A 55-year-old man was referred to the outpatient pulmonary department of our hospital because of dyspnoea during exertion and when bending forward, which had been present for at least 6 months. He reported experiencing severe symptoms of breathlessness and many of his daily activities had to be adapted or interrupt due to symptoms (as documented by the Baseline Dyspnea Index (BDI)) (table 1). Recent infectious episodes or episodes of neck or shoulder pain were absent. His medical history included systemic arterial hypertension, obesity (body mass index (BMI) 36 kg·m−2), and obstructive sleep apnoea for which he was treated with night-time continuous positive airway pressure therapy (8 cmH2O). He was a former smoker (18 pack-years) who quit smoking 15 years ago. 8 months ago, he underwent abdominal surgery (transabdominal epigastric hernia repair). The presence of cardiopulmonary disease and other aetiologies, such as neuromuscular disease, was excluded. Chest radiograph showed an elevated left hemidiaphragm and impaired left phrenic nerve conduction (i.e. increased latency and compound muscle action potential (CMAP) duration) after electrical stimulation (table 1) [1].
Short abstract
Patients with diaphragm dysfunction experience exertional dyspnoea. Respiratory muscle function assessments can identify breathing abnormalities and IMT might help to reduce symptoms (mostly via improvements in non-diaphragmatic muscles). http://bit.ly/2QdxNFP
Case history
A 55-year-old man was referred to the outpatient pulmonary department of our hospital because of dyspnoea during exertion and when bending forward, which had been present for at least 6 months. He reported experiencing severe symptoms of breathlessness and many of his daily activities had to be adapted or interrupt due to symptoms (as documented by the Baseline Dyspnea Index (BDI)) (table 1). Recent infectious episodes or episodes of neck or shoulder pain were absent. His medical history included systemic arterial hypertension, obesity (body mass index (BMI) 36 kg·m−2), and obstructive sleep apnoea for which he was treated with night-time continuous positive airway pressure therapy (8 cmH2O). He was a former smoker (18 pack-years) who quit smoking 15 years ago. 8 months ago, he underwent abdominal surgery (transabdominal epigastric hernia repair). The presence of cardiopulmonary disease and other aetiologies, such as neuromuscular disease, was excluded. Chest radiograph showed an elevated left hemidiaphragm and impaired left phrenic nerve conduction (i.e. increased latency and compound muscle action potential (CMAP) duration) after electrical stimulation (table 1) [1].
Table 1.
Demographic data, dyspnoea, lung function tests and phrenic nerve conductance assessments pre- and post-inspiratory muscle training (IMT)
| Baseline (pre-IMT) | Post-IMT | Reference value | |
| Demographic data | |||
| Sex | Male | ||
| Age years | 55 | ||
| Height cm | 187 | ||
| Weight kg | 127 | ||
| BMI kg·m−2 | 36 | ||
| BDI, score/total score | 5/12 | ||
| Lung function | |||
| FEV1 sitting L (% pred) | 2.62 (62) | 2.75 (65) | |
| FVC sitting L (% pred) | 3.29 (60) | 3.65 (68) | |
| FEV1/FVC sitting % | 80 | 75 | |
| FEV1 supine L (% pred) | 1.85 (44) | 1.84 (44) | |
| FVC supine L (% pred) | 2.50 (46) | 2.61 (48) | |
| FEV1/FVC supine % | 74 | 70 | |
| ΔFVC sitting versus supine % | −24 | −28 | |
| MVV L·min−1 (% pred) | 98.3 (72) | 103.1 (75) | |
| IC L (% pred) | 3.07 (75) | 3.33 (82) | |
| TLC L (% pred) | 5.43 (69) | 5.80 (74) | |
| FRC L (% pred) | 2.36 (63) | 2.47 (66) | |
| RV L (% pred) | 1.89 (79) | 2.12 (88) | |
| DLCO mmol·min−1·kPa−1 (% pred) | 9.20 (83) | 9.94 (89) | |
| Phrenic nerve conductance | |||
| Latency ms | 8 | ||
| Right | 7.6 | 8.6 | |
| Left | 15.4 | 17.6 | |
| CMAP duration ms | 15 | ||
| Right | 8.9 | 16 | |
| Left | 21 | 25 | |
| Amplitude mV | 0.46 | ||
| Right | 0.60 | 0.60 | |
| Left | 0.50 | 0.20 | |
MVV: maximum voluntary ventilation; IC: inspiratory capacity; TLC: total lung capacity; FRC: functional residual capacity; RV: residual volume; DLCO: diffusing capacity of the lung for carbon monoxide. Reference values for phrenic nerve conductance based on [1].
During baseline assessments, upright forced vital capacity (FVC) was 60% of predicted, while the ratio between forced expiratory volume in 1 s and FVC (FEV1/FVC) was 80% [2]. Static lung volumes and capacities were also decreased [3]. Taken together the results of these assessments suggest a restrictive pulmonary function impairment without expiratory flow limitation. When the FVC manoeuvre was performed in the supine position, a reduction by 24% was observed relative to sitting without severe symptoms of orthopnoea occurring during the supine assessment (table 1).
The maximal inspiratory (PImax) and expiratory pressures (PEmax) were −108 cmH2O (97% predicted) and 213 cmH2O (176% predicted), respectively (table 2) [4, 5].
Table 2.
Respiratory muscle strength assessment pre- and post-IMT
| Baseline (pre-IMT) | Post-IMT | |
| PImax cmH2O (% pred) | −108 (97) | −133 (120) |
| PEmax cmH2O (% pred) | 213 (176) | 285 (236) |
| Sniff Poes cmH2O | −93 | −124 |
| Sniff Pga cmH2O | −19 | −23 |
| Sniff Pdi cmH2O (% pred) | 74 (51) | 101 (69) |
| Twitch Pdi (right) cmH2O | 6.4 | 8.5 |
| Twitch Pdi (left) cmH2O | 2.4 | 4.6 |
| Twitch Pdi (bilateral) cmH2O | 10.8 | 12.7 |
Sniff Poes: oesophageal pressure during sniff manoeuvre; Sniff Pga: gastric pressure during sniff manoeuvre; Sniff Pdi: transdiaphragmatic pressure during sniff manoeuvre; Twitch Pdi: transdiaphragmatic pressure during bilateral phrenic nerve magnetic stimulation.
Based on these findings the diagnosis of unilateral diaphragm dysfunction (UDD) was made.
Question
Could an inspiratory muscle training (IMT) programme, in this patient with almost normal PImax, be indicated to improve diaphragm function and exertional dyspnoea?
Answer
To answer this question, additional assessments were performed both before and after a 6-month IMT period.
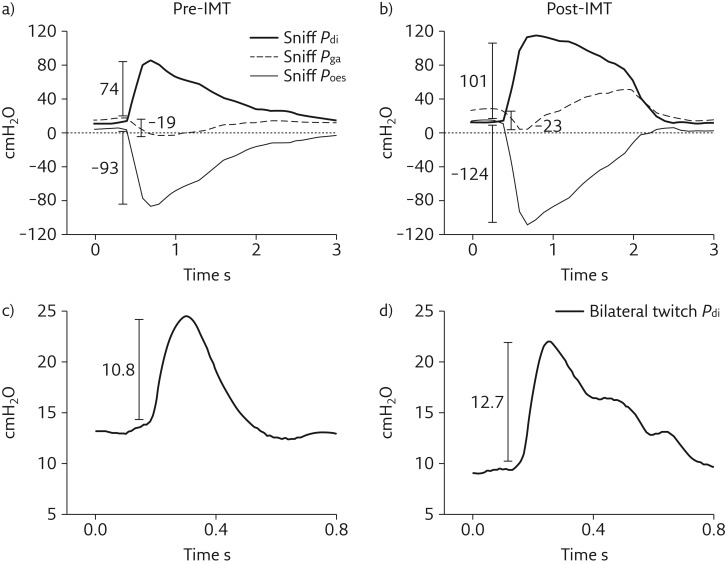
Transdiaphragmatic pressure (Pdi) obtained during maximal voluntary sniff manoeuvres (sniff Pdi) was 74 cmH2O (51% predicted) (table 2 and figure 1a) [5]. A sniff Pdi of <80 cmH2O is indicative of significant diaphragmatic weakness [6]. Bilateral magnetic phrenic nerve stimulation resulted in a twitch Pdi of 10.8 cmH2O (figure 1b). During unilateral stimulation the obtained twitch Pdi values were 2.4 and 6.4 (for left and right stimulation, respectively) (table 2). Twitch Pdi values of <20 cmH2O during bilateral stimulation or <10 cmH2O during unilateral stimulation are indicative of significant diaphragmatic weakness [6]. In addition, a paradoxical response in gastric pressure (Pga) (i.e. negative relative to end-expiratory Pga) was observed during the maximal sniff manoeuvres (sniff Pga) (table 2 and figure 1a).
Figure 1.
Illustration of Pdi, Pga and Poes pressure curves during a sniff manoeuvre a) pre- and b) post-IMT; and Pdi during bilateral magnetic phrenic stimulation c) pre- and d) post-IMT.
Furthermore, a maximal incremental (10 W·min−1) cardiopulmonary exercise test (CPET) (Vmax229d; SensorMedics, Yorba Linda, CA, USA) was performed on a cycle ergometer (Ergometrics 800S; SensorMedics). The patient achieved a peak work rate of 160 W (65% predicted), maximal oxygen consumption (V′O2max) of 20.8 mL·kg−1·min−1 (69% predicted) and a maximal heart rate of 140 beats per min (82% of predicted maximum) [7, 8]. The peak minute ventilation (V′E) was 59 L·min−1 (60% MVV). The patient rated the intensity of his perceived “breathing discomfort” (dyspnoea) and leg discomfort on a modified Borg scale (0–10) every minute during the test [9]. He indicated 9 for dyspnoea and 7 for leg effort at peak exercise and selected dyspnoea as the main reason (80% relative to 20% leg effort) for stopping the test.
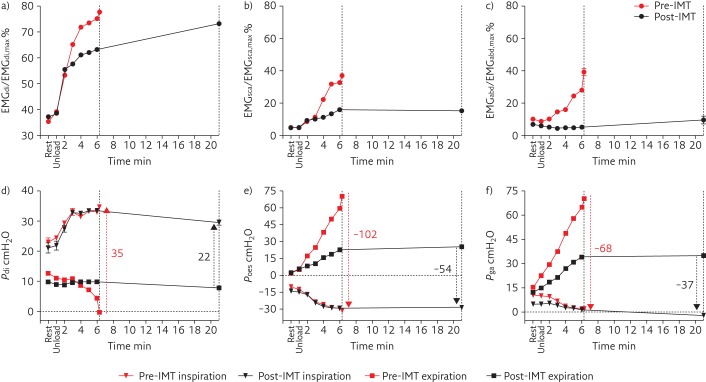
A subsequent constant work rate (CWR) endurance cycling test was performed at 70% of the peak work rate achieved during the CPET. The duration of loaded cycling was 5 min and 30 s and, again, the main reason for stopping exercise was dyspnoea (80% relative to 20% leg effort). During the CWR test, a combined diaphragm electromyogram (EMGdi)-electrode catheter with oesophageal and gastric balloons was used to assess the diaphragm activation (EMGdi/EMGdi,max), oesophageal (Poes), gastric (Pga) and transdiaphragmatic (Pdi) pressures [10]. In addition, surface EMG signals of scalene (EMGsca/EMGsca,max) and rectus abdominus (EMGabd/EMGabd,max) muscles were recorded. The EMGdi/EMGdi,max and EMGsca/EMGsca,max were expressed relative to maximal activation obtained during inspiratory capacity manoeuvres. The EMGabd/EMGabd,max was expressed relative to the maximal activation obtained during the expiratory phase of a FVC manoeuvre [11]. The measures were assessed continuously and breath-by-breath measurements were averaged for each minute. At the end of the endurance test the EMGdi/EMGdi,max, EMGsca/EMGsca,max and EMGabd/EMGabd,max were 78%, 37% and 39%, respectively (figure 2 a–c). The Pdi, Poes and Pga swings were 35 cmH2O, −102 cmH2O and −68 cmH2O, respectively (figure 2 d–f). Of note, the Pga swings were negative, indicating paradoxical Pga responses during tidal (exercise) breathing.
Figure 2.
Percentage of maximal muscle activation of a) diaphragm (EMGdi/EMGdi, max), b) scalene (EMGsca/EMGsca, max), c) abdominal (EMGabd/EMGSabd, max) muscles, and d) Pdi, e) Poes and f) Pga, during constant work rate exercise pre- and post-IMT. Dotted lines indicate the end of the endurance test for pre- and post-IMT. Red arrows indicate swing pressures pre-IMT and black arrows indicate swing pressures post-IMT.
These additional assessments further supported the diagnosis of UDD, identified specific diaphragm weakness and quantified the degree of exertional dyspnoea.
Treatment
IMT was performed using an electronic tapered flow resistive loading device (POWERbreathe®KH1, HaB International Ltd., Southam, UK). The programme was based on a high intensity IMT protocol as described previously [11–13]. It consisted of two to three daily sessions of 30 full vital capacity inspirations (4–5 min per session), performed 7 days a week for 6 months. The patient was able to start the training against an external load of 45 cmH2O (peak pressure during inspiration, ∼40% PImax), which was initially selected as the highest load that allowed him to perform nearly full vital capacity inspirations. Once a month supervised training at the outpatient clinic was performed. This allowed review of the stored data on quantity and quality of home-based training sessions and comparison of those data with the results obtained during the supervised session. Evaluated training parameters included average mean pressure generated per breath (cmH2O), average mean power per breath (W), average inspiratory volume per breath (L) and total external mechanical work of breathing per session (J) [14]. The training load was progressively increased over the course of the training period from 45 cmH2O up to 62 cmH2O. The patient was very motivated to perform his breathing exercises and the perceived improvement in symptoms during the initial weeks of his training period probably contributed to increase his motivation. He completed a total of 420 sessions over the 6-month period (2.3 sessions per day).
Post-IMT assessment
Lung function tests and phrenic nerve conductance
All lung volumes and capacities increased slightly after training. The differences in FVC between upright and supine performance were unchanged, indicating the persistence of UDD after the intervention period (table 1). Phrenic nerve conduction tests also did not show evidence of improvements in any of the recorded parameters. This suggests the absence of spontaneous nerve recovery [15, 16] during the study period.
Respiratory muscle strength
Bilateral and unilateral twitch Pdi (diaphragm muscle contraction) were still indicative of significant diaphragm weakness after IMT (table 2). However, sniff Pdi improved by 27 cmH2O. This improvement was mostly due to increases in Poes (global inspiratory strength) during the inspiratory sniff manoeuvre (from −93 to −124 cmH2O). The inspiratory sniff Pga remained paradoxical (figure 1). PImax (global inspiratory strength) increased by 25 cmH2O achieving 120% of predicted normal values (table 2). These results suggest that the improvements in inspiratory muscle function were not related to improvements in diaphragm contractility but mostly to improvements in non-diaphragmatic inspiratory muscles [5].
Symptoms
A one-unit change in the transitional dyspnoea index focal score is considered clinically important [17]. After the intervention period the patient presented a change of +6 (out of scores that could range from −9 to +9). The patient reported that he could perform activities such as stair climbing at higher effort levels without breathlessness and that he could perform daily activities more vigorously with less interruptions and less symptoms of breathlessness.
Exercise capacity
During the CPET the patient achieved the same peak work rate (160 W) and similar V′O2max (20.6 mL·kg−1·min−1, 68% predicted) [8] in comparison to baseline assessments.
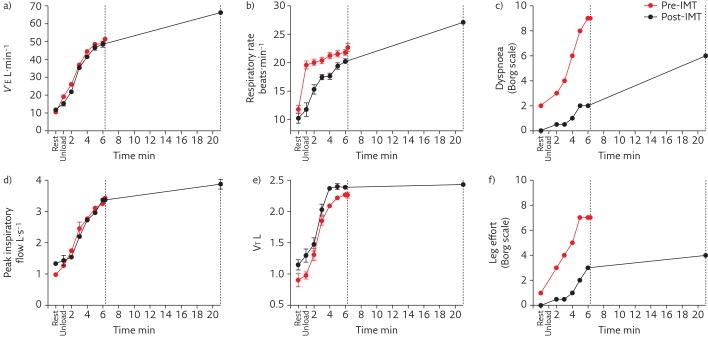
The endurance cycling test was repeated at the same work rate as at baseline. Exercise time increased from 6 to 20 min, thereby reaching the maximal pre-specified duration of the post-intervention test. The dyspnoea perception decreased from 9 to 2 at the timepoint at which the baseline test had to be interrupted by the patient (figure 3). For a similar V′E at iso-work rate during cycling, less diaphragm (EMGdi/EMGdi,max) and scalene (EMGsca/EMGsca,max) activation were observed to generate a similar inspiratory effort (i.e. inspiratory Poes and Pdi) (figures 2 and 3). This comparable inspiratory effort resulted in higher tidal volume and lower respiratory frequency throughout exercise (figures 2 and 3). Both the reduction in the activation for a given inspiratory effort and improved mechanical response (slower and deeper breathing pattern) for a given inspiratory effort contributed to improve neuromechanical coupling. This probably contributed to reduce the intensity of perceived breathing discomfort (dyspnoea) during exercise [18]. In addition, inspiratory Pga remained paradoxical during endurance cycling, indicating the persisting diaphragm defect.
Figure 3.
a) V′E, b) Respiratory rate, c) Borg score for dyspnoea, d) Peak inspiratory flow, e) tidal volume (VT) and f) Borg score for leg effort during constant work rate exercise pre- and post-IMT. Dotted lines indicate the end of the endurance test for pre- and post-IMT.
On the expiratory side, a decrease in EMGabd/EMGabd,max was observed post-IMT and both expiratory Poes and Pga swings decreased by about 50%, mainly due to the decrease in positive expiratory pressure generation (figure 2). This reduced reliance on abdominal muscle recruitment for active expiration suggests decreased reliance on passive recoil forces during inspiration after the intervention [19].
Considering these results, we can conclude that, for this patient, the IMT programme was effective to reduce exertional dyspnoea without improving diaphragm function. Although the IMT did not affect isolated diaphragm function, the improvements in global inspiratory muscle function and breathing pattern during exercise decreased the symptoms, indicating that this patient has benefited from the IMT programme despite his almost normal baseline PImax and persistent UDD. In the initial assessment, the PImax measurement did not detect respiratory weakness. The PImax manoeuvre is a global measurement reflecting global inspiratory muscle strength (combined measurement of diaphragmatic and non-diaphragmatic inspiratory muscle strength). This fact reinforces the need for specific assessments of diaphragmatic strength to identify UDD. Twitch Pdi is therefore the preferred assessment to confirm diagnosis of diaphragm weakness [5].
Additional discussion points
The patient self-reported that he had neither consciously increased sport participation nor his participation in physical activities at work or during leisure time during the study period. In line with this, the absence of changes in peak work rate and VO2max during the incremental cycling test, and similar V′E at a given work rate during the CWR endurance cycling test, make it unlikely that improvements in cardiopulmonary fitness might have contributed to his reductions in exertional dyspnoea symptoms. In addition, no changes in body weight were observed, excluding this factor as a contributor to improvements in respiratory muscle function and symptoms.
Given the patient's continuing motivation and the fact that we observed persisting improvements in both training parameters and respiratory muscle function it was collaboratively decided to continue with the IMT intervention after the 6-month study period. In case a plateau in the training parameters or decrease in motivation are observed, it can be decided to either reduce the frequency to once daily or once every 2 days while maintaining intensity (i.e. external load) in order to maintain benefits.
How the specific effects of IMT that were observed in this specific case relate to the general population of patients with UDD is unclear. Since symptoms vary according to clinical presentation, associated conditions and the possibility of spontaneous recovery in some cases, the respiratory muscle responses to training may also vary. Despite the promising findings in this case, further studies, in addition to the few (mostly case) studies that have been published to date [20–22], are needed to elucidate the specific mechanisms by which IMT can impact on the interaction between the diaphragm and non-diaphragmatic respiratory muscles during exercise breathing in patients with UDD.
Key points:
Patients with diaphragm dysfunction experience symptoms of exertional dyspnoea.
Assessment of pulmonary and respiratory muscle function at rest and during exercise can help to identify abnormalities in breathing characteristics underlying exertional symptoms.
IMT might help these patients to improve respiratory muscle function and reduce symptoms on exertion.
The effects of IMT on exercise capacity and symptoms in the presented case of a patient with persisting UDD were most probably caused by improvements in non-diaphragmatic respiratory muscle function.
Self-evaluation questions
- 1) Which of the listed measurements is the best option to assess isolated diaphragm strength?
- a) Twitch Pdi
- b) PImax
- c) Sniff Pdi
- d) CMAP
- 2) Which of the following observations is typically observed in a patient with UDD?
- a) Important decrease (>40%) in FVC measured in supine position relative to FVC obtained in upright position
- b) A sniff Pdi <80 cmH2O and a bilateral twitch Pdi <20 cmH2O
- c) Severe symptoms of orthopnoea
- d) PImax of <70% of predicted normal value
- 3) IMT may improve exertional dyspnoea in patients with UDD by:
- a) Improving phrenic nerve conduction
- b) Increasing isolated diaphragm contractility
- c) Increasing non-diaphragmatic inspiratory muscle function
- d) Reducing the decrease in FVC in supine position relative to assessments performed in the upright position
Suggested answers
1) a.
2) b.
3) c.
Footnotes
Author contributions D. Langer, R. Gosselink, D. Testelmans and M. Caleffi Pereira contributed to the concept and writing of the manuscript. S. Dacha contributed to the acquisition and data analysis. All authors approved the final manuscript.
Conflict of interest: M. Caleffi Pereira has nothing to disclose.
Conflict of interest: S. Dacha has nothing to disclose.
Conflict of interest: D. Testelmans has nothing to disclose.
Conflict of interest: R. Gosselink has nothing to disclose.
Conflict of interest: D. Langer has nothing to disclose.
Support statement This study was financed by Research Foundation – Flanders (FWO) (grant GOA4516N) and KU Leuven (grant C22/15/035) and, in part, by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance code 001.
References
- 1.Resman-Gaspersc A, Podnar S. Phrenic nerve conduction studies: technical aspects and normative data. Muscle Nerve 2008; 37: 36–41. [DOI] [PubMed] [Google Scholar]
- 2.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26: 511–522. [DOI] [PubMed] [Google Scholar]
- 4.Neder JA, Andreoni S, Lerario MC, et al. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res 1999; 32: 719–727. [DOI] [PubMed] [Google Scholar]
- 5.ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002; 166: 518–624. [DOI] [PubMed] [Google Scholar]
- 6.McCool FD, Manzoor K, Minami T. Disorders of the diaphragm. Clin Chest Med 2018; 39: 345–360. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 2001; 37: 153–156. [DOI] [PubMed] [Google Scholar]
- 8.Blackie SP, Fairbarn MS, McElvaney GN, et al. Prediction of maximal oxygen uptake and power during cycle ergometry in subjects older than 55 years of age. Am Rev Respir Dis 1989; 139: 1424–1429. [DOI] [PubMed] [Google Scholar]
- 9.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. [PubMed] [Google Scholar]
- 10.Luo YM, Moxham J, Polkey MI. Diaphragm electromyography using an oesophageal catheter: current concepts. Clin Sci 2008; 115: 233–244. [DOI] [PubMed] [Google Scholar]
- 11.Langer D, Ciavaglia C, Faisal A, et al. Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD. J Appl Physiol 2018; 125: 381–392. [DOI] [PubMed] [Google Scholar]
- 12.Charususin N, Gosselink R, McConnell A, et al. Inspiratory muscle training improves breathing pattern during exercise in COPD patients. Eur Respir J 2016; 47: 1261–1264. [DOI] [PubMed] [Google Scholar]
- 13.Langer D, Charususin N, Jacome C, et al. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Phys Ther 2015; 95: 1264–1273. [DOI] [PubMed] [Google Scholar]
- 14.Langer D, Jacome C, Charususin N, et al. Measurement validity of an electronic inspiratory loading device during a loaded breathing task in patients with COPD. Respir Med 2013; 107: 633–635. [DOI] [PubMed] [Google Scholar]
- 15.McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl J Med 2012; 366: 932–942. [DOI] [PubMed] [Google Scholar]
- 16.Gayan-Ramirez G, Gosselin N, Troosters T, et al. Functional recovery of diaphragm paralysis: a long-term follow-up study. Respir Med 2008; 102: 690–698. [DOI] [PubMed] [Google Scholar]
- 17.Witek TJ J, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J 2003; 21: 267–272. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell DE, Banzett RB, Carrieri-Kohlman V, et al. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc 2007; 4: 145–168. [DOI] [PubMed] [Google Scholar]
- 19.Bonnevie T, Gravier FE, Ducrocq A, et al. Exercise testing in patients with diaphragm paresis. Respir Physiol Neurobiol 2018; 248: 31–35. [DOI] [PubMed] [Google Scholar]
- 20.Chatham K, Gelder CM, Lines TA, et al. Suspected statin-induced respiratory muscle myopathy during long-term inspiratory muscle training in a patient with diaphragmatic paralysis. Phys Ther 2009; 89: 257–266. [DOI] [PubMed] [Google Scholar]
- 21.Kodric M, Trevisan R, Torregiani C, et al. Inspiratory muscle training for diaphragm dysfunction after cardiac surgery. J Thorac Cardiovasc Surg 2013; 145: 819–823. [DOI] [PubMed] [Google Scholar]
- 22.Petrovic M, Lahrmann H, Pohl W, et al. Idiopathic diaphragmatic paralysis–satisfactory improvement of inspiratory muscle function by inspiratory muscle training. Respir Physiol Neurobiol 2009; 165: 266–267. [DOI] [PubMed] [Google Scholar]