Abstract
Background
Schizophrenia is a debilitating psychiatric disorder characterized by molecular and anatomical abnormalities of multiple brain regions. Our recent study showed that dysbiosis of the gut microbiota contributes to the onset of schizophrenia-relevant behaviors, but the underlying mechanisms remain largely unknown.
Purpose
This study aimed to investigate how gut microbiota shapes metabolic signatures in multiple brain regions of schizophrenia microbiota recipient mice.
Methods
Gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) were used to compare the metabolic signatures in the cortex, cerebellum and striatum of schizophrenia microbiota and healthy microbiota recipient mice. Enrichment analysis was further conducted to uncover the crucial metabolic pathways related to schizophrenia-relevant behaviors.
Results
We found that the metabolic phenotypes of these three regions were substantially different in schizophrenia microbiota recipient mice from those in healthy microbiota recipient mice. In total, we identified 499 differential metabolites that could discriminate the two groups in the three brain regions. These differential metabolites were mainly involved in glycerophospholipid and fatty acyl metabolism. Moreover, we found four of fatty acyl metabolites that were consistently altered in the three brain regions.
Conclusion
Taken together, our study suggests that alterations of glycerophospholipid and fatty acyl metabolism are implicated in the onset of schizophrenia-relevant behaviors, which may provide a new understanding of the etiology of schizophrenia.
Keywords: schizophrenia, gut microbiota, metabolomics, glycerophospholipids, fatty acyls, fecal microbiota transplantation
Introduction
Schizophrenia is a chronic neurodevelopmental disorder characterized by abnormal mental activities such as impaired cognition, emotion and intention.1 It places a large economic burden on health systems, families and society.2 Increasing lines of evidence have suggested that social, environmental and genetic factors are implicated in the onset of this disease.3,4 Several classical theories, such as neurotransmitter alterations, gene loci and epigenetic abnormalities and dysregulation of inflammation, have attempted to explain the pathogenesis of schizophrenia.5–8 However, its underlying mechanisms remain largely unknown.
The gut microbiota is a large ecosystem that inhabits in the human intestine.9,10 Many studies have shown that the gut microbiota can modulate brain functions and behaviors through the microbiota-gut-brain axis.11–14 Clinical research has reported that schizophrenia is correlated with anatomical abnormalities of the gastrointestinal system.15 Moreover, our previous study showed that the gut microbial composition was significantly altered in schizophrenia patients relative to healthy controls, and dysbiosis of the gut microbiota contributed to the onset of schizophrenia-relevant behaviors through moudlation of the hippocampal glutamate-glutamine-GABA cycle.16 However, how the gut microbiota systematically shapes brain metabolic signatures remains unclear.
Abnormalities of neural circuits have been widely recognized as a hallmark of schizophrenia.17–19 Previous research demonstrated that brain regions such as the hippocampus, cortex and striatum are involved in the pathophysiology of schizophrenia.20,21 Additionally, clinical investigations have also shown that the cerebellum is associated with imparied cognition and emotion in schizophrenia patients.22–24 In this study, two metabolomics approaches, gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS), were combined to analyze the metabolic changes of three brain regions (cortex, cerebellum and striatumin) of schizophrenia microbiota and healthy microbiota recipient mice. Moreover, we mapped these differential metabolites to their respective biochemical pathways and further performed intersection metabolic analysis to determine how dysbiosis of the gut microbiota paticipates in the onset of schizophrenia.
Materials And Methods
Animals And Sample Collection
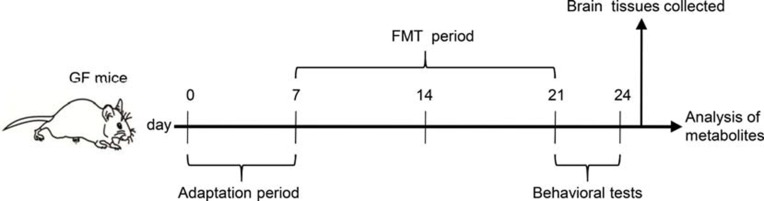
This animal study was approved by the Ethics Committee of Army Medical University (China) and Chongqing Medical University (2011002). All experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The details of our workflow are shown in Figure 1. The samples were derived from our previous research.16 The model was constructed by orally transplanting a liquid fecal suspension into germ-free mice. Briefly, the fecal microbiota transplantation (FMT) experiment was conducted by obtaining 0.2 g of feces from schizophrenia patients (n=5) or healthy individuals (n=5). These fecal samples were mixed with 3 mL of reduced sterile phosphate-buffered saline to obtain a suspension. Then, each mouse was randomly administered 200 μl of the fecal suspension by gavage in a flexible-film gnotobiotic isolator. Behavioral tests including open-field, sociability and social novelty preference, prepulse inhibition, Y-maze and forced swimming tests were performed after 14 days of colonization. Consequently, we found that the germ-free mice that received schizophrenia microbiota transplants displayed schizophrenia-relevant behaviors relative to healthy microbiota recipient mice. All mice were sacrificed after the behavioral tests, and cortex, cerebellum and striatum tissues were collected and stored at −80°C until metabolite detection.
Figure 1.
Experimental procedure. Day 0–7, animal adaptation time; animals were grouped on the last day of the first week; Day 8–21, FMT period; Day 22–24, behavioral tests.
Abbreviations: GF mice, germ-free mice; FMT, fecal microbiota transplantation.
Gas Chromatography-Mass Spectrometry
GC-MS metabolomics analysis was used to characterize the metabolic signatures of cortex, cerebellum and striatum tissues obtained from schizophrenia microbiota (n=8) and healthy microbiota recipient mice (n=8). Briefly, tissue samples (30 mg) were placed in an Eppendorf tube (1.5 mL) with 20 µl of an internal standard (0.006 mg of L-2-chloro-phenylalanine and 20 µl of methanol) and 600 μl of a mixture containing 480 μl of methanol and 120 μl of water. The samples were extracted by ultrasonication for 600 s after homogenization and centrifuged at 4°C and 10,000 g for 900 s to obtain the dried extracts used for derivatization. Quality control (QC) samples were obtained by mixing all tissue samples and were analyzed using the same method used for the other samples. Every eighth QC sample was injected to assess the repeatability of the experiment. The tissue samples were analyzed by a gas chromatography system coupled to an MSD system (Agilent, CA). The derivative samples were separated using an HP-5MS capillary column made of fused silica. Highly purified helium with a steady speed of 6.0 mL/min was used as the carrier gas. The temperature of the injection port was kept at 280°C. ChromaTOF software was used to analyze the GC-MS data. Then, the Fiehn database was used to quantify the metabolites. Partial least-squares discriminant analysis (PLS-DA) was performed to visually discriminate the samples from schizophrenia microbiota and healthy microbiota recipient mice using SIMCA software (version 14.0, Umetrics, Umea, Sweden).25
Liquid Chromatography-Mass Spectrometry
Samples (15 mg) were placed in an Eppendorf tube (1.5 mL) with 20 μl of internal standard (0.006 mg of L-2-chloro-phenylalanine and 20 μl of methanol) and 600 μl of a mixture containing 480 μl of methanol and 120 μl of water. The samples were placed at −80°C for 600 s and then centrifuged at 4°C and 10,000 g for 900 s after homogenization. Then, 150 μl of supernatant fluid was used for the subsequent LC-MS analysis. QC samples were obtained by mixing all tissue samples and were analyzed using the same approach used for the other samples. Every eighth QC sample was injected to assess the repeatability of the experiment. The mobile phase consisted of aqueous formic acid (0.1% (v/v) formic acid) and acetonitrile (0.1% (v/v) formic acid); the injection volume was 3.00 μl, and the flow rate was set at 0.40 mL/min. A Waters mass spectrometer (VION IMS Q-TOF) was used to collect mass spectrometric data. The source and desolvation temperatures were set to 120°C and 500°C, respectively. The desolvation gas flow was set at 900 l/h. The range of centroid data was 50–1000 m/z. The scan time was set to 0.1 s, and the inter scan time was set to 0.02 s. The data were analyzed using Progenesis QI software before visualization. The SIMCA-P+14.0 software package was used to analyze the data set, which consisted of positive and negative data. PLS-DA was used to visualize the metabolic differences between the two groups.26 In this study, a seven-round cross-validation was performed to avoid model overfitting. The orthogonal projections to latent structures discriminant analysis models were also validated by a 200-iteration permutation analysis. Detailed LC-MS analysis was performed according to our previously published study.13
Metabolic Pathway Analysis And Functional Annotation
Variables with a p value < 0.05 and a variable importance in projection (VIP) score >1.0 were considered differential variables. Metabolic annotation was performed using the LIPID MAPS system (http://www.lipidmaps.org/). Pathway enrichment analysis was carried out using the MetaboAnalyst database (https://www.metaboanalyst.ca/) and the Kyoto Encyclopedia of Genes and Genomes database (https://www.kegg.jp/). The Human Metabolome Database was also used for relevant analyses (http://www.hmdb.ca/).
Statistical Analysis
Statistical analysis was performed using SPSS 20.0 (Chicago, USA). Comparisons between two groups were performed using student’s t-test. The statistical significance level was set at a p value < 0.05. Data were visualized by Cytoscape 3.7.0 and GraphPad Prism 8.0 (San Diego, California, USA).
Results
Significant Differences In Metabolic Phenotypes Between Schizophrenia Microbiota And Healthy Microbiota Recipient Mice
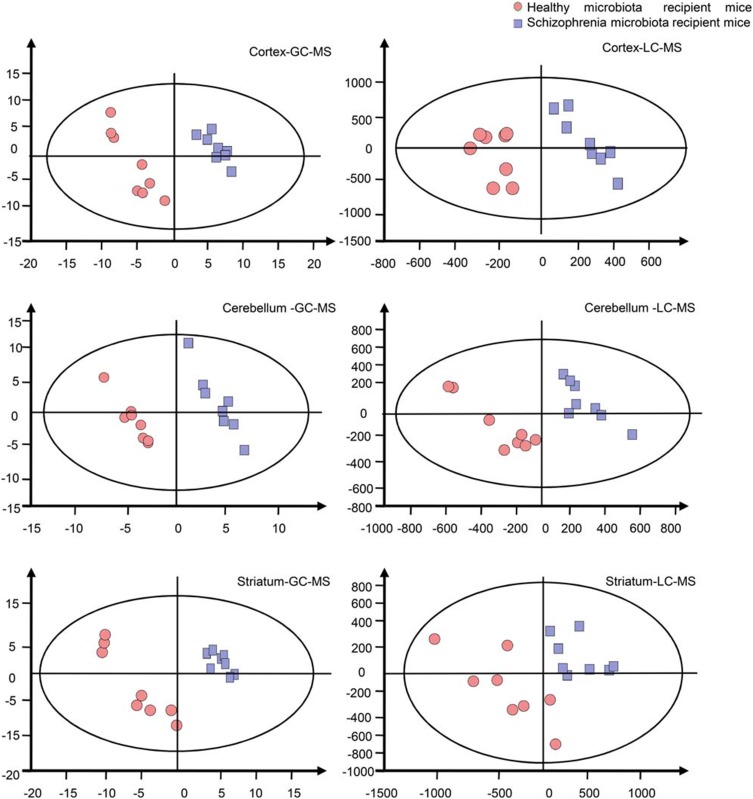
Previously, in an FMT experiment, we found that schizophrenia microbiota recipient mice displayed schizophrenia-relevant behaviors including hyperactivity (greater total distance in the open-field test), increased startle responses (exaggerated startle response to high-decibel tones), decreased anxiety (greater distance traveled in the center region in the open-field test) and depressive-like behaviors (decreased immobility in a forced swimming test).16 Tissue samples, including cortex, cerebellum and striatum tissue, derived from our previous study were used. The metabolic signatures of the three brain regions were systematically compared between schizophrenia microbiota and healthy microbiota recipient mice by both GC-MS and LC-MS. The typical base peak intensity and original total ion chromatograms are shown in Figures S1 and S2, respectively. PLS-DA showed clear discrimination between the two groups (Figure 2). R2Y and Q2 represent variation of the cumulative model and the cumulative predicted variation, respectively. The R2Y and Q2 values indicate a robust metabolic difference between the two groups. Furthermore, the 200-iteration permutation test suggested that the possibility of overfitting was avoided, confirming the reliability of these generated models.
Figure 2.
Metabonomic analysis of brain samples. Partial least-squares discriminant analysis score plots showing clear discrimination between schizophrenia microbiota recipient mice (blue boxes) and healthy microbiota recipient mice (red dots) (n=8 each group) by gas chromatography-mass spectrometry (GC-MS) (cortex: R2Y=0.997, Q2=0.894; cerebellum: R2Y=0.989, Q2=0.578; striatum: R2Y=0.976, Q2=0.881) and liquid chromatography-mass spectrometry (LC-MS) (cortex: R2Y=0.941, Q2=0.15; cerebellum: R2Y=0.953, Q2=0.139; striatum: R2Y=0.963, Q2=0.699).
Classification Of The Differential Metabolites
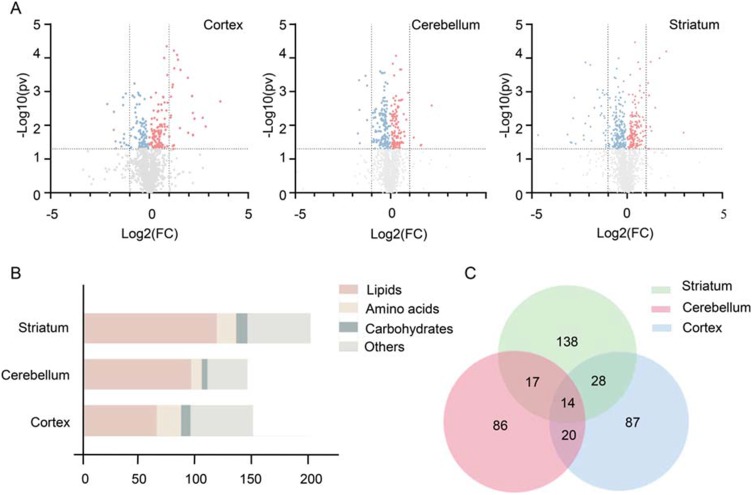
Initially, by comparing the levels of metabolites, we identified 213, 255 and 428 significantly altered metabolites (p value < 0.05) in the cortex, cerebellum and striatum, respectively, between the two groups (Figure 3A). Addtionlly, the VIP score (>1.0) was further used to define the differential metabolites. Consequently, we identified a total of 499 differential metabolites between schizophrenia microbiota and healthy microbiota recipient mice in the three brain regions (VIP score > 1.0 and p value < 0.05). Additionally, 263 of the 499 metabolites were up-regulated, and the remaining metabolites were down-regulated in the schizophrenia microbiota recipient mice relative to the healthy microbiota recipient mice. The detailed data are shown in Table S1. Functionally, the differential metabolites are involved in lipid, amino acid and carbohydrate metabolism (Figure 3B). Interestingly, more than half of the differential metabolites (56.1%) were lipids. Compared with healthy microbiota recipient mice, most of the metabolites in the three pathways were down-regulated in the cerebellum and striatum but up-regulated in the cortex in schizophrenia microbiota recipient mice (Table S2).
Figure 3.
(A) Volcano plot of metabolites. Metabolites were identified by gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry in the three brain regions. The red dots (up-regulation) and blue dots (down-regulation) represent 213, 255 and 428 metabolites selected from 2415, 2373 and 2416 identified metabolites in the cortex, cerebellum and striatum, respectively (p value < 0.05). (B) Number of differential metabolites. Variables with a VIP score >1 and p value < 0.05 were considered differential variables. In total, 499 differential metabolites were identified in the cortex, cerebellum and striatum. (C) Venn diagram of differential metabolites. The numbers of altered metabolites in the three brain regions in schizophrenia microbiota recipient mice are shown. We found that 14 differential metabolites overlapped among the three brain regions.
Intersection Metabolic Analysis Of Differential Metabolites
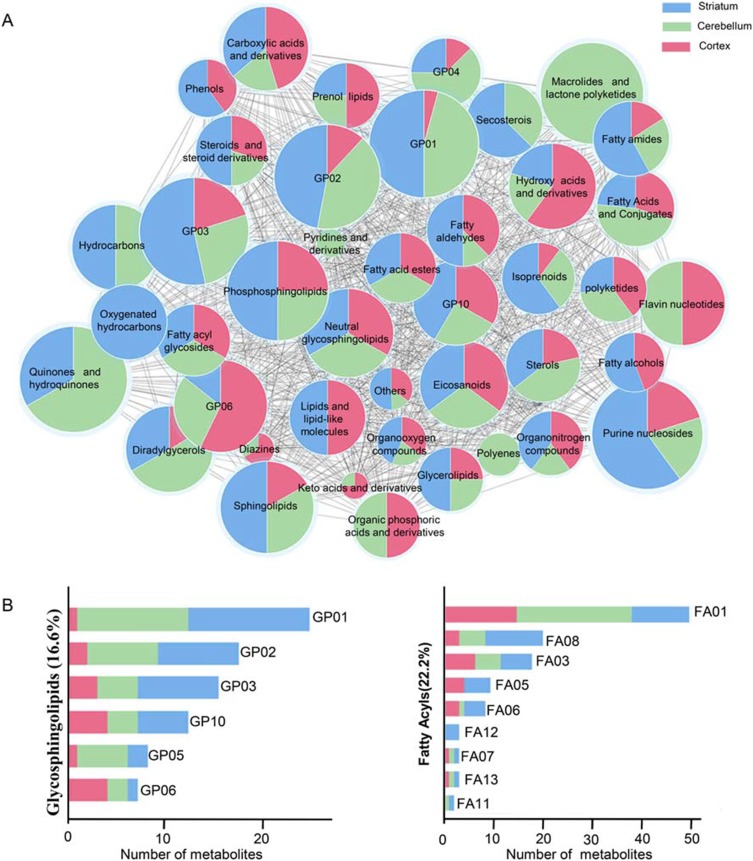
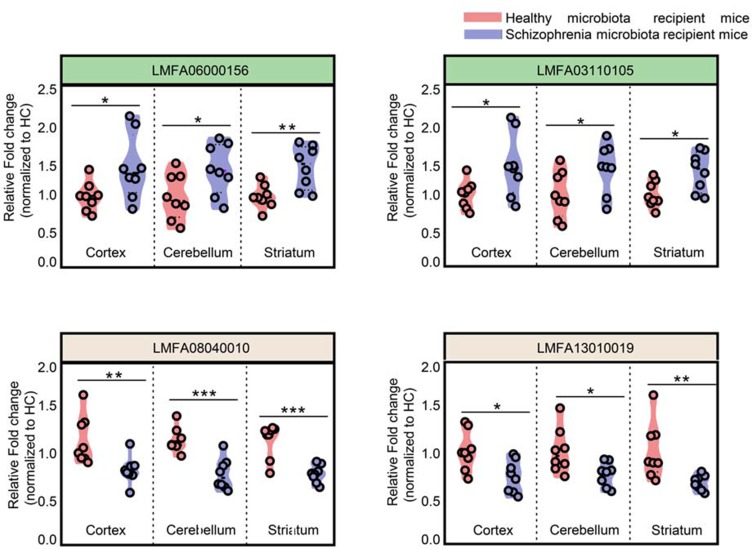
Intersection metabolic analysis showed that at the class level, the differential metabolites were evenly distributed in the cortex, cerebellum and striatum, suggesting that the gut microbiota broadly influences multiple brain regions in schizophrenia microbiota recipient mice (Figure 4A). In addition, we found that the majority of the differential metabolites were involved in lipid metabolism, especially glycerophospholipid and fatty acyl metabolism (Figure 4B). Compared with healthy microbiota recipient mice, glycerophospholipid and fatty acyl metabolism was mainly down-regulated in the cerebellum and striatum of schizophrenia microbiota recipient mice, but the changes in these metabolites were diverse in the cortex (Table S3). Moreover, we found that 14 differential metabolites were consistently altered in the three regions (Figure 3C). Most of these metabolites were classified as lipids including fatty acyls, sterol lipids, glycerolipids, prenollipids, polyketides and neutral glycosphingolipids (Table S4). Specifically, four metabolites including “3,7-Dimethyl-8.11-dioxo-2E,6E,9E-dodecatrienal”, ‘15-Deoxy-d-12.14-PGJ2ʹ, “anandamide (20:l,n-9)” and “1-(O-alpha-D-glucopyranosyl)-3-keto-(1, 27R)-octacosanediol”, which are fatty acyls, were consistently altered (Figure 5). Together, these results indicated that disruptions in glycerophospholipid and fatty acyl metabolism are involved in the pathophysiology of schizophrenia.
Figure 4.
(A) Intersection metabolic analysis of differential metabolites at the class level. Intersection analysis was displayed by a network of nodes and lines using Cytoscape. The majority of metabolites were evenly distributed in the three regions at the class level. The size of the node represents the relative abundance of metabolites, and the lines represent the correlations between metabolites. (B) Proportion of differential metabolites of fatty acyls and glycerophospholipids in the three brain regions. GP01, Glycerophosphocholines; GP02, Glycerophosphoethanolamines; GP10, Glycerophosphates; GP04, Glycerophosphoglycerols; GP06, Glycerophosphoinositols; GP03, Glycerophosphoserines; FA01, Fatty acids and conjugates; FA08, Fatty amides; FA03, Eicosanoids; FA05, Fatty alcohols; FA06, Fatty aldehydes; FA12, Oxygenated hydrocarbons; FA07, Fatty acid esters; FA13, Fatty acyl glycosides; FA11, Hydrocarbons.
Figure 5.
Overlapping differential metabolites in the three brain regions were fatty acyls. Fatty acyl metabolites were consistently altered in the cortex, cerebellum and striatum. (n=8 subjects per group. *p<0.05; **p<0.01; ***p<0.001), LMFA06000156, “3,7-Dimethyl-8.11-dioxo-2E,6E,9E-dodecatrienal”; LMFA03110105, ‘15-Deoxy-d-12.14-PGJ2ʹ; LMFA08040010, “anandamide(20:l,n-9)”; LMFA13010019, “1-(O-alpha-D-glucopyranosyl)-3-keto- (1,27R)-octacosanediol”.
Pathway Analysis Of Lipid Metabolites
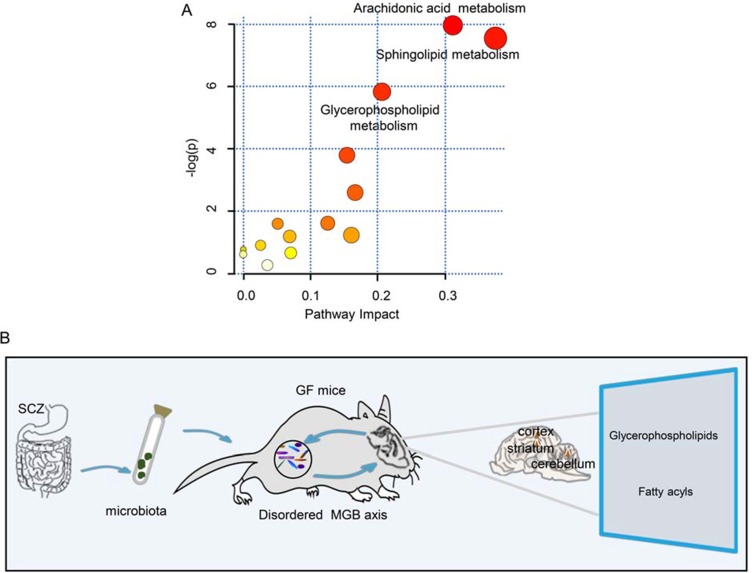
As the majority of the differential metabolites were lipids, we performed a functional analysis of lipid metabolites in the three brain regions. We observed that these metabolic pathways, including glycerophospholipid, sphingolipid and arachidonic acid metabolism, were significantly altered in the schizophrenia microbiota recipient mice relative to the healthy microbiota recipient mice (Figure 6A). This finding also confirms that disruptions of glycerophospholipid and fatty acyl metabolism are a hallmark of schizophrenia microbiota recipient mice.
Figure 6.
(A) Metabolite pathways of lipids. Color variations (from yellow to red) indicate metabolites from the database with different levels of significance. (B) Schematic representation of the findings. The gut microbiota may be associated with the onset of schizophrenia-relevant behaviors via regulation of glycerophospholipid and fatty acyl metabolism.
Discussion
In this study, the metabolic signatures of the cortex, cerebellum and striatum were systematically analyzed in schizophrenia microbiota and healthy microbiota recipient mice by two complementary metabolic approaches. We found that the metabolite signatures of these three regions were substaintially different between the two groups. Interestingly, we found that disruptions of glycerophospholipid and fatty acyl metabolism were mainly involved in the onset of schizophrenia-related behaviors. These findings provide a new perspective for understanding of the pathogenesis of schizophrenia (Figure 6B).
We showed that dysbiosis of the gut microbiota could significantly affect the metabolic phenotypes of the cortex, cerebellum and striatum in schizophrenia microbiota recipient mice. Consistent with these findings, previous neuroimaging studies have shown that these regions are highly correlated with the onset of schizophrenia.27–30 Additionally, at a functional level, alterations of lipid, amino acid and carbohydrate metabolism were also observed in schizophrenia microbiota recipient mice.16 We also observed that the altered metabolites were mainly involved in lipid metabolism, and a majority of the consistently altered metabolites were involved in lipid metabolism in the three brain regions. These findings help to increase the understanding the metabolic mechanisms of schizophrenia.
In the three brain regions, we found that glycerophospholipid metabolism was down-regulated in schizophrenia microbiota recipient mice compared with healthy microbiota recipient mice. Emerging studies have demonstrated that glycerophospholipids are involved in the initiation and elongation of dendritic spines.31,32 Our findings suggest that dysbiosis of the gut microbiota may participate in the pathogenesis of schizophrenia by modulating the growth of dendritic spines. Consistent with our hypothesis, previous studies have observed a marked reduction in dendritic spines in human postmortem brain tissue from schizophrenia patients.33,34 Further studies are required to investigate how the gut microbiota modulates the genes related to dendritic spine development.
Fatty acyls are the basic building blocks of complex lipids, which play a crucial role in sustaining the physiological function of neurons.35–37 We found that the levels of major fatty acyls were decreased in the cerebellum and striatum but increased in the cortex in schizophrenia microbiota recipient mice. Studies have shown that some fatty acyls can increase synaptic protein levels and the number of c-Fos-positive neurons and thus enhance synaptic plasticity in the hippocampus.38–40 Our findings indicate that disturbances of gut microbiota may be associated with the onset of schizophrenia-relevant behaviors via regulation of synaptic plasticity. Consistent with our speculation, synaptic plasticity was significantly reduced in the hippocampus of a neurodevelopmental model of schizophrenia. Moreover, treatment of these rats with fatty acid could effectively alleviate the synaptic plasticity deficits.41–43 These diverse changes of fatty acyls in different brain regions suggest that fatty acyls may play a complicated role in the onset of schizophrenia-relevant behaviors through modulating the functions of neural circuits, which requires further investigation.
Previously, we found that schizophrenia microbiota recipient mice were characterized by disrupted serum lipid metabolism, including phosphatidylcholine (PC) and phosphatidylethanolamine (PE).16 Interestingly, similar findings were also observed in the cortex, cerebellum and striatum. PC and PE are the most abundant phospholipids in cell organelles.44,45 A previous study reported that the PC and PE levels were decreased in platelet membranes of schizophrenia patients.46 Moreover, another study observed that PC and PE were significantly altered in the white matter of schizophrenic patients.47 These previous findings suggest that the alterations in PC and PE may be a common molecular basis of schizophrenia in the peripheral and central compartments.
Our research has the following limitations: (i) based on animal research, we found that alterations in glycerophospholipid and fatty acyl metabolism were implicated in the onset of schizophrenia-relevant behaviors. However, recent meta-analysis of clinical trials showed that supplementation with probiotics may not be effective in the treatment of schizophrenia or depression.48,49 Therefore, further studies aimed at identifying key gut species and their modulation of metabolic pathways are required, which may provide effective treatment targets for schizophrenia; (ii) a non-targeted metabolomics study was used to characterize the altered metabolic signatures of three brain regions in schizophrenia microbiota recipient mice. Further studies using gas chromatography-quadrupole time of flight mass spectrometry should be considered due to its high resolution, sensitivity and selectivity.50 Additionally, targeted lipidomics studies should be used to clarify the detailed metabolic changes.
Conclusion
In this study, using systematic, comparative metabonomic analyses, we observed that dysbiosis of the gut microbiota may participate in the onset of schizophrenia-relevant behaviors by modulating glycerophospholipid and fatty acyl metabolism. Our findings provide novel clues for investigating the pathological mechanisms of schizophrenia.
Acknowledgement
This work was supported by the National Key R&D Program of China (2016YFC1307200, 2017YFA0505700, 2017YFD0500503 and 2017YFD0501000), the Natural Science Foundation Project of China (81771490, 81371310, 31271189, 81200899, 81601207 and 31300917), institutional funds from the Chongqing Science and Technology Commission (cstc2014kjrc-qnrc10004).
Disclosure
Hong Wei and Peng Xie are co-senior authors. The authors report no conflicts of interest in this work.
References
- 1.Lysaker PH, Gumley A, Luedtke B, et al. Social cognition and metacognition in schizophrenia: evidence of their independence and linkage with outcomes. Acta Psychiatr Scand. 2013;127(3):239–247. doi: 10.1111/acps.2013.127.issue-3 [DOI] [PubMed] [Google Scholar]
- 2.Jin H, Mosweu I. The societal cost of schizophrenia: a systematic review. PharmacoEconomics. 2017;35(1):25–42. doi: 10.1007/s40273-016-0444-6 [DOI] [PubMed] [Google Scholar]
- 3.Mumtaz F, Khan MI, Zubair M, Dehpour AR. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed Pharmacother. 2018;105:1205–1222. doi: 10.1016/j.biopha.2018.05.086 [DOI] [PubMed] [Google Scholar]
- 4.Zhuo C, Yao Y, Xu Y, et al. Schizophrenia and gut-flora related epigenetic factors. Prog Neuropsychopharmacol Biol Psychiatry. 2019;90:49–54. doi: 10.1016/j.pnpbp.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 5.Prata J, Santos SG, Almeida MI, Coelho R, Barbosa MA. Bridging Autism Spectrum Disorders and Schizophrenia through inflammation and biomarkers - pre-clinical and clinical investigations. J Neuroinflammation. 2017;14(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang AC, Tsai S-J. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int J Mol Sci. 2017;18(8):1689. doi: 10.3390/ijms18081689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitton L, Cosgrove D, Clarkson C, et al. Cognitive analysis of schizophrenia risk genes that function as epigenetic regulators of gene expression. Am J Med Genet B Neuropsychiatr Genet. 2016;171(8):1170–1179. doi: 10.1002/ajmg.b.32503 [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Qi X, Zhu C, Wan L. Activation of IL-8 and its participation in cancer in schizophrenia patients: new evidence for the autoimmune hypothesis of schizophrenia. Neuropsychiatr Dis Treat. 2018;14:3393–3403. doi: 10.2147/NDT.S188210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosa CP, Brancaglion GA, Miyauchi-Tavares TM, Corsetti PP, de Almeida LA. Antibiotic-induced dysbiosis effects on the murine gastrointestinal tract and their systemic repercussions. Life Sci. 2018;207:480–491. doi: 10.1016/j.lfs.2018.06.030 [DOI] [PubMed] [Google Scholar]
- 10.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–938. doi: 10.1172/JCI76304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan JX, Deng FL, Zeng BH, et al. Absence of gut microbiota during early life affects anxiolytic behaviors and monoamine neurotransmitters system in the hippocampal of mice. J Neurol Sci. 2019;400:160–168. doi: 10.1016/j.jns.2019.03.027 [DOI] [PubMed] [Google Scholar]
- 13.Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21(6):786–796. doi: 10.1038/mp.2016.44 [DOI] [PubMed] [Google Scholar]
- 14.Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs. 2016;30(11):1019–1041. doi: 10.1007/s40263-016-0370-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312–1313. doi: 10.1016/S0140-6736(04)17181-X [DOI] [PubMed] [Google Scholar]
- 16.Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi: 10.1126/sciadv.aau8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigurdsson T. Neural circuit dysfunction in schizophrenia: insights from animal models. Neuroscience. 2016;321:42–65. doi: 10.1016/j.neuroscience.2015.06.059 [DOI] [PubMed] [Google Scholar]
- 18.Cao H, Dixson L, Meyer-Lindenberg A, Tost H. Functional connectivity measures as schizophrenia intermediate phenotypes: advances, limitations, and future directions. Curr Opin Neurobiol. 2016;36:7–14. doi: 10.1016/j.conb.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 19.Lett TA, Voineskos AN, Kennedy JL, Levine B, Daskalakis ZJ. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biol Psychiatry. 2014;75(5):361–370. doi: 10.1016/j.biopsych.2013.07.026 [DOI] [PubMed] [Google Scholar]
- 20.Viana J, Hannon E, Dempster E, et al. Schizophrenia-associated methylomic variation: molecular signatures of disease and polygenic risk burden across multiple brain regions. Hum Mol Genet. 2017;26(1):210–225. doi: 10.1093/hmg/ddw373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Errico F, Napolitano F, Squillace M, et al. Decreased levels of D-aspartate and NMDA in the prefrontal cortex and striatum of patients with schizophrenia. J Psychiatr Res. 2013;47(10):1432–1437. doi: 10.1016/j.jpsychires.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 22.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20(3):236–260. doi: 10.1007/s11065-010-9142-x [DOI] [PubMed] [Google Scholar]
- 23.Sathyanesan A, Zhou J, Scafidi J, Heck DH, Sillitoe RV, Gallo V. Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat Rev Neurosci. 2019;20(5):298–313. doi: 10.1038/s41583-019-0152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung US, Han DH, Shin YJ, Renshaw PF. A prosocial online game for social cognition training in adolescents with high-functioning autism: an fMRI study. Neuropsychiatr Dis Treat. 2016;12:651–660. doi: 10.2147/NDT.S94669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei Y, Li D, Deng J, et al. Metabolomic profiling of three brain regions from a postnatal infected Borna disease virus Hu-H1 rat model. Metabolomics. 2013;10(3):484–495. doi: 10.1007/s11306-013-0593-y [DOI] [Google Scholar]
- 26.Zheng P, Wei Y-D, Yao G-E, et al. Novel urinary biomarkers for diagnosing bipolar disorder. Metabolomics. 2013;9(4):800–808. doi: 10.1007/s11306-013-0508-y [DOI] [Google Scholar]
- 27.Sui J, Pearlson GD, Du Y, et al. In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol Psychiatry. 2015;78(11):794–804. doi: 10.1016/j.biopsych.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carass A, Cuzzocreo JL, Han S, et al. Comparing fully automated state-of-the-art cerebellum parcellation from magnetic resonance images. NeuroImage. 2018;183:150–172. doi: 10.1016/j.neuroimage.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 2019;42(3):205–220. doi: 10.1016/j.tins.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuo K, Tang Y, Song Z, et al. Repetitive transcranial magnetic stimulation as an adjunctive treatment for negative symptoms and cognitive impairment in patients with schizophrenia: a randomized, double-blind, sham-controlled trial. Neuropsychiatr Dis Treat. 2019;15:1141–1150. doi: 10.2147/NDT.S196086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziegler AB, Tavosanis G. Glycerophospholipids - emerging players in neuronal dendrite branching and outgrowth. Dev Biol. 2018. [DOI] [PubMed] [Google Scholar]
- 32.Meltzer S, Bagley JA, Perez GL, et al. Phospholipid homeostasis regulates dendrite morphogenesis in drosophila sensory neurons. Cell Rep. 2017;21(4):859–866. doi: 10.1016/j.celrep.2017.09.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71(12):1323–1331. doi: 10.1001/jamapsychiatry.2014.1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11(6):557–566. doi: 10.1038/sj.mp.4001792 [DOI] [PubMed] [Google Scholar]
- 35.Fahy E, Subramaniam S, Brown HA, et al. A comprehensive classification system for lipids. J Lipid Res. 2005;46(5):839–861. doi: 10.1194/jlr.E400004-JLR200 [DOI] [PubMed] [Google Scholar]
- 36.Xicoy H, Wieringa B, Martens GJM. The role of lipids in Parkinson’s disease. Cells. 2019;8(1):27. doi: 10.3390/cells8010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Huang S, Lam SM, Liu Z, Shui G, Zhang YQ. Acsl, the Drosophila ortholog of intellectual-disability-related ACSL4, inhibits synaptic growth by altered lipids. J Cell Sci. 2016;129(21):4034–4045. doi: 10.1242/jcs.195032 [DOI] [PubMed] [Google Scholar]
- 38.Su HM. Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance. J Nutr Biochem. 2010;21(5):364–373. doi: 10.1016/j.jnutbio.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 39.Yamada D, Wada K, Sekiguchi M. Modulation of long-term potentiation of cortico-amygdala synaptic responses and auditory fear memory by dietary polyunsaturated fatty acid. Front Behav Neurosci. 2016;10:164. doi: 10.3389/fnbeh.2016.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saumweber T, Weyhersmuller A, Hallermann S, et al. Behavioral and synaptic plasticity are impaired upon lack of the synaptic protein SAP47. J Neurosci. 2011;31(9):3508–3518. doi: 10.1523/JNEUROSCI.2646-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanderson TM, Cotel MC, O’Neill MJ, Tricklebank MD, Collingridge GL, Sher E. Alterations in hippocampal excitability, synaptic transmission and synaptic plasticity in a neurodevelopmental model of schizophrenia. Neuropharmacology. 2012;62(3):1349–1358. doi: 10.1016/j.neuropharm.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 42.Patten AR, Sickmann HM, Dyer RA, Innis SM, Christie BR. Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure. Neurosci Lett. 2013;551:7–11. doi: 10.1016/j.neulet.2013.05.051 [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi K, Takagi T, Ishii S, Suzuki H, Miyakawa T. Attenuated bidirectional short-term synaptic plasticity in the dentate gyrus of Schnurri-2 knockout mice, a model of schizophrenia. Mol Brain. 2018;11(1):56. doi: 10.1186/s13041-018-0400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khot V, Chavan-Gautam P, Joshi S. Proposing interactions between maternal phospholipids and the one carbon cycle: a novel mechanism influencing the risk for cardiovascular diseases in the offspring in later life. Life Sci. 2015;129:16–21. doi: 10.1016/j.lfs.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 45.Rangel-Huerta OD, Pastor-Villaescusa B, Gil A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics. 2019;15(6):93. doi: 10.1007/s11306-019-1553-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt A, Maras A, Petroianu G, Braus DF, Scheuer L, Gattaz WF. Effects of antipsychotic treatment on membrane phospholipid metabolism in schizophrenia. J Neural Transm. 2001;108(8–9):1081–1091. doi: 10.1007/s007020170026 [DOI] [PubMed] [Google Scholar]
- 47.Ghosh S, Dyer RA, Beasley CL. Evidence for altered cell membrane lipid composition in postmortem prefrontal white matter in bipolar disorder and schizophrenia. J Psychiatr Res. 2017;95:135–142. doi: 10.1016/j.jpsychires.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 48.Ng QX, Soh AYS, Venkatanarayanan N, Ho CYX, Lim DY, Yeo WS. A systematic review of the effect of probiotic supplementation on schizophrenia symptoms. Neuropsychobiology. 2019;78(1):1–6. doi: 10.1159/000498862 [DOI] [PubMed] [Google Scholar]
- 49.Ng QX, Peters C, Ho CYX, Lim DY, Yeo WS. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord. 2018;228:13–19. doi: 10.1016/j.jad.2017.11.063 [DOI] [PubMed] [Google Scholar]
- 50.Zhang B, Zhang H, Du C, et al. Metabolic responses of the growing Daphnia similis to chronic AgNPs exposure as revealed by GC-Q-TOF/MS and LC-Q-TOF/MS. Water Res. 2017;114:135–143. doi: 10.1016/j.watres.2017.02.046 [DOI] [PubMed] [Google Scholar]