Abstract
Background: Ganoderma lucidum has been used in Chinese medicine for thousands years to improve health and to promote longevity. One important function of G lucidum is to modulate the immune system. However, the underlying mechanism is not well understood. Programmed cell death protein 1 (PD-1) is a cell surface protein present in certain immune cells (eg, B- and Tcells) and plays an important role in modulating the immune response. The role of PD-1 protein in G lucidum–mediated immunomodulation is unknown. Methods: Cultured human Blymphocytes and extract prepared from G lucidum spores (GLE) were used to determine PD-1 protein in G lucidum–mediated immunomodulation. Both western blotting and immunofluorescence (IF) microscopy assays were used to determine the effect of GLE treatment on PD-1 protein expression. A reverse transcription-based quantitative polymerase chain reaction (real-time PCR) assay was used to determine the effect of GLE on transcription of pdcd-1 gene. Results: Both our western blotting and IF staining results demonstrated great reduction in PD-1 protein and in proportion of PD-1+ cells in these B-lymphocytes. Our real-time PCR results indicated that this PD-1 protein reduction was not caused by a transcriptional inhibition of the gene. In addition, our western blotting study further revealed that the GLE treatment caused an increase in expression of CCL5 chemokine in the cultured B-lymphocytes. Conclusions: PD-1 protein is an important target of G lucidum–mediated immunomodulation. G lucidum and its bioactive compounds can be developed into novel immunomodulators for prevention and treatment of cancer and many other diseases.
Keywords: Ganoderma lucidum, Ganoderma lucidum spore extract, GLE, immunomodulation, PD-1, B-lymphocytes, protein reduction, transcription inhibition, novel immunomodulators
Introduction
Fungi have been used in traditional Chinese medicine for thousands of years to prevent and treat a variety of diseases including allergies, arthritis, hypertension, hepatitis, and inflammation, among others.1-8 Ganoderma lucidum, which also is known as Lingzhi (in China) or Reishi (in Korea and Japan), is a well-known medicinal fungus that has been used to improve health and to promote longevity.1,2,4 Recent studies demonstrated the effects of G lucidum on immunomodulation and antitumor properties,9-15 although the underlying molecular mechanisms are not fully understood.
Many bioactive compounds are carried by G lucidum.2,3,7,13,16 Based on their chemical structures, these compounds can be categorized into 2 major groups: triterpenoids and polysaccharides.2,5-7,11-15 There are over 150 different triterpenoid products, including ganoderic acids and ganoderma alcohols (Ganoderiols), identified in G lucidum.13,17 The polysaccharides carried by G lucidum contain many different sugar compositions (eg, glucose, mannose, galactose, and glucuronic acid) and are generally in the range of 13 kDa to 23 kDa.11,15,18 It is generally believed that the polysaccharides function as immunomodulators, whereas the triterpenoids inhibit cancer cell proliferation and metastasis.3,10 Both fruit body and spores of G lucidum carry polysaccharides and triterpenoids, although high levels of these contents are found mostly in the spores of G lucidum. The contents of spores cannot be easily released because of a 2-layer wall structure unless the wall structure is removed or punctured.
Programmed cell death protein 1, also known as PD-1 and CD279, is a cell membrane protein encoded by pdcd-1 gene.19 PD-1 protein is expressed in many types of immune cells, including B- and T-lymphocytes.20-26 PD-1 protein interacts with programmed death ligand 1 (PD-L1), which is expressed in dendritic cells, macrophages, and many types of cancer cells, to regulate the immune system.20-26 The interaction of PD-1 with PD-L1 inhibits T-cell proliferation, suppresses T-cell inflammatory activity, and prevents autoimmune diseases.20-28 However, this interaction also prevents the immune system from killing tumor cells, leading to cancer development. Therefore, strategies to block the PD-1/PD-L1 interaction have attracted great attention in cancer prevention and treatment. Antibodies that target PD-1 and PD-L1 have been used in treating many types of cancer.29-33 However, many challenges exist in applying the antibody-based immunotherapy to cancer treatment.34 Developing alternative approaches or identifying new compounds that can more effectively disrupt the PD-1/PD-L1 interactions, therefore, would be important for PD-1/PD-L1–based cancer therapy in the future.
In our recent studies, we investigated the possible role of PD-1 protein in G lucidum–mediated immunomodulation. Using extract prepared from wall-broken G lucidum spores (GLE), the results of our western blotting demonstrated that treatment of GM00130 and GM02248 human B-lymphocytes with GLE caused a great reduction of PD-1 protein in these cells. The results of our immunofluorescence (IF) microscopy study revealed that the GLE treatment caused a reduction of PD-1+ cells in the cultured GM00130 B-lymphocytes. However, the results of our real-time polymerase chain reaction (PCR) studies indicated that the transcription level of pdcd-1 gene was not inhibited by the GLE treatment in these cells, which rules out the possibility of transcriptional inhibition of pdcd-1 gene for GLE-mediated reduction of PD-1 protein. In addition, the results of our western blotting studies revealed that the GLE treatment caused an increase of chemokine CCL5 in both GM00130 and GM02248 human B-lymphocytes. These results suggest that reducing PD-1 protein is an important mechanism for G lucidum–mediated immunomodulation, and the GLE-mediated PD-1 protein reduction is not caused by a transcriptional inhibition of the pdcd-1 gene. These results further suggest that G lucidum and its contents can be developed into novel immunomodulators to target the PD-1 protein for treatment of cancer and many other diseases.
Materials and Methods
Cells, Chemicals, and Oligonucleotides Used in the Study
The GM00130 and GM02248 cells were purchased from the Coriell Institute for Medical Research (Camden, NJ). Both GM00130 and GM02248 cells were Epstein-Barr virus–transformed human B-lymphocytes. The GM00130 B-lymphocytes were derived from a normal individual, whereas the GM02248 B-lymphocytes were derived from a patient who carried a mutant XPC protein, which is required for nucleotide excision repair process.35-37 Both GM00130 and GM02248 B-lymphocytes were maintained in RPMI1640 medium supplemented with 15% fetal bovine serum at 37°C with 5% CO2.
The phorbol 12-myristate 13-acetate (PMA) was purchased from the Sigma Aldrich Inc (St. Louis, MO). The ionomycin (Io) was purchased from Cayman Chemical (Ann Arbor, MI). All other chemicals used in this study were purchased from the Sigma Aldrich Inc. The PMA was prepared as 5 mg/mL stock in dimethyl sulfoxide and stored at −80°C. The Io was purchased as 10 mM stock in ethanol and stored at −80°C. The Io was diluted to 1 mM in 1× phosphate-buffered saline (PBS) and used for cell treatment immediately.
The oligonucleotides used in this study were synthesized by Midland Certified Reagent Company (Midland, TX), and the sequences of these oligonucleotides are listed in Table 1.
Table 1.
Primers Used in the Real-Time PCR Study.
| pdcd-1 forward primer | 5′-CAGCCTGGTGCTGCTAGTCTG-3′ |
| pdcd-1 reverse primer | 5′-GTCCACAGAGAACACAGGCAC-3′ |
| PD-L1 forward primer | 5′-GTGTACCGCTGCATGATCAG-3′ |
| PD-L1 reverse primer | 5′-GGTAGCCCTCAGCCTGACATG-3′ |
| Bcl-XL forward primer | 5′-GAGGTGATCCCCATGGCAGC-3′ |
| Bcl-XL reverse primer | 5′-CCAAGCTGCGATCCGACTCAC-3′ |
| CCL5 forward primer | 5′-CTGCGCTCCTGCATCTGCCTC-3′ |
| CCL5 reverse primer | 5′-GAGTTGATGTACTCCCGAAC-3′ |
| p16 forward primer | 5′-GAGCCTTCGGCTGACTGGCTG-3′ |
| p16 reverse primer | 5′-CCGTGGAGCAGCAGCAGCTC-3′ |
| p53 forward primer | 5′-AGCAGTCACAGCACATGACG-3′ |
| p53 reverse primer | 5′-CACTCGGATAAGATGCTGAG-3′ |
Abbreviation: PCR, polymerase chain reaction.
Preparation of G lucidum Spore Extract
The wall-broken G lucidum spores powder was obtained from Longevity Valley Pharmaceuticals Inc (People’s Republic of China). The GLE was prepared as follows: the G lucidum spore powder was weighed and resuspended in 10 times 95% ethanol (w/v) and incubated at 85°C for 2 hours. The supernatant was collected after centrifugation (10 000 rpm × 10 minutes at 4°C). The pellets were resuspended into another 10 times 95% ethanol (w/v) and incubated at 85°C for 2 hours. The supernatant was collected by centrifugation. The supernatants were combined, filtered through a 0.22µm filter, and dried in CentriVap concentrator (Labconco). The dried pellets were weighted and resolved into 1× PBS at a concentration of 100 mg/mL and filtered through a 0.22 µm filter. The solution was designated as GLE and stored at −80°C for further use.
Inducing Expression of PD-1 Protein
The cultured GM00130 and GM02248 B-lymphocytes were treated with both PMA (50 ng/mL) and Io (1 µM) by adding the stock solutions directly to the culture medium and incubated at 37°C cell culture incubator for 48 hours. Levels of pdcd-1 mRNA and PD-1 protein were determined by reverse transcription–based quantitative PCR (qPCR, real-time PCR) and western blotting, respectively.
GLE Treatment
The cultured GM00130 and GM02248 human B-lymphocytes were treated with PMA/Io for 24 hours to induce expression of the PD-1 protein. The PMA/Io-treated cells were then treated with GLE by adding the GLE directly into the cell growth medium. For the real-time PCR study, cells were collected 24 hours after the GLE treatment and total RNA was isolated from the harvested cells using the RNeasy Mini Kit (Qiagen Inc, Valencia, CA). For the immunoblotting study, cells were collected 48 hours after the GLE treatment and lysed in RIPA cell lysis buffer.
Cell Growth Curve Assay
For the cell growth study, both GM00130 and GM02248 human B-lymphocytes were treated with GLE at indicated concentrations (0.25 mg/mL, 0.5 mg/mL, and 1 mg/mL) in the presence or absence of PMA (50 ng/mL)/Io (1 µM). The cell density was determined for each treatment at various time points after the GLE treatment (days 0, 1, 2, 3, and 4) using Invitrogen’s Countess automated cell counter. The cell growth study was performed in 3 independent experiments, and the cell growth curve was determined for each treatment using a GraphPad Prism software (La Jolla, CA).
Fluorescence-Activated Cell Sorting (FACS) Assay
For FACS study, cells were collected 48 hours after the GLE treatment. Cells were washed once in 10 mL 1× PBS/1% bovine serum albumin buffer and then resuspended into 100 µL flow cytometry staining buffer (1× PBS, 1% bovine serum albumin, and 0.1% sodium azide). The cells were stained with allophycocyanin (APC)-labeled mouse anti-human CD279 (PD-1) antibody (MIH4, eBioscience) by mixing the cells with 5 µL APC-labeled PD-1 antibody and incubating on ice for 30 minutes. The cells were washed 3 times in flow cytometry staining buffer (1 mL × 3) to remove any free PD-1 antibody. The cells were finally resuspended in 1 mL flow cytometry staining buffer and counterstained with a DAPI reagent for visualizing nuclei of live cells. The cells were analyzed by flow cytometry to determine the percentage of PD-1+ cell population in each treatment.
Immunofluorescence Microscopy Assay
For IF microscopy study, the GM00130 human B-lymphocytes were stained with APC-labeled mouse antihuman CD279 (PD-1) antibody and resuspended into 1 mL flow cytometry staining buffer. The nuclei of cells were counterstained with a DAPI reagent. Twenty microliters of cells was placed onto Invitrogen’s Countess cell counting chamber slide. The PD-1+ cells were visualized by a Zeiss LSM 780 fluorescence microscope using red light (630 nm), and the nuclei of cells were visualized by blue light (480 nm). A total of 250 to 300 cells were counted for each treatment, and the percentage of PD-1+ cells were determined for each treatment.
Reverse Transcription-Based qPCR (Real-Time PCR) Assay
RNA isolated from both untreated and treated GM00130 and GM02248 human B-lymphocytes were used in the real-time PCR assay. A reverse transcription reaction was used to generate cDNA from the RNA samples using a High-Capacity cDNA Reverse transcription Kit (Applied Biosystems Inc, Foster City, CA). The cDNAs were then used as templates in a qPCR protocol to determine the mRNA levels of pdcd-1, PD-L1, Bcl-XL, CCL5, p16, and p53 using a Power Sybr Green PCR Master Mix (Applied Biosystems Inc) with primers designed to bind to the coding region sequences of these genes. The mRNA level of β-actin was also determined from each cDNA sample and used as an internal control for the real-time PCR study. The mRNA level of β-actin in the untreated GM00130 B-lymphocytes was used as a standard, and the levels of pdcd-1, PD-L1, Bcl-XL, CCL5, p16, and p53 mRNAs in each RNA sample were calculated as fold change compared with that of the untreated GM00130 B-lymphocytes. Triplicates were used for each sample in the real-time PCR study, and 3 independent experiments were done for the real-time PCR study. The mRNA level of each target gene was calculated as mean fold + standard deviation (SD) to that of the GM00130 B-lymphocytes. Statistical analysis was done to determine if any significant difference in the level of mRNA exists for each tested gene between untreated and treated cells for GM00130 and GM02248 B-lymphocytes (*P ≤ .05; **P ≤ .01).
Western Blotting Assay
The immunoblotting study was performed using SDS-PAGE with 10% or 12% gel, and 15 µg total protein/lane was used for each cell lysate. After transferring the proteins to polyvinylidene difluoride membranes, the membranes were hybridized with antibodies against PD-1 (AF1086, R & D System), RANTES (CCL5; C-12, Santa Cruz Biotechnology, Dallas, TX), CD19 (PDR134, Santa Cruz Biotechnology), AKT1 (B1, Santa Cruz Biotechnology), or β-actin (C4, Santa Cruz Biotechnology). Quantification of the proteins bands on X-ray films was done using an ImageJ software (https://imagej.nih.gov/ij/).
Data Analysis
All data were presented as mean + SD. Statistically significant differences were determined using a Student’s t test with 95% confidence interval (CI). The data were obtained from at least 3 independent experiments.
Results
The Effect of GLE Treatment on Proliferation of GM00130 and GM02248 Human B-Lymphocytes
Many studies have demonstrated the effects of G lucidum on immunomodulation.10,11,15 Therefore, we first determined the effect of GLE on proliferation of immune cells. Since our work was focused on determining the role of PD-1 protein in G lucidum–mediated immunomodulation and results of studies have demonstrated expression of the PD-1 protein in various types of immune cells, including B- and T-cells,20-26 we chose both GM00130 and GM02248 human B-lymphocytes in this study as a model. We also used both PMA and Io to induce expression of PD-1 in cultured B-lymphocytes in this work because the work of others has demonstrated the induced expression of PD-1 in B- and T-cells by PMA/Io.38-40
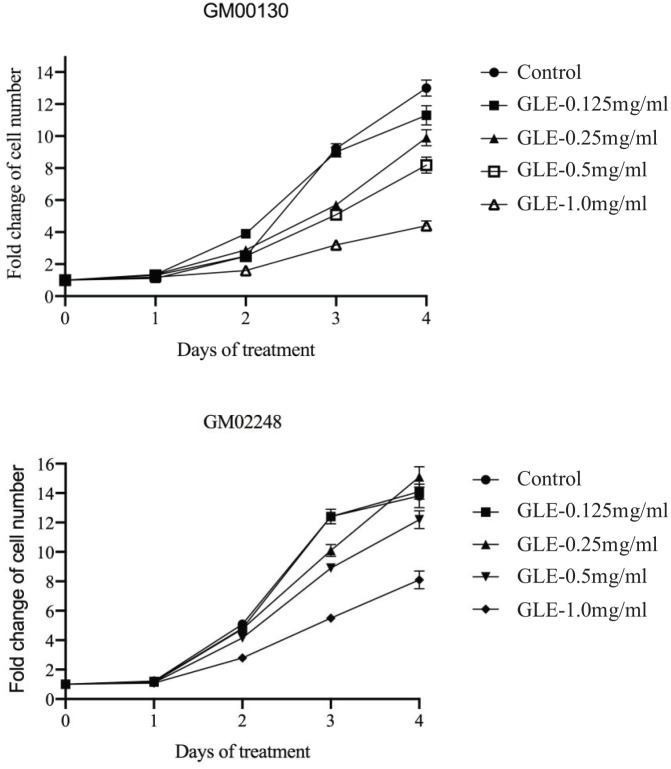
Both GM00130 and GM02248 B-lymphocytes were treated with GLE at various concentrations (0.125 mg/mL, 0.25 mg/mL, 0.5 mg/mL, and 1 mg/mL) by adding the GLE directly into the cell growth medium. At various time points (days 0, 1, 2, 3, and 4), cell density was determined and the cell growth curve was determined for each treatment (Figure 1). The results of our cell proliferation studies indicated that the cell growth of both GM00130 and GM02248 human B-lymphocytes were not greatly affected by the GLE at concentrations as high as 0.5 mg/mL (Figure 1). At 1 mg/mL GLE concentration, however, some clear inhibitory effects were observed in proliferation of these cells (Figure 1). Therefore, the 0.5 mg/mL GLE was chosen for our PD-1 protein study.
Figure 1.
The growth curve of GM00130 and GM02248 human B-lymphocytes in the presence of various concentrations of GLE. Cells were treated with GLE at indicated concentration by adding the GLE directly into the cell growth medium. The cell density was determined at various time points after the GLE treatment (days 0, 1, 2, 3, and 4), and the cell growth curve was generated from the cell density obtained from this study (mean ± SD). The results were based on 3 independent experiments. The cell growth curve was generated by GraphPad Prism software (La Jolla, CA).
The GLE Treatment Caused Reduction of the PD-1 Protein in Both GM00130 and GM02248 Human B-Lymphocytes
To study a mechanism through which the presence of GLE causes immunomodulation, we focused on determining the role of PD-1 protein on this action because of its important function in immunomodulation.20-26 The clinical implication of PD-1/PD-L1–based immunotherapy in cancer treatment29-33 also prompted us in studying the role of PD-1 in G lucidum–mediated immunomodulation.
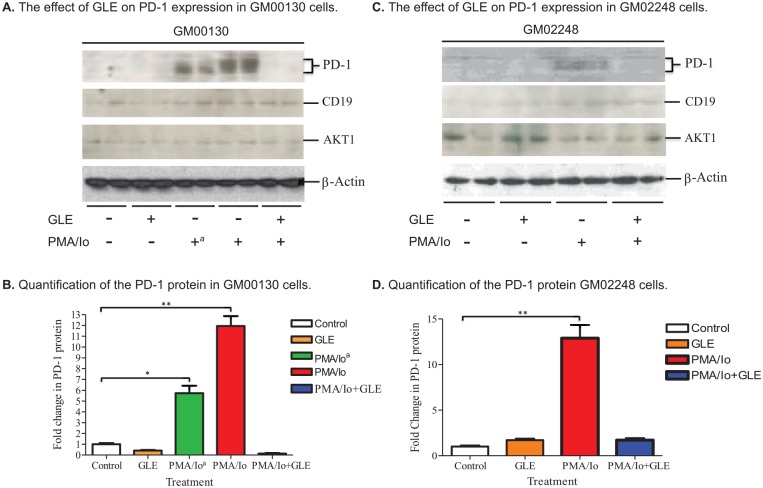
Both GM00130 and GM02248 human B-lymphocytes were treated with PMA/Io to induce expression of PD-1 protein in these cells. The cells were then treated with GLE (0.5 mg/mL) for 48 hours. The cells were harvested and lysed in RIPA cell lysis buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 25 mM Tris, pH 7.4). The level of PD-1 protein was determined for each cell lysate by an immunoblotting assay (Figure 2). As controls, the protein levels of CD19, which is a cell membrane protein expressed on B-lymphocytes, and AKT1, which is an intracellular protein expressed in most cell types including B cells, were also determined from the cell lysates (Figure 2). Low levels of PD-1 protein were detected from both GM00130 and GM02248 human B-lymphocytes (Figure 2A and B). The treatment of PMA/Io increased the PD-1 protein level to 11.9-fold in GM00130 and 12.9 fold in GM02248 B-lymphocytes (Figure 2B and D). The treatment of GM00130 and GM02248 human B-lymphocytes with GLE, however, greatly reduced the level of PD-1 protein in the PMA/Io-treated cells to 0.14 fold and 1.71 fold to that of the untreated GM00130 and GM02248 B-lymphocytes, respectively (Figure 2B and D). As controls, the protein levels of CD19 and AKT1 were not affected by the GLE treatment in these cells (Figure 2A and C). (The Supplement Figure 1 contains more controls, including cells treated with DMSO alone as a nagative control). These results clearly demonstrated that PD-1 protein was an important target for GLE treatment and the GLE treatment caused great reduction of the PD-1 protein in the human B-lymphocytes.
Figure 2.
The effect of GLE on expression of PD-1 protein in both GM00130 and GM02248 human B-lymphocytes. The cells were first treated with PMA/Io for 24 hours. Some of the cells were then treated with GLE (0.5 mg/mL) for 48 hours. Cells lysates prepared from the cells (15 µg protein/lane) were analyzed by western blotting. Quantification of the PD-1 protein was the result of 4 independent western blots (*P ≤ .05; **P ≤ .01).
The GLE Treatment Resulted in a Decrease of PD-1+-Cells in GM00130 Human B-Lymphocytes
The results of our immunoblotting studies demonstrated the greatly reduced levels of PD-1 protein in the GLE-treated GM00130 and GM02248 human B-lymphocytes. To further evaluate the effect of GLE treatment on expression of PD-1 protein, we determined the status of PD-1+ cells in both untreated and GLE-treated GM00130 human B-lymphocytes.
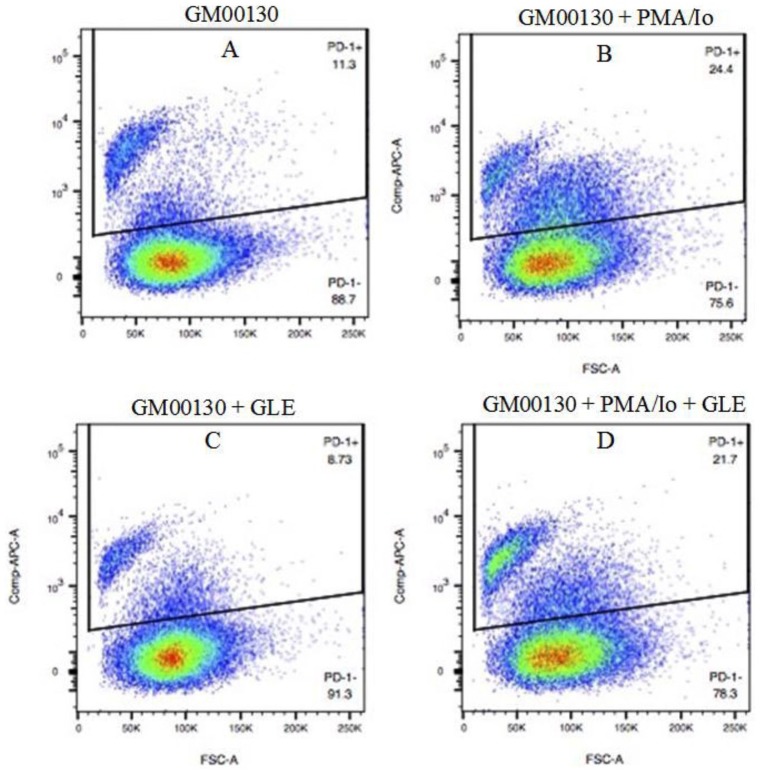
The effect of GLE treatment on the number of PD-1+ cells was first determined by a FACS assay. The GM00130 human B-lymphocytes were treated with GLE in the presence or absence PMA/Io for 48 hours. The cells were harvested and stained with an APC-labeled mouse anti-human PD-1 antibody. The cells were analyzed by a FACS assay to determine the proportion of PD-1+ cells in each treatment (Figure 3). A low percentage of PD-1+ cells (11.3%) was detected in the cultured GM00130 human B-lymphocytes in our FACS assay (Figure 3A). When treated with PMA/Io, the PD-1+ cells were increased to 24.4% (Figure 3B). The GLE treatment decreased the PD-1+ cells from 11.3% to 8.54% in cultured GM00130 cells and from 24.4% to 21.7% in PMA/Io-treated GM00130 cells, respectively (Figure 3C vs A and D vs B).
Figure 3.
The effect of GLE on PD-1+ cells in GM00130 human B-lymphocytes by FACS. The GM00130 human B-lymphocytes were treated with GLE (0.5 mg/mL) in the absence or presence of PMA (50 ng/mL)/Io (1 µM) at 37°C in cell culture incubator for 48 hours. The cells were harvested and stained with APC-labeled mouse antihuman PD-1 antibody (MIH4, eBioscience) and counterstained with a DAPI reagent. The cells were analyzed by FACS to determine the percentage of PD-1+ cells in each treatment. The flow cytometry data were from 3 independent experiments.
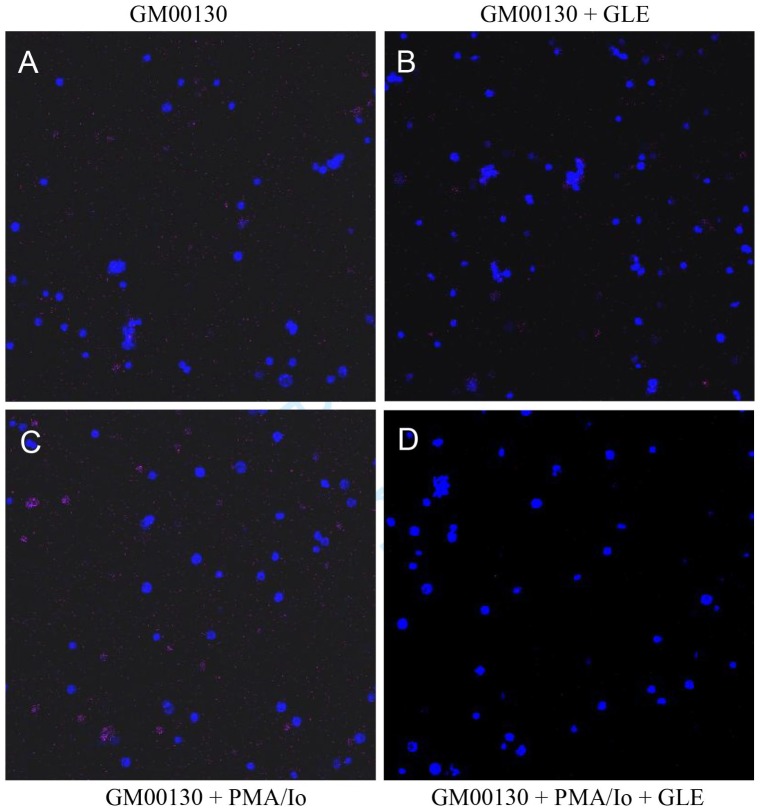
Although some decrease of PD-1+ cells was observed in the GLE-treated GM00130 B-lymphocytes in our FACS study, this rate of decrease was not as great as we anticipated based on our western blot results. To further evaluate the effect of GLE treatment on the number of PD-1+ cells, we also performed an IF microscopy assay to determine the effect of GLE treatment on the number of PD-1+ cells using the cells prepared for our FACS assay (Figure 4 and Table 2). The results of our IF microscopy study revealed that 3.7% of PD-1+ cells exist in the cultured GM00130 human B-lymphocytes (Figure 4 and Table 2). The PMA/Io treatment increased the PD-1+ cells to 19.2% in the cultured GM00130 human B-lymphocytes (Figure 4 and Table 2). When treated with GLE, however, the percentage of PD-1+ cells was decreased to 1.8% and 4.5% in GM00130 and PMA/Io-treated GM00130 B-lymphocytes, respectively (Figure 4 and Table 2). The results of this IF microscopy analysis clearly demonstrated that the GLE treatment caused a reduction of PD-1+ cells in the GM00130 human B-lymphocytes.
Figure 4.
The effect of GLE on PD-1+ cells in GM00130 human B-lymphocytes by immunofluorescence (IF) microscopy assay. The GM00130 human B-lymphocytes were treated with GLE (0.5 mg/mL) in the absence or presence of PMA (50 ng/mL)/Io (1 µM) at 37°C for 48 hours. The cells were harvested, stained with an APC-labeled mouse antihuman PD-1 antibody, and counterstained with a DAPI reagent. The PD-1+ cells were visualized by a Zeiss LSM 780 fluorescence microscope using red light (630 nm). The nuclei of cells were visualized under the fluorescence microscope using a blue light (480 nm). The IF microscopy study was done in 3 independent experiments.
Table 2.
The Effect of GLE Treatment on PD-1+ Cells in the Cultured GM00130 Human B-Lymphocytes. The Number of Both PD-1+ and PD-1− Cells Were Counted From 3 Views/Reactions.
| Treatment | No. of PD-1− Cells | No. of PD-1+ Cells | Total No. of Cells | % of PD-1+ Cells |
|---|---|---|---|---|
| Control | 258 | 10 | 268 | 3.7% |
| PMA/Io | 232 | 55 | 287 | 19.2% |
| GLE | 219 | 4 | 223 | 1.8% |
| PMA/Io + GLE | 276 | 13 | 289 | 4.5% |
Abbreviations: GLE, Ganoderma lucidum spores extract; PMA, phorbol 12-myristate 13-acetate; Io, ionomycin.
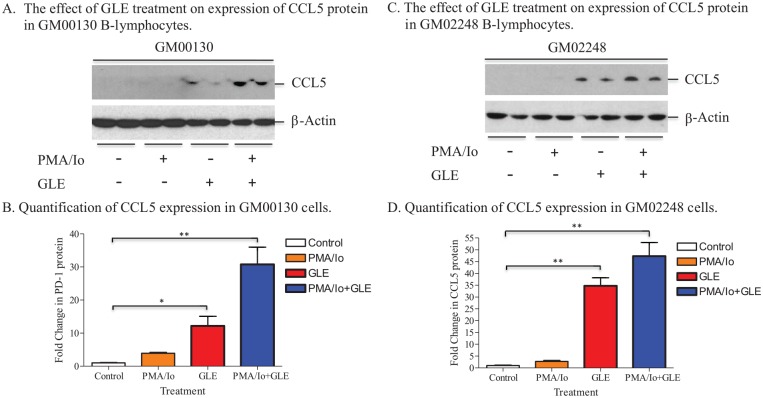
The GLE Treatment Resulted in an Increase in Expression of CCL5 Protein in Both GM00130 and GM02248 Human B-Lymphocytes
The results of both our immunoblotting and IF microscopy studies demonstrated the effect of GLE on reducing PD-1 protein in both GM00130 and GM02248 human B-lymphocytes. To further understand the effect of G lucidum–mediated immunomodulation, we determined the level of chemokine C-C motif ligand 5 (CCL5) in both untreated and GLE-treated GM00130 and GM02248 human B-lymphocytes by western blotting assay. CCL5 is a chemokine that plays a very important role in recruiting immune cells (eg, T-cells and eosinophils) to tumors and in proliferating immune cells.41-44 Recent work of others demonstrated that blockade of PD-1 protein with a PD-1 antibody resulted in an increase in expression of CCL5 and enhanced migration of T-cells to tumors.42,44
The results of our western blotting revealed that the CCL5 chemokine was expressed at very low levels in both untreated GM00130 and GM02248 human B-lymphocytes (Figure 5A/B and C/D). The PMA/Io treatment had little effect on CCL5 protein expression in both GM00130 and GM02248 human B-lymphocytes (Figure 5A/B and C/D). In the presence of GLE, however, the level of CCL5 was significantly more increased, especially in the cells that were treated with both PMA/Io and GLE (Figure 5A/B and C/D). This result suggests that the GLE treatment caused significantly more increase of the CCL5 chemokine in both GM00130 and GM02248 human B-lymphocytes.
Figure 5.
The effect of GLE on expression of CCL5 protein in both GM00130 and GM02248 human B-lymphocytes. Both GM00130 and GM02248 human B-lymphocytes were treated with GLE (0.5 mg/mL) in the presence or absence of PMA (50 ng/mL)/Io (1 µM) at 37°C cell culture incubator for 48 hours. The cells were collected, and 15 µg total protein/lane was used in the western blotting with 13% gel. The CCL5 (C-12) antibody was purchased from Santa Cruz Biotechnologies Inc. Quantification of the CCL5 protein was the result of 4 independent western blots (*P ≤ .05; **P ≤ .01).
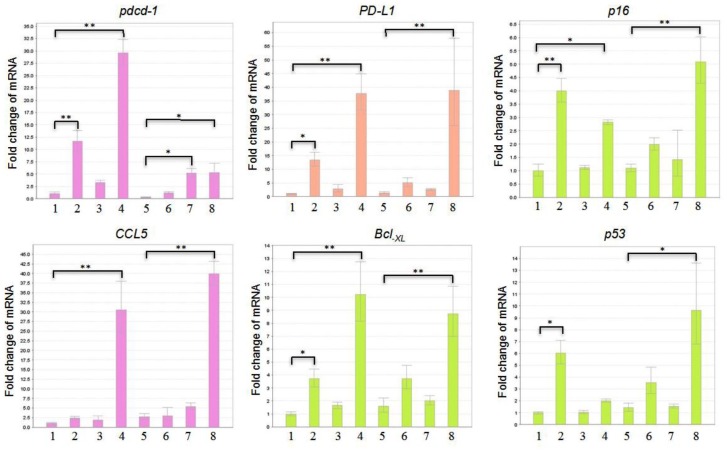
The GLE Treatment Did Not Inhibit Transcription of pdcd-1 Gene in Either GM00130 or GM02248 Human B-Lymphocytes
The results of our immunoblotting studies demonstrated the effect of GLE treatment on reducing expression of PD-1 protein in both cultured GM00130 and GM02248 human B-lymphocytes. To define a mechanism through which the GLE treatment causes decreased PD-1 protein in these cells, we further performed a reverse transcription-based qPCR (real-time PCR) study to determine the mRNA level of pdcd-1 genes, which encodes the PD-1 protein, in both untreated and GLE-treated GM00130 and GM02248 human B-lymphocytes (Figure 6). As controls, the mRNA levels of several genes, including Bcl-XL, CCL5, p16, p53, and PD-L1, were also determined from the RNA samples by the real-time PCR assay (Figure 6). The results of our real-time PCR study indicated that the PMA/Io treatment caused a moderate increase of pdcd-1 mRNA in both GM00130 and GM02248 human B-lymphocytes (Figure 6). Interestingly, the GLE treatment caused significant more increases in the level of pdcd-1 mRNA in both GM00130 and GM02248 human B-lymphocytes (Figure 6), and the combinational treatment of both PMA/Io and GLE caused even more increases of the pdcd-1 mRNA in both GM00130 and GM02248 human B-lymphocytes (Figure 6). The mRNA levels of the Bcl-XL, CCL5, p16, p53, and PD-L1 genes were also significantly more increased in the GLE-treated GM00130 and GM02248 B-lymphocytes than those untreated or PMA/Io-treated GM00130 or GM02248 B-lymphocytes (Figure 6). These results strongly suggest that transcriptional inhibition of the pdcd-1 gene was not a mechanism for GLE-mediated PD-1 reduction in GM00130 or GM2248 human B-lymphocytes.
Figure 6.
The effect of GLE on transcriptions of pdcd-1, Bcl-XL, CCL5, p16, p53, and PD-L1 genes in both GM00130 and GM02248 human B-lymphocytes. The cells were treated with GLE (0.5 mg/mL) in the absence or presence of PMA (50 ng/mL)/Io (1 µM) for 24 hours, and total RNA was isolated. A reverse transcription-based quantitative PCR (real-time PCR) assay was performed to determine the mRNA levels of the indicated genes in each RNA sample, and 40 ng total RNA/reaction was used in the real-time PCR study. (1) Untreated GM00130 cells; (2) GLE-treated GM00130 cells; (3) PMA/Io-treated GM00130 cells; (4) PMA/Io + GLE-treated GM00130 cells; (5) untreated GM02248 cells; (6) GLE-treated GM02248 cells; (7) PMA/Io-treated GM02248 cells; (8) PMA/Io + GLE-treated GM02248 cells (*P ≤ .05; **P ≤ .01).
Discussion
Ganoderma lucidum has been used in traditional Chinese medicine for thousands of years to promote better health and to treat various diseases.1-8,45,46 One important benefit of G lucidum consumption is its ability to modulate the immune system, which enhances the immune response to fight against development of many diseases.10-15 Although many studies have been done in determining the medicinal effects of G lucidum, the mechanism that leads to the immunomodulating effect of G lucidum is largely unknown. In this work, we focused on determining the role of PD-1 protein in GLE-mediated immunomodulation using cultured human B-lymphocytes as a model. The results of our immunoblotting studies demonstrated that the GLE treatment caused a great reduction of the PD-1 protein in both GM00130 and GM02248 human B-lymphocytes. The results of our FACS and IF microscopic studies revealed that the GLE treatment led to a decrease in PD-1+ cells in GM00130 human B-lymphocytes. However, the results of our real-time PCR studies indicated that the GLE treatment did not inhibit transcription of the pdcd-1 gene in either GM00130 or GM02248 human B-lymphocytes, eliminating the possible role of transcriptional inhibition for GLE-mediated PD-1 protein reduction in these human B-lymphocytes. In addition, the results of our immunoblotting studies further revealed that the GLE treatment caused a great increase of the CCL5 chemokine in both GM00130 and GM02248 human B-lymphocytes. Considering the important function of PD-1 protein in immunomod-ulation, these results suggest that reducing PD-1 protein is an important mechanism for G lucidum–mediated immunomodulation.
The results of our western blotting studies demonstrated that the GLE treatment caused a large decrease in the levels of PD-1 protein in cultured GM00130 and GM02248 human B-lymphocytes. However, the results of our real-time PCR studies revealed that the GLE treatment did not cause any decrease in transcription of the pdcd-1 gene in these cells. Therefore, transcriptional inhibition of the pdcd-1 gene is not a likely mechanism for GLE-mediated reduction of the PD-1 protein in these human B-lymphocytes. The mechanism that leads to a reduced PD-1 protein in the GLE-treated GM00130 and GM02248 human B-lymphocytes is unknown. However, the work of a recent report suggests that ubiquitination of PD-1 protein is an important mechanism for modulating the anticancer immunity of T-cells.47 It is possible that the G lucidum modulates the immune system (eg, T- and B-lymphocytes) through ubiquitination and subsequent proteasomal degradation of the PD-1 protein. Although GLE might cause PD-1 protein reduction through enhancing membrane internalization and subsequent ubiquitination of the PD-1 protein, the results of our western blotting study on CD19 are strongly against this possibility since the levels of CD19 B-cell membrane protein remains unchanged under the GLE treatment. Another possible mechanism is that certain compound(s) contained in the GLE may selectively bind to the PD-1 protein, which cause conformation change of the protein and lead to ubiquitination and degradation of the PD-1 protein. Further studies need to determine if ubiquitination indeed occurs in the GLE-treated GM00130 and GM02248 human B-lymphocytes, and the compound(s) that selectively bind to the PD-1 protein also need to be identified.
The results of our real-time PCR studies revealed that the GLE treatment did not inhibit transcription of the pdcd-1 gene but, instead, caused increased transcriptions of the pdcd-1 gene in both GM00130 and GM02248 human B-lymphocytes. Interestingly, increased transcriptions were also observed for several other tested genes, including Bcl-XL, CCL5, p16, p53, and PD-L1, when the GM00130 and GM02248 human B-lymphocytes were treated with GLE in the absence or presence of PMA/Io. Although the mechanisms that lead to increased transcriptions of these genes are unknown, it is possible that some compounds contained in the GLE may possess activities to induce global gene transcription. Therefore, further studies are needed to identify the compounds of the GLE that cause increased transcriptions of these genes. Future studies also need to identify all the genes affected by the GLE treatment.
The protein-protein interaction of PD-1 with PD-L1 prevents T-cells from killing tumor cells by inhibiting T-cells from producing cytokines and chemokines that help recruit immune cells (eg, T-lymphocytes) to the tumor sites and kill tumor cells.41,42,44 Works of others demonstrate that blockade of PD-1 protein with PD-1 antibodies increases expression of the CCL5 chemokine and enhances recruitment of T-lymphocytes to tumor sites.42,44 The results of our studies also revealed that the GLE treatment caused an increase in expression of the CCL5 chemokine. Therefore, G lucidum may modulate immune response by reducing PD-1 protein and enhancing immune response (eg, infiltration of T-cells) to fight disease development such as tumor growth. Therefore, G lucidum and its bioactive compounds could be developed into novel immunomodulators for both prevention and treatment of many diseases.
It is well known that the interaction of PD-1 and PD-L1 causes T-cell exhaustion and inhibits T-cell proliferation, resulting in an increased risk in cancer development.26,34,48,49 Clinical studies have also demonstrated that many types of cancer cells express high level of PD-L1 protein.26,49 Therefore, blockade of PD-1/PD-L1 interaction provides an attractive strategy in cancer treatment. Antibodies that target PD-1 and PD-L1 have been used in immunotherapy to treat many types of cancer.29-33 However, the PD-1/PD-L1 antibody–based immunotherapy also faces many challenges.34 Other alternative approaches that can effectively disrupt the PD-1/PD-L1 interaction need to be explored. Small molecule immunomodulators that can disrupt the PD-1/PD-L1 interactions have attracted great attention in cancer drug design and development.50-53 Although many clinical trials are currently ongoing, no small molecule immunomodulator drugs that target the PD-1 protein are currently used in clinical application.53 The results of our studies demonstrate that the GLE selectively reduces PD-1 protein in the cultured GM00130 and GM02248 human B-lymphocytes. Therefore, G lucidum and its bioactive contents could be developed into novel immunomodulators to target the PD-1 protein for treatment of many diseases, including cancer. Future studies need to determine if the results obtained in the cultured human B-lymphocytes can be achieved in animal studies and, more important, in human clinical studies.
In this work, we used 95% ethanol extract of GLE in our studies. The 95% ethanol extract of G lucidum was used in many studies.54-57 The 95% ethanol GLE is known to contain higher levels of triterpenoids than water GLE of G lucidum.54-57 Therefore, the observed effect of GLE on reducing PD-1 protein is likely caused by triterpenoids, although the contribution of polysaccharides on reducing PD-1 protein level could not be excluded in this work. Further identifying the compounds of GLE that are responsible for GLE-mediated PD-1 protein reduction would be important in understanding the mechanisms of G lucidum–mediated immunomodulation and anticancer activities.
In conclusion, the results of our studies suggest that reducing PD-1 protein is an important mechanism for G lucidum–mediated immunomodulation, and this reduction was not caused by a transcriptional inhibition of the pdcd-1 gene. In addition, the results of our studies further demonstrate that the GLE treatment causes an increase in expression of chemokine CCL5. Therefore, G lucidum and its bioactive compounds can be developed into novel immunomodulators to target the PD-1 protein for prevention and treatment of many diseases, including cancer.
Supplemental Material
Supplemental material, Supplement_Figure for The Possible Role of PD-1 Protein in Ganoderma lucidum–Mediated Immunomodulation and Cancer Treatment by Gan Wang, Le Wang, Jianlong Zhou and Xiaoxin Xu in Integrative Cancer Therapies
Acknowledgments
We thank Mr Eric Van Buren and Mr Daniel DeSantis of the Microscopy, Imaging, and Cytometry Resources Core of Wayne State University for their technical assist in FACS and IF-microscopy studies.
Footnotes
Authors’ Note: The data generated or analyzed during this study are included in the published article.
Author Contributions: GW designed and carried out most of the studies. GW also drafted the manuscript. LW was involved in manuscript preparation. JZ provided the wall-broken Ganoderma lucidum spores. XX was involved in design of the study. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Performance of this work was facilitated by the Cell Culture Facility Cores of the Institute of Environmental Health Sciences (IEHS), Wayne State University, and the Microscopy, Imaging, and Cytometry Resources Core of School of Medicine, Wayne State University. The Cell Culture Facility Core is supported, in part, by NIH Center Grant P30 ES020957 to the IEHS, Wayne State University. The Microscopy, Imaging, and Cytometry Resources Core is supported, in part, by NIH Center grant P30 CA22453 to the Karmanos Cancer Institute, Wayne State University, and the Perinatology Research Branch of the Nation Institutes of Child Health and Development. The work was supported by an internal fund from the Wayne State University.
ORCID iD: Gan Wang  https://orcid.org/0000-0002-4981-8845
https://orcid.org/0000-0002-4981-8845
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Chen K, Li C. Recent advances in studies on traditional Chinese anti-aging materia medica. J Tradit Chin Med. 1993;13:223-226. [PubMed] [Google Scholar]
- 2. Shiao MS. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Rec. 2003;3:172-180. [DOI] [PubMed] [Google Scholar]
- 3. Yuen JW, Gohel MD. Anticancer effects of Ganoderma lucidum: a review of scientific evidence. Nutr Cancer. 2005;53:11-17. [DOI] [PubMed] [Google Scholar]
- 4. Sanodiya BS, Thakur GS, Baghel RK, Prasad GB, Bisen PS. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol. 2009;10:717-742. [DOI] [PubMed] [Google Scholar]
- 5. Benzie IFF, Wachtel-Galor S. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. Boca Raton, FL: CRC Press; 2011. [PubMed] [Google Scholar]
- 6. Lee KH, Morris-Natschke SL, Yang X, et al. Recent progress of research on medicinal mushrooms, foods, and other herbal products used in traditional Chinese medicine. J Tradit Complement Med. 2012;2:84-95. [PMC free article] [PubMed] [Google Scholar]
- 7. Soccol CR, Bissoqui LY, Rodrigues C, et al. Pharmacological properties of biocompounds from spores of the Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes): a review. Int J Med Mushrooms. 2016;18:757-767. [DOI] [PubMed] [Google Scholar]
- 8. Money NP. Are mushrooms medicinal? Fungal Biol. 2016;120:449-453. [DOI] [PubMed] [Google Scholar]
- 9. Wang SY, Hsu ML, Hsu HC, et al. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer. 1997;70:699-705. [DOI] [PubMed] [Google Scholar]
- 10. Sliva D. Ganoderma lucidum (Reishi) in cancer treatment. Integr Cancer Ther. 2003;2:358-364. [DOI] [PubMed] [Google Scholar]
- 11. Xu Z, Chen X, Zhong Z, Chen L, Wang Y. Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am J Chin Med. 2011;39:15-27. [DOI] [PubMed] [Google Scholar]
- 12. Radwan FF, Perez JM, Haque A. Apoptotic and immune restoration effects of ganoderic acids define a new prospective for complementary treatment of cancer. J Clin Cell Immunol. 2011;S3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu GS, Guo JJ, Bao JL, et al. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum—a review. Expert Opin Investig Drugs. 2013;22:981-992. [DOI] [PubMed] [Google Scholar]
- 14. Suarez-Arroyo IJ, Rosario-Acevedo R, Aguilar-Perez A, et al. Anti-tumor effects of Ganoderma lucidum (Reishi) in inflammatory breast cancer in in vivo and in vitro models. PLoS One. 2013;8:e57431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li LF, Liu HB, Zhang QW, et al. Comprehensive comparison of polysaccharides from Ganoderma lucidum and G sinense: chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci Rep. 2018;8:6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paterson RR. Ganoderma—a therapeutic fungal biofactory. Phytochemistry. 2006;67:1985-2001. [DOI] [PubMed] [Google Scholar]
- 17. Peng XR, Liu JQ, Han ZH, Yuan XX, Luo HR, Qiu MH. Protective effects of triterpenoids from Ganoderma resinaceum on H2O2-induced toxicity in HepG2 cells. Food Chem. 2013;141:920-926. [DOI] [PubMed] [Google Scholar]
- 18. Liang CJ, Lee CW, Sung HC, et al. Ganoderma lucidum polysaccharides reduce lipopolysaccharide-induced interleukin-1β expression in cultured smooth muscle cells and in thoracic aortas in mice. Evid Based Complement Alternat Med. 2014;2014:305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics. 1994;23:704-706. [DOI] [PubMed] [Google Scholar]
- 20. Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol. 2013;25:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yao S, Chen L. PD-1 as an immune modulatory receptor. Cancer J. 2014;20:262-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gianchecchi E, Fierabracci A. Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in Treg development and their involvement in autoimmunity onset and cancer progression. Front Immunol. 2018;9:2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106. [DOI] [PubMed] [Google Scholar]
- 27. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217:45-59. [DOI] [PubMed] [Google Scholar]
- 29. Emens LA, Ascierto PA, Darcy PK, et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017;81:116-129. [DOI] [PubMed] [Google Scholar]
- 30. Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med. 2018;283:110-120. [DOI] [PubMed] [Google Scholar]
- 31. Fucà G, de Braud F, Di Nicola M. Immunotherapy-based combinations: an update. Curr Opin Oncol. 2018;30:345-351. [DOI] [PubMed] [Google Scholar]
- 32. Salmaninejad A, Valilou SF, Shabgah AG, et al. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234:16824-16837. doi: 10.1002/jcp.28358 [DOI] [PubMed] [Google Scholar]
- 33. Pawłowska A, Suszczyk D, Okła K, Barczyński B, Kotarski J, Wertel I. Immunotherapies based on PD-1/PD-L1 pathway inhibitors in ovarian cancer treatment. Clin Exp Immunol. 2019;195:334-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Botticelli A, Zizzari I, Mazzuca F, et al. Cross-talk between microbiota and immune fitness to steer and control response to anti PD-1/PDL-1 treatment. Oncotarget. 2017;8:8890-8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39-85. [DOI] [PubMed] [Google Scholar]
- 36. Errol CF, Graham CW, Wolfram S, Richard DW, Roger AS, Tom E. DNA Repair and Mutagenesis. 2nd ed. Washington, DC: ASM Press; 2006. [Google Scholar]
- 37. Hu J, Selby CP, Adar S, Adebali O, Sancar A. Molecular mechanisms and genomic maps of DNA excision repair in Escherichia coli and humans. J Biol Chem. 2017;292:15588-15597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269-274. [DOI] [PubMed] [Google Scholar]
- 39. Ikebuchi R, Konnai S, Okagawa T, et al. Blockade of bovine PD-1 increases T cell function and inhibits bovine leukemia virus expression in B cell in vitro. Vet Res. 2013;44:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bally AP, Austin JW, Boss JM. Genetic and epigenetic regulation of PD-1 expression. J Immunol. 2016;196:2431-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sullivan NL, Eickhoff CS, Zhang X, Giddings OK, Lane TE, Hoft DF. Importance of the CCR5-CCL5 axis for mucosal Trypanosoma cruzi protection and B cell activation. J Immunol. 2011;187:1358-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng W, Liu C, Xu C, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Waele J, Marcq E, Van Audenaerde JR, et al. Poly(I:C) primes primary human glioblastoma cells for an immune response invigorated by PD-L1 blockade. Oncoimmunology. 2017;7:e1407899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Araujo JM, Gomez AC, Aguilar A, et al. Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer. Sci Rep. 2018;8:4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang M, Huang J, Xie X, Holman CD. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int J Cancer. 2009;124:1404-1408. [DOI] [PubMed] [Google Scholar]
- 46. Jin X, Beguerie JR, Sze DM, Chan GC. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst Rev. 2012;(6):CD007731. doi: 10.1002/14651858.CD007731.pub2 [DOI] [PubMed] [Google Scholar]
- 47. Meng X, Liu X, Guo X, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 2018;564:130-135. [DOI] [PubMed] [Google Scholar]
- 48. Kelley MC. Immune responses to BRAF-targeted therapy in melanoma: is targeted therapy immunotherapy? Crit Rev Oncog. 2016;21:83-91. [DOI] [PubMed] [Google Scholar]
- 49. Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Skalniak L, Zak KM, Guzik K, et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget. 2017;8:72167-72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guzik K, Zak KM, Grudnik P, et al. Small-molecule inhibitors of the programmed cell death-1/programmed death-ligand 1 (PD-1/PD-L1) interaction via transiently induced protein states and dimerization of PD-L1. J Med Chem. 2017;60:5857-5867. [DOI] [PubMed] [Google Scholar]
- 52. Shaabani S, Huizinga HPS, Butera R, et al. A patent review on PD-1/PD-L1 antagonists: small molecules, peptides, and macrocycles (2015-2018). Expert Opin Ther Pat. 2018;28:665-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen S, Song Z, Zhang A. Small-molecule immuno-oncology therapy: advances, challenges and new directions. Curr Top Med Chem. 2019;19:180-185. [DOI] [PubMed] [Google Scholar]
- 54. Chen Y, Xie MY, Gong XF. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J Food Eng. 2007;81:162-170. [Google Scholar]
- 55. Guo WL, Pan YY, Li L, Li TT, Liu B, Lv XC. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018;9:3419-3431. [DOI] [PubMed] [Google Scholar]
- 56. Liu J, Kurashiki K, Shimizu K, Kondo R. 5alpha-reductase inhibitory effect of triterpenoids isolated from Ganoderma lucidum. Biol Pharm Bull. 2006;29:392-395. [DOI] [PubMed] [Google Scholar]
- 57. Lu QY, Jin YS, Zhang Q, et al. Ganoderma lucidum extracts inhibit growth and induce actin polymerization in bladder cancer cells in vitro. Cancer Lett. 2004;216:9-20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement_Figure for The Possible Role of PD-1 Protein in Ganoderma lucidum–Mediated Immunomodulation and Cancer Treatment by Gan Wang, Le Wang, Jianlong Zhou and Xiaoxin Xu in Integrative Cancer Therapies