Subsets of highly invasive, therapy-resistant tumor cells contribute to the development of metastasis and treatment failures. Recent evidence suggests that these tumor cell subsets are enriched for cancer stem cells (CSCs) (1–3). Similar to nonneoplastic stem cells, CSCs express specific markers and transcription factors and can self-renew or differentiate. For example, breast CSCs are identified as positive for CD44, ALDH1 activity, and/or expressing SOX2, OCT4, or Nanog (4). Compared to bulk tumor cells, CSCs are often more resistant to cell death, including that induced by chemo- or radiotherapy. Furthermore, CSCs are metabolically plastic with different redox states associated with epithelial- or mesenchymal-like breast CSCs (5). Hypoxia is an important inducer of CSC phenotypes, and hypoxia-inducible factor (HIF) 2α is known to mediate OCT4 up-regulation (6–9). Importantly, CSCs are enriched for the ability to propagate tumors in immunocompromised mice, accounting for their alternative designation as tumor-initiating cells. However, this name should not imply that the CSC/tumor-initiating cell is the cell of origin for the cancer. While it is true that stem cell acquisition of mutations can lead to tumorigenesis, the CSC hypothesis indicates the importance of targeting the existing population of cancer cells with stem cell-like characteristics in order to prevent disease recurrence. Thus, understanding how CSC function and survival are controlled is important. In PNAS, He et al. (10) extend previous findings (11, 12) and further establish a causal relationship between manganese superoxide dismutase (SOD2) overexpression and CSC formation, and provide a mechanism that explains this association involving acetylated SOD2, increased mitochondrial H2O2, and HIF2α expression.
Changes in metabolism, bioenergetics, and redox signaling are established hallmarks of cancer. Within this framework, the role of SOD2, which works in the mitochondria to catalyze the oxidation and reduction (dismutase activity) of 2 superoxide anion molecules to O2 and H2O2, respectively, is complex: Both tumor-suppressive and -promoting functions have been described (13). At physiologic levels, SOD2 is antitumorigenic; lower dismutase activity leads to stabilization of HIF1α and underlies cancer cell adaptation to hypoxia (14). However, SOD2 expression is elevated in many cancers, and sites of metastasis have higher SOD2 levels compared to primary tumors (15–18). If SOD2 is an antioxidant, why does its overexpression not confer greater protection, but instead result in a flipping of its function to a procancer role? Insights into this conundrum are provided by He et al. (10). They show that CSC gene expression signatures were greater in SOD2-overexpressing MCF7 cells (breast cancer-derived epithelial cells) compared to parental controls. SOD2-overexpressing cells were more mesenchymal-like and displayed increased growth and invasiveness in vitro, key functional end points supporting prometastatic and tumorigenic potential.
To determine how SOD2 overexpression could promote reprogramming toward a CSC-like state, He et al. (10) focus on HIF2α as a critical downstream mediator. HIF2α, but not HIF1α, protein was increased in SOD2-overexpressing MCF-7 cells. EPAS1 (HIF2α) messenger RNA (mRNA) was also increased, indicating that changes in EPAS1 transcription contribute to and/or are a product of SOD2-mediated CSC phenotypes. Targeting EPAS1 but not HIF1A in cells with SOD2 overexpression decreased expression of POU5F1 (Oct4) and Nanog transcripts, demonstrating the importance for HIF2α in breast CSC maintenance. As HIF2α is suggested to be stabilized at higher oxygen tensions than HIF1α (8, 19) and the majority of He et al.’s (10) experiments were performed in normoxia, the results suggest that a SOD2/HIF2α axis could increase stem cell/hypoxia signals even when oxygen tensions are high, as in a perivascular niche. It will be interesting to determine whether there are synergistic effects under hypoxia, particularly for well-established hypoxia-induced phenotypes such as invasion and stem cell maintenance.
Next, He et al. (10) address how SOD2 overexpression results in elevation of HIF2α in relation to SOD2 catalytic activity. A compelling set of data indicate that SOD2-dependent induction of the CSC-like phenotype is not related to superoxide dismutation per se but a peroxidase activity that is associated with SOD2 acetylation. The authors conclude that it is not the fact that SOD2 expression is higher in cancer that is important, but that acetylation of SOD2 is also elevated coincidentally with higher protein expression. Previous studies, by this group and others, demonstrate that SOD acetylation on lysine 68 (SOD2K68) results in a loss of dismutase activity and a gain of peroxidase activity (20–22). In enzymes, whose primary function is as a peroxidase, substrate H2O2 is reduced to water and coupled to the oxidation of a specific substrate. With SOD2, like other pseudoperoxidases, oxidation of nonspecific substrates may occur, and the result is oxidative damage. Indeed, the peroxidase activity of SOD2 has been shown to increase oxidative damage to mitochondria and sensitize cells to peroxide stress (23, 24). He et al. (10) demonstrate that scavenging of H2O2 prevented the increase in OCT4 and Nanog mRNA, as well as HIF2α and cancer stemness in SOD2-overexpressing cells. This sets up an interesting proposition whereby SOD2K68 uses H2O2 as a substrate for the peroxidase reaction, and somehow this leads to further H2O2 generation that selects for surviving CSC-like cells and/or is key for reprogramming. This model raises a number of questions and warrants consideration of redox signaling and oxidative stress paradigms. Is formation of H2O2 via SOD2-peroxidase activity a direct effect of peroxidase activity, or indirect? Presumably it is the latter and involves damage to endogenous mitochondrial components that then results in increased H2O2. How SOD2K68Ac-derived H2O2 activates HIF2α is unclear; is this selective for SOD2K68Ac-derived H2O2, or can other sources of H2O2 also mediate this response? Recent advances in redox signaling paradigms reveal key roles for relays mediated by protein–protein interactions whereby the initial oxidation occurs with high-reactive protein thiols (e.g., on peroxiredoxins), which then transmit the signal by a series of thiol–disulfide exchange processes with target proteins (25). Whether such redox relays play a role in modulating how H2O2 derived from the peroxidase activity of SOD2K68Ac activates HIF2α will be interesting to determine. If SOD2 acetylation loses the ability to make H2O2 (dismutation), from where does H2O2 for peroxidase activity originate? Is the latter derived from noncatalyzed superoxide dismutation, which occurs at an appreciable rate, and/or or are there different pools of SOD2, nonacetylated vs. acetylated, with the first providing substrate for the latter? The model proposed by He et al. (10) could involve H2O2-induced H2O2 formation, a feed-forward pathway for which there is precedent. For example, endothelial NOX4-derived H2O2 promotes subsequent NOX2-derived H2O2 in the mitochondria to regulate angiogenesis (26). Such data are leading to a deeper appreciation that mitochondria are hubs that integrate redox-signaling networks, via retrograde mechanisms, across the cell. Identification of SOD2K68Ac and its role in profoundly altering cell phenotype adds to a growing list of examples.
The conclusion that SOD2K68 links variations in SOD2 activity to SOD2 expression to HIF2α and a CSC phenotype was derived from 3 key complementary experiments. First, down-regulation of the mitochondrial deacetylase, Sirt3, increased SOD2 acetylation and the molecular signatures indicative of more cancer stemness. Critically, these responses were attenuated with concomitant SOD2 knockdown, strongly supporting SOD2 acetylation as the crucial step. Second, silencing the mitochondrial acetyl transferase, GCN5L1, to decrease acetylation, led to lower CSC markers. Finally, expression of an acetylation mimic or resistant mutant, SOD2K68Q and SOD2K68R, respectively, altered the CSC phenotype in a manner consistent with SOD2 acetylation leading to HIF2α expression. Underscoring the translational relevance of the proposed mechanism, key insights from in vitro cell function and phenotyping experiments were verified in mouse models and human tissue. Further studies evaluating how the balance between acetyltransferase and deacetylase activity is regulated by or in coordination with SOD2 expression are needed. Interestingly, Sirt3 silencing, while increasing acetylation, also changed SOD2 expression, suggesting a coordinated mechanism. Acetylation will also be modulated by metabolic status and supply of substrate acetyl CoA. Whether differential acetylation of SOD2 is a general mechanistic mediator linking altered metabolism and cancer also warrants further investigation. Notably, recent data suggest a similar paradigm whereby the function of key modulator of metabolism, AMPK, switches from suppressing to promoting tumor formation (27).
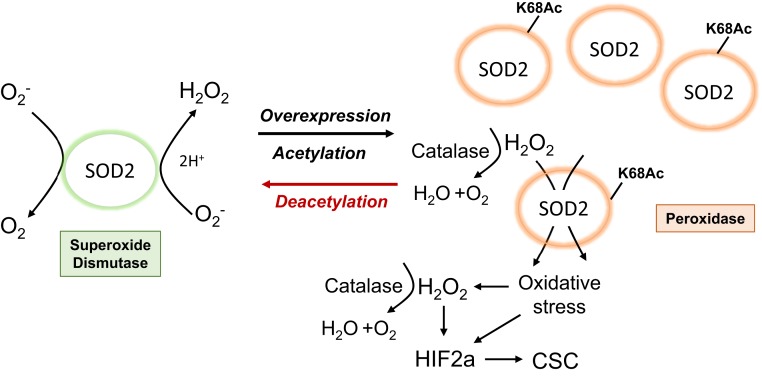
Taken together, He et al. (10) support a model whereby acetylation of SOD2 is the critical switch that converts SOD2 from an antioxidant (dismutase) to prooxidant (peroxidase) and pro-HIF2α mediator associated with increased CSC maintenance. This acetylation-dependent, “Jekyll–Hyde” function of SOD2 is illustrated in Fig. 1. Finally, it is important to note that other mechanisms may operate to mediate protumorigenic effects of SOD2 overexpression [e.g., proteotoxicity (28)] and other posttranslational modifications to SOD2 have been reported. SOD2 nitration in inflammatory states inactivates the enzyme (29). Given the role of inflammation in cancer, it would be interesting to determine how such SOD2 modifications lead to alterations in the balance of dismutase vs. peroxidase activities. In turn, it will be important to define how SOD2 modifications including SOD2K68Ac impact the immune system and whether they regulate CSC-mediated immune evasion and/or sensitivity to immunotherapy (30). Thus, many future directions remain to be explored to fully understand how SOD2 impacts tumor growth and maintenance.
Fig. 1.
Acetylated (Jekyll) and deacetylated (Hyde) SOD2 and cancer stem cell formation. Acetylated SOD2 loses dismutase activity and acquires peroxidase activity that promotes an H2O2-dependent feed-forward mechanism for generated further H2O2, activating HIF2α and cancer stem cell formation.
Acknowledgments
A.B.H. acknowledges support from NIH grant R01 NS104339.
Footnotes
The authors declare no competing interest.
See companion article on page 23534.
References
- 1.Batlle E., Clevers H., Cancer stem cells revisited. Nat. Med. 23, 1124–1134 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Meacham C. E., Morrison S. J., Tumour heterogeneity and cancer cell plasticity. Nature 501, 328–337 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valent P., et al. , Cancer stem cell definitions and terminology: The devil is in the details. Nat. Rev. Cancer 12, 767–775 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Tang B., et al. , A flexible reporter system for direct observation and isolation of cancer stem cells. Stem Cell Reports 4, 155–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo M., et al. , Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab. 28, 69–86.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forristal C. E., Wright K. L., Hanley N. A., Oreffo R. O., Houghton F. D., Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 139, 85–97 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson E., et al. , CD44 interacts with HIF-2α to modulate the hypoxic phenotype of perinecrotic and perivascular glioma cells. Cell Reports 20, 1641–1653 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Li Z., et al. , Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15, 501–513 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Y., et al. , HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J. Exp. Clin. Cancer Res. 37, 256 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He C., et al. , SOD2 acetylation on lysine 68 promotes stem cell reprogramming in breast cancer. Proc. Natl. Acad. Sci. U.S.A. 116, 23534–23541 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z., et al. , SOD2 is a C-myc target gene that promotes the migration and invasion of tongue squamous cell carcinoma involving cancer stem-like cells. Int. J. Biochem. Cell Biol. 60, 139–146 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Bamodu O. A., et al. , 4-Acetyl-antroquinonol B suppresses SOD2-enhanced cancer stem cell-like phenotypes and chemoresistance of colorectal cancer cells by inducing hsa-miR-324 re-expression. Cancers (Basel) 10, E269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayo J. C., Sainz R. M., Quiros-Gonzalez I. (2018) MnSOD/SOD2 in cancer: The story of a double agent. Reactive Oxygen Species 5, 86–106. [Google Scholar]
- 14.Kaewpila S., Venkataraman S., Buettner G. R., Oberley L. W., Manganese superoxide dismutase modulates hypoxia-inducible factor-1 alpha induction via superoxide. Cancer Res. 68, 2781–2788 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamarajugadda S., et al. , Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 4, e504 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemachandra L. P., et al. , Mitochondrial superoxide dismutase has a protumorigenic role in ovarian clear cell carcinoma. Cancer Res. 75, 4973–4984 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye H., et al. , Proteomic based identification of manganese superoxide dismutase 2 (SOD2) as a metastasis marker for oral squamous cell carcinoma. Cancer Genomics Proteomics 5, 85–94 (2008). [PMC free article] [PubMed] [Google Scholar]
- 18.Hart P. C., et al. , MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 6, 6053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmquist-Mengelbier L., et al. , Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 10, 413–423 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Ganini D., Santos J. H., Bonini M. G., Mason R. P., Switch of mitochondrial superoxide dismutase into a prooxidant peroxidase in manganese-deficient cells and mice. Cell Chem. Biol. 25, 413–425.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y., et al. , Lysine 68 acetylation directs MnSOD as a tetrameric detoxification complex versus a monomeric tumor promoter. Nat. Commun. 10, 2399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y., et al. , Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 12, 534–541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansenberger-Fricano K., et al. , The peroxidase activity of mitochondrial superoxide dismutase. Free Radic. Biol. Med. 54, 116–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weydert C. J., et al. , Increased oxidative stress created by adenoviral MnSOD or CuZnSOD plus BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea) inhibits breast cancer cell growth. Free Radic. Biol. Med. 44, 856–867 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stöcker S., Van Laer K., Mijuskovic A., Dick T. P., The conundrum of hydrogen peroxide signaling and the emerging role of peroxiredoxins as redox relay hubs. Antioxid. Redox Signal. 28, 558–573 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Kim Y. M., et al. , ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am. J. Physiol. Cell Physiol. 312, C749–C764 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vara-Ciruelos D., Russell F. M., Hardie D. G., The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? Open Biol. 9, 190099 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Chen C. L., Kang P. T., Jin Z., Chen Y. R., Differential protein acetylation assists import of excess SOD2 into mitochondria and mediates SOD2 aggregation associated with cardiac hypertrophy in the murine SOD2-tg heart. Free Radic. Biol. Med. 108, 595–609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y. S., Gupta Vallur P., Phaëton R., Mythreye K., Hempel N., Insights into the dichotomous regulation of SOD2 in cancer. Antioxidants 6, E86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H., et al. , HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc. Natl. Acad. Sci. U.S.A. 112, E6215–E6223 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]