Abstract
Background:
The aim of the current systematic review and network meta-analysis (NMA) was to assess the diagnostic characteristics of the gastroesophageal reflux disease questionnaire (GERDQ), proton-pump inhibitor (PPI) test, baseline impedance, mucosal impedance, dilated intercellular spaces (DIS), salivary pepsin, esophageal pH/pH impedance monitoring and endoscopy for gastroesophageal reflux disease (GERD).
Methods:
We searched PubMed and the Cochrane Controlled Trial Register database (from inception to 10 April 2018) for studies assessing the diagnostic characteristics of the GERDQ, PPI test, baseline impedance, mucosal impedance, DIS, or salivary pepsin and esophageal pH/pH impedance monitoring/endoscopy in patients with GERD. Direct pairwise comparison and a NMA using Bayesian methods under random effects were performed. We also assessed the ranking probability.
Results:
A total of 40 studies were identified. The NMA found no significant difference among the baseline impedance, mucosal impedance, and esophageal pH/pH impedance monitoring and endoscopy in terms of both sensitivity and specificity. It was also demonstrated that the salivary pepsin detected by the Peptest device had comparable specificity to esophageal pH/pH impedance monitoring and endoscopy. Results of ranking probability indicated that esophageal pH/pH impedance monitoring and endoscopy had highest sensitivity and specificity, followed by mucosal impedance and baseline impedance, whereas GERDQ had the lowest sensitivity and PPI test had the lowest specificity.
Conclusions:
In a systematic review and NMA of studies of patients with GERD, we found that baseline impedance and mucosal impedance have relatively high diagnostic performance, similar to esophageal pH/pH impedance monitoring and endoscopy.
Keywords: baseline impedance, dilated intercellular space, GERDQ, mucosal impedance, network meta-analysis, proton-pump inhibitor test, salivary pepsin
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common healthcare issues, with an estimated worldwide prevalence of up to 33%1,2 resulting in a heavy economic burden of approximately $13.0 billion/year to the healthcare system in the USA alone, due to the different diagnostic testing and overuse of proton-pump inhibitors (PPIs) to a great extent.3 Albeit with the advances of diagnostic tests for GERD, the lack of a ‘gold standard’ has made identifying patients with GERD one of the biggest dilemmas in clinical practice.
Although GERD is generally empirically diagnosed based on typical reflux symptoms (heartburn and regurgitation),4,5 the sensitivity and specificity of the symptom-based diagnosis of GERD is limited due to the complex symptom spectrum for GERD.6 Patients with suspected GERD symptoms are often first tested for a response to PPI therapy, which definitely results in unnecessary overuse of PPIs because of its high placebo effect and low specificity.7 So far, upper endoscopy and esophageal pH/pH impedance testing are usually performed to detect GERD complications, as well as documentation of the presence of reflux for an objective GERD diagnosis.8 In order to develop a better understanding of the pathophysiology and improve appropriate GERD diagnosis, several new diagnostic tests, such as baseline impedance, esophageal mucosal impedance, salivary pepsin, and histopathology have been developed in recent years.9
To the best of our knowledge, there has been little published information regarding the comparison of diagnostic performance among individual tests. Therefore, we performed a systematic review and network meta-analysis (NMA) to assess the diagnostic characteristics of the GERDQ questionnaire, PPI test, baseline impedance, mucosal impedance, dilated intercellular spaces (DIS), salivary pepsin, esophageal pH/pH impedance monitoring and endoscopy for GERD.
Methods
Search strategy
An electronic and manual search of PubMed and the Cochrane Controlled Trial Register database for relevant articles from inception to April 2018 was performed by two authors independently. The combination of keywords and free text including: GERD Questionnaires; omeprazole, lansoprazole, pantoprazole, rabeprazole, esomeprazole, and PPI test; baseline impedance; mucosal impedance; dilated intercellular spaces; pepsin; esophageal pH/pH impedance monitoring; and endoscopy were used as search terms of different diagnostic tests for GERD. Additional search terms were: GERD or GORD, expanded to diagnosis, screening, reproducibility of results, sensitivity and specificity, false-negative reactions, false-positive reactions, predictive value, accuracy, and likelihood ratio.10
The searches were limited to English- or Chinese-language studies performed in adults (age > 18 years). Only data accessible in peer-reviewed journals were included to minimize potential sources of bias and inaccuracy.11
Inclusion criteria and exclusion criteria
Studies were screened for inclusion and final decisions on exclusion were made by two authors independently. Studies assessing the diagnostic characteristics of the GERDQ, PPI test, baseline impedance, mucosal impedance, DIS, salivary pepsin, esophageal pH/pH impedance monitoring, or endoscopy in adults with presumptive GERD were screened to be included. Studies were excluded if they focused only on children (age < 18 years) or patients who have specific diseases (such as cardiovascular disease) or who have had operations, if they focused exclusively on patients with extraesophageal GERD symptoms (such as asthma or laryngitis).
Data extraction
Data from the included studies were extracted by two authors independently. Information extracted included patient characteristics, study design, setting, gold standard and diagnostic modalities, and definitions of outcomes. Numbers were extracted directly from the tables or derived from percentages if only the total number of patients was available. Discrepancies were resolved by discussion until consensus was achieved for all data.
Quality assessment of studies
The quality of all included studies was assessed by researchers, according to the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool.12 The QUADAS-2 tool included the following four key domains: patient selection, index test, reference standard, and flow of patients through the study, and timing of the index tests and reference standard (flow and timing). Review Manager 5 (RevMan 5.2.3, Cochrane Collaboration, Oxford, UK) statistical computing software was used to carry out quality assessment and investigation of publication bias.
The positive standard of diagnostic tests for GERD
The following standards for GERD were used in the current study, all of which were based on commonly accepted measures (Table 1).
Table 1.
The information of included studies.
| Comparison (author) | Evaluable patients (n) | Men (%) | Average age | Prevalence (%) of esophagitis | Reference tests |
Study test | Outcome measure extracted | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper endoscopy | pH/pH impedance monitoring |
||||||||||
| AET | Number of reflux events | DeMeester score | Symptom reflux association | ||||||||
| A versus B | |||||||||||
| Frazzoni et al.13 | 121 | 51.89 | NA | 42.33 | Erosion | Total AET > 3.2% | >48 | — | SAP ⩾ 95% or SI ⩾ 50% | MNBI | A cut-off value of 2292 Ω |
| Ravi et al.14 | 55 | 41.38 | 60 | 22.73 | — | Total AET ⩾ 5.0% | — | — | — | Baseline impedance | A cut-off value of 2268 Ω |
| Frazzoni et al.15 | 289 | 45.67 | 50 | 23.53 | Erosion | Total AET >3.2% | — | — | SAP ⩾ 95% or SI ⩾ 50% | MNBI | A cut-off value of 2292 Ω |
| Kandulski et al.16 | 36 | 30.77 | 55 | 30.77 | Erosion | Total AET > 4.2% | — | — | SAP ⩾ 95% or SI ⩾ 50% | Baseline impedance | A cut-off value of 2100 Ω |
| A versus C | |||||||||||
| Hayat et al.17 | 198 | 44.14 | 49.7 | NA | — | Total AET > 4.2% | — | — | SAP ⩾ 95% | Salivary pepsin | At least one sample +ve > 16 ng/ml |
| Saritas Yuksel et al.18 | 47 | 65.52 | 50 | 23.4 | Erosion | Total AET > 4.2% | — | — | — | Salivary pepsin | A cut-off value of +1.0 or greater |
| A versus D | |||||||||||
| Cui et al.19 | 565 | 40.7 | 49.4 | NA | Erosion | — | — | ⩾14.7 | SAP ⩾ 95% | DIS | A cut-off value of 0.9 μm |
| Zhou et al.20 | 576 | 41.67 | 49.3 | 27.04 | Erosion | — | — | ⩾14.7 | SAP ⩾ 95% | DIS | A cut-off value of 0.9 μm |
| Cui et al.21 | 161 | 59.66 | NA | 48.74 | Erosion | — | — | ⩾14.7 | — | DIS | A cut-off value of 0.85 μm |
| Mastracci et al.22 | 139 | 57.14 | 52 | 40.34 | Erosion | Total AET > 5.5% | — | — | — | DIS | Mild and marked DIS |
| Vela et al.23 | 26 | NA | NA | 11.54 | Erosion | Total AET > 5.3% | — | — | SAP ⩾ 95% | DIS | A cut-off value of 0.68 μm |
| A versus E | |||||||||||
| Zhou et al.20 | 636 | 41.67 | 49.3 | 27.04 | Erosion | — | — | ⩾14.7 | SAP ⩾ 95% | GERDQ | A cut-off score of 8 |
| Zavala-Gonzales et al.24 | 252 | 37 | 49.49 | 43.65 | Erosion | Total AET > 4.2% | — | — | SI ⩾ 50% | GERDQ | A cut-off score of 8 |
| Jonasson et al.25 | 169 | 53 | 47.4 | 81.07 | Erosion | Total AET > 5.5%, or upright AET > 6.7%, or supine AET > 6.9% | — | — | SAP ⩾ 95% | GERDQ | A cut-off score of 8 |
| Lacy et al.26 | 177 | 28.2 | 51.00 | 16.87 | — | Total AET > 5.3% | — | — | — | GERDQ | A cut-off score of 8 |
| Jones et al.27 | 308 | 46.43 | 47 | 37.66 | Erosion | Total AET > 5.5% | — | — | SAP ⩾ 95% | GERDQ | A cut-off score of 8 |
| Bai et al.28 | 8065 | 50.1 | 46.3 | 17.79 | Erosion | — | — | — | — | GERDQ | A cut-off score of 8 |
| A versus F | |||||||||||
| Zhou et al.20 | 636 | 41.67 | 49.3 | 27.04 | Erosion | — | — | ⩾14.7 | SAP ⩾ 95% | Esomeprazole, 20 mg BID for 2 weeks | Complete symptom relief |
| Jonasson et al.25 | 124 | 53 | 47.4 | 81.07 | Erosion | Total AET > 5.5%, or upright AET > 6.7%, or supine AET > 6.9% | — | — | SAP ⩾95% | PPI for 4 weeks | Symptoms decrease to 1 day/week |
| Lee et al.29 | 188 | 41.49 | 49.9 | 37.23 | Erosion | — | — | — | — | Lansoprazole, 15 mg/30 mg/60 mg once daily for 2 weeks | Symptom score improved by more than 50% |
| Dent et al.30 | 296 | 46.43 | 47 | 37.66 | Erosion | Total AET > 5.5% | — | — | SAP ⩾ 95% | Esomeprazole, 40 mg daily for 14 ± 3 days | Complete symptom relief |
| Cho et al.31 | 73 | 57.53 | 47 | 63.01 | Erosion | Total AET > 4.4% | — | — | SAP ⩾ 95% | Lansoprazole, 30 mg BID for 2 weeks | Symptom score improved by more than 50% |
| Zheng et al.32 | 27 | 29.63 | 57 | 3.7 | Erosion | Total AET > 4.0% | — | ⩾ 14.0 | SI ⩾ 50% | Esomeprazole, 20 mg BID for 2 weeks | NA |
| Lee et al.33 | 164 | 43.29 | NA | 42.07 | Erosion | — | — | — | — | Rabeprazole, 20 mg/40 mg for 2 weeks | 50% symptom reduction |
| Fan et al.34 | 32 | 37.5 | 47.3 | 0 | — | — | — | ⩾14.7 | SI ⩾ 50% | Rabeprazole, 10 mg BID for 2 weeks | Symptom score reduced by more than a third |
| des Varannes et al.35 | 29 | 40.28 | NA | 31.94 | Erosion | Total AET > 4.2% | — | — | SAP ⩾ 95% or SI ⩾ 50% | Rabeprazole, 20 mg BID for 1 week | The cut-off descriptor of at least a ‘clear improvement’ |
| Aanen et al.36 | 67 | 62.16 | 51 | NA | — | Total AET > 4.2% | — | — | SAP ⩾ 95% or SI ⩾ 50% | Esomeprazole, 40 mg daily for 2 weeks | Adequate symptom suppression |
| Dickman et al.37 | 35 | 65.71 | 55.6 | 34.29 | Erosion | Total AET > 4.2%, or upright AET > 6.0%, or supine AET > 1.2% | Rabeprazole, 20 mg BID for 1 week | Symptom score improved by more than 50% | |||
| Juul-Hansen et al.38 | 52 | 33.85 | 47 | 0 | — | Total AET > 4.0% | — | — | — | Lansoprazole, 60 mg for 1 week | Symptom relief: yes/no |
| Pandak et al.39 | 38 | 42.86 | NA | 26.19 | Erosion | Total AET > 4.2% | — | — | — | Omeprazole, 40 mg/d orally BID for 2 weeks | Symptom score improved by more than two points |
| Juul-Hansen et al.40 | 56 | 31.25 | 54 | 0 | — | Total AET > 4.2% | — | — | — | Lansoprazole 60 mg daily for 1 week | Antacid use decreased by more than 75% |
| Fass et al.41 | 35 | 94.29 | 55 | 100 | Erosion | Total AET > 4.2% | — | — | — | Omeprazole (40 mg a.m. plus 20 mg p.m.) for 1 week | Heartburn score improved by more than 50% |
| Fass et al.42 | 42 | 76.19 | 55.2 | 50 | Erosion | Total AET > 4.2% | — | — | — | Omeprazole (40 mg a.m. plus 20 mg p.m.) for 1 week | Heartburn score improved by more than 50% |
| Bate et al.43 | 58 | 55.07 | 47.4 | 49.28 | — | Total AET > 4.0% | — | — | — | Omeprazole, 40 mg o.m. for 2 weeks | Symptoms improved by at least two grades (or from mild to none) |
| Johnsson et al.44 | 160 | NA | NA | 57.5 | Erosion | Total AET > 4.0% | — | — | — | Omeprazole, 20 mg BID for 1 week | Symptoms decreased by at least one grade |
| Fass et al.45 | 37 | 97.44 | 60.1 | 43.24 | Erosion | Total AET > 4.2% | — | — | — | Omeprazole (40 mg a.m. plus 20 mg p.m.) for 1 week | More than 50% reduction in symptoms |
| Carlsson et al.46 | 225 | 50.1 | 50 | 61 | Erosion | — | — | — | — | Omeprazole, 20 mg for 4 weeks | Complete symptom relief |
| Galmiche, Barthelemy and Hamelin47 | 141 | 44.7 | 50 | 26 | Erosion | — | — | — | — | Omeprazole, 20 mg for 4 weeks | Symptoms decrease to 1 day/week, less than mild |
| Hatlebakk et al.48 | 161 | 57 | 49 | 48 | Erosion | — | — | — | — | Omeprazole, 20 mg for 4 weeks | Symptoms decrease to 1 day/week, less than mild |
| Venables et al.49 | 330 | 52 | 52 | 31 | Erosion | — | — | — | — | Omeprazole, 20 mg for 4 weeks | Symptoms decrease to 1 day/week, less than mild |
| A versus G | |||||||||||
| Ates et al.50 | 268 | NA | NA | 22.76 | Erosion | Total AET > 5.3% | — | — | — | Mucosal impedance | A cut-off value of 2019 Ω |
| Saritas Yuksel et al.51 | 69 | NA | NA | 27.54 | Erosion | Total AET > 5.3% | Mucosal impedance | A cut-off value of 3200 Ω | |||
| F versus E | |||||||||||
| Xu et al.52 | 2014 | 126 | 46.83 | 45.6 | NA | 8-week PPI treatment | GERDQ | A cut-off score of 8 | |||
A: esophageal pH/pH impedance monitoring and/or endoscopy; B: baseline impedance; C: salivary pepsin; D: DIS; E: GERDQ; F: PPI test; G: mucosal impedance.
AET, acid exposure time; BID, twice daily; DIS, dilated intercellular space; GERD, gastroesophageal reflux disease; GERDQ, GERD questionnaire; MNBI, mean nocturnal baseline impedance; NA, not applicable; o.m., every morning; SAP, symptom association probability; SI, symptom index; PPI, proton-pump inhibitor.
Upper endoscopy or esophageal pH/pH impedance monitoring
Esophageal mucosal breaks on upper endoscopy suggest the presence of GERD. GERD was diagnosed in studies when patients had esophagitis of any grade in one of the commonly used classification systems, such as the Los Angeles or Hetzel–Dent grading systems.
Ambulatory esophageal pH/pH impedance monitoring is generally considered to provide the most objective evidence for pathologic reflux. The results were considered abnormal based on criteria defined in the individual studies. Whenever possible, we chose definitions that would reasonably be interpreted as abnormal in clinical practice, including: (a) acid exposure time (AET) ⩾ 3.2%–5.5% of the monitoring time; (b) DeMeester score ⩾ 14; (c) positive symptom reflux association, such as symptom-associated probability (SAP) ⩾ 95% or symptom index (SI) ⩾ 50%.
Baseline impedance
The baseline impedance was assessed on the pH/impedance system, and the cut-off value ranging from 2100 Ω to 2292 Ω was established and used in individual studies.
Salivary pepsin
Salivary pepsin was detected and quantitatively/semiquantitatively measured by non-invasive rapid salivary pepsin lateral flow device (LFD) (Peptest, RDBiomed, Hull, UK). The cut-off value was used based on criteria defined in individual studies.
Dilated intercellular space
The quantitative or semiquantitative measurement of DIS under light microscopy was performed, and the cut-off value was used based on criteria defined in individual studies.
GERDQ
GERDQ was considered positive if the score was >8.
Proton-pump inhibitor test
The definition of ‘a positive PPI test’ was based on criteria defined in individual studies. Whenever possible, we chose definitions that would reasonably be interpreted as representing success in clinical practice, and ‘complete relief of heartburn’ is the most commonly adopted criteria.
Mucosal impedance
The cut-off value of mucosal impedance was also established and used in the individual studies.
Statistical analysis
Firstly, traditional pairwise meta-analyses were performed for studies to compare different diagnostic modalities using the Stata version 12.0 software (StataCorp, College Station, TX, USA). The pooled estimates of odd ratios (ORs) and 95% confidence intervals (CIs) for sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), and diagnostic odds ratio of GERD were calculated if there were at least four studies included that had no threshold effect. Area under receiver operating characteristic (AUROC) was also calculated. When the AUROC is closer to 1, the clinical value is greater. When the AUROC is between 0.5 and 0.7, the clinical value is lower. An AUROC value > 0.7 indicates that the clinical value is good. Heterogeneity among studies was tested using the I2 and Chi-square tests.53 Secondly, the evidence network structure was drawn using the R version 3.5.1 statistical computing software and network package. Each node represents different diagnostic tests, with the node size reflecting the number of patients, and the thickness of lines between nodes indicating the number of included studies. Thirdly, Bayesian network meta-analyses were performed to combine the effective sizes of direct and indirect comparisons. Lack of autocorrelation and convergence were checked and confirmed by four chains and a 20,000-simulation burn-in phase; finally, direct probability statements were derived from an additional 50,000-simulation phase.54 The consistency between direct and indirect evidence was assessed with the node-splitting method, and the consistency or inconsistency model was selected accordingly.55 The ranking probability was then used to calculate the probability of each diagnostic test being the most effective diagnostic method based using a Bayesian approach, and the bar charts of the ranking probability were also produced; the larger the value is, the better the rank of the diagnostic test.56,57 Comparison-adjusted funnel plots were performed to detect the small study effects on data.56,58 R (version 3.5.1) package GeMTC was used for this network meta-analysis.
Results
Study search flow
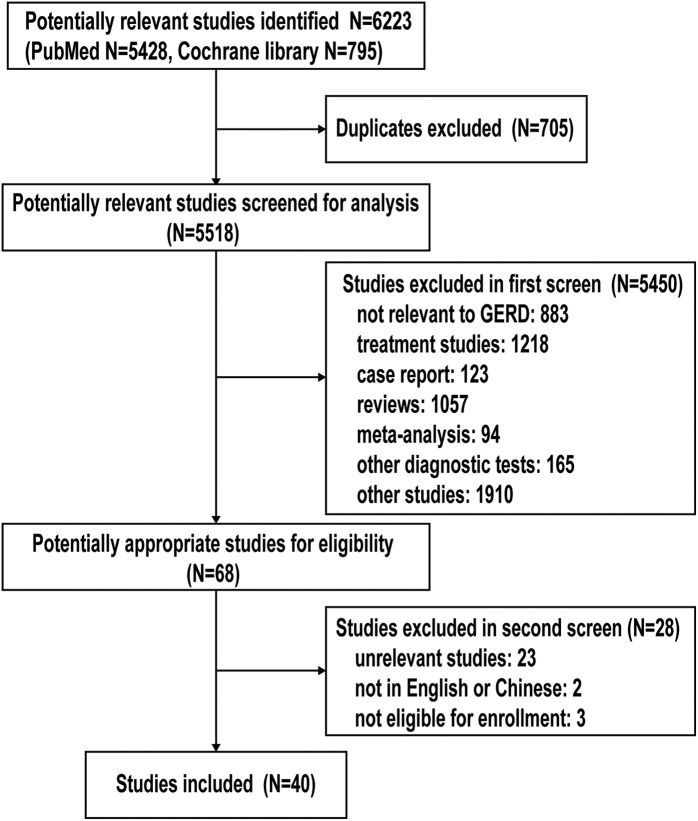
A total of 6223 potentially relevant studies were initially retrieved and identified, of which 705 duplicate studies were excluded. Of the 5518 citations, 5450 citations were ruled out during the first screen by abstract review. A total of 68 studies were then evaluated for eligibility by full-text review. After full-text review, studies unrelated to diagnostic tests for GERD (n = 23), studies not in English/Chinese language (n = 2), and studies not eligible for enrollment (n = 3) were excluded. Altogether, a total of 40 published studies meeting the predetermined inclusion criteria were identified 13–52 (Figure 1).
Figure 1.
The PRISMA study search flow.
PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis.
GERD, gastroesophageal reflux disease.
Study characteristics and qualities
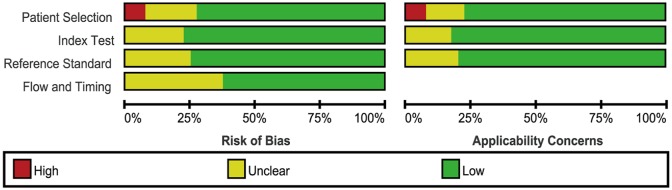
Of 40 included studies, the evaluation of esophageal impedance for GERD diagnosis was performed in 4 studies, salivary pepsin by Peptest in 2 studies, DIS in 5 studies, GERDQ in 6 studies, and PPI test in 23 studies when compared with esophageal pH/pH impedance monitoring or endoscopy. One study compared the diagnostic accuracy of GERDQ and PPI test. The clinical information of the included studies is shown in Table 1. The diagnostic characteristic of diagnostic tests for GERD varied across studies (Table 2). The evaluation of the risk of bias and applicability concerns using the QUADAS-2 was shown in Figure 2 and Supplementary Figure 1.
Table 2.
Diagnostic evaluation of seven diagnostic tests for gastroesophageal reflux disease.
| Comparison (author) | Patients |
Prevalence of GERD according to reference test | SEN | SPE | PPV | NPV | LR+ | LR− | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | Total | ||||||||
| A versus B | ||||||||||||
| Frazzoni et al.13 | 60 | 3 | 20 | 38 | 121 | 0.66 | 0.75 | 0.93 | 0.95 | 0.66 | 10.25 | 0.27 |
| Ravi et al.14 | 25 | 5 | 4 | 21 | 55 | 0.53 | 0.86 | 0.81 | 0.83 | 0.84 | 4.48 | 0.17 |
| Frazzoni et al.15 | 62 | 31 | 6 | 190 | 289 | 0.24 | 0.91 | 0.86 | 0.67 | 0.97 | 6.50 | 0.10 |
| Kandulski et al.16 | 15 | 5 | 4 | 12 | 36 | 0.53 | 0.79 | 0.71 | 0.75 | 0.75 | 2.68 | 0.30 |
| A versus C | ||||||||||||
| Hayat et al.17 | 66 | 40 | 18 | 74 | 198 | 0.42 | 0.79 | 0.65 | 0.62 | 0.80 | 2.24 | 0.33 |
| Saritas Yuksel et al.18 | 11 | 2 | 11 | 23 | 47 | 0.47 | 0.50 | 0.92 | 0.85 | 0.68 | 6.25 | 0.54 |
| A versus D | ||||||||||||
| Cui et al.19 | 186 | 123 | 111 | 145 | 565 | 0.53 | 0.63 | 0.54 | 0.60 | 0.57 | 1.36 | 0.69 |
| Zhou et al.20 | 188 | 118 | 119 | 151 | 576 | 0.53 | 0.61 | 0.56 | 0.61 | 0.56 | 1.40 | 0.69 |
| Cui et al.21 | 111 | 0 | 8 | 42 | 161 | 0.74 | 0.93 | 1.00 | 1.00 | 0.84 | NA | 0.07 |
| Mastracci et al.22 | 102 | 6 | 17 | 14 | 139 | 0.86 | 0.86 | 0.70 | 0.94 | 0.45 | 2.86 | 0.20 |
| Vela et al.23 | 9 | 1 | 6 | 10 | 26 | 0.58 | 0.60 | 0.91 | 0.90 | 0.63 | 6.60 | 0.44 |
| A versus E | ||||||||||||
| Zhou et al.20 | 203 | 145 | 149 | 139 | 636 | 0.55 | 0.58 | 0.49 | 0.58 | 0.48 | 1.13 | 0.86 |
| Zavala-Gonzales et al.24 | 129 | 20 | 51 | 52 | 252 | 0.71 | 0.72 | 0.72 | 0.87 | 0.50 | 2.58 | 0.39 |
| Jonasson et al.25 | 115 | 11 | 32 | 11 | 169 | 0.87 | 0.78 | 0.50 | 0.91 | 0.26 | 1.56 | 0.44 |
| Lacy et al.26 | 77 | 41 | 31 | 28 | 177 | 0.61 | 0.71 | 0.41 | 0.65 | 0.47 | 1.20 | 0.71 |
| Jones et al.27 | 125 | 33 | 69 | 81 | 308 | 0.63 | 0.64 | 0.71 | 0.79 | 0.54 | 2.23 | 0.50 |
| Bai et al.28 | 620 | 1405 | 815 | 5225 | 8065 | 0.18 | 0.43 | 0.79 | 0.31 | 0.87 | 2.04 | 0.72 |
| A versus F | ||||||||||||
| Zhou et al.20 | 248 | 158 | 104 | 126 | 636 | 0.55 | 0.70 | 0.44 | 0.61 | 0.55 | 1.27 | 0.67 |
| Jonasson et al.25 | 90 | 4 | 27 | 3 | 124 | 0.94 | 0.77 | 0.43 | 0.96 | 0.10 | 1.35 | 0.54 |
| Lee et al.29 | 55 | 82 | 15 | 36 | 188 | 0.37 | 0.79 | 0.31 | 0.40 | 0.71 | 1.13 | 0.70 |
| Dent et al.30 | 106 | 35 | 91 | 64 | 296 | 0.67 | 0.54 | 0.65 | 0.75 | 0.41 | 1.52 | 0.71 |
| Cho et al.31 | 49 | 4 | 15 | 5 | 73 | 0.88 | 0.77 | 0.56 | 0.92 | 0.25 | 1.72 | 0.42 |
| Zheng et al.32 | 5 | 11 | 4 | 7 | 27 | 0.33 | 0.56 | 0.39 | 0.31 | 0.64 | 0.91 | 1.14 |
| Lee et al.33 | 52 | 28 | 17 | 67 | 164 | 0.42 | 0.75 | 0.71 | 0.65 | 0.80 | 2.56 | 0.35 |
| Fan et al.44 | 20 | 2 | 5 | 5 | 32 | 0.78 | 0.80 | 0.71 | 0.91 | 0.50 | 2.80 | 0.28 |
| Des Varannes et al.35 | 15 | 6 | 3 | 5 | 29 | 0.62 | 0.83 | 0.45 | 0.71 | 0.63 | 1.53 | 0.37 |
| Aanen et al.36 | 41 | 17 | 5 | 4 | 67 | 0.69 | 0.89 | 0.19 | 0.71 | 0.44 | 1.10 | 0.57 |
| Dickman et al.37 | 12 | 2 | 4 | 17 | 35 | 0.46 | 0.75 | 0.89 | 0.86 | 0.81 | 7.13 | 0.28 |
| Juul-Hansen et al.38 | 34 | 17 | 0 | 1 | 52 | 0.65 | 1.00 | 0.06 | 0.67 | 1.00 | 1.06 | 0.00 |
| Pandak et al.39 | 19 | 7 | 1 | 11 | 38 | 0.53 | 0.95 | 0.61 | 0.73 | 0.92 | 2.44 | 0.08 |
| Juul-Hansen et al.40 | 29 | 11 | 5 | 11 | 56 | 0.61 | 0.85 | 0.50 | 0.73 | 0.69 | 1.71 | 0.29 |
| Fass et al.41 | 21 | 8 | 0 | 6 | 35 | 0.60 | 1.00 | 0.43 | 0.72 | 1.00 | 1.75 | 0.00 |
| Fass et al.42 | 28 | 3 | 7 | 4 | 42 | 0.83 | 0.80 | 0.57 | 0.90 | 0.36 | 1.87 | 0.35 |
| Bate et al.43 | 22 | 11 | 10 | 15 | 58 | 0.55 | 0.69 | 0.58 | 0.67 | 0.60 | 1.63 | 0.54 |
| Johnsson et al.44 | 100 | 16 | 35 | 9 | 160 | 0.84 | 0.74 | 0.36 | 0.86 | 0.20 | 1.16 | 0.72 |
| Fass et al.45 | 18 | 2 | 5 | 12 | 37 | 0.62 | 0.78 | 0.86 | 0.90 | 0.71 | 5.48 | 0.25 |
| Carlsson et al.46 | 66 | 25 | 72 | 62 | 225 | 0.61 | 0.48 | 0.71 | 0.73 | 0.46 | 1.66 | 0.73 |
| Galmiche et al.47 | 27 | 65 | 10 | 39 | 141 | 0.26 | 0.73 | 0.38 | 0.29 | 0.80 | 1.17 | 0.72 |
| Hatlebakk et al.48 | 55 | 59 | 22 | 25 | 161 | 0.48 | 0.71 | 0.30 | 0.48 | 0.53 | 1.02 | 0.96 |
| Venables et al.49 | 80 | 120 | 21 | 109 | 330 | 0.31 | 0.79 | 0.48 | 0.40 | 0.84 | 1.51 | 0.44 |
| A versus G | ||||||||||||
| Ates et al.50 | 108 | 6 | 34 | 120 | 268 | 0.53 | 0.76 | 0.95 | 0.95 | 0.78 | 15.97 | 0.25 |
| Saritas Yuksel et al.51 | 37 | 9 | 5 | 18 | 69 | 0.61 | 0.88 | 0.67 | 0.80 | 0.78 | 2.64 | 0.18 |
| F versus E | ||||||||||||
| Xu et al.52 | 68 | 2 | 34 | 22 | 126 | 0.81 | 0.67 | 0.92 | 0.97 | 0.39 | 8.00 | 0.36 |
A: esophageal pH/pH impedance monitoring and/or endoscopy; B: baseline impedance; C: salivary pepsin; D: DIS; E: GERDQ; F: PPI test; G: mucosal impedance.
AET, acid exposure time; DIS, dilated intercellular space; FN, false negative; FP, false positive; GERD, gastroesophageal reflux disease; GERDQ, GERD questionnaire; LR, likelihood ratio; MNBI, mean nocturnal baseline impedance; NPV, negative-predictive value; PPI, proton-pump inhibitor; PPV, positive-predictive value; SAP, symptom association probability; SEN, sensitivity; SI, symptom index; SPE, specificity; TP, true positive; TN, true negative.
Figure 2.
The evaluation of risks of bias of included studies.
Pairwise meta-analysis for diagnostic tests for GERD
A direct pairwise meta-analysis of the diagnostic performance of six different tests for GERD diagnosis was conducted. The results revealed that the baseline impedance, GERDQ and PPI test exhibited lower sensitivity and specificity when compared with esophageal pH/pH impedance monitoring or endoscopy. We also calculated the AUROC for each diagnostic test and found that the esophageal impedance and PPI test were higher than 0.70, indicating that they had relatively high diagnostic value (Supplementary Table 1). The pairwise meta-analysis of DIS could not be performed successfully due to a threshold effect. The pairwise meta-analysis of salivary pepsin and mucosal impedance could not be performed either because there were only two studies included.
Evidence network of diagnostic tests for GERD
The evidence network structure included seven diagnostic tests. The highest number of evaluable patients performed the esophageal pH/pH impedance monitoring or endoscopy, and most studies compared PPI test with esophageal pH/pH impedance monitoring or endoscopy for GERD diagnosis (Figure 3).The effect of the direct comparison of different tests with esophageal pH/pH impedance monitoring or endoscopy had similar effect on the entire network meta-analysis (Supplementary Figure 2).
Figure 3.
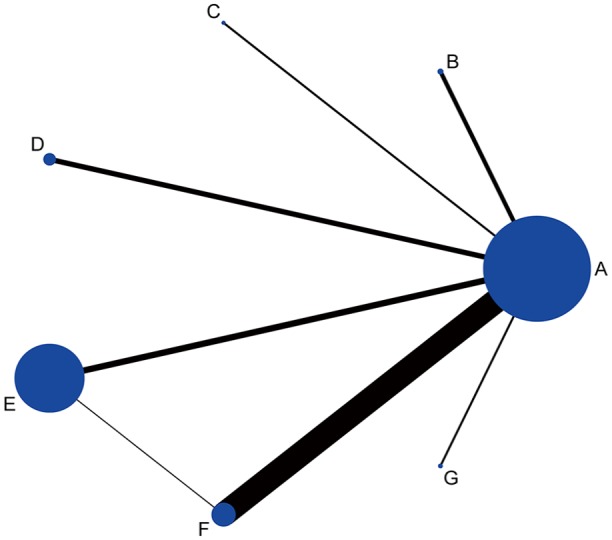
The evidence network structure.
A: esophageal pH/pH impedance monitoring or endoscopy; B: baseline impedance; C: salivary pepsin; D: DIS; E: GERDQ; F: PPI test; G: mucosal impedance.
DIS, dilated intercellular spaces; GERD, gastroesophageal reflux disease; GERDQ, GERD questionnaire; PPI, proton-pump inhibitor.
Main results of network meta-analysis of diagnostic tests for GERD
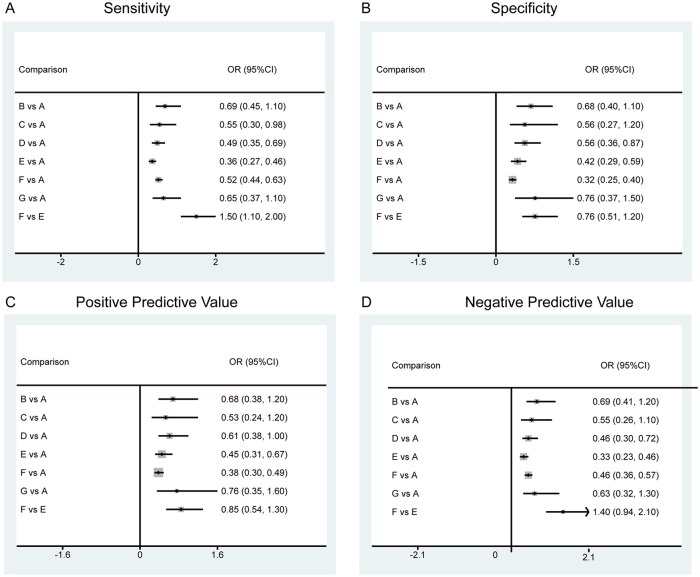
The NMA found no significant difference among the baseline impedance, mucosal impedance, and esophageal pH/pH impedance monitoring or endoscopy in terms of both sensitivity and specificity. It was also demonstrated that the salivary pepsin detected by Peptest had comparable specificity with esophageal pH/pH impedance monitoring or endoscopy (Figure 4).
Figure 4.
The forest plots based on sensitivity, specificity, positive-predictive value and negative-predictive value of different diagnostic tests for GERD.
A: esophageal pH/pH impedance monitoring or endoscopy; B: baseline impedance; C: salivary pepsin; D: DIS; E: GERDQ; F: PPI test; G: mucosal impedance.
DIS, dilated intercellular spaces; GERD, gastroesophageal reflux disease; GERDQ, GERD questionnaire; PPI, proton-pump inhibitor.
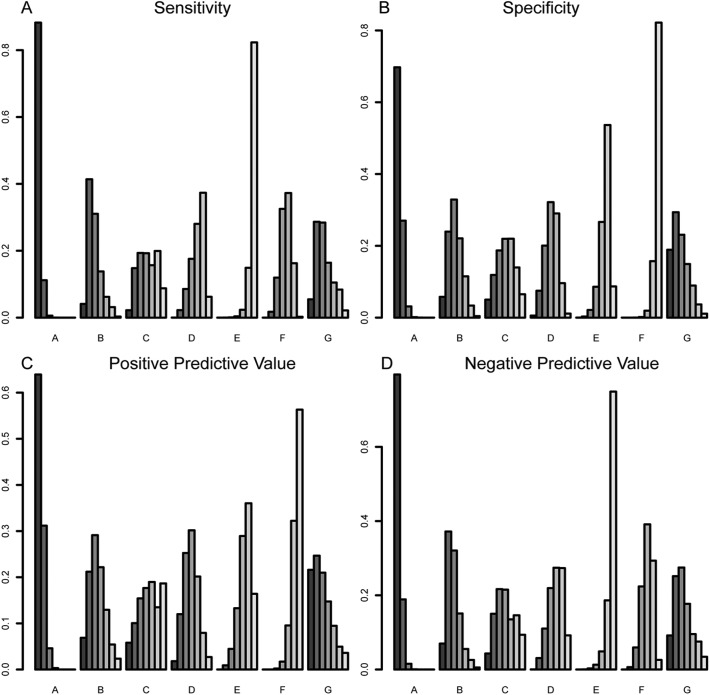
Ranking probability of diagnostic tests for GERD
Ranking probability indicated that esophageal pH/pH impedance monitoring and/or endoscopy had the highest sensitivity, followed by baseline impedance or mucosal impedance, PPI test, salivary pepsin or DIS, and GERDQ [Figure 5(a)]. Moreover, esophageal pH/pH impedance monitoring and/or endoscopy also had the highest specificity, followed by the mucosal impedance, baseline impedance, DIS or salivary pepsin, GERDQ, and PPI test [Figure 5(b)]. The ranking probability of positive-predictive value and negative-predictive value was also provided in Figure 5(c) and 5(d).
Figure 5.
The ranking probability based on sensitivity, specificity, positive-predictive value and negative-predictive value of different diagnostic tests for GERD.
A: esophageal pH/pH impedance monitoring and/or endoscopy; B: baseline impedance; C: salivary pepsin; D: DIS; E: GERDQ; F: PPI test; G: mucosal impedance.
DIS, dilated intercellular spaces; GERD, gastroesophageal reflux disease; GERDQ, GERD questionnaire; PPI, proton-pump inhibitor.
Assessment of publication bias
The results of assessment of publication bias demonstrated symmetrical distribution, indicating no small sample effect or publication bias in this NMA (Supplementary Figure 3).
Discussion
We present the first systematic review and NMA comparing the diagnostic performance of GERDQ questionnaire, PPI test, baseline impedance, mucosal impedance, DIS, salivary pepsin and esophageal pH/pH impedance monitoring/endoscopy for GERD. The NMA and ranking probabilities reveal that the mucosal impedance and baseline impedance had comparable sensitivity and specificity with esophageal pH/pH impedance monitoring or endoscopy. GERDQ had the lowest sensitivity, and PPI test had the lowest specificity.
The results of direct pairwise comparison and NMA shows that esophageal reflux monitoring or endoscopy is superior to other diagnostic tests for GERD, which is in accordance with the recommendation in current guidelines.59 In the current meta-analysis, the criteria for abnormal reflux varied across the included studies which may add clinical heterogeneity to our analysis to some extent. However, this heterogeneity couldn’t be avoided, since the understanding toward GERD pathophysiology and the diagnostic criteria for GERD had developed and changed during the past several decades. For instance, the latest Lyon Consensus lists conclusive evidence, including Los Angeles grade C and D erosive esophagitis from upper endoscopy or AET > 6% from pH/pH impedance monitoring for the definitive diagnosis of GERD.60 However, most previous studies diagnosed GERD based on lower AET thresholds and esophageal mucosal breaks, regardless of grades. Further studies are needed to compare different grades of esophagitis and different criteria for reflux for the diagnosis and management of GERD.
We also found that the esophageal mucosal impedance as well as the baseline impedance had comparable diagnostic performance with esophageal reflux monitoring and endoscopy. Baseline impedance reflects the integrity of the esophageal mucosa,61 with low values observed in patients having GERD, and associated with increased acid reflux, as well as DIS.62,63 It is reported that mucosal impedance can help differentiate GERD from eosinophilic esophagitis (EoE), achalasia, and healthy controls with higher specificity (95%) when compared with reflux testing (64%).50 Given its high diagnostic performance, the esophageal mucosal impedance, as well as the baseline impedance, may become promising diagnostic tools for GERD in the future. However, normative values for them still need to be determined. Also, mucosa impedance is currently not commercialized and widely used despite high diagnostic utility.
Additionally, the salivary pepsin detection was initially proposed as a non-invasive method for the diagnosis of GERD. Our results demonstrated that the measurement of salivary pepsin detected by the Peptest device had comparable specificity to esophageal pH/pH impedance monitoring or endoscopy. Nevertheless, Sifrim and coworkers failed to reproduce good specificity of salivary pepsin to diagnose GERD, and they found that salivary pepsin could not differentiate GERD from functional heartburn.64 Therefore, salivary pepsin detection cannot be recommended for clinical application at present. Besides, our results found that the measurement of DIS only had only modest diagnostic characteristics. It has been reported that esophageal mucosal changes such as DIS may help differentiate GERD from other disorders.65,66 Even so, the measurement of DIS on electron microscopy is not ready for clinical practice yet.
There are limitations to these findings. First, esophageal pH/pH impedance monitoring and endoscopy were examined together in the current NMA, which were found to have highest sensitivity and specificity. However, upper endoscopy alone has, actually, low sensitivity for GERD, so we should interpret results with caution. Second, we only analyzed GERDQ, while there are several other questionnaires for GERD diagnosis. However, all of them have different scoring systems and putting them together may lead to high heterogeneity in the NMA. And the GERDQ is a questionnaire derived from validated questionnaires including the Reflux Disease Questionnaire, Gastrointestinal Symptom Rating Scale, and the GERD Impact Scale. It is the most commonly used questionnaire for GERD. Thus, we had to choose only one questionnaire to be examined. Also, we did not include the postreflux swallow-induced peristaltic wave (PSPW) index in the current NMA because studies evaluating the diagnostic value of the PSPW index are limited at present; future NMA studies can include it, since it is also a promising metric for GERD diagnosis. Third, the rankings and probabilities can sometimes be misleading, ignoring the basic principles of certainty evaluation in evidence during NMA. We should analyze the results of NMA with caution and take the results of pairwise comparison into account. Furthermore, there exists high heterogeneity in the study populations, including various baseline characteristics, different procedure of the testing, and different outcome measure, etc., which may make the results incomparable, to an extent. Finally, the results of pairwise comparison and NMA were associated with wide confidence intervals and some included studies were of moderate-to-low quality.
In conclusion, the current systematic review and NMA shows that esophageal mucosal impedance and baseline impedance has a high diagnostic performance similar to esophageal reflux monitoring or endoscopy. The future direction of GERD management should be developing and improving techniques with high diagnostic performance; not only for a precision phenotype definition but also for a tailored treatment strategy.
Supplemental Material
Supplemental material, Supplementary_Fig._1._risk_of_bias_for_each_study for Assessing different diagnostic tests for gastroesophageal reflux disease: a systematic review and network meta-analysis by Mengyu Zhang, John E. Pandolfino, Xuyu Zhou, Niandi Tan, Yuwen Li, Minhu Chen and Yinglian Xiao in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_Fig._2._the_effect_of_evidence for Assessing different diagnostic tests for gastroesophageal reflux disease: a systematic review and network meta-analysis by Mengyu Zhang, John E. Pandolfino, Xuyu Zhou, Niandi Tan, Yuwen Li, Minhu Chen and Yinglian Xiao in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_Fig._3._Publication_Bias for Assessing different diagnostic tests for gastroesophageal reflux disease: a systematic review and network meta-analysis by Mengyu Zhang, John E. Pandolfino, Xuyu Zhou, Niandi Tan, Yuwen Li, Minhu Chen and Yinglian Xiao in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_Table1._pairwise_meta_analysis-supplementary for Assessing different diagnostic tests for gastroesophageal reflux disease: a systematic review and network meta-analysis by Mengyu Zhang, John E. Pandolfino, Xuyu Zhou, Niandi Tan, Yuwen Li, Minhu Chen and Yinglian Xiao in Therapeutic Advances in Gastroenterology
Acknowledgments
Mengyu Zhang: data acquisition and analysis, manuscript drafting; John E Pandolfino: critical revision of the manuscript; Xuyu Zhou: statistical analysis; Niandi Tan: data acquisition and analysis; Yuwen Li: data acquisition and analysi;, Minhu Chen and Yinglian Xiao: study design, data analysis, study supervision, and finalizing the manuscript.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants from the National Natural Science Foundation of China (81770544).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Mengyu Zhang  https://orcid.org/0000-0002-2838-1103
https://orcid.org/0000-0002-2838-1103
Minhu Chen  https://orcid.org/0000-0001-9925-135X
https://orcid.org/0000-0001-9925-135X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mengyu Zhang, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
John E. Pandolfino, Department of Medicine, Northwestern University, Chicago, IL, USA
Xuyu Zhou, Medical Information Research Institute, Sun Yat-sen University, Guangzhou, China.
Niandi Tan, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Yuwen Li, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Minhu Chen, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Yinglian Xiao, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, China.
References
- 1. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920; quiz 1943. [DOI] [PubMed] [Google Scholar]
- 2. El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014; 63: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012; 143: 1179–1187.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108: 308–328; quiz 29. [DOI] [PubMed] [Google Scholar]
- 5. Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135: 1392–1413, 1413.e1-5. [DOI] [PubMed] [Google Scholar]
- 6. Richter JE, Rubenstein JH. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology 2018; 154: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bytzer P, Jones R, Vakil N, et al. Limited ability of the proton-pump inhibitor test to identify patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2012; 10: 1360–1366. [DOI] [PubMed] [Google Scholar]
- 8. Roman S, Gyawali CP, Savarino E, et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil 2017; 29: 1–15. [DOI] [PubMed] [Google Scholar]
- 9. Vaezi MF, Sifrim D. Assessing old and new diagnostic tests for gastroesophageal reflux disease. Gastroenterology 2018; 154: 289–301. [DOI] [PubMed] [Google Scholar]
- 10. Deville WL, Bezemer PD, Bouter LM. Publications on diagnostic test evaluation in family medicine journals: an optimal search strategy. J Clin Epidemiol 2000; 53: 65–69. [DOI] [PubMed] [Google Scholar]
- 11. Ioannidis JP, Chew P, Lau J. Standardized retrieval of side effects data for meta-analysis of safety outcomes. A feasibility study in acute sinusitis. J Clin Epidemiol 2002; 55: 619–626. [DOI] [PubMed] [Google Scholar]
- 12. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 13. Frazzoni M, de Bortoli N, Frazzoni L, et al. The added diagnostic value of postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance in refractory reflux disease studied with on-therapy impedance-pH monitoring. Neurogastroenterol Motil 2017; 29. [DOI] [PubMed] [Google Scholar]
- 14. Ravi K, Geno DM, Vela MF, et al. Baseline impedance measured during high-resolution esophageal impedance manometry reliably discriminates GERD patients. Neurogastroenterol Motil 2017. May; 29(5). DOI: 10.1111/nmo.12974. Epub 2016 Oct 24. [DOI] [PubMed] [Google Scholar]
- 15. Frazzoni M, Savarino E, De Bortoli N, et al. Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease. Clin Gastroenterol Hepatol 2016; 14: 40–46. [DOI] [PubMed] [Google Scholar]
- 16. Kandulski A, Weigt J, Caro C, et al. Esophageal intraluminal baseline impedance differentiates gastroesophageal reflux disease from functional heartburn. Clin Gastroenterol Hepatol 2015; 13: 1075–1081. [DOI] [PubMed] [Google Scholar]
- 17. Hayat JO, Gabieta-Somnez S, Yazaki E, et al. Pepsin in saliva for the diagnosis of gastro-oesophageal reflux disease. Gut 2015; 64: 373–380. [DOI] [PubMed] [Google Scholar]
- 18. Saritas Yuksel E, Hong SK, et al. Rapid salivary pepsin test: blinded assessment of test performance in gastroesophageal reflux disease. Laryngoscope 2012; 122: 1312–1316. [DOI] [PubMed] [Google Scholar]
- 19. Cui R, Zhang H, Zhou L, et al. Diagnostic value of dilated intercellular space and histopathologic scores in gastroesophageal reflux disease. Dis Esophagus 2015; 28: 530–537. [DOI] [PubMed] [Google Scholar]
- 20. Zhou LY, Wang Y, Lu JJ, et al. Accuracy of diagnosing gastroesophageal reflux disease by GerdQ, esophageal impedance monitoring and histology. J Dig Dis 2014; 15: 230–238. [DOI] [PubMed] [Google Scholar]
- 21. Cui R, Zhou L, Lin S, et al. The feasibility of light microscopic measurements of intercellular spaces in squamous epithelium in the lower-esophagus of GERD patients. Dis Esophagus 2011; 24: 1–5. [DOI] [PubMed] [Google Scholar]
- 22. Mastracci L, Spaggiari P, Grillo F, et al. Microscopic esophagitis in gastro-esophageal reflux disease: individual lesions, biopsy sampling, and clinical correlations. Virchows Archiv 2009; 454: 31–39. [DOI] [PubMed] [Google Scholar]
- 23. Vela MF, Craft BM, Sharma N, et al. Refractory heartburn:comparison of intercellular space diameter in documented GERD vs. functional heartburn. Am J Gastroenterol 2011; 106: 844–850. [DOI] [PubMed] [Google Scholar]
- 24. Zavala-Gonzales MA, Azamar-Jacome AA, et al. Validation and diagnostic usefulness of gastroesophageal reflux disease questionnaire in a primary care level in Mexico. J Neurogastroenterol Motil 2014; 20: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jonasson C, Wernersson B, Hoff DA, et al. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2013; 37: 564–572. [DOI] [PubMed] [Google Scholar]
- 26. Lacy BE, Chehade R, Crowell MD. A prospective study to compare a symptom-based reflux disease questionnaire to 48-h wireless pH monitoring for the identification of gastroesophageal reflux (revised 2-26-11). Am J Gastroenterol 2011; 106: 1604–1611. [DOI] [PubMed] [Google Scholar]
- 27. Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2009; 30: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 28. Bai Y, Du Y, Zou D, et al. Gastroesophageal Reflux Disease Questionnaire (GerdQ) in real-world practice: a national multicenter survey on 8065 patients. J Gastroenterol Hepatol 2013; 28: 626–631. [DOI] [PubMed] [Google Scholar]
- 29. Lee SH, Jang BI, Jeon SW, et al. A multicenter, randomized, comparative study to determine the appropriate dose of lansoprazole for use in the diagnostic test for gastroesophageal reflux disease. Gut Liver 2011; 5: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dent J, Vakil N, Jones R, Bytzer P, et al. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: the Diamond study. Gut 2010; 59: 714–721. [DOI] [PubMed] [Google Scholar]
- 31. Cho YK, Choi MG, Lim CH, et al. Diagnostic value of the PPI test for detection of GERD in Korean patients and factors associated with PPI responsiveness. Scand J Gastroenterol 2010; 45: 533–539. [DOI] [PubMed] [Google Scholar]
- 32. Zheng J, Du ZM, Chen MH, et al. [Diagnosis of gastroesophageal reflux disease-related noncardiac chest pain]. Zhonghua yi xue za zhi 2008; 88: 1390–1393. [PubMed] [Google Scholar]
- 33. Lee YC, Lin JT, Wang HP, et al. Influence of cytochrome P450 2C19 genetic polymorphism and dosage of rabeprazole on accuracy of proton-pump inhibitor testing in Chinese patients with gastroesophageal reflux disease. J Gastroenterol Hepatol 2007; 22: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 34. Fan YH, Lu B, Zhan LX, et al. [Oesophageal acid exposure test in non-erosive gastroesophageal reflux disease and the diagnostic value of rabeprazole]. Zhonghua nei ke za zhi 2007; 46: 475–477. [PubMed] [Google Scholar]
- 35. Des Varannes SB, Sacher-Huvelin S, Vavasseur F, et al. Rabeprazole test for the diagnosis of gastro-oesophageal reflux disease: results of a study in a primary care setting. World J Gastroenterol 2006; 12: 2569–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aanen MC, Weusten BL, Numans ME, et al. Diagnostic value of the proton pump inhibitor test for gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2006; 24: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 37. Dickman R, Emmons S, Cui H, et al. The effect of a therapeutic trial of high-dose rabeprazole on symptom response of patients with non-cardiac chest pain: a randomized, double-blind, placebo-controlled, crossover trial. Aliment Pharmacol Ther 2005; 22: 547–555. [DOI] [PubMed] [Google Scholar]
- 38. Juul-Hansen P, Rydning A. Endoscopy-negative reflux disease: what is the value of a proton-pump inhibitor test in everyday clinical practice? Scand J Gastroenterol 2003; 38: 1200–1203. [DOI] [PubMed] [Google Scholar]
- 39. Pandak WM, Arezo S, Everett S, et al. Short course of omeprazole: a better first diagnostic approach to noncardiac chest pain than endoscopy, manometry, or 24-hour esophageal pH monitoring. J Clin Gastroenterol 2002; 35: 307–314. [DOI] [PubMed] [Google Scholar]
- 40. Juul-Hansen P, Rydning A, Jacobsen CD, et al. High-dose proton-pump inhibitors as a diagnostic test of gastro-oesophageal reflux disease in endoscopic-negative patients. Scand J Gastroenterol 2001; 36: 806–810. [DOI] [PubMed] [Google Scholar]
- 41. Fass R, Ofman JJ, Sampliner RE, et al. The omeprazole test is as sensitive as 24-h oesophageal pH monitoring in diagnosing gastro-oesophageal reflux disease in symptomatic patients with erosive oesophagitis. Aliment Pharmacol Ther 2000; 14: 389–396. [DOI] [PubMed] [Google Scholar]
- 42. Fass R, Ofman JJ, Gralnek IM, et al. Clinical and economic assessment of the omeprazole test in patients with symptoms suggestive of gastroesophageal reflux disease. Arch Intern Med 1999; 159: 2161–2168. [DOI] [PubMed] [Google Scholar]
- 43. Bate CM, Riley SA, Chapman RW, et al. Evaluation of omeprazole as a cost-effective diagnostic test for gastro-oesophageal reflux disease. Aliment Pharmacol Ther 1999; 13: 59–66. [DOI] [PubMed] [Google Scholar]
- 44. Johnsson F, Weywadt L, Solhaug JH, et al. One-week omeprazole treatment in the diagnosis of gastro-oesophageal reflux disease. Scand J Gastroenterol 1998; 33: 15–20. [DOI] [PubMed] [Google Scholar]
- 45. Fass R, Fennerty MB, Ofman JJ, et al. The clinical and economic value of a short course of omeprazole in patients with noncardiac chest pain. Gastroenterology 1998; 115: 42–49. [DOI] [PubMed] [Google Scholar]
- 46. Carlsson R, Dent J, Watts R, et al. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol 1998; 10: 119–124. [PubMed] [Google Scholar]
- 47. Galmiche JP, Barthelemy P, Hamelin B. Treating the symptoms of gastro-oesophageal reflux disease: a double-blind comparison of omeprazole and cisapride. Aliment Pharmacol Ther 1997; 11: 765–773. [DOI] [PubMed] [Google Scholar]
- 48. Hatlebakk JG, Hyggen A, Madsen PH, et al. Heartburn treatment in primary care: randomised, double blind study for 8 weeks. BMJ (Clinical research ed) 1999; 319: 550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Venables TL, Newland RD, Patel AC, et al. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol 1997; 32: 965–973. [DOI] [PubMed] [Google Scholar]
- 50. Ates F, Yuksel ES, Higginbotham T, et al. Mucosal impedance discriminates GERD from non-GERD conditions. Gastroenterology 2015; 148: 334–343. [DOI] [PubMed] [Google Scholar]
- 51. Saritas Yuksel E, Higginbotham T, Slaughter JC, et al. Use of direct, endoscopic-guided measurements of mucosal impedance in diagnosis of gastroesophageal reflux disease. Clin Gastroenterol 2012; 10: 1110–1116. [DOI] [PubMed] [Google Scholar]
- 52. Xu X, Chen Q, Liang S, et al. Comparison of gastroesophageal reflux disease questionnaire and multichannel intraluminal impedance pH monitoring in identifying patients with chronic cough responsive to antireflux therapy. Chest 2014; 145: 1264–1270. [DOI] [PubMed] [Google Scholar]
- 53. Chen LX, Li YL, Ning GZ, et al. Comparative efficacy and tolerability of three treatments in old people with osteoporotic vertebral compression fracture: a network meta-analysis and systematic review. PloS One 2015; 10: e0123153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tu YK, Needleman I, Chambrone L, et al. A Bayesian network meta-analysis on comparisons of enamel matrix derivatives, guided tissue regeneration and their combination therapies. J Clin Periodontol 2012; 39: 303–314. [DOI] [PubMed] [Google Scholar]
- 55. Zhu GQ, Shi KQ, Huang S, et al. Systematic review with network meta-analysis: the comparative effectiveness and safety of interventions in patients with overt hepatic encephalopathy. Aliment Pharmacol Ther 2015; 41: 624–635. [DOI] [PubMed] [Google Scholar]
- 56. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PloS One. 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64: 163–171. [DOI] [PubMed] [Google Scholar]
- 58. Mavridis D, Giannatsi M, Cipriani A, et al. A primer on network meta-analysis with emphasis on mental health. Evid Based Ment Health 2015; 18: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aziz Q, Fass R, Gyawali CP, et al. Functional esophageal disorders. Gastroenterology 2016; 150: 1368–1379. [DOI] [PubMed] [Google Scholar]
- 60. Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018; 67: 1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Farre R, Blondeau K, Clement D, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut 2011; 60: 885–892. [DOI] [PubMed] [Google Scholar]
- 62. Kessing BF, Bredenoord AJ, Weijenborg PW, et al. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol 2011; 106: 2093–2097. [DOI] [PubMed] [Google Scholar]
- 63. Zhong C, Duan L, Wang K, et al. Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J Gastroenterol 2013; 48: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Woodland P, Singendonk MMJ, Ooi J, et al. Measurement of salivary pepsin to detect gastroesophageal reflux disease is not ready for clinical application. Clin Gastroenterol Hepatol 2019. February; 17(3): 563–565. doi: 10.1016/j.cgh.2018.05.016. Epub 2018 May 18 [DOI] [PubMed] [Google Scholar]
- 65. Savarino E, Zentilin P, Mastracci L, et al. Microscopic esophagitis distinguishes patients with non-erosive reflux disease from those with functional heartburn. J Gastroenterol 2013; 48: 473–482. [DOI] [PubMed] [Google Scholar]
- 66. Van Malenstein H, Farre R, Sifrim D. Esophageal dilated intercellular spaces (DIS) and nonerosive reflux disease. Am J Gastroenterol 2008; 103: 1021–1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Fig._1._risk_of_bias_for_each_study for Assessing different diagnostic tests for gastroesophageal reflux disease: a systematic review and network meta-analysis by Mengyu Zhang, John E. Pandolfino, Xuyu Zhou, Niandi Tan, Yuwen Li, Minhu Chen and Yinglian Xiao in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Fig._2._the_effect_of_evidence for Assessing different diagnostic tests for gastroesophageal reflux disease: a systematic review and network meta-analysis by Mengyu Zhang, John E. Pandolfino, Xuyu Zhou, Niandi Tan, Yuwen Li, Minhu Chen and Yinglian Xiao in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Fig._3._Publication_Bias for Assessing different diagnostic tests for gastroesophageal reflux disease: a systematic review and network meta-analysis by Mengyu Zhang, John E. Pandolfino, Xuyu Zhou, Niandi Tan, Yuwen Li, Minhu Chen and Yinglian Xiao in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Table1._pairwise_meta_analysis-supplementary for Assessing different diagnostic tests for gastroesophageal reflux disease: a systematic review and network meta-analysis by Mengyu Zhang, John E. Pandolfino, Xuyu Zhou, Niandi Tan, Yuwen Li, Minhu Chen and Yinglian Xiao in Therapeutic Advances in Gastroenterology