Abstract
The aim of the present study was to examine the association between the migration of breast cancer cells in vitro and radiosensitivity by establishing a breast cancer cell model with different migratory capacities. Transwell chambers in a 24-well plate were used to separate MDA-MB-231 and ZR-7530 cells and to establish cell models with different migratory capacities. Subsequently, the radiosensitivity of the cell models was measured using a radiation clone formation assay. Furthermore, differential gene expression was determined using gene microarray analysis. The protein expression levels of the differentially expressed genes (DEGs) were assessed using western blot analysis. From each parental cell line, a pair of daughter cell lines were established in with differing migratory abilities. These daughter cell lines were named MDA-MB-231 UP-10 (231 UP-10), MDA-MB-231 Down-10 (231 Down-10), ZR-75-30 UP-10 (7530 UP-10) and ZR-75-30 Down-10 (7530 Down-10). Radiation clone formation assays revealed that the cell lines with increased migratory abilities (231 Down-10 and 7530 Down-10) demonstrated higher radio-resistance compared with the cell lines with decreased migratory abilities (231 UP-10 and 7530 UP-10). Gene microarrays identified numerous DEGs between the pairs of UP and Down cell lines. A focus was placed on genes associated with cell adhesion and it was identified that phosphorylated Fak and phosphorylated EGFR expression levels were increased in 231 Down-10 and 7530 Down-10 cells, compared with the 231 UP-10 and 7530 UP-10 cells. Other genes including ZO-1, FN1 and SOX9 expression were also increased in the 231 Down-10 and 7530 Down-10 cells compared with 231 UP-10 and 7530 UP-10 cells. Cell lines with increased migratory capacities may be more radio-resistant compared with cell lines with a decreased migratory capabilities. The mechanism may be associated with changes in the expression of cell adhesion molecules and epithelial-mesenchymal transition (EMT). Therapeutic strategies targeting cell adhesion or EMT may increase the radiation sensitivity of breast cancer cells, in addition to improving the effect of radiation therapy.
Keywords: breast cancer, cell migration, radiosensitivity, cell adhesion, epithelial-mesenchymal transition
Introduction
Breast cancer is one of the most common types of cancer worldwide. In recent years, in China, the mortality rate of patients with breast cancer has continuously increased. As such, breast cancer is a significant cause of mortality in females (1). As the result of multidisciplinary collaborations, there have been significant advances in the treatment of patients with breast cancer, of which radiotherapy is a key strategy (2,3). Postoperative radiotherapy decreases the lymph node recurrence rate by approximately two-thirds. Clinical data has demonstrated that breast-conserving surgery combined with radiotherapy decreases the local recurrence rate from 18–35 to 2–10% (4); however, certain patients do experience relapse, suggesting that their tumors may be resistant to radiotherapy. Therefore, it is important to examine the mechanism underlying this phenomenon and determine the intrinsic radiosensitivity of cells and the cellular microenvironment (5,6). Studies regarding radiosensitivity have progressed through 3 different stages; from the tissue to the cellular level and subsequently to the molecular level. The third stage concerns how biological processes may affect cell radiosensitivity, including hypoxia, cell cycle distribution, cell proliferation, apoptosis, DNA damage repair and more (7–9), and these mechanisms involve genetic variation and epigenetic modification. Therefore, it may be possible to determine the effect of cell radiosensitivity by examining the regulatory mechanisms underlying these processes.
Invasion and metastasis are important behavioral characteristics of cancer cells, and acquisition of these processes endows cells with a variety of properties. For example, epithelial-mesenchymal transition (EMT) is associated with cell invasion and metastasis, cell stemness, and radiotherapy and chemotherapy tolerance (10). Numerous transcription factors regulate EMT, including Zinc finger E-box-binding homeobox protein, Snail, Slug, and Twist. These factors downregulate epithelial cadherin (E-cadherin), which is pivotal in the EMT process (11). E-cadherin participates in the regulation of cell adhesion, which serves a critical role in cancer cell invasion and metastasis (12). Our previous study indicated that human epidermal growth factor receptor 2 (HER2) decreased breast cancer cell radiosensitivity by activating focal adhesion kinase in vitro and in vivo (13). Therefore, cell adhesion processes, and invasion and metastatic processes may be associated with the response to radiotherapy. To determine whether there was an association between migration and metastasis, radiosensitivity, daughter cell lines with differing migratory capabilities from 2 parent cell lines were established in the present study using Transwell chambers in a 24-well plate. There was a negative association between migration and radiosensitivity and this may be associated with the expression of cell adhesion molecules and/or EMT.
Materials and methods
Cell lines and cell culture
The breast cancer MDA-MB-231 and ZR-75–30 cell lines were purchased from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 units/ml), streptomycin (100 µg/ml) 2 mM l-glutamine and 1 mM sodium pyruvate. All cells were incubated in a 37°C in a humidified atmosphere with 5% CO2.
Migration assays
The migration assay was performed as previously described (13,14). Briefly, a total of 2×104 of MDA-MB-231 or ZR-75–30 were placed in the upper chamber of a Transwell chamber (BD Biosciences) with an 8-µm pore filter between the chambers. The cells were allowed to migrate at 37°C for 8 h toward the chamber of medium supplemented with 2.5% fetal bovine serum. Non-migrating cells on the upper side of the insert were removed and the migrated cells on the lower side of the insert were fixed with ice-cold methanol for 10 min at room temperature, stained with 0.1% crystal violet for 20 min at room temperature, imaged and counted at magnification ×200 under a light microscope. The assay was repeated 3 times in duplicate.
Establishment of the cell model
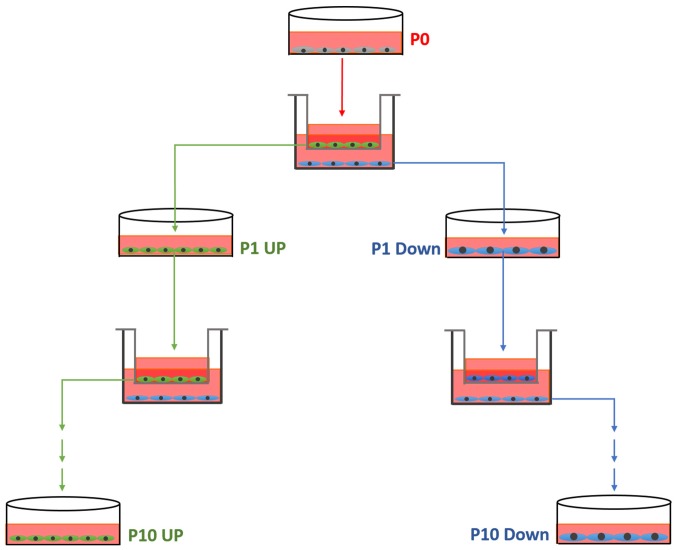
From each cell line, a pair of cell lines differing in migratory ability was established, according to the schematic diagram in Fig. 1. Initially, a migration assay was performed as aforementioned (named P0). The cells from the upper chamber, which had not migrated, were collected and cultured in new dishes, termed 231 Down-1 (P1). The cells which had migrated through the insert after 8 h were also collected and cultured. These cells were termed 231 UP-1 (P1). This process was repeated 10 times, each time using the collected cells that had or had not migrated, until the MDA-MB-231 UP-10, MDA-MB-231 Down-10, ZR-75-30-UP-10 and ZR-75-30-Down-10 cell cultures were established.
Figure 1.
Schematic diagram of the process of the establishment of the cell model.
Genes microarray
Total RNA was isolated from 2×106 target cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and was treated with DNase I to remove any contaminating genomic DNA. Microarray analysis was used to screen changes in genome-wide gene expression patterns in the MDA-MB-231 UP-10 and MDA-MB-231 Down-10 cells, the ZR-75–30 UP-10 and ZR-75–30 Down-10 cell line. The changes in human gene expression patterns were assessed using Affymetrix gene microarrays (CapitalBio Technology Co., Ltd.) and 3 replicates were used for microarrays analysis. Gene ontology (GO) enrichment analysis (14,15) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis (16–18) were performed to analyze the pathways involved.
Irradiation and clone formation assay
MDA-MB-231 UP-10, MDA-MB-231 Down-10 cells, ZR-75–30 UP-10 and ZR-75–30 Down-10 cells were seeded into 6 cm cell culture dishes at a density of 1×106 cells. A total of 24 h later, the cells were irradiated with a single dose of X-rays (0, 2, 4, 6 and 8 Gy), using a linear accelerator (Varian Medical Systems) at 3 Gy/min and then seeded into 6 cm petri dishes and incubated for 10–14 days to allow colonies to form. The cell inoculum of the single cell suspension was determined for each sample according to the expected number of colony formation (30–100). The colonies were fixed with 4% paraformaldehyde for 20 min at room temperature and stained with 0.1% crystal violet for 20 min at room temperature. The number of colonies with ≥50 cells were counted under a microscope. The plating efficiency (PE) and survival fraction (SF) were calculated using the following equations: PE = number of colonies formed without irradiation/the number of cells inoculated ×100%; and SF = colonies counted/cells seeded × PE. All assays were performed independently at least 3 times.
Western blot analysis
MDA-MB-231 UP-10, MDA-MB-231 Down-10 cells, ZR-75–30 UP-10 and ZR-75–30 Down-10 cells were seeded into 10-cm culture dishes. Cell lysates were harvested at 75% confluence using 500 µl cell lysis buffer (25 mmol/l Tris-HCl at pH 7.6, 150 mmol/l NaCl, 1.0% Nonidet P-40, 1.0% sodium deoxycholate and 0.1% SDS) with a 30 min incubation on ice. The supernatant was collected under rotation and was centrifuged at 13,000 × g for 30 min at 4°C. The protein concentrations were determined using a BCA Protein Assay Reagent (Thermo Fisher Scientific, Inc.). Western blot analyses were performed as described previously (19). The primary antibodies included anti-fibronectin 1 (FN1; cat. No sc69681; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-β-actin (cat. no. 3700s; 1:1,000; Cell Signaling Technology, Inc.), anti-tight junction protein ZO-1 (ZO-1; cat. no. 8193s; 1:1,000; Cell Signaling Technology, Inc.), anti-focal adhesion kinase (FAK; cat. no. 9330T; 1:1,000; Cell Signaling Technology, Inc.), anti-epidermal growth factor receptor (EGFR; cat. no. 4267s; 1:1,000; Cell Signaling Technology, Inc.), anti-pEGFR (cat. nos. Y1068 and 3777s; 1:1,000; Cell Signaling Technology, Inc.), anti-pEGFR (cat. nos. Y1173 and 4407s; 1:1,000; Cell Signaling Technology, Inc.) and transcription factor SOX-9 (SOX9; cat. no. 82630s; 1:1,000; Cell Signaling Technology, Inc.). Horseradish peroxidase-conjugated secondary antibodies included donkey anti-mouse immunoglobulin (cat. no. NA931; 1:2,000; GE Healthcare) and donkey anti-rabbit immunoglobulin (cat. no. NA9340; 1:2,000; GE Healthcare).
Statistical analysis
Statistical analysis was performed using the SPSS Statistics version 22.0 (IBM Corp.). Quantitative data were presented as the mean ± standard deviation. Comparisons between the means of two groups were conducted using a unpaired Student's t-test. The experimental data were analyzed and plotted using GraphPad Prism (v6.0; GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Establishment and verification of the cell model
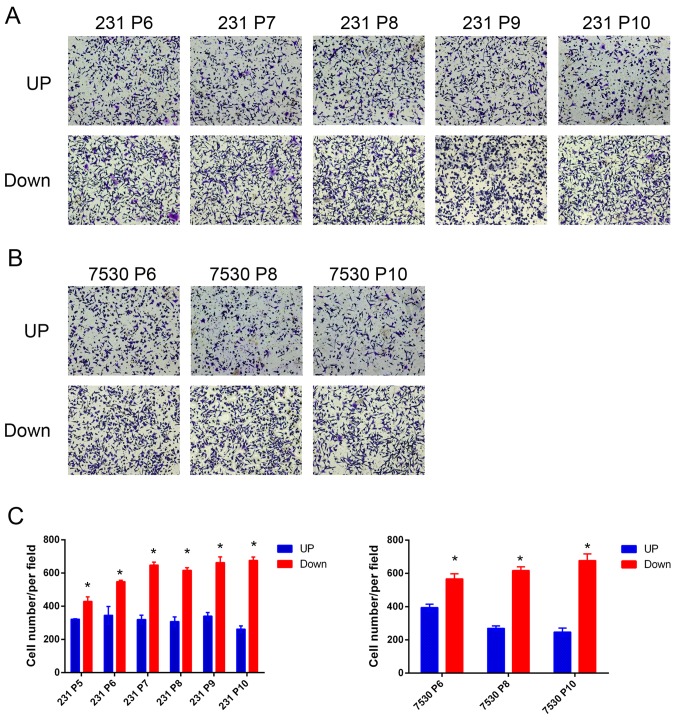
To accurately determine whether there was any association between migratory capacity and radiosensitivity, the differences in genetic background between different cells must be removed. Therefore, cell models from the same parent cell line that exhibited different degrees of migratory capabilities was established. In this model, the Transwell chamber served as a ‘selection’ tool, which separated MDA-MB-231 or ZR-75–30 cells into two groups of cells based on their migratory capacities. For each parent cell line, 2 daughter cell lines were established, MDA-MB-231 UP, MDA-MB-231 Down cell line, ZR-75–30 UP and ZR-75–30 Down cell line. To examine the model, a migration assay was performed and the number of cells that had migrated through the membrane was quantified. The results indicated that a greater number of MDA-MB-231 Down cells migrated through the membrane compared with the MDA-MB-231 UP cells after the 5th repetition (P5). A similar effect was observed in the ZR-75–30 daughter cell lines. Furthermore, the cells in the later generations tended to exhibit increased migratory capabilities (Fig. 2). Following the 10th repetition (P10), the resultant daughter cell lines were used for further study.
Figure 2.
Validation of the cell model establishment using a Transwell assay. (A and B) Representative images of the migrated (A) MDA-MB-231 and (B) ZR-75–30 cell line generations. (C) Quantitative analysis of the number of migrated cells. A total of 5 images were captured at random locations for each experiment. *P<0.05 vs. UP cells. 231, MDA-MB-231; 7530, ZR-75-30.
A high migratory capacity is associated with increased radio-resistance
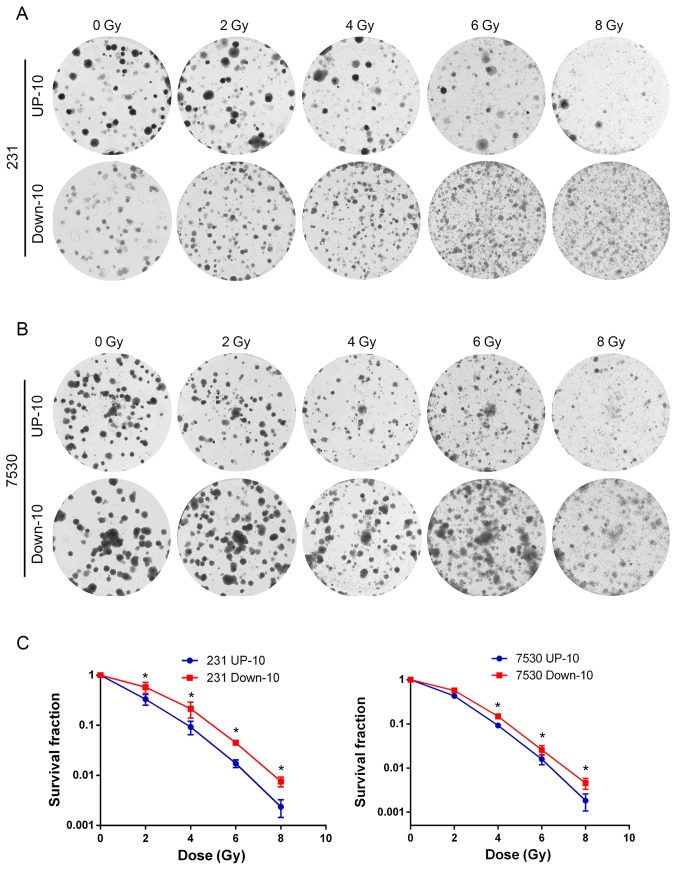
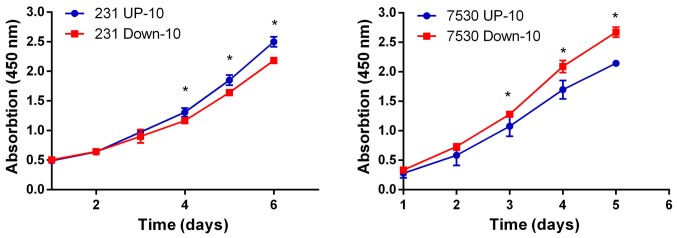
To investigate the association between the migratory capacity and radiosensitivity, irradiation and colony formation assays were performed to determine the response to irradiation in the 4 daughter cell lines. The survival curve indicated that the MDA-MB-231 Down-10 and ZR-75–30 Down-10 cells were less sensitive to radiation compared with the corresponding UP-10 cells when treated with a dose between 2–8 Gy (Fig. 3). Although the MDA-MB-231 Down-10 cells exhibited a greater migratory capacity compared with the MDA-MB-231 UP-10 cells, the proliferative capacity of the MDA-MB-231 UP-10 cells was increased. However, the proliferative capacity was lower in the ZR-75–30 UP-10 compared with the ZR-75–30 Down-10 cells (Fig. 4).
Figure 3.
Cells with an increased migratory capacity are more radio-resistant. (A and B) Representative images of irradiation and colony formation in (A) MDA-MB-231 and (B) ZR-75–30 cell line generations. (C) Survival curves of 231 UP-10, 231 Down-10, 7530 UP-10 and 7530 Down-10 cells following different doses of X-ray irradiation. 231 Down-10 and 7530 Down-10 cells were less sensitive to radiation compared with their respective UP cells when treated with a dose of X-ray between 2–8 Gy. 231, MDA-MB-231; 7530, ZR-75-30.
Figure 4.
Proliferation assay of cells with increased and decreased migratory capacities. The proliferative rate was determined using a Cell Counting Kit-8 assay. P<0.05. 231, MDA-MB-231; 7530, ZR-75-30.
Bioinformatics analysis
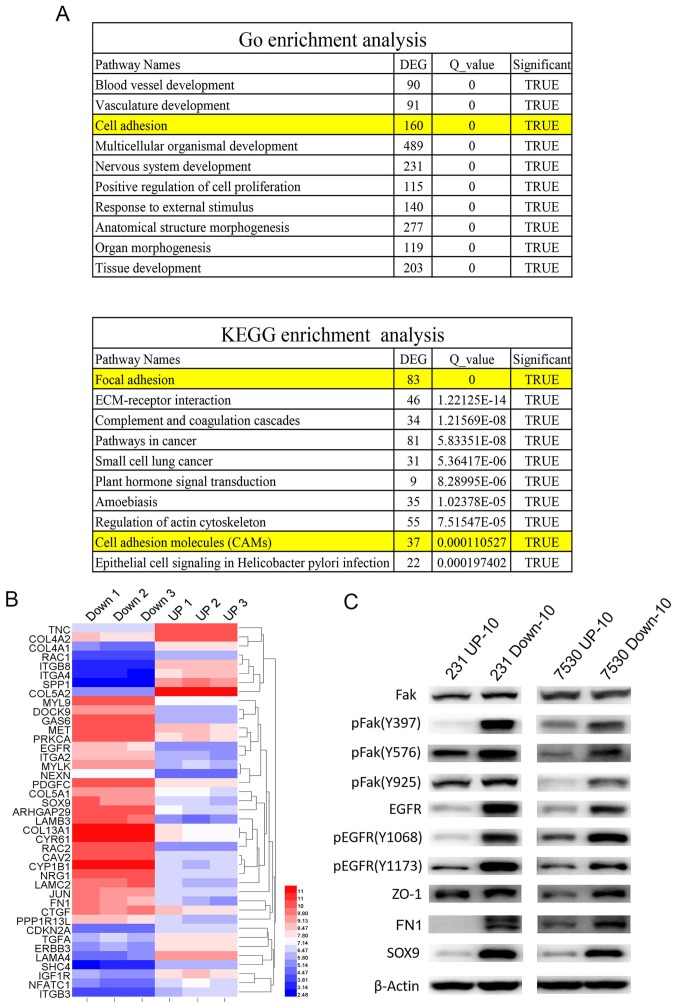
To examine the mechanism underlying cells with an increased migratory capacity increased radio-resistance, genome-wide gene profiling was performed to determine the differentially expressed genes in the MDA-MB-231 UP-10 compared with MDA-MB-231 Down-10 cells, and in the ZR-75–30 UP-10 compared with the ZR-75–30 Down-10 cells. Affymetrix gene microarrays indicated that numerous genes were upregulated or downregulated in the UP-10 cells compared with the Down-10 cells. GO and KEGG pathway assays demonstrated that a number of pathways were involved. Amongst the 10 of the most significantly altered pathways, both GO and KEGG pathway assays suggested that the cell adhesion pathway was involved (Fig. 5A and B). Our previous study demonstrated that Fak-associated genes were important in HER2-mediated radio-resistance (13). Therefore, the expression of Fak was determined. Western blot analysis results demonstrated that total Fak expression levels were similar in the UP-10 cells and Down-10 cells. However, the expression of (pFak), including Y397, Y576 and Y925 sites, were significantly increased in the Down-10 cells compared with the UP-10 cells. Furthermore, EGFR, pEGFR, ZO-1, FN1 and SOX9 were also expressed robustly in the Down-10 cells (Fig. 5C).
Figure 5.
Cell adhesion pathway is associated with radio-resistance of cells with high migratory capacities. (A) GO and KEGG pathway analyses indicated that the cell adhesion pathway was involved in radio-resistance of cells with an increased migratory capacity (231 Down-10 and 7530 Down-10 cells). (B) Cluster map of microarray-identified DEGs, demonstrating a notable change in cell adhesion associated genes. The left panel represents the gene expression ratio of 231 UP-10 cells with 231 Down-10 cells. Right panel represents the gene expression ratio of 7530 UP-10 cells with 7530 Down-10 cells. (C) Western blot analysis of molecules associated with cell adhesion signaling pathway and the epithelial-mesenchymal transition. Fak, pFak, EGFR, pEGFR, ZO-1, FN1 SOX9 were protein expression levels were measured. β-actin was used as the loading control. DEG, differentially expressed genes; 231, MDA-MB-231; 7530, ZR-75-30; Fak, focal adhesion kinase 1; p, phosphorylated; EGFR, epidermal growth factor receptor; ZO-1, tight junction protein ZO-1; FN1, fibronectin 1; SOX9, transcription factor SOX-9; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Discussion
In recent years, as a result of multidisciplinary research approaches, there has been a significant advancement in the diagnosis and treatment of breast cancer, and the overall survival of patients continues to improve. The contribution of radiotherapy to this has been indispensable. However, certain patients may experience relapse following radiotherapy, suggesting that these tumors may be radio-resistant. The intrinsic sensitivity of radiotherapy is important for the radio-response, therefore, examining the mechanism of radiosensitivity of breast cancer cells may improve the radiotherapeutic effect. A number of biological processes may affect cell radiosensitivity, including hypoxia, cell cycle distribution, cell proliferation, apoptosis, DNA damage repair and others. Invasion and metastasis are important characteristics of cancer cells and acquisition of these processes endow cells with a variety of properties. For example, EMT is associated with cell invasion and metastasis (10). A number of studies have demonstrated an association between EMT and the treatment tolerance of cancer, which have provided evidence to support the investigation of the association between cell invasiveness and radiosensitivity (20–22). Certain studies have demonstrated that genes regulating cell invasiveness can affect cell radiosensitivity (23–25). However, the direct evidence concerning the effects of cell invasiveness on radiosensitivity remain scarce. The present study focused on the association between cell migration and radiosensitivity. To investigate this issue, a cell model was established from the same parent cell line, with different migratory capacities, by using Transwell chambers as a selection pressure. The results indicated that, following 5 rounds of screening, the ‘Down’ groups of cells exhibited increased levels of migration compared with the ‘Up’ groups of cells. The effect was more evident as the selection process continued. Therefore, cells obtained following the 10th round of selection were used for further investigation. The result of the clone formation assay verified the hypothesis that cells with higher migratory capacities were associated with increased levels of radio-resistance. The proliferative capacity of the MDA-MB-231 UP-10 cells was increased compared with MDA-MB-231 Down-10 cells, but was reduced in the ZR-75–30 UP-10 compared with the ZR-75–30 Down-10 cells, suggesting that the proliferation process was not associated with the change in radiosensitivity.
As cell invasion was associated with radio-resistance, the underlying molecular mechanisms governing this association were examined. By doing so, it is possible to improve the understanding of the underlying mechanism regulating cell invasion and radiosensitivity. Additionally, it may unveil potential therapeutic strategies that simultaneously solve multiple issues (metastases and radio-resistance). Therefore, genome-wide gene profiling was performed to screen differentially expressed genes between the UP-10 and respective Down-10 cells. Numerous genes were differentially expressed and a number of associated pathways were identified. Analysis of these pathways revealed that the cell adhesion pathway was highly importance. Our previous study demonstrated that HER2 decreased the radiosensitivity of breast cancer cells by activating Fak in vitro and in vivo, and by inhibiting Fak activity using a Fak inhibitor (PF-562281), the radiosensitivity in HER2-overexpressing breast cancer cells was restored (13). As Fak serves a central role in the cell adhesion process, the expression of Fak in the cells lines in the present study was determined and it was identified that pFak, including Y397, Y576 and Y925 sites, were significantly increased in the Down-10 cells compared with the respective UP-10 cells. The expression Fak-associated genes was also identified. The results indicated that the expression of cell adhesion-associated genes, including EGFR, pEGFR, ZO-1, FN1 and SOX9 were significantly increased in the Down-10 cells compared with the respective UP-10 cells.
Fak is a 125 kD protein that is recruited as a participant in focal adhesion dynamics between cells, and has a role in motility and cell survival (26,27). It is typically located at structures known as focal adhesions, which are multi-protein structures that are connected to the extracellular matrix. It has been demonstrated that when Fak was inhibited, breast cancer cells became less metastatic as a result of a decrease in mobility (28). Numerous previous studies have indicated that Fak may participate in regulating cell proliferation, migration, anchoring and apoptosis (29,30). Certain studies have indicated that Fak is also involved in radiosensitivity regulation, although there are conflicting data on this issue: For example, Cordes et al (23) described a type of cell adhesion-mediated radio-resistance. Such a phenomenon was associated with the cell adhesion network regulated by integrin and Fak. Furthermore, a second study indicated that overexpression of Fak in the lung cancer A549 cell line resulted in cell radio-resistance, while knockdown of Fak in pancreatic cancer cells led to radiosensitization (24,25). A recent study identified that Fak may mediate the radioresistance of HIEC cells. Fak knockdown markedly increased the radiosensitivity of HIEC cells, and mice treated with FAK inhibitors exhibited aggravated mice rectal damage (31). In addition, Zhang et al (32) identified that β1-integrin-blocking antibody and FAK inhibitor-enhanced radiation induced apoptosis in esophageal cancer cells. In addition, Fak is associated with cell stemness and may initiate the EMT process. These phenotypes have been suggested to increase cell radio-resistance (33). However, contradictory results have been described by Graham et al (34), who revealed that the loss of Fak expression led to cell radio-resistance, and increasing expression of Fak in these cells restored radiosensitivity.
In conclusion, invasion and metastasis are hallmarks of cancer cells and these processes infer a variety of properties in cells. The association between migration and radiosensitivity is not fully understood. A cell model was established in which daughter cell lines with either increased or decreased migratory capacities were derived from the same parent cell line. Cell lines with an increased migratory capacity were more radio-resistant, potentially due to the upregulation of factors involved in cell adhesion and EMT. Additionally, Fak may serve a central role in radiosensitization and may be an important potential therapeutic target. A limitation of the present study was that the conclusion was based on only two pairs of cell lines. Additional cell lines are required to validate the conclusion and may provide an improved understanding of the specific pathways and components involved.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Nature Science Foundation of China (grant nos. 81702640 and 81660439) and The Guizhou provincial science and technology project [grant nos. (2017)1114 and (2017)1104].
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the author on reasonable request.
Authors' contributions
JH, LL, HZ, HC, NW and MD designed and performed experiments, prepared the figures and interpreted the data. LL and HZ drafted the results section of the manuscript. NW verified the statistical analysis and revised the manuscript. XG, QN and JH conceived and designed the study, interpreted the data, wrote and revised the manuscript and obtained funding to support the research. All authors have read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Li H, Zheng RS, Zhang SW, Zeng HM, Sun XK, Xia CF, Yang XZ, Chen WQ, He J. Incidence and mortality of female breast cancer in china, 2014. Zhonghua Zhong Liu Za Zhi. 2018;40:166–171. doi: 10.3760/cma.j.issn.0253-3766.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EBCTCG (Early Breast Cancer Trialists' Collaborative Group) McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, Gray R, Mannu G, Peto R, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;47:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 5.Joubert A, Foray N. Intrinsic radiosensitivity and DNA double-strand breaks in human cells. Cancer Radiother. 2007;11:129–142. doi: 10.1016/j.canrad.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marples B, Collis SJ. Low-dose hyper-radiosensitivity: Past, present, and future. Int J Radiat Oncol Biol Phys. 2008;70:1310–1318. doi: 10.1016/j.ijrobp.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 8.Zhivotovsky B, Joseph B, Orrenius S. Tumor radiosensitivity and apoptosis. Exp Cell Res. 1999;248:10–17. doi: 10.1006/excr.1999.4452. [DOI] [PubMed] [Google Scholar]
- 9.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–942. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Meng J, Li P, Zhang Q, Yang Z, Fu S. A radiosensitivity gene signature in predicting glioma prognostic via EMT pathway. Oncotarget. 2014;5:4683–4693. doi: 10.18632/oncotarget.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anzai E, Hirata K, Shibazaki M, Yamada C, Morii M, Honda T, Yamaguchi N, Yamaguchi N. FOXA1 induces E-cadherin expression at the protein level via suppression of slug in epithelial Breast cancer cells. Biol Pharm Bull. 2017;40:1483–1489. doi: 10.1248/bpb.b17-00307. [DOI] [PubMed] [Google Scholar]
- 12.Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]
- 13.Hou J, Zhou Z, Chen X, Zhao R, Yang Z, Wei N, Ni Q, Feng Y, Yu X, Ma J, Guo X. HER2 reduces breast cancer radiosensitivity by activating focal adhesion kinase in vitro and in vivo. Oncotarget. 2016;7:45186–45198. doi: 10.18632/oncotarget.9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: Tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Gene Ontology Consortium: The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–D338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanehisa M, Sato Y, Furumichi M, Morishima K, Tanabe M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019;47:D590–D595. doi: 10.1093/nar/gky962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou J, Wang Z, Xu H, Yang L, Yu X, Yang Z, Deng Y, Meng J, Feng Y, Guo X, Yang G. Stanniocalicin 2 suppresses breast cancer cell migration and invasion via the PKC/claudin-1-mediated signaling. PLoS One. 2015;10:e122179. doi: 10.1371/journal.pone.0122179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santamaría PG, Moreno-Bueno G, Cano A. Contribution of Epithelial plasticity to therapy resistance. J Clin Med. 2019;8:676. doi: 10.3390/jcm8050676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie P, Yu H, Wang F, Yan F, He X. Inhibition of LOXL2 enhances the radiosensitivity of castration-resistant prostate cancer cells associated with the reversal of the EMT process. BioMed Res Int. 2019;27:4012590. doi: 10.1155/2019/4012590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordes N, Meineke V. Cell adhesion-mediated radioresistance (CAM-RR). Extracellular matrix-dependent improvement of cell survival in human tumor and normal cells in vitro. Strahlenther Onkol. 2003;179:337–344. doi: 10.1007/s00066-003-1074-4. [DOI] [PubMed] [Google Scholar]
- 24.Cordes N, Frick S, Brunner TB, Pilarsky C, Grützmann R, Sipos B, Klöppel G, McKenna WG, Bernhard EJ. Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin-1. Oncogene. 2007;26:6851–6862. doi: 10.1038/sj.onc.1210498. [DOI] [PubMed] [Google Scholar]
- 25.Beinke C, Van Beuningen D, Cordes N. Ionizing radiation modules of the expression and tyrosine phosphorylation of the focal adhesion-associated proteins focal adhesion kinase (FAK) and its substrates p130cas and paxillin in A549 human lung carcinoma cells in vitro. Int J Radiat Biol. 2003;79:721–731. doi: 10.1080/09553000310001610231. [DOI] [PubMed] [Google Scholar]
- 26.Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172:151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanks SK, Ryzhova L, Shin NY, Brábek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 28.Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–370. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han EK, Mcgonigal T, Wang J, Giranda VL, Luo Y. Functional analysis of focal adhesion kinase (FAK) reduction by small inhibitory RNAs. Anticancer Res. 2004;24:3899–3905. [PubMed] [Google Scholar]
- 30.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: Mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li JJ, Tu WZ, Chen XM, Ying HY, Chen Y, Ge YL, Wang J, Xu Y, Chen TF, Zhang XW, et al. FAK alleviates radiation-induced rectal injury by decreasing apoptosis. Toxicol Appl Pharmacol. 2018;360:131–140. doi: 10.1016/j.taap.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Deng X, Qiu L, Peng F, Geng S, Shen L, Luo Z. Knockdown of C1GalT1 inhibits radioresistance of human esophageal cancer cells through modifying β1-integrin glycosylation. J Cancer. 2018;9:2666–2677. doi: 10.7150/jca.25252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L, Tripodi M. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res. 2008;314:143–152. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Graham K, Moran-Jones K, Sansom OJ, Brunton VG, Frame MC. FAK deletion promotes p53-mediated induction of p21, DNA-damage responses and radio-resistance in advanced squamous cancer cells. PLoS One. 2011;6:e27806. doi: 10.1371/journal.pone.0027806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the author on reasonable request.