Significance
Identification of enhancers responsible for tissue-specific expression is essential for an in-depth understanding of developmental gene regulation and of the etiology of congenital malformation caused by defective gene function. Shh is indispensable for forebrain and hypothalamic development, and its deletion causes severe craniofacial defects observed in human holoprosencephaly (HPE). SHH signaling starts with Shh expression in the prechordal plate (PrCP), and this SHH then induces SHH signaling in the forebrain. Despite its importance, no enhancer responsible for Shh expression in the PrCP has yet been identified. We report here a brain enhancer, named SBE7. Mice lacking SBE7 lost Shh expression in both the PrCP and the ventral midline of the forebrain, exhibiting developmental defects similar to HPE.
Keywords: Shh, enhancer, prechordal plate, HPE, SOD
Abstract
Sonic hedgehog (SHH) signaling plays a pivotal role in 2 different phases during brain development. Early SHH signaling derived from the prechordal plate (PrCP) triggers secondary Shh induction in the forebrain, which overlies the PrCP, and the induced SHH signaling, in turn, directs late neuronal differentiation of the forebrain. Consequently, Shh regulation in the PrCP is crucial for initiation of forebrain development. However, no enhancer that regulates prechordal Shh expression has yet been found. Here, we identified a prechordal enhancer, named SBE7, in the vicinity of a cluster of known forebrain enhancers for Shh. This enhancer also directs Shh expression in the ventral midline of the forebrain, which receives the prechordal SHH signal. Thus, the identified enhancer acts not only for the initiation of Shh regulation in the PrCP but also for subsequent Shh induction in the forebrain. Indeed, removal of the enhancer from the mouse genome markedly down-regulated the expression of Shh in the rostral domains of the axial mesoderm and in the ventral midline of the forebrain and hypothalamus in the mouse embryo, and caused a craniofacial abnormality similar to human holoprosencephaly (HPE). These findings demonstrate that SHH signaling mediated by the newly identified enhancer is essential for development and growth of the ventral midline of the forebrain and hypothalamus. Understanding of the Shh regulation governed by this prechordal and brain enhancer provides an insight into the mechanism underlying craniofacial morphogenesis and the etiology of HPE.
An early event of organization of the vertebrate central nervous system is the inductive action of the axial mesoderm on differentiation of the neural ectoderm (1, 2). An anterior part of the axial mesoderm referred to as the prechordal plate (PrCP) is crucial for formation of the forebrain (3–5), which consists of 2 subdivisions, the telencephalon and diencephalon. Sonic hedgehog (SHH) is a major signaling molecule that promotes regionalization of the embryonic brain along the anteroposterior axis (6–8) as well as the dorsoventral axis (9–12). Shh is expressed throughout the axial mesoderm, including the PrCP and the notochord. Surgical removal of the PrCP from chick, mouse, and amphibian embryos revealed that prechordal Shh expression is necessary for differentiation and growth of the forebrain, suggesting that the PrCP is an early organizing center for brain development (4, 13–15). SHH protein produced in the PrCP is secreted dorsally to induce Shh expression in the ventral midline of the forebrain (6). Transition of the signal from the prechordal SHH to the neuronal secondary source of SHH is an essential event in the cascade of brain formation (6, 13).
Six brain enhancers for Shh, named SBE1 to SBE6, have been identified in the genomic region spanning the Shh and Lmbr1 coding sequences (7, 16–19). Two of these, SBE1 and SBE5, located in an intron of Shh and Lmbr1, direct Shh expression in the ventral midline of the posterior forebrain and midbrain, respectively (18, 20). A screen for enhancers upstream of the Shh coding sequence uncovered a cluster of forebrain enhancers, SBE2, SBE3, and SBE4. When a transgenic LacZ reporter is flanked by SBE2 and SBE3, the enhancers drive reporter expression in the anterior diencephalon and the anterior portion of the telencephalon, respectively, while SBE4 drives the transgenic reporter expression in both diencephalon and telencephalon (17). These nested expressions driven by the 3 forebrain enhancers recapitulate the endogenous expression of Shh in the forebrain (17). Although the enhancers that direct neuronal Shh expression in telencephalon and diencephalon have been identified, and some of the upstream transcription factors (TFs) for these enhancers have been elucidated (21, 22), the entire spatiotemporal regulation of Shh is not yet fully understood. In particular, enhancer(s) that regulate Shh expression in the axial mesoderm including the PrCP remain to be elucidated. Recent genome-wide screenings around the Shh locus suggested the presence of 4 notochord enhancers in the vicinity of the known forebrain enhancers and in more-upstream regions of the Shh locus (23).
In the present study, we identified a forebrain enhancer in the vicinity of the forebrain enhancer cluster, and named it SBE7. It directs Shh expression not only in the ventral midline of the forebrain but also in the PrCP. When SBE7 was eliminated from the mouse genome, Shh expression in both the anterior forebrain and the PrCP was significantly reduced. As a consequence, the mutant embryos exhibited severe malformation of diencephalon-derived brain structures such as the hypothalamus and the midline structure of the forebrain, which are similar to a mild form of human holoprosencephaly (HPE) (24). Our results showed that the identified enhancer is essential to induce Shh expression in the PrCP, and, in turn, to induce secondary Shh expression in the rostral and ventral midline of the forebrain overlying the PrCP, and that SBE7-mediated SHH signaling from the PrCP is crucial for development of the rostral and midline structures of the forebrain and hypothalamus.
Results
A Brain Enhancer in the Shh Regulatory Region.
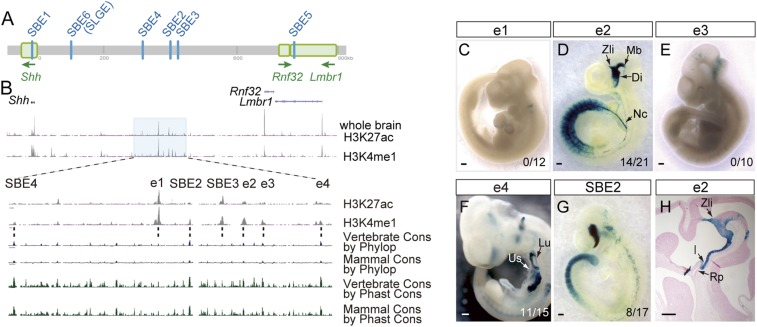
The University of California, Santa Cruz (UCSC) public database provides epigenetic information on active enhancer markers (http://genome.ucsc.edu/) (25, 26). Chromatin immunoprecipitation sequencing (ChIP-seq) data for the mouse embryonic brain at E14.5 using histone H3K4me1 and H3K27ac antibodies revealed crowded signal peaks in a region between the Rnf32 and Shh coding sequences (Fig. 1A); this region overlaps a forebrain enhancer cluster of Shh (Fig. 1 A and B). Three known forebrain enhancers, SBE2, SBE3, and SBE4, which are known to act in the early brain around E10.5 (17), still keep active marks even in the later brain. In addition to these known forebrain enhancers, some regions carrying active chromatin marks were observed within the cluster. Their sequences also showed evolutionary conservation among vertebrates (Fig. 1B). We cloned regions with the 4 highest signal peaks of the histone mark H3K4me1 at E14.5, and evaluated regulatory activity of these sequences in transgenic mice carrying a LacZ reporter flanked by each of the 4 regions. Reporter expression was not specifically observed in the E10.5 brain of transgenic mice with regions e1, e3, or e4 (Fig. 1 C, E, and F), although e4 drove reporter expression in the lung and surrounding epithelial tissues (Fig. 1F and SI Appendix, Fig. S1 A–H). When we generated the e4 deletion mutant using the CRISPR-Cas9 system (SI Appendix, Fig. S1 I–L), homozygotes of the e4 deletion mutant were viable and did not show any significant outward signs of morphological defects.
Fig. 1.
Identification of a forebrain enhancer. (A) Genomic locations of the 6 known forebrain enhancers in the Shh regulatory region. SBE1 and SBE5 are intronic enhancers in the Shh and Lmbr1 genes, respectively. The other 4 enhancers are located in the intergenic region. (B) ChIP-seq data on the embryonic brain at E14.5 with anti-H3K27ac and anti-H3K4me1 antibodies uploaded in the UCSC genome browser. Several signal peaks were found upstream of the Shh locus. (Lower) 6 plots showing an enlarged view of the cluster harboring 3 forebrain enhancers, shaded in 2 first (Upper) plots. (Bottom) 4 plots showing phylogenetically conserved regions detected by Phylop and PhastCons. (C–G) Transgenic reporter expression driven by e1, e2, e3, e4, and SBE2 genome fragments at E10.5. The number of LacZ-positive embryos among the total number of embryos carrying a transgene is indicated (Bottom Right). (H) A sagittal section of the e2 transgenic brain. Di, diencephalon; Mb, midbrain; Nc, notochord; Lu, lung; Us, upper stomach; Rp, Rathke’s pouch; I, infundibulum. (Scale bar, 0.5 mm.)
In contrast, e2 drove strong transgenic reporter expression on the ventral side of the embryonic brain at E10.5 (Fig. 1D). The regulatory activity of e2 overlaps equally with that of SBE2 in the rostral diencephalon and with those of SBE1 and SBE5 in the caudal diencephalon and midbrain (Fig. 1G) (17, 18). In sections of the forebrain, the e2-driven expression was observed in the ventral midline immediately caudal to the infundibulum (Fig. 1H). Although the expression of endogenous Shh and the reporter expression driven by SBE2 are known to form bilateral stripes adjacent to the ventral midline of diencephalon (17), the e2-driven expression remained at the ventral midline and did not fully recapitulate the endogenous Shh expression (SI Appendix, Fig. S2). The LacZ reporter signal of e2 was also observed in the axial mesoderm, and the tail mesoderm where Shh is not normally expressed.
Thus, it appeared that the e2 region residing in the forebrain enhancer cluster contains enhancer activity for the axial mesoderm as well as for the ventral forebrain. We named e2 Shh brain enhancer 7 (SBE7). Since the active histone mark profile was obtained from the whole brain of later-stage embryos at E14.5, genomic regions within the forebrain enhancer cluster may have a potential to regulate the expression of Shh at E10.5.
SBE7 Drives Reporter Expression in the Axial Mesoderm Including the Prechordal Plate.
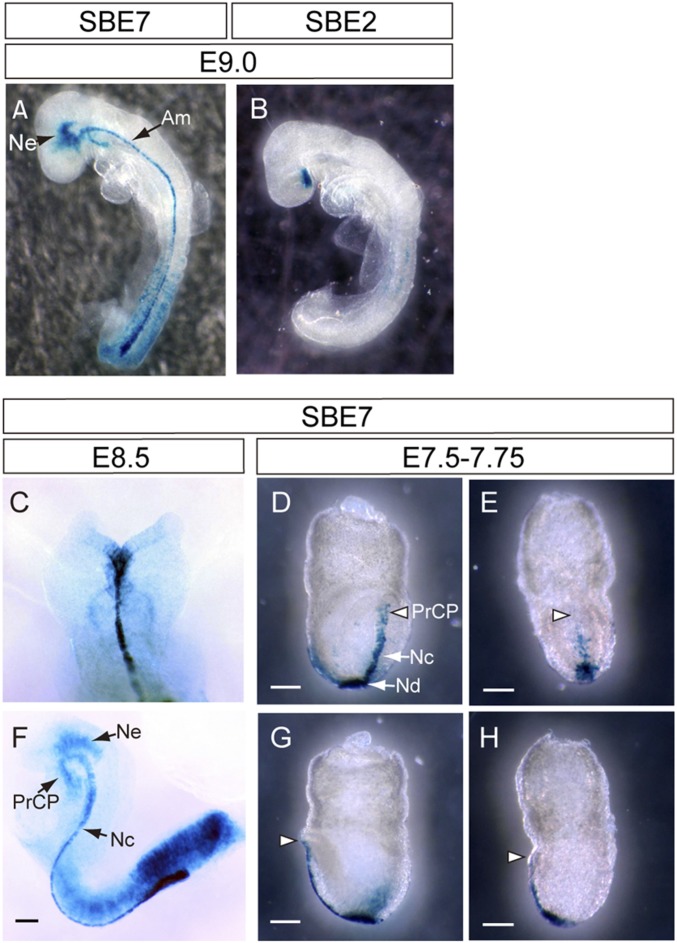
Shh expression in the PrCP is essential to induce Shh in the ventral midline of the forebrain (4, 6, 13). To determine whether SBE7 acts to initiate Shh expression in the forebrain, we tested its regulatory activity at different embryonic stages in comparison with that of SBE2. At E9.0, SBE7 drove reporter expression in the notochord, PrCP, and ventral diencephalon, whereas SBE2 did not drive expression in the axial mesoderm (Fig. 2 A and B). Regulatory activity of SBE7 was observed in both axial mesoderm and diencephalon at E8.5 (Fig. 2 C and F). In contrast, no reproducible expression of LacZ was observed in the SBE2-LacZ transgenic mouse at the same stage, suggesting that SBE2 begins to direct endogenous Shh expression after E8.5 (SI Appendix, Fig. S3). Given that SBE7 drives reporter expression at early stages in the mouse embryo, we investigated expression at earlier embryonic stages. From the early bud stage (E7.5) to the late head fold stage (E7.75), reporter expression driven by SBE7 was observed in the caudal axial mesoderm and node (Fig. 2 D–H). Moreover, 2 of the LacZ-positive embryos showed the prechordal expression of LacZ. The SBE7 regulatory activity recapitulated the endogenous expression at early stages (27).
Fig. 2.
Regulatory activity mediated by SBE7. (A and B) Comparison of reporter expression driven by SBE7 (n = 10/13) and SBE2 at E9.0 (n = 4/6). (C–H) LacZ reporter expression driven by SBE7 at early embryonic stages. (C) Dorsal and (F) lateral views of SBE7-driven reporter expression at E8.5 (n = 3/6). (D and E) Frontal and (G and H) lateral views of reporter expression driven by SBE7 from E7.5 (early bud stage) to E7.75 (late head fold stage). Five out of 11 embryos show a detectable reporter expression. Two show LacZ expression in the PrCP (D and G; open arrowhead) and the other 3 do not (E and H; open arrowhead). Am, axial mesoderm; Ne, neuroepithelium; Nd, node. (Scale bars, 0.2 mm.)
SBE7 Is Essential for Shh Expression in the Prechordal Plate and Ventral Midline of the Forebrain.
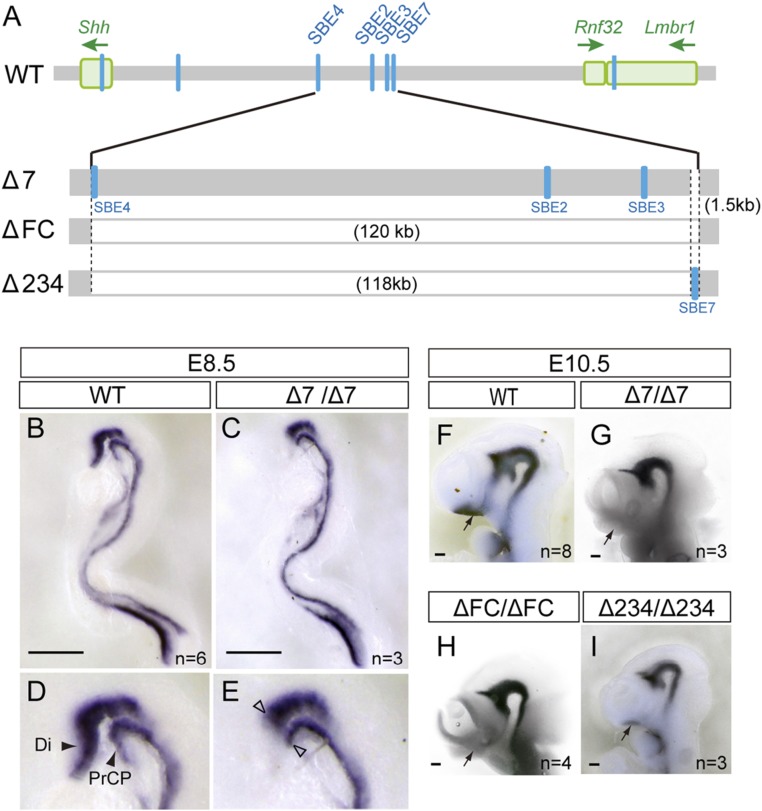
To directly examine the role of SBE7 in Shh regulation and forebrain development, we first eliminated SBE7 alone from the mouse genome using the CRISPR-Cas9 system (Fig. 3A and SI Appendix, Fig. S4A), and established 2 SBE7 deletion mouse lines (Δ7). Endogenous Shh expression was detected in both the PrCP and the ventral midline of the forebrain of wild-type embryos at E8.5 (Fig. 3 B and D). In the Δ7 homozygote, Shh expression clearly diminished in the PrCP and the overlying neuroepithelium, suggesting that SBE7 is essential as a prechordal enhancer for Shh expression (Fig. 3 C and E). To elucidate the demarcation of Shh regulation between SBE7 and other forebrain enhancers including SBE2, we further generated 2 mouse lines with deletions in the genomic region around the forebrain enhancer cluster. One line, named ΔFC, had a 120-kb deletion including all of the forebrain enhancers, and the other, named Δ234, had a 118-kb deletion encompassing SBE2, SBE3, and SBE4, but retained SBE7 (Fig. 3A and SI Appendix, Fig. S4 B and C). The Δ7 and ΔFC homozygous embryos showed abrogation of Shh expression in the telencephalon and the rostral diencephalon (Fig. 3 G and H). These 2 deletion mutant lines retained endogenous Shh expression in the ventral midbrain and the caudal diencephalon including the zona limitans intrathalamica (ZLI), although SBE7 can drive reporter expression in these regions. Although Δ234 embryos also showed a down-regulation of Shh in the telencephalon, a detectable level of the Shh expression was shown in the rostral diencephalon (Fig. 3I and SI Appendix, Fig. S5 B and D).
Fig. 3.
Mutant genomes with 3 types of deletion around the forebrain enhancer cluster. (A) Diagrams of the mutant genomes with deletion of 1.5 kb encompassing SBE7 (Δ7), a 120-kb region harboring the whole forebrain enhancer cluster (ΔFC), and a 118-kb region excluding SBE7 from the 120-kb region (Δ234). (B–E) Whole-mount in situ hybridization with an Shh riboprobe in the (B and D) wild-type (WT) and (C and E) Δ7 homozygous embryo at E8.5. D and E are enlargements of B and C, respectively. Filled arrowheads in D indicate Shh expression in the rostral tip of the Di and PrCP. Open arrowheads in E depict boundaries of loss of Shh expression in the rostral tip of Di and also PrCP. (F–I) Shh expression in a lateral view of the E10.5 wild type and homozygotes of the 3 types of deletion. Arrow indicates the diencephalic region where Shh is normally expressed. (Scale bars, 0.5 mm.)
Elimination of SBE7 Causes Severe Morphological Defects in the Ventral Midline of the Forebrain.
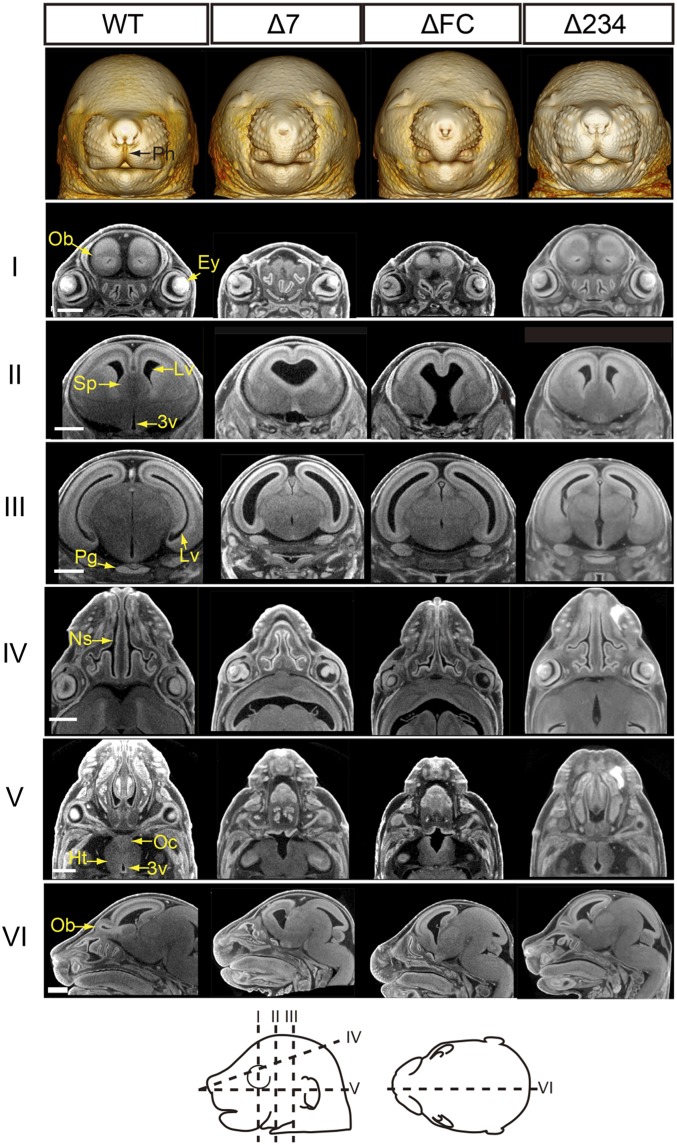
Homozygotes of Δ7 died within 2 d after birth (SI Appendix, Table S1), whereas heterozygotes of Δ7 were viable and did not show any outward abnormalities. To establish whether down-regulation of Shh in the forebrain impacts normal brain formation, we performed a 3D morphological analysis of E16.5 mouse brains using X-ray microcomputed tomography (micro-CT). A 3D reconstruction of micro-CT images revealed that homozygotes of Δ7 and ΔFC showed facial hypoplasia with reduced binocular distance, nostril obstruction, loss of philtrum, and cleft lip (Fig. 4 and SI Appendix, Fig. S6). These phenotypes are typical midline defects observed in HPE. In contrast, facial structures of the Δ234 mouse were virtually normal at E16.5.
Fig. 4.
Micro-CT images of mutant embryos with deleted forebrain enhancers. A facial view of 3D reconstruction of micro-CT images of E16.5 embryos (Top). Virtual sections of 3D reconstructed heads are aligned in rows with Roman numerals to the left. Positions of vertical and horizontal sections are illustrated by dotted lines with corresponding Roman numerals in the diagrams (Bottom); 3v, third ventricle; Ey, eye; Ht, hypothalamus; Lv, lateral ventricle; Ns, nasal septum; Ob, olfactory bulb; Oc, optic chiasma; Pg, pituitary gland; Ph, philtrum; Sp, septum pellucidum. (Scale bars, 1.0 mm.)
Virtual sections of the CT images revealed that brains of the Δ7 and ΔFC homozygotes had expanded third and lateral ventricles, with deformation of the septum and the midline of the hypothalamus (Fig. 4 and SI Appendix, Fig. S7 A and B). Furthermore, we observed absence or hypoplasia of the olfactory bulb and the pituitary gland in both mutants. In facial organs, the Δ7 and ΔFC homozygous embryos showed a thin nasal septum, a short nasal cavity, and abnormally shaped eyes with absence of the optic chiasma. These phenotypes were variable in the Δ7 homozygous embryo (SI Appendix, Table S2). Three out of 8 Δ7 homozygous embryos showed the severe phenotype, nearly equivalent to that of the ΔFC homozygous embryo. The remaining 5 showed no abnormality in the brain septum, the lateral ventricles, the nasal septum, or the olfactory bulb (SI Appendix, Table S2). In contrast, Δ234 homozygous embryos showed little abnormality in the brain and facial structures, but their third ventricle was slightly expanded (Fig. 4 and SI Appendix, Fig. S7C and Table S2).
Since the Δ234 homozygote was viable after birth and showed far less severe abnormality in craniofacial development at E16.5, we further analyzed the phenotype of adult Δ234 mice (n = 3) (SI Appendix, Fig. S8). The Δ234 homozygote had a significantly smaller body size than the heterozygote and wild-type mouse at 1 mo of age (SI Appendix, Fig. S8A). Micro-CT analysis revealed that the Δ234 homozygote has a hypoplastic pituitary gland and a bent nasal septum (SI Appendix, Fig. S8 B and C), but no visible defects in the septum, olfactory bulb, eyes, or optic chiasma (SI Appendix, Fig. S8 B and C). Therefore, one or a combination of the 3 enhancers, SBE2, SBE3, and SBE4 (or perhaps some unknown regulatory element residing within the 118-kb deletion), probably provides the Shh regulation required for pituitary and nasal development.
SIX3 Is Not Required for SBE7-Mediated Shh Regulation in the PrCP or the Hypothalamus.
It is known from previous studies that SIX3, SOX2, and SOX3 are upstream TFs of the Shh expression through SBE2 binding (21, 22). Inactivation of Six3 results in a failure to activate Shh expression in the ventral forebrain, and consequently causes HPE (28). To test the possibility that SIX3 and SOX2 and SOX3 also regulates SBE7 activity, we first searched for potential TF-binding sites in the SBE7 sequence by using the JASPAR database (29). We could not find typical SIX3-binding sites but found 3 potential sites for SIX1 and SIX2 and one binding site for SOX2 and SOX3 (SI Appendix, Fig. S9A). We introduced a dysfunctional mutation into all 3 potential SIX-binding sites or the SOX2 and SOX3 binding site, respectively. Transgenic reporter assays revealed that the SBE7-driven reporter expression was not affected by mutations in these binding sites (SI Appendix, Fig. S9 A and B). In addition, the Six3 expression level was unchanged in the Δ7 homozygote embryo (SI Appendix, Fig. S9C). SBE7 is unlikely regulated by direct binding of SIX3, and thereby may not be involved in the feedback regulation between Shh and Six3 in the forebrain (SI Appendix, Fig. S10). To functionally dissect SBE7, we generated 2 deletion constructs of SBE7 and carried out transgenic reporter assay (SI Appendix, Fig. S9A). A 514-bp SBE7 construct drove reporter expression similar to that driven by the full-length SBE7 (SI Appendix, Fig. S9B), whereas a 486-bp SBE7 construct did not show specific expression in the forebrain (SI Appendix, Fig. S9B). The result suggested that a 190-bp fragment at the right side of the 514-bp SBE7 construct, which contains an evolutionary conserved sequence block across mammals (SI Appendix, Fig. S9A), is required for regulatory activity in the forebrain. To understand interactions between SBE7 and HPE- and SOD-causative genes other than Six3, Sox2, and Sox3, we sought potential TF-binding sites for ZIC2, TGIF, HESX1, and OTX2 within the 190 bp. Although a binding site for HESX1 was found in the 190 bp, abolishment of the binding site by introducing a mutation did not influence the reporter expression in transgenic mice (SI Appendix, Fig. S9 A and B).
Discussion
SBE7 Is an Enhancer That Regulates Shh Expression in the Diencephalon, Mesencephalon, and Prechordal Plate.
SBE7 is located within the forebrain enhancer cluster, and turns out to possess a broad regulatory activity from the diencephalon to mesencephalon (SI Appendix, Fig. S11). We note that SBE7 directs Shh expression in the PrCP, which is the most anterior tip of the axial mesoderm, as well as in the diencephalon. A deletion of SBE7 strikingly diminished Shh expression in the PrCP, but not in the notochord; this indicates that SBE7 is indispensable for Shh regulation in the PrCP, and that there is another enhancer for Shh expression in the notochord. SHH protein produced in the PrCP is secreted dorsally and induces an Shh-expressing cell population at the rostral and ventral midline of the neuroepithelium (6, 13, 30). This induced cell population acts as the secondary signaling center for forebrain organization. Indeed, the single deletion of SBE7 resulted in abrogation of Shh expression in the midline of the diencephalon overlying the PrCP, leading to marked morphological defects in the ventral midline of the forebrain, and eventually developmental anomalies of the hypothalamus.
In contrast to the phenotype of SBE7 deficiency, the brain of Δ234 exhibited far less severe morphological anomalies (Fig. 4). Likewise, it is known that a mutant with a deletion of SBE1, which directs Shh expression in the mesencephalon and in the posterior diencephalon including ZLI, shows no obvious defects in brain formation, although Shh was down-regulated in a part of the mesencephalon and diencephalon (20). Loss of SBE1 may be compensated by one or more redundant enhancers including SBE5 (18). Taking these observations together, it appears that the forebrain enhancers including SBE7 have redundant Shh-regulating activity. Notably, SBE7 is necessary for the normal expression of Shh in the ventral diencephalon. It is possible that the SHH protein produced by SBE7 in the PrCP, the first signaling center, is indispensable for induction of Shh in the forebrain. Alternatively, SBE7 may also play a pivotal role in induction of Shh in the forebrain cooperating with SBE2. In the present study, we could not dissect the enhancer function of SBE7 into the prechordal- and diencephalic-specific activity. The necessity of diencephalic function of SBE7 for the normal brain development is still unclear.
SBE7 Deficiency Models Semilobar-Type HPE.
Abrogation of Shh expression in the PrCP and the overlying diencephalon caused morphological defects in the midline brain and facial structures, including the hypothalamus, septum, optic chiasma, pituitary, olfactory bulb, and nasal septum (Fig. 4 and SI Appendix, Fig. S6), which are also observed in HPE (3, 13) and partially in septooptic dysplasia (SOD) (31). HPE is a midline defect with incomplete separation of the brain hemispheres, and is classified into alobar, semilobar, and lobar types according to the degree of severity. In the alobar type, there is no brain division at all, with one eye, a tubular nose, and cleft lip. The most serious symptom is known as cyclopia (3, 4). Many genetic factors are involved in the onset of HPE. Mutations in SHH, ZIC2, TGIF, and SIX3 are listed as genetic causalities of HPE (32–36). It is well known that Six3 regulates Shh expression through its direct binding to one of the forebrain enhancers, SBE2 (21). The common features of SOD are abnormal structures along the midline of the brain, optic nerve, pituitary, septum pellucidum, and corpus callosum (31, 37). Mutations of HESX1, OTX2, SOX2, and SOX3 are known to cause SOD (38). Mouse mutants generated by conditional gene targeting with Cre drivers of Foxb1 (39), Nkx2.1 (40), and SBE2 (22) displayed abrogated Shh expression specifically in the ventral midline of the rostral diencephalon, including the hypothalamus primordium, while leaving Shh expression in the PrCP intact (41, 42). All of these mutants showed defects in growth of the hypothalamus and in specification of the basal portion of the forebrain (43). Notably, the mutant mouse lacking Shh expression by SBE2-mediated Cre recombination is reported as a model of SOD, in which Shh regulation by the SoxB1 family members Sox2 and Sox3 is impaired specifically in the ventral diencephalon (22). The homozygous SBE7 deletion mutant shows cleft lip, orbital hypotelorism (narrow space between the eyes), and no philtrum, in addition to defects in the pituitary, optic nerve, and midline forebrain that are commonly found in SOD. It is most likely that Shh down-regulation in the ventral midline of the forebrain is at least one of the common etiologies of HPE and SOD. Considering the broader spectrum of the SOD model and SBE7-deficient mice, the phenotypic difference between HPE and SOD may be due to a spatiotemporal difference in Shh down-regulation: Early and prechordal down-regulation is responsible for the incomplete separation of the 2 cerebral hemispheres in HPE, and late and diencephalic down-regulation is responsible for the hypothalamic and midline forebrain defects in SOD. Taking all of these observations into account, it is appropriate to classify the phenotype observed in the SBE7-deficient mutant as semilobar-type HPE (24, 44). It will be intriguing to test whether familial semilobar-type HPE patients have mutations in their SBE7 sequences.
Homozygotes of Δ7 lost Shh expression in the PrCP and the overlying diencephalon (Fig. 3), and Δ7 and ΔFC homozygotes develop semilobar-type HPE, but not alobar-type HPE (Fig. 4). This result is not consistent with a previous report that about half of the mice whose PrCP was surgically removed developed alobar-type HPE (13). Large phenotypic variability is a marked feature of HPE and mouse models of HPE. Multiple genetic factors are involved in HPE pathogenesis, and different genetic backgrounds in human patients and mouse mutant lines may explain the penetrance of HPE and its phenotypic variability (24, 35). Several Six3 conditional mouse mutants exhibited semilobar-type HPE in the genetic background of C57BL/6 strain, but a far more severe form of HPE was observed in embryos with mixed genetic backgrounds (28). Given that the genetic background of Δ7 and ΔFC is C57BL/6, even more severe phenotypes may be seen when the deletion is placed in a mixed genetic background. Alternatively, the PrCP may provide a signaling molecule other than SHH that is required for normal forebrain and facial development. For instance, BMP7 secreted from the PrCP is involved in positioning of the hypothalamus along the anteroposterior axis in the diencephalon, in combination with SHH signaling (6, 45). Therefore, it is possible that another unknown factor(s) is responsible for proper development of the ventral midline of the rostral diencephalon. Notably, the Δ7 homozygote retains Shh expression in the notochord, suggesting at least one other enhancer to regulate the Shh expression in the axial mesoderm posterior to the PrCP. Thus, we cannot rule out the possibility that an unknown enhancer has activity to regulate the Shh expression at the anterior or posterior tip of the PrCP, which is necessary for normal development of the ventral midline of the rostral diencephalon.
Context-Dependent Regulation of Shh by Brain Enhancers.
Six3 knockout mice show down-regulation of Shh in the ventral diencephalon and human HPE-like defects in the brain (21, 28). Considering that SHH signaling is necessary for the maintenance of Six3 expression, there is a positive feedback loop between Shh and Six3 that is crucial for normal development of the hypothalamus (28). Shh expression in the diencephalon is down-regulated in a compound mutant of Sox2 and Sox3 (22), whereas the Shh expression is up-regulated by deficiency of Tbx3, which is a negative regulator for Sox2 (46). A ChIP study revealed that SOX2 binds to a Six3 forebrain enhancer located upstream of the Six3 locus (47). Furthermore, compound knockout of Sox2 and Sox3 markedly down-regulated Six3 expression in the diencephalon (47). These findings suggest that SOX2 and SOX3 are TFs to link the feedback loop between Shh and Six3. Since SIX3 and SOX2 bind directly to SBE2 (21, 22), SBE2 is a key mediator of Shh regulation by SIX3 and SOXs in the diencephalon. During brain development, the expression domain of Shh at the midline of ventral brain becomes bilateral at E10.5 onward (17). T-box TFs are involved in lateralization of the medial Shh expression through direct binding to SFPE2, which is an intronic brain enhancer of Shh, or are involved in blocking SOX2 activity to suppress the Shh expression at the ventral midline (46, 48). In this study, we did not find any evidence that SIX3 or SOX proteins act as TFs in the regulation of Shh through SBE7 in forebrain development. In fact, SBE7 itself did not reproduce the lateralized expression of Shh, probably due to the absence of functional SOX2 binding sites within the sequence. Therefore, our results indicate that Shh expression in the forebrain is regulated in 2 distinct contexts, one mediated by SBE2 and SFPE2 and the other by SBE7. SBE7 mainly contributes to the early prechordal expression of Shh and the subsequent induction of neural Shh at the midline of forebrain. SBE2 plays a central role in the diencephalic expression of Shh, especially in the mediolateral transition of Shh to form bilateral stripes at later stages, which is necessary for the growth and differentiation of bilateral hypothalamic areas (17, 21, 22, 46). The 2 modes of enhancer function may act in a coordinated fashion for regulatory dynamics of Shh in the mouse forebrain.
Materials and Methods
The sequences of SBE7 and e4 were ascribed to DNA Data Bank of Japan with Accession Numbers LC461025 and LC461026, respectively. An extended description of the materials and methods is presented in SI Appendix, SI Materials and Methods.
Animal experiments in this study were performed in accordance with the approved guidelines by the Animal Care and Use Committee of National Institute of Genetics (NIG).
Supplementary Material
Acknowledgments
We thank Dr. A. McMahon for the Shh probes, and Dr. P. Gruss for the Six3 probe. We thank the ENCODE Consortium and Dr. B. Ren laboratory for the Encyclopedia of DNA Elements (ENCODE) dataset. We thank the Mouse Research Supporting Unit at NIG. We thank H. Nakazawa and N. Yamatani for embryonic manipulation for generation of transgenic and mutant mice; and Y. Sakakibara for whole-mount in situ hybridization at early embryonic stage. This work was supported by KAKENHI (Grants-in-Aid for Scientific Research) Grants JP24247002 and JP15H02412 from JSPS (Japan Society for the Promotion of Science).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. A.L.J. is a guest editor invited by the Editorial Board.
Data deposition: The sequences of SBE7 and e4 were submitted to DNA Data Bank of Japan (DDBJ) with accession nos. LC461025 and LC461026, respectively.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901732116/-/DCSupplemental.
References
- 1.Rowan A. M., Stern C. D., Storey K. G., Axial mesendoderm refines rostrocaudal pattern in the chick nervous system. Development 126, 2921–2934 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Tanabe Y., Jessell T. M., Diversity and pattern in the developing spinal cord. Science 274, 1115–1123 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Chiang C., et al. , Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Pera E. M., Kessel M., Patterning of the chick forebrain anlage by the prechordal plate. Development 124, 4153–4162 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Shimamura K., Rubenstein J. L., Inductive interactions direct early regionalization of the mouse forebrain. Development 124, 2709–2718 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Dale J. K., et al. , Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell 90, 257–269 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Epstein D. J., McMahon A. P., Joyner A. L., Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development 126, 281–292 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Ye W., Shimamura K., Rubenstein J. L., Hynes M. A., Rosenthal A., FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93, 755–766 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Ericson J., Muhr J., Jessell T. M., Edlund T., Sonic hedgehog: A common signal for ventral patterning along the rostrocaudal axis of the neural tube. Int. J. Dev. Biol. 39, 809–816 (1995). [PubMed] [Google Scholar]
- 10.Ericson J., et al. , Sonic hedgehog induces the differentiation of ventral forebrain neurons: A common signal for ventral patterning within the neural tube. Cell 81, 747–756 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Martí E., Bumcrot D. A., Takada R., McMahon A. P., Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature 375, 322–325 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Roelink H., et al. , Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell 81, 445–455 (1995). [DOI] [PubMed] [Google Scholar]
- 13.Aoto K., et al. , Mouse Shh is required for prechordal plate maintenance during brain and craniofacial morphogenesis. Dev. Biol. 327, 106–120 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Li H., Tierney C., Wen L., Wu J. Y., Rao Y., A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate. Development 124, 603–615 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider V. A., Mercola M., Spatially distinct head and heart inducers within the Xenopus organizer region. Curr. Biol. 9, 800–809 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Benabdallah N. S., et al. , SBE6: A novel long-range enhancer involved in driving sonic hedgehog expression in neural progenitor cells. Open Biol. 6, 160197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong Y., A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development 133, 761–772 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Yao Y., et al. , Cis-regulatory architecture of a brain signaling center predates the origin of chordates. Nat. Genet. 48, 575–580 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukiji N., Amano T., Shiroishi T., A novel regulatory element for Shh expression in the lung and gut of mouse embryos. Mech. Dev. 131, 127–136 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Jeong Y., et al. , Spatial and temporal requirements for sonic hedgehog in the regulation of thalamic interneuron identity. Development 138, 531–541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong Y., et al. , Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat. Genet. 40, 1348–1353 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L., et al. , Disruption of SoxB1-dependent Sonic hedgehog expression in the hypothalamus causes septo-optic dysplasia. Dev. Cell 22, 585–596 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Symmons O., et al. , The Shh topological domain facilitates the action of remote enhancers by reducing the effects of genomic distances. Dev. Cell 39, 529–543 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter T. C., Kennedy A. M., Woodward P. J., Holoprosencephaly: A survey of the entity, with embryology and fetal imaging. Radiographics 35, 275–290 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Kent W. J., et al. , The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.E. P. Consortium; ENCODE Project Consortium , An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Echelard Y., et al. , Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Geng X., et al. , Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev. Cell 15, 236–247 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan A., et al. , JASPAR 2018: Update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 46, D260–D266 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohyama K., Ellis P., Kimura S., Placzek M., Directed differentiation of neural cells to hypothalamic dopaminergic neurons. Development 132, 5185–5197 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Kelberman D., Dattani M. T., Septo-optic dysplasia–Novel insights into the aetiology. Horm. Res. 69, 257–265 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Houtmeyers R., et al. , Zic2 mutation causes holoprosencephaly via disruption of NODAL signalling. Hum. Mol. Genet. 25, 3946–3959 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Nanni L., Schelper R. L., Muenke M. T., Molecular genetics of holoprosencephaly. Front. Biosci. 5, D334–D342 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Paulussen A. D., et al. , The unfolding clinical spectrum of holoprosencephaly due to mutations in SHH, ZIC2, SIX3 and TGIF genes. Eur. J. Hum. Genet. 18, 999–1005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roessler E., Hu P., Muenke M., Holoprosencephaly in the genomics era. Am. J. Med. Genet. C. Semin. Med. Genet. 178, 165–174 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi K., Anderson A. E., Sutherland A. E., Wotton D., Loss of Tgif function causes holoprosencephaly by disrupting the SHH signaling pathway. PLoS Genet. 8, e1002524 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb E. A., Dattani M. T., Septo-optic dysplasia. Eur. J. Hum. Genet. 18, 393–397 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelberman D., Dattani M. T., Genetics of septo-optic dysplasia. Pituitary 10, 393–407 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Zhao T., Zhou X., Szabó N., Leitges M., Alvarez-Bolado G., Foxb1-driven Cre expression in somites and the neuroepithelium of diencephalon, brainstem, and spinal cord. Genesis 45, 781–787 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Xu Q., Tam M., Anderson S. A., Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol. 506, 16–29 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Shimogori T., et al. , A genomic atlas of mouse hypothalamic development. Nat. Neurosci. 13, 767–775 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabó N. E., et al. , Role of neuroepithelial Sonic hedgehog in hypothalamic patterning. J. Neurosci. 29, 6989–7002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaess S., Szabó N., Haddad-Tóvolli R., Zhou X., Álvarez-Bolado G., Sonic hedgehog signaling in the development of the mouse hypothalamus. Front. Neuroanat. 8, 156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geng X., Acosta S., Lagutin O., Gil H. J., Oliver G., Six3 dosage mediates the pathogenesis of holoprosencephaly. Development 143, 4462–4473 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manning L., et al. , Regional morphogenesis in the hypothalamus: A BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev. Cell 11, 873–885 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Trowe M. O., et al. , Inhibition of Sox2-dependent activation of Shh in the ventral diencephalon by Tbx3 is required for formation of the neurohypophysis. Development 140, 2299–2309 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee B., et al. , Genomic code for Sox2 binding uncovers its regulatory role in Six3 activation in the forebrain. Dev. Biol. 381, 491–501 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Jeong Y., Epstein D. J., Distinct regulators of Shh transcription in the floor plate and notochord indicate separate origins for these tissues in the mouse node. Development 130, 3891–3902 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.