Significance
Activated naïve T cells acquire a memory state that improves their ability to respond to second antigen encounter. Whether activated T cells in tolerant settings can develop memory is less clear. Using a mouse model of cardiac transplantation, we show that alloreactive T cells failed to acquire phenotypic and functional characteristics of memory cells, but instead developed cell-intrinsic hyporesponsiveness. Acquiring the dysfunctional state depended on alloantigen persistence and duration of alloantigen exposure. Following infection-dependent abrogation of established tolerance, alloreactive T cells remained dysfunctional. The resilience of the hyporesponsive state, including the inability to differentiate into memory T cells, may explain why episodes of transplant rejection in the clinic do not preclude subsequent successful weaning from immunosuppressive drugs.
Keywords: T cells, transplantation, tolerance, exhaustion, memory
Abstract
Following antigen stimulation, naïve T cells differentiate into memory cells that mediate antigen clearance more efficiently upon repeat encounter. Donor-specific tolerance can be achieved in a subset of transplant recipients, but some of these grafts are rejected after years of stability, often following infections. Whether T cell memory can develop from a tolerant state and whether these formerly tolerant patients develop antidonor memory is not known. Using a mouse model of cardiac transplantation in which donor-specific tolerance is induced with costimulation blockade (CoB) plus donor-specific transfusion (DST), we have previously shown that systemic infection with Listeria monocytogenes (Lm) months after transplantation can erode or transiently abrogate established tolerance. In this study, we tracked donor-reactive T cells to investigate whether memory can be induced when alloreactive T cells are activated in the setting of tolerance. We show alloreactive T cells persist after induction of cardiac transplantation tolerance, but fail to acquire a memory phenotype despite becoming antigen experienced. Instead, donor-reactive T cells develop T cell-intrinsic dysfunction evidenced when removed from the tolerant environment. Notably, Lm infection after tolerance did not rescue alloreactive T cell memory differentiation or functionality. CoB and antigen persistence were sufficient together but not separately to achieve alloreactive T cell dysfunction, and conventional immunosuppression could substitute for CoB. Antigen persistence was required, as early but not late surgical allograft removal precluded the acquisition of T cell dysfunction. Our results demonstrate transplant tolerance-associated T cell-intrinsic dysfunction that is resistant to memory development even after Lm-mediated disruption of tolerance.
Development of adaptive immunological memory ensures more rapid clearance of antigen upon recurrent encounters and therefore protects the host from reinfection; it also forms the basis of vaccination (1). The enhanced ability of memory T cells to respond to repeated antigen challenge is due both to their presence at a higher frequency than in naïve hosts, and to the fact that they are transcriptionally poised to produce cytokines and proliferate faster than naïve T cells (2). Similarly, memory to alloantigen in transplantation results in faster rejection of a subsequent transplant and is a barrier to costimulation blockade (CoB)-induced transplantation tolerance, whether memory is secondary to rejection of an earlier transplant, to a previous semiallogeneic pregnancy, to homeostatic proliferation, or to cross-reactivity with past infections (3–6).
Transplant rejection can also occur in patients after years of graft tolerance in the absence of immunosuppression, sometimes following infections (7, 8). Whether memory of alloreactive T cells can develop following rejection from this state of operational tolerance is not known and is the focus of our study. Development of T cell memory following graft loss in tolerant recipients could limit acceptance of a new graft as memory T cells are more resistant to CoB and suppression by regulatory T cells (Tregs) (9–11). Conversely, if T cell memory does not develop from a state of tolerance, it may explain why episodes of acute rejection in the clinic do not necessarily preclude subsequent successful weaning from immunosuppression (7, 12).
We have modeled infection-mediated abrogation of tolerance in mice transplanted with cardiac allografts in which tolerance is induced by donor-specific transfusion (DST) with splenocytes on the day of transplantation, as a circulating source of alloantigen, in addition to administration of blocking anti-CD154 antibody on d0, d7, and d14. This regimen results in a state of donor-specific tolerance rather than chronic rejection of cardiac allografts, as recipients remain immunocompetent against third party antigens but spontaneously accept a second donor-matched cardiac allograft transplanted many weeks after the first (13, 14); the allografts display minimal T cell infiltrate with a high percentage of FoxP3+ Tregs, and alloantibodies are not generated (13, 15–17). Tolerance in these animals is associated with reduced expansion of conventional T cells (Tconvs) (18, 19), but not of Tregs (20), with a small percentage of Tconvs converting into induced Tregs for some T cell specificities (21, 22). Anti-CD154/DST is also thought to induce dysfunction of alloreactive Tconvs (exhaustion or anergy) when tested early after alloantigen encounter (18), although this remains controversial (23) and has not been examined at the maintenance phase of tolerance. Thus, the consequences of this tolerance-inducing regimen are a high ratio of Tregs:Tconvs and the speculation that the function of these Tconvs may also be intrinsically reduced. Using this tolerance model, we previously reported that a systemic infection with Listeria monocytogenes (Lm) breaks established tolerance when the infection occurs at the maintenance phase of tolerance, resulting in the rejection of previously stable allografts (17), thus mirroring the rejection that sometimes happens after infections in tolerant patients (7, 8). Infection-dependent transplant rejection occurred in the absence of detectable cross-reactivity by anti-Lm T cells on alloantigen, and instead was due to bystander activation of alloreactive T cells by Lm-induced production of type I IFN and IL-6 (17). Intriguingly, mice in which tolerance had been broken with Lm resulting in the rejection of a primary cardiac allograft did not develop a memory of the rejection event but instead retained a memory of the tolerance. Accordingly, second donor-matched cardiac allografts transplanted after the infection had cleared were accepted spontaneously (13). Importantly, depletion of Tregs prevented the spontaneous acceptance of the second hearts, demonstrating that alloreactive Tconvs were present in this setting and that they were suppressed by Tregs. In the current study, we tested whether alloreactive Tconvs were able to differentiate into memory T cells either during establishment of transplantation tolerance or following their infection-dependent bystander transient reactivation.
Following cardiac transplantation, CD4+ T cells appear to play a more important role than CD8+ T cells for allograft rejection, as their depletion but not that of CD8+ T cells prevents acute heart transplant rejection (24). Thus, to track the function of alloreactive Tconvs at the maintenance phase of tolerance and after the return of tolerance following Lm infection, we seeded C57BL/6 mice (H-2b) before transplantation with syngeneic CD4+ TCR75 TCR-transgenic T cells that recognize a peptide from Kd presented by host I-Ab. In parallel, we used fluorescently labeled peptide:MHC tetramers to track endogenous CD4+ and CD8+ responses specific to model antigens expressed in the donor grafts. We show that tolerance induction prevented the development of canonical memory in donor-reactive T cells >1 mo after transplantation, but instead resulted in Tconv-intrinsic dysfunction akin to exhaustion. Notably, Lm infection induced graft rejection in tolerant mice but did not restore memory development of alloreactive T cells, consistent with the return of tolerance after infection. Cell-intrinsic Tconv dysfunction was not specific to our tolerizing protocol as CoB could be substituted by conventional immunosuppression, but required a source of persistent alloantigen.
These data support the conclusion that long-term graft acceptance prevents the development of classical T cell memory and, instead, persistent alloantigen, in conjunction with CoB or conventional immunosuppression, leads to cell-intrinsic Tconv dysfunction that resists subsequent memory differentiation upon antigen reencounter in the absence of CoB or donor-specific regulation. However, cell-intrinsic dysfunction of alloreactive Tconvs is not sufficient to establish transplantation tolerance, supporting our published data that multiple mechanisms concurrently promote the maintenance of transplantation tolerance (14). These dysfunctional Tconvs from tolerant hosts fail to acquire a memory phenotype even after bystander activation by Lm infection and rejection of the transplanted organ, thus clarifying the long-term fate of alloreactive T cells during the abrogation and reestablishment of tolerance. The inability of dysfunctional T cells to differentiate into memory T cells may explain why episodes of acute rejection in transplant patients do not preclude their subsequent weaning from immunosuppression to reveal a state of tolerance to allografts (7, 12).
Results
Graft-Reactive Tconvs in Tolerant Mice Are Antigen Experienced but Fail to Acquire a Memory Phenotype and Are Functionally Impaired.
We have previously shown that cardiac allograft rejection in wild-type mice depends on CD4+ T cells (24). To understand the fate of graft-reactive CD4+ T cells following transplant rejection or tolerance, and in order to have a fixed traceable population of alloreactive T cells that is not confounded by new thymic emigrants, we injected into congenic mice (CD45.2+, H-2b) CD45.1+CD4+ TCR75 T cells that bear a transgenic TCR recognizing a Kd-derived peptide presented by I-Ab (Fig. 1A). Whereas we have shown that seeding of TCR75 T cells at the time of BALB/c (H-2d) cardiac transplantation helps expand peripheral endogenous allogeneic T cells in untreated mice that undergo acute rejection (25), we chose a number of TCR75 cells (0.5 to 1 × 105) that did not prevent tolerance induction (SI Appendix, Fig. S1 and ref. 14). Tolerance was induced with a CoB regimen consisting of anti-CD154 on d0, d7, and d14 and DST on d0. We first investigated whether alloreactive T cells persisted after induction of transplantation tolerance. TCR75 cells (see gating strategy in SI Appendix, Fig. S2A) were present in the spleen and lymph nodes (LNs) of tolerant mice d35 to d60 posttransplantation in similar numbers as TCR75 cells recovered d35 to d60 posttransfer into untransplanted naïve mice (Fig. 1B). They also retained a Tconv phenotype as fewer than 2% up-regulated FoxP3 (SI Appendix, Fig. S2B). Thus, TCR75 cells survive long term following the induction of transplantation tolerance.
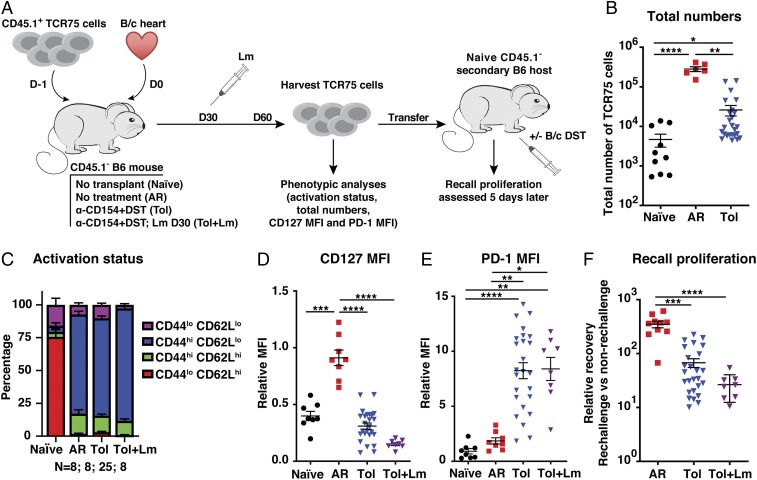
Fig. 1.
Lm infection after establishment of tolerance fails to rescue memory differentiation and function of alloreactive T cells. (A) Experiment design. (B) Total numbers of TCR75 cells isolated from spleen and lymph nodes on d30 to d80 posttransfer from naïve untransplanted mice (n = 10), mice having undergone acute rejection (AR) of a BALB/c (B/c) graft (n = 6), tolerant mice (Tol) transplanted with a B/c graft and treated with anti-CD154 + DST (n = 25), and tolerant mice infected with Lm-GFP on d30 (n = 8). A subset of the cells isolated d30 to d80 posttransfer were phenotyped for their activation status measured by CD44 and CD62L (C) and expression of CD127 (D) and PD-1 (E). A group receiving DST only was included in each experiment, and MFI values in D and E were normalized to this DST-only group such that all experiments could be analyzed together (DST = 1). (F) CD44hiTCR75 cells sorted from primary hosts were pooled by group and adoptively transferred into new naïve mice (secondary hosts). The next day a subset of these mice was challenged with DST and recall proliferation was measured d5 to d6 post rechallenge by quantifying the ratio of the number of cells recovered in rechallenged mice to the average number of cells in nonrechallenged mice. AR n = 10, Tol n = 26, Tol + Lm = 8. Data were pooled from 3 to 8 independent experiments. Mean values were compared by Brown–Forsythe and Welsh ANOVA tests. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To determine whether seeded TCR75 cells differentiated into effector/memory T cells in tolerant animals, we analyzed their expression of CD44 and CD62L. Whereas the majority of sorted TCR75 cells parked in untransplanted naïve mice retained a naïve CD44loCD62Lhi phenotype, the majority of TCR75 cells isolated at >35 d posttransplantation from the periphery of either untreated mice that acutely rejected their allograft or from tolerant mice exhibited marks of antigen experience and expressed the CD44hiCD62Llo phenotype of effector/memory T cells (Fig. 1C). To distinguish between effector and memory phenotypes, we analyzed expression of CD127 (IL-7Rα) and programmed death-1 (PD-1), as effector T cells down-regulate CD127 and up-regulate PD-1 downstream of TCR signaling, whereas quiescent memory T cells express higher levels of CD127 than naïve T cells and low levels of PD-1 (26, 27). To enable comparison between independent experiments, since a limited number of cardiac allografts can be transplanted each day due to the technical complexity of the surgery, the CD127 and PD-1 mean fluorescence intensities (MFIs) were normalized to naïve C57BL/6 mice seeded with TCR75 cells and then immunized with BALB/c DST, in the absence of transplantation. The CD127 and PD-1 MFIs of each experimental mouse were normalized to the average MFI of TCR75 cells in this DST-immunized group (assigned a value of 1), and the data reported as relative MFI. Peripheral TCR75 cells from mice that had undergone acute rejection >30 d prior expressed a CD127hiPD-1lo phenotype (Fig. 1 D and E and SI Appendix, Fig. S2C), as expected for conventional memory T cells, with MFI values similar to memory TCR75 cells from mice immunized with DST alone >30 d prior (i.e., relative MFI close to 1). In contrast, peripheral TCR75 cells from tolerant animals expressed a CD127loPD-1hi phenotype (Fig. 1 D and E and SI Appendix, Fig. S2C). The levels of PD-1 on TCR75 cells varied between individual tolerant mice, perhaps reflecting the duration since last alloantigen encounter since PD-1 is up-regulated upon T cell activation, but all tolerant mice retained their grafts until sacrifice irrespective of PD-1 MFI. Taken together, peripheral TCR75 cells in tolerant mice did not acquire a canonical memory T cell phenotype but resembled chronically stimulated or exhausted T cells (27). To exclude that this tolerant phenotype was unique to TCR75 cells, we tracked polyclonal endogenous T cells reactive to donor antigens using fluorescently labeled donor-peptide:MHC tetramers. Untransplanted mice immunized with 2W/ovalbumin (OVA)-expressing DST, or infected with Lm engineered to express the model antigens 2W and OVA, generated CD127hiPD-1lo canonical memory 2W-reactive CD4+ and OVA-reactive CD8+ T cells, when compared to the phenotype of naïve T cells normalized to 1 (SI Appendix, Fig. S3). In contrast, following transplantation with BALB/cxC57BL/6 F1 hearts transgenic for 2W and OVA, the anti-CD154 + DST tolerance induction regimen resulted in CD127loPD-1hi 2W-reactive CD4+ T cells, similar to TCR75 cells in tolerant hosts. An intermediate phenotype was observed with OVA-reactive CD8+ T cells in tolerant hosts, where expression of CD127 was depressed but PD-1 expression was highly variable and not significantly increased compared to memory CD8+ T cells induced by DST or Lm-2W-OVA. Thus, tolerance induction results in antigen experience and acquisition of a phenotype in peripheral donor-reactive CD4+ T cells more reminiscent of chronic stimulation than of memory.
Memory and exhausted T cells are not only distinguished phenotypically but also functionally. Memory T cells have high proliferative capacity upon repeat stimulation but exhausted T cells are impaired in their recall proliferation (28). To test whether T cells from tolerant mice behaved functionally as memory or exhausted T cells, we sorted the CD44hiCD45.1+ TCR75 cells harvested on >30 d posttransplantation from the spleen and lymph nodes of their nontolerant and tolerant hosts and adoptively transferred similar numbers into new naïve congenic (CD45.2+) hosts (Fig. 1A). This allowed us to compare on a per-cell basis the functionality of TCR75 populations from acute rejection versus tolerant primary hosts, eliminating bias from higher TCR75 cell frequency in acute rejection primary hosts. Adoptively transferring the TCR75 cells into new naïve hosts also removed the alloreactive T cells from the tolerant environment of the primary host, away from regulatory cells and inhibitory ligands, to assess their Tconv-intrinsic function. One day after transferring the TCR75 cells to secondary hosts, we rechallenged a subset of those mice with alloantigen in the form of DST and assessed the cell recovery in DST-challenged versus unchallenged hosts 5 to 6 d later as a measure of recall proliferation. Cell recovery in secondary hosts was much greater in recipients of TCR75 cells obtained from rejecting than tolerant primary hosts. Indeed, we recovered 300 to 400× as many cells from rechallenged mice as from nonrechallenged hosts of rejecting TCR75 cells but 10-fold fewer from hosts of tolerant TCR75 cells (Fig. 1F). This was a consequence of abortive proliferation rather than lack of proliferation by TCR75 cells from tolerant hosts, as nearly 100% of T cells in all groups up-regulated the proliferation marker Ki67 in challenged but not in unchallenged secondary hosts, consistent with antigen reexposure (SI Appendix, Fig. S4). Of note, higher PD-1 expression on TCR75 cells at the time of harvest from tolerant primary hosts was not correlated with greater reduction in recall proliferation in secondary hosts (r = −0.05298, P [2 tailed] = 0.8149). This is consistent with reports that although higher expression of PD-1 marks exhausted T cells, its expression is not necessary for the T cell dysfunction observed in chronic lymphocytic choriomeningitis virus (LCMV) infection (29). Our data indicate that phenotypically and functionally, T cells from tolerant mice behaved less like memory cells and more like exhausted T cells.
Lm Infection at the Maintenance Phase of Tolerance Fails to Convert Dysfunctional T Cells into Memory T Cells.
We have previously shown that a systemic Lm infection can transiently reactivate alloreactive T cells in a bystander manner via infection-triggered type I IFN and IL-6, resulting in graft rejection in ∼50% of recipients and a rejection crisis with enlarged allografts resulting in erosion of tolerance in the nonrejecting mice (17, 30). To test whether tolerant alloreactive Tconvs activated in this manner became memory T cells, 8 tolerant mice harboring seeded TCR75 cells from d(−1) were infected with Lm i.p. on d30 posttransplantation, resulting in rapid rejection in 4 mice and a rejection crisis with enlargement of the hearts in the other 4, consistent with our previous reports (17, 30). Analysis of TCR75 cells at d60 posttransplantation, the memory phase following the d30 infection, revealed a doubling in TCR75 cell numbers compared with uninfected mice (SI Appendix, Fig. S5), confirming the bystander activation of the TCR75 cells following the infection. However, their phenotype and function was indistinguishable from that of TCR75 cells from uninfected tolerant animals (Fig. 1 B–F and SI Appendix, Figs. S3 and S4). Thus, similarly to what has been recently reported for exhausted CD8+ T cells following chronic LCMV infection and transiently reinvigorated by blockade of the PD-1/PD-L1 axis (31), tolerant alloreactive T cells exposed to an Lm infection failed to become memory T cells and displayed reduced accumulation upon alloantigen rechallenge. This is consistent with the return of tolerance and spontaneous acceptance of a second donor-matched cardiac allograft we previously reported at the same time point after infection (13). Notably, the residual function of these Tconvs, as they still expanded moderately following alloantigen rechallenge, may explain why Tregs were required for the restoration of tolerance in postinfection mice (13).
The Combination of Costimulation Blockade and Chronic Antigen Exposure Results in Impaired Recall Proliferation.
We next investigated the mechanism by which alloreactive Tconvs acquire this dysfunctional phenotype. In in vitro studies with CD4+ T cell clones, TCR engagement in the absence of costimulation was reported to induce anergy (32), which resulted in the cells then remaining unresponsive to secondary stimulation even if in the presence of costimulation. In transplant-tolerant mice, graft-reactive T cells are initially exposed to antigen in the presence of CoB, and they also have continuous access to alloantigen from the surviving graft, potentially leading to chronic stimulation similar to the exhaustion reported in models of chronic viral infections and tumors (33–36). Indeed, we have previously reported that naïve TCR75 cells injected into tolerant mice 2 mo after the induction of cardiac transplantation tolerance undergo abortive proliferation (cell division but minimal accumulation) (14), demonstrating the persistence of antigen, along with a suppressive environment. To test whether CoB in vivo was sufficient to induce anergy of TCR75 cells manifesting as reduced recall proliferation in secondary hosts, naïve C57BL/6 mice were seeded with sorted congenic TCR75 cells, and immunized with BALB/c DST in the presence or absence of anti-CD154, but without an allograft. Anti-CD154 effectively limited expansion of TCR75 cells in primary hosts on d6 (SI Appendix, Fig. S6A) and d35 (Fig. 2A) post-DST immunization, as well as prevented the development of anti-donor IgM and IgG antibodies (SI Appendix, Fig. S6B). However, both TCR75 cells from mice immunized with DST and with anti-CD154 + DST exhibited a CD127hiPD-1lo memory phenotype at d35 post-DST (Fig. 2 C and D). Moreover, having encountered alloantigen in the presence of anti-CD154 did not impair TCR75 recall proliferation in secondary hosts (Fig. 2E), suggesting that CoB alone is not sufficient to drive alloreactive Tconv dysfunction in vivo. To determine the duration of alloantigen persistence after anti-CD154 + DST immunization, BALB/c and C57BL/6 splenocytes were coinjected at a 1:1 ratio into C57BL/6 naïve recipients treated or not with anti-CD154. Although BALB/c cells were present in the secondary lymphoid organs 1 d later in both anti–CD154-treated and untreated hosts, they were absent from both groups on d6, in contrast to persistence of syngeneic cells (Fig. 2B).
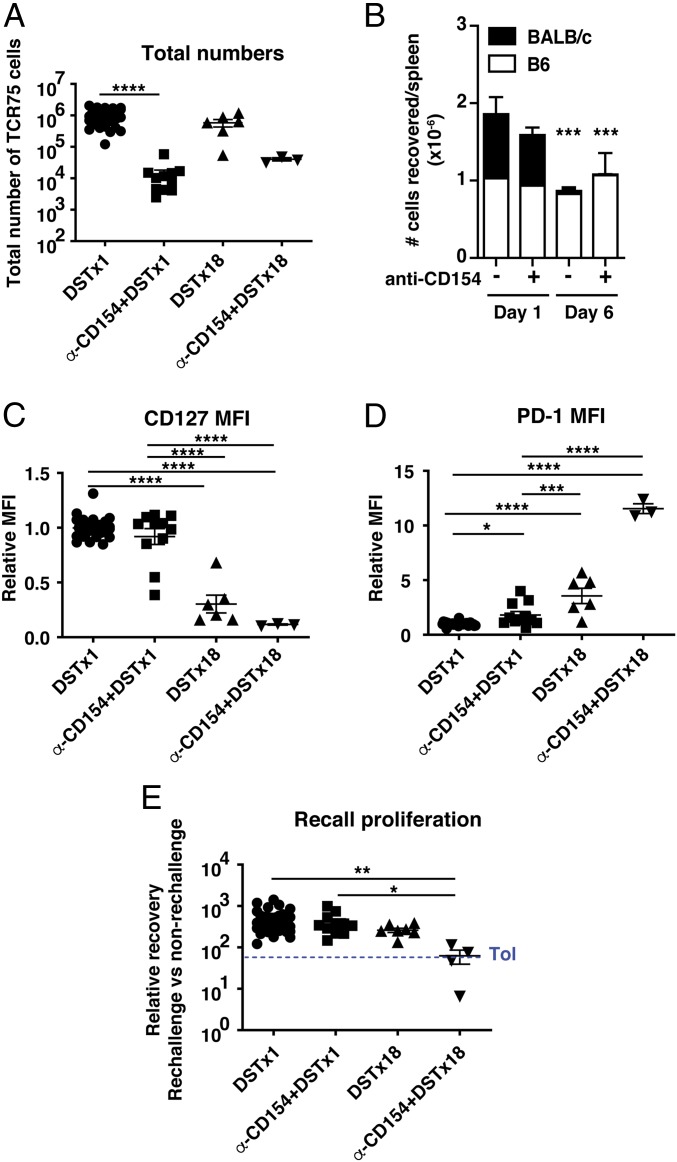
Fig. 2.
The combination of costimulation blockade and chronic antigen exposure results in impaired recall proliferation. C57BL/6 mice were seeded with TCR75 cells (105) on d(−1) and immunized with either a single injection of DST on d0 ± anti-CD154 on d0, d7, and d14 (DST×1 and α-CD154 + DST×1), or 18 doses of DST administered every other day for the duration of the experiment ± anti-CD154 on d0, d7, and d14 (DST×18 and α-CD154 + DST×18). (A) Total numbers of TCR75 cells isolated from spleen and lymph nodes on d35 posttransfer. DST×1 n = 35, α-CD154 + DST×1 n = 11, DST×18 n = 6, α-CD154 + DST×18 n = 3. (B) BALB/c (CD45.2/.2) and B6 (CD45.1/.1 or CD45.1/.2) splenocytes were injected in an equal ratio into B6 (CD45.1/.2 or CD45.1/.1) mice ± anti-CD154 on d0 and the numbers of transferred cells in the spleen were quantified 1 or 6 d later. The d1 group of mice (@) did not have seeded TCR75 cells. d1: DST n = 4; α-CD154 + DST n = 4; d6: DST n = 4; α-CD154 + DST n = 3. (C and D) MFI of CD127 and PD-1 on TCR75 cells isolated from spleen and lymph nodes on d35. Values were normalized to DST only (MFI of DST group = 1). (E) TCR75 cells sorted from d35 primary hosts were pooled by group and adoptively transferred into new naïve mice (secondary hosts). Challenge and analysis as in Fig. 1F. The mean of the tolerant group in Fig. 1F is shown as a dashed blue line for reference. DST×1 n = 43, α-CD154 + DST×1 n = 11, DST×18 n = 7, α-CD154 + DST×18 n = 4. Data from the first 3 groups were pooled from 2 to 11 independent experiments. Mean values were compared by Kruskal–Wallis with Dunn’s correction for multiple pairwise comparisons (A and C–E) and by 2-way ANOVA with Bonferroni posttests (B). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Therefore, to test whether chronic antigen stimulation was sufficient to drive impaired recall proliferation, C57BL/6 mice seeded with TCR75 cells received 18 injections of BALB/c splenocytes every other day until killing on d35, with or without injections of anti-CD154 on d0, d7, and d14, as in our transplant tolerance protocol. TCR75 cells from animals injected with DST×18 displayed an intermediate phenotype with lower CD127 and higher PD-1 than animals injected with DST once (Fig. 2 C and D), but did not show reduced recall proliferation when restimulated with alloantigen in secondary hosts (Fig. 2E). In contrast, TCR75 cells from animals that had received anti-CD154 + DST×18 displayed the CD127loPD-1hi phenotype (Fig. 2 C and D) and impaired recall proliferation (Fig. 2E) reminiscent of the TCR75 cells from tolerant animals. These results suggest that chronic alloantigen exposure in the form of repeated DST immunizations until killing is not sufficient to drive T cell dysfunction, unless combined with CoB, highlighting cooperation between antigen persistence and CoB.
Persistence of Antigen Is Necessary for Alloreactive T Cells to Acquire Impaired Recall Proliferation.
The previous experiments demonstrated that persistence of antigen in the presence of CoB was sufficient to drive T cell dysfunction even in the absence of an allograft. To address whether persistence of antigen (the cardiac allograft), in the presence of CoB, was necessary to drive impaired recall proliferation, BALB/c allografts transplanted into C57BL/6 mice seeded with naïve TCR75 cells and treated with the DST + anti-CD154 tolerogenic regimen were surgically removed either d7 or d21 posttransplantation and TCR75 cells were analyzed on d35 posttransplantation. TCR75 cells did not expand more in primary tolerant hosts whose graft was removed on d7, but retained a CD127 and PD-1 phenotype that was closer to that of memory TCR75 cells from mice that had undergone acute rejection (Fig. 3 A–C). Most notably, graft resection on d7 but not on d21 posttransplantation restored recall proliferation of TCR75 cells in secondary hosts (Fig. 3D), demonstrating that persistence of antigen beyond 7 d is required for alloreactive Tconv dysfunction to take place. This is reminiscent of the requirement by virus-specific CD8+ T cells to be exposed to antigen for at least 2 wk to become exhausted following chronic LCMV infection (37).
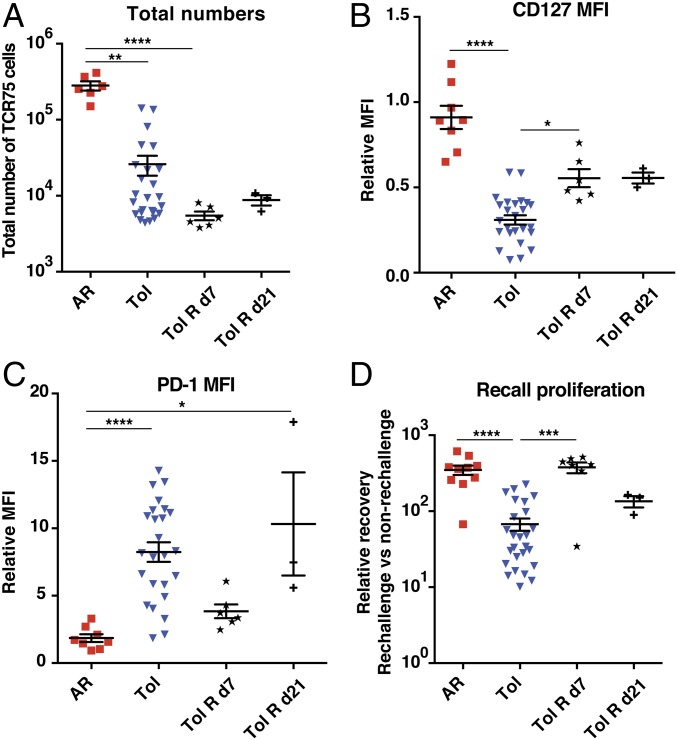
Fig. 3.
Surgical graft removal early but not late posttransplantation rescues recall proliferation of graft-reactive T cells in tolerant animals. C57BL/6 mice were seeded with TCR75 cells (105) on d(−1), transplanted with BALB/c hearts on d0, and either left untreated (AR) or treated with anti-CD154 on d0, d7, d14 and DST on d0 (Tol). In a subset of the Tol mice grafts were surgically removed on d7 (Tol R d7) or d21 (Tol R d21). (A) Total numbers of TCR75 cells isolated from spleen and lymph nodes on d35 through d60. AR n = 6; Tol n = 25; Tol R d7 n = 6; Tol R d21 n = 3. A subset of the cells isolated d35 through d80 posttransfer were phenotyped for their expression of CD127 (B) and PD-1 (C) relative to that in mice injected with DST alone on d0 (MFI of DST group = 1). AR n = 8; Tol n = 25; Tol R d7 n = 6; Tol R d21 n = 3. (D) TCR75 cells sorted from primary hosts were pooled by group and adoptively transferred into new naïve mice (secondary hosts). Challenge and analysis as in Fig. 1F. AR n = 10, Tol n = 26, Tol R d7 n = 7; Tol R d21 n = 3. Data from the first 3 groups were pooled from 2 to 8 independent experiments. In all panels, AR and Tol groups are the same as those presented in Fig. 1. Mean values were compared by Kruskal–Wallis with Dunn’s correction for multiple pairwise comparisons. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Conventional Immunosuppression Can Drive Alloreactive T Cell Dysfunction.
Whereas donor-specific tolerance is rare in transplant patients, many transplant recipients have stable long-lasting grafts while treated with conventional immunosuppression. To determine whether the 2-wk treatment with CoB was uniquely required to drive alloreactive T cell dysfunction or whether other therapies that control initial T cell expansion and maintain long-term graft acceptance could also enable development of T cell dysfunction, we assessed the impact of conventional immunosuppression on the phenotype and function of alloreactive T cells. C57BL/6 mice seeded with TCR75 cells were transplanted with a BALB/c heart and received DST and either injections of cyclosporine A (CsA), mycophenolate mofetyl (MMF) or rapamycin (Rapa) alone at doses that did not prolong graft survival beyond d17, or a combination of MMF + Rapa or of MMF + Rapa + CsA, which prolonged graft survival beyond d35. Analysis of TCR75 cells at >35 d posttransplantation revealed that MMF alone but not CsA or Rapa alone at the doses utilized trended to partially control TCR75 expansion (Fig. 4A), but that none of the single immunosuppressive agents prevented the acquisition of a CD127hiPD1lo memory phenotype (Fig. 4 B and C). Moreover, MMF alone did not result in impaired recall proliferation (CsA alone or Rapa alone were not tested) (Fig. 4D). In contrast, the combination therapies that significantly reduced the numbers of alloreactive T cells in primary hosts and prolonged graft survival >35 d, prevented acquisition of a memory phenotype and resulted in impaired recall proliferation in secondary hosts (Fig. 4 A–D). These data demonstrate that short-term blockade of costimulation per se is not unique in inducing cell-intrinsic Tconv dysfunction; rather, dysfunction can develop with immunosuppressive therapies utilized in patients. Thus, cell-intrinsic Tconv dysfunction is a feature of graft persistence rather than exclusive to tolerance.
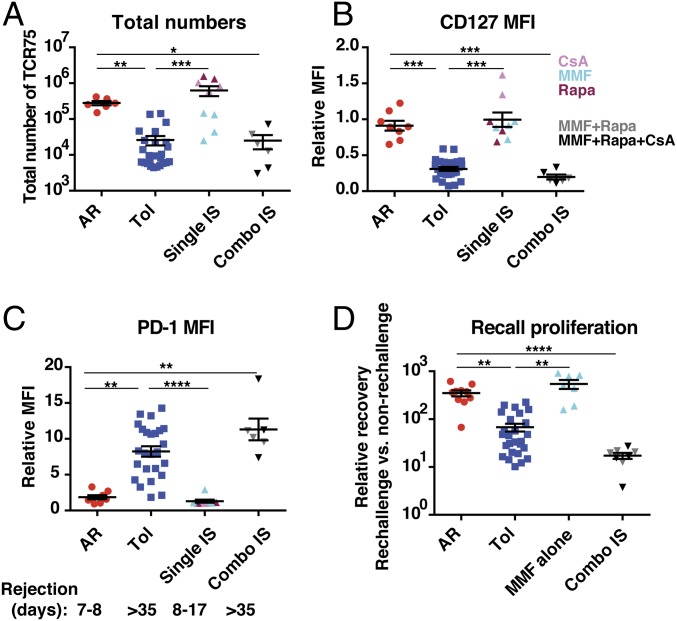
Fig. 4.
Impaired recall proliferation of graft-reactive T cells can occur with conventional immunosuppression. C57BL/6 mice were seeded with TCR75 cells (105) on d(−1), transplanted with BALB/c hearts on d0, and either left untreated (AR) n = 6, treated with anti-CD154 on d0, d7, and d14 and DST on d0 (Tol) n = 25, or treated with daily administration of individual immunosuppressive drugs on d0 to d14 of rapamycin n = 3, cyclosporine n = 2, or mycophenolate mofetil n = 4, collectively single immunosuppression (IS) n = 9, or with combinations on d0 to d14 of mycophenolate mofetil and rapamycin n = 3 or mycophenolate mofetil, rapamycin, and cyclosporine n = 3, collectively combo IS n = 6. (A) Total numbers of TCR75 cells isolated from spleen and lymph nodes on d35 to d60. (B) A subset of the cells isolated d35 to d80 posttransfer were phenotyped for their expression of CD127 (B) and PD-1 (C), relative to that in the group immunized with DST alone (MFI of DST group = 1), AR n = 8; Tol n = 25; single IS n = 9; combo IS n = 6. Median graft survival times are listed below C. (D) TCR75 cells sorted from primary hosts were pooled by group and adoptively transferred into new naïve mice (secondary hosts). Challenge and analysis as in Fig. 1F. AR n = 10, Tol n = 26, MMF alone n = 7, combo IS n = 8. Data were pooled from 2 to 8 independent experiments. In all panels, AR and Tol groups are the same as those presented in Figs. 1 and 3. Mean values were compared by Kruskal–Wallis with Dunn’s correction for multiple pairwise comparisons. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
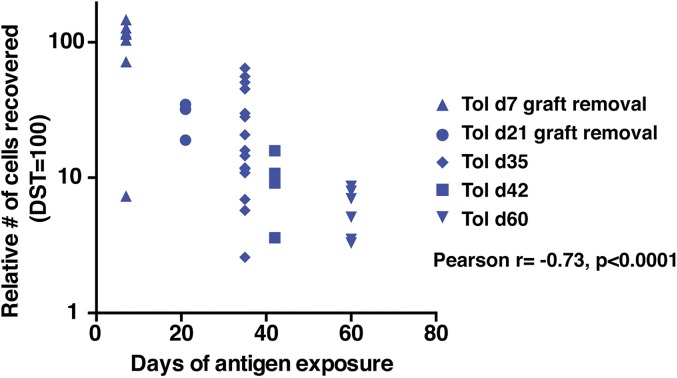
Time elapsed since transplantation is the parameter that appears most predictive of whether liver transplant recipients have developed operational tolerance and can be weaned from their conventional immunosuppressive drugs (12). To determine whether there was a correlation between the duration of alloantigen persistence and the level of impaired recall proliferation, we compared the recall proliferation by TCR75 cells from all our tolerant animals based on the duration of TCR75 cell exposure to the allograft. To compare more rigorously between independent experiments and eliminate the possible confounder of T cell senescence, recall proliferation by TCR75 cells harvested from tolerant primary hosts at different time points posttransplantation was normalized to recall proliferation by time-matched memory TCR75 cells from untransplanted primary hosts immunized with DST, a control group performed in each experiment. As shown in Fig. 5, we observed a highly significant progressive reduction in recall proliferation by tolerant TCR75 cells in secondary hosts proportional to their increasing time of alloantigen exposure in primary hosts and relative to time-matched memory TCR75 cells from DST-immunized hosts. Altogether, our results demonstrate that chronic alloantigen exposure, along with impaired T cell signaling, either from anti-CD154 or conventional immunosuppression, is necessary and sufficient for alloreactive Tconvs to display cell-intrinsic impaired recall proliferation and for their failure to become bona fide memory T cells.
Fig. 5.
Time-dependent loss of recall proliferation by donor-reactive tolerant T cells with prolonged graft antigen exposure. Relative recovery of TCR75 cells in rechallenged vs. nonrechallenged mice 5 to 6 d post rechallenge with DST in secondary naïve hosts. To account for any possible impact of senescence, all groups were normalized to recall proliferation by TCR75 cells harvested from time-matched untransplanted controls that received DST on the same day their matched experimental group received a transplant (DST = 100). These values are plotted against the number of days that the hearts (and DST for the respective control mice) were present in the animals. Tolerant animals are the same as those shown in Figs. 1, 3, and 4. Tol d7 graft removal (7-d exposure to alloantigen, analysis on d35) n = 7; Tol d21 graft removal (21-d exposure to alloantigen, analysis on d35) n = 3; Tol d35 (35-d exposure to alloantigen, analysis on d35) n = 15; Tol d42 (42-d exposure to alloantigen, analysis on d42) n = 4; Tol d60 (60-d exposure to alloantigen, analysis on d60) n = 7. These data are from 8 independent experiments. The relative recovery of the cells was negatively correlated with the duration of antigen exposure, Pearson’s r = −0.73, ****P < 0.0001.
Discussion
Unlike naïve T cells that differentiate into memory cells following antigen encounter, our results show that naïve alloreactive CD4+ Tconvs that persisted following exposure to an allograft under a short-term tolerogenic regimen became antigen experienced but did not differentiate into memory T cells. They instead displayed phenotypic signs of chronic stimulation and developed a T cell-intrinsic dysfunctional state, characterized by impaired recall proliferation still apparent when the cells were removed from the tolerant environment and were sorted away from potential extrinsic regulatory cells and inhibitory ligands. Moreover, a systemic infection with Lm that transiently reactivated alloimmunity and overrode transplantation tolerance failed to rescue memory development or restore recall proliferation. This state of cell-intrinsic dysfunction was programmed during the 2-wk induction of tolerance with CoB but was not specific to anti-CD154 or tolerance and could be induced with conventional immunosuppression.
The finding that tolerant alloreactive CD4+ T cells fail to develop memory after systemic infection is reminiscent of the recent discovery that CD8+ T cells exhausted following chronic LCMV infection failed to recover memory differentiation and functionality following reinvigoration by blockade of the PD-1/PD-L1 axis (31). In this setting, the return to T cell exhaustion after checkpoint blockade was due to epigenetic imprinting during tolerance induction. Another setting of return to tolerance was described in self–antigen-specific CD8+ T cells following transient activation by homeostatic proliferation in lymphopenic hosts and was also ascribed to epigenetic imprinting (38). In transplantation, a T cell-restricted deficiency in IRF4 was recently reported to result in spontaneous donor-specific tolerance of cardiac allografts associated with impaired memory development and hyporesponsiveness of alloreactive CD4+ T cells, even if the animals received anti-PD1 and anti–CTLA-4 at the time of initial cardiac transplantation, which prevented graft acceptance (39, 40). A similar phenomenon of return of tolerance may have been observed in kidney allograft recipient monkeys in which tolerance was induced by a regimen resulting in transient mixed chimerism, as injections of IL-2 resulted in rapid rejection of previously tolerated allografts, but cessation of IL-2 aborted the rejection process and restored kidney function (41). Our data indicate that therapeutic induction of transplantation tolerance by CoB also generates a resilient T cell dysfunctional state. Whether the return to tolerance and T cell dysfunction after Lm infection is due to epigenetic imprinting in alloreactive Tconvs remains to be tested.
Although Lm infection can occur in transplant recipients (42), it is rare. Whether more common viral infections such as cytomegalovirus (CMV), Epstein-Barr virus (EBV), and BK can also break tolerance and/or transiently reinvigorate T cells is not known. Acute and persistent (LCMV clone 13) viral infections incurred before or at the time of transplantation have been shown to prevent the induction of transplantation tolerance (4, 43, 44). However, to our knowledge, LCMV Armstrong (acute strain) is the only viral infection that has been reported to abrogate transplantation tolerance when administered after transplantation (albeit shortly after transplantation), whereas murine CMV and vaccinia virus did not acutely break tolerance (45). This acute LCMV infection might be a setting in which to test whether a virus can, like Lm infection, reinvigorate dysfunctional alloreactive T cells. Additionally, a recent report has shown that latent murine CMV in preinfected mice becomes reactivated following the inflammation of transplantation, and reactivation is further amplified by immunosuppression (46). It might be of interest to study the impact of this reactivation on alloreactive Tconv dysfunction, especially if dysfunction of alloreactive T cells has time to develop before CMV is reactivated.
When TCR75 T cells were challenged with DST in secondary hosts, a functional defect in recall proliferation was observed in tolerant T cells obtained before or after Lm infection, when compared with memory T cells obtained from rejecting mice. The inability of DST immunization to reinvigorate tolerant T cells is probably not because DST immunization is a weak stimulus, as DST injection induced a robust expansion and memory phenotype acquisition by naïve TCR75 cells in primary hosts, similar to that observed following cardiac transplantation (see similar total numbers in Fig. 1A for acute rejection (AR) versus Fig. 2B for DST, and see the relative MFI of AR:DST close to 1 for CD127 and PD-1 in Fig. 1 D and E). Nevertheless, whether exposure to alloantigen in other forms, such as in a skin graft, or when the antigen is shared between an infectious agent and an allograft, would successfully reinvigorate tolerant T cells, remains to be determined.
Whereas cell-intrinsic dysfunction of alloreactive CD4+ Tconvs is clearly a feature of donor-specific transplantation tolerance, according to the strict definition of tolerance as a state in which a second donor-matched allograft is spontaneously accepted and a third party allograft is rejected as we have previously shown in noninfected hosts (13), tolerance is not required to achieve T cell dysfunction and T cell dysfunction is not sufficient to achieve transplantation tolerance. Indeed, CoB could be replaced with conventional immunosuppression that can prolong graft survival while administered, but does not induce transplantation tolerance. Consistent with this result, altered memory development of alloreactive CD4+ Tconvs was also reported in 2 models of chronic cardiac allograft rejection by C57BL/6 hosts, using either Bm12 cardiac allografts transgenic for the expression of Kd and I-Ed in the absence of immunosuppression, or BALB/c hearts with a suboptimal anti-CD154 regimen (47). In these chronic rejection models, alloreactive T cells resembled effector/memory T cells, rather than central memory T cells observed after acute rejection of BALB/c hearts. However, it was suggested that chronic rejection resulted in dysfunctional alloreactive CD4+ T cells, as transfer of polyclonal splenic CD4+ T cells from Bm12.Kd.I-Ed heart-transplanted mice into secondary T cell-deficient mice challenged with B6.Kd cardiac allografts resulted in less B cell help, as measured by reduced levels of alloantibodies, than transfer of cells from primary hosts that had acutely rejected a BALB/c heart (47). Similarly, exhaustion of CD4+ T cells has been invoked in a model of spontaneous chronic cardiac allograft rejection in mice deficient in fucosyltransferase VII (Fut7), an enzyme important for selectin biosynthesis (48). Although alloreactive T cells were not tracked, Fut7-deficient polyclonal CD4+ T cells expressed an exhausted CD127loPD-1hi phenotype in untreated transplanted hosts with long-term allografts (48). Together, these data, along with our results on mice treated with conventional immunosuppression, suggest that long-term exposure to antigen in the form of chronic rejection may also lead to T cell exhaustion/dysfunction.
Persistence of antigen for ∼3 wk, rather than tolerance, was necessary for programming cell-intrinsic Tconv dysfunction in our model as removal of the allograft on d7 but not d21 prevented acquisition of impaired recall proliferation. In Ali et al. (47), impaired memory development during chronic cardiac allograft rejection correlated with persistence of antigen, with donor MHC class I persisting long term following transplantation. Conversely, expression of donor class II is thought to wane shortly after transplantation, presumably because of the short half-life of passenger hematopoietic cells that express MHC class II (47). Whether donor MHC class II persists long enough following transplantation to trigger dysfunction of host T cells with that alloreactivity is unclear. Donor class II-reactive TCR transgenic CD4+ TEa cells isolated from mice transplanted with a skin graft and treated with anti-CD154 + DST were shown to be dysfunctional when transferred into secondary lymphopenic hosts (18), but these cells were harvested from the primary hosts only 7 d posttransplantation when the alloreactive T cells are still likely coated with anti-CD154. The function of TEa cells harvested after discontinuation of the treatment was not performed. Conversely, donor MHC class I-reactive TCR75 cells did not become dysfunctional after the induction of tolerance with anti-CD154 + DST (23), but those experiments were performed without an allograft. Our data are consistent with the latter study in that in anti-CD154 + DST-immunized untransplanted mice, alloreactive T cells did not become dysfunctional, but introduction of an allograft to this regimen resulted in T cell dysfunction. Of note, both TEa and TCR75 cells are CD4+ T cells that recognize donor MHC indirectly, after processing and presentation by host antigen-presenting cells. Whether CD4+ Tconvs that recognize donor MHC directly develop a dysfunctional phenotype remains to be tested. Our observation of a CD127loPD-1hi phenotype on 2W-reactive endogenous CD4+ T cells from tolerant mice, which resembles that of dysfunctional TCR75 cells, suggests that dysfunction may also occur in CD4+ T cells reactive to minor donor antigens, though the small number of these cells in tolerant mice precluded their isolation and transfer into secondary hosts to directly test their recall proliferation capability. Finally, an intermediate CD127loPD-1int phenotype was observed in OVA-reactive CD8+ T cells, but whether this is unique to the OVA specificity or generalizable to all donor-reactive CD8+ T cells, and whether the intermediate phenotype in CD8+ T cells has functional implications remains to be elucidated.
Our results are consistent with the clinical observation that the time elapsed since transplantation is the best parameter to predict successful weaning from immunosuppression in liver transplant patients (12), as we show a positive correlation between the length of time that alloreactive T cells were exposed to the graft and the degree of reduced recall proliferation. Whether cell-intrinsic dysfunction of alloreactive Tconvs occurs in liver transplant patients in which tolerance was not deliberately induced, and whether it occurs in patients on clinical trials for the deliberate induction of transplantation tolerance, or even in stable immunosuppressed patients remains to be determined. Partial deletion and regulation of alloreactive T cells has been observed in transplant patients that became tolerant following treatment with combined donor bone marrow and kidney transplantation to induce mixed chimerism (49, 50). Whether cell-intrinsic dysfunction of remaining alloreactive T cells also occurs, and whether it can return in the tolerant patients who go on to lose their grafts after infections are open questions. This is important because the return to T cell dysfunction after transient reinvigoration in the absence of alloreactive T cell memory development following rejection would avoid the barrier that T cell memory poses to the induction of tolerance to a subsequent allograft (51).
Overall, our data demonstrate the development of Tconv-intrinsic dysfunction of alloreactive CD4+ T cells in settings of long-term allograft acceptance, a state that depends on initial persistence of antigen enabled by immunosuppression and that is not rescued by systemic infections that erode or break established transplantation tolerance. Tconv-intrinsic dysfunction is not sufficient for transplantation tolerance, as it occurs in nontolerant animals treated by conventional immunosuppression. Along with the observation that multiple mechanisms of Tconv control cooperate to maintain transplantation tolerance (14) and with the importance of Tregs to the return of tolerance after infection (13), our data support the speculation that dysfunctional Tconvs may be more susceptible to Treg suppression than memory T cells, thus facilitating the postinfection return of tolerance.
Materials and Methods
Mice.
Six to 8-week-old BALB/c and C57BL/6 mice were purchased from Envigo RMS, Inc. (Indianapolis, IN). TCR75 CD4+ TCR-transgenic mice were obtained from R. Pat Bucy, University of Alabama, Birmingham, AL, and crossed to CD45.1 homozygous C57BL/6 mice purchased from The Jackson Laboratory (Bar Harbor, ME). The resulting mice were also crossed to RAG1-deficient mice. TCR75 and TCR75/RAG-KO mice were used where indicated. 2W/OVA-Tg mice on the C57BL/6 background that constitutively express the 2W and OVA model antigens under the control of the actin promoter (52) were obtained from James Moon, Harvard Medical School, Charlestown, MA, and crossed once to BALB/c mice to generate BALB/cxC57BL/6 F1 mice. A subset of mice received DST with conventional immunosuppression (3 mg/kg Rapa, 160 mg/kg MMF, 20 mg/kg CsA) through daily oral gavage or 80 mg/kg MMF in Alzet osmotic pumps for 2 wk. Mice were housed under specific pathogen-free conditions and used in agreement with the University of Chicago’s Institutional Animal Care and Use Committee, according to the National Institutes of Health guidelines for animal use.
Heart Transplantation.
Cardiac transplantation was performed as previously described (13) using a technique adapted from Corry et al. (53). For induction of tolerance, mice were treated with 600 μg of anti-CD154 (BioXCell) on d0, d7, and d14 posttransplantation and DST of splenocytes on d0. In a subset of mice, heart grafts were removed on d7 or d21 posttransplantation.
Lm Infection.
Lm engineered to express GFP, or Act A-deficient Lm expressing OVA peptides and 2W (54) was grown overnight, rediluted 1:50 and regrown for 1.5 h for cultures in early log phase growth before enumeration of colony forming units (CFU) (absorbance at OD600) (16). Doses of 106 (Lm-GFP) to 108 (Lm-2W-OVA) CFU (i.p.) were chosen for highest rejection rate with minimal lethality. Animals were infected with Lm-GFP d30 through d60 following transplantation to break established tolerance or with Lm-2W-OVA on d0 in untransplanted mice to generate endogenous T cell memory to 2W and OVA.
Adoptive Transfer of TCR75 Cells into Primary Hosts.
Cells were isolated from spleen and lymph nodes of donor TCR75 transgenic mice and counted (Accuri C6 flow cytometer; BD Biosciences). A subset of cells was stained for expression of CD4, Vβ8.3, CD45.1, and CD44. The percentage of CD44loCD45.1+CD4+Vβ8.3+ TCR75 T cells was used to calculate the total number of cells for the adoptive transfer. Cells were resuspended in 200 μL of PBS and injected retroorbitally.
Donor Splenocyte Transfusion.
C57BL/6 mice received retroorbitally or intraperitoneally one-quarter spleen from BALB/c, C57BL/6, or 2W/OVA-transgenic F1 mice, washed, twice filtered, and resuspended in 200 μL PBS. In some experiments, C57BL/6 CD45.1/CD45.2 heterozygous or CD45.1 homozygous mice received an equal ratio of BALB/c CD45.2 homozygous splenocytes with CD45.1 homozygous or CD45.1/CD45.2 heterozygous C57BL/6 splenocytes. The presence of the transferred cells in spleen and lymph nodes was analyzed by flow cytometry at d1 and d6.
Alloantibody Determination.
For donor-specific antibody detection, serum was collected before and after transplantation, diluted (1:50), and incubated with 106 BALB/c splenocytes resuspended at 20 × 106 cells/mL for 1 h at 4 °C. After washing twice, the cells were incubated with anti-IgG (Southern Biotech, Cat. No. 1030-02), anti-IgM (eBioscience, Cat. No. 125790-82), or anti-CD19 (eBioscience, Cat. No. 17-0193-82) and anti-B220 (BD Biosciences, Cat. No. 561226) for 30 min. MFI was measured by flow cytometry gating on B220− or CD19− cells.
Magnetic Enrichment, Cell Sorting, and Flow Cytometry for Transfer into Secondary Hosts.
CD45.1+ TCR75 cells from spleen and lymph nodes harvested >d35 following transfer into primary hosts, mice were stained with anti-CD45.1-bio (eBioscience) and incubated with streptavidin magnetic beads (Miltenyi) for magnetic enrichment with an AutoMACS machine (Miltenyi).
For analysis of TCR75 cells harvested from the primary host, single-cell suspensions of lymphocytes were prepared from isolated spleens. Cells were stained with a fixable live/dead stain (Aqua, Invitrogen) for 30 min at room temperature and then with antibodies to CD4 (L3T4), CD45.1 (A20), Vβ8.3 (1B3.3), PD-1 (J43), CD127 (A7R34), CD62L (MEL-14), and CD44 (IM7). In some experiments, cells were stained with anti-Ki67 (SolA15) and anti-FoxP3 (JFK-16s) following permeabilization with the FoxP3/Transcription Factor Staining Buffer (eBioscience). All mAbs were from BD Biosciences or eBioscience.
For analysis of endogenous polyclonal T cells reactive to donor 2W or OVA harvested from the primary host, pMHC multimers were used to stain magnetically enriched T cells as previously described (55).
For transfer of TCR75 cells into secondary hosts, magnetically enriched CD45.1+ cells were stained with fluorescently coupled antibodies against CD45.1, CD4, Vβ8.3, and CD44, as well as fluorescently labeled streptavidin and further sorted for CD45.1+CD4+Vβ8.3+CD44hi cells (FACSAria, BD Biosciences). Cells (2 × 103) were transferred into secondary hosts 1 d before injection or not of DST and animals were killed 5 d later to assess TCR75 cell recovery and expression of Ki67 from the spleen of immunized versus nonimmunized secondary hosts.
Data Analysis.
Flow cytometry data were analyzed using FlowJo (TreeStar). Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc).
Data Availability.
Data, associated protocols, and materials in the paper will be made available to readers upon request to the corresponding author.
Supplementary Material
Acknowledgments
We thank the University of Chicago flow cytometry core for help with cell sorting. M.L.M. was funded by American Heart Association Pre-Doctoral Fellowships (13PRE14550022 and 15PRE22180007), a Cardiovascular Pathophysiology and Biochemistry Training Grant (T32 HL07237), and a Howard Hughes Medical Institute Med-into-Grad Program Training Grant (56006772). C.M.M. was funded by the Growth Development and Disabilities Training Program (T32 HD007009). The work was supported by NIAID Grant P01AI-97113 (to M.-L.A. and A.S.C.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910298116/-/DCSupplemental.
References
- 1.Jaigirdar S. A., MacLeod M. K., Development and function of protective and pathologic memory CD4 T cells. Front. Immunol. 6, 456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youngblood B., Hale J. S., Ahmed R., T-cell memory differentiation: Insights from transcriptional signatures and epigenetics. Immunology 139, 277–284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton B. M., Xu R., Wherry E. J., Porrett P. M., Pregnancy promotes tolerance to future offspring by programming selective dysfunction in long-lived maternal T cells. J. Leukoc. Biol. 101, 975–987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams A. B., et al. , Heterologous immunity provides a potent barrier to transplantation tolerance. J. Clin. Invest. 111, 1887–1895 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford M. L., Larsen C. P., Overcoming the memory barrier in tolerance induction: Molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr. Opin. Organ Transplant. 15, 405–410 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z., et al. , Homeostatic proliferation is a barrier to transplantation tolerance. Nat. Med. 10, 87–92 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouard S., et al. , The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am. J. Transplant. 12, 3296–3307 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Kawai T., et al. , Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am. J. Transplant. 14, 1599–1611 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J., et al. , Allograft rejection mediated by memory T cells is resistant to regulation. Proc. Natl. Acad. Sci. U.S.A. 104, 19954–19959 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes-Cerisuelo M., et al. , Increased pretransplant frequency of CD28+ CD4+ TEM predicts belatacept-resistant rejection in human renal transplant recipients. Am. J. Transplant. 17, 2350–2362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathews D. V., et al. , Belatacept-resistant rejection is associated with CD28+ memory CD8 T cells. Am. J. Transplant. 17, 2285–2299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benítez C., et al. , Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 58, 1824–1835 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Miller M. L., et al. , Spontaneous restoration of transplantation tolerance after acute rejection. Nat. Commun. 6, 7566 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller M. L., et al. , Tracking of TCR-transgenic T cells reveals that multiple mechanisms maintain cardiac transplant tolerance in mice. Am. J. Transplant. 16, 2854–2864 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., et al. , TLR signals promote IL-6/IL-17-dependent transplant rejection. J. Immunol. 182, 6217–6225 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T., et al. , Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J. Immunol. 180, 5991–5999 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T., et al. , Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am. J. Transplant. 10, 1524–1533 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quezada S. A., et al. , Mechanisms of donor-specific transfusion tolerance: Preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood 102, 1920–1926 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Quezada S. A., et al. , Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: The interplay of clonal anergy and immune regulation. J. Immunol. 175, 771–779 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Young J. S., Yin D., Vannier A. G. L., Alegre M. L., Chong A. S., Equal expansion of endogenous transplant-specific regulatory T cell and recruitment into the allograft during rejection and tolerance. Front. Immunol. 9, 1385 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochando J. C., et al. , Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7, 652–662 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Ferrer I. R., et al. , Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc. Natl. Acad. Sci. U.S.A. 108, 20701–20706 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chai J. G., et al. , Allospecific CD4(+) T cells retain effector function and are actively regulated by Treg cells in the context of transplantation tolerance. Eur. J. Immunol. 45, 2017–2027 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Szot G. L., et al. , Different mechanisms of cardiac allograft rejection in wildtype and CD28-deficient mice. Am. J. Transplant. 1, 38–46 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Miller M. L., et al. , Adoptive transfer of tracer-alloreactive CD4+ T cell receptor transgenic T cells alters the endogenous immune response to an allograft. Am. J. Transplant. 16, 2842–2853 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaech S. M., et al. , Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Wherry E. J., et al. , Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Wherry E. J., Kurachi M., Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odorizzi P. M., Pauken K. E., Paley M. A., Sharpe A., Wherry E. J., Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young J. S., et al. , Erosion of transplantation tolerance after infection. Am. J. Transplant. 17, 81–90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pauken K. E., et al. , Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins M. K., Schwartz R. H., Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 165, 302–319 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller S. N., Ahmed R., High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U.S.A. 106, 8623–8628 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller M. J., Zajac A. J., Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170, 477–486 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Zajac A. J., et al. , Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadzadeh M., et al. , Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angelosanto J. M., Blackburn S. D., Crawford A., Wherry E. J., Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 86, 8161–8170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schietinger A., Delrow J. J., Basom R. S., Blattman J. N., Greenberg P. D., Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science 335, 723–727 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J., et al. , Ablation of transcription factor IRF4 promotes transplant acceptance by driving allogenic CD4+ T cell dysfunction. Immunity 47, 1114–1128.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H., et al. , Ablation of interferon regulatory factor 4 in T cells induces “memory” of transplant tolerance that is irreversible by immune checkpoint blockade. Am. J. Transplant. 19, 884–893 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada Y., et al. , Repeated injections of IL-2 break renal allograft tolerance induced via mixed hematopoietic chimerism in monkeys. Am. J. Transplant. 15, 3055–3066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fishman J. A., Infection in solid-organ transplant recipients. N. Engl. J. Med. 357, 2601–2614 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Dangi A., Zhang L., Zhang X., Luo X., Murine CMV induces type 1 IFN that impairs differentiation of MDSCs critical for transplantation tolerance. Blood Adv. 2, 669–680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams M. A., et al. , Cutting edge: Persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J. Immunol. 169, 5387–5391 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Welsh R. M., et al. , Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J. Virol. 74, 2210–2218 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z., et al. , A clinically relevant murine model unmasks a “two-hit” mechanism for reactivation and dissemination of cytomegalovirus after kidney transplant. Am. J. Transplant. 19, 2421–2433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali J. M., et al. , Diversity of the CD4 T cell alloresponse: The short and the long of it. Cell Rep. 14, 1232–1245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarraj B., et al. , Impaired selectin-dependent leukocyte recruitment induces T-cell exhaustion and prevents chronic allograft vasculopathy and rejection. Proc. Natl. Acad. Sci. U.S.A. 111, 12145–12150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris H., et al. , Tracking donor-reactive T cells: Evidence for clonal deletion in tolerant kidney transplant patients. Sci. Transl. Med. 7, 272ra10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savage T. M., et al. , Early expansion of donor-specific Tregs in tolerant kidney transplant recipients. JCI Insight 3, 124086 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su C. A., Fairchild R. L., Memory T cells in transplantation. Curr. Transplant. Rep. 1, 137–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moon J. J., et al. , Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc. Natl. Acad. Sci. U.S.A. 108, 14602–14607 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corry R. J., Winn H. J., Russell P. S., Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 16, 343–350 (1973). [DOI] [PubMed] [Google Scholar]
- 54.Ertelt J. M., et al. , Selective priming and expansion of antigen-specific Foxp3−CD4+ T cells during Listeria monocytogenes infection. J. Immunol. 182, 3032–3038 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller M. L., et al. , Distinct graft-specific TCR avidity profiles during acute rejection and tolerance. Cell Rep. 24, 2112–2126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, associated protocols, and materials in the paper will be made available to readers upon request to the corresponding author.