Abstract
This study evaluated the effects of 6 weeks of whole-body vibration (WBV) exercise on flexibility and the rating of perceived exertion (RPE) in metabolic syndrome (MetS) individuals using 2 biomechanical conditions (fixed frequency [FF] and variable frequency [VF]). Nineteen MetS individuals were randomly allocated in FF-WBV (n = 9, 7 women and 2 men) and VF-WBV (n = 10, 8 women and 2 men) groups. Anterior trunk flexion (ATF) and RPE were determined before and after each session. The acute cumulative exposure effects were analyzed. The FF-WBV group was exposed to 5 Hz on a side alternating vibrating platform (SAVP), exposed to 10 and 50 seconds with the SAVP turned off. The VF-WBV group individuals were intermittently exposed (1 minute WBV exercise/1 minute rest) to 5 to 16 Hz, increased by 1 Hz per session and the peak-to-peak displacement (PPD) were 2.5, 5.0, and 7.5 mm. Regarding to ATF, significant improvements (P < .05) were observed in the in the acute (VF group) and cumulative intervention (FF and VF-WBV groups). The RPE significantly (P < .05) improved only in VF-WBV (cumulative intervention). In conclusion, WBV exercise improved the flexibility and decreased the RPE in MetS individuals. These findings suggest that WBV exercise can be incorporated into physical activities for MetS individuals.
Keywords: metabolic syndrome, whole-body vibration exercise, flexibility, rating of perceived exertion
Introduction
Metabolic syndrome (MetS) is a common and serious clinical disorder that represents a challenge to professionals in public health.1 Overweight and obesity are among the major determinants of MetS.2 As defined by the International Diabetes Federation (IDF), MetS involves 3 or more risk factors, which include increase in the abdominal waist and 2 other factors of high triglycerides, low- and high-density lipoprotein cholesterol, high blood pressure, and elevated fasting blood glucose.3 Metabolic syndrome is a major contributor to the epidemic of cardiovascular disease in the United States as well as the emerging pandemic. It has been identified that (1) more than 1 in 3 adults and (2) about 40% of adults aged 40 years and older have MetS and (3) MetS individuals are largely asymptomatic but have a 10-year risk of a first coronary event, based on the Framingham Risk Score of 16% to 18%, which is as high as a patient who has experienced a prior coronary event. Moreover, it is suggested that there is underdiagnosis and undertreatment of MetS4 and MetS increases the cardiovascular risk and type 2 diabetes mellitus (T2DM).3 The Global Burden of Diseases, Injuries and Risk Factors Study 2016 listed a ranking of a risk factor exposure and attributable burden of disease. The metabolic risks, such as body mass index (BMI) and high fasting plasma glucose, showed the most significant increase.2 Levels of adiposity and blood pressure have also a strong association with morbidity and mortality.5
Insulin resistance is a metabolic disorder that reflects a low ability to displace glucose into target tissues, which raises the risk of cardiovascular complications.6 One of the complications of T2DM is diabetic neuropathy that can affect peripheral nerves, including pain fibers.7 Following the International Association for the Study of Pain,8 neuropathic pain is caused by a lesion of the somatosensory nervous system due to a consequence of a disease, such as diabetes mellitus. In addition, musculoskeletal pain can be found in individuals with MetS9; and obese people have more pain than individuals with normal body mass.10 Duruoz et al11 reported that low back pain can also be found in individuals with MetS.11 In general, the mechanisms underlying the association between these metabolic disturbances and pain are still unknown. Stefani and Galanti12 showed that the occurrence of osteoarthritis can be associated with chronic inflammation in MetS individuals and the excess of body mass can alter cartilage metabolism. It is suggested that inflammation and the mechanical overload seem to have important roles in these relations.12 In addition, the reduction in the joint amplitude is positively associated with MetS.13
Flexibility is related to the ability of a joint to perform a complete movement. It is dependent on the distensibility of the articular capsule, adequate warming, and muscle viscosity.14 Elderly women with MetS reported reduced flexibility and difficulty in performing daily activities compared to those without the disease.15
Regular flexibility exercises can develop range of motion in the major muscle-tendon groups in accordance with individualized goals.16 In addition, there is evidence supporting that exercise is the first-line therapeutic treatment of all forms of chronic pain.17
Scherr et al18 reported that rating of perceived exertion (RPE) is a widely used psychophysical tool to assess subjective perception of effort during exercise. The RPE scale is, in general, used to aid in prescribing exercise intensity. It is a reliable indicator of physical discomfort and is strongly correlated with several other physiological measures of exertion.19,20 Ekkekakis and Lind21 reported an increase in RPE in overweight individuals during exercise compared to normal weight individuals.
Whole-body vibration (WBV) exercise is generated in an individual exposed to mechanical vibration produced in a vibrating platform (VP).22 A protocol involving WBV exercise must consider parameters, such as frequency, peak-to-peak displacement (PPD), intensity, number of sessions and number of bouts, work and rest time in each session, and position of the individual during the WBV exercise.23 Whole-body vibration exercise can be useful and suitable intervention for treating musculoskeletal disorders, reducing pain, and improving back function in individuals with chronic low back pain.24 Studies have also reported that WBV exercise decreases the risk of falls and enhances the strengh25 and flexibility26 in elderly individuals.
Previous researches have investigated the effects of WBV exercise in MetS individuals.27,28 However, it is desirable to uncover new knowledge with respect to WBV exercise protocols considering additional sessions, position of the individual on the base of the side alternating VP (SAVP), length of the intervention, and their effects on MetS. Therefore, the aim of the current study is to evaluate the acute and cumulative effects from 6 weeks of WBV exercise using 2 biomechanical conditions (fixed frequency [FF] and variable frequency [VF]) on flexibility and RPE in MetS individuals.
As hypothesis, it is expected that the WBV exercise improves the flexibility and decreases the RPE in MetS individuals.
Material and Methods
Study Design
A randomized controlled trial was approved by the Ethical Committee, registered on the Plataforma Brasil with the number CAAE: 19826413800005259 and registration in the REBEC RBR-2BGHMH. This study was conducted in accordance with Resolution No. 466/12 of the National Health Council, the recommendations of the Declaration of Helsinki were followed, and all participants signed the informed consent form.
The recruitment of the participants occurred from January 2018 to January 2019 in the Hospital Universitário Pedro Ernesto, Universidade do Estado do Rio de Janeiro. The WBV exercise protocol was performed in the Laboratório de Vibrações Mecânicas e Práticas Integrativas. The Consolidated Standards of Reporting Trials was used to report all the different steps of the interventions utilized in this study.29 No changes occurred to methods after trial commencement.
Study Population
This study recruited 31 participants and 19 performed the protocol (58.79 ± 12.55 years old, 1.62 ± 0.09 m height, and 86.27 ± 15.03 kg body mass). The inclusion criteria were individuals of both sexes, older than 18 years, with MetS (according to IDF), independently of the level of physical activity. The exclusion criteria were (1) individuals with systolic blood pressure ≥180 mm Hg and diastolic blood pressure ≥110 mm Hg; (2) cardiovascular disease clinically evident in the past 6 months manifested by myocardial infarction or stroke; (3) neurological, muscular, or rheumatologic disease that limits the ability to undertake the WBV exercise protocol; (4) disabling clinical symptom reported during the WBV exercise intervention; (5) BMI >40 kg/m2; and (6) individuals who refuse to sign the consent form.
Metabolic syndrome individuals were divided in 2 groups: FF-WBV (n = 9, 7 women and 2 men) and VF-WBV (n = 10, 8 women and 2 men). The randomization procedure was carried out from a blinded envelope that paired the participant with the group.
Participants declared that they were sedentary and were instructed to continue their daily activities and medications during the investigation. In general, the drugs used by the participants were diuretics, β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor antagonists.
Clinical Evaluation
A clinical evaluation was performed to collect data such as age, height, body mass, BMI, and waist circumference (WC). The analysis of the plasma concentration of blood biomarkers was performed to confirm the diagnostics of MetS. The cardiometabolic risk factors were defined as following the IDF MetS definition.3 People with 3 or more factors were defined as having MetS. The blood biomarkers analysis confirmed MetS.
Anthropometric Measurements and Physiological Parameters
Height and body mass were measured on a digital balance (MIC 200 PPA, Micheletti, São Paulo, Brazil) to calculate the BMI.30 The WC was obtained applying tape around the abdomen at the level of umbilicus.31
Anterior Trunk Flexion
In the standing position, each individual flexed their trunk as much as possible, without flexing the knees and/or extending their neck. The distance from the middle finger to the floor was measured.13 The anterior trunk flexion (ATF) was determined before and after each session, during 6 weeks.
The acute effect analyzed the mean measurement between the end and the beginning of the first session of each protocol. The cumulative effect analyzed the mean of ATF before the first session and the mean of ATF before the intervention in the last session (6 weeks).
Rating of Perceived Exertion Scale
Each individual reported the RPE by observing a numerical scale from 6 to 20, ranging from “light” (6 points) to “maximal effort” (20 points),32 performing the ATF test, before and after the interventions, of the FF-WBV or the VF-WBV groups.
The acute effect was determined by analyzing the mean of RPE before and after the first session of each protocol. The cumulative effect was considered by averaging the RPE before the first session and before the intervention in the last session (6 weeks).
Intervention Groups
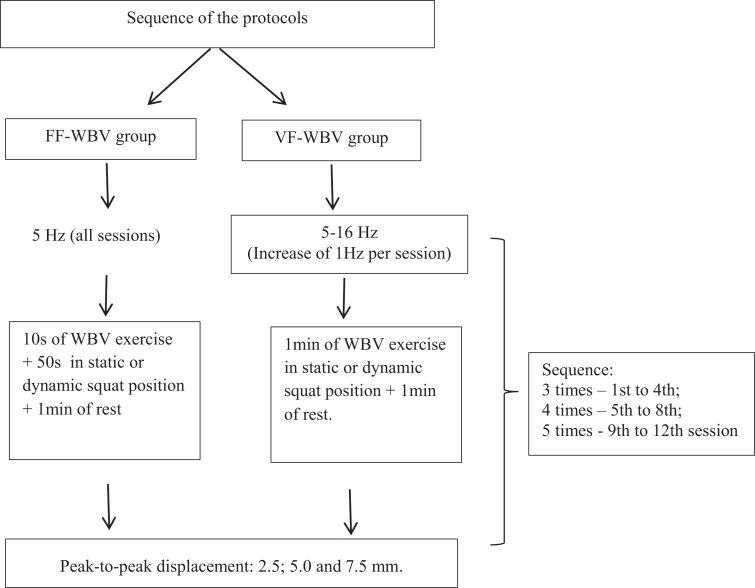
The individuals who were exposed to WBV exercise with FF (FF-WBV) during 12 sessions (twice a week) on an SAVP (Novaplate fitness evolution, DAF Produtos Hospitalares Ltda, from Estek, São Paulo, Brazil). In a session, the individuals were exposed to a bout with the frequency of 5 Hz with PPD of 2.5 mm for 10 plus 50 seconds on SAVP, with the vibration machine turned off in the squat position and rested for 1 minute. These steps were used, in this same session, in 2 other bouts with the PPD of 5.0 and 7.5 mm. This same sequence was performed 3 times in the first and second weeks (total time of 18 minutes). In the third and fourth weeks, the sequence was performed 4 times (total time of 24 minutes) and in the fifth and sixth weeks, 5 times (total time of 30 minutes).
The individuals were exposed to WBV exercise with VF (VF-WBV) during 12 sessions (twice a week) on an SAVP that commenced at 5 Hz in the first session and ended at 16 Hz in the last session. In a session, the individuals were exposed to a bout with PPD of 2.5 mm for 1 minute on SAVP, with the vibration machine turned on and rested for 1 minute. These steps were used, in this same session, in 2 other bouts with the PPD of 5.0 and 7.5 mm. This sequence was performed 3 times in the first week (total time of 18 minutes), 5 Hz in the first session, and 6 Hz in the second session; in the second week (total time of 18 minutes), 7 Hz in the third session and 8 Hz in the fourth session; in the third week (total time of 24 minutes), 9 Hz in the fifth session and 10 Hz in the sixth session; in the fourth week (total time of 24 minutes), 11 Hz in the seventh session and 12 Hz in the eighth session; in the fifth week (total time of 30 minutes), 13 Hz in the 9th session and 14 Hz in the 10th session; and in the sixth week (total time of 30 minutes), 15 Hz in the 11th session and 16 Hz in the 12th session. The position of the individuals in the 1st, 3rd, 5th, 7th, 9th, and 11th sessions was static squat and in the 2nd, 4th, 6th, 8th, 10th, and 12th session was dynamic squat.
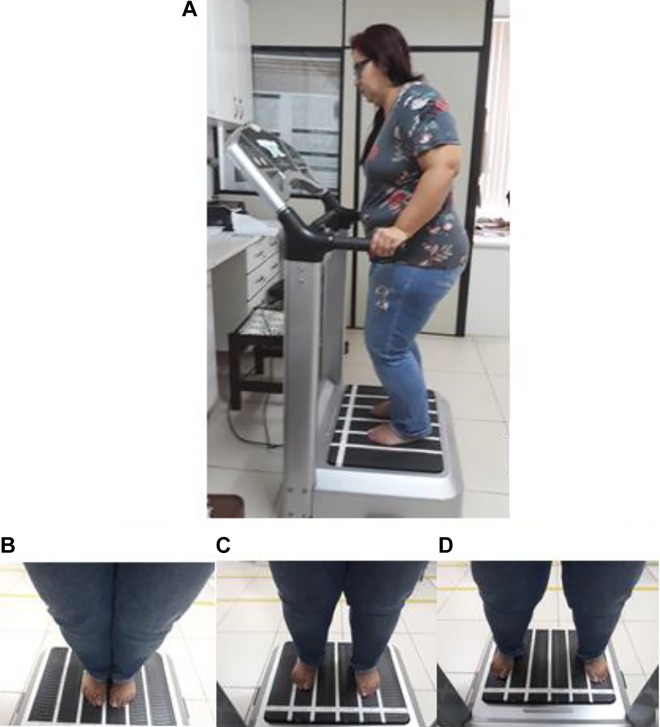
Figure 1 illustrates the PPD (2.5, 5.0, and 7.5 mm). This sequence was performed 3 times from the first to the fourth session, 4 times from the fifth to the eighth session, and 5 times from the 9th to the 12th session. All individuals were instructed to adopt the standing position (barefoot), holding on the platform side rails and with 130° knees flexion. Static and dynamic squat positions were alternated from one session to another. Figure 2 illustrates the sequence of the protocols that were used in the FF-WBV and VF-WBV groups.
Figure 1.
The peak-to-peak displacement used in this study. A, Individual in a stand position, 130° knee flexion, and barefoot; (B) individual in 2.5 mm of PPD on the base of the SAVP; (C) individual in 5 mm of PPD on the base of the SAVP; and (D) individual in 7.5 mm of PPD on the base of the SAVP. PPD indicates peak-to-peak displacement; SAVP, side alternating vibrating platform.
Figure 2.
The WBV exercise protocol sequence.
Statistical Analysis
The sample size was calculated using the parameter flexibility.27 For a statistical power of 80%, a standard deviation (SD) of 10.31 and significance level of 5%, a sample size (www.lee.dante.br/) of 10 participants in each group was calculated.
The data of the present study were analyzed using the statistical analysis software of GraphPad Prism 6.0 for Windows (GraphPad Software, San Diego, California). A non-normal distribution was confirmed. The nonparametric Wilcoxon test was applied to compare variables before and after intervention in each group and Mann-Whitney test to compare intergroup outcomes. The significance level was set at P ≤ .05, and data are presented as mean and SD. The coefficient of variation and the effect sizes were used to express the reliability of the functional tests determined (Cohen d), considering small effect sizes with d ≤ 0.2; moderate effect sizes with 0.2 < d < 0.8, and large effects sizes with d ≥ 0.8 for parametric data.28
Results
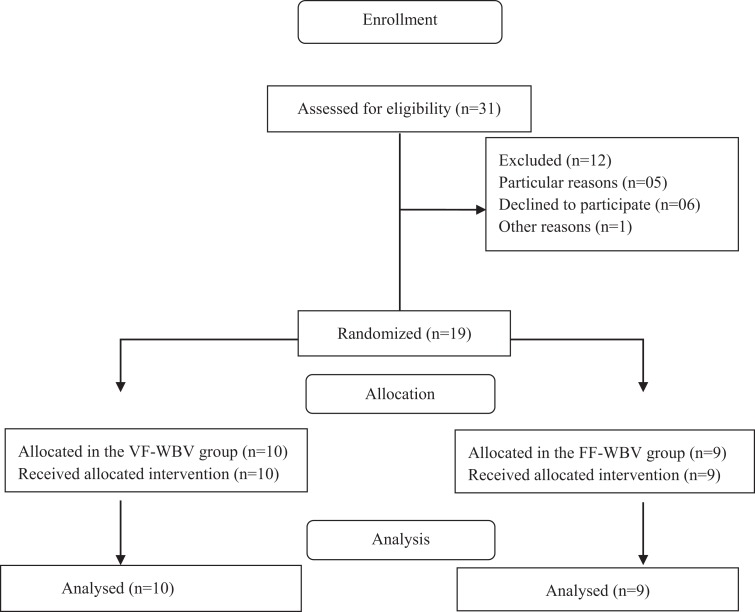
The enrollment flow diagram is shown in Figure 3. Thirty-one individuals were recruited, 5 individuals were excluded due particular reasons involving costs with the transportation, 6 declined to participate, and 1 individual declined due other reasons.
Figure 3.
The flow diagram of the study.
The characteristics of the individuals (baseline) in both the FF-WBV and VF-WBV groups are presented in Table 1, as the anthropometric data and the values of the RPE and ATF. No significant differences were found between these 2 groups, except for BMI values.
Table 1.
Characteristics of the Individuals (Baseline).a
| Characteristics | FF-WBV, n = 9, mean ± SD | VF-WBV, n = 10, mean ± SD | P Value |
|---|---|---|---|
| Age (years) | 58 ± 13 | 57 ± 12 | .94 |
| Body mass (kg) | 82 ± 10.9 | 88.8 ± 11.9 | .21 |
| Height (cm) | 1.65 ± 0.1 | 1.63 ± 0.09 | .63 |
| WC (cm) | 103.8 ± 6.66 | 109.03 ± 8.7 | .17 |
| BMI (kg/m2) | 29.8 ± 2.07 | 33.2 ± 3.50 | .02 |
| RPE (score) | 8.89 ± 2.14 | 10.78 ± 2.10 | .54 |
| ATF (cm) | 18.94 ± 8.70 | 16.94 ± 10.05 | .71 |
Abbreviations: ATF, anterior trunk flexibility, BMI, body mass index; FF-WBV, fixed frequency whole-body vibration; RPE, rating of perceived exertion; SD, standard deviation; VF-WBV, variable frequency whole-body vibration; WC, waist circumference.
a P value ≤.05.
Acute Exposure
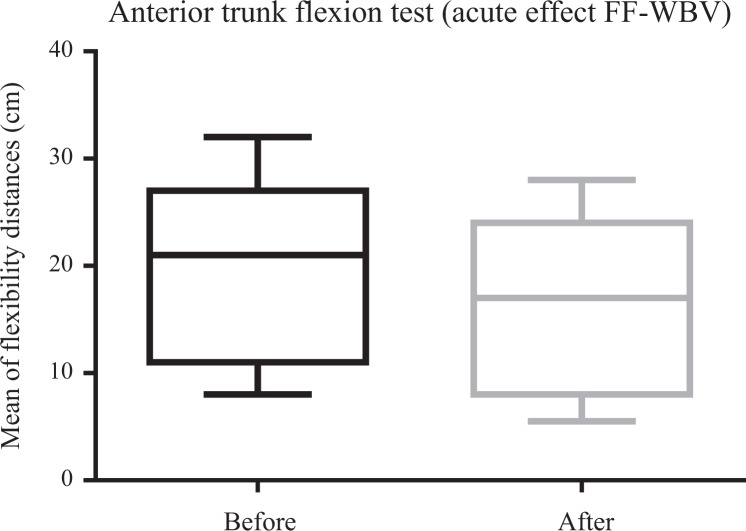
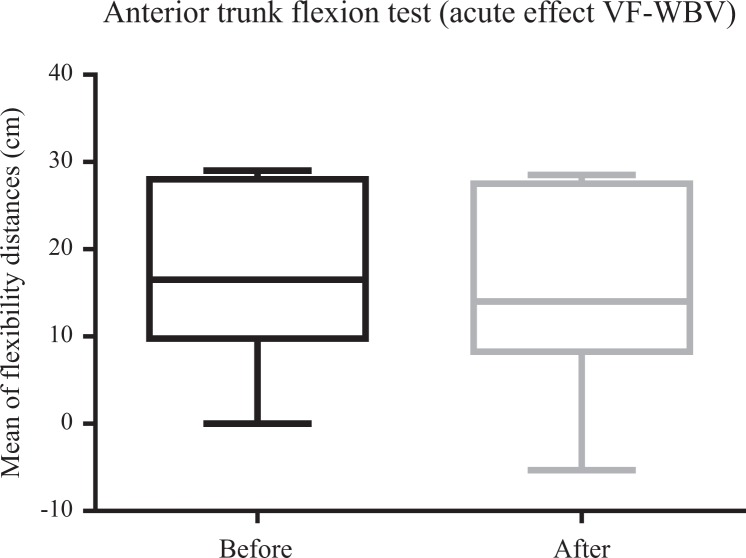
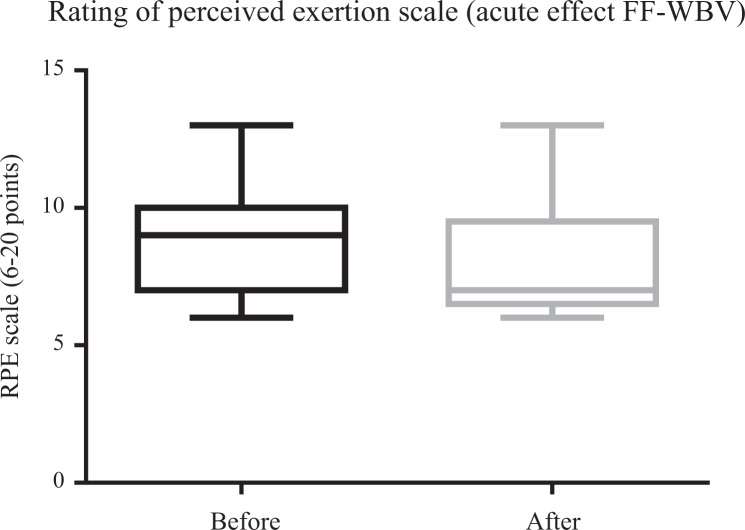
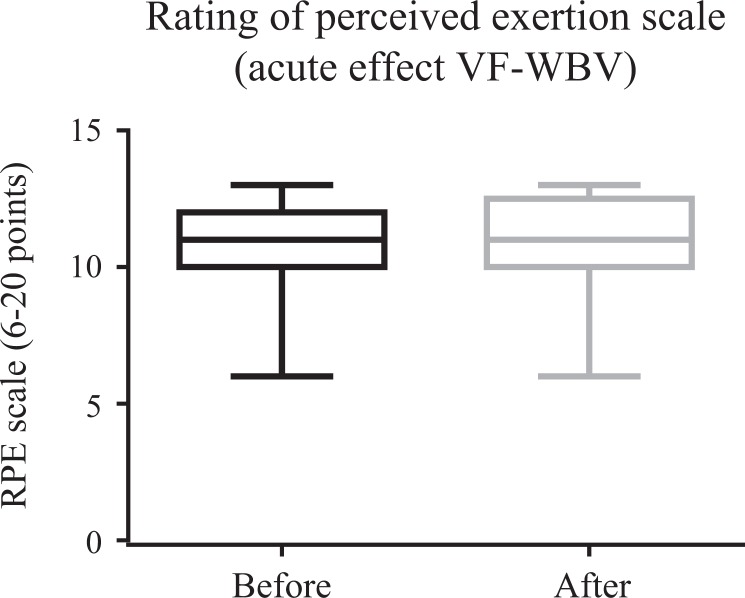
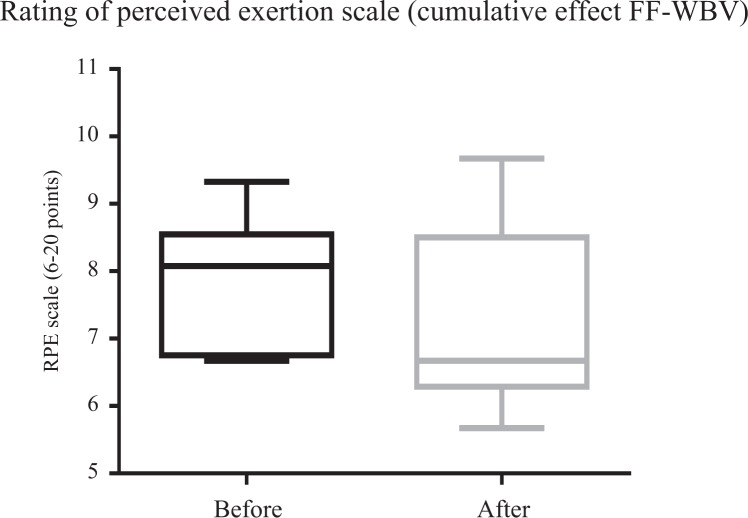
Figures 4 and 5 illustrate a significant (P ≤ .05) improvement in ATF for VF-WBV (P = .03) and a no significant improvement for FF-WBV (P = .29) groups, with a small and moderate effect size of d = 0.32 and d = 0.13, respectively. No significant (P > .05) improvement in RPE was evident in FF-WBV (P = .09) and VF-WBV (P = .90; Figures 6 and 7) groups, with a large and moderate effect size of d = 1.24 and d = 0.61, respectively.
Figure 4.
Mean of distances between middle finger to the floor of fixed frequency whole-body vibration group to perform anterior trunk flexion.
Figure 5.
Mean of distances between middle finger to the floor of variable frequency whole-body vibration group to perform anterior trunk flexion.
Figure 6.
Mean of rating of perceived exertion scale of fixed frequency whole-body vibration group.
Figure 7.
Mean of rating of perceived exertion scale of variable frequency whole-body vibration group.
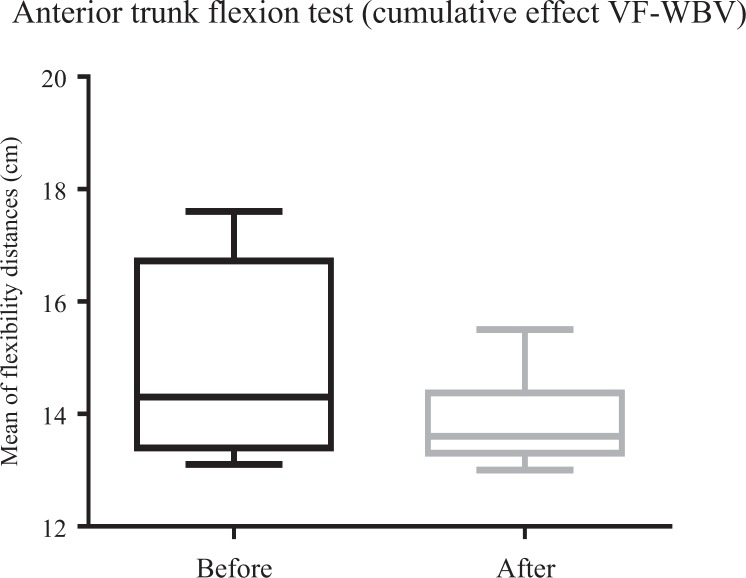
Cumulative Exposure
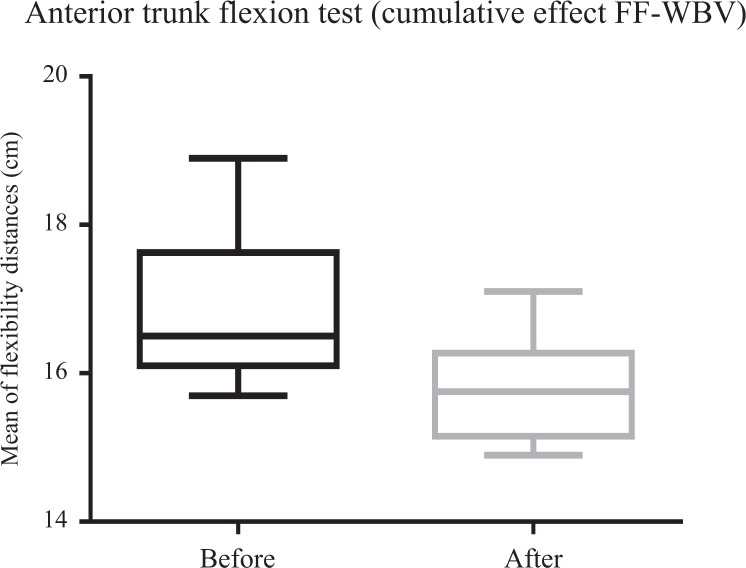
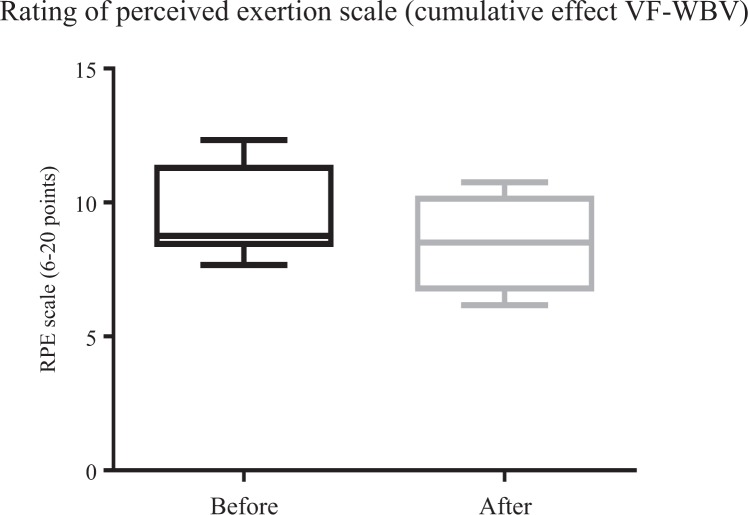
Figures 8 and 9 show a significant (P ≤ .05) improvement of ATF for FF-WBV (P = .002) and VF-WBV (P = .003) groups, with a small and moderate effect size of d = 0.13 and d = 0.32, respectively. No significant (P > .05) improvement in RPE for FF-WBV group (P = .59) was evident, but ATF showed an improvement for VF-WBV (P = .023) group (Figures 10 and 11), with a small and moderate effect size of d = 0.07 and d = 0.78, respectively.
Figures 8.
Mean of distances between middle finger to the floor of fixed frequency whole-body vibration during anterior trunk flexion (flexibility).
Figures 9.
Mean of distances between middle finger to the floor of variable frequency whole-body vibration during anterior trunk flexion (flexibility).
Figures 10.
Mean of rating of perceived exertion scale of fixed frequency whole-body vibration group.
Figures 11.
Mean of rating of perceived exertion scale of variable frequency whole-body vibration group.
Discussion
The purpose of this study is to evaluate the effects of WBV exercise using 2 biomechanical conditions on the flexibility and on the RPE to perform ATF in individuals with MetS. Previous research had demonstrated that patients with MetS also report lower levels of physical activity, causing poorer cardiorespiratory endurance and decreased muscular strength.33,34 Obese can present higher risk of joint lesions and the excess of abdominal fat can alter the levels of trunk flexibility.35 In elderly populations with MetS, lower functional capacity, muscular strength, and flexibility have been reported.36
WBV exercise could be considered a safe and reliable strategy in the management of individuals with MetS. It is relevant to indicate that no significant differences were found between the individuals of the 2 groups when some anthropometric characteristics were considered (such as age, height, body mass, and WC). The current study demonstrated that significant improvements in the ATF were verified (Figures 5, 8, and 9). This was observed in individuals exposed to (1) 5 Hz for 60 seconds (VF-WBV) in acute effect, (2) only 5 Hz for 10 seconds (FF-WBV) after 6 weeks in cumulative effect, and (3) VFs from 5 to 16 Hz for 60 seconds (VF-WBV), after 6 weeks in cumulative effect.
The improvement of ATF represents an interesting result. An increase in flexibility means more efficiency on individual’s movement. In fact, the trunk represents 60% of the total mass of the body with a high position above the support base constituted by the feet, which raises the position of the center of gravity and therefore poses a challenge to balance. In addition, the trunk has a complex structure, with a highly articulated skeleton and dense muscle networks, which gives it sufficient versatility to perform a large number of different tasks while maintaining the body in balance. This versatility, which is an asset, is also a challenge to balance, especially during locomotion, where trunk movements must be regulated in a complex way according to the combination of anticipatory and reactive muscular actions. The trunk is equipped to perform this role as a central part of the body. The role of the trunk muscles is then limited to a stabilizing effect on the body and head in response to movements induced by the movements of the lower limbs. Furthermore, considering the RPE to perform ATF, an improvement was only found in the VF-WBV group in the cumulative effect after 6 weeks (Figure 11). Chang et al13 reported the effect of WBV exercise on improving skeletal muscle mass index, physical fitness, and quality of life of sarcopenic older people living in institutions in a 12-week WBV intervention, including also action on the flexibility (shoulder).
The study results showed the effect of WBV intervention on improving the skeletal muscle mass index, physical fitness, and quality of life of sarcopenic older people living in institutions and could serve as a crucial reference to health-care professionals.
Considering the ATF after the interventions (FF-WBV and VF-WBV) in acute effect, the intervention showed a significant change (P ≤ .05) in VF-WBV group (Figure 5). These findings were in agreement with Sá-Caputo et al27 who showed acute effects improvements in trunk’s flexibility after the WBV exercise protocol in individuals with MetS. Dallas et al37 examined the acute effects of different vibration loads of WBV exercise on flexibility in springboard divers. They concluded that WBV exercise can be a strategy to increase flexibility. It is important to point out that a slight exposition (5 Hz for 10 seconds) in the FF-WBV was also capable to improve the ATF, as the VF-WBV after 6 weeks (cumulative effect; Figures 8 and 9). Cochrane et al38 have performed a study with 9 healthy males in 2 conditions on a Galileo vibration machine (alternating platform) for 15 seconds at 0 Hz (resting) and 6 Hz. It was shown that at 6 Hz, the amplitude of medial gastrocnemius (MG) muscle–tendon complex (375 μm) was significantly greater (P < .05) compared to 0 Hz (35 μm). The MG contractile length amplitude at 6 Hz (176 μm) was significantly greater (P < .05) compared to 0 Hz (4 μm). Significant increases (P < .05) in electromyography modulation were found for all muscles during the 6 Hz compared to the 0 Hz condition. It is suggested that low-frequency WBV results in small muscle length changes and increases muscle activation. In addition, the improvement of the ATF could be related to a decrease in the level of the pain, as reported by Neto et al, who found a significant decrease in knee osteoarthritis pain level in acute and cumulative effects after intervention with WBV exercise,39 and Kim et al40 who elucidated the effect of a 12-week horizontal vibration exercise in chronic low back pain patients as compared to vertical vibration exercise. They concluded that horizontal is as effective as vertical vibration in reducing pain and strengthening the lumbar muscle in chronic low back pain patients. It is also possible to speculate that the repetition of the ATF test per se may lead to improvements of the flexibility, especially if it is considered the viscoelastic properties of the musculoskeletal system.41
In MetS individuals, in the current study, although the findings related to ATF showed an improvement in the VF-WBV group, this did not reflect in an immediate alteration of the RPE in the FF-WBV (5 Hz, 10 seconds) neither in the VF-WBV group (5 Hz, 60 second). Similarly, Duc et al42 have investigated the effect of performing a preconditioning exercise with or without WBV on a subsequent cycling sprint performance. After a warmup, the preconditioning exercise (body-loaded half squats) was applied: 30 seconds of half-squats with WBV (40 Hz, 2 mm) or 30 seconds of half-squats without WBV with a 10-second all-out sprint performed after 1 minute. No differences were observed between the conditions for RPE after the sprints. However, performing preconditioning exercise with WBV resulted in superior peak and mean power output compared to preconditioning exercise without WBV.42 This last statement is in agreement with our findings related to the improvement of the ATF (Figures 5, 8, and 9). In the current work, a decrease (improvement) in the RPE was only found in the individuals of the groups exposed to VF-WBV exercise in the cumulative effect (Figure 11).
This research had some limitations and must be improved, such as the total of 6 weeks of the protocol of the WBV exercise and the small sample size. Moreover, it will be interesting to evaluate the effect of mechanical vibration with different frequencies in the acute effect using the same protocol. In addition, participants were recruited irrespectively of physical activity level and the study does not include a control group.
Conclusion
Considering the small sample size and the lack of a control group, the results suggest that the 2 protocols (FF 5 Hz), related to cumulative effect, and (VF from 5 up to 16 Hz), related to acute and cumulative effect, might improve the trunk flexibility (even with reduced times, 10 or 60 seconds). A decrease in the RPE was only observed in the cumulative effect with VF. The practical application of the current study is that WBV exercise can be incorporated into physical activities for MetS individuals. The WBV exercise would be a feasible strategy to increase flexibility, but larger trials, with an adequate control group, are needed to clearly assess the usefulness of WBV in the intervention of patients with MetS.
Supplemental Material
Supplemental Material, CONSORT_2010_Checklist for Acute and Cumulative Effects With Whole-Body Vibration Exercises Using 2 Biomechanical Conditions on the Flexibility and Rating of Perceived Exertion in Individuals With Metabolic Syndrome: A Randomized Clinical Trial Pilot Study by P. C. Paiva, C. A. Figueiredo, A. Reis-Silva, A. Francisca-Santos, L. L. Paineiras-Domingos, E. Martins-Anjos, M. E. S. Melo-Oliveira, G. M. G. Lourenço-Revelles, E. Moreira-Marconi, E. O. Guedes-Aguiar, A. A. Brandão, M. F. T. Neves, V. L. Xavier, D. L. Borges, A. C. R. Lacerda, V. A. Mendonça, A. Sonza, H. Quinart, F. C. Boyer, R. Taiar, A. Sartorio, D. J. Cochrane, M. Bernardo-Filho and D. C. Sá-Caputo in Dose-Response
Footnotes
Authors' Note: M E S Melo-Oliveira and G M G Lourenço-Revelles are also affiliated with Mestrado Profissional em Saúde, Medicina Laboratorial e Tecnologia Forense, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES; Finance Code 001); the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
ORCID iD: L. L. Paineiras-Domingos  https://orcid.org/0000-0003-3451-5056
https://orcid.org/0000-0003-3451-5056
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Sherling DH, Perumareddi P, Hennekens CH. Metabolic syndrome: clinical and policy implications of the new silent killer. J Cardiovasc Pharmacol Ther. 2017;22(4):365–367. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2016 Risk Factors Collaborators. Global, regional and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks on clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2017;390(10100):1345–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Federation (IDF); 2006;4–7. [Google Scholar]
- 4. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313(19):1973–1974. [DOI] [PubMed] [Google Scholar]
- 5. Gnatiuc L, Alegre-Diaz J, Halsey J, et al. Adiposity and blood pressure in 110000 Mexican adults. Hypertension. 2017;69(4):608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hwang M, Lee S. Insulin resistance: vascular function and exercise. Integr Med Res. 2016;5(3):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. I Diretriz Brasileira de Diagnóstico e Tratamento de Síndrome Metabólica. Arq Bras Cardiol. 2005;84(suppl 1):3–5. [PubMed] [Google Scholar]
- 8. International Association for the Study of Pain. Part III: Pain Terms: A Current List with Definitions and Notes on Usage. 2011. www.iasp-pain.org. Updated 2018. Accessed October 10, 2019.
- 9. Briggs MS, Spees C, Bout-Tabaku S, Taylor CA, Eneli I, Schmitt LC. Cardiovascular risk and metabolic syndrome in obese youth enrolled in a multidisciplinary medical weight management program: implications of musculoskeletal pain, cardiorespiratory fitness, and health-related quality of life. Metab Syndr Relat Disord. 2015;13(3):102–109. [DOI] [PubMed] [Google Scholar]
- 10. Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the southern pain prevalence study. J Pain. 2007;8(5):430–436. [DOI] [PubMed] [Google Scholar]
- 11. Duruoz MT, Turan Y, Gurgan A, Deveci H. Evaluation of metabolic syndrome in patients with chronic low back pain. Rheumatol Int. 2012;32(3):663–667. [DOI] [PubMed] [Google Scholar]
- 12. Stefani L, Galanti G. Physical exercise prescription in metabolic chronic disease. Adv Exp Med Biol. 2017;1005:123–141. [DOI] [PubMed] [Google Scholar]
- 13. Chang KV, Hung CY, Li CM, et al. Reduced flexibility associated with metabolic syndrome in community-dwelling elders. PLoS One. 2015;10(1):e0117167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 6th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2000;85–86. [Google Scholar]
- 15. Vieira LD, Tibana RA, Tajra V, et al. Decreased functional capacity and muscle strength in elderly women with metabolic syndrome. Clin Interv Aging. 2013;8:1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. [DOI] [PubMed] [Google Scholar]
- 17. Polaski AM, Phelps AL, Kostek MC, Szucs KA, Kolber BJ. Exercise-induced hypoalgesia: a meta-analysis of exercise dosing for the treatment of chronic pain. PLoS One. 2019;14(1):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013;113(1):147–155. [DOI] [PubMed] [Google Scholar]
- 19. Balasekaran G, Loh MK, Govindaswamy VV, Cai SJ. Omni Scale Perceived Exertion responses in obese and normal weight male adolescents during cycle exercise. J Sports Med Phys Fitness. 2014;54(2):186–196. [PubMed] [Google Scholar]
- 20. Day ML, McGuigan MR, Brice G, Foster C. Monitoring exercise intensity during resistance training using the session RPE scale. J Strength Cond Res. 2004;18(2):353–358. [DOI] [PubMed] [Google Scholar]
- 21. Ekkekakis P, Lind E. Exercise does not feel the same when you are overweight: the impact of self-selected and imposed intensity on affect and exertion. Int J Obes (Lond). 2006;30(4):652–660. [DOI] [PubMed] [Google Scholar]
- 22. Rittewger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108(5):877–904. [DOI] [PubMed] [Google Scholar]
- 23. Rauch F, Sievanen H, Boonen S, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–198. [PubMed] [Google Scholar]
- 24. Wang XQ, Pi YL, Chen PJ, Chen BL, et al. Whole body vibration exercise for chronic low back pain: study protocol for a single-blind randomized controlled trial. Trials. 2014;15:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goudarzian M, Ghavi S, Shariat A, et al. Effects of whole body vibration training and mental training on mobility, neuromuscular performance, and muscle strength in older men. J Exerc Rehabil. 2017;13(5):573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang SF, Lin PC, Yang RS, Yang RJ. The preliminary effect of whole-body vibration intervention on improving the skeletal muscle mass index, physical fitness, and quality of life among older people with sarcopenia. Geriatrics. 2018;18(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sá-Caputo DC, Paineiras-Domingos LL, Oliveira R, Mario FT, et al. Acute Effects of whole-body vibration on the pain level, flexibility, and cardiovascular responses in individuals with metabolic syndrome. Dose Response. 2018;16(4). doi:10.1177/1559325818802139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sá-Caputo DC, Paineiras-Domingos LL, Francisca-Santos A, Martins dos Anjos E, et al. Whole-body vibration improves the functional parameters of individuals with metabolic syndrome: an exploratory study. BMC Endocr Disord. 2019;19(1):6 doi:10.1186/s12902-018-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. [DOI] [PubMed] [Google Scholar]
- 30. Casadei K, Kiel J. Anthropometric Measurement. Treasure Island, FL: StatPearls Publishing; 2018. http://www.ncbi.nlm.nih.gov/books/NBK537315/. 2019 Jan 1. StatPearls [Internet]. Updated 2017. Accessed January 1, 2019. [PubMed] [Google Scholar]
- 31. Heyward VH, Stolarczyk LM. Applied Body Composition Assessment. Champaign, IL: Human Kinetics; 1996, 76–77. [Google Scholar]
- 32. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 33. Jurca R, Lamonte MJ, Church TS, et al. Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc. 2004;36(8):1301–1307. [DOI] [PubMed] [Google Scholar]
- 34. Farias DL, Tibana RA, Teixeira TG, et al. Elderly women with metabolic syndrome present higher cardiovascular risk and lower relative muscle strength. Einstein (Sao Paulo). 2013;11(2):174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghezelbash F, Shirazi-Adl A, Plamandona A, Arjmand N, Parnianpour M. Obesity and obesity shape markedly influence spine biomechanics: a subject-specific risk assessment model. Ann Biomed Eng. 2017;45(10):2373–2382. [DOI] [PubMed] [Google Scholar]
- 36. Choi M, Yeom HA, Jung D. Association between physical activity and metabolic syndrome in older adults in Korea: analysis of data from the Korean National Health and Nutrition Examination Survey IV. Nurs Health Sci. 2013:15(3):379–386. [DOI] [PubMed] [Google Scholar]
- 37. Dallas G, Paradisis G, Kirialanis P, Mellos V, Argitaki P, Smirniotou A. The acute effects of different training loads of whole body vibration on flexibility and explosive strength of lower limbs in divers. Biol Sport. 2015;32(3):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cochrane DJ, Loram ID, Stannard SR, Rittweger J. Changes in joint angle, muscle-tendon complex length, muscle contractile tissue displacement, and modulation of EMG activity during acute whole-body vibration. Muscle Nerve. 2009;40(3):420–429. [DOI] [PubMed] [Google Scholar]
- 39. Neto S, Marconi E, Kutter C, et al. Beneficial effects of whole body mechanical vibration alone or combined with auriculotherapy in the pain and in flexion of knee of individuals with knee osteoarthritis. Acupunct Electrother Res. 2017;42(3):185–201. [Google Scholar]
- 40. Kim H, Kwon BS, Park JWM, et al. Effect of whole body horizontal vibration exercise in chronic low back pain patients: vertical versus horizontal vibration exercise. Ann Rehabil Med. 2018;42(6):804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gordon R, Bloxham S. A systematic review of the effects of exercise and physical activity on non-specific chronic low back pain. Healthcare (Basel). 2016;4(2). pii: E22. doi:10.3390/healthcare4020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Duc S, Rønnestad BR, Bertucci W. Adding whole body vibration to preconditioning squat exercise increases cycling sprint performance. J Strength Cond Res. 2017. doi:10.1519/JSC.0000000000002236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, CONSORT_2010_Checklist for Acute and Cumulative Effects With Whole-Body Vibration Exercises Using 2 Biomechanical Conditions on the Flexibility and Rating of Perceived Exertion in Individuals With Metabolic Syndrome: A Randomized Clinical Trial Pilot Study by P. C. Paiva, C. A. Figueiredo, A. Reis-Silva, A. Francisca-Santos, L. L. Paineiras-Domingos, E. Martins-Anjos, M. E. S. Melo-Oliveira, G. M. G. Lourenço-Revelles, E. Moreira-Marconi, E. O. Guedes-Aguiar, A. A. Brandão, M. F. T. Neves, V. L. Xavier, D. L. Borges, A. C. R. Lacerda, V. A. Mendonça, A. Sonza, H. Quinart, F. C. Boyer, R. Taiar, A. Sartorio, D. J. Cochrane, M. Bernardo-Filho and D. C. Sá-Caputo in Dose-Response