where you can't do the job anymore so you've got to get out of there and let someone else do the job.”
Furthermore, functional limitations in physical activity are also experienced by severe asthma patients, who perform less moderate and vigorous activity [29] and only walk an average of 5362 steps per day [30]. Although advancement in the medications used to manage severe asthma show promise and allow patients to continue participating in physical activities, prescribed daily maintenance therapies can be expensive and have known side-effects, leading to suboptimal treatment adherence and medication-related burdens.
Side-effects and intimacy: medication-related burden
To effectively manage symptoms in any chronic disease, optimal treatment adherence is required. However, in a severe asthma population this is challenging. In an Australian study, suboptimal adherence was reported in 44% of severe asthma patients [31]. This is problematic as suboptimal adherence increases the risk of acute attack and is associated with symptoms of anxiety and fear [32]. Understanding the reasons for suboptimal adherence in severe asthma is key to implementing behaviour change strategies.
It is also important to recognise the medication-related side-effects that cause significant burden to many severe asthma patients. Long-term OCS use causes notable side-effects, such as weight gain (73%), sleep disturbance (41%) and thinning skin/stretch marks (41%) [33], among others. Furthermore, patients voiced major concerns about future risk of eye problems/cataracts (48%), further weight gain (48%) and fragile bones (47%) [33]. Neuropsychiatric symptoms also occur when OCSs are used in doses of 6 mg or above [34]. Hyland et al. [19] sought to understand the impact of pharmacological treatment on severe asthma patients. They conclude that the burden of OCSs in severe asthma is poorly addressed in both policy and in practice. The impact of weight gain and depression from OCSs was illustrated in this study, with a 53-year-old male reporting: “My weight thing is the big thing. It sounds so stupid, but I've had years of people going on about how fat I am.” Another male participant, aged 66 years, states: “I can't tell you how much I hate being on them because of the depression.”
Relationships and intimacy are also affected by medications used in severe asthma. Some patients expressed themselves as feeling “encaged” by their asthma [35], whilst others reported feeling like a “third party” was present in their relationship [18]. Holmes et al. [18] sought to explore the impact of severe asthma on intimacy, and four emergent themes were reported. These related to the domains of physical intimacy, emotional intimacy, image of self, and the role of the health provider. The need for and impact of short-acting β2-agonists during periods of intimacy was articulated by a 32-year-old female who stated: “My Bricanyl pump's [sic] … a big part of our relationship as well, we have to manoeuvre the pump around the actions in the bedroom. There's times when he has to hold the pump, so it's like three people in the relationship.”
Overall, severe asthma has an impact on many aspects of patients' lives, but these may not emerge during standard asthma consultations. Developing an understanding of the patient's perspective and their well-being from a multidimensional viewpoint is desirable [17], and may improve patient–clinician partnerships and identify areas to target with self-management strategies.
Strategies to improve quality of life
While there have been major advances in severe asthma management over the past decade, particularly in terms of patient phenotyping, the emergence of new monoclonal antibody therapies and improved models of care, data from the “Still Fighting for Breath” publication indicate that the experience of patients living with severe asthma has not significantly improved during this time [20]. “Fighting for Breath”, a large-scale survey conducted in 2005 by the European Federation of Allergy and Airways Diseases Patients' Associations, reported that patients with severe asthma had inadequately controlled symptoms and disturbed sleep, missed opportunities to socialise with friends and go on holidays, and missed out on job opportunities [36]. A decade later, “Still Fighting for Breath”, a large global real-life online follow-up survey, was conducted to evaluate the current impact of severe persistent asthma on patients' daily lives and activities [20]. Comparison of the 2005 and 2016 surveys revealed that severe asthma patients still live with uncontrolled symptoms, have disturbed sleep (97% versus 66%), miss going out with friends (52% versus 38%), miss holidays (39% versus 28%), and miss out on job opportunities (24% versus 21%) [20]. These results identify the need to improve the overall management of patients with severe asthma [20]. Improved management strategies are required to reduce the burden from severe uncontrolled symptoms, fear of acute attacks and treatment burden.
Progress has been made in terms of pharmacological management, notably monoclonal antibody therapies and long-term macrolide antibiotics [37, 38]. Randomised controlled trials of currently available monoclonal antibody therapies approved in some countries (omalizumab, mepolizumab, benralizumab) have demonstrated efficacy of these treatments in reducing acute asthma exacerbations [39–42]. Furthermore, in the largest randomised controlled trial of long-term macrolide antibiotics in asthma, exacerbations were reduced by 40% [38]. Effects of these treatments on QoL have not been as dramatic. Overall, they have resulted in clinically and statistically significant improvements from baseline to trial end-point in the intervention conditions (using the Juniper Asthma Quality of Life Questionnaire [43]) [33, 39, 41, 42, 44–47]; however, differences between the intervention and control conditions have largely not reached the clinically important difference of 0.5 units.
However, looking beyond this, nonpharmacological interventions such as education sessions, written asthma action plans, active symptom monitoring, physical activity and psychological interventions may promote living well with severe disease (table 2). Unfortunately, despite the evidence for the effectiveness of nonpharmacological approaches in asthma, these may not have been tested in severe populations, are not always implemented or may not be feasible in all clinical settings. For example, a population study examining nonpharmacological care in asthma found that of patients who had a moderate to severe asthma diagnosis, only 19% had ever received a written asthma action plan, just over half (56%) had ever received verbal action plans, and doctor assessment of inhaler technique occurred in 58% of patients [48]. These data suggest the need to improve the implementation of evidence-based self-management strategies in severe asthma. There is also a need to establish efficacy data for interventions that target outcomes that are highly important to patients.
Table 2.
Summary of severe asthma burden domains, characteristics, impacts and nonpharmacological strategies
| Burden | Characteristic | Impact | Strategy |
| Emotional | Feeling alone | Personal relationships | Education sessions |
| Guilt | Everyday activities/roles | Psychotherapeutic intervention | |
| Anxiety and/or depression | Physical activity | Exercise intervention | |
| Panic | Treatment adherence | Multidimensional assessment | |
| Financial | Worsening of symptoms | Healthcare services | Written asthma action plan |
| Poor treatment adherence | Inability to purchase medications | Symptom monitoring | |
| Loss of productivity at work | Career choice/longevity | Psychotherapeutic intervention | |
| Absenteeism | Employment | Psychosocial and family support | |
| Functional | Fatigue | Employment/social engagements | Multidimensional assessment |
| Inability to participate in desired activities/lifestyle | Relationships/travel | Education sessions | |
| Decrease in moderate and vigorous physical activity | Weight gain/obesity/deconditioning | Exercise interventions | |
| Lack of personal and emotional intimacy | Personal relationships | Psychotherapeutic intervention | |
| Medication | Nonadherence | Symptom control | Multidimensional assessment |
| Comorbidities | Disease management complexity | Assessment and guideline-based treatment | |
| Neuropsychiatric symptoms | Treatment adherence | Written asthma action plan | |
| Lack of intimacy | Personal relationships | Psychotherapeutic intervention | |
| Weight gain | Physical activity/self-esteem | Exercise intervention |
Health literacy: education sessions
The overall goal of asthma education is to provide skills and knowledge, and promote partnerships between multidisciplinary teams and severe asthma patients that optimise symptom control [49]. Optimal symptom control may be achieved when patients are actively involved in disease management; however, limited health literacy levels may prevent the goal being attained [49]. With this in mind, education sessions for patients and their families or carers may be structured as interactive or non-interactive sessions with written, verbal, visual and/or audio elements [50]. However, these must align with health literacy levels of individuals. Daly et al. [51] conducted a study to evaluate the effectiveness of a 12-week severe asthma education programme and reported significant improvements beyond the minimal clinically important difference in asthma control and HRQoL. For every patient diagnosed with severe asthma, education should commence on diagnosis and continue at each review. The importance of review should never be discounted as it provides the opportunity for continued education and appraisal of personalised written asthma action plans, adherence, inhaler technique, symptoms and coping strategies.
When to modify treatment: written asthma action plan
Written asthma action plans in severe asthma allow patients to modify their treatment in response to worsening symptoms [52]. Severe asthma patients should liaise with their treating clinician to determine the level of symptom severity (action point) or peak flow reading that prompts the initiation of urgent action in prevention of serious outcomes [52]. A systematic review published in 2004 identified 17 randomised control trials that evaluated written asthma action plans compared to usual care [53]. Highly significant improvements in asthma health outcomes were reported, with the risk of being admitted to hospital decreasing by over 40% and visits to the emergency department falling by over 20% (relative risk 0.78, 95% CI 0.67–0.91) [53]. However, despite established longstanding evidence, 81% of moderate-to-severe asthma patients have never received a written asthma action plan [48]. These findings highlight the need for improved engagement from treating clinicians, as written asthma action plans not only facilitate early detection and treatment of acute attacks, but also increase the level of autonomy held by severe asthma patients.
Symptoms or peak expiratory flow: symptom monitoring
The day-to-day monitoring of symptoms increases well-being and includes measurement of symptoms and medications or measurement of airway function by peak expiratory flow (PEF) [52]. Buist et al. [54] conducted a randomised clinical trial to evaluate the effectiveness of symptom and PEF monitoring. The results concluded that there were no significant differences between the types of monitoring with respect to accessing healthcare services, with Asthma QoL Questionnaire (AQLQ) scores and lung function both improving [54]. The significant difference in AQLQ scores between baseline and 6-month follow-up (mean 0.4 units, 95% CI 0.3–0.5 units; p<0.0001) suggests that symptom monitoring either via symptoms or PEF increases well-being in severe asthma [54]. Engaging patients in self-monitoring requires regular discussion and review.
Physical activity: exercise intervention
Exercise, in conjunction with dietary and psychological support, has been reported to reduce body weight by 7% in moderate-to-severe asthma patients [55]. Exercise training and weight-loss programmes, when combined, result in increased aerobic capacity and strength, leading to better clinical control of asthma and improved HRQoL [55]. Additional benefits of exercise include a reduction in pro-inflammatory mediators (C-reactive protein, interleukin-6 and -8, tumour necrosis factor-α, monocyte chemoattractant protein-1 and leptin) associated with severe asthma, and reductions in metabolic dysfunction and obesity [55]. With described benefits to overall health and QoL, it is important to consider why sedentary lifestyles are reported in severe asthma patients [30].
Reasons for reduced physical activity have been explored in an adolescent population with asthma [56]. The main barrier to participants undertaking exercise was fear of an asthma attack, and as a result many adolescents withdrew from exercise as a coping strategy. Despite this, exercise was seen as important, with 81% of participants reporting it as the most enjoyable activity [56]. This is a poorly explored area in severe asthma, but in qualitative studies patients frequently discuss the disappointment they experience in relation to the limits severe asthma places on their ability to exercise or engage in activity with friends and family [4]. Few studies have investigated interventions designed to improve activity in severe asthma. This is an important area for future research.
Cognitive behavioural therapy/psycho-educational interventions: psychotherapeutic interventions
In patients with severe asthma, there are few efficacy studies reporting on psychological interventions, although favourable improvements in QoL and asthma control in patients with persistent asthma have been reported in a systematic review [57]. A recent feasibility trial of a depression and anxiety intervention using group-based cognitive behavioural therapy in people with severe asthma has been reported [58]. The study showed improvements in breathlessness, but was burdened by high drop-out rates, as participants were unable to attend the scheduled group sessions due to illness. Both anxiety and depression are common in severe asthma patients, with prevalence rates of 38% and 25%, respectively, compared to those with milder disease (30% and 9%) [24], resulting in a 2.4-fold increased risk of poor asthma control [59]. Whilst more research is required, the impact of these interventions on asthma outcomes should not be discounted. Research has demonstrated that psycho-educational interventions may reduce hospital admissions, improve QoL and possibly decrease psychological morbidity in patients with severe asthma, although to date high-quality research remains limited [60].
Improving health-related quality of life: multidimensional assessment
In the best efforts to deliver optimal management in severe asthma, healthcare providers and patients must overcome challenges of the current healthcare systems regarding fragmentation of care within multiple settings [61]. To navigate this, international experts have proposed a multidimensional or systematic assessment [62]. Multidimensional assessment (MDA) in the context of severe asthma is a coordinated series of investigations and assessments, designed to confirm diagnosis or highlight mechanisms of persisting symptoms (such as phenotyping), identify comorbidities relevant to severe asthma and identify risk factors [63]. Factors that form part of the MDA include airway assessments such as bronchodilator reversibility, airflow limitation and airway inflammation [63]. Assessment of relevant comorbidities, such as anxiety, depression, gastro-oesophageal reflux disease [64] and sinusitis, also forms part of the MDA [63]. Risk factors such as smoking, physical inactivity and obesity, and self-management skills such as inhaler device technique, medication adherence and inhaler device polypharmacy are also assessed [63]. The MDA approach is a method of implementing a precision medicine model of care, which targets treatment to an individual patient on the basis of genetic, phenotypic, biomarker and psychosocial characteristics [65]. MDA in severe asthma has been credited with improvements in HRQoL, improved control of symptoms and a reduction in frequency of acute attacks [13]. MDA may therefore be considered a model of care to increase well-being and QoL in severe asthma.
Conclusion: future needs
While severe asthma management has advanced in terms of phenotyping patients, new monoclonal antibody therapies and improved models of care, the burden of severe disease, and its impacts on HRQoL, remains a major problem. Conventional strategies and guideline-based treatments alone are ineffective for some patients, with excessive emotional, financial, functional and medication-related burdens continually being described by patients. We have presented some strategies that may promote living well with severe asthma. It is important to note that this is not an exhaustive list of strategies, and other nonpharmacological interventions exist, such as diet and weight-loss interventions, which have shown promising results in a general asthma population [66]. To help increase well-being in severe asthma patients, their perspective should be understood. Only then can targeted interventions be identified and implemented. To guarantee continued improvements in well-being, evolving nonpharmacological therapies and interventions require further investigation and testing, specifically in severe asthma populations.
Self-evaluation questions
- Health-related quality of life is described as:
- a) Part of a person's overall quality of life, determined primarily by their health status
- b) How frequently they administer bronchodilators
- c) Satisfaction with interactions between health staff and pharmacists
- d) Patient engagement and participation in treatment
- Self-management in severe asthma should include:
- a) Education sessions, written asthma action plans and symptom monitoring
- b) Physical activity and psychological intervention
- c) Positive patient perspectives
- d) All of the above
- High levels of burden in severe asthma have an impact upon:
- a) Motivation and levels of physical activity
- b) Social life, intimacy and mental health
- c) Employment and travel
- d) All of the above
- A multidimensional assessment is based on:
- a) Airway limitation and inflammation
- b) Genetic, phenotypic, biomarker and psychosocial characteristics
- c) Pulmonary comorbidities
- d) Risk factors
Suggested answers
a.
d.
d.
b.
Footnotes
Conflict of interest: M.A. Stubbs has nothing to disclose.
Conflict of interest: V.L. Clark receives a fellowship from the National Health and Medical Research Council, Centre of Research Excellence in Severe Asthma, and also reports a grant from AstraZeneca to cover research-related costs, received for providing education to AstraZeneca staff, outside the submitted work.
Conflict of interest: V.M. McDonald reports grants and speaker fees from GlaxoSmithKline and AstraZeneca, and personal fees for educational symposia from Menarini, all outside the submitted work.
References
- 1.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 2.Hekking PP, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. [DOI] [PubMed] [Google Scholar]
- 3.von Bülow A, Kriegbaum M, Backer V, et al. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract 2014; 2: 759–767. [DOI] [PubMed] [Google Scholar]
- 4.Foster JM, McDonald VM, Guo M, et al. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J 2017; 50: 1700765. [DOI] [PubMed] [Google Scholar]
- 5.Hyland ME, Jones RC, Lanario JW, et al. The construction and validation of the Severe Asthma Questionnaire. Eur Respir J 2018; 52: 1800618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald VM, Hiles SA, Jones KA, et al. Health-related quality of life burden in severe asthma. Med J Aust 2018; 209: S28–S33. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med 2010; 181: 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc Sci Med 1995; 41: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 9.Spilker B, ed. Quality of Life and Pharmacoeconomics in Clinical Trials. 2nd Edn Philadelphia, Lippincott-Raven, 1996. [Google Scholar]
- 10.Hossny E, Caraballo L, Casale T, et al. Severe asthma and quality of life. World Allergy Organ J 2017; 10: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volmer T, Effenberger T, Trautner C, et al. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J 2018; 52: 1800703. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishna N, Tay TR, Hore-Lacy F, et al. Profile of difficult to treat asthma patients referred for systematic assessment. Respir Med 2016; 117: 166–173. [DOI] [PubMed] [Google Scholar]
- 13.Clark VL, Gibson PG, Genn G, et al. Multidimensional assessment of severe asthma: a systematic review and meta-analysis. Respirology 2017; 22: 1262–1275. [DOI] [PubMed] [Google Scholar]
- 14.Bardin PG, Rangaswamy J, Yo SW. Managing comorbid conditions in severe asthma. Med J Aust 2018; 209: S11–S17. [DOI] [PubMed] [Google Scholar]
- 15.Centre of Excellence in Severe Asthma. Severe Asthma Toolkit. https://toolkit.severeasthma.org.au/ [Google Scholar]
- 16.Karam C. The evolution of patient satisfaction to patient experience. Front Health Serv Manage 2017; 33: 30–34. [DOI] [PubMed] [Google Scholar]
- 17.Eassey D, Reddel HK, Foster JM, et al. “…I've said I wish I was dead, you'd be better off without me”: a systematic review of people's experiences of living with severe asthma. J Asthma 2019; 56: 311–322. [DOI] [PubMed] [Google Scholar]
- 18.Holmes LJ, Yorke JA, Dutton C, et al. Sex and intimacy in people with severe asthma: a qualitative study. BMJ Open Respir Res 2019; 6: e000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyland ME, Whalley B, Jones RC, et al. A qualitative study of the impact of severe asthma and its treatment showing that treatment burden is neglected in existing asthma assessment scales. Qual Life Res 2015; 24: 631–639. [DOI] [PubMed] [Google Scholar]
- 20.Katsaounou P, Odemyr M, Spranger O, et al. Still Fighting for Breath: a patient survey of the challenges and impact of severe asthma. ERJ Open Res 2018; 4: 00076-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cacioppo JT, Hawkley LC, Thisted RA. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol Aging 2010; 25: 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ten Brinke A, Ouwerkerk ME, Zwinderman AH, et al. Psychopathology in patients with severe asthma is associated with increased health care utilization. Am J Respir Crit Care Med 2001; 163: 1093–1096. [DOI] [PubMed] [Google Scholar]
- 23.Valença AM, Falcão R, Freire RC, et al. The relationship between the severity of asthma and comorbidites with anxiety and depressive disorders. Braz J Psychiatry 2006; 28: 206–208. [DOI] [PubMed] [Google Scholar]
- 24.McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology 2019; 24: 37–47. [DOI] [PubMed] [Google Scholar]
- 25.Hiles SA, Harvey ES, McDonald VM, et al. Working while unwell: workplace impairment in people with severe asthma. Clin Exp Allergy 2018; 48: 650–662. [DOI] [PubMed] [Google Scholar]
- 26.Sadatsafavi M, Lynd L, Marra C, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J 2010; 17: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Australian Institute of Health and Welfare. Asthma snapshot. https://www.aihw.gov.au/reports/chronic-respiratory-conditions/asthma/formats Date last updated: July 24, 2018. Date last accessed: June 18, 2019.
- 28.Dockrell M, Partridge MR, Valovirta E. The limitations of severe asthma: the results of a European survey. Allergy 2007; 62: 134–141. [DOI] [PubMed] [Google Scholar]
- 29.Aparecido da Silva R, Rocco PGL, Cukier A, et al. Patients with asthma moderate and severe presents reduced moderate levels of physical activity, less aerobic conditioning and poorly control of the asthma. Eur Respir J 2015; 46: Suppl. 59, PA3560. [Google Scholar]
- 30.Cordova-Rivera L, Gibson PG, Gardiner PA, et al. Physical activity and exercise capacity in severe asthma: key clinical associations. J Allergy Clin Immunol Pract 2018; 6: 814–822. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Tay TR, Radhakrishna N, et al. Nonadherence in the era of severe asthma biologics and thermoplasty. Eur Respir J 2018; 51: 1701836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baiardini I, Sicuro F, Balbi F, et al. Psychological aspects in asthma: do psychological factors affect asthma management? Asthma Res Pract 2015; 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prazma CM, Wenzel SE, Nelsen LM, et al. Perception of oral corticosteroid side effects in patients with corticosteroid-dependent asthma. J Allergy Clin Immunol 2017; 139: Suppl. 2, AB96. [Google Scholar]
- 34.Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol 2015; 136: 1488–1495. [DOI] [PubMed] [Google Scholar]
- 35.Partridge MR. Examining the unmet need in adults with severe asthma. Eur Respir Rev 2007; 16: 67–72. [Google Scholar]
- 36.European Federation of Allergy and Airways Diseases Patients’ Associations (EFA). Fighting for Breath. Brussels, EFA, 2005. Available from: www.efanet.org/resources/library/1441-58fighting-for-breath-a-european-patient-perspective-on-severe-asthma [Google Scholar]
- 37.Grainge CL, Maltby S, Gibson PG, et al. Targeted therapeutics for severe refractory asthma: monoclonal antibodies. Expert Rev Clin Pharmacol 2016; 9: 927–941. [DOI] [PubMed] [Google Scholar]
- 38.Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 659–668. [DOI] [PubMed] [Google Scholar]
- 39.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 40.Christensen MA, Ott M. Innovative therapies for severe asthma. Fed Pract 2017; 34: 25–31. [PMC free article] [PubMed] [Google Scholar]
- 41.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. [DOI] [PubMed] [Google Scholar]
- 42.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–2127. [DOI] [PubMed] [Google Scholar]
- 43.Juniper EF, Svensson K, Mörk AC, et al. Modification of the asthma quality of life questionnaire (standardised) for patients 12 years and older. Health Qual Life Outcomes 2005; 3: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortega AN, Goodwin RD, McQuaid EL, et al. Parental mental health, childhood psychiatric disorders, and asthma attacks in island Puerto Rican youth. Ambul Pediatr 2004; 4: 308–315. [DOI] [PubMed] [Google Scholar]
- 45.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355–366. [DOI] [PubMed] [Google Scholar]
- 46.Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med 2017; 377: 936–946. [DOI] [PubMed] [Google Scholar]
- 47.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 48.Tan DJ, Burgess JA, Perret JL, et al. Non-pharmacological management of adult asthma in Australia: cross-sectional analysis of a population-based cohort study. J Asthma 2018; in press [ 10.1080/02770903.2018.1545030]. [DOI] [PubMed] [Google Scholar]
- 49.Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int 2014; 111: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goeman D, Jenkins C, Crane M, et al. Educational intervention for older people with asthma: a randomised controlled trial. Patient Educ Couns 2013; 93: 586–595. [DOI] [PubMed] [Google Scholar]
- 51.Daly RD, Holmes LJ, Scanlon H, et al. P133: A multidisciplinary patient education programme significantly improves asthma control and quality of life in patients with severe asthma. Thorax 2015; 70: Suppl. 3, A143. [Google Scholar]
- 52.Andrews KL, Jones SC, Mullan J. Asthma self management in adults: a review of current literature. Collegian 2014; 21: 33–41. [DOI] [PubMed] [Google Scholar]
- 53.Gibson PG, Powell H. Written action plans for asthma: an evidence-based review of the key components. Thorax 2004; 59: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buist AS, Vollmer WM, Wilson SR, et al. A randomized clinical trial of peak flow versus symptom monitoring in older adults with asthma. Am J Respir Crit Care Med 2006; 174: 1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freitas PD, Ferreira PG, da Silva A, et al. The effects of exercise training in a weight loss lifestyle intervention on asthma control, quality of life and psychosocial symptoms in adult obese asthmatics: protocol of a randomized controlled trial. BMC Pulm Med 2015; 15: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fereday J, MacDougall C, Spizzo M, et al. “There's nothing I can't do – I just put my mind to anything and I can do it”: a qualitative analysis of how children with chronic disease and their parents account for and manage physical activity. BMC Pediatr 2009; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kew KM, Nashed M, Dulay V, et al. Cognitive behavioural therapy (CBT) for adults and adolescents with asthma. Cochrane Database Syst Rev 2016; 9: CD011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yorke J, Adair P, Doyle AM, et al. A randomised controlled feasibility trial of group cognitive behavioural therapy for people with severe asthma. J Asthma 2017; 54: 543–554. [DOI] [PubMed] [Google Scholar]
- 59.Luyster FS, Strollo PJ Jr, Holguin F, et al. Association between insomnia and asthma burden in the Severe Asthma Research Program (SARP) III. Chest 2016; 150: 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith JR, Mugford M, Holland R, et al. Psycho-educational interventions for adults with severe or difficult asthma: a systematic review. J Asthma 2007; 44: 219–241. [DOI] [PubMed] [Google Scholar]
- 61.Chung LP, Hew M, Bardin P, et al. Managing patients with severe asthma in Australia: current challenges with the existing models of care. Intern Med J 2018; 48: 1536–1541. [DOI] [PubMed] [Google Scholar]
- 62.Gibson PG, McDonald VM. Management of severe asthma: targeting the airways, comorbidities and risk factors. Intern Med J 2017; 47: 623–631. [DOI] [PubMed] [Google Scholar]
- 63.Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet 2010; 376: 803–813. [DOI] [PubMed] [Google Scholar]
- 64.Ortega H, Li H, Suruki R, et al. Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc 2014; 11: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 65.Jameson JL, Longo DL. Precision medicine – personalized, problematic, and promising. N Engl J Med 2015; 372: 2229–2234. [DOI] [PubMed] [Google Scholar]
- 66.Scott HA, Gibson PG, Garg ML, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy 2013; 43: 36–49. [DOI] [PubMed] [Google Scholar]