Abstract
Hexavalent chromium [Cr(VI)] is one of the most common environmental carcinogen causing lung cancer in humans; however, the mechanism of Cr(VI) carcinogenesis remains elusive. Cancer stem cells (CSCs) are considered as cancer initiating and maintaining cells. Ours and other recent studies showed that chronic Cr(VI) exposure induces CSC-like property representing an important mechanism of Cr(VI) carcinogenesis. However, how Cr(VI) exposure induces CSC-like property remains largely unknown. In this study, we found that stably knocking down the expression of c-Myc, a proto-oncogene and one of key stemness factors playing critical roles in cancer initiation and progression, in Cr(VI)-transformed human bronchial epithelial cells [BEAS-2B-Cr(VI)] significantly decreased their CSC-like property and tumorigenicity in mice. Moreover, stably knocking down c-Myc expression in parental nontransformed BEAS-2B cells significantly impaired the capability of chronic Cr(VI) exposure to induce CSC-like property and cell transformation. It was also found that stably overexpressing c-Myc alone in parental nontransformed BEAS-2B cells is capable of causing CSC-like property and cell transformation. Mechanistic studies showed that chronic Cr(VI) exposure increases c-Myc expression by down-regulating the level of microRNA-494 (miR-494). It was further determined that overexpressing miR-494 significantly reduces Cr(VI)-induced CSC-like property, cell transformation, and tumorigenesis mainly through down-regulating c-Myc expression. Together, these findings indicate that chronic low dose Cr(VI) exposure induces CSC-like property and tumorigenesis by increasing c-Myc expression through down-regulating the level of miR-494, revealing an important role of the proto-oncogene c-Myc in Cr(VI) carcinogenesis.
Keywords: hexavalent chromium, c-Myc, miR-494, cancer stem cell-like property, cell transformation, carcinogenesis
The widespread usage of chromium (Cr) and its compounds in the manufacture of many consumer products plus the presence of Cr in particulate matters from automobile emissions and other sources make Cr be one of the most common environmental pollutants (International Agency for Research on Cancer [IARC], 1990). Cr exists in several valence and the common forms of Cr found in occupational and general environment include Cr(0), Cr(III), and Cr(VI); however, only Cr(VI) (hexavalent chromium) was recognized as a carcinogen being capable of causing lung cancer and other types of cancers (IARC, 1990; Stout et al., 2009). Although Cr(VI) is a well-known human carcinogen and epidemiological studies also revealed a link between Cr(VI) exposure and increased risk of lung cancer, the mechanism of Cr(VI) carcinogenesis has not been well understood (Urbano et al., 2012; Wang and Yang, 2019).
Cancer stem cells (CSCs) generally refer to a small population of cancer cells possessing characteristics associated with normal stem cells, especially the capability of self-renewal and generation of different types of cells found in a tumor (Nguyen et al., 2012; Wang and Yang, 2019). The CSC theory proposes that cancers are originated from CSCs. In other words, CSCs or CSC-like cells are considered as cancer initiating and maintaining cells (Nguyen et al., 2012). However, one of key questions in CSC field that remain to be answered is where CSCs come from. It has been proposed that CSCs (i) may come from adult tissue stem cells that are malignantly transformed by endogenous factors or environmental carcinogens; (ii) may be converted from the “ordinary” cancer cells; or (iii) may come from cells residing in a special compartment termed stem cell or CSC niche (Batlle and Clevers, 2017; Plaks et al., 2015).
Studies showed that chronic metal carcinogen exposure is capable of malignantly transforming normal stem cells and producing CSC-like cells (Wang and Yang, 2019). For example, Tokar et al. reported that chronic arsenic exposure is able to transform human normal prostate stem/progenitor cell line WPE-stem cells and normal rat kidney stem/progenitor cell line RIMM-18 cells into CSC-like cells (Tokar et al., 2010, 2013). Ours and other recent studies showed that chronic Cr(VI) exposure to immortalized human bronchial epithelial cells is also capable of producing CSC-like cells (Dai et al., 2017; Wang et al. 2018). Given the well-agreed role of CSCs or CSC-like cells in cancer initiation and progression, the findings from these studies strongly suggest that the capability of chronic metal carcinogen exposure to induce CSC-like cells is a novel mechanism of metal carcinogenesis (Wang and Yang, 2019). However, it remains largely unknown how metal carcinogen including Cr(VI) exposure produces CSC-like cells.
The c-Myc gene is a proto-oncogene and its abnormal expression and activation play important roles in the initiation, maintenance, and progression of many types of human cancers (Dang, 2012; Gabay et al., 2014). It has been proposed that c-Myc contributes to cell malignant transformation and tumorigenesis by promoting protein synthesis, uncontrolled cell proliferation, genomic instability, dysregulated tumor cell metabolism, and tumor angiogenesis (Majello and Perini, 2015; Stine et al., 2015; Wahlström and Henriksson, 2015). Moreover, studies showed that c-Myc is also one of four Yamanaka factors that collectively produce induced-pluripotent stem (iPS) cells from fibroblasts; and subcutaneous injection of these iPS cells into nude mice generates tumors (Takahashi and Yamanaka, 2006), implying that c-Myc could be critically involved in cancer stemness (Chappell and Dalton, 2013; Yoshida, 2018). Interestingly, ours and other previous studies showed that c-Myc expression level is upregulated in metal carcinogen-transformed cells (Chen et al., 2001; Joseph et al., 2001; Pratheeshkumar et al., 2016; Wang et al., 2013); however, it is unknown whether c-Myc plays a role in metal carcinogenesis. The goal of this study was to determine how chronic Cr(VI) exposure increases c-Myc level and whether c-Myc contributes causally to chronic Cr(VI) exposure-induced CSC-like property, cell malignant transformation, and tumorigenesis.
MATERIALS AND METHODS
Cell culture
Immortalized parental human bronchial epithelial BEAS-2B cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). Both parental BEAS-2B cells and chronic low dose Cr(VI) exposure (0.25 µM of K2Cr2O7 × 20 weeks)-transformed BEAS-2B cells [BEAS-2B-Cr(VI)] generated in our recent study (Wang et al., 2018) were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum (FBS).
Generation of miR-494 stable overexpression, c-Myc stable knockdown, and stable overexpression cells
To generate vector control and miR-494 stable expression cells, parental BEAS-2B, passage-matched Control BEAS-2B, and Cr(VI)-transformed BEAS-2B cells were transduced with vector control (pMIRNA-GFP) or miR-494 precursor-expressing (pMIRNA-GFP-494) lentiviral particles (System Biosciences, Mountain View, CA). Cells were passaged 48 h after lentiviral particle transduction. Fluorescence-activated cell sorting was carried out to sort GFP positive cells after 48 h culture. The overexpression of miR-494 was determined by quantitative PCR (Q-PCR) analysis. To generate c-Myc stable knockdown cells, parental BEAS-2B and Cr(VI)-transformed BEAS-2B cells were transduced with nontargeting control small hairpin RNA (shRNA) lentiviral (pZIP-hCMV-ZsGreen-puro-Control shRNA) or c-Myc targeting shRNA lentiviral (pZIP-hCMV-ZsGreen-puro-c-Myc shRNA) particles (transOMIC Technologies Inc., Huntsville, AL). Forty-eight hours after lentiviral particle transduction, cells were selected with puromycin (1 µg/ml). The knockdown of c-Myc expression was confirmed by Western blot analysis. To generate c-Myc stable overexpression cells, parental BEAS-2B cells and chronic low dose Cr(VI)-exposed miR-494 overexpression cells were transduced with vector control (pTOL-hCMV) or c-Myc overexpressing (pTOL-hCMV-c-Myc) lentiviral particles (transOMIC Technologies Inc.) and selected with puromycin (1 µg/ml) following the procedures described in our previous studies (Wang et al., 2014, 2011; Xiao et al., 2018). The overexpression of c-Myc was confirmed by Western blot.
Chronic cell transformation experiments by a low dose Cr(VI) (0.25 µM of K2Cr2O7) exposure
The control shRNA and c-Myc stable knockdown (c-Myc shRNA) BEAS-2B cells, the vector control and miR-494 stable expression BEAS-2B cells were continuously exposed to 0.25 µM of Cr(VI) (K2Cr2O7) for 20 weeks as described in our recent study (Wang et al., 2018). At the end of 20-week Cr(VI) exposure, cells were used for soft agar colony formation assay and suspension culture sphere formation assay as described below to assess Cr(VI)-induced cell transformation and CSC-like property, respectively.
Soft agar colony formation assay
The soft agar colony formation assay was performed in 6-cm cell culture dishes in triplicates for each group as previously described (Yang et al., 2005). Briefly, cells were harvested by trypsinization and suspended in DMEM containing 10% FBS at a concentration of 0.5 × 104 cells/ml. Normal melting point agar (5 ml of 0.6% agar in DMEM) was placed into each 60-mm cell culture dish as the bottom agar. After solidification of the bottom agar, 4 ml of cell mixture consisting of 2 ml of cell suspension (0.5 × 104 cells/ml) and 2 ml of 0.8% lower melting point agar in DMEM containing 10% FBS were poured over the bottom agar. After solidification of the upper agar, 3 ml of DMEM containing 10% FBS were added, and dishes were incubated at 37°C in a humidified 5% CO2 atmosphere. Soft agar colonies were stained with 0.003% crystal violet, photographed and counted (if >100 μm) after 4-week incubation.
Suspension culture sphere formation assay
The first generation sphere formation under serum-free suspension culture conditions reflecting the stem cell property was determined following the published protocol (Dontu et al., 2003) with minor modifications. Briefly, single cells were plated in ultralow attachment 24-well culture plates (Corning, Corning, NY) at a density of 2.5 × 103 cells/well suspended in serum-free DMEM containing human recombinant basic fibroblast growth factor (bFGF, 20 ng/ml), human recombinant epidermal growth factor (EGF, 20 ng/ml) (R&D, Minneapolis, MN), B27 (50 times diluted from the original 50× stock solution, Invitrogen, Carlsbad, CA), and heparin (4 μg/ml, Sigma). Plates were incubated at 37°C in a humidified 5% CO2 atmosphere. Spheres were viewed, photographed and counted (if >100 μm) under a phase-contrast microscope after 10-day culture. The second generation sphere formation assay was performed according to the published protocol (Garcia et al., 2018). Briefly, all spheres in each group from the first generation sphere assays were collected, pooled together and incubated with trypsin at 37°C for 5 min to disperse spheres into single cells. Cells were then counted and 1000 cells from each group were seeded for the second generation sphere formation assay following the procedures described earlier.
Side population analysis and ALDEFLUOR assay
The side population (SP) analysis was performed following the reported protocol (Ho et al., 2007). Verapamil (an inhibitor of ABC transporters causing Hoechst dye extrusion from cells) treatment (100 µM) group was included to verify that the gated population of cells is diminished upon verapamil treatment and thus indeed SP cells. The ALDEFLUOR Kit from Stem Cell Technologies (Vancouver, BC, Canada) was used for performing ALDEFLUOR assay according to instructions from the manufacturer. The ALDH inhibitor DEAB treatment group was included to verify that the gated ALDEFLUOR positive cells are diminished upon DEAB treatment and thus indeed ALDEFLUOR positive cells.
Nude mouse xenograft tumorigenesis study
Two nude mouse tumorigenesis model studies were performed and animal protocols were reviewed and approved by the University of Kentucky Institutional Animal Care and Use Committee. In the first nude mouse tumorigenesis study, shRNA vector control and c-Myc stable knockdown BEAS-2B-Cr(VI) [Cr(VI)-transformed BEAS-2B] cells (1.5 × 106 cells in 0.1 ml of 1:1 growth factor-reduced matrigel and PBS) were injected subcutaneously into the right flank of female nude mice (Nu/Nu, Charles River laboratories, five mice in each group). In the second nude mouse tumorigenesis study, miR-494 overexpression and chronic low dose Cr(VI)-exposed [BEAS-2B-GFP-miR-494-Cr(VI)] vector control [BEAS-2B-GFP-miR-494-Cr(VI)-vector control] and c-Myc overexpression [BEAS-2B-GFP-miR-494-Cr(VI)-c-Myc OE] cells (1.5 × 106 cells in 0.1 ml of 1:1 growth factor-reduced matrigel and PBS) were injected subcutaneously into the right flank of female nude mice. After cell injection, nude mice were maintained under specific pathogen-free conditions. All animals were euthanized 12 weeks after injection, and the xenograft tissues were harvested and fixed with 10% formalin solution. These two nude mouse tumorigenesis studies were repeated with another five mice in each group under similar conditions. The presented results are combined results from the original animal studies and the repeated animal studies and total number of mice in each group is ten.
Western blot
Cells were harvested and washed with PBS and lysed using cell lysis buffer following our published protocol (Wang et al., 2014; Yang et al., 2005) and subjected to SDS-polyacrylamide gel electrophoresis (10–30 µg of protein/lane). The following primary antibodies were used: anti-c-Myc (Cat. #: 5605; dilution: 1:1000), anti-Nanog (Cat. #: 4903; dilution: 1:2000), Anti-KLF4 (Cat. #: 4038; dilution: 1:1000) (Cell Signaling Technology, Beverly, MA), and anti-β-actin (Cat. #: 5441; dilution: 1:8000) (Millipore Sigma, St. Louis, MO). Western blot was quantitated using Image J software and the quantitated results are presented as the ratio of a specific protein band intensity divided by the corresponding β-actin band intensity.
The miR-494 Q-PCR analysis
Cellular total RNAs were extracted using QIAGEN miRNeasy mini kit and used for Q-PCR analysis of miR-494 level carried out in ABI 7500 Fast Real Time PCR System using the TaqMan miR-494 miRNA assay (Applied Biosystems, Inc., Foster City, CA). U6 snRNA was analyzed by TaqMan PCR assay and used as the internal control for normalizing relative miR-494 expression level (Wang et al., 2011).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Cell proliferation was determined using the tetrazolium dye colorimetric assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide [MTT] assay) following the procedures described in our previous study (Zhao et al., 2011). Briefly, 1000 cells were plated in 96-well plates. After overnight, 24, 48, and 72 h culture, the culture medium was replaced with 50 μl of MTT-serum-free fresh medium (MTT final concentration: 0.5 mg/ml) and incubated for 4 h. Then, 200 μl of dimethylsulfoxide was added to dissolve the formazan crystals. The MTT absorbance was measured with a microplate reader (Molecular Devices, Sunnyvale, CA) at a wavelength of 570 nm.
H&E and IF staining of human lung tissue sections
The H&E and immunofluorescence (IF) staining of c-Myc in human lung tissue sections was carried out following our previous procedures (Zhao et al., 2010). The IF staining pictures taken under a Nikon fluorescent microscope are the overlaid images of c-Myc staining in red fluorescence with nuclear 4′,6-diamidino-2-phenylindole (DAPI) staining in blue fluorescence. The images were overlaid using Nikon NIS-Elements software.
Statistical analysis
The statistical analyses for the significance of differences between different groups (mean ± SD) were carried out by testing different treatment effects using two-tailed t-tests for a comparison of two data sets or one-way analysis of variance (ANOVA) for multiple data sets. A p-value of <.05 was considered statistically significant. The significance of difference in nude mouse xenograft tumor incidence was tested using Fisher’s exact test. Because the final data for the xenograft tumor incidence were combined from two separate studies (the original animal studies and the repeated animal studies), the significance level was adjusted using the Bonferroni correction to control false positive rate. A p-value of <.025 was considered statistically significant for the xenograft tumor incidence rate analysis.
RESULTS
Stably Knocking Down c-Myc Expression Level in Cr(VI)-Transformed Human Bronchial Epithelial Cells Significantly Reduces Their CSC-Like Property and Tumorigenicity in Mice
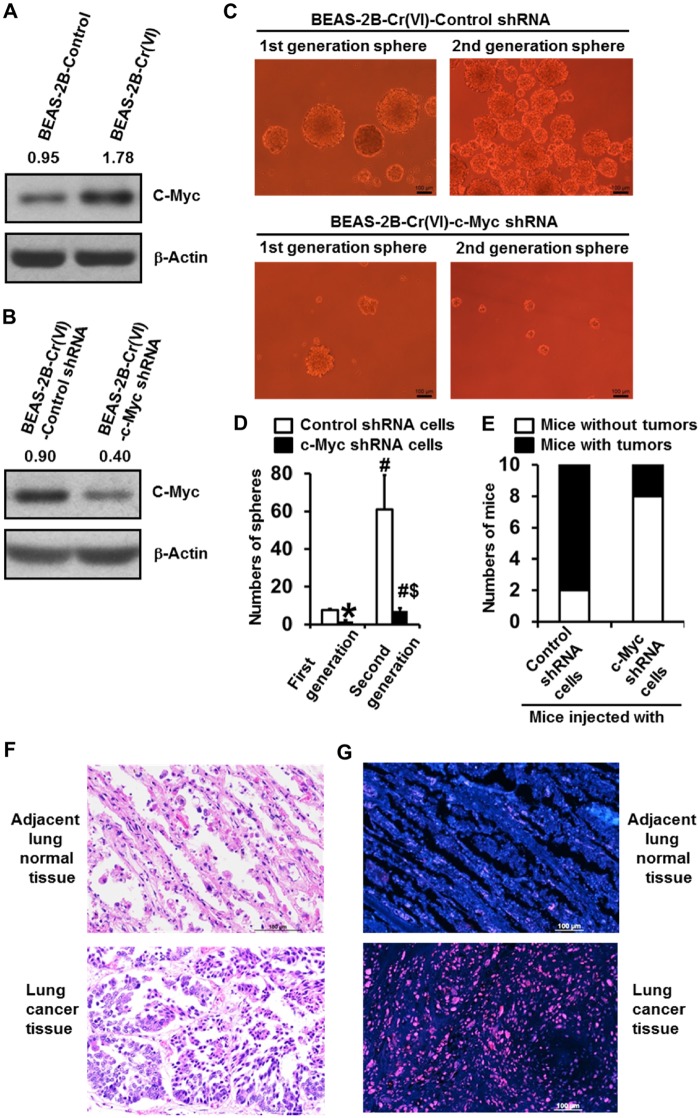
Ours and other previous studies showed that c-Myc expression level is upregulated in metal carcinogen-transformed cells (Chen et al., 2001; Joseph et al., 2001; Pratheeshkumar et al., 2016; Wang et al., 2013); however, it remains to be determined whether the upregulated c-Myc plays a role in metal carcinogenesis. Our Western blot analysis showed that c-Myc expression level is dramatically higher in Cr(VI)-transformed BEAS-2B cells [BEAS-2B-Cr(VI)] than passage-matched control BEAS-2B cells (BEAS-2B-Control) (Figure 1A). In addition, increased c-Myc expression level was also observed in Cr(VI)-transformed 16HBE cells (Supplementary Figure 1A). To investigate whether the upregulated c-Myc plays an important role in Cr(VI) carcinogenesis, we first stably knocked down c-Myc expression in Cr(VI)-transformed cells for determining the role of c-Myc in the malignant phenotypes of Cr(VI)-transformed cells. A great knockdown of c-Myc expression level in Cr(VI)-transformed cells was confirmed by Western blot (Figure 1B). MTT assays showed that Cr(VI)-transformed cell grow faster than the control cells (Supplementary Figure 1B). Stably knocking down c-Myc reduced Cr(VI)-transformed cell proliferation (Supplementary Figure 1C). In our recent study (Wang et al., 2018), we showed that Cr(VI)-transformed cells display CSC-like property as evidenced by forming significantly more suspension culture spheres. In this study, we used two additional well-established assays for analyzing CSC-like cells including flow cytometry-based Side Population analysis and ALDEFLUOR assay (Sugihara and Saya, 2013) to further demonstrate that Cr(VI)-transformed cells exhibit CSC-like property. As shown in Supplementary Figures 2A–D, Cr(VI)-transformed cells had significantly more side population cells and ALDEFLUOR positive cells than the control cells, which are consistent with our previous results using the suspension culture sphere formation assay (Wang et al., 2018). Moreover, we also analyzed other CSC markers and found that the levels of Nanog and KLF4 are greatly upregulated in Cr(VI)-transformed cells (Supplementary Figure 3). These findings along with the previous sphere formation results provide convincing evidence demonstrating that Cr(VI)-transformed cells have CSC-like property.
Figure 1.
Stably knocking down c-Myc expression level in Cr(VI)-transformed human bronchial epithelial cells significantly reduces their CSC-like property and tumorigenicity in mice. A, Western blot analysis of c-Myc expression level in passage-matched control cells and Cr(VI)-transformed cells. B, Western blot analysis of c-Myc expression level in Cr(VI)-transformed cells stably expressing a control shRNA or c-Myc knockdown shRNA. C, Representative images of the first and second generation suspension spheres formed by Cr(VI)-transformed cells stably expressing a control shRNA or c-Myc knockdown shRNA. D, Quantitation of the first and second generation suspension spheres formed by Cr(VI)-transformed cells stably expressing a control shRNA [BEAS-2B-Cr(VI)-Control shRNA] or c-Myc knockdown shRNA [BEAS-2B-Cr(VI)-c-Myc shRNA]. The results are presented as means ± standard deviations (n = 3). *p < .05, compared with the first generation spheres formed by BEAS-2B-Cr(VI)-Control shRNA cells; #p < .05, compared with the corresponding first generation spheres formed by BEAS-2B-Cr(VI)-Control shRNA cells or BEAS-2B-Cr(VI)-c-Myc shRNA cells, respectively; $p < .05, compared with the second generation spheres formed by BEAS-2B-Cr(VI)-Control shRNA cells. E, Effect of stably knocking down c-Myc expression level in Cr(VI)-transformed cells on their tumor forming capability in nude mice (n = 10). F and G, The level of c-Myc is drastically higher in chromate exposure-caused human lung cancer tissue than that in the adjacent normal lung tissue. Representative images of H&E staining (F) and c-Myc immunofluorescence (IF) staining (G) in the squamous cell lung carcinoma tissue and the adjacent normal lung tissue from a 40-year-old nonsmoker worker exposed to chromate for 22 years. The IF staining images (G) are the overlaid images of c-Myc (red) and DNA DAPI (blue) staining. Similar staining results were obtained in lung cancer tissues from a 69-year-old smoker worker exposed to chromate for 11 years. Scale bar: 100 μm.
Further analysis using suspension culture sphere formation assay showed that Cr(VI)-transformed cells with c-Myc stable knockdown formed significantly less spheres than the shRNA control cells (Figs. 1C and 1D), suggesting that upregulation of c-Myc expression plays a critical role in maintaining the CSC-like property of Cr(VI)-transformed cells. Self-renewal property is a unique feature of CSCs or CSC-like cells (Nguyen et al., 2012; Wang and Yang, 2019). To further determine the role of c-Myc in Cr(VI)-induced CSC-like property, we also analyzed the effect of c-Myc knockdown on the self-renewal capability of Cr(VI)-transformed cells by determining the sphere forming ability of cells from the first generation spheres. It was found that cells from the first generation control shRNA group spheres are capable of forming drastically more spheres (Figs. 1C and 1D), demonstrating their strong self-renewal ability. In contrast, cells from the first generation c-Myc stable knockdown group spheres formed significantly less spheres (Figs. 1C and 1D), revealing their impaired self-renewal ability. The importance of c-Myc upregulation in Cr(VI) exposure-induced CSC-like property is further supported by our Western blot analysis showing that knocking down c-Myc expression greatly reduces the level of other CSC marker such as Nanog in Cr(VI)-transformed cells (Supplementary Figure 3).
CSCs or CSC-like cells are considered as tumor initiating cells (Nguyen et al., 2012; Wang and Yang, 2019). Ours and other studies showed that Cr(VI)-transformed cells are capable of forming tumors when inoculated into nude mice (Pratheeshkumar et al., 2016; Wang et al., 2018). To determine the role of c-Myc in tumor forming capability of Cr(VI)-transformed cells, we injected subcutaneously Cr(VI)-transformed cells with shRNA control or c-Myc stable knockdown to nude mice and found that eight of ten mice injected with shRNA control cells grow tumors (tumor incidence rate: 80%), but only two of ten mice injected with c-Myc stable knockdown cells generate tumors (tumor incidence rate: 20%, p < .025 compared with shRNA control cell group) (Figure 1E). Together, these findings strongly suggest that knockdown c-Myc expression in Cr(VI)-transformed cells significantly impairs their CSC-like property and tumorigenicity.
To further demonstrate the important role of c-Myc in Cr(VI) carcinogenesis, we examined the levels of c-Myc in Cr(VI) exposure-caused human lung cancer tissue sections and the matched-adjacent normal lung tissue sections from chromate workers. The chromate exposure history of workers was reported in our collaborator Dr. Kazuya Kondo’s previous publications (Ali et al., 2011; Kondo et al., 2006). Our IF staining of c-Myc revealed that c-Myc level is drastically higher in Cr(VI) exposure-caused human lung cancer tissues than that in the adjacent normal lung tissues. The representative H&E histology and IF staining images of c-Myc from a 40-year-old nonsmoker worker exposed to chromate for 22 years are shown in Figures 1F and 1G.
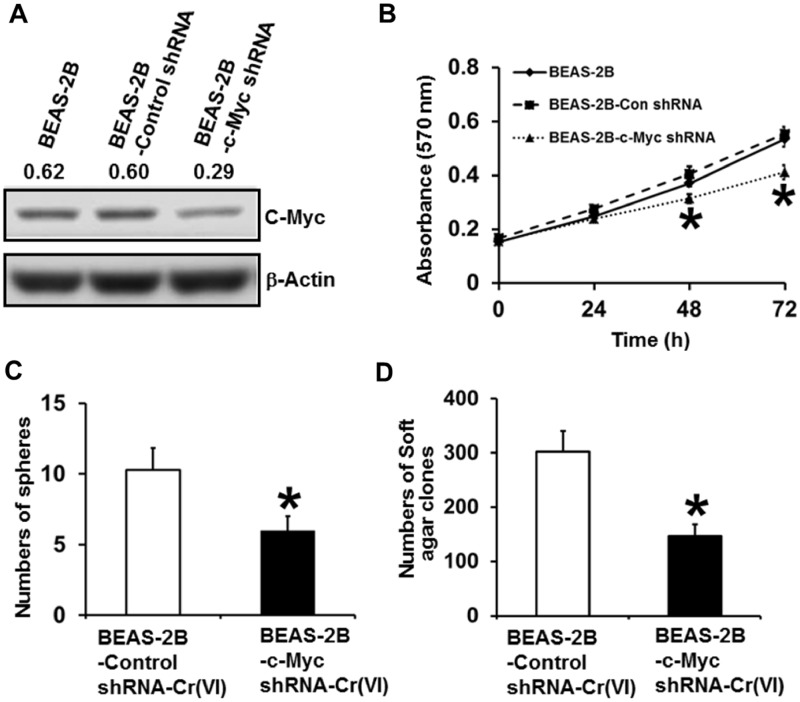
Stably Knocking Down c-Myc Expression Level in Parental BEAS-2B Cells Significantly Reduces the Capability of Chronic Cr(VI) Exposure to Induce CSC-Like Property and Cell Transformation
Next, we wanted to determine whether c-Myc upregulation plays a role in chronic Cr(VI) exposure to induce CSC-like property and cell transformation. We generated shRNA control and c-Myc stable knockdown parental BEAS-2B cells and Western blot analysis confirmed the dramatic lower c-Myc expression level in c-Myc knockdown cells (BEAS-2B-c-Myc shRNA) (Figure 2A). MTT analysis showed that stably knocking down c-Myc expression in parental BEAS-2B cells significant reduces cell proliferation (Figure 2B). The shRNA control and c-Myc stable knockdown BEAS-2B cells were then continuously exposed to 0.25 µM of Cr(VI) (K2Cr2O7) for 20 weeks for cell transformation experiment following the protocol described in our recent study (Wang et al., 2018). At the end of 20 weeks Cr(VI) exposure, suspension culture sphere formation assay and soft agar colony formation assay revealed that stably knocking down c-Myc expression significantly reduces the numbers of chronic low dose Cr(VI) exposure-induced suspension spheres and soft agar colonies (Figs. 2C and 2D). These results suggest that knocking down c-Myc expression impairs the capability of chronic Cr(VI) exposure to induce CSC-like property and cell transformation, implying that c-Myc is critically involved in Cr(VI)-induced cell transformation process.
Figure 2.
Stably knocking down c-Myc expression level in parental BEAS-2B cells significantly reduces chronic Cr(VI) exposure inducing CSC-like property and cell transformation. A, Western blot analysis of c-Myc expression level in parental BEAS-2B cells and parental BEAS-2B cells stably expressing a control shRNA or c-Myc knockdown shRNA. B, MTT analysis of the growth curve of parental BEAS-2B cells and parental BEAS-2B cells stably expressing a control shRNA or c-Myc knockdown shRNA. The results are presented as means ± standard deviations (n = 6). Quantitation of suspension culture sphere formation (C) and soft agar colony formation (D) by chronic low dose Cr(VI)-exposed control shRNA and c-Myc stable knockdown cells. After exposure to 0.25 µM of Cr(VI) (K2Cr2O7) for 20 weeks, control shRNA [BEAS-2B-Control shRNA-Cr(VI)] and c-Myc stable knockdown [BEAS-2B-c-Myc shRNA-Cr(VI)] cells were harvested for suspension culture spheroid formation (C) and soft agar colony formation (D) assays as described in Methods. The results are presented as means ± standard deviations (n = 3). *p < .05, compared with BEAS-2B-Control shRNA-Cr(VI) cells.
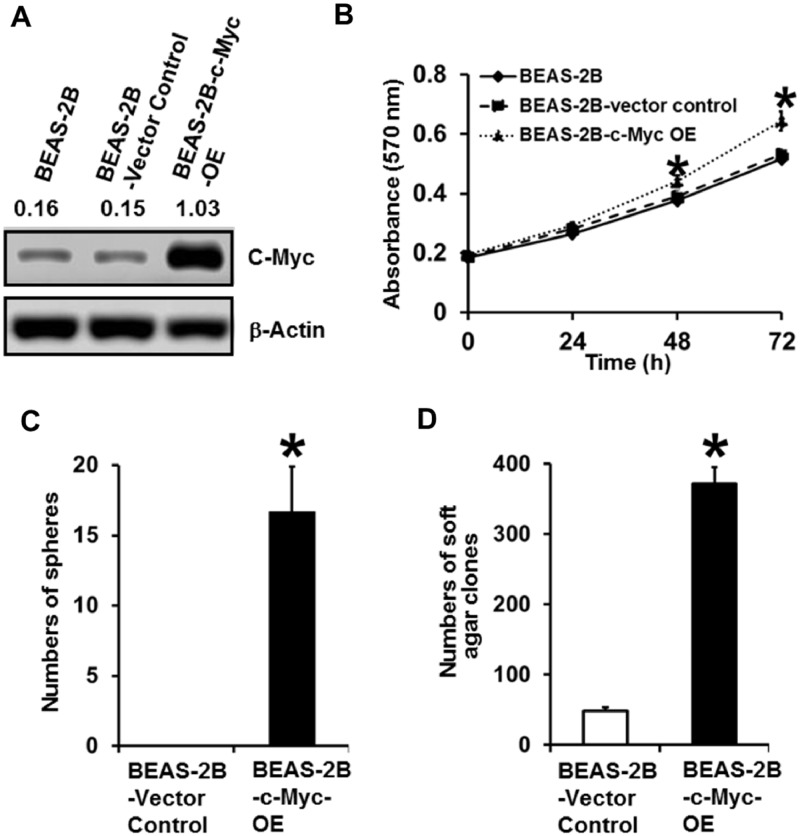
Stably Overexpressing c-Myc Alone in Parental BEAS-2B Cells Induces CSC-Like Property and Cell Transformation
The c-Myc is a proto-oncogene and previous studies showed overexpressing c-Myc alone in immortalized nontransformed cells may or may not cause cell transformation (Dang, 2012). To further determine the role of c-Myc upregulation in Cr(VI)-induced transformation of BEAS-2B cells, we next investigated whether overexpressing c-Myc alone is capable of causing transformation of BEAS-2B cells. We generated vector control and c-Myc stable overexpression (OE) parental BEAS-2B cells (BEAS-2B-c-Myc-OE). The c-Myc overexpression in c-Myc stable OE cells was confirmed by Western blot analysis (Figure 3A). MTT analysis showed that stably overexpressing c-Myc in parental BEAS-2B cells significantly increases cell proliferation (Figure 3B). Under the suspension and serum-free culture condition, although vector control cells did not form spheres, c-Myc overexpressing BEAS-2B cells were capable of forming a significant number of spheres (Figure 3C), which is comparable to the number of spheres formed by chronic Cr(VI) exposure-transformed BEAS-2B cells (Wang et al., 2018). Moreover, the c-Myc overexpressing BEAS-2B cells also grew a significant number of soft agar colonies (Figure 3D), which is also close to the number of soft agar colonies produced by Cr(VI)-transformed BEAS-2B cells (Wang et al., 2018). These results suggest that stably overexpressing c-Myc alone in BEAS-2B cells is sufficient to cause CSC-like property and cell transformation. These findings support our conclusion that c-Myc upregulation by Cr(VI) exposure plays an important role in Cr(VI) exposure-induced cell transformation and in maintaining the malignant phenotypes of Cr(VI)-transformed cells.
Figure 3.
Stably overexpressing c-Myc alone in parental BEAS-2B cells induces CSC-like property and cell transformation. A, Western blot analysis of c-Myc expression level in parental BEAS-2B cells and parental BEAS-2B vector control or c-Myc stable overexpression (BEAS-2B-c-Myc OE) cells. B, MTT analysis of the growth curve of parental BEAS-2B cells and parental BEAS-2B vector control or c-Myc stable overexpression (BEAS-2B-c-Myc OE) cells. The results are presented as means ± standard deviations (n = 6). Quantitation of suspension culture sphere formation (C) and soft agar colony formation (D) by parental BEAS-2B vector control and c-Myc stable overexpression (BEAS-2B-c-Myc OE) cells. The results are presented as means ± standard deviations (n = 3). *p < .05, compared with BEAS-2B-Vector Control cells.
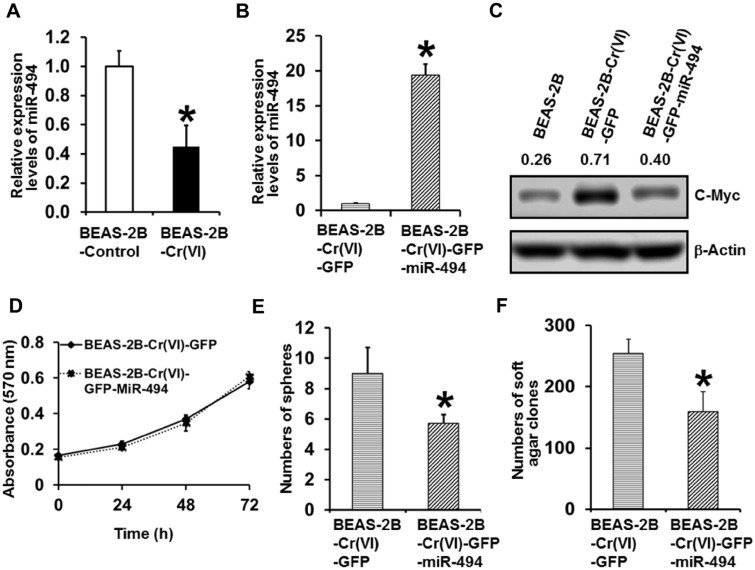
The Expression Level of miR-494 Is Down-Regulated in Cr(VI)-Transformed Cells and Stably Overexpressing miR-494 in Cr(VI)-Transformed Cells Significantly Reduces Their Malignant Phenotypes
We next started exploring the mechanism by which chronic Cr(VI) exposure upregulates c-Myc expression. Regulation of c-Myc expression is complex and one of the well-characterized mechanisms is the microRNA (miRNA) regulation of c-Myc expression (Jackstadt and Hermeking, 2015; Mei and Wu, 2016). Although c-Myc is able to regulate many miRNA expressions, the expression of c-Myc itself is also regulated by miRNAs. One of these well-characterized miRNAs being capable of down-regulating c-Myc expression is miR-494 and c-Myc is a validated target of miR-494 (Jackstadt and Hermeking, 2015). Our Q-PCR analysis showed that miR-494 expression level is significantly down-regulated in Cr(VI)-transformed cells compared with passage-matched control cells (Figure 4A). To determine whether c-Myc upregulation in Cr(VI)-transformed cells is caused by miR-494 down-regulation, we stably re-expressed miR-494 in Cr(VI)-transformed cells and miR-494 overexpression was confirmed by Q-PCR analysis (Figure 4B). Western blot analysis showed that stably overexpressing miR-494 in Cr(VI)-transformed cells dramatically reduces c-Myc expression level (Figure 4C), indicating an important role of miR-494 down-regulation in c-Myc upregulation in Cr(VI)-transformed cells.
Figure 4.
The expression level of miR-494 is down-regulated in Cr(VI)-transformed cells and stably overexpressing miR-494 in Cr(VI)-transformed cells significantly reduces their malignant phenotypes. A, Q-PCR analysis of the relative expression levels of miR-494 in passage-matched control cells and Cr(VI)-transformed cells. The miR-494 level in Cr(VI)-transformed cells was expressed relative to passage-matched control cells (mean ± standard deviations, n = 3). *p < .05, compared with passage-matched control cells. B, Q-PCR analysis of the relative expression levels of miR-494 in Cr(VI)-transformed cells with GFP vector control [BEAS-2B-Cr(VI)-GFP] or GFP-miR-494 stable overexpression [BEAS-2B-Cr(VI)-GFP-miR-494]. The miR-494 level in miR-494 stable overexpression cells was expressed relative to GFP vector control cells (mean ± standard deviations, n = 3). *p < .05, compared with the GFP vector control cells. C, Western blot analysis of c-Myc expression level in parental BEAS-2B cells, Cr(VI)-transformed cells with GFP vector control or GFP-miR-494 stable overexpression. D, MTT analysis of the growth curve of Cr(VI)-transformed cells with GFP vector control or GFP-miR-494 stable overexpression. The results are presented as means ± standard deviations (n = 6). Quantitation of suspension culture sphere formation (E) and soft agar colony formation (F) by Cr(VI)-transformed cells with GFP vector control or GFP-miR-494 stable overexpression. The results are presented as means ± standard deviations (n = 3). *p < .05, compared with BEAS-2B-Cr(VI)-GFP vector control cells.
The expression level of miR-494 is often dysregulated in various cancer; however, the reported role of miR-494 in cancer is controversial (Chen et al., 2017; Han et al., 2019; Macedo et al., 2017; Yang et al., 2018; Yu et al., 2018; Zhang et al., 2018). We then further characterized the effect of overexpressing miR-494 on the malignant phenotypes of Cr(VI)-transformed cells. Although overexpressing miR-494 in Cr(VI)-transformed cells did not display a significant effect on cell proliferation under the standard monolayer culture condition (Figure 4D); however, miR-494 overexpression significantly reduced the numbers of spheres formed under suspension culture condition and the number of colonies formed in soft agar (Figs. 4E and 4F). These results suggest that miR-494 down-regulation, leading to upregulation of c-Myc, plays an important role in the malignant phenotypes of Cr(VI)-transformed cells.
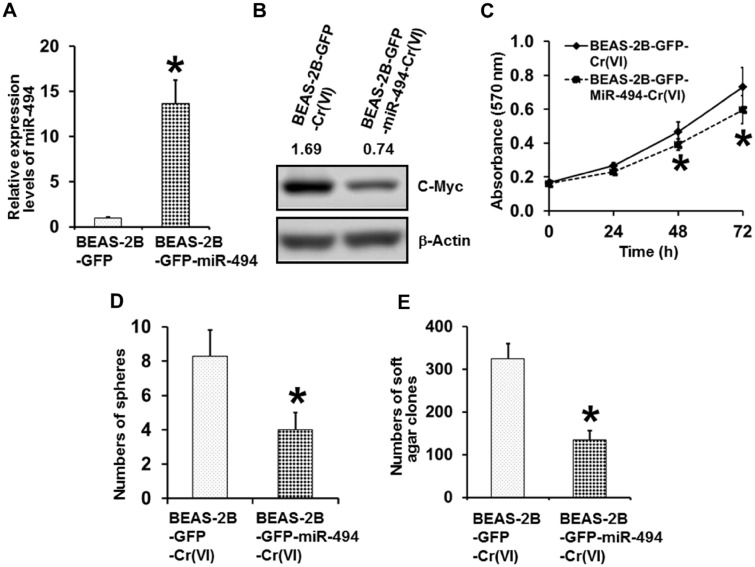
Stably Overexpressing miR-494 in Parental BEAS-2B Cells Significantly Impairs the Capability of Chronic Cr(VI) Exposure to Induce CSC-Like Property and Cell Transformation
Next, we wanted to further determine whether stably overexpressing miR-494 in parental BEAS-2B cells is capable of preventing chronic Cr(VI) exposure from upregulating c-Myc expression level and thus reducing Cr(VI) exposure-induced CSC-like property and cell transformation. We generated vector control (BEAS-2B-GFP) and miR-494 stable overexpression parental BEAS-2B (BEAS-2B-GFP-miR-494) cells; and miR-494 overexpression in miR-494 stable expression cells was confirmed by Q-PCR analysis (Figure 5A). These vector control and miR-494 stable overexpression BEAS-2B cells were then continuously exposed to 0.25 µM of Cr(VI) (K2Cr2O7) for 20 weeks for cell transformation experiment following the protocol described in our recent study (Wang et al., 2018). At the end of 20 weeks Cr(VI) exposure, Western blot analysis revealed that Cr(VI)-exposed miR-494 stable overexpression cells have dramatically lower c-Myc expression level than Cr(VI)-exposed vector control cells (Figure 5B), indicating that stably overexpressing miR-494 impaired the ability of chronic Cr(VI) exposure upregulating c-Myc level. MTT analysis showed that Cr(VI)-exposed miR-494 stable overexpression cells grow significantly slower than Cr(VI)-exposed vector control cells (Figure 5C). Moreover, further functional studies using suspension culture sphere formation assay and soft agar colony formation assay revealed that stably overexpressing miR-494 significantly reduces the numbers of chronic Cr(VI) exposure-induced suspension spheres and soft agar colonies (Figs. 5D and 5E). These results suggest that stably overexpressing miR-494 significantly reduces the capability of chronic Cr(VI) exposure to induce CSC-like property and cell transformation, implying that miR-494 down-regulation plays a critical role in Cr(VI)-induced cell transformation process.
Figure 5.
Stably overexpressing miR-494 in parental BEAS-2B cells significantly reduces chronic Cr(VI) exposure inducing CSC-like property and cell transformation. A, Q-PCR analysis of the relative expression levels of miR-494 in parental BEAS-2B GFP vector control [BEAS-2B-GFP] cells or GFP-miR-494 stable overexpression [BEAS-2B-GFP-miR-494] cells. The miR-494 level in miR-494 stable overexpression cells was expressed relative to GFP vector control cells (mean ± standard deviations, n = 3). *p < .05, compared with the GFP vector control cells. B, Western blot analysis of c-Myc expression level in chronic low dose Cr(VI)-exposed GFP vector control and GFP-miR-494 stable overexpression cells. After exposure to 0.25 µM of Cr(VI) (K2Cr2O7) for 20 weeks, GFP vector control [BEAS-2B-GFP-Cr(VI)] and miR-494 stable overexpression cells [BEAS-2B-GFP-miR-494-Cr(VI)] were harvested for Western blot analysis. C, MTT analysis of the growth curve of chronic low dose Cr(VI)-exposed GFP vector control and GFP-miR-494 stable overexpression cells. The results are presented as means ± standard deviations (n = 6). *p < .05, compared with the chronic low dose Cr(VI)-exposed GFP vector control cells. Quantitation of suspension culture sphere formation (D) and soft agar colony formation (E) by chronic low dose Cr(VI)-exposed GFP vector control cells or GFP-miR-494 stable overexpression cells. The results are presented as means ± standard deviations (n = 3). *p < .05, compared with the chronic low dose Cr(VI)-exposed GFP vector control cells.
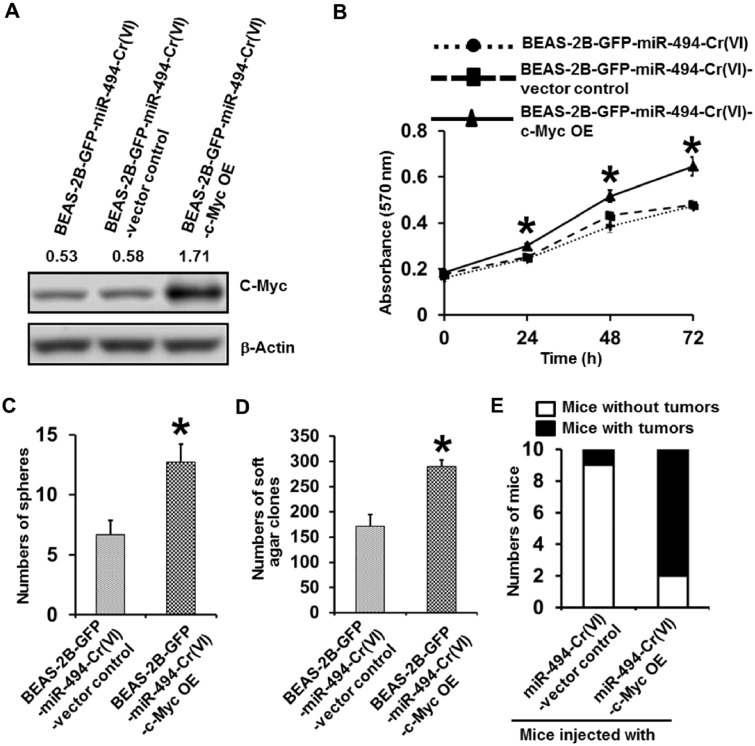
Stably Overexpressing c-Myc in Chronic Cr(VI)-Exposed miR-494 Stable Overexpression Cells Reverses the Inhibitory Effect of miR-494 on Cr(VI)-Induced CSC-Like Property, Cell Transformation and Tumorigenesis
After demonstrating that stably overexpressing miR-494 is able to significantly reduce Cr(VI) exposure-induced CSC-like property and cell transformation, we next wanted to further determine whether miR-494 overexpression inhibits Cr(VI) exposure-induced CSC-like property and cell transformation mainly through down-regulating c-Myc expression. To address this point, we generated vector control and nontargetable c-Myc stable overexpression cells in chronic Cr(VI)-exposed miR-494 stable overexpression cells [BEAS-2B-GFP-miR-494-Cr(VI)-vector control; BEAS-2B-GFP-miR-494-Cr(VI)-c-Myc OE]. The c-Myc overexpression in the double (c-Myc and miR-494) overexpression cells was confirmed by Western blot analysis (Figure 6A). MTT analysis showed that overexpressing nontargetable c-Myc in chronic Cr(VI)-exposed miR-494 stable expression cells significantly increases cell proliferation (Figure 6B). Moreover, suspension culture sphere formation assay showed that c-Myc and miR-494 double overexpression cells formed significantly more spheres than the corresponding vector control cells (Figure 6C), which is comparable to the number of spheres formed by Cr(VI)-exposed control cells (Figure 5D). Similarly, the c-Myc and miR-494 double overexpression cells also grew significantly more colonies in soft agar (Figure 6D), which is also comparable to the number of soft agar colonies formed by Cr(VI)-exposed control cells (Figure 5E). Furthermore, mouse tumorigenesis experiment showed while only one of ten mice injected with BEAS-2B-GFP-miR-494-Cr(VI)-vector control cells has tumor (tumor incidence rate: 10%); however, eight of ten mice injected with the c-Myc and miR-494 double overexpression cells grow tumors (tumor incidence rate: 80%, p < .025 compared with miR-494-Cr(VI)-vector control cell group) (Figure 6E). Together, these results suggest that stably overexpressing c-Myc is capable of reversing the inhibitory effect of miR-494 overexpression on Cr(VI) exposure-induced CSC-like property, cell transformation and tumorigenesis. These results also provide evidence showing that miR-494 overexpression reduces Cr(VI) exposure-induced CSC-like property, cell transformation and tumorigenesis mainly through down-regulating c-Myc expression.
Figure 6.
Stably overexpressing nontargetable c-Myc in chronic low dose Cr(VI)-exposed miR-494 stable overexpression cells reverses the inhibitory effect of miR-494 on Cr(VI)-induced CSC-like property, cell transformation and tumorigenesis. A, Western blot analysis of c-Myc expression level in chronic low dose Cr(VI)-exposed miR-494 stable expression (BEAS-2B-GFP-miR-494-Cr(VI)), vector control [BEAS-2B-GFP-miR-494-Cr(VI)-vector control], and c-Myc overexpression [BEAS-2B-GFP-miR-494-Cr(VI)-c-Myc OE] cells. B, MTT analysis of the growth curve of chronic low dose Cr(VI)-exposed miR-494 stable expression, vector control and c-Myc overexpression cells. The results are presented as means ± standard deviations (n = 6). *p < .05, compared with the chronic low dose Cr(VI)-exposed miR-494 stable expression with c-Myc overexpression cells. Quantitation of suspension culture sphere formation (C) and soft agar colony formation (D) by chronic low dose Cr(VI)-exposed miR-494 stable expression vector control and miR-494 stable expression with c-Myc overexpression cells. The results are presented as means ± standard deviations (n = 3). *p < .05, compared with the chronic low dose Cr(VI)-exposed miR-494 stable expression vector control cells. E, Effect of overexpressing c-Myc in chronic low dose Cr(VI)-exposed miR-494 stable expression cells on their tumor forming capability in nude mice (n = 10).
DISCUSSION
The mechanism of the carcinogenicity of Cr(VI), one of the most common and well-recognized Group I environmental carcinogen causing lung cancer and other types of cancers in humans, has not been well understood. Ours and other recent studies showed that chronic Cr(VI) exposure is capable of inducing CSC-like property (Dai et al., 2017; Wang et al., 2018). Interestingly, chronic exposure to other common metal carcinogens such as arsenic, cadmium, and nickel has also been shown to be able to produce CSC-like cells (Wang and Yang, 2019). Given the well-accepted role of CSCs or CSC-like cells in initiating and maintaining cancer, the capability of metal carcinogen exposure producing CSC-like cells may represent an important mechanism of metal carcinogenesis (Wang and Yang, 2019). Nonetheless, the mechanism of how metal carcinogen exposure induces CSC-like property remains largely unknown (Wang and Yang, 2019). In this study we determined that chronic Cr(VI) exposure induces CSC-like property and tumorigenesis by increasing c-Myc expression through down-regulating the level of miR-494.
The c-Myc gene is a proto-oncogene and its expression and activity are highly upregulated in more than half of human cancers; as a result, high c-Myc expression or activation is considered as a hallmark of both cancer initiation and maintenance (Dang, 2012; Gabay et al., 2014). The role of c-Myc in metal carcinogenesis was initially explored by analyzing its expression level in metal carcinogen-transformed cells. It was found that c-Myc expression level was upregulated in arsenic-transformed rat liver epithelial cells (TRL 1215) and human bronchial epithelial cells (HBEC), cadmium-transformed mouse fibroblast cells (BALB/c-3T3), and Cr(VI)-transformed human bronchial epithelial cells (BEAS-2B) (Chen et al., 2001; Joseph et al., 2001; Pratheeshkumar et al., 2016; Wang et al., 2013). However, whether c-Myc upregulation plays an important role in the malignant phenotypes of metal carcinogen-transformed cells or contributes significantly to metal carcinogens-caused CSC-like property, cell malignant transformations and tumorigenesis has not been determined.
Our recent study showed that chronic Cr(VI) exposure induces CSC-like property, cell malignant transformation and tumorigenesis (Wang et al., 2018). In current study, we also found that c-Myc protein level in Cr(VI)-transformed human bronchial epithelial cells is dramatically higher than that in passage-matched control cells. By using shRNA to stably knock down c-Myc expression in Cr(VI) transformed cells, we demonstrated that down-regulation of c-Myc expression significantly reduces CSC-like property of Cr(VI)-transformed cells as evidenced by their reduced capability of forming suspension spheres and tumorigenicity in nude mice. These findings indicate that high c-Myc level in Cr(VI)-transformed cells plays an important role in maintaining their CSC-like property and tumor initiating capability. In addition, we also stably knocked down c-Myc expression in parental human bronchial epithelial cells and the c-Myc knockdown cells were chronically exposed to Cr(VI) for assessing cell transformation. The finding that c-Myc stable knockdown significantly decreases the ability of Cr(VI) exposure to induce CSC-like property and cell transformation provides evidence showing that c-Myc upregulation contributes significantly to Cr(VI)-induced CSC-like property and cell transformation.
To further demonstrate an important role of c-Myc upregulation in Cr(VI) inducing CSC-like property and cell transformation, we asked whether stably overexpressing c-Myc alone in parental BEAS-2B cells is able to induce CSC-like property and cell transformation. To answer this question, we stably overexpressed c-Myc in parental BEAS-2B cells. The results from suspension culture sphere formation assay and soft agar colony formation assay showed that overexpressing c-Myc alone significantly increases the capability of parental BEAS-2B cells forming suspension spheres and soft agar colonies, suggesting that overexpressing c-Myc alone in BEAS-2B cells is capable of inducing CSC-like property and cell transformation. These findings support our conclusion that upregulation of c-Myc by Cr(VI) exposure plays an important role in chronic Cr(VI) exposure-induced CSC-like property and cell transformation.
How does chronic Cr(VI) exposure upregulate the level of c-Myc expression? The proto-oncogene c-Myc functions as a transcription factor regulating thousands of gene expression including those critically involved in stemness and carcinogenesis (Chappell and Dalton, 2013; Dang, 2012; Yoshida, 2018). On the other hand, the expression and activity of c-Myc are strictly regulated in normal cells by multiple mechanisms to ensure normal cell functions (Dang, 2012; Stine et al., 2015). Among many mechanisms that tightly control c-Myc expression, the epigenetic regulation of c-Myc expression level mediated by noncoding RNAs such as microRNAs (miRNAs) has been well-documented (Jackstadt and Hermeking, 2015; Mei and Wu, 2016). One of the miRNAs that is able to down-regulate c-Myc expression and has anti-CSC-like property is the miR-494 (Chen et al., 2017; Yu et al., 2018). C-Myc has been shown to be a validated target of miR-494 (Jackstadt and Hermeking, 2015). In this study we found that the expression level of miR-494 is significantly down-regulated in Cr(VI)-transformed cells. Re-expressing miR-494 in Cr(VI)-transformed cells greatly reduced c-Myc protein level and their transformed phenotypes. Moreover, stable overexpression of miR-494 in parental human bronchial epithelial cells significantly impaired the ability of chronic Cr(VI) exposure to upregulate c-Myc level and induce CSC-like property, cell malignant transformation and tumorigenesis. Furthermore, overexpressing a nontargetable c-Myc overcome the inhibitory effect of miR-494 on Cr(VI)-induced CSC-like property and tumorigenesis. Together, these findings indicate that chronic Cr(VI) exposure increases c-Myc expression by down-regulating the level of miR-494. Ours and previous other studies showed chronic arsenic or Cr(VI) exposure may upregulate c-Myc expression by activating the Wnt/β-catenin pathway (Pratheeshkumar et al., 2016; Wang et al., 2013). It is likely that Cr(VI) activation of other signaling pathways such as the Wnt/β-catenin pathway may also contribute to c-Myc upregulation by Cr(VI) exposure.
How does chronic Cr(VI) exposure reduce miR-494 expression? Our recent study showed that upregulation of histone repressive methylations mediated by increasing expression of histone methyltransferases contributes causally to chronic Cr(VI) exposure-induced CSC-like property and cell transformation (Wang et al., 2018); however, how increased histone repressive methylations promote Cr(VI)-caused CSC-like property and cell transformation remains to be determined. Because repressive histone methylation modifications inhibit gene expression, we hypothesize that one mechanism of increased histone repressive methylations promoting Cr(VI)-induced CSC-like property and cell transformation is to down-regulate miR-494 expression and increase c-Myc expression. Our ongoing research is testing this hypothesis.
It is interesting to note while down-regulating c-Myc level by using c-Myc shRNA in Cr(VI)-transformed cells significantly reduces their proliferation (Supplementary Figure 1C), but c-Myc down-regulation by overexpressing miR-494 in Cr(VI)-transformed cells does not display a significant effect on cell proliferation (Figure 4D). However, stably expressing miR-494 in parental nontransformed cells could prevent chronic Cr(VI) exposure-induced cell transformation and reduce cell proliferation (Figure 5C). The underlying mechanisms responsible for these inconsistent observations are currently not clear. One potential reason could be that miR-494 may also target other genes important for cell proliferation in addition to c-Myc and may exhibit cellular context-dependent effects on cell proliferation when overexpressed in Cr(VI)-transformed cells or in parental nontransformed cells followed by chronic Cr(VI) exposure.
In summary, we showed that the proto-oncogene c-Myc expression level is dramatically increased in chronic low dose Cr(VI) exposure-transformed human bronchial epithelial BEAS-2B cells. By using shRNA stable knockdown approach, we demonstrated that downregulation of c-Myc in Cr(VI)-transformed cells significantly reduces their CSC-like property and tumorigenicity. Moreover, stable knockdown of c-Myc expression in parental BEAS-2B cells significantly impaired the ability of chronic Cr(VI) exposure to induce CSC-like property and cell transformation. We also determined that stably overexpressing c-Myc alone in parental BEAS-2B cells is capable of inducing CSC-like property and cell transformation, providing additional support to our conclusion that upregulation of c-Myc by Cr(VI) exposure plays an important role in Cr(VI) exposure-induced CSC-like property and cell transformation. Further mechanistic and functional studies indicated that chronic Cr(VI) exposure increases c-Myc expression by down-regulating the level of miR-494. Taken together, this study reveals an important role of c-Myc upregulation in Cr(VI)-induced CSC-like property, cell malignant transformation and tumorigenesis.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Dr Jianrong Wu at the Shared Resources Facility on Biostatistics and Bioinformatics at University of Kentucky Markey Cancer Center for his assistance with statistical analysis.
FUNDING
This research was supported by National Institute of Environmental Health Sciences grants 5R01ES026151, 1R01ES028256, 1P30ES026529-01A1 and The University of Kentucky Center for Appalachian Research in Environmental Sciences Career Development Award. This research was also supported by the Shared Resources Facilities on Biostatistics and Bioinformatics and Biospecimen Procurement and Translational Pathology at University of Kentucky Markey Cancer Center (P30CA177558).
REFERENCES
- Ali A. H., Kondo K., Namura T., Senba Y., Takizawa H., Nakagawa Y., Toba H., Kenzaki K., Sakiyama S., Tangoku A. (2011). Aberrant DNA methylation of some tumor suppressor genes in lung cancers from workers with chromate exposure. Mol. Carcinog. 5, 89–99. [DOI] [PubMed] [Google Scholar]
- Batlle E., Clevers H. (2017). Cancer stem cells revisited. Nat. Med. 23, 1124–1134. [DOI] [PubMed] [Google Scholar]
- Chappell J., Dalton S. (2013). Roles for MYC in the establishment and maintenance of pluripotency. Cold Spring Harb. Perspect. Med. 3, a014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Liu J., Zhao C. Q., Diwan B. A., Merrick B. A., Waalkes M. P. (2001). Association of c-myc overexpression and hyperproliferation with arsenite-induced malignant transformation. Toxicol. Appl. Pharmacol. 175, 260–268. [DOI] [PubMed] [Google Scholar]
- Chen S. M., Wang B. Y., Lee C. H., Lee H. T., Li J. J., Hong G. C., Hung Y. C., Chien P. J., Chang C. Y., Hsu L. S., et al. (2017). Hinokitiol up-regulates miR-494-3p to suppress BMI1 expression and inhibits self-renewal of breast cancer stem/progenitor cells. Oncotarget 8, 76057–76068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Ji Y., Wang W., Kim D., Fai L. Y., Wang L., Luo J., Zhang Z. (2017). Loss of fructose-1, 6-bisphosphatase induces glycolysis and promotes apoptosis resistance of cancer stem-like cells: An important role in hexavalent chromium-induced carcinogenesis. Toxicol. Appl. Pharmacol. 331, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V. (2012). MYC on the path to cancer. Cell 149, 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G., Abdallah W. M., Foley J. M., Jackson K. W., Clarke M. F., Kawamura M. J., Wicha M. S. (2003). In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17, 1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay M., Li Y., Felsher D. W. (2014). MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect. Med. 4, a014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D. A., Baek C., Estrada M. V., Tysl T., Bennett E. J., Yang J., Chang J. T. (2018). USP11 enhances TGFβ-induced epithelial-mesenchymal plasticity and human breast cancer metastasis. Mol. Cancer Res. 16, 1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Li Z. J., Sun P. (2019). MicroRNA-494 promotes the proliferation and migration of human glioma cancer cells through the protein kinase B/mechanistic target of rapamycin pathway by phosphatase and tensin homolog expression. Oncol. Rep. 41, 351–360. [DOI] [PubMed] [Google Scholar]
- Ho M. M., Ng A. V., Lam S., Hung J. Y. (2007). Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 67, 4827–4833. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (1990). Chromium. IARC Monogr. Eval. Carcinog. Risks Hum. 49, 49–256. [PMC free article] [PubMed] [Google Scholar]
- Jackstadt R., Hermeking H. (2015). MicroRNAs as regulators and mediators of c-MYC function. Biochim. Biophys. Acta 1849, 544–553. [DOI] [PubMed] [Google Scholar]
- Joseph P., Muchnok T. K., Klishis M. L., Roberts J. R., Antonini J. M., Whong W. Z., Ong T. (2001). Cadmium-induced cell transformation and tumorigenesis are associated with transcriptional activation of c-fos, c-jun, and c-myc proto-oncogenes: Role of cellular calcium and reactive oxygen species. Toxicol. Sci. 61, 295–303. [DOI] [PubMed] [Google Scholar]
- Kondo K., Takahashi Y., Hirose Y., Nagao T., Tsuyuguchi M., Hashimoto M., Ochiai A., Monden Y., Tangoku A. (2006). The reduced expression and aberrant methylation of p16(INK4a) in chromate workers with lung cancer. Lung Cancer 53, 295–302. [DOI] [PubMed] [Google Scholar]
- Macedo T., Silva-Oliveira R. J., Silva V. A. O., Vidal D. O., Evangelista A. F., Marques M. M. C. (2017). Overexpression of mir-183 and mir-494 promotes proliferation and migration in human breast cancer cell lines. Oncol. Lett. 14, 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majello B., Perini G. (2015). Myc proteins in cell biology and pathology. Biochim. Biophys. Acta 1849, 467–468. [DOI] [PubMed] [Google Scholar]
- Mei Y., Wu M. (2016). Noncoding RNAs regulating p53 and c-Myc signaling. Adv. Exp. Med. Biol. 927, 337–365. [DOI] [PubMed] [Google Scholar]
- Nguyen L. V., Vanner R., Dirks P., Eaves C. J. (2012). Cancer stem cells: An evolving concept. Nat. Rev. Cancer 12, 133–143. [DOI] [PubMed] [Google Scholar]
- Plaks V., Kong N., Werb Z. (2015). The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 5, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratheeshkumar P., Son Y. O., Divya S. P., Turcios L., Roy R. V., Hitron J. A., Wang L., Kim D., Dai J., Asha P., et al. (2016). Hexavalent chromium induces malignant transformation of human lung bronchial epithelial cells via ROS-dependent activation of miR-21-PDCD4 signaling. Oncotarget 7, 51193–51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine Z. E., Walton Z. E., Altman B. J., Hsieh A. L., Dang C. V. (2015). MYC, metabolism, and cancer. Cancer Discov. 5, 1024–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout M. D., Herbert R. A., Kissling G. E., Collins B. J., Travlos G. S., Witt K. L., Melnick R. L., Abdo K. M., Malarkey D. E., Hooth M. J. (2009). Hexavalent chromium is carcinogenic to F344/N rats and B6C3F1 mice after chronic oral exposure. Environ. Health Perspect. 117, 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara E., Saya H. (2013). Complexity of cancer stem cells. Int. J. Cancer 132, 1249–1259. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Tokar E. J., Diwan B. A., Waalkes M. P. (2010). Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ. Health Perspect. 118, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar E. J., Person R. J., Sun Y., Perantoni A. O., Waalkes M. P. (2013). Chronic exposure of renal stem cells to inorganic arsenic induces a cancer phenotype. Chem. Res. Toxicol. 26, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano A. M., Ferreira L. M., Alpoim M. C. (2012). Molecular and cellular mechanisms of hexavalent chromium-induced lung cancer: An updated perspective. Curr. Drug Metab. 13, 284–305. [DOI] [PubMed] [Google Scholar]
- Wahlström T., Henriksson M. A. (2015). Impact of MYC in regulation of tumor cell metabolism. Biochim. Biophys. Acta 1849, 563–569. [DOI] [PubMed] [Google Scholar]
- Wang Z., Yang C. (2019). Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: A novel mechanism of metal carcinogenesis. Semin. Cancer Biol. 57, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Humphries B., Xiao H., Jiang Y., Yang C. (2013). Epithelial to mesenchymal transition in arsenic-transformed cells promotes angiogenesis through activating β-catenin-vascular endothelial growth factor pathway. Toxicol. Appl. Pharmacol. 271, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Humphries B., Xiao H., Jiang Y., Yang C. (2014). MicroRNA-200b suppresses arsenic-transformed cell migration by targeting protein kinase Cα and Wnt5b-protein kinase Cα positive feedback loop and inhibiting Rac1 activation. J. Biol. Chem. 289, 18373–18386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wu J., Humphries B., Kondo K., Jiang Y., Shi X., Yang C., (2018). Upregulation of histone-lysine methyltransferases plays a causal role in hexavalent chromium-induced cancer stem cell-like property and cell transformation. Toxicol. Appl. Pharmacol. 342, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhao Y., Smith E., Goodall G. J., Drew P. A., Brabletz T., Yang C. (2011). Reversal and prevention of arsenic-induced human bronchial epithelial cell malignant transformation by microRNA-200b. Toxicol. Sci. 121, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Li Y., Tao H., Humphries B., Li A., Jiang Y., Yang C., Luo R., Wang Z. (2018). Integrin α5 down-regulation by miR-205 suppresses triple negative breast cancer stemness and metastasis by inhibiting the Src/Vav2/Rac1 pathway. Cancer Lett. 433, 199–209. [DOI] [PubMed] [Google Scholar]
- Yang C., Liu Y., Leskow F. C., Weaver V. M., Kazanietz M. G. (2005). Rac-GAP-dependent inhibition of breast cancer cell proliferation by {beta}2-chimerin. J. Biol. Chem. 280, 24363–24370. [DOI] [PubMed] [Google Scholar]
- Yang C., Wu J., Zhang R., Zhang P., Eckard J., Yusuf R., Huang X., Rossman T. G., Frenkel K. (2005). Caffeic acid phenethyl ester (CAPE) prevents transformation of human cells by arsenite (As) and suppresses growth of As-transformed cells. Toxicology 213, 81–96. [DOI] [PubMed] [Google Scholar]
- Yang Y., Tao X., Li C. B., Wang C. M. (2018). MicroRNA-494 acts as a tumor suppressor in pancreatic cancer, inhibiting epithelial-mesenchymal transition, migration and invasion by binding to SDC1. Int. J. Oncol. 53, 1204–1214. [DOI] [PubMed] [Google Scholar]
- Yoshida G. J. (2018). Emerging roles of Myc in stem cell biology and novel tumor therapies. J. Exp. Clin. Cancer Res. 37, 173.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Yu X., Liu H., Song Q., Yang Y. (2018). miR-494 inhibits cancer-initiating cell phenotypes and reverses resistance to lapatinib by downregulating FGFR2 in HER2-positive gastric cancer. Int. J. Mol. Med. 42, 998–1007. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Guo L., Li Y., Feng G. H., Teng F., Li W., Zhou Q. (2018). MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer. Mol. Cancer 17, 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Tan Y. S., Haslam S. Z., Yang C., (2010). Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol. Sci. 115, 1. 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wang Z., Jiang Y., Yang C. (2011). Inactivation of Rac1 reduces Trastuzumab resistance in PTEN deficient and insulin-like growth factor I receptor overexpressing human breast cancer SKBR3 cells. Cancer Lett. 313, 54–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.