Abstract
Introduction:
To facilitate initial clinical decision whether to use esophagectomy alone or neoadjuvant therapy in surgical care for individual patients with adenocarcinoma of the esophagus and esophagogastric junction—information not available from randomized trials—a machine-learning analysis was performed using worldwide real-world data on patients undergoing different therapies for this rare adenocarcinoma.
Methods:
Using random forest technology in a sequential analysis, we 1) identified eligibility for each of 4 therapies among 13,365 patients: esophagectomy alone (n=6,649), neoadjuvant therapy (n=4,706), esophagectomy and adjuvant therapy (n=998), and neoadjuvant and adjuvant therapy (n=1,022); 2) performed survival analyses incorporating interactions of patient and cancer characteristics with therapy; 3) determined optimal therapy as that predicted to maximize lifetime within 10 years (restricted mean survival time, RMST) for each patient; and 4) compared lifetime gained from optimal versus actual therapies.
Results:
Actual therapy was optimal in 61% of those receiving esophagectomy alone; neoadjuvant therapy was optimal for 36% receiving neoadjuvant therapy. Many patients were predicted to benefit from postoperative adjuvant therapy. Total RMST for actual therapy received was 58,825 years. Had patients received optimal therapy, total RMST was predicted to be 62,982 years, a 7% gain.
Conclusions:
Average treatment effect for adenocarcinoma of the esophagus yields only crude evidence-based therapy guidelines. However, patient response to therapy is widely variable, and survival after data-driven predicted optimal therapy often differs from actual therapy received. Therapy must address an individual patient’s cancer and clinical characteristics to provide precision surgical therapy for adenocarcinoma of the esophagus and esophagogastric junction.
Keywords: Real-world data, machine learning, artificial intelligence, survival analysis
INTRODUCTION
Since the randomized ChemoRadiotherapy for Oesphageal Cancer followed by Surgery Study (CROSS) demonstrated a survival advantage of neoadjuvant therapy for locally advanced cancer of the esophagus and esophagogastric junction compared with esophagectomy alone, it has become the standard of care.1,2 However, in CROSS, this survival advantage was not universal. Average treatment effect for patients with adenocarcinoma was smaller than that for squamous cell carcinoma, and even smaller for those with regional lymph node metastases (cN+). Women and those with poor performance status also benefited less. Decision-making is further confounded by multiple factors including the poor, unpredictable response to neoadjuvant therapy (29% in CROSS), a worse survival of complete neoadjuvant responders than that of patients undergoing esophagectomy alone for early-stage cancers,3–6 and a paucity of specific biomarkers, genetic markers,7–9 and gene therapies for this cancer. These survival differences, the uncertain benefit, and toxicity of neoadjuvant therapy, make decision-making for an individual patient with this rare adenocarcinoma problematic. Despite this, neoadjuvant therapy is now extrapolated to less advanced cancers confined to the muscularis propria (cT2).10–14
Randomized trials, the foundation of evidence-based care, provide an average treatment effect; they do not guide individually tailored (precision) therapy—the right therapy, for the right patient, at the right time. The strategy that “future trials should focus on identification of the optimum regimen and should attempt to identify and select patients most likely to benefit from specific therapy options”15 is not an expectation of the highly controlled, non–real world randomized trial paradigm. An alternative is developing individual treatment effects from real-world data,16–18 generated by machine learning,19,20 that identify the specific therapy most likely to benefit an individual patient. That is the objective of this study, which capitalizes on availability of worldwide real-world data of patients who underwent different therapies for adenocarcinoma of the esophagus or esophagogastric junction.3,4,21–25
Its specific objectives were to 1) identify therapies for which each patient is plausibly eligible, 2) perform a survival analysis incorporating these therapies, 3) identify the therapy predicted to maximize survival for an individual patient (optimal therapy), and 4) compare survival of optimal to actual therapy received.
MATERIALS AND METHODS
Patients and Therapies
At 33 Worldwide Esophageal Cancer Collaboration (WECC) institutions (Supplemental Appendix E1, a list of WECC participating institutions and investigators), 22,123 patients had clinical staging real-world data available for epithelial cancers of the esophagus and esophagogastric junction as part of the effort to provide clinical,21,22 pathologic,23,24 and post-neoadjuvant staging data3,4 for the American Joint Commission on Cancer (AJCC), Cancer Staging Manual, 8th Edition.6 Of these, 13,930 had adenocarcinoma or adenosquamous carcinoma and 13,365 underwent 1) esophagectomy alone (n=6,649), 2) neoadjuvant therapy (n=4,706), 3) esophagectomy and adjuvant therapy (n=998), or 4) neoadjuvant and adjuvant therapy (n=1,022).
Data
This study used 36 variables representing patient, cancer, and treatment characteristics (Supplemental Appendix E2, WECC data elements for the AJCC 8th Edition Cancer Staging Manual and data elements used in random forest analysis), with site and continent excluded to contain dimensionality of data and reduce confounding with treatment.21,22 Variables were obtained after local ethics-board approval of databases and data-use agreements with Cleveland Clinic. Data were requested in completely de-identified form (Health Insurance Portability and Accountability Act research standards) for analysis, using required variables with standard definitions including demographics, comorbidities, cancer characteristics, cancer treatment, and time-related mortality (Tables 1 and 2). The Case Cancer Institutional Review Board of Case Western Reserve University and Cleveland Clinic Institutional Review Board approved the entire project and use of these data for research, with patient consent waived.
Table 1.
Baseline characteristics of patients with adenocarcinoma of the esophagus, stratified by therapy received
| Esophagectomy Alone n = 6649 | Neoadjuvant n = 4706 | Esophagectomy + Adjuvant n = 988 | Neoadjuvant + Adjuvant n = 1022 | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | na | No. (%) or Mean ± SD | na | No. (%) or Mean ± SD | na | No. (%) or Mean ± SD | na | No. (%) or Mean ± SD |
| Demographics | ||||||||
| Age (y) | 6370 | 65 ± 11 | 4529 | 61 ± 9.8 | 988 | 60 ± 10 | 1022 | 59 ± 9.4 |
| Female | 6647 | 1029 (15) | 4706 | 539 (11) | 988 | 106 (11) | 1022 | 124 (12) |
| Body mass index (kg/m2) | 3569 | 27 ± 5.1 | 2811 | 28 ± 5.2 | 481 | 25 ± 4.7 | 432 | 27 ± 5.3 |
| Weight loss (kg) | 3644 | 0/0/5b | 2067 | 0/1/10b | 598 | 0/0/6b | 483 | 0/1/8.8b |
| Comorbidities | ||||||||
| ECOG performance status | 1024 | 1492 | 270 | 409 | ||||
| 0 | 376 (37) | 568 (38) | 114 (42) | 97 (24) | ||||
| 1 | 489 (48) | 879 (59) | 91 (34) | 289 (71) | ||||
| 2 | 103 (10) | 37 (2.5) | 39 (14) | 17 (4.2) | ||||
| 3 | 49 (4.8) | 8 (0.53) | 24 (8.9) | 4 (0.97) | ||||
| 4 | 7 (0.68) | 0 (0) | 2 (0.74) | 2 (0.48) | ||||
| Diabetes | 5339 | 666 (12) | 4468 | 570 (13) | 673 | 57 (8.5) | 655 | 71 (11) |
| IDDM | 5087 | 99 (1.9) | 4271 | 60 (1.4) | 659 | 12 (1.8) | 635 | 8 (1.3) |
| NIDDM | 5087 | 315 (6.2) | 4271 | 313 (7.3) | 659 | 31 (4.7) | 635 | 43 (6.8) |
| Coronary artery disease | 2121 | 435 (21) | 2855 | 431 (15) | 277 | 25 (9.0) | 489 | 59 (12) |
| Arrhythmia | 1579 | 55 (3.5) | 1795 | 40 (2.2) | 204 | 10 (4.9) | 193 | 3 (1.6) |
| Hypertension | 4407 | 1340 (30) | 3164 | 922 (29) | 599 | 164 (27) | 555 | 165 (30) |
| Peripheral arterial disease | 2806 | 133 (4.7) | 2857 | 76 (2.7) | 422 | 13 (3.1) | 527 | 8 (1.5) |
| Smoker | 4548 | 3118 (69) | 3810 | 2572 (68) | 641 | 465 (73) | 546 | 351 (64) |
| Past | 3522 | 1239 (35) | 3049 | 1347 (44) | 546 | 219 (40) | 508 | 220 (43) |
| Current | 3522 | 853 (24) | 3049 | 464 (15) | 546 | 151 (28) | 508 | 93 (18) |
| FEV1 (% of predicted) | 2652 | 95 ± 21 | 2026 | 97 ± 19 | 459 | 96 ± 19 | 521 | 93 ± 18 |
| FVC (% of predicted) | 2206 | 102 ± 19 | 1154 | 100 ± 17 | 341 | 106 ± 19 | 265 | 99 ± 14 |
| Creatinine (mg/dL) | 593 | 71 ± 37 | 437 | 76 ± 21 | 197 | 78 ± 18 | 237 | 79 ± 18 |
| Bilirubin (mg/dL) | 399 | 11 ± 6.3 | 166 | 10 ± 7.8 | 210 | 12 ± 5.7 | 253 | 9.6 ± 7.2 |
| Decade | 6649 | 4706 | 988 | 1022 | ||||
| 1970–1979 | 22 (0.33) | 5 (0.10) | 18 (1.8) | 1 (0.098) | ||||
| 1980–1989 | 206 (3.1) | 79 (1.7) | 95 (9.6) | 59 (5.8) | ||||
| 1990–1999 | 2220 (33) | 665 (14) | 319 (32) | 164 (16) | ||||
| 2000–2009 | 3596 (54) | 2792 (59) | 438 (44) | 556 (54) | ||||
| 2010–2014 | 605 (9.1) | 1165 (25) | 118 (12) | 242 (24) | ||||
| Continent | 6649 | 4706 | 988 | 1022 | ||||
| North America | 3388 (51) | 3091 (66) | 576 (58) | 811 (79) | ||||
| Europe | 2571 (39) | 1215 (26) | 238 (24) | 170 (17) | ||||
| Asia | 190 (2.9) | 14 (0.30) | 154 (16) | 39 (3.8) | ||||
| Australia | 326 (4.9) | 379 (8.1) | 0 (0) | 0 (0) | ||||
| South America | 172 (2.6) | 6 (0.13) | 20 (2.0) | 0 (0) | ||||
| Africa | 2 (0.030) | 1 (0.021) | 0 (0) | 2 (0.20) | ||||
Patients with data available.
15th/50th/85th percentiles.
Key: ECOG, Eastern Cooperative Oncology Group; FEV21 (%), forced expiratory volume in 1 second (percent of predicted); FVC (%), forced vital capacity (percent of predicted); IDDM, insulin-dependent diabetes mellitus; NIDDM, non–insulin-dependent diabetes mellitus; SD, standard deviation.
Table 2.
Clinical cancer characteristics of patients with adenocarcinoma of the esophagus, stratified by therapy received
| Esophagectomy Alone n = 6649 | Neoadjuvant n = 4706 | Esophagectomy + Adjuvant n = 988 |
Neoadjuvant + Adjuvant n = 1022 | |
|---|---|---|---|---|
| Characteristic | No. (%) | No. (%) | No. (%) | No. (%) |
| cT | ||||
| cT0 | 143 (3.1) | 9 (0.23) | 5 (0.71) | 3 (0.32) |
| cTis | 201 (4.3) | 5 (0.13) | 5 (0.71) | 3 (0.32) |
| cT1 | 1235 (26) | 130 (3.4) | 50 (7.1) | 17 (1.8) |
| cT2 | 1136 (24) | 737 (19) | 169 (24) | 160 (17) |
| cT3 | 1798 (38) | 2879 (74) | 418 (60) | 720 (78) |
| cT4 | 164 (3.5) | 118 (3.0) | 53 (7.6) | 25 (2.7) |
| cTX | 1972 | 828 | 288 | 94 |
| cN | ||||
| cN0 | 2909 (61) | 1397 (35) | 278 (41) | 231 (27) |
| cN+ | 1857 (39)a | 2562 (65)b | 397 (59)c | 615 (73)d |
| cN1 | 94 (80) | 55 (73) | 78 (68) | 43 (73) |
| cN2 | 17 (15) | 18 (24) | 32 (28) | 15 (25) |
| cN3 | 6 (5.1) | 2 (2.7) | 5 (4.3) | 1 (1.7) |
| cNX | 1883 | 747 | 313 | 176 |
| cM | ||||
| cM0 | 6442 (97) | 4494 (95) | 945 (96) | 947 (93) |
| cM1 | 207 (3.1) | 212 (4.5) | 43 (4.4) | 75 (7.3) |
| Gradee | ||||
| cG1 | 273 (27) | 41 (2.7) | 21 (12) | 10 (14) |
| cG2 | 362 (35) | 705 (47) | 61 (36) | 28 (39) |
| cG3 | 366 (36) | 747 (50) | 86 (51) | 32 (45) |
| cG4 | 24 (2.3) | 10 (0.67) | 2 (1.2) | 1 (1.4) |
| cGX | 5624 | 3203 | 818 | 951 |
| Location | ||||
| cUpper | 53 (0.94) | 23 (0.58) | 4 (0.46) | 8 (1.1) |
| cMiddle | 243 (4.3) | 135 (3.4) | 32 (3.7) | 12 (1.6) |
| cLower | 5231 (95) | 3832 (96) | 828 (96) | 724 (97) |
| cLocationX | 1032 | 716 | 124 | 278 |
Data available for 4766 patients.
Data available for 3959 patients.
Data available for 675 patients.
Data available for 846 patients.
G1, well differentiated; G2, moderately well differentiated; G3, poorly differentiated; G4, undifferentiated.
Missing data for independent variables were imputed using “on the fly” random forest imputation26 implemented in the open-source randomForestSRC R-software27 under default settings.
Endpoint
The endpoint was all-cause mortality from first management decision. Median potential follow-up28 was 9.8 years (25% >14.3 years, 10% >19.8 years) if there had been no deaths, but considering deaths in this elderly population with a rapidly lethal cancer, median follow-up was 1.6 years. Median follow-up for surviving patients was 2.4 years, with 25% followed more than 5.1 years and 10% more than 8.5 years.
Statistical Analysis
Because the initial therapy decision is whether to use esophagectomy alone or neoadjuvant therapy, and use of adjuvant therapy is a decision made after pathologic characteristics of adenocarcinoma are determined, these two therapies are the focus of this paper. The primary objective of the analysis was to identify for a given patient the therapy predicted to maximize length of life. This was determined in four steps.
Although theoretically all patients could receive any of the four therapies, clinical practice precludes certain therapies for particular patients. For example, maximal therapy—neoadjuvant and adjuvant therapy—would not plausibly be prescribed for clinical high-grade dysplasia (cTis) or cT1 cancers, which would be treated by ablation or resection, typically endoscopically. Thus, for each patient, we identified the therapies for which he or she was plausibly eligible using nonparametric random forest classification (Supplemental Appendix E3, plausible therapy eligibility).27 Forty-five patients were excluded because their estimated probability of receiving their actual therapy was less than 5%.
A survival analysis was performed using random forests for survival that incorporated interactions of all variables with the four therapies (Supplemental Appendix E4, survival analysis).27 From this analysis, a survival curve was generated for the therapy actually received. Predicted survival curves for alternative therapy scenarios (that is, for therapies a patient did not receive, but for which the patient was eligible) were generated using the same patient data, but substituting these alternative therapies for actual therapy received. This produced up to four survival curves for each patient, one for each therapy for which the patient was eligible.
Length of life over the subsequent 10 years from first management decision was predicted for therapies for which each patient was eligible. Length of life for each therapy was estimated by restricted mean survival time (RMST),29–31 which is equivalent to the area beneath a survival curve from first management decision up to a specific time point. We chose 10 years as that specific time point and expressed RMST in months. RMST was calculated for each therapy for which a patient was eligible. We also calculated the difference in RMST between pairs of therapies to estimate the amount of survival time gained (positive number) or lost (negative number) by being treated with one therapy versus the other. Up to six differences were obtained among the four eligible therapies. We defined optimal therapy as the eligible therapy yielding the maximum RMST.
Predicted outcome of optimal therapy was compared with actual therapy received. Specifically, for each patient, the difference in RMST for optimal versus actual therapy was calculated, producing lifetime gained from optimal therapy.
Illustrative Scenarios
To illustrate how we envision results of this study will be used in precision cancer care decisions, we selected five patients from the WECC database with a) early stage, b) intermediate stage, and c) advanced stage adenocarcinomas (Table 3). Predicted survival curves were generated by “sending the patient’s characteristics up through the random forest” for each plausible treatment.32 RMST at 10 years was calculated for each scenario.
Table 3.
Patient and cancer characteristics in five patient scenarios
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Clinical Cancer Characteristics | |||||
| cT | cT1 | cT4 | cT2 | cT2 | cT2 |
| cN | cN0 | cN1 | cN1 | cN0 | cN0 |
| cM | cM0 | cM0 | cM0 | cM0 | cM0 |
| cG | cG3 | cG2 | cG3 | cG3 | cG2 |
| cNodes | 0 | 1 | 1 | 0 | 0 |
| Distance from incision (cm) | 35 | 36 | 35 | 37 | 35 |
| Location | L | L | L | L | L |
| Lymphovascular invasion | No | Yes | No | No | No |
| Extracapsular invasion | No | No | No | No | No |
| Length (cm) | 2.5 | 5.1 | 5.9 | 5.4 | 5.2 |
| Patient Characteristics | |||||
| Age (y) | 67 | 65 | 60 | 74 | 44 |
| Sex | Male | Male | Male | Male | Female |
| Race | White | White | White | White | White |
| Body mass index (kg/m2) | 26 | 25 | 22 | 27 | 24 |
| Weight loss (kg) | 2.6 | 2.0 | 7.6 | 5.8 | 12 |
| ECOG performance status | 0 | 1 | 0 | 1 | 1 |
| Barrett esophagus | No | No | No | No | No |
| Other cancers | No | No | No | No | No |
| Diabetes | No | No | No | NIDDM | No |
| Coronary artery disease | No | No | No | No | No |
| Arrhythmia | No | No | No | No | No |
| Hypertension | Yes | No | No | No | Yes |
| Peripheral arterial disease | No | No | No | No | No |
| Smoker | Past | Current | Past | No | Past |
| FEV1 (% of predicted) | 97 | 101 | 97 | 98 | 92 |
| Creatinine (mg/dL) | 83 | 72 | 67 | 72 | 68 |
| Bilirubin (mg/dL) | 11.7 | 12.6 | 11.2 | 11.0 | 10.6 |
Key: ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 second; NIDDM, non–insulin dependent diabetes mellitus.
RESULTS
Plausible Therapy Eligibility
Of 13,365 patients, 45 were less than 5% eligible for actual therapy received, and were not considered in analysis of optimal therapy. Of the remaining 13,320 patients, 12,421 (93%) were plausibly eligible for esophagectomy alone, 10,900 (82%) for neoadjuvant therapy, and 10,001 (75%) for either; 3,816 (29%) were eligible for all therapies (see Supplemental Appendix E3).
Survival Analysis
For each cTNM classification and therapy, individual patient survival estimates were highly variable (Figure 1). For patients with cT1N0M0 adenocarcinoma eligible for esophagectomy alone, average 5-year survival was 70%, but variability occurred across the entire range such that many patients experienced survival well beyond 10 years, but elderly patients and those with many non-cancer comorbidities and poor performance status were predicted to have poor survival (Figure 1A). For those eligible for neoadjuvant therapy, average 5-year survival was 36%, with predicted survival curves displaced downward such that few were predicted to survive beyond 10 years despite response to therapy (Figure 1B). For patients with cTanyN+M0 adenocarcinoma eligible for esophagectomy alone, average 5-year survival was 25%, but with variability across the entire range (Figure 1C). For those eligible for neoadjuvant therapy, average 5-year survival was also 25%, with predicted survival curves displaced downward (Figure 1D). For patients with cT2N0M0 adenocarcinoma, average 5-year survival was 41% for esophagectomy alone, with many displaying predicted survival at 10 years greater than 80% (Figure 1E), and 37% for neoadjuvant therapy (Figure 1F), but with no curves displaying predicted survival at 10 years of more than 80%.
Figure 1:
Individual survival estimates for patients eligible for esophagectomy alone or neoadjuvant therapy. Bolded line is average treatment effect. N+ means lymph node–positive cancers. A, cT1N0M0 adenocarcinoma eligible for esophagectomy alone. B, cT1N0M0 adenocarcinoma eligible for neoadjuvant therapy. C, cTanyN+M0 adenocarcinoma eligible for esophagectomy alone. D, cTanyN+M0 adenocarcinoma eligible for neoadjuvant therapy. E, cT2N0M0 adenocarcinoma eligible for esophagectomy alone. F, cT2N0M0 adenocarcinoma eligible for neoadjuvant therapy.
Optimal Therapy
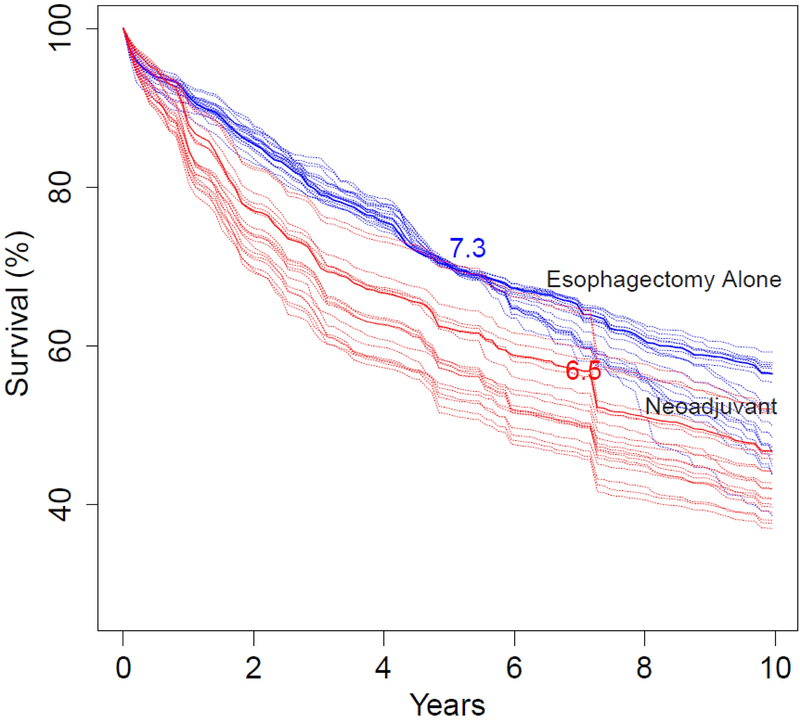
Optimal survival is illustrated for the five therapy scenarios (see Table 3). The first consists of patients with early cT1N0M0 adenocarcinoma who were predicted to have a survival of 70% ± 0.5% at 5 years and approximately 55% at 10 years after esophagectomy alone (Figure 2A). A representative patient, a 67-year-old man (Table 3, Patient 1), is depicted by the solid blue line. He had an RMST of 7.3 years and 5- and 10-year survival of 70% and 56%, respectively. With neoadjuvant therapy, RMST was 6.5 years, a reduction of 9.6 months, and predicted 5- and 10-year survival was 62% and 47%, respectively.
Figure 2:
Individual patient survival predictions for five clinical scenarios (Table 3). A, Predicted survival for patients with early-stage (cT1N0M0) adenocarcinoma of the esophagus with survival of 70% ± 0.5% at 5 years and 55% ± 0.5% at 10 years after esophagectomy alone (blue lines). Red lines are survival had the same patients undergone neoadjuvant therapy. Solid lines are for a 67-year-old man (Table 3, Patient 1) with restricted mean survival times (RMST) of 7.3 years for esophagectomy alone and 6.5 years for neoadjuvant therapy. B, Patient is a 65-year-old man with advanced-stage (cG2T4N1M0) adenocarcinoma (Table 3, Patient 2), with RMSTs of 4.7 years for esophagectomy alone (blue line) and 6 years for neoadjuvant therapy (red line). C, Patient is a 60-year-old man with advanced-stage (cG3T2N1M0) adenocarcinoma (Table 3, Patient 3), with RMSTs of 4.7 years for esophagectomy alone (blue line) and 3.6 years for neoadjuvant therapy (red line). D, Patient is a 74-year-old man with intermediate-stage (cG3T2N0M0) adenocarcinoma (Table 3, Patient 4), with RMSTs of 4.7 years for esophagectomy alone (blue line) and 4.1 years for neoadjuvant therapy (red line). E, Patient is a 44-year-old woman with intermediate-stage (cG2T2N1M0) adenocarcinoma (Table 3, Patient 5) with RMSTs of 4.8 years for esophagectomy alone (blue line) and 5.5 years for neoadjuvant therapy (red line).
The second and third scenarios consist of two patients with advanced cTanyN+M0 adenocarcinoma. The first is a 65-year-old man with cG2T4N1M0 adenocarcinoma (Table 3, Patient 2). With neoadjuvant therapy, predicted 5- and 10-year survival was 55% and 39%, respectively, with an RMST of 6 years (Figure 2B). With esophagectomy alone, predicted 5- and 10-year survival was 40% and 29%, respectively, with an RMST of 4.7 years, a reduction of 15.6 months. The second was a 60-year-old man (Table 3, Patient 3) with cG3T2N1M0 adenocarcinoma. With neoadjuvant therapy, predicted 5- and 10-year survival was 27% and 19%, respectively, with an RMST of 3.6 years (Figure 2C). With esophagectomy alone, predicted 5- and 10-year survival was 40% and 29%, respectively, with an RMST of 4.7 years, an improvement of 13.2 months over neoadjuvant therapy.
The fourth and fifth scenarios consist of two patients with intermediate-stage cT2N0M0 adenocarcinoma. The first is a 74-year-old man with diabetes who had cG3T2N0M0 adenocarcinoma (Table 3, Patient 4). With neoadjuvant therapy, predicted 5- and 10-year survival was 33% and 21%, respectively, with an RMST of 4.1 years (Figure 2D). With esophagectomy alone, predicted 5- and 10-year survival was 40% and 28%, respectively, with an RMST of 4.7 years, an improvement of 7.2 months. The second patient, a 44-year-old woman with a history of smoking, had a cG2T2N0M0 adenocarcinoma (Table 3, Patient 5). With neoadjuvant therapy, predicted 5- and 10-year survival was 49% and 35%, respectively, with an RMST of 5.5 years (Figure 2E). With esophagectomy alone, predicted 5- and 10-year survival was 40% and 28%, respectively, with an RMST of 4.8 years, a reduction of 8.4 months over neoadjuvant therapy.
These scenarios focused on patients with typical survival for the given therapy and cancer. However, the interaction of clinical characteristics, cancer characteristics, and therapies produced a wide array of patient-specific predicted survival for this often rapidly lethal cancer in these elderly patients (see Figure 1).
Optimal versus Actual Therapy
Esophagectomy alone was optimal in 61% of patients whose actual therapy was esophagectomy alone (Figure 3). Of the remaining 39% of patients undergoing esophagectomy alone, approximately half (20%) would have benefited from the addition of adjuvant therapy and approximately half (19%) from neoadjuvant therapy (approximately half of this 19% [9%] with adjuvant therapy).
Figure 3:
Mirrored histogram of number of patients who received optimal therapy based on RMST and those who would have benefited from an alternative therapy.
Neoadjuvant therapy was optimal in 36% of patients whose actual therapy was neoadjuvant therapy (see Figure 3). Of the remaining 64% of patients receiving neoadjuvant therapy, approximately one quarter (17%) would have benefited from adjuvant therapy and three quarters (47%) from esophagectomy, either alone (34%) or with adjuvant therapy (13%).
Among the 13,320 patients meeting plausibility therapy eligibility, total RMST for actual therapy received was 58,825 years. Had these patients received optimal therapy, total RMST was predicted to be 62,982 years, a 7% gain.
DISCUSSION
Principal Findings
Based on real-world worldwide data, most patients with adenocarcinoma of the esophagus and esophagogastric junction are eligible for either esophagectomy alone or neoadjuvant therapy. Survival is highly variable for each therapy, depending on both patient and clinical cancer characteristics. Esophagectomy alone was optimal therapy in nearly two thirds of patients receiving this therapy, but neoadjuvant therapy was optimal in only about a third. Many patients would have benefited from additional adjuvant therapy based on pathologic cancer characteristics determined after esophagectomy (see Figure 3). Although optimal therapy was predicted to lengthen life only modestly in the overall cohort for this highly lethal cancer, lifetime gained was predicted to be substantial for some individual patients, particularly with esophagectomy alone.
Average Treatment Effect
The CROSS study showed that, on average, overall survival was better after neoadjuvant therapy compared with esophagectomy alone.1 In that study, the ideal patient for neoadjuvant therapy was a male with excellent performance status (WHO 0) and a squamous-cell carcinoma without regional lymph metastases. However, a female patient with a mildly reduced performance status (WHO 1) and an adenocarcinoma with regional lymph node metastases was likely to fare equally well with esophagectomy alone. A recent meta-analysis of 22 randomized trials also reported improved survival with neoadjuvant therapy versus esophagectomy alone for patients with locally advanced esophageal cancer, but more for adenocarcinoma than squamous-cell carcinoma.33 However, response to neoadjuvant therapy is unpredictable and low—29% in CROSS—and survival of complete responders is worse than that of patients undergoing esophagectomy for early-stage cancers, the hysteresis effect of down-staging.5
The conclusions of CROSS,1 that preoperative chemoradiotherapy (five courses of carboplatin and paclitaxel, with 41.4 Gy of concurrent radiotherapy) is safe and leads to a significant increase in overall survival among patients with adenocarcinoma or squamous-cell carcinoma of the esophagus or esophagogastric junction, are based on evidence-based average treatment effect that does not identify optimal therapy for an individual patient. Further, in clinical practice, recommendations based on average treatment effect in CROSS have been extrapolated to esophageal cancers confined to the wall (cT2). cT2N0M0 cancers thus represent just such a clinical challenge: Theoretically still confined to the esophageal wall, they are rarely confirmed to be pT2N0M0 cancers. In our experience, the majority of these patients were overstaged, and a minority understaged.10 Evidence-based attempts to define the optimal therapy for this cancer have reported variable outcomes with esophagectomy alone and neoadjuvant therapy.10–14,34
Individual Treatment Effect
In contrast to decision for therapy based on average treatment effect, the Obama Precision Medicine Initiative represents an emerging approach for disease treatment that accounts for individual variability and demographics, comorbid condition, lifestyle, socioeconomic position, genes, environment, and myriad other factors, ranging from genetics to environment, that distinguish the individual patient and his or her response to disease and its treatment.7–9,35–38 As stated by Tonelli and Shirts,39 precision medicine explicitly prioritizes the individualization of care and focuses attention on unique characteristics of a particular patient. In this fashion, [precision medicine] differs greatly from [evidence-based medicine], which seeks to determine the best course of action for a patient with an appeal to generalizable knowledge gained from population-based studies … To realize the goals of [precision medicine], the hierarchy of evidence pyramid must yield to a more horizontal conception of medical knowledge.
There is scant available evidence for treatable cancer-specific biomarkers and genetic abnormalities in adenocarcinoma of the esophagus7–9; however, there are well-established regional differences in the world, with adenocarcinoma more prevalent in the West and squamous cell cancers in the East, along with many other epidemiologic variables that are associated with as yet incompletely defined -omics.37,38 In this study, we have demonstrated wide variability of survival for all treatments across all stages of adenocarcinoma of the esophagus. We found that optimal therapy depends on patient, cancer, and clinical characteristics, and their interactions with specific therapies. We have exploited this wide variability to permit more precise cancer care based on these individual treatment effects, as opposed to average treatment effects: precision cancer care.
Strengths and Weaknesses
Worldwide real-world data provide a large number of patients with variable clinical and cancer characteristics, along with variability in therapy, all of which facilitated exploration of complex clinical interactions with various therapies. This variability in managing esophageal cancer is disappearing in this era of evidence-based medicine that focuses on average treatment effect. Machine-learning statistical techniques are capable of using the heterogeneous practices employed around the world for identifying the complex interactions among patient, clinical, and cancer characteristics that generate a wide spectrum of predicted survival.
Nevertheless, despite considerable data per patient, and more patients and locations worldwide than were available for analysis for the 7th Edition of the AJCC Staging Manual,40 more detailed patient and cancer characteristics could have provided more refined survival predictions. Such data would include specific biomarkers and gene characteristics,7–9 environmental exposure,37 and socioeconomic position.38
It may be argued that the WECC data is outdated because it contains many patients across all cancer stages who underwent only esophagectomy alone in the pre-CROSS era. However, these real-world data provided us the opportunity to identify patients who would benefit from esophagectomy alone across cancer stage. Further, neoadjuvant therapy was available across the entire period of patient accrual in WECC and was well represented. In addition, despite absence of randomized trials of adjuvant therapy, it also identified patients for whom additional post-esophagectomy therapy was of value.
Because of dimensionality constraints, we did not separate induction or adjuvant chemotherapy from chemoradiotherapy or radiotherapy only. However, although chemotherapy may provide local control, studies indicate no difference in survival.41–44
Due to considerable, but to an extent systematic, institutional variability in therapy worldwide, therapeutic preference was confounded with therapy received. Therefore, institution was suppressed in the analysis. In addition, therapy was delivered in a non-standardized, institution-specific manner, rendering therapies comparable only as a general class.
Accurate and precise clinical staging is a universal problem, in part because of cost limitations and regional availability of staging modalities.21 This reduced accuracy of cancer characteristics at the time of therapeutic decision-making.7–9
Finally, despite data representing all inhabited continents, WECC did not capture the entire denominator of patients diagnosed with adenocarcinoma of the esophagus or esophagogastric junction. More institutions were invited to send data than did so, and institutions doing a very small number of cases are not represented. Therefore, we do not know what biases there may be in the data based on response to the invitation to join WECC or our failure to invite sites to participate. Due to inclusive dates of the study, endoscopic treatment of early-stage mucosal cancers are poorly represented, and minimally invasive procedures are today more commonly performed than present in the WECC dataset.
Clinical Implications
Each patient and his or her esophageal adenocarcinoma is different. The average treatment effect is too coarse for optimal clinical decision-making. Identical therapy for every patient with advanced adenocarcinoma (or even intermediate-stage cancers), as suggested by evidence-based data from randomized trials, is not an optimal therapeutic strategy. Optimal therapy to maximize survival is specific for each adenocarcinoma patient, based on how patient, cancer, and clinical characteristics interact with alternative therapies. Even in patients with the most advanced-stage adenocarcinoma, neoadjuvant therapy may not be optimal; patients may also benefit from additional adjuvant therapy following either esophagectomy alone or neoadjuvant therapy, based on pathologic cancer characteristics.45,46
Prescription of surgical therapy for a patient with adenocarcinoma of the esophagus or esophagogastric junction should include consideration of patient, clinical, and cancer characteristics. Machine-learning modeling of outcomes will allow individualization of therapy, a significant step from evidence-based decision-making toward precision cancer care, even in the absence of biomarker and other –omic data.
Conclusions
Average treatment effect for adenocarcinoma of the esophagus yields only crude evidence-based guidelines for therapy. Real-world worldwide data demonstrate that patient response to therapy is widely variable, and survival after data-driven predicted optimal therapy often differs from actual therapy received. Therefore, treatment decisions must account for patient and clinical cancer characteristics to maximize survival for the individual patient—precision cancer care for adenocarcinoma of the esophagus and esophagogastric junction.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Marie Semple, MPH, for statistical programming, Carolyn Apperson-Hansen, MStat, for assistance with Institutional Review Board and data-use agreements, and Gina Ventre, MFA, and Tess Parry, BS, for manuscript editing.
Funding: This work was supported by the 1) International Society for Diseases of the Esophagus (ISDE), 2) Daniel and Karen Lee Chair in Thoracic Surgery at Cleveland Clinic (TWR), 3) Drs. Sidney and Becca Fleischer Heart and Vascular Education Chair (EHB), 4) Clinical and Translational Science Collaborative at the Case Western Reserve University School of Medicine from the National Institutes of Health (NIH) National Center for Advancing Translational Sciences and NIH Roadmap for Medical Research [grant number UL1TR000439], 5) National Institute of General Medical Sciences [grant number R01GM125072 (HI)], and 6) Gus P. Karos Registry Fund at Cleveland Clinic. These funding sources played no role in the collection, analysis, or interpretation of data; writing of the report; or decision to submit the article for publication. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the ISDE, Cleveland Clinic, or NIH.
Abbreviations:
- AJCC
American Joint Committee on Cancer
- CROSS
ChemoRadiotherapy for Oesphageal Cancer followed by Surgery Study
- RMST
restricted mean survival time
- WECC
Worldwide Esophageal Cancer Collaboration
Footnotes
Conflicts of interest: The authors have no relevant financial activities outside the submitted work to report.
DECLARATION OF INTERESTS
Authors have nothing to disclose.
REFERENCES
- 1.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro J, van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8. [DOI] [PubMed] [Google Scholar]
- 3.Rice TW, Lerut TE, Orringer MB, Chen LQ, Hofstetter WL, Smithers BM, et al. Worldwide Esophageal Cancer Collaboration: neoadjuvant pathologic staging data. Dis Esophagus. 2016;29:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice TW, Ishwaran H, Kelsen DP, Hofstetter WL, Apperson-Hansen C, Blackstone EH. For the Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanoni A, Verlato G, Giacopuzzi S, Motton M, Casella F, Weindelmayer J, et al. ypN0: does it matter how you get there? Nodal downstaging in esophageal cancer. Ann Surg Oncol. 2016;23:998–1004. [DOI] [PubMed] [Google Scholar]
- 6.American Joint Committee on Cancer. Cancer Staging Manual. 8th ed. Amin MB, Edge SB, Greene FL, et al. , eds. New York, NY: Springer; 2017. [Google Scholar]
- 7.Wang C, Wang J, Chen Z, Gao Y, He J. Immunohistochemical prognostic markers of esophageal squamous cell carcinoma: a systematic review. Chinese Journal of Cancer. 2017;36:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purim O, Beny A, Inbar M, Shulman K, Brenner B, Dudnik E, et al. Biomarker-driven therapy in metastatic gastric and esophageal cancer: real-life clinical experience. Target Oncol. 2018;13:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Secrier M, Li X, de Silva N, Eldridge MD, Contino G, Bornschein J, et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet. 2016;48:1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice TW, Mason DP, Murthy SC, Zuccaro G Jr., Adelstein DJ, Rybicki LA, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg. 2007;133:317–24. [DOI] [PubMed] [Google Scholar]
- 11.Markar SR, Gronnier C, Pasquer A, Duhamel A, Beal H, Thereaux J, et al. Role of neoadjuvant treatment in clinical T2N0M0 oesophageal cancer: results from a retrospective multi-center European study. Eur J Cancer. 2016;56:59–68. [DOI] [PubMed] [Google Scholar]
- 12.Goense L, Visser E, Haj Mohammad N, Mook S, Verhoeven RHA, Meijer GJ, et al. Role of neoadjuvant chemoradiotherapy in clinical T2N0M0 esophageal cancer: a population-based cohort study. Eur J Surg Oncol. 2018;44:620–5. [DOI] [PubMed] [Google Scholar]
- 13.Hardacker TJ, Ceppa D, Okereke I, Rieger KM, Jalal SI, LeBlanc JK, et al. Treatment of clinical T2N0M0 esophageal cancer. Ann Surg Oncol. 2014;21:3739–43. [DOI] [PubMed] [Google Scholar]
- 14.Mota FC, Cecconello I, Takeda FR, Tustumi F, Sallum RAA, Bernardo WM. Neoadjuvant therapy or upfront surgery? A systematic review and meta-analysis of T2N0 esophageal cancer treatment options. Int J Surg. 2018;54:176–81. [DOI] [PubMed] [Google Scholar]
- 15.Duan XF, Tang P, Yu ZT. Neoadjuvant chemoradiotherapy for resectable esophageal cancer: an in-depth study of randomized controlled trials and literature review. Cancer Biol Med. 2014;11:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirklin JW, Akins CW, Blackstone EH, Booth DC, Califf RM, Cohen LS, et al. ACC/AHA guidelines and indications for coronary artery bypass graft surgery--a report of the American College of Cardiology/American Heart Association task force on assessment of diagnostic and therapeutic cardiovascular procedures (subcommittee on coronary artery bypass graft surgery). J Am Coll Cardiol. 1991;17:543–89. [PubMed] [Google Scholar]
- 17.Yoon DY, Smedira NG, Nowicki ER, Hoercher KJ, Rajeswaran J, Blackstone EH, et al. Decision support in surgical management of ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2010;139:283–93. [DOI] [PubMed] [Google Scholar]
- 18.Makady A, de Boer A, Hillege H, Klungel O, Goettsch W. What is real-world data? A review of definitions based on literature and stakeholder interviews. Value Health. 2017;20:858–65. [DOI] [PubMed] [Google Scholar]
- 19.Shen L, Ding Y, Battioui C. A framework of statistical methods for identification of subgroups with differential treatment effects in randomized trials Applied Statistics in Biomedicine and Clinical Trials Design: Selected Papers from 2013 ICSA/ISBS Joint Statistical Meetings. New York, NY: Springer; 2015. p. 411–25. [Google Scholar]
- 20.Vittinghoff E, McCulloch CE, Woo C, Cummings SR. Estimating long-term effects of treatment from placebo-controlled trials with an extension period, using virtual twins. Stat Med. 2010;29:1127–36. [DOI] [PubMed] [Google Scholar]
- 21.Rice TW, Apperson-Hansen C, DiPaola LM, Semple ME, Lerut TE, Orringer MB, et al. Worldwide Esophageal Cancer Collaboration: clinical staging data. Dis Esophagus. 2016;29:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice TW, Ishwaran H, Blackstone EH, Hofstetter WL, Kelsen DP, Apperson-Hansen C. For the Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for clinical staging (cTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice TW, Chen LQ, Hofstetter WL, Smithers BM, Rusch VW, Wijnhoven BP, et al. Worldwide Esophageal Cancer Collaboration: pathologic staging data. Dis Esophagus. 2016;29:724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice TW, Ishwaran H, Hofstetter WL, Kelsen DP, Apperson-Hansen C, Blackstone EH. For the Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang F, Ishwaran H. Random forest missing data algorithms. Stat Analysis Data Mining. 2017;10:363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishwaran H, Kogalur UB. RandomForestSRC: Random forests for survival, regression and classification (RF-SRC). R package version 2.6.0.13. http://cran.r-project.org, 2018. [Google Scholar]
- 28.Goldman AI. Eventcharts: visualizing survival and other timed-event data. Am Statistician. 1992;46:13–8. [Google Scholar]
- 29.Jiang R, Lu W, Song R, Hudgens MG, Naprvavnik S. Doubly robust estimation of optimal treatment regimes for survival data--with application to an HIV/AIDS study. Ann Appl Stat. 2017;11:1763–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pak K, Uno H, Kim DH, Tian L, Kane RC, Takeuchi M, et al. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017;3:1692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang B, Kuan PF. Comparison of the restricted mean survival time with the hazard ratio in superiority trials with a time-to-event end point. Pharm Stat. 2018;17:202–13. [DOI] [PubMed] [Google Scholar]
- 32.Lu M, Sadiq S, Feaster DJ, Ishwaran H. Estimating individual treatment effect in observational data using random forest methods. J Comput Graph Stat. 2018;27:209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng H, Zhao Y, Jing T, Ma J, Zhang J, Wang C, et al. Traditional and cumulative meta-analysis: chemoradiotherapy followed by surgery versus surgery alone for resectable esophageal carcinoma. Mol Clin Oncol. 2018;8:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atay SM, Blum M, Sepesi B. Adjuvant chemotherapy following trimodality therapy for esophageal carcinoma--Is the evidence sufficient? J Thorac Dis. 2017;9:3626–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley WT, Nilsen WJ, Manolio TA, Masys DR, Lauer M. News from the NIH: potential contributions of the behavioral and social sciences to the precision medicine initiative. Transl Behav Med. 2015;5:243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrams J, Conley B, Mooney M, Zwiebel J, Chen A, Welch JJ, et al. National Cancer Institute’s precision medicine initiatives for the new National Clinical Trials Network. Am Soc Clin Oncol Educ Book. 2014:71–6. [DOI] [PubMed] [Google Scholar]
- 37.Coleman HG, Xie SH, Lagergren J. The epidemiology of esophageal adenocarcinoma. Gastroenterology. 2018;154:390–405. [DOI] [PubMed] [Google Scholar]
- 38.Eng OS, Nelson RA, Konstantinidis I, Chao J, Erhunmwunsee L, Raz DJ, et al. Disparities in survival after trimodality therapy for esophageal adenocarcinoma. Dis Esophagus. 2018;31. [DOI] [PubMed] [Google Scholar]
- 39.Tonelli MR, Shirts BH. Knowledge for precision medicine: mechanistic reasoning and methodological pluralism. JAMA. 2017;318:1649–50. [DOI] [PubMed] [Google Scholar]
- 40.American Joint Committee on Cancer. Cancer Staging Manual. 7th ed. Edge SB, Byrd DR, Compton CC, et al. , eds. New York, NY: Springer-Verlag, 2010, p. 103–115. [Google Scholar]
- 41.Munch S, Habermehl D, Agha A, Belka C, Combs SE, Eckel R, et al. Perioperative chemotherapy vs. neoadjuvant chemoradiation in gastroesophageal junction adenocarcinoma: a population-based evaluation of the Munich Cancer Registry. Strahlenther Onkol. 2018;194:125–35. [DOI] [PubMed] [Google Scholar]
- 42.Favi F, Bollschweiler E, Berlth F, Plum P, Hescheler DA, Alakus H, et al. Neoadjuvant chemotherapy or chemoradiation for patients with advanced adenocarcinoma of the oesophagus? A propensity score-matched study. Eur J Surg Oncol. 2017;43:1572–80. [DOI] [PubMed] [Google Scholar]
- 43.Deng HY, Wang WP, Wang YC, Hu WP, Ni PZ, Lin YD, et al. Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur J Cardiothorac Surg. 2017;51:421–31. [DOI] [PubMed] [Google Scholar]
- 44.Visser E, Edholm D, Smithers BM, Thomson IG, Burmeister BH, Walpole ET, et al. Neoadjuvant chemotherapy or chemoradiotherapy for adenocarcinoma of the esophagus. J Surg Oncol. 2018;117:1687–96. [DOI] [PubMed] [Google Scholar]
- 45.Glatz T, Bronsert P, Schafer M, Kulemann B, Marjanovic G, Sick O, et al. Perioperative platin-based chemotherapy for locally advanced esophagogastric adenocarcinoma: postoperative chemotherapy has a substantial impact on outcome. Eur J Surg Oncol. 2015;41:1300–7. [DOI] [PubMed] [Google Scholar]
- 46.Moorcraft SY, Smyth EC, Cunningham D. Adjuvant or neoadjuvant therapy for operable esophagogastric cancer? Gastric Cancer. 2015;18:1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.