Abstract
The aim of the present study was to use a competing risk model to analyze the prognostic value of mucinous adenocarcinoma (MAC) in patients with colorectal cancer (CRC). An additional aim was to construct nomograms for estimating the 3- and 5-year overall survival (OS) and cancer specific survival (CSS) rates of patients with primary CRC with MAC. The data were extracted from the Surveillance, Epidemiology, and End Results database, and a Multivariate Cox model and competing risk model were applied to assess the OS and CSS. Cox-based and competing risk-based nomograms were constructed and internally validated by discrimination and calibration, using the bootstrapping method with 1,000 times replicates. A total of 13,035 MAC and 61,958 non-mucinous adenocarcinoma (NMAC) CRC patients were enrolled in the present study. Compared with NMAC, MAC patients had a poorer OS and CSS time in the overall population, and in subgroups that comprised metastatic, non-metastatic, male, site of sigmoid colon, rectosigmoid junction and rectal CRC cases (HR>1; P<0.05). The Cox and competing risk-based nomograms showed effective discrimination and calibration. In conclusion, MAC was associated with poor OS and CSS in patients with CRC of the distal colon and rectum. The nomograms of primary CRC patients with MAC may aid the identification of individual patients with a high risk of overall mortality and cancer-associated mortality within 3 or 5 years.
Keywords: MAC, CRC, nomograms, prognosis, competing risk
Introduction
Worldwide, colorectal cancer (CRC) is the second and third most commonly occurring cancer in women and men, respectively, and the number of cancer-associated mortalities due to CRC ranked fourth among all cancer patients in 2012 (1). Mucinous adenocarcinoma (MAC) is a specific histological subtype of CRC, which occurs in 1.6–25.4% of all CRC cases (2). MAC is defined by the World Health Organization as ‘an adenocarcinoma in which a substantial amount of mucin (>50% of the tumor) is retained within the tumor’ (3).
When compared with non-mucinous adenocarcinoma (NMAC), the prognostic significance of MAC remains uncertain. Previous studies have reported that MAC has a worse overall survival (OS) rate than NMAC (4,5). In addition, MAC was identified to be associated with chemotherapy (6,7) and chemo-radiotherapy resistance (8). However, other studies have stated that there was no prognostic difference between MAC and NMAC (9,10). Population-based studies revealed that MAC resulted in a poorer outcome in rectal, but not colon cancer (11,12). Of note, despite being an aggressive disease, the median age at diagnosis for colon cancer is 68 in men and 72 in women, and for rectal cancer, 63 years in males and females (13). It was therefore established that the competing risk model should be used as part of the aged-patient assessment, as these patients may succumb to other non-cancerous diseases, such as cardiovascular disease (14). However, no competing risk-based analysis was conducted for the prognosis of MAC of CRC in this particular referenced study.
The nomogram is a valuable quantitative tool that utilizes biological or clinical variables to determine a statistical prognostic model, which could indicate the probability of the clinical outcome for a particular individual (15). Nomograms have been reported to compare favorably to the TNM staging systems in a variety of cancers such as renal cell cancer and bladder cancer (16,17). To the best of our knowledge, until now, there was no competing risk-based nomogram for the prognosis of CRC patients of the MAC subtype.
The present population-based study was performed to evaluate the prognostic significance of MAC when compared with NMAC in patients with CRC, using Cox and Fine-Gray regression for proportional hazards modeling of subdistribution hazard (SH) models (18). The prognostic values of different clinical variables for MAC and NMAC CRC patients were also determined. Finally, nomograms were constructed for the prognostic prediction of CRC patients with the MAC subtype within 3 or 5 years.
Materials and methods
Cohort selection
The cohort was obtained from 18 registries of Surveillance, Epidemiology, and End Results (SEER) program, using SEER*Stat 8.3.5 software (SEER ID: daid). Patients who registered on or after the year 2004 were included in the present study if they met the following criteria: i) A primary patient with CRC who had received surgery; ii) a patient with >3 months of follow-up to exclude the bias of perioperative death; iii) an MAC or NMAC patient, where MAC and NMAC were defined by the third edition of the International Classification of Diseases for Oncology (ICD-O-3). The histology codes for MAC were 8480 and 8481, and the codes for NMAC were 8140, 8144, 8210, 8211, 8221, 8261, 8262 and 8263; and iv) a sufficient follow-up time (in the present study, the group with a similar median survival time was selected as the single 2004 group) which to capture enough events to reveal meaningful analysis patterns.
Study variables and endpoints
The following patient variables were extracted: Age at diagnosis, sex, race (Caucasian, African-American, other races or unknown), tumor location (cecum, appendix, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, rectum and unknown site), tumor size, 6th American Joint Committee on Cancer (AJCC) tumor stage (19), grade (well-differentiated, moderately differentiated, poorly differentiated and undifferentiated or anaplastic, and unknown), number of positive regional nodes, radiotherapy and chemotherapy experience, marital status, and carcinoembryonic antigen (CEA) status (positive/elevated, negative/normal and unknown/borderline). The widowed or single (never married or having a domestic partner), or divorced or separated patients were defined as unmarried. The value of age at diagnosis, tumor size and number of positive regional nodes was classified into small categorical variables to fit the linear assumption. The median observed survival time was referred to as ‘median follow-up’.
Statistical analysis
The differences between each clinical factor and MAC or NMAC of CRC were analyzed using the χ2 test. The Kaplan-Meier (KM) method was used to construct the survival curves, and the log-rank P-value was calculated. Cumulative incidences of death (CID) was estimated for mortalities caused by cancer or any other reason. The multivariate Cox proportional hazards regression model and multivariate SH model were used to assess patient OS and CSS, respectively. All of the aforementioned clinical factors were considered during the multivariate analyses. The hazard ratio (HR) and 95% confidence interval (95% CI) were also calculated.
Cox and SH model-based nomograms were constructed using a multivariate logistic regression model to predict the 3- and 5-year survival or cancer specific death rates of patients with MAC, respectively. The internal validation of the nomograms was performed by discrimination and calibration, using the bootstrapping method with 1,000 times replicates (15). The area under the curve (AUC) of the receiver operating characteristic curve (which is also referred to as C-statistics) was assessed to evaluate the discrimination of nomograms, which ranged from 0.5–1.0; 0.5 indicated that the outcome was completely random, and 1.0 indicated perfect discrimination. Calibration curves were also drawn in order to assess how close the nomogram-estimated risk was to the observed risk.
All statistical analyses were performed using R software (version 3.5.1; http://cran.r-project.org/bin/windows/base/old/3.5.1). The R package ‘MASS’ was used to perform the χ2 test. The KM analysis and log-rank P-value were tested using the R package ‘survival’. The R package ‘cmprsk’ was used to perform the CIF test and multivariate SH analysis. The competing risk nomogram was plotted by R packages ‘mstate’ and ‘regplot’. The AUC and calibration curve were plotted by R package ‘riskRegression’. P<0.05 was considered to indicate a statistically significant difference.
Results
Cohort selection
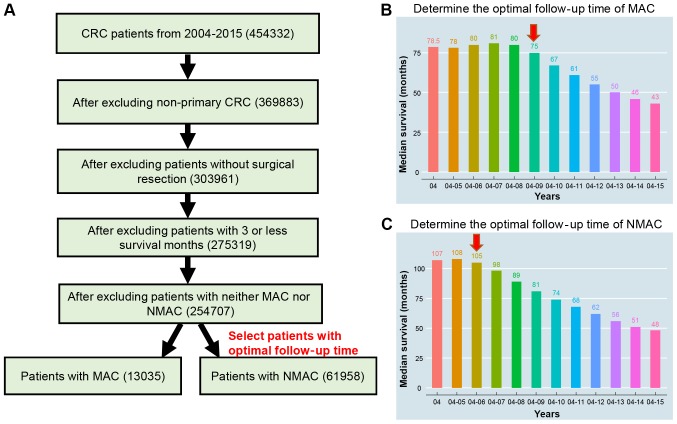
The detailed selection procedure is illustrated in Fig. 1. To ensure that the obtained data also included a sufficient follow-up time, data were obtained from patients diagnosed with MAC between 2004 and 2009, and NMAC between 2004 and 2006, with had similar median survival times as the single 2004 group. Collectively, 13,035 MAC patients and 61,958 NMAC patients were recruited. The median age of MAC and NMAC was 68 and 67 years, respectively. The median survival time of MAC and NMAC were 75 months and 105 months, respectively. The death rates from cancer and any other reason were 35.08 and 20.14% for MAC, and 28.81 and 23.18% for NMAC. It was also observed that the MAC CRC patients were of an older age with an increased number of female patients, were predominantly Caucasian with right-side colon incidence, a larger tumor size, higher AJCC 6th stage and grade, and a higher rate of positive CEA status (P<0.001; Table I).
Figure 1.
Data selection process for the present study. (A) Selection process. (B) Median survival time of different groups (by year) in MAC, following selection. (C) Median survival time of different groups (by year) in NMAC, following selection. MAC, mucinous adenocarcinoma; NMAC, non-mucinous adenocarcinoma.
Table I.
Characteristic of the selected variables.
| MAC | NMAC | ||
|---|---|---|---|
| Characteristics | Patients, n (%) | Patients, n (%) | P-value |
| Age (years) | <0.001 | ||
| <50 | 1,744 (13.38) | 6,864 (11.08) | |
| 50–59 | 2,359 (18.10) | 12,719 (20.53) | |
| 60–69 | 2,984 (22.89) | 15,221 (24.57) | |
| 70–79 | 3,293 (25.26) | 15,874 (25.62) | |
| ≥80 | 2,655 (20.37) | 11,280 (18.21) | |
| Sex | <0.001 | ||
| Female | 6,738 (51.69) | 30,108 (48.59) | |
| Male | 6,297 (48.31) | 31,850 (51.41) | |
| Race | <0.001 | ||
| Caucasian | 10,705 (82.13) | 49,913 (80.56) | |
| African American | 1,473 (11.30) | 6,623 (10.69) | |
| Others or unknown | 857 (6.57) | 5,422 (8.75) | |
| Location | <0.001 | ||
| Cecum | 3,045 (23.36) | 9,740 (15.72) | |
| Appendix | 824 (6.32) | 241 (0.39) | |
| Ascending colon | 2,544 (19.52) | 8,018 (12.94) | |
| Hepatic flexure | 707 (5.42) | 2,191 (3.54) | |
| Transverse colon | 1,018 (7.81) | 3,882 (6.27) | |
| Splenic flexure | 377 (2.89) | 1,654 (2.67) | |
| Descending colon | 503 (3.86) | 2,835 (4.58) | |
| Sigmoid colon | 1,695 (13.00) | 15,104 (24.38) | |
| Rectosigmoid | 645 (4.95) | 5,716 (9.23) | |
| Junction | |||
| Rectum | 1,464 (11.23) | 11,738 (18.95) | |
| Unknown | 213 (1.63) | 839 (1.35) | |
| Tumor size (cm) | <0.001 | ||
| ≤2 | 1,817 (13.94) | 9,167 (14.80) | |
| >6 | 3,036 (23.29) | 7,393 (11.93) | |
| 2–4 | 3,105 (23.82) | 19,393 (31.30) | |
| 4–6 | 3,756 (28.81) | 14,707 (23.74) | |
| Unknown | 1,321 (10.13) | 11,298 (18.23) | |
| AJCC 6th stage (19) | <0.001 | ||
| 0/(Tis) | 51 (0.39) | 2,823 (4.56) | |
| I | 1,677 (12.87) | 16,873 (27.23) | |
| IIA | 3,792 (29.09) | 14,999 (24.21) | |
| IIB | 862 (6.61) | 1,960 (3.16) | |
| IIIA | 318 (2.44) | 2,177 (3.51) | |
| IIIB | 2,315 (17.76) | 9,378 (15.14) | |
| IIIC | 1,629 (12.50) | 5,326 (8.60) | |
| IV | 2,148 (16.48) | 7,141 (11.53) | |
| Unknown | 243 (1.86) | 1,281 (2.07) | |
| Grade | <0.001 | ||
| Low grade (I and II) | 9,283 (71.22) | 47,290 (76.33) | |
| High grade | 2,612 (20.04) | 10,176 (16.42) | |
| (III and IV) | |||
| Unknown | 1,140 (8.75) | 4,492 (7.25) | |
| Regional nodes positive | <0.001 | ||
| ≥10 | 6600 (50.63) | 33151 (53.51) | |
| 0 | 3015 (23.13) | 12971 (20.94) | |
| 1–3 | 1824 (13.99) | 6545 (10.56) | |
| 4–9 | 1596 (12.24) | 9291 (15.00) | |
| Chemotherapy | <0.001 | ||
| No | 7,352 (56.40) | 39,410 (63.61) | |
| Yes | 5,683 (43.60) | 22,548 (36.39) | |
| Radiotherapy | <0.001 | ||
| No | 11,474 (88.02) | 53,499 (86.35) | |
| Yes | 1,520 (11.66) | 8,241 (13.30) | |
| Unknown | 41 (0.31) | 218 (0.35) | |
| Marital status | <0.001 | ||
| Married | 7,242 (55.56) | 35,752 (57.70) | |
| Unmarried | 5,413 (41.53) | 23,799 (38.41) | |
| Unknown | 380 (2.92) | 2,407 (3.88) | |
| CEA status | <0.001 | ||
| Negative/normal | 3,776 (28.97) | 18,665 (30.13) | |
| Positive/elevated | 3,619 (27.76) | 12,903 (20.83) | |
| Unknown/borderline | 5,640 (43.27) | 30,390 (49.05) | |
MAC, mucinous adenocarcinoma; NMAC, non-mucinous adenocarcinoma; CEA, carcinoembryonic antigen. Significant results are in bold.
Patients with MAC exhibit poorer outcomes than patients with NMAC
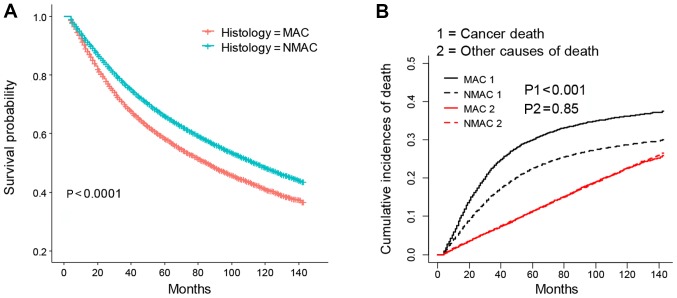
KM analysis revealed that MAC patients possessed poorer OS times than those with NMAC (P<0.001; Fig. 2A). The CID plot also identified an increased risk of cancer-associated mortality for MAC patients (P<0.001; Fig. 2B). However, no significant competing risk was observed when comparing the prognosis between MAC and NMAC patients (P=0.85; Fig. 2B). Indeed, few differences were identified between the results of the multivariate Cox and SH models. As listed in Table II, multivariate Cox model and SH model analysis of the entirety of both patient groups revealed that MAC patients had poorer OS (HR=1.07; 95% CI=1.05–1.10) and CSS (HR=1.07, 95% CI=1.03–1.11) times than patients with NMAC, respectively. Further subgroup analysis revealed that the MAC cohort had worse OS and CSS than those in the NMAC groups, in both metastatic or non-metastatic or male patients with CRC (HR>1, P<0.05). The results indicated that MAC resulted in poorer OS (HR>1, P<0.05), but not CSS (P>0.05) when compared with NMAC in females. In addition, when assessing the location differences, the results indicated that patients with MAC had shorter OS and CSS times than NMAC patients when cancer occurred in the sigmoid colon, rectosigmoid junction or rectum (HR>1, P<0.05), and that MAC patients also exhibited poorer OS times than NMAC patients with cancer in the descending colon (HR>1, P<0.05). Collectively, the results suggest that the MAC cohort possessed worse outcomes than the NMAC cohort when the cancer was located in the distal colon or rectum, but not the proximal colon.
Figure 2.
KM and CID plots of patients with MAC and NMAC CRC. (A) KM and (B) CIF plots of MAC and NMAC CRC patients. KM, Kaplan-Meier; MAC, mucinous adenocarcinoma; NMAC, non-mucinous adenocarcinoma; CRC, colorectal cancer; CID, cumulative incidence of death.
Table II.
Multivariate analysis of the prognostic value of MAC and NMAC status and the prognosis of patients with CRC.
| MAC vs. NMAC | |||
|---|---|---|---|
| Variable | Patients amount | Cox model HR (95% CI) | SH model HR (95% CI) |
| All | 74,993 | 1.07 (1.05–1.10) | 1.07 (1.03–1.11) |
| Non-metastatic CRC | 9,289 | 1.11 (1.08–1.14) | 1.16 (1.11–1.21) |
| Metastatic CRC | 64,180 | 1.11 (1.05–1.18) | 1.11 (1.04–1.18) |
| Female | 36,846 | 1.07 (1.03–1.11) | 1.03 (0.98–1.08) |
| Male | 38,147 | 1.09 (1.05–1.13) | 1.12 (1.06–1.18) |
| Cecum | 12,785 | 1.02 (0.97–1.08) | 1.00 (0.93–1.08) |
| Appendix | 1,065 | 0.87 (0.68–1.10) | 0.98 (0.74–1.29) |
| Ascending colon | 10,562 | 1.06 (0.99–1.13) | 1.06 (0.97–1.16) |
| Hepatic flexure | 2,898 | 1.02 (0.91–1.15) | 0.91 (0.77–1.08) |
| Transverse colon | 4,900 | 0.99 (0.90–1.09) | 0.89 (0.77–1.02) |
| Splenic flexure | 2,031 | 1.02 (0.87–1.20) | 1.10 (0.89–1.35) |
| Descending colon | 3,338 | 1.20 (1.05–1.37) | 1.01 (0.84–1.22) |
| Sigmoid colon | 16,799 | 1.19 (1.11–1.27) | 1.25 (1.14–1.37) |
| Rectosigmoid junction | 6,361 | 1.23 (1.10–1.38) | 1.20 (1.04–1.39) |
| Rectum | 13,202 | 1.18 (1.09–1.27) | 1.22 (1.11–1.34) |
HR; hazard ratio; CI, confidence interval; MAC, mucinous adenocarcinoma; NMAC, non-mucinous adenocarcinoma; SH, subdistribution hazard. Bold results indicate stasitical significance P<0.05.
Independent prognostic factors of OS and CSS for MAC and NMAC
The independent prognostic value of the selected variables for MAC and NMAC patients are listed in Table III. Multivariate Cox and SH models were used. With both Cox and SH models, the results indicated that increased age, male sex, African-American race, higher tumor stage and grade, a greater number of positive regional lymph nodes, unmarried status and CEA-positive status were risk factors for MAC and NMAC patients (HR>1; P<0.05). Tumor size was revealed to be a risk factor for NMAC (HR>1; P<0.05), but not MAC (P>0.05). The use of chemotherapy was associated with a longer OS time in both MAC and NMAC patients (HR<1; P<0.05). However, there was no association between chemotherapy and CSS in MAC and NMAC patients using the SH model (P>0.05). The use of radiotherapy was associated with a worse OS in MAC and NMAC patients (HR>1; P<0.05). Additionally, radiotherapy decreased the CSS of NMAC patients (HR>1; P<0.05), yet not those with MAC CRC (P>0.05). Interestingly, it was found that primary CRC of the appendix was associated with longer OS and CSS in MAC patients (other parts vs. appendix; HR>1; P<0.05); however, not NMAC patients (P>0.05). To further confirm the role that tumor location served in the prognosis of MAC and NMAC, the locations were divided into four groups that included the right colon (Cecum, appendix, ascending colon, hepatic flexure and transverse colon), left colon (splenic flexure, descending colon, sigmoid colon and rectosigmoid junction), rectum and unknown locations. The results revealed that cancer of the left colon resulted in a worse OS and CSS than that of the right colon in MAC patients (HR>1; P<0.05; Table IV), and conversely, that CRC in the left colon resulted in an improved OS and CSS in patients with NMAC (HR<1, P<0.05; Table IV). The analysis also indicated that rectal cancer had an inferior CSS than right-sided colon cancer among patients with MAC and NMAC (HR>1, P<0.05; Table IV).
Table III.
Multivariate Cox and SH analyses of each variable in MAC and NMAC CRC patients.
| MAC | NMAC | |||
|---|---|---|---|---|
| Characteristics | COX model HR (95% CI) | SH model HR (95% CI) | COX model HR (95% CI) | SH model HR (95% CI) |
| Age (years) | ||||
| <50 | References | References | References | References |
| 50–59 | 1.08 (0.98–1.18) | 1.04 (0.94–1.14) | 1.09 (1.04–1.14) | 1.01 (0.96–1.07) |
| 60–69 | 1.17 (1.07–1.28) | 1.03 (0.94–1.14) | 1.46 (1.39–1.53) | 1.12 (1.06–1.18) |
| 70–79 | 1.94 (1.78–2.12) | 1.28 (1.16–1.42) | 2.47 (2.36–2.58) | 1.38 (1.31–1.46) |
| ≥80 | 3.34 (3.05–3.66) | 1.54 (1.37–1.72) | 4.58 (4.37–4.80) | 1.69 (1.59–1.80) |
| Sex | ||||
| Female | References | References | References | References |
| Male | 1.26 (1.20–1.32) | 1.20 (1.13–1.28) | 1.29 (1.26–1.32) | 1.14 (1.11–1.18) |
| Race | ||||
| Caucasian | References | References | References | References |
| Black | 1.07 (1.00–1.16) | 1.15 (1.05–1.26) | 1.19 (1.15–1.23) | 1.25 (1.19–1.31) |
| Others or unknown | 0.90 (0.82–1.00) | 1.06 (0.94–1.19) | 0.82 (0.78–0.85) | 0.95 (0.90–1.01) |
| Location | ||||
| Appendix | References | References | References | References |
| Cecum | 2.18 (1.92–2.49) | 1.87 (1.59–2.20) | 1.06 (0.89–1.28) | 0.89 (0.72–1.10) |
| Ascending colon | 2.11 (1.85–2.41) | 1.69 (1.43–1.99) | 1.01 (0.84–1.21) | 0.76 (0.61–0.94) |
| Hepatic flexure | 2.18 (1.86–2.55) | 1.69 (1.38–2.07) | 1.06 (0.88–1.28) | 0.88 (0.70–1.10) |
| Transverse colon | 2.07 (1.79–2.40) | 1.62 (1.34–1.95) | 1.05 (0.87–1.27) | 0.85 (0.68–1.06) |
| Splenic flexure | 2.05 (1.71–2.46) | 1.92 (1.53–2.40) | 0.99 (0.82–1.20) | 0.84 (0.67–1.06) |
| Descending colon | 2.22 (1.88–2.63) | 1.75 (1.42–2.16) | 0.96 (0.80–1.16) | 0.83 (0.66–1.03) |
| Sigmoid colon | 2.26 (1.98–2.59) | 2.00 (1.70–2.36) | 0.96 (0.80–1.15) | 0.79 (0.63–0.97) |
| Rectosigmoid junction | 2.29 (1.95–2.70) | 1.93 (1.58–2.35) | 0.98 (0.82–1.18) | 0.84 (0.67–1.04) |
| Rectum | 2.30 (1.97–2.68) | 2.04 (1.68–2.47) | 1.06 (0.88–1.28) | 0.93 (0.75–1.16) |
| Unknown | 2.65 (2.17–3.25) | 2.24 (1.76–2.85) | 1.10 (0.90–1.35) | 0.97 (0.76–1.23) |
| Tumor size (cm) | ||||
| ≤2 | References | References | References | References |
| >6 | 1.00 (0.92–1.08) | 0.96 (0.86–1.07) | 1.02 (0.98–1.07) | 1.02 (0.96–1.09) |
| 2–4 | 1.08 (0.99–1.17) | 0.98 (0.88–1.09) | 1.05 (1.01–1.09) | 1.05 (0.99–1.11) |
| 4–6 | 1.06 (0.98–1.14) | 1.01 (0.91–1.12) | 1.06 (1.01–1.10) | 1.06 (1.01–1.13) |
| Unknown | 1.02 (0.92–1.13) | 1.02 (0.89–1.17) | 0.98 (0.94–1.03) | 1.04 (0.97–1.11) |
| Stage | ||||
| 0/(Tis) | References | References | References | References |
| I | 1.20 (0.76–1.90) | 0.98 (0.45–2.14) | 1.33 (1.23–1.43) | 2.24 (1.89–2.65) |
| IIA | 1.51 (0.96–2.37) | 1.85 (0.86–4.01) | 1.78 (1.65–1.93) | 4.68 (3.93–5.58) |
| IIB | 2.35 (1.48–3.73) | 4.68 (2.15–10.17) | 2.72 (2.47–2.99) | 9.98 (8.27–12.05) |
| IIIA | 1.05 (0.64–1.73) | 1.21 (0.53–2.77) | 1.37 (1.23–1.53) | 3.58 (2.93–4.37) |
| IIIB | 1.85 (1.16–2.95) | 3.12 (1.43–6.81) | 2.21 (2.02–2.42) | 7.46 (6.24–8.91) |
| IIIC | 2.17 (1.37–3.44) | 4.17 (1.92–9.05) | 2.70 (2.46–2.96) | 10.18 (8.51–12.17) |
| IV | 7.13 (4.51–11.28) | 13.07 (6.03–28.31) | 9.35 (8.62–10.16) | 34.27 (28.82–40.75) |
| Unknown | 2.21 (1.37–3.58) | 3.90 (1.77–8.60) | 1.97 (1.78–2.18) | 6.17 (5.11–7.45) |
| Grade | ||||
| Low grade (I and II) | References | References | References | References |
| High grade (III and IV) | 1.20 (1.14–1.27) | 1.18 (1.09–1.27) | 1.13 (1.10–1.16) | 1.22 (1.17–1.27) |
| Unknown | 1.01 (0.93–1.10) | 1.02 (0.92–1.14) | 0.98 (0.93–1.04) | 1.03 (0.95–1.11) |
| Regional nodes positive | ||||
| 0 | References | References | References | References |
| 1–3 | 1.31 (1.16–1.47) | 1.53 (1.33–1.76) | 1.24 (1.18–1.30) | 1.39 (1.30–1.48) |
| 4–9 | 1.73 (1.55–1.93) | 1.89 (1.65–2.16) | 1.41 (1.34–1.49) | 1.56 (1.46–1.67) |
| ≥10 | 2.14 (1.95–2.36) | 2.32 (2.05–2.63) | 1.56 (1.50–1.63) | 1.84 (1.74–1.95) |
| Chemotherapy | ||||
| No | References | References | References | References |
| Yes | 0.83 (0.78–0.88) | 1.00 (0.92–1.07) | 0.84 (0.81–0.86) | 0.98 (0.94–1.02) |
| Radiotherapy | ||||
| No | References | References | References | References |
| Yes | 1.13 (1.02–1.26) | 1.14 (1.00–1.30) | 1.15 (1.10–1.20) | 1.15 (1.08–1.22) |
| Unknown | 1.24 (0.84–1.84) | 1.18 (0.66–2.11) | 1.08 (0.91–1.29) | 1.16 (0.91–1.48) |
| Marital status | ||||
| Married | References | References | References | References |
| Unmarried | 1.22 (1.16–1.28) | 1.12 (1.05–1.20) | 1.28 (1.25–1.31) | 1.15 (1.12–1.19) |
| Unknown | 1.11 (0.96–1.27) | 1.00 (0.82–1.20) | 0.99 (0.94–1.06) | 0.93 (0.85–1.02) |
| CEA status | ||||
| Negative/normal | References | References | References | References |
| Positive/elevated | 1.41 (1.32–1.50) | 1.43 (1.31–1.55) | 1.50 (1.46–1.55) | 1.53 (1.47–1.60) |
| Unknown/borderline | 1.19 (1.12–1.26) | 1.19 (1.10–1.29) | 1.17 (1.14–1.20) | 1.20 (1.15–1.25) |
‘References’ refers to this group being the variable with which the other age groups were compared. Hazard ratio (HR) and 95% confidence index (95% CI) were calculated; MAC, mucinous adenocarcinoma; NMAC, non-mucinous adenocarcinoma; CEA, carcinoembryonic antigen. Bold results indicate statistical significance P<0.05.
Table IV.
Additional subgroup prognostic analysis for MAC and NMAC patients by different location.
| MAC | NMAC | |||
|---|---|---|---|---|
| Location | Cox model HR (95% CI) | SH model HR (95% CI) | Cox model HR (95% CI) | SH model HR (95% CI) |
| Right | References | References | References | References |
| Left | 1.13 (1.07–1.19) | 1.18 (1.10–1.27) | 0.93 (0.90–0.95) | 0.96 (0.92–0.99) |
| Rectum | 1.16 (1.05–1.29) | 1.25 (1.09–1.43) | 1.02 (0.98–1.06) | 1.10 (1.04–1.17) |
| Unknown | 1.37 (1.15–1.62) | 1.40 (1.15–1.72) | 1.06 (0.97–1.16) | 1.15 (1.03–1.30) |
‘References’ means this group is the variable with which the other age groups were compared. Hazard ratio (HR) and 95% confidence index (95% CI) were calculated; MAC, mucinous adenocarcinoma; NMAC, non-mucinous adenocarcinoma. Bold results indicate stasitical significant P<0.05.
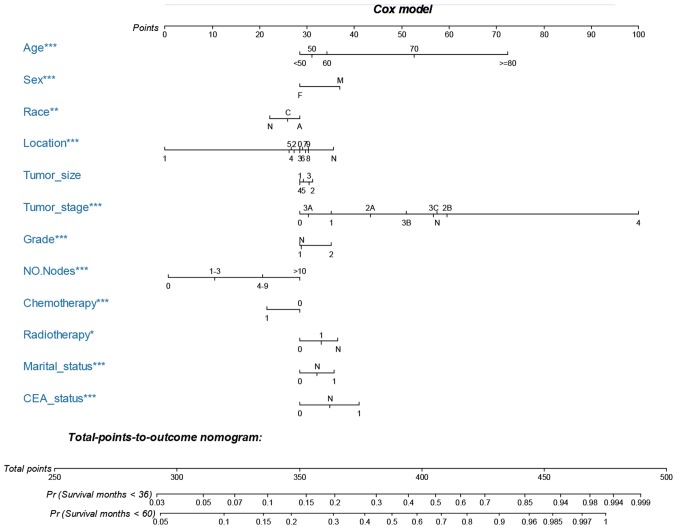
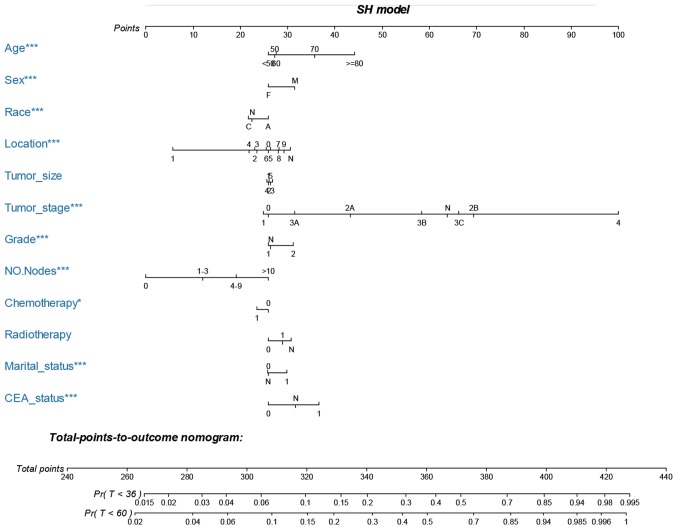
Nomogram development and validation
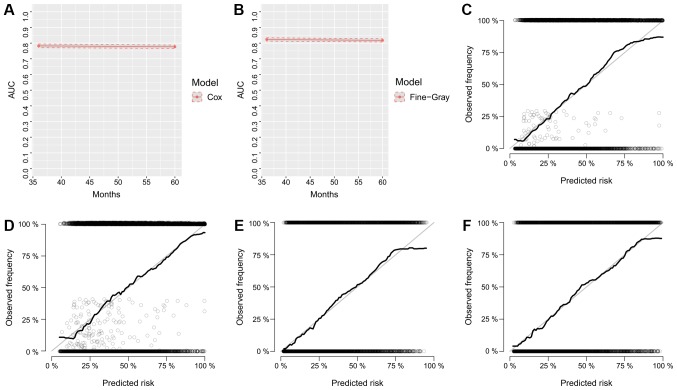
The nomograms were constructed using multivariate Cox-based and SH-based models. A weighted total score calculated from each variable was used to estimate the 3- and 5-year OS and cancer-specific mortality prediction (Figs. 3 and 4). Time dependent AUC plots suggested effective discrimination (AUC>0.75; Fig. 5A-B). The calibration plots illustrated a positive correlation between the observed OS and CSS and the nomogram-predicted OS and CSS (Fig. 5C-F).
Figure 3.
Nomogram for predicting 3- and 5-year OS of patients with MAC CRC. The nomogram is used by summing the points identified on the top scale for each independent variable and drawing a vertical line from the total points scale to the 3- and 5-year OS to obtain the probability of survival. The total points projected to the bottom scale indicate the % probability of the 3- and 5-year survival. Sex: M = male, F = female; race: C = caucasian, A = African-American, N = other race or unknown race; location, 0, cecum; 1, appendix; 2, ascending colon; 3, hepatic flexure; 4, transverse colon; 5, splenic flexure; 6, descending colon; 7, sigmoid colon; 8, rectosigmoid junction; 9, rectum; and N, unknown; tumor size: 1, 0–2 cm; 2, 2–4 cm; 3, 4–6 cm; 4, >6 cm; and 5, unknown size; tumor stage, N, unknown stage; grade: 1, well differentiated; grade I and moderately differentiated; grade II; 2, poorly differentiated; grade III and undifferentiated; anaplastic; grade IV, and N, unknown grade; NO. Nodes, the number of positive regional lymph nodes; chemotherapy, 0, none/unknown and 1, yes; radiotherapy, 0, none/unknown or refused,; 1, beam radiation or a combination of beam with implants or isotopes or radiation with method or source not specified or radioactive implants or radioisotopes, and N, recommended, unknown if administered; marital status, 0, married; 1, widowed or single (never married or having a domestic partner) or divorced or separated; CEA status, 0, negative/normal; 1, positive/elevated; and N, unknown/borderline. MAC, mucinous adenocarcinoma; CRC, colorectal cancer; OS, overall survival; CEA, carcinoembryonic antigen. *P<0.05, **P<0.01, ***P<0.001.
Figure 4.
Nomogram for predicting 3- and 5-year cancer specific mortality of MAC CRC patients. The nomogram is used by summing the points identified on the top scale for each independent variable and drawing a vertical line from the total points scale to the 3- and 5-year OS to obtain the probability of survival. The total points projected to the bottom scale indicate the % probability of the 3- and 5-year survival. Sex: M, male; F, female; race: C, Caucasian; A, African-American; N, other race or unknown race; location, 0, cecum; 1, appendix; 2, ascending colon; 3, hepatic flexure; 4, transverse colon; 5, splenic flexure; 6, descending colon; 7, sigmoid colon; 8, rectosigmoid junction; 9, rectum; and N, unknown; tumor size: 1, 0–2 cm; 2, 2–4 cm; 3, 4–6 cm; 4, >6 cm; and 5, unknown size; tumor stage; N, unknown stage; grade: 1, well differentiated; grade I and moderately differentiated; grade II, 2, poorly differentiated; grade III and undifferentiated; anaplastic; grade IV, and N, unknown grade; NO. nodes, the number of positive regional lymph nodes; chemotherapy, 0, none/unknown and 1, yes; radiotherapy, 0, none/unknown or refused; 1, beam radiation or combination of beam with implants or isotopes or radiation with method or source not specified or radioactive implants or radioisotopes and N, recommended, unknown if administered; marital status, 0, married; 1, widowed or single (never married or having a domestic partner) or divorced or separated; CEA status, 0 = negative/normal, 1, positive/elevated, and N, unknown/borderline. MAC, mucinous adenocarcinoma; CRC, colorectal cancer; OS, overall survival; CEA, carcinoembryonic antigen. *P<0.05, **P<0.01, ***P<0.001.
Figure 5.
Calibration curves and AUC plots. Calibration for internal validation of Cox-based and SH based nomograms. Time-dependent AUC plots of (A) Cox-based and (B) SH-based nomograms. Calibration plots for 3-year (C) and 5-year (D) Cox-based nomograms. Calibration plots for 3-year (E) and 5-year (F) SH-based nomograms. AUC, area under the curve; SH, subdistribution hazard.
Discussion
Conventional prognostic methods, such as the KM method and standard Cox proportional hazard regression may be inappropriate in the presence of competing risks (20). To the best of our knowledge, the present study was the first competing risk analysis-based investigation on the prognosis of patients with primary CRC with MAC. An increasing concern regarding competing risk resulted in the development of competing-risk nomograms for a group of cancers such as renal cell cancer and melanoma (21–25). The present study established the first competing-risk nomogram for patients with primary CRC with the MAC subtype, who had undergone surgery.
The results obtained herein indicated that MAC may reduce the OS and CSS times of CRC patients, which was supported by previous studies (4,5). Further subgroup analysis revealed that patients with MAC had worse OS and CSS times than NMAC patients in either metastatic CRC or non-metastatic CRC, or in male patients. Of note, it was observed that MAC had an inferior outcome only when located in the distal colon (the descending and the sigmoid colon) and rectum. In addition, the results indicated that left-sided colon cancer resulted in reduced OS and CSS times compared with right-sided cancer in patients with MAC, yet improved the OS and CSS in left-sided colon cancer patients with NMAC (compared with right-sided cancer patients).
Age was previously identified as a risk factor for the OS of patients with CRC (26,27), which was supported by the results of the present study; the competing risk model also identified increased age as a detrimental factor for MAC and NMAC patients. Over 20% of patients succumbed to pathologies than cancers (which may have been age-related) in the present study. The results revealed that age was indeed a risk factor for the cancer-associated mortality of patients with CRC.
It was observed that male CRC patients had poorer prognoses in MAC and NMAC, which was consistent with the results of previous studies (28,29). The results herein demonstrated that African-Americans subjects had worse outcomes than Caucasians, and that African-American patients with CRC were observed to have higher frequencies of somatic genetic alterations such as those in the KRAS proto-oncogene, GTPase (KRAS), B-Raf proto-oncogene, serine/threonine kinase (BRAF), and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α, which may contribute to these outcomes (30).
A notable result of the present study was the prognostic differences of patients with MAC and NMAC at different locations. MAC CRC in the appendix bestowed an improved outcome compared with mucinous cancers in any other part of the colon and rectum. The incidence of appendiceal cancer has increased in recent years (31), and a previous study reported that mucinous appendiceal cancer exhibited an improved survival rate compared with other histological types of appendiceal cancer (31). Mucinous appendiceal cancer was also observed to have a considerably promising outcome, even in disseminated disease (32). The present study provides novel information for the prognosis of mucinous appendiceal cancer.
A previous study reported that left-sided colon cancer was more sensitive to anti- epidermal growth factor receptor (EGFR)-targeted therapy than right-sided colon cancer (33). In addition, patients with right-sided colon cancer were observed to have an increased number of BRAF mutations than those with left-sided colon cancer, which may contribute to anti-EGFR therapy-associated resistance (34) and therefore, poor prognosis (35). The results of the present study indicated that left-sided colon cancer resulted in a worse outcome than right-sided colon cancer in patients with MAC. However, left-sided cancer exhibited better outcomes than right-sided cancer in patients with NMAC. This may have been caused by the improved prognosis of the mucinous appendiceal cancer in right-sided colon MAC. Nevertheless, following the exclusion of mucinous appendiceal cancer patients in the MAC group, there was no significant difference between left- and right-side colon MAC patients (Data not shown); this suggested that location-based prognostic differences exist in MAC and NMAC. In addition, MAC patients were also found to have worse outcomes than those with NMAC in left-side colon cancers.
Interestingly, AJCC 6th IIB CRC patients had worse OS and CSS time than those with IIIA and IIIB CRC (MAC and NMAC patients). In patients with MAC, IIB CRC resulted in a worse OS and CSS time than IIIC CRC. Additionally IIB CRC patients were observed to have worse OS times than IIIC CRC patients. Previous studies revealed that colorectal cancer patients with stage II often exhibited a worse prognosis than stage III patients (36–38). Patients with stage IIA rectal cancer were identified to have poorer CSS times than patients with stage IIIA rectal cancer (36). A previous study also reported that patients with stage IIB/C colon cancer had a worse OS time than those with stage IIIA colon cancer, even after adjusting for adequate lymph nodes retrieved and receipt of adjuvant chemotherapy (37). Another SEER-based study revealed that patients with stage IIB CRC had a poorer OS time than those with stage IIIA and IIIB colorectal cancer (38). These results indicated that the local factors may be as important as local lymph node metastasis, as AJCC 6th stage IIB colorectal cancers directly invade other organs or structures and/or perforate the visceral peritoneum, while many III stage colorectal cancers remain within the bowel wall, but also spread to the lymph nodes (19). The results of the present study partially confirmed the findings of these previous studies.
The present study had several strengths. Firstly, it was a larger population-based study, and large-scale samples of patients with MAC were involved. Secondly, compared with former population-based studies (11,12), an appropriate follow up time was decided when selecting the cohort to achieve more reliable results (Fig. 1). Thirdly, to the best of our knowledge, no nomograms for patients with MAC have been constructed in previous studies. Additionally the Cox and SH models-based nomograms were well validated by discrimination and calibration. Finally, the predictive factors used to construct these nomograms were easily obtained, allowing a feasible translation into clinical use.
There were also a number of limitations to the present study. Firstly, CRC is a heterogeneous disease with frequent genetic variations, including KRAS and BRAF mutations, and microsatellite instability, which are associated with prognosis (39,40). Unfortunately, these were not taken into consideration in the present study. Secondly, the detailed therapy data for patients with CRC in the current study were limited, such as anti-EGFR therapy, immunotherapy and detailed strategies of chemotherapy. Thirdly, despite the nomograms being well validated by internal validation of discrimination and calibration, the external validation from other cohorts is required in the future, in order to test their accuracy.
In conclusion, to the best of our knowledge, the present study is the first population-based study to use a competing risk model to estimate the CSS of primary CRC patients with MAC. It was observed that patients with MAC exhibited worse OS and CSS times than patients with NMAC. This is the first study to construct OS- and CSS-based nomograms for estimating the prognosis of CRC patients with MAC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- MAC
mucinous adenocarcinoma
- NMAC
non-mucinous adenocarcinoma
- CRC
colorectal cancer
- OS
overall survival
- CSS
cancer specific survival
- SEER
Surveillance, Epidemiology, and End Results
- HR
hazard ratio
- SH
subdistribution hazard
- 95% CI
95% confidence interval
- KM
Kaplan-Meier
- CID
cumulative incidences of death
- CEA
carcinoembryonic antigen
- EGFR
epidermal growth factor receptor
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81372178, 81502386 and 81772944) and the Zhejiang Provincial Program for High-level Innovative Healthcare talents.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
Authors' contributions
DD, BZ, YZ and HJ collected and analyzed the data. DD and BZ reviewed the data analysis and drafted the manuscript. XW designed the study and reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This is not applicable since the corresponding data were obtained from a public database.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Hugen N, van Beek JJ, de Wilt JH, Nagtegaal ID. Insight into mucinous colorectal carcinoma: Clues from etiology. Ann Surg Oncol. 2014;21:2963–2970. doi: 10.1245/s10434-014-3706-6. [DOI] [PubMed] [Google Scholar]
- 3.Jass JR, Sobin LH, Watanabe H. The World Health Organization's histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer. 1990;66:2162–2167. doi: 10.1002/1097-0142(19901115)66:10<2162::AID-CNCR2820661020>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 4.Kanemitsu Y, Kato T, Hirai T, Yasui K, Morimoto T, Shimizu Y, Kodera Y, Yamamura Y. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum. 2003;46:160–167. doi: 10.1007/s10350-004-6518-0. [DOI] [PubMed] [Google Scholar]
- 5.Green JB, Timmcke AE, Mitchell WT, Hicks TC, Gathright JB, Jr, Ray JE. Mucinous carcinoma-just another colon cancer? Dis Colon Rectum. 1993;36:49–54. doi: 10.1007/BF02050301. [DOI] [PubMed] [Google Scholar]
- 6.Catalano V, Loupakis F, Graziano F, Torresi U, Bisonni R, Mari D, Fornaro L, Baldelli AM, Giordani P, Rossi D, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009;100:881–887. doi: 10.1038/sj.bjc.6604955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol: 2005;16:1305–1310. doi: 10.1093/annonc/mdi244. [DOI] [PubMed] [Google Scholar]
- 8.Sengul N, Wexner SD, Woodhouse S, Arrigain S, Xu M, Larach JA, Ahn BK, Weiss EG, Nogueras JJ, Berho M. Effects of radiotherapy on different histopathological types of rectal carcinoma. Colorectal Dis. 2006;8:283–288. doi: 10.1111/j.1463-1318.2005.00934.x. [DOI] [PubMed] [Google Scholar]
- 9.Farhat MH, Barada KA, Tawil AN, Itani DM, Hatoum HA, Shamseddine AI. Effect of mucin production on survival in colorectal cancer: A case-control study. World J Gastroenterol. 2008;14:6981–6985. doi: 10.3748/wjg.14.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Consorti F, Lorenzotti A, Midiri G, Di Paola M. Prognostic significance of mucinous carcinoma of colon and rectum: A prospective case-control study. J Surg Oncol. 2000;73:70–74. doi: 10.1002/(SICI)1096-9098(200002)73:2<70::AID-JSO3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Warschkow R, Tarantino I, Huttner FJ, Schmied BM, Guller U, Diener MK, Ulrich A. Predictive value of mucinous histology in colon cancer: A population-based, propensity score matched analysis. Br J Cancer. 2016;114:1027–1032. doi: 10.1038/bjc.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, Rodriguez-Bigas MA, Cormier JN, Chang GJ, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: Analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19:2814–2821. doi: 10.1245/s10434-012-2321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Cancer Society, corp-author. Atlanta, GA: 2017. Colorectal Cancer Facts and Figures 2017–2019. [Google Scholar]
- 14.Zhang Z. Survival analysis in the presence of competing risks. Ann Transl Med. 2017;5:47. doi: 10.21037/atm.2017.07.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015;16:e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 17.Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol. 2006;24:3819–3820. doi: 10.1200/JCO.2006.07.1290. [DOI] [PubMed] [Google Scholar]
- 18.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: An in depth guide for clinicians. Bone Marrow Transplant. 2010;45:1388–1395. doi: 10.1038/bmt.2009.359. [DOI] [PubMed] [Google Scholar]
- 19.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 20.Noordzij M, Leffondre K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670–2677. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 21.Shen W, Sakamoto N, Yang L. Melanoma-specific mortality and competing mortality in patients with non-metastatic malignant melanoma: A population-based analysis. BMC Cancer. 2016;16:413. doi: 10.1186/s12885-016-2438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brockman JA, Alanee S, Vickers AJ, Scardino PT, Wood DP, Kibel AS, Lin DW, Bianco FJ, Jr, Rabah DM, Klein EA, et al. Nomogram predicting prostate cancer-specific mortality for men with biochemical recurrence after radical prostatectomy. Eur Urol. 2015;67:1160–1167. doi: 10.1016/j.eururo.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Shen W, Sakamoto N. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol. 2013;31:468–474. doi: 10.1200/JCO.2012.42.4457. [DOI] [PubMed] [Google Scholar]
- 24.Kutikov A, Egleston BL, Wong YN, Uzzo RG. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol. 2010;28:311–317. doi: 10.1200/JCO.2009.22.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kattan MW, Heller G, Brennan MF. A competing-risks nomogram for sarcoma-specific death following local recurrence. Stat Med. 2003;22:3515–3525. doi: 10.1002/sim.1574. [DOI] [PubMed] [Google Scholar]
- 26.Charlton ME, Kahl AR, Greenbaum AA, Karlitz JJ, Lin C, Lynch CF, Chen VW. KRAS testing, tumor location, and survival in patients with stage IV colorectal cancer: SEER 2010–2013. J Natl Compr Canc Netw. 2017;15:1484–1493. doi: 10.6004/jnccn.2017.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Eeghen EE, Bakker SD, van Bochove A, Loffeld RJ. Impact of age and comorbidity on survival in colorectal cancer. J Gastrointest Oncol. 2015;6:605–612. doi: 10.3978/j.issn.2078-6891.2015.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quirt JS, Nanji S, Wei X, Flemming JA, Booth CM. Is there a sex effect in colon cancer? Disease characteristics, management, and outcomes in routine clinical practice. Curr Oncol. 2017;24:e15–e23. doi: 10.3747/co.24.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majek O, Gondos A, Jansen L, Emrich K, Holleczek B, Katalinic A, Nennecke A, Eberle A, Brenner H, GEKID Cancer Survival Working Group et al. Sex differences in colorectal cancer survival: Population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS One. 2013;8:e68077. doi: 10.1371/journal.pone.0068077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang M, Shen XJ, Kim S, Araujo-Perez F, Galanko JA, Martin CF, Sandler RS, Keku TO. Somatic gene mutations in African Americans may predict worse outcomes in colorectal cancer. Cancer Biomark. 2013;13:359–366. doi: 10.3233/CBM-130366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marmor S, Portschy PR, Tuttle TM, Virnig BA. The rise in appendiceal cancer incidence: 2000–2009. J Gastrointest Surg. 2015;19:743–750. doi: 10.1007/s11605-014-2726-7. [DOI] [PubMed] [Google Scholar]
- 32.Dulskas A, Poskus T, Poskus E, Strupas K. Long-term outcomes after surgery for appendiceal mucinous tumours. Visc Med. 2018;34:151–155. doi: 10.1159/000485092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH, Atkins JN, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA. 2017;317:2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, Santini D, Masi G, Stasi I, Canestrari E, Rulli E, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101:715–721. doi: 10.1038/sj.bjc.6605177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salem ME, Weinberg BA, Xiu J, El-Deiry WS, Hwang JJ, Gatalica Z, Philip PA, Shields AF, Lenz HJ, Marshall JL, et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8:86356–86368. doi: 10.18632/oncotarget.21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mo S, Dai W, Xiang W, Huang B, Li Y, Feng Y, Li Q, Cai G, et al. Survival contradiction between stage IIA and stage IIIA rectal cancer. A retrospective study. 2018;9:1466–1475. doi: 10.7150/jca.23311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu QD, Zhou M, Medeiros KL, Peddi P, Kavanaugh M, Wu XC. Poor survival in stage IIB/C (T4N0) compared to stage IIIA (T1-2 N1, T1N2a) colon cancer persists even after adjusting for adequate lymph nodes retrieved and receipt of adjuvant chemotherapy. BMC Cancer. 2016;16:460. doi: 10.1186/s12885-016-2446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YC, Lee YL, Chuang JP, Lee JC. Differences in survival between colon and rectal cancer from SEER data. PLoS One. 2013;8:e78709. doi: 10.1371/journal.pone.0078709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bogaert J, Prenen H. Molecular genetics of colorectal cancer. Ann Gastroenterol. 2014;27:9–14. [PMC free article] [PubMed] [Google Scholar]
- 40.Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012:19–27. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).