Abstract
Background
Dietary fluoride supplements were first introduced to provide systemic fluoride in areas where water fluoridation is not available. Since 1990, the use of fluoride supplements in caries prevention has been re‐evaluated in several countries.
Objectives
To evaluate the efficacy of fluoride supplements for preventing dental caries in children.
Search methods
We searched the Cochrane Oral Health Group's Trials Register (to 12 October 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 3), MEDLINE via Ovid (1950 to 12 October 2011), EMBASE via Ovid (1980 to 12 October 2011), WHOLIS/PAHO/MEDCARIB/LILACS/BBO via BIREME (1982 to 12 October 2011), and Current Controlled Trials (to 12 October 2011). We handsearched reference lists of articles and contacted selected authors.
Selection criteria
We included randomised or quasi‐randomised controlled trials comparing, with minimum follow‐up of 2 years, fluoride supplements (tablets, drops, lozenges) with no fluoride supplement or with other preventive measures such as topical fluorides in children less than 16 years of age at the start. The main outcome was caries increment measured by the change in decayed, missing and filled tooth surfaces (DMFS).
Data collection and analysis
Two review authors, independently and in duplicate, assessed the eligibility of studies for inclusion, and carried out risk of bias assessment and data extraction. In the event of disagreement, we sought consensus and consulted a third review author. We contacted trial authors for missing information. We used the prevented fraction (PF) as a metric for evaluating the efficacy of the intervention. The PF is defined as the mean caries increment in controls minus mean caries increment in the treated group divided by mean caries increment in controls. We conducted random‐effects meta‐analyses when data could be pooled. We assessed heterogeneity in the results of the studies by examining forest plots and by using formal tests for homogeneity. We recorded adverse effects (fluorosis) when the studies provided relevant data.
Main results
We included 11 studies in the review involving 7196 children.
In permanent teeth, when fluoride supplements were compared with no fluoride supplement (three studies), the use of fluoride supplements was associated with a 24% (95% confidence interval (CI) 16 to 33%) reduction in decayed, missing and filled surfaces (D(M)FS). The effect of fluoride supplements was unclear on deciduous or primary teeth. In one study, no caries‐inhibiting effect was observed on deciduous teeth while in another study, the use of fluoride supplements was associated with a substantial reduction in caries increment.
When fluoride supplements were compared with topical fluorides or with other preventive measures, there was no differential effect on permanent or deciduous teeth.
The review found limited information on the adverse effects associated with the use of fluoride supplements.
Authors' conclusions
This review suggests that the use of fluoride supplements is associated with a reduction in caries increment when compared with no fluoride supplement in permanent teeth. The effect of fluoride supplements was unclear on deciduous teeth. When compared with the administration of topical fluorides, no differential effect was observed. We rated 10 trials as being at unclear risk of bias and one at high risk of bias, and therefore the trials provide weak evidence about the efficacy of fluoride supplements.
Plain language summary
Fluoride supplements (tablets, drops, lozenges or chewing gums) for preventing tooth decay in children
Tooth decay (dental caries) can cause pain and lead to loss of teeth. In most developed countries, the prevalence of dental caries has decreased in the past 30 years in child populations. Nevertheless, some individuals or populations experience an increased caries challenge and are considered as being at 'high caries risk'.
Fluoride is a mineral that prevents tooth decay. Fluoride can be administered in different ways, either topically (toothpastes, mouth rinses, varnishes, gels) or systemically (fluoride supplements, fluoridated water, salt). Today, posteruptive (topical) preventive effect of fluoride is considered as being more important than the pre‐eruptive (systemic) effect. Topical fluorides have been shown to be highly effective and the use of fluoride‐containing toothpastes is now almost universal. When daily toothbrushing with a fluoridated toothpaste is not carried out or when the caries‐risk is increased, additional sources of fluoride could be recommended.
Fluoride supplements are administered in the form of lozenges, tablets or liquids. In this review, we only considered fluoride administered through supplements.
The review indicates that in schoolchildren (greater than 6 years of age), fluoride supplements when compared with no fluoride supplementation had a preventive effect on caries in permanent teeth. There was no differential effect between fluoride supplements and topical fluorides for preventing dental caries. Many of the studies included in the review had been conducted at a time when topical fluorides were not widely used. There is thus a lack of evidence from the review to make actual good recommendations. Today, the effect of fluoride supplements in children using fluoride toothpastes on a regular basis would probably be limited.
In the review, no conclusion could be reached about the effectiveness of fluoride supplements in preventing tooth decay in young children (less than 6 years of age) with deciduous teeth. Moreover, insufficient evidence exists to show whether or not using fluoride supplements in young children (less than 6 years of age) could mottle teeth (fluorosis), an effect of chronic ingestion of excessive amounts of fluoride.
Summary of findings
Background
Dental caries is a multifactorial disease due to "an imbalance in physiologic equilibrium between tooth mineral and biofilm fluid". Cariogenic bacteria can produce acids when they metabolise fermentable carbohydrates. These acids dissolve the calcium phosphate mineral of the tooth enamel or dentin (this is demineralisation). If the process is not halted, the carious lesion progresses leading eventually to a cavity. Protective factors such as salivary calcium, phosphate and proteins, salivary flow and fluoride in saliva can prevent or reverse the carious process by inhibiting demineralisation or enhancing remineralisation (Featherstone 1999; Fejerskov 2004).
Dental caries is a controllable disease and a public health problem because it affects a large number of people around the world. The prevalence of dental caries among adults is high and the disease affects nearly 100% of the population. In most developed countries, the prevalence and severity of dental caries have decreased in the past 30 years in child populations. The repartition of carious lesions has changed with most disease now found in a small number of children often characterised by a low socioeconomic status. Exposure to fluoride is usually seen as the principal reason for this caries decline together with improving living conditions (Marthaler 2004; Petersen 2005).
Fluorides play a key role in the prevention and control of dental caries. Initially, it was believed that fluoride had to be ingested to increase intake of fluoride during tooth formation in order to improve caries resistance. This paradigm of an important pre‐eruptive preventive effect of fluoride has influenced caries prevention and research during the last 50 years. Fluoride had to be taken systemically through fluoridation of drinking water or ingestion of supplements. In this context, the risk associated with ingestion of fluoride in children was linked to acute and chronic toxicity of fluoride. Caries prevention had to be balanced against increasing dental fluorosis. The 'topical' preventive effect of fluoride was, for a long time, claimed to be minor compared with the 'systemic' effect. The new paradigm emphasising on the posteruptive preventive effect of fluoride evolved based on research findings conducted in the 1970s. Laboratory studies showed that fluoride is able to influence chemical exchanges between the tooth mineral and the surrounding plaque fluid even at very low concentrations. Emphasis was then made on topical fluoride treatments such as fluoridated toothpastes. Today, fluoride is considered as a key protective factor which interacts directly on the tooth surface. The posteruptive effect is now considered as major compared to the pre‐eruptive one (Featherstone 1999; Fejerskov 2004).
The pre‐eruptive and posteruptive effects of fluoride are not easy to separate when analysing results of clinical and epidemiological studies. This is due to different factors. It is impossible to conduct randomised controlled trials of fluoride supplementation or water fluoridation to determine how much of the anti‐caries effect was obtained from pre‐ or posteruptive effect. Additionally, what complicates this issue is that maximum protection against caries is obtained when teeth erupt into an environment with low concentrations of fluoride in the mouth; and hence systemic or pre‐eruptive effects are not mutually exclusive phases. The context of eruption is also an important factor; teeth emerging in a caries‐free mouth are at lower caries risk. There is a cumulative effect of fluoride with an increased preventive effect for longer exposures (Limeback 1999; Thylstrup 1990). Given all of these factors, it is not possible in any one study to define clearly the posteruptive effect of fluoride on dental caries.
Topically applied fluorides are not intended for ingestion and thus act mainly posteruptively. Numerous clinical trials have investigated the anti‐caries effect of topical fluoride interventions and several Cochrane systematic reviews have been conducted confirming the efficacy of topical fluorides as toothpastes, mouth rinses, gels and varnish for preventing dental caries in children and adolescents (Marinho 2002a; Marinho 2002b; Marinho 2003a; Marinho 2003b). Concerning systemic intake of fluoride, it is difficult as stated above to ascertain whether there is a real pre‐eruptive effect. Water fluoridation has been the principal approach for community caries prevention. A systematic review reported that water fluoridation is associated with an increased proportion of children without caries and a reduction in the number of teeth affected by caries. A dose‐dependent increase in dental fluorosis was also found (McDonagh 2000). In many countries, water fluoridation has not been implemented. Alternative sources of systemic fluoride have thus been introduced, such as fluoridated salt or fluoride supplements. Salt fluoridation is used in 30 countries worldwide, mainly in Europe and in Central and South America. A Cochrane systematic review evaluating the impact of salt fluoridation in reducing caries levels and its potential harms is being conducted (Gillespie 2007). Systematic reviews are available on the effects of milk fluoridation (Yeung 2005) and salt fluoridation (Yeung 2011). Some attempts have also been made to add fluoride to sugar, bread and cereals.
Numerous clinical studies on the caries preventive effect of dietary fluoride supplements are available. They have been conducted in various countries in Western, Eastern and Northern Europe as well as in North America (Strean 1946) as early as the 1940s and recently in China. Earlier studies (before 1970 to 1980) were conducted under 'ideal' conditions as fluoridated toothpastes were not widespread. They have been conducted in a period when it was assumed that the cariostatic effect of fluoride was largely pre‐eruptive. Incorporation of fluoride in the forming enamel was seen as essential and those studies were not intended to distinguish between pre‐ and posteruptive effect. The early studies on fluoride supplements were reviewed by Birch in 1969 (Birch 1969) and by Binder et al in 1978 (Binder 1978). Later studies (after 1980) were conducted in a context where many topical and systemic fluoride sources co‐existed. Children living in communities without water fluoridation might receive significant amounts of systemic fluoride from foods and drinks processed in fluoridated communities, from other sources of systemic fluoride such as fluoridated salt or from involuntary ingestion of fluoride toothpastes. Those more recent studies often focused on the posteruptive effect of fluoride. They were conducted on schoolchildren who were asked to chew or suck the supplements before ingestion.
Later reviews published by Riordan (Riordan 1993; Riordan 1996; Riordan 1999), Ismail (Ismail 1994; Ismail 2008) and Burt (Burt 1999) made a critical analysis of the literature to determine the efficacy of fluoride supplements in caries prevention. Those reviews stated that the evidence for efficacy of fluoride supplements when used from birth was poor, that compliance with fluoride supplement recommendations was low making them a poor public health measure and that supplements use was a risk factor for dental fluorosis (Ismail 1999). Since then, the place of fluoride supplements in caries prevention has been re‐evaluated in several countries. Recommendations about their use have been modified. The age of initial use of supplements was delayed, the doses recommended for different age groups were reduced and the use of fluoride supplements was limited to high risk children (Adair 1999; Banting 1999). No meta‐analysis has been conducted to evaluate the efficacy of fluoride supplements. Recommendations for the use of fluoride supplements vary around the world. The caries preventive advice is often confusing to both dental public health and private dental practitioners. This confusion explains the fact that primary care physicians and paediatricians do not follow completely the current fluoride supplementation guidelines (Sohn 2007).
Objectives
To evaluate the effects of fluoride supplements in the form of tablets (chewable or not), drops, lozenges and chewing gums for preventing dental caries in children.
To examine whether the effects of fluoride supplements varies according to the age of administration, background exposure to topical fluoride and type of supplements used.
To evaluate whether there is a differential effect between fluoride supplements and topical fluorides.
To evaluate whether there is a differential effect between fluoride supplements and other caries preventive measures.
We considered fluoride supplements to include fluoride tablets (chewable or not), drops, lozenges and chewing gums.
We excluded slow release devices, fluoridated toothpicks and generally nutritional fluoridation such as wheat, sugar, salt and water fluoridation.
Fluoridated chewing gums are usually not considered as being fluoride supplements. Nevertheless, we decided to include them in this review for two reasons: firstly fluoride in chewing gums is partly ingested; secondly chewable tablets and chewing gums could be difficult to distinguish during the process of searching for eligible studies.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised (or quasi‐randomised) controlled clinical trials (RCTs) with randomisation at the level of the child or at the level of a group (cluster).
We excluded other study designs such as non‐randomised controlled clinical trials, controlled before/after studies, prospective cohort studies, single group before/after designs, historical control studies, interrupted time series, observational and retrospective epidemiological studies.
We excluded studies with an intervention or follow‐up period of less than 2 years. We considered that carious lesions preferably take at least 2 years to develop or to be visible during a clinical examination, if the primary outcome is the number of newly developed cavitated lesions (particularly when the D3MFT metric is used).
Types of participants
We included children or adolescents aged 16 or less at the start of the study (irrespective of initial level of dental caries, background exposure to fluorides, dental treatment level, nationality, setting where intervention is received or time when it started).
We excluded older participants in order to avoid the selection of studies concerning the use of fluoride supplements to prevent root caries or to improve bone density.
We excluded studies including only participants aged 16 years and older at baseline.
Types of interventions
Active intervention/test group
Fluoride supplements in the form of tablets, drops, lozenges (or chewing gums):
with or without the use of vitamins;
using any fluoride agent, at any concentration, amount, frequency of use, duration of application, and with any technique of application (sucked or not, chewed or not);
with or without the use of topical fluorides (fluoride rinse, topical fluoride application, fluoride varnish or fluoride toothpaste) or non‐fluoride based measures (chlorhexidine, xylitol, sealants, oral hygiene interventions, etc).
Control group
No fluoride supplements:
no treatment;
use of a placebo supplement (with or without the use of vitamins);
use of topical fluorides (fluoride rinse, topical fluoride application, fluoride varnish or fluoride toothpaste);
use of other preventive measures (chlorhexidine, xylitol, sealants, oral hygiene interventions, etc).
Other criteria
We excluded studies when the active intervention consisted of any other systemically delivered fluoride (water, milk, salt) provided in addition to fluoride supplements.
We excluded studies when a topical fluoride based measure or a non‐fluoride based preventive measure applied in a control group was different from the one administered in the intervention group in addition to fluoride supplements.
Types of outcome measures
Primary outcomes
For permanent and deciduous dentition, changes in caries increment, as measured by the difference between the number of decayed, missing and filled teeth (dmft/DMFT) or surfaces (dmfs/DMFS) at baseline and at the time of final evaluation for the same children.
Secondary outcomes
For permanent and deciduous dentition:
Differences in final caries experience as measured by the final number of decayed, missing and filled teeth (dmft/DMFT) or surfaces (dmfs/DMFS) in the treatment and control groups (if the groups were comparable at baseline).
Any other measures of dental caries such as proportion of children developing new caries or changes in caries‐free subjects.
Caries assessed clinically at the dentin level. If a combined clinical and radiographic assessment had been used, we recorded and noted this.
We excluded studies with no caries assessment and also studies reporting only on changes in plaque/salivary bacterial counts, fluoride uptake by enamel or dentin or fluoride salivary secretion.
Adverse effects
We recorded adverse effects when reported (dental fluorosis when assessed with a specific index and any other possible negative effects). A full investigation of adverse effects was not possible as we excluded observational and retrospective epidemiological studies.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
The Cochrane Oral Health Group's Trials Register (to 12 October 2011) (see Appendix 5);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 3, 2011) (see Appendix 4);
MEDLINE via Ovid (1950 to 12 October 2011) (see Appendix 1);
EMBASE via Ovid (1974 to 12 October 2011) (see Appendix 2);
LILACS via BIREME Virtual Health Library (1982 to 12 October 2011) (see Appendix 3);
PanAmerican via BIREME Virtual Health Library (1982 to 12 October 2011) (see Appendix 3);
WHOLIS via BIREME Virtual Health Library (1982 to 12 October 2011)(see Appendix 3);
MedCarib via BIREME Virtual Health Library (1982 to 12 October 2011) (see Appendix 3);
Brazilian Bibliography of Dentistry (BBO) via BIREME Virtual Health Library (1982 to 12 October 2011) (see Appendix 3);
Current Controlled Trials (www.controlled‐trials.com/) (to 12 October 2011) (see Appendix 6).
We used a combination of controlled vocabulary and free text terms for searching MEDLINE via Ovid (Appendix 1). We decided not to use the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE, as published in Box 6.4c in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011), because many of the trials eligible for this review were older and did not have an abstract, and there was a risk of losing these potentially important studies.
We developed detailed search strategies for each database searched. These were based on the search strategy developed for MEDLINE but revised appropriately for each database to take account of differences in controlled vocabulary and syntax rules (Appendix 2, Appendix 3, Appendix 4, Appendix 5, Appendix 6).
Searching other resources
We checked bibliographic references of identified trials and review articles for additional studies.
We contacted organisations and experts known to be involved in the field when necessary to find unpublished studies. We sent letters to authors of selected studies asking them for clarifications and other known unpublished or ongoing research.
We identified journals in which trials in this field are likely to be reported: Journal of Dental Research, Acta Odontologica Scandinavica, Journal of the American Dental Association, Swedish Dental Journal, British Dental Journal, ASDC Journal of Dentistry for Children, Archives of Oral Biology, Caries Research, Community Dentistry and Oral Epidemiology, Community Dental Health, Journal of Public Health Dentistry. They have been handsearched as part of The Cochrane Collaboration's handsearching programme, thus we did not need to handsearch them as part of the review process.
There was no restriction regarding language or date of publication or publication status. We were able to translate non‐English papers for languages such as French, German, Spanish and Russian. Cochrane Collaboration translators carried out translations for any other languages.
Data collection and analysis
Selection of studies
We imported records resulting from the searching process into a single database in the bibliographic software package Endnote. We removed duplicates in order to facilitate the retrieval of relevant articles.
Two review authors independently examined the title, keywords and abstract of all reports identified by the search, taking into account inclusion and exclusion criteria. The review authors were not blinded with respect to authors' names, journal or date of publication. If, in the opinion of both review authors, an article clearly did not fulfil the defined inclusion criteria, we considered it ineligible. For studies appearing to meet the inclusion criteria, or for which there was insufficient data in the title and abstract to make a clear decision, we obtained the full report. On receipt of the full articles, the two review authors checked that each study fulfilled the inclusion criteria. A third review author was consulted to resolve any disagreement. Cochrane Collaboration translators assessed trial reports in languages other than French, German, Russian or Spanish for eligibility. When these studies were considered eligible, a review author completed the inclusion form with the help of the translator.
Data extraction and management
Two review authors extracted the data independently, using data extraction forms. In case of discrepancy, we sought consensus. We piloted the data extraction forms on 10 articles and made modifications where necessary. For each trial we recorded the following data.
Author(s), year of publication, number of reports on the study, year/study began, country.
Methods: study design, research objective, study duration, method of allocation, randomisation/quasi‐randomisation, unit (individual/cluster), comparability of baseline characteristics, blindness of participants, blindness in outcome assessment, reliability of primary outcome measurement, co‐intervention and/or contamination, institutions and manufacturers involved, local characteristics.
Participants: setting where participants were recruited, criteria for inclusion, demographic characteristics (age, gender, socioeconomical status), caries severity, exposure to fluoride, number at start and at the end of the study.
Intervention: type of supplement used (tablet, lozenge, drop, other), modalities of administration (chewing, etc), treatment duration and application frequency, fluoride doses, fluoride agents, combination of methods, compliance (supervision of participants).
Details of the outcomes: method of assessment (clinical/radiographic, diagnostic thresholds used, account for reversals), mean duration of study.
Primary outcome measures (caries increment): units measured (tooth/surface), index used (DMFT/S, DF/T, etc), types of tooth/surface considered (deciduous, permanent), state of tooth/surface eruption (erupted/erupting).
Secondary outcome measures (variation of DMF index, percentage of children with caries).
Adverse effects (fluorosis) if recorded.
Details of analysis: measures of effect, confidence intervals, crude/adjusted results.
Disagreements between the two review authors were discussed and a third review author was consulted when necessary. We contacted the trial authors to find missing information. Studies rejected at this stage were recorded in the 'Characteristics of excluded studies' table. The 'Characteristics of included studies' tables provide a description of the data reported from each study.
Assessment of risk of bias in included studies
Two review authors independently carried out risk of bias assessment following the domain‐based evaluation described in Chapter 8 of the Cochrane Handbook (Higgins 2011). The evaluations were compared and any inconsistencies were discussed and resolved. We contacted the study author(s) to seek clarification in case of uncertainty over data.
In this two‐part tool we assessed the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants, personnel and outcome assessors (performance bias and detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
Each domain in the tool includes one or more specific entries in a 'Risk of bias' table. Within each entry, the first part of the tool describes what was reported to have happened in the study, in sufficient detail to support a judgement about the risk of bias. The second part of the tool assigns a judgement relating to the risk of bias for that entry. This is achieved by assigning a judgement of 'Low risk' of bias, 'High risk' of bias, or 'Unclear risk' of bias.
After taking into account the additional information provided by the authors of the trials, we graded studies into the following categories.
Low risk of bias: low risk of bias for all key domains.
Unclear risk of bias: unclear risk of bias for one or more key domains.
High risk of bias: high risk of bias for one or more key domains.
A risk of bias table was completed for each included study (see 'Characteristics of included studies'). Results are presented graphically by study (Figure 1) and by domain over all studies (Figure 2).
1.
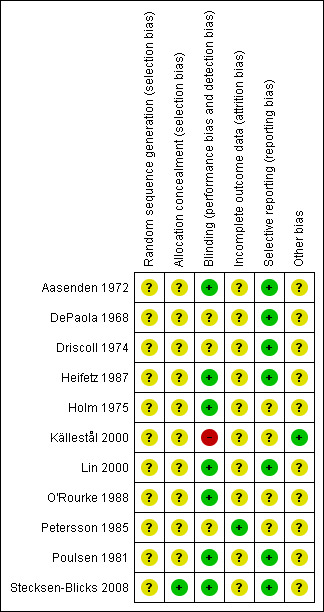
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
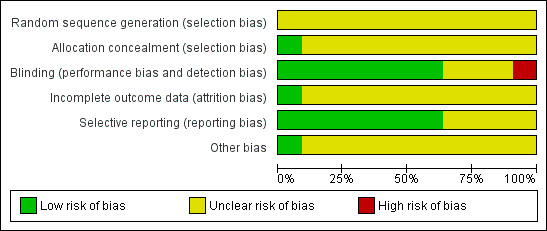
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Measures of treatment effect
For the main outcome variable, we estimated the treatment effect in each study by the prevented fraction (PF): mean caries increment in controls minus mean caries increment in the treated group divided by mean caries increment in controls. The PF is considered to be more appropriate than the standardised mean difference (SMD) because it allows to combine different types of caries increments data. We calculated the 95% confidence interval of the PF using Stata following the formula of Dubey (Dubey 1965). We calculated PFs by combining, when possible, several indexes. We calculated the PF values separately for caries increment data at the surface and tooth level and for deciduous and permanent teeth. We conducted random‐effects meta‐analyses when data could be pooled and we produced forest plot graphs. We used Review Manager (RevMan 2011) and STATA software to conduct the statistical analysis.
Dealing with missing data
We calculated missing caries increment values when necessary. Depending on the studies, we calculated caries increment either by subtracting initial DMFS (or DMFT) to final DMFS (or DMFT) or by adding caries increment for erupting teeth and for already erupted teeth.
We imputed missing standard deviations that were not obtained by contacting the original researchers (Van Rijkom 1998). Expecting increment to be approximately a Poisson variable, we supposed the log of standard deviation to be a linear function of the log of the mean. We estimated the parameters of the function by means of a simple regression over all the studies included in the analysis. We decided to estimate two separate regression lines for the increments in surface and the increments in number of teeth (there were actually no studies with missing standard deviation for increment of teeth). We also estimated two separate regression lines for the intervention and control groups. We did not separate permanent and deciduous teeth. We included results of all follow‐ups.
Assessment of heterogeneity
We assessed heterogeneity in the studies' results by examining forest plots and by using formal tests for homogeneity based on the I² statistics.
Assessment of reporting biases
We explored publication biases by drawing funnel plots and by investigating their degrees of asymmetry.
Data synthesis
We calculated estimates of treatment effects (PFs) using the Stata software package. We conducted meta‐analyses with Revman (RevMan 2011), using a random‐effects model for the PF data.
We conducted four different types of comparisons.
We first estimated treatment effects for studies or study groups comparing the administration of fluoride supplements with no treatment or with a placebo.
Then, we estimated treatment effects for studies or study groups comparing the administration of fluoride supplements with the application of topical fluorides. Some studies were considered in two different types of comparisons (1 and 2) when they included several control groups with and without the use of topical fluorides.
We examined studies which compared the effects of fluoride supplements to other preventive measures separately.
We conducted a complementary comparison to explore variations in PF values calculated for teeth already erupted at the start, and teeth erupting during, the study period.
For each type of comparison, we estimated the combined effect separately using different outcome categories for deciduous and permanent teeth. We also considered caries increments calculated at the tooth level and at the surface level separately. We calculated PFs by combining several indexes as DS (decayed surfaces), DFS (decayed and filled surfaces), and DMFS (decayed, missing and filled surfaces). We considered some studies in two different subgroups when they included several types of outcome on permanent or deciduous teeth, or at the tooth or surface levels.
We carried out main analyses for a length of follow‐up of 24 to 36 months, which was the more frequent duration of the studies. We performed complementary analyses for data in studies including longer follow‐ups. We estimated PFs separately for the different lengths of follow‐up extracted from the same studies.
In the studies with more than one intervention group, such as those comparing different frequencies of application or different types of supplements, we considered the results (numbers, mean caries increments and standard deviations) from all relevant experimental groups separately in the meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We assessed heterogeneity by inspection of forest plots of the estimates and confidence intervals of treatment effects.
The following variables were taken into account to explore the differences in PF values: type of control group (placebo, no treatment), type of topical fluoride used in the control group if any (fluoride toothpaste, varnish, mouthwash), children's age at start, type of supplements used (tablets, drops, lozenges, sodium fluoride (NaF), acidulated phosphate fluoride (APF), dosage), year when the study began (which indicated background exposure to fluoridated toothpastes) and (oral) health status of the children (special needs children, children with high caries risk). We also assessed the influence of some study characteristics such as randomisation, blindness in caries assessment or percentage of drop out when possible. Due to the small number of PF values, it was not possible to create subgroups or to conduct a meta‐regression to formally explore the influence of those study characteristics.
Results
Description of studies
Results of the search
The results of the different electronic searches are presented in Table 4. Following the removal of duplicates, 1416 records were retrieved from the electronic database search. The search of non‐electronic resources retrieved 28 other potentially relevant records. We screened records on the basis of the title, keywords and abstract. We arranged the translation of non‐English articles when necessary and we used English abstracts, when available, to identify if they were eligible studies. The members of the review group translated reports in French, Spanish, German and Russian. Personal contacts or members of the Cochrane Collaboration translated other reports.
1. Results of the electronic searches.
| Database | Date | Number of reports |
| MEDLINE via Ovid | 12 October 2011 | 1148 |
| CENTRAL | 12 October 2011 | 152 |
| OHG Register | 12 October 2011 | 264 |
| EMBASE via Ovid | 12 October 2011 | 248 |
| LILACs/PanAmerican/WHOLIS/MedCarib /Brazilian Bibliography of Dentistry |
12 October 2011 | 25 |
| Current Controlled Trials | 12 October 2011 | 1 |
After this initial screening, we considered 79 records to be potentially eligible, and proceeded with a more detailed assessment. This resulted in 11 included studies (23 reports + 3 postintervention reports) and 38 excluded studies (49 reports). Two reports from the PAHO database related to the evaluation of the Bermuda dietary supplement program could not be found and we added them to the 'Studies awaiting classification' section (Horowitz 1994). We added one study with no information about the treatment administered to the control group to the same section (Niedenthal 1957). We also added one Thai study for which the characteristics and quality were difficult to evaluate to this section (Prasertsom 1992). We found few relevant reports relating to the use of fluoride chewing gums (n = 3) and we excluded these.
Included studies
See 'Characteristics of included studies' tables for details of included studies.
We included 11 studies in the review, of which three have more than one publication giving results for different follow‐ups. Reports were published between 1968 and 2008 and referred to studies conducted mainly in Sweden and USA, but also in UK, Denmark and Taiwan.
Design and methods
The review includes placebo controlled trials but also trials comparing the treatment group to other active interventions or to no treatment. In the control groups, placebo supplements were administered in three studies (Aasenden 1972; DePaola 1968; Driscoll 1974) and no treatment in two other studies (Lin 2000; O'Rourke 1988). In one study (Källestål 2000), a letter with toothbrushing instructions was sent to the parents of the children in the control group; this group was not considered in the analysis as a 'no treatment group'. In five studies, the effect of fluoride supplements was compared with the use of topical fluoride: fluoride rinse (Heifetz 1987; Holm 1975; Poulsen 1981), fluoride varnish (Källestål 2000; Petersson 1985) or fluoridated toothpaste (Petersson 1985). In one study, the effect of xylitol and xylitol/fluoride‐containing lozenges was compared (Stecksen‐Blicks 2008).
The review includes trials with two to five arms. Three studies had more than one treatment group in addition to a control group. In those three studies, the effect of the sodium fluoride (NaF) tablets was compared with NaF drops (Lin 2000), acidulated phosphate fluoride (APF) tablet once a day with APF tablets twice a day (Driscoll 1974) and APF supplements with NaF supplements (Aasenden 1972). Two studies used more than one control group. In one of those studies the fluoride supplements were compared with the application of fluoride varnish and use of fluoridated toothpastes (Petersson 1985), while in the other study the prescription of fluoride lozenges was compared with parental information, fluoride varnish applications or individual prevention (Källestål 2000). In this latter study, comparisons other that the one made between fluoride supplements and fluoride varnish applications were not considered in the meta‐analysis.
Studies were generally large with only two studies allocating less than 100 children to relevant study groups (Lin 2000; Stecksen‐Blicks 2008). The total number of children participating in the trials was 7196 (number of children at start), and ranged from 140 in the smallest trial (Lin 2000) to 1640 in the largest trial (Heifetz 1987), with an average of 654 participants per trial.
Participants
Participants were recruited from school settings in seven studies and were patients of selected dental clinics in the four other studies (Källestål 2000; Lin 2000; Petersson 1985; Stecksen‐Blicks 2008).
The ages of the children at the start ranged from 2 to 12 years. Two trials included children who were aged 2 to 3 years (Lin 2000; Petersson 1985) and three included children aged 5 to 6 years (Driscoll 1974; Heifetz 1987; O'Rourke 1988). The participants of the five other studies were older, aged from 7 to 12 years.
In two studies, participants were children with high caries risk (Källestål 2000; Stecksen‐Blicks 2008), and in one study, participants were children with cleft lip and/or palate (Lin 2000).
Decayed, (missing) and filled surfaces (DMFS) data at baseline were reported in eight studies and ranged from 0.24 DMFS (Heifetz 1987) to 8.6 DFS (Aasenden 1972). Baseline data for deciduous tooth surface (dmfs) were reported in three studies varying from 0.9 dfs to 4.73 dmfs (Heifetz 1987; Lin 2000; Petersson 1985).
Information on 'background exposure to other fluoride sources' was not always available. All the studies were conducted in communities with no water fluoridation (< 0.1 ppm) except in one study (Källestål 2000) where parents answered a questionnaire and indicated the fluoride content of the water they consumed. Generalised use of fluoridated toothpastes was reported in three studies (Heifetz 1987; Källestål 2000; Stecksen‐Blicks 2008). In one study, the use of topical fluoride was indicated: "many schools at that time got fluoride mouthwash on a weekly basis" (Holm 1975). In the study conducted in Taiwan in children aged 2 to 3 years (Lin 2000), the authors indicated that toothbrushing was done without fluoridated toothpastes. The absence of exposure to fluoridated toothpastes could be assumed based on year of publication (before 1975) for three studies (Aasenden 1972; DePaola 1968; Driscoll 1974). Nevertheless, in one study conducted in 1972 in the USA, the authors stated that the majority of the children had a history of some kind of topical fluoride exposure (Aasenden 1972). No information was available concerning exposure to topical fluorides in three trials conducted in Europe during the 1980s (O'Rourke 1988; Petersson 1985; Poulsen 1981). Thus some form of fluoride exposure could be considered for five trials and no exposure for one, with the information not available for the remaining five trials.
Interventions
Four of the included trials (Källestål 2000; Lin 2000; Petersson 1985; Stecksen‐Blicks 2008) reported unsupervised use of fluoride supplements at home while in the remaining seven trials, supplements were used under supervision at school. The compliance has been evaluated in two of the four studies where supplements were given at home (Källestål 2000; Stecksen‐Blicks 2008).
Fluoride supplements were administered through different forms: drops in one study (Lin 2000), tablets in seven studies (DePaola 1968; Driscoll 1974; Heifetz 1987; Holm 1975; Lin 2000; O'Rourke 1988; Petersson 1985), tablets diluted in a solution in one study (Aasenden 1972) and lozenges in three studies (Källestål 2000; Poulsen 1981; Stecksen‐Blicks 2008).
Two types of fluoride agents were tested, including neutral sodium fluoride (NaF) in 10 trials and acidulated phosphate fluoride (APF) in three trials (Aasenden 1972; DePaola 1968; Driscoll 1974).
The fluoride dosages of the supplements ranged from 0.25 mg to 1 mg of fluoride (F). The daily administration of 1 mg F was tested in five trials (Aasenden 1972; DePaola 1968; Heifetz 1987; O'Rourke 1988; Poulsen 1981). In one study, the administration of tablets with 1 mg F once or twice a day was compared (Driscoll 1974). Three studies investigated daily administration of supplements with lower fluoride levels (0.4 to 0.5 mg F) (Holm 1975; Lin 2000; Petersson 1985). In one of those studies (Petersson 1985), tablets with 0.25 mg F were given twice a day. In two studies, one to two lozenges with 0.25 mg F were administered three times a day (Källestål 2000; Stecksen‐Blicks 2008).
Outcome measures
Ten studies reported caries increment data at the surface level which was the primary outcome measure. In one study, caries increment was recorded at the tooth level only (O'Rourke 1988). The majority of studies (n = 9) reported results for the permanent dentition. Four trials gave data about caries increment for deciduous teeth: dmfs increment was reported in two studies (Heifetz 1987; Lin 2000), dfs in one study (Petersson 1985) and dmft in two studies (Lin 2000; O'Rourke 1988). Two trials reporting effects on the deciduous dentition also assessed effects on permanent teeth (Heifetz 1987; O'Rourke 1988). With regard to the components of the DMFS index used, five trials reported DMFS data (Driscoll 1974; Heifetz 1987; Källestål 2000; Poulsen 1981; Stecksen‐Blicks 2008), two reported DFS data (Aasenden 1972; DePaola 1968), and one trial reported DS data only (Holm 1975). Results based on all tooth/surface types were reported in nine trials. In one study, caries increment was available only for approximal surfaces (Stecksen‐Blicks 2008). In two studies, caries increment was given separately for teeth erupting during the study and teeth present at baseline (Poulsen 1981) or per age group (Poulsen 1981).
Two studies reported other dental caries data as the frequency distribution of new manifest carious surfaces and the distribution of the children according to the number of erupted surfaces, group, baseline DMFS and caries increment (Petersson 1985; Poulsen 1981). Caries increment has been reported for all teeth/surfaces assessed but also according to the type of surface (occlusal, approximal, buccal/lingual) in three studies (Heifetz 1987; Holm 1975; Petersson 1985) and according to the status of eruption (erupted at baseline versus erupting during the study) in four studies (Aasenden 1972; Driscoll 1974; Heifetz 1987; Poulsen 1981).
Diagnostic methods used were described in all studies, but thresholds used for caries detection and monitoring of caries incidence were not always clearly described. Three studies took into account the reversals (DePaola 1968; Driscoll 1974; Källestål 2000). One examiner made the dental examinations in four studies (Aasenden 1972; DePaola 1968; O'Rourke 1988; Poulsen 1981) while several examiners conducted the evaluation in the other studies. Only four studies reported some data about examiners' reproducibility (Heifetz 1987; Källestål 2000; Lin 2000; Stecksen‐Blicks 2008). Clinical examinations (Driscoll 1974; Heifetz 1987; Lin 2000; O'Rourke 1988) or clinical and radiographic examinations (Aasenden 1972; DePaola 1968; Holm 1975; Källestål 2000; Petersson 1985; Poulsen 1981; Stecksen‐Blicks 2008) were conducted to determine dental status and calculate caries increment.
Some studies reported other data: carious risk factors (Källestål 2000), costs (Källestål 2000; O'Rourke 1988), number of children experiencing pain, anaesthesia and fear (O'Rourke 1988) and oral hygiene status (Holm 1975). Enamel biopsies were made on children from one study (Aasenden 1972). In two trials (Driscoll 1974; Heifetz 1987), assessments of DMFS increments or adverse effects (fluorosis) were made during a postintervention follow‐up period (Driscoll 1979; Driscoll 1981; Nowjack‐Raymer 1995).
Adverse effects were unreported in the majority of studies. Data on fluorosis were reported in one study (Driscoll 1974).
Follow‐ups of 24 to 36 months were the most common (reported in all 11 trials). Three trials presented also DMFS/T data for longer follow‐ups (Driscoll 1974; Heifetz 1987; Källestål 2000). Analysis was undertaken on results nearest to 24 to 36 months follow‐up. We conducted complementary analysis for longer follow‐ups.
Excluded studies
Reasons for exclusion of the studies are given in the 'Characteristics of excluded studies' table. The 38 studies (49 reports) in this section were excluded for a variety of reasons: non‐random allocation; randomisation not stated or indicated; administration of additional preventive agents; insufficient length of follow‐up; lack of longitudinal follow‐up; fluoride agent which did not fulfil the definition of fluoride supplements; lack of data (no values for caries indexes). A trial could be excluded for more than one reason.
Risk of bias in included studies
The results of the assessment of the risk of bias in included studies are summarised in Figure 1 and Figure 2.
Many aspects of the quality of the studies were unclear as insufficient information was available in the reports. The assessment of blinding and absence of selective reporting was easier as more information was given in the manuscripts.
Only one study (Stecksen‐Blicks 2008) had three domains of the risk of bias assessment (allocation concealment, blinding, free of selective reporting) rated as being at low risk of bias. Four studies were rated as having a low risk of bias for blinding and absence of selective reporting (Aasenden 1972; Heifetz 1987; Lin 2000; Poulsen 1981). Other studies had only one domain rated as being at low risk of bias.
Overall, we rated 10 trials as being at unclear risk of bias and one at high risk of bias (Källestål 2000).
Allocation
Random sequence generation
None of the included studies clearly reported the randomisation process. In nine trials, statements such as "were randomised" or "randomly assigned" appeared but there was no description of the process of randomisation.
In one study (Holm 1975), children were not allocated individually to the study groups. School classes were randomly divided into the two study groups.
In another study (Petersson 1985), sequence generation was not described as being randomised but we judged it as being quasi‐randomised because it was stated that: "children were listed in official population list and numbered I to IV consecutively and in this way 4 groups were formed".
Allocation concealment
In all the studies except one (Stecksen‐Blicks 2008), there was no information about the way the generated randomisation sequence was concealed from individuals involved in the enrolment and assignment of participants. We therefore considered allocation concealment to be at low risk of bias for one study (Stecksen‐Blicks 2008) and unclear risk of bias for the remaining 10 studies.
Blinding
Double‐blinding with blind outcome assessment and use of a placebo was described in five trials (Aasenden 1972; DePaola 1968; Driscoll 1974; Poulsen 1981; Stecksen‐Blicks 2008). In two of those trials (DePaola 1968; Driscoll 1974), the product used as control was not identical to the test product as colour coded bottles were used. Hence blinding of participants and examiners could have been compromised and we rated these studies as 'unclear' for this domain.
Single‐blinding (blind dental caries assessment) with no placebo use was described in four trials (Heifetz 1987; Holm 1975; Lin 2000; O'Rourke 1988).
In one trial (Petersson 1985), blind outcome assessment was unclear as "examiners were not aware to which group the child belonged but clinical examinations were made by two dentists who also introduced the prophylactic programs and conducted necessary restorative treatments".
In another study (Källestål 2000), blind outcome assessment was not achieved as it is stated that: "The collaboration with the clinicians and their crucial contribution to the data collection made it impossible to do the caries registration in a blinded fashion."
Incomplete outcome data
Participants included in the final analysis (24 to 36 months follow‐up) as a proportion of the participants present at the start in all studies was 72.4% (5210 analysed out of 7196 randomised). There was considerable variation in drop‐out rates ranging from 5% (Petersson 1985) to 29.6% at 2 years (Heifetz 1987). A common reason for attrition was that participants were not available for follow‐up examination at the end of the study. Authors frequently stated that children moved from the area or the school for reasons unrelated to the study. The number of children lost or excluded, by reason for attrition or by study group, was not reported. There was therefore not enough information to determine the level of risk of bias (high or low). We judged 10 studies as being at unclear risk of bias for this domain due to a lack of information about attrition rates by group. We evaluated one study (Petersson 1985), with a very low drop‐out rate of 5% after 2 years, as having a low risk of bias for this domain.
Selective reporting
We considered selective reporting to be at low risk of bias for seven trials as data on caries increment were reported in the results section in accordance with the prespecified indexes announced in the methods section.
In four studies (Holm 1975; Källestål 2000; O'Rourke 1988; Petersson 1985), methods for the evaluation of outcomes were insufficiently described and caries increment data were scarce, so these studies were judged as being at unclear risk of bias for this domain.
Other potential sources of bias
Baseline characteristics
In almost all the trials, it was stated that study groups were comparable at baseline for the initial caries levels. Slight differences indicating some degree of imbalance were noted in only one trial (Driscoll 1974).
Free of contamination or co‐intervention
All the studies were judged free from the possibility of the administration of the intervention to children in the control group (contamination) or of the application of an additional treatment to one of the groups (co‐intervention). They were judged to be at low risk of bias for this.
Reliability and validity of caries assessment
Only one study (Källestål 2000) presented data on the reliability and validity of caries assessments. Also, reversals were not adjusted for in the calculation of increments in all the studies. Overall, there may be significant inconsistencies in how the outcome measures were measured and analysed.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Fluoride supplements compared to no fluoride supplement for preventing dental caries.
| Fluoride supplements compared to no fluoride supplement for preventing dental caries | ||||||
| Patient or population: Children and adolescents Settings: Supplements administered at school or at home in North America, United Kingdom and Taiwan Intervention: Fluoride supplements Comparison: No fluoride supplement (placebo or no treatment) | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1,2 | Corresponding risk1,2 | |||||
| No treatment | Fluoride supplements | |||||
| Caries increment on permanent tooth surfaces (D(M)FS increment) Follow‐up: 24‐36 months | The mean caries increment on permanent tooth surfaces ranged across control groups from 2.64 to 12.29 surfaces | The mean caries increment on permanent tooth surfaces in the intervention groups ranged from 1.92 to 8.98 surfaces | 0.24 (0.16 to 0.33) | 1240 (3 studies) | ⊕⊕⊕⊝ moderate | Random sequence generation, allocation concealment rated as unclear in those 3 studies3,4 |
| Caries increment on deciduous tooth surfaces (dmfs increment) Follow‐up: 24‐36 months | The mean caries increment on deciduous tooth surfaces in the control group was 8.35 surfaces | The mean caries increment on deciduous tooth surfaces in the intervention groups ranged from 1.55 to 4.1 surfaces | 0.73 (0.46 to 0.99) | 115 (1 study) | ⊕⊝⊝⊝ very low | Only one study with a small sample size and an important effect3. Random sequence generation, allocation concealment rated as unclear in this study |
| Caries increment in permanent teeth (D(M)FT increment) Follow‐up: 24‐36 months | The mean caries increment in permanent teeth ranged across control groups from 0.52 to 5.64 teeth | The mean caries increment in permanent teeth in the intervention groups ranged from 0.32 to 3.83 teeth | 0.29 (0.19 to 0.39) | 1208 (3 studies) | ⊕⊕⊕⊝ moderate | Random sequence generation, allocation concealment rated as unclear in those 3 studies3,4 |
| Caries increment in deciduous teeth (dmft increment) Follow‐up: 24‐36 months | The mean caries increment in deciduous teeth ranged across control groups from 1.02 to 4.24 teeth | The mean caries increment in deciduous teeth in the intervention groups ranged from 0.89 to 2.02 teeth | 0.46 (0.08 to 0.83) | 696 (2 studies) | ⊕⊝⊝⊝ very low | Only two studies with high heterogeneity. Confidence interval, wide. Random sequence generation, allocation concealment rated as unclear in those 2 studies3 |
| Fluorosis (adverse effect) % of children with fluorotic teeth (quoted as questionable to severe) Follow‐up: 55 months | 32/212 = 15% | 40/202 = 20% (APF once a day) 43/197 = 22% (APF twice a day) |
Not estimable | 611 (1 study) | ⊕⊝⊝⊝ very low | Only one study. Fluorosis evaluated on teeth that erupted lately during the study period. Random sequence generation, allocation concealment rated as unclear in this study3,4 |
| *The basis for the assumed risk (mean caries increment values in control groups)and corresponding risk (mean caries increment values in intervention groups) is provided in footnotes. The relative effect (95% confidence interval) is evaluated by calculating the prevented fraction = mean caries increment in controls minus mean caries increment in the treated group divided by mean caries increment in controls. APF: Acidulated phosphate fluoride; CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1DMFS/T: Number of tooth surfaces (S) or number or teeth (T) decayed, missing or filled due to dental caries. 2Caries increment = final DMFS/T minus baseline DMFS/T. 3Many studies have been excluded from the review due to a lack of information concerning the allocation process. 4Studies conducted at a time when the use of topical fluoride was limited. Today, the effect of fluoride supplements would be different due to the widespread use of fluoridated toothpastes.
Summary of findings 2. Fluoride supplements compared to topical fluoride for preventing dental caries.
| Fluoride supplements compared to topical fluoride for preventing dental caries | ||||||
| Patient or population: Children and adolescents Settings: Supplements administered at school or at home in Sweden, North America and Danemark Intervention: Fluoride supplements Comparison: Topical Fluoride | ||||||
| Outcomes | Illustrative comparative risks | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1,2 | Corresponding risk1,2 | |||||
| Topical fluoride | Fluoride supplements | |||||
| Caries increment on permanent tooth surfaces (D(MF)S increment) Follow‐up: 24‐36 months | The mean caries increment on permanent tooth surfaces ranged across control groups from 0.9 to 5.4 surfaces | The mean caries increment on permanent tooth surfaces in the intervention groups ranged from 0.8 to 6.1 surfaces | ‐0.10 (‐0.25 to 0.05) | 2047 (5 studies) | ⊕⊕⊕⊝ moderate | Random sequence generation, allocation concealment rated as unclear in those 5 studies3 |
|
Caries increment on deciduous tooth surfaces (d(m)fs increment) Follow‐up: 24‐36 months |
The mean caries increment on deciduous tooth surfaces in the control group ranged from 1.7 to 2.5 surfaces | The mean caries increment on deciduous tooth surfaces in the intervention groups ranged from 1.8 to 2.06 surfaces | 0.13 (‐0.07 to 0.33) | 1051 (2 studies) | ⊕⊕⊕⊝ moderate | Random sequence generation, allocation concealment rated as unclear in those 2 studies3 |
| Fluorosis (adverse effect) % of children with fluorotic teeth | See comment | See comment | Not estimable | See comment | See comment | Not estimated |
| *The basis for the assumed risk (mean caries increment values in control groups)and corresponding risk (mean caries increment values in intervention groups) is provided in footnotes. The relative effect (95% confidence interval) is evaluated by calculating the prevented fraction = mean caries increment in controls minus mean caries increment in the treated group divided by mean caries increment in controls. CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1DMFS/T: Number of tooth surfaces (S) or number or teeth (T) decayed, missing or filled due to dental caries. 2Caries increment = final DMFS/T minus baseline DMFS/T. 3Many studies have been excluded from the review due to a lack of information concerning the allocation process.
Summary of findings 3. Fluoride supplements compared to other preventive measures for preventing dental caries.
| Fluoride supplements compared to other preventive measures for preventing dental caries | ||||||
| Patient or population: Patients with preventing dental caries Settings: Children and adolescents Intervention: Fluoride supplements Comparison: Other preventive measures | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1,2 | Corresponding risk1,2 | |||||
| Other preventive measures | Fluoride supplements | |||||
| Caries increment on permanent tooth surfaces (DMFS increment on proximal surfaces) Follow‐up: 24‐36 months | The mean caries increment on permanent tooth surfaces in the control group was 2.7 surfaces | The mean caries increment on permanent tooth surfaces in the intervention group was 2.7 surfaces | 0.00 (‐0.59 to 0.59) | 115 (1 study) | ⊕⊕⊝⊝ low | One study3, small sample, large confidence interval. Caries increment measured only on approximal surface |
| *The basis for the assumed risk (mean caries increment values in control groups)and corresponding risk (mean caries increment values in intervention groups) is provided in footnotes. The relative effect (95% confidence interval) is evaluated by calculating the prevented fraction = mean caries increment in controls minus mean caries increment in the treated group divided by mean caries increment in controls. CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1DMFS/T: Number of tooth surfaces (S) or number or teeth (T) decayed, missing or filled due to dental caries. 2Caries increment = final DMFS/T minus baseline DMFS/T. 3Many studies have been excluded from the review due to a lack of information concerning the allocation process.
Effect of fluoride supplements on dental caries increment
We have reported the results separately for permanent and deciduous teeth. We calculated the prevented fractions (PFs) separately for decayed, missing and filled surfaces (DMFS) and for decayed, missing and filled teeth (DMFT). We calculated PFs by combining when possible several indexes such as DMFS and DFS (D(M)FS), DMFS, DFS and DS (D(MF)S), DMFT and DFT (D(M)FT). Data issued from follow‐ups ranging from 24 to 36 months were grouped and this length was the reference period used for all the analyses. We calculated PF values separately for longer lengths of follow‐up.
In two studies, some caries increment data were not available. In one study (Källestål 2000), caries increment was calculated by subtracting initial DMFS or DMFT to final DMFS or DMFT for follow‐ups other than 48 months. In one study (Poulsen 1981), caries increment was calculated by adding caries increment for teeth erupting during the study and caries increment for teeth erupted at start. Standard deviations (SDs) of mean caries increments were missing in one study (Petersson 1985). We calculated missing standard deviations using a linear regression (Table 5).
2. Data available in the studies and data used in the analyses.
| Study | Available data | Data extracted and used in the analyses |
| Aasenden 1972 | Caries increment (DFS, DFT) after 12, 24, 36 months Mean and SEM available | Caries increment (DFS, DFT) after 36 months SD calculated from SEM Number of controls divided per 2 |
| De Paola 1968 | Caries increment (DFS, DFT) after 10 and 24 months Mean and SD available |
Caries increment (DFS, DFT) after 24 months |
| Driscoll 1974 | Caries increment (DMFS) after 30, 55 and 72 months Caries increment (DMFS) after 30 months given separately for teeth present at baseline and teeth erupting during the study Mean and SEM available |
Caries increment (DMFS) after 30 months calculated by adding caries increment (DMFS) for teeth erupted at baseline + caries increment (DMFS) for teeth erupting during the study Caries increment (DMFS) after 55 months Caries increment (DMFS) after 72 months SD calculated with SEM Number of controls divided per 2 |
| Heifetz 1987 | Caries increment (DMFS, dmfs) after 24, 60 and 96 months Mean and SD available |
Caries increment (DMFS, dmfs) after 24, 60 and 96 months |
| Holm 1975 | Caries increment (DS) after 24 months Mean and SD available |
Caries increment (DS) after 24 months |
| Kallestal 2000 | Caries increment (DMFS, DMFSe) after 48 months
Mean and SD available Mean DMFS, DMFSe (enamel) at baseline Mean DMFS, DMFSe for each of the 5 years of study |
Caries increment (DMFS) after 48 months Calculation of caries increment (DMFS) after 24 and 60 months by subtracting baseline DMFS to final DMFS SD estimated 24 months = length close/other follow‐ups in the same comparison group |
| Lin 2000 | Caries increment (dmft, dmfs) after 24 months Mean and SD available |
Caries increment (dmft, dmfs) after 24 months Number of controls divided per 2 |
| O Rourke 1988 | Caries increment (dmft, DMFT ) after 12, 24, 36 months Mean and SD available |
Caries increment (dmft, DMFT) after 24 months 24 months = length close to other follow‐ups in the same comparison group |
| Petersson 1985 | Caries increment (ds) after 12 and 24 months Mean available, SD not available |
Caries increment (ds) after 24 months SD estimated Number of controls divided per 3 |
| Poulsen 1981 | Caries increment (DMFS) after 36 months Caries increment (DMFS) given per age (7, 11 years) and separately for teeth erupted at baseline or for teeth erupting during the study Mean available, SD not available |
Caries increment (DMFS) calculated by adding caries increment (DMFS) for teeth erupted at baseline and caries increment (DMFS) for teeth erupting during the study Caries increment (DMFS) calculated separately per age (7, 11 years) SD estimated |
| Stecksen Blicks 2008 | Caries increment (DMFSa: approximal caries and DSe: enamel lesions on approximal surfaces) after 24 months Mean and SD available |
Caries increment (DMFSa) after 24 months |
SD = standard deviation; SEM = standard error of the mean.
1. Effect of fluoride supplements when compared with no fluoride supplement
1.1. Effect on permanent tooth surfaces: D(M)FS PFs for a follow‐up of 24 to 36 months (Figure 3)
(seeFigure 3)
3.
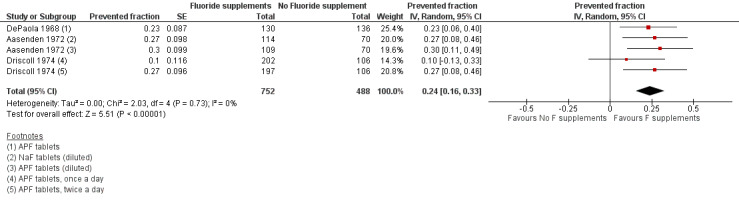
Forest plot of comparison: 1 Fluoride supplements vs no fluoride supplement ‐ outcome: 1.1 D(M)FS (24‐36 months) PFs
The D(M)FS PF pooled estimate was 0.24 (95% confidence interval (CI) 0.16 to 0.33) suggesting a benefit from the use of fluoride supplements (P < 0.00001). No heterogeneity was observed. We extracted data from three studies conducted in the period 1968 to 1974 which included schoolchildren aged from 6 to 11 years at baseline. In those three studies, the effect of NaF or APF tablets (1 mg F), used once or twice a day and diluted or chewed was compared to placebo tablets through five treatment groups.
1.2. and 1.3. Effect on permanent tooth surfaces: DMFS PFs for longer follow‐ups (55 and 72 months)
Results for other follow‐ups were available from one study (Driscoll 1974). The DMFS PFs varied from 0.25 (95% CI 0.12 to 0.38) after 55 months of follow‐up to 0.28 (95% CI 0.16 to 0.41) after 72 months, indicating a benefit from the use of fluoride supplements (P < 0.0001). This study began in 1969 and concerned children aged 6 years, and evaluated the effect of APF tablets (1 mg F) administered once or twice a day.
1.4. Effect on permanent teeth: D(M)FT PFs for a follow‐up of 24 to 36 months
For three trials combined, the D(M)FT PF pooled estimate was 0.29 (95% CI 0.19 to 0.39) suggesting a substantial benefit from the use of fluoride supplements (P < 0.00001). No heterogeneity was observed. We extracted data from studies conducted in the period 1968 to 1988 which included children aged from 5 to 11 years at baseline (Aasenden 1972; DePaola 1968; O'Rourke 1988). The effect of APF and NaF tablets (1 mg F), diluted or not, used once a day at school was compared with placebo tablets or no treatment.
1.5. and 1.6. Effect on deciduous tooth surfaces: dmfs PFs and dmft PFs for a follow‐up of 24 to 36 months
Heterogeneity was important when pooling the dmft PF values of two studies (Chi² = 14.54 (df = 2); P < 0.0007).
No significant effect was found for one study with a dmft PF of 0.13 (95% CI ‐0.09 to 0.35) (O'Rourke 1988). Children were 5 years of age at the start and the administration of fluoride tablets (1 mg F) at school was compared with no treatment.
A strong beneficial effect was observed in the other study which included children with cleft lip and/or palate for dmft PF (0.65; 95% CI 0.47 to 0.84) (P < 0.00001) and for dmfs PF (0.73; 95% CI 0.46 to 0.99) (P < 0.00001). The number of children studied was small in this study (n = 115). Children were aged 22 to 26 months at the start and two types of fluoride supplements (tablets and drops, 0.5 mg F) were tested versus no treatment. Children did not use topical fluoride in all the study groups.
2. Effect of fluoride supplements when compared with topical fluoride (fluoride rinse, fluoride varnish, fluoridated toothpastes)
2.1. Effect on permanent tooth surfaces: D(MF)S PFs for a follow‐up of 24 to 36 months (Figure 4)
(seeFigure 4)
4.
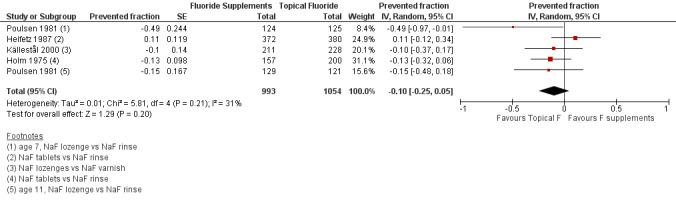
Forest plot of comparison: 2 Fluoride supplements vs topical fluoride ‐ outcome: 2.1 D(MF)S (24‐36 months) PFs
Four trials were combined (Heifetz 1987; Holm 1975; Källestål 2000; Poulsen 1981). The D(MF)S PF pooled estimate was ‐0.10 (95% CI ‐0.25 to 0.05) suggesting no benefit from the use of fluoride supplements when compared with the use of topical fluoride. No heterogeneity in the results was observed. In these studies, the fluoride supplements (tablets or lozenges) the children's ages (5 to 12 years), the study periods (1975 to 2000) and the topical fluorides used (rinses or varnishes) were different but this did not seem to influence the D(MF)S PFs.
2.2. and 2.3. and 2.4. Effect on permanent tooth surfaces: DMFS PFs for longer follow‐ups (48, 60 and 96 months)
Results for other follow‐ups were available from two studies (Heifetz 1987; Källestål 2000). No effect from the use of fluoride supplements when compared with the use of topical fluoride was observed after 48 or 60 months of follow‐up. There was heterogeneity between the two studies for the 60 months follow‐up (Chi² = 3.01 (df = 1); P = 0.08; I² = 67%). A beneficial effect of fluoride supplements was noticed with a DMFS PF of 0.21 (95% CI 0.04 to 0.38) (P = 0.02) for the longer follow‐up (96 months). It must be noted that a very high level of drop outs (> 60%) was observed in this study for this length of follow‐up (Heifetz 1987).
2.5. Effect on deciduous tooth surfaces: d(m)fs PFs for a follow‐up of 24 to 36 months (Figure 5)
(seeFigure 5)
5.
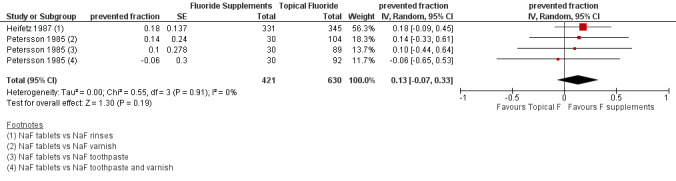
Forest plot of comparison: 2 Fluoride supplements vs topical fluoride, outcome 2.5 d(m)fs (24‐36 months) PFs
No significant effect or heterogeneity was observed in this analysis which concerned two studies (four groups) (Heifetz 1987; Petersson 1985). For all trials combined, the d(m)fs PF pooled estimate was 0.13 (95% CI ‐0.07 to 0.33). In these studies, the children's ages (3 and 6 years) and the topical fluorides used (varnishes, toothpastes, rinses) were different but this did not seem to influence the d(m)fs PFs.
3. Effect of fluoride supplements when compared with other preventive measures
3.1. Effect on permanent tooth surfaces: DMFS approximal PFs for a follow‐up of 24 to 36 months
No significant effect was observed in this analysis which concerned only one study (Stecksen‐Blicks 2008). For this trial, the DMFS approximal PF was 0.00 (95% CI ‐0.59 to 0.59) when fluoride given in addition to xylitol in lozenges was compared with xylitol alone. This 2‐year study started in 2001 and concerned children aged 10 to 12 years at the start.
4. Subgroup analysis and investigation of heterogeneity
We were not able to conduct a meta‐regression due to the small number of studies available for each outcome and for a length of follow‐up of 24 to 36 months (9 PF values for dmfs/d(m)ft, 2 PF values for D(M)FT and 11 PF values for D(MF)S).
Due to the small number of studies, it was not possible to examine the effects of some study characteristics such as randomisation, blindness in caries assessment or percentage of drop out.
Due to the small number of studies, it was not possible to examine the effects of fluoride supplements according to the types of supplements, age of the children or background exposure to topical fluorides.
The influence of some explanatory variables on caries increments by study group was explored in two studies. In one trial, the number of erupted teeth, age and baseline DMFS were taken into account. The effect of fluoride supplements was higher for children with caries at baseline in the younger age group (Poulsen 1981). In another study (Källestål 2000), a multidimensional analysis was conducted and the variables socioeconomic status, ethnicity, earlier preventive program, sealants, self‐administration of fluoride, eating sweets and toothbrushing frequency significantly influenced caries increment in addition to the study group. In this multidimensional analysis, no significant effect was found for the group with fluoride supplements when compared with the reference group (with toothbrushing information).
5. Funnel plot and test for funnel plot asymmetry: D(M)FS PF
Due to the small number of studies, it was not possible to assess publication bias except for analysis (1‐1) Effect on permanent tooth surfaces D(M)FS (24 to 36 months) PFs (Figure 6) and analysis (2‐1) Effect on permanent tooth surfaces D(MF)S (24 to 36 months) PFs (Figure 7). No publication bias was apparent but these results must be considered with caution as the number of studies was very small.
6.
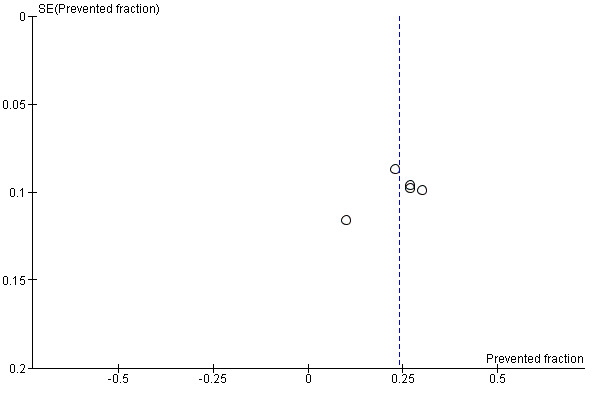
Funnel plot of comparison: Fluoride supplements vs no fluoride supplement ‐ outcome D(M)FS (24‐36 months) PFs
7.
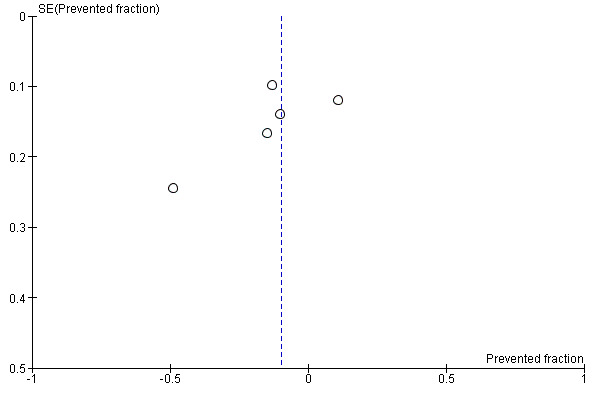
Funnel plot of comparison: Fluoride supplements vs topical Fluoride ‐ outcome: D(MF)S (24‐36 months) PFs
Effect of fluoride supplements on other outcomes
We did not conduct a meta‐analysis for secondary outcomes because data were scarce and non‐homogenous.
Caries increments per type of surfaces (occlusal, bucco‐lingual, mesio‐distal) were given in three studies. No difference in caries increments per type of surface was observed in two studies (Holm 1975; Petersson 1985). In another study, the effect of fluoride supplements (when compared to fluoride rinse) was higher for occlusal surfaces (Heifetz 1987).
In one study, the effect of fluoride supplements on plaque and gingivitis was evaluated. There was no difference between the groups (fluoride supplements versus fluoride rinses) for the mean plaque and gingivitis scores after 2 years (Holm 1975).
Costs were studied in two trials. In one study, no cost‐effectiveness analysis was conducted for the group with fluoride supplements because there was no significant effect when compared to the reference group (with toothbrushing information) (Källestål 2000). In another study, a reduction of 19% in the cost of treatments (for both dentitions) was found for the group with fluoride supplements when compared to a control group. In the group with fluoride supplements, there was a lower number of children undergoing general anaesthesia after 2 years (O'Rourke 1988).
A complementary descriptive analysis was conducted to assess the effect of fluoride supplements applied posteruptively and pre‐ and posteruptively. Caries increments given for "teeth already erupted at the beginning of the study" evaluated the posteruptive effect of fluoride supplements. Caries increments given for "teeth erupting during the study" evaluated the pre‐ and posteruptive effect of fluoride supplements. Data were available from two studies where the effects of fluoride supplements have been compared to placebos (Aasenden 1972; Driscoll 1974). These studies were conducted among children aged 6 to 11 years at baseline and followed during 2 to 6 years. The total and subtotals D(M)FS PF pooled estimates were not calculated because data were obtained for different follow‐ups of the same studies. Results indicate that the PF values tended to be higher for teeth erupting lately than for teeth already erupted at the beginning of the study period. For teeth erupted at start, the PF values varied from a minimum of ‐0.06 (95% CI ‐0.16 to 0.28) to a maximum of 0.27 (95% CI 0.13 to 0.41) according to the length of follow‐up, the type and the frequency of use of fluoride supplements. For teeth erupting lately during the study period, the PF values varied from a minimum of 0.27 (95% CI 0.13 to 0.41) to a maximum of 0.50 (95% CI 0.22 to 0.78).
In one study (Driscoll 1974), data were given concerning the distribution of children according to Dean's fluorosis classification after 55 months of study. Fluorosis was recorded on teeth that erupted lately during the study period. For all study groups, 18.9% of the children showed signs of dental fluorosis (questionable to severe). The percentages varied slightly from 15% in the placebo control group, 20% in the group with one APF tablet per day and 22% in the group with two APF tablets per day.
Discussion
Summary of main results
The main question addressed by this review was the efficacy of fluoride supplements in the form of tablets, drops, lozenges or chewing gums for preventing dental caries in children. A total of 7196 children (aged 2 to 12 years) participated in the 11 included trials. In those studies, fluoride supplements were administered in various forms (tablets, lozenges, drops) using two types of fluoride agents: acidulated phosphate fluoride (APF) and sodium fluoride (NaF). On the permanent dentition, the pooled results from the three trials assessing the effect of fluoride supplements suggested that the use of this intervention was associated with a 24% (95% confidence interval 16% to 33%) reduction on average in decayed, missing and filled tooth surfaces (D(M)FS) when compared with no fluoride supplement. On deciduous teeth, one study was unclear about the evidence that fluoride supplements have a caries‐inhibiting effect when compared with no fluoride supplement. In another study, the use of fluoride supplements was associated on average with a 65% reduction in decayed, missing and filled teeth (dmft) with a 95% confidence interval of 47% to 84%. There was therefore only weak evidence that the use of fluoride supplements prevents dental caries in deciduous teeth. When fluoride supplements were compared with the use of topical fluorides in six trials (varnish, rinses, toothpastes) or with the use of other preventive measures in one trial (Xylitol lozenges), there was no clear evidence of a differential effect on permanent dentition nor on deciduous teeth whatever the studies' characteristics.
A secondary aim of this review was to examine whether there was any relationship between the caries‐preventive effect of fluoride supplements and the age of administration, initial level of caries severity, background exposure to topical fluorides or type of supplements used. The influence of the type of supplements could not be explored due to the large variation in types of supplements, fluoride dosages, fluoride agents and methods of administration used in the 11 included studies. Due to the small number of studies, we were not able to study the relationship between the age of administration, the initial level of caries severity, background exposure to topical fluorides and the magnitude of the treatment effect.
Overall completeness and applicability of evidence
We aimed to identify randomised controlled trials evaluating the use of fluoride supplements in the prevention of caries.
The fewer trials in the deciduous dentition compared to the mixed or permanent dentition is of particular concern. This review included mainly studies conducted among older children (> 6 years) and which have studied the efficacy of fluoride supplements on permanent teeth. A small number of studies concerned children under the age of 5 to 6 years. We also found that there is little evidence about the efficacy of fluoride supplements on deciduous teeth when administered to very young children. No data were available concerning adverse effects related to fluoride supplementation in children aged less than 6 years. The ratio benefit /risk of fluoride supplementation was thus unknown for young children. Moreover, we could not explore the effect of different dosages of fluoride supplementation. We were unable to obtain valuable information about the effectiveness of fluoride supplements for infants and preschool children.
This review provides little information about the risk of adverse effects. Only one of the trials reported data about risk of fluorosis where a slight increase in fluorosis prevalence was observed with an increase in fluoride interventions in the study groups. No information was reported on other adverse effects. The lack of data on enamel fluorosis makes it difficult to evaluate the effectiveness of fluoride supplements (the benefits of fluoride supplements use in preventing caries against potential negative effects). This situation can be explained by the type of studies considered (clinical trials), the age of the participants at baseline in seven trials (7 to 12 years), and a short length of follow‐up for many trials (2 to 3 years). A report (not included in the review) gives data about the prevalence and severity of dental fluorosis in children who participated in one of the clinical trials included in this review (Nowjack‐Raymer 1995). Fluorosis was evaluated 3 years after the discontinuation of the program on 448 (out of 1640) remaining children. Overall, the prevalence of fluorosis was 4.4%. No statistically significant differences existed in the prevalence or severity of fluorosis among the preventive regimens, among children who began the regimens at ages 5, 6, or 7 or by eruptive status of teeth.
For a long time, and especially in the USA, systemic fluorides have been claimed to have pre‐ and posteruptive effects on dental caries. This position is not widely shared as it is now widely considered that the primary mode of action of fluoride is through topical mechanisms when the fluoride ion is in the biofilm or is deposited on the outer surface of enamel. Recent recommendations or reviews have emphasised the importance of the posteruptive effect of fluorides and encouraged that fluoride supplements should be kept as long as possible in the mouth before swallowing (CDC 2001). In this review, it has not been possible to clearly distinguish the pre‐eruptive and posteruptive effect of fluoride supplements. Many of the studies included in the review have evaluated the posteruptive effect of fluoride supplements on older children (> 6 years) and on teeth that had already erupted in the oral cavity and terminated the process of enamel mineralisation. In some studies, caries increments were calculated separately for teeth already erupted at baseline and teeth erupting during the study. We found that PF values tended to be higher for teeth erupting lately than for teeth already erupted at the beginning of the study period. Nevertheless, this trend did not allow any definitive conclusion concerning a pre‐ or posteruptive effect of fluoride supplements.
The review does not address cost‐effectiveness in terms of the potential reduction in financial cost associated with caries prevention. We found some cost data in several included studies but it was not possible to address cost‐effectiveness in the review.
In one study, children in both groups (intervention and control) received an additional non‐fluoride agent (xylitol) which is known to have an anticaries effect. The addition of xylitol in supplements resulted in an additional preventive benefit. The influence of fluoride could have thus been difficult to highlight in this study.
All the included studies were conducted in communities with no water fluoridation. Exposure to topical fluoride was identified when possible but this information was not always available. Exposure to topical fluoride, mainly from toothpastes, was thus estimated by considering the year of the study. In several recent studies, the exposure to topical fluorides was clearly stated. The absence of exposure was assumed for studies conducted before 1975. Thus, in some trials conducted during the 1980s, it was not possible to determine the level of exposure to fluoridated toothpastes. This could be identified as a limitation of the review, nevertheless this has not impacted greatly on the results of the analyses.
Fluoride supplements are considered as being as systemic source of fluoride along with population level fluoride interventions such as water, salt and milk fluoridation. Fluoride supplements differ from other systemic sources as they are often prescribed at the level of the individual and are dependant on patient compliance for their effect. In this sense they fit more naturally with topical fluorides such as toothpaste or mouthrinse. Compliance is a key element which may influence the efficacy of fluoride supplements. In the studies included in this review, the fluoride supplements were distributed mainly in schools. Thus, the ability of families to administer fluoride supplements to their child on a regular basis could not be assessed. The compliance with fluoride supplements administered at home was assessed in only two studies. In these two studies, different criteria were used to assess compliance. Forty‐one to 62% of the children were considered as having a good compliance.
Quality of the evidence
The publication date of the studies included in this review vary from 1968 to 2008. The quality of conduct and reporting of clinical studies has improved during this time. This is clearly apparent in the review given that a lot of information was lacking in earlier studies.
Included studies and particularly older ones lacked information on the methods of randomisation and on the process of allocation concealment. They were thus considered as 'unclear' for these domains.
Blinding of participants was done in a few trials. In trials where the effect of fluoride supplements was compared with topical fluorides, double blinding was not possible. In some trials, blinding of participants was stated but products were not similarly packaged. The lack of blinding for participants has probably had minimal consequences on outcome assessment.
The outcome assessment was carried out by examiners blinded to treatment allocation in the majority of the included studies. Nevertheless, blindness in outcome assessment could have been compromised in two studies.
The primary outcome measure used in the included studies was caries increment. Almost all studies have reported the caries increment at a surface level but caries indexes used varied across the studies. Some studies used global DMFS data but others reported data with partial indexes such as DFS, DS, DMFS for approximal surfaces, DMFS for teeth erupting or yet erupted at baseline. The calculation of PF values allowed us to pool together those various indexes.
In many studies, the reliability and validity of caries assessment was not ensured. The reproducibility of caries assessment was not verified and diagnostic thresholds for caries detection were not clearly defined. Errors or imprecise evaluations might have occurred during caries assessments leading to a high risk of bias.
Risk of bias arising from imbalance of baseline caries levels across groups was low. Stratification according to initial caries level was employed in some trials. Moreover, the study groups were comparable at baseline for the initial caries levels in the majority of the included studies.
We included studies with a follow‐up period of at least 2 years. A follow‐up period of 2 to 3 years is considered as optimal for studies which report caries increment data at the dentinal level. This was the case in this review because many studies were old and did not report data at the enamel level. In studies with several follow‐ups, caries increment reported closest to this time (24 to 36 months) was chosen as the outcome measure for this review.
The proportion of drop out after a 2 to 3 years period was not negligible in the included studies. A common reason for attrition was that participants were not available for follow‐up examination at the end of the study. Authors frequently stated that children moved from the area or the school for reasons unrelated to the study. The number of children lost or excluded by study group and by reason for attrition was not reported. The risk of bias of this domain was therefore unclear for the majority of studies.
A potential source of bias in the review was the contamination from other sources of fluoride or co‐intervention. For studies which took place in school settings, the risk of contamination was low because the administration of fluoride supplements was carefully supervised. A possible source of contamination was the use of fluoride supplements in the control group and this might have happened in studies where supplements were prescribed for home use.
The risk of bias of the included studies was difficult to evaluate and we frequently assessed the various domains as being at unclear risk of bias. The studies included can not be considered to be free from bias particularly for randomisation, allocation concealment and quality of caries assessment.
Potential biases in the review process
The results of this review help understand the effect of fluoride supplements in the prevention of dental caries in children. Nevertheless, there are several limitations that must be addressed.
The results of a meta‐analysis depend on the studies included. We have conducted a thorough search for studies but it is possible that we did not locate all relevant studies, particularly those that were unpublished. Studies with positive results favouring treatment may be more likely to be published; this could introduce bias into the results.
Given that we chose to include only randomised or quasi‐randomised controlled trials (RCTs or quasi‐RCTs), we excluded studies that used less stringent designs. The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) recommends to consider as randomised studies, reports in which the word 'randomised' is clearly written. In older studies, the process of randomisation was not clearly described. We excluded several studies from this review because of a lack of information about the process of randomisation. In several studies, the word 'randomisation' was not used and the process of randomisation was poorly described ("Children divided into 2 groups"). Many years ago the use of the word randomisation was not frequently used particularly in non‐English speaking countries. This might have introduced an inclusion bias in a review where many studies were conducted in the 1960s and 1970s. In order to conduct sensitivity analyses where the impact of including or excluding the non‐randomised studies can be evaluated, The Cochrane Collaboration's protocol may need to be revised.
We also tried to retrieve unreported data by contacting study authors but we were not able to include 14 studies for which information on the randomisation process or main results was lacking. Those papers were published during the 60s and 70s, before the publication of the CONSORT Statement, and so important data were omitted. The many years since publication made it impossible to obtain information from authors. If data from these studies were available, the results of this review would have been more powerful and informative.
Finally, it is also important to note that the overall study quality ratings fell in the low range; this could also have introduced a bias into the results. The lack of important information in many trial reports has resulted in categorisations of 'unclear' risk of bias.
Studies did not use the exact same interventions, follow the same protocol or report the same outcome. Thus, we restricted our pooling to interventions that were very similar, and could appropriately be pooled. Moreover, we calculated prevented fractions (PFs) by combining several indexes; two different measures may not be exactly alike and this may have introduced a measurement bias.
The external validity of the review can be considered as good; the included studies gave data for various participant ages, baseline levels of caries or countries.
We have investigated sources of heterogeneity in this review, examining factors related to participants and study characteristics. The calculation of the estimated PFs was done separately for different lengths of follow‐up. Nevertheless, due to a small number of data we were not able to conduct a meta‐regression or to create other subgroups.
A final comment is that the reported studies did not conduct intention‐to‐treat (ITT) analyses. This recent approach to analysis of randomised controlled trials may assist in reducing bias created when only those subjects who completed the trial are included in the final analysis.
Agreements and disagreements with other studies or reviews
The results of the present review are in accordance with the conclusion of recent reviews (Espelid 2010; Ismail 2008) that have examined the evidence regarding the effectiveness of fluoride supplements in preventing caries. In a recent review (Ismail 2008), 12 trials evaluating the preventive effect of fluoride supplements were considered. The authors concluded that there is weak evidence that the use of fluoride supplements prevents dental caries in primary teeth. They found some evidence that fluoride supplements prevent caries in permanent teeth. The Swedish Council on Technology Assessment in Health Care has also recently conducted a systematic review on the effectiveness of different measures for caries prevention. Five studies related to the effect of fluoride supplements on permanent teeth were included in this review. The authors concluded that there was no clear evidence that the use of fluoride supplements prevents dental caries on permanent teeth. They noticed that the only study that found a significant preventive effect of fluoride supplements was an old study conducted during the 70s (Swedish Council 2002). The American Center for Disease Control (CDC) also has published in 2001 recommendations for using fluoride to prevent and control dental caries. They concluded that the quality of evidence to support use of fluoride supplements by children aged less than 6 years was low. They selected three randomised controlled trials and concluded that they provided good evidence about the preventive effect of fluoride supplements on dental caries among children aged between 6 and 16 years in programs conducted in schools (CDC 2001).
This review provides little information about the risk of adverse effects. However, the use of fluoride supplements by young children is usually known to be a risk factor for dental fluorosis. A systematic review has investigated the impact of the use of fluoride supplements in communities without water fluoridation during the period of tooth development (< age 6) on the risk of dental fluorosis (Ismail 1999). Twenty‐four studies that assessed dental fluorosis in children who had used fluoride supplements earlier in their life were included. Among them, 14 studies (10 cross‐sectional and 4 follow‐up studies) had data that allowed a quantitative estimation of the risk of dental fluorosis. A consistent association between the use of fluoride supplements and dental fluorosis was noticed in the 24 studies. The meta‐analyses of the cross‐sectional studies estimated that the odds ratio of dental fluorosis in users of fluoride supplements compared with non‐users ranged between 2.4 and 2.6. The meta‐analyses of the follow‐up studies estimated that the risk ratio in long‐term users was between 5.5 and 12.2. This review stated that in communities with no water fluoridation, the use of fluoride supplements during the first 6 years of life was associated with a significant increase in the risk of developing dental fluorosis. Evidence is weak as this statement is derived mainly from the results of cross‐sectional surveys. Retrospective and cohort studies have found a strong link between regular intake of fluoride supplements and the risk of developing fluorosis. Conversely, monitoring of children who participated in clinical studies evaluating the preventive effect of fluoride supplements showed almost no difference in the prevalence and severity of fluorosis between the children from the intervention or the control group. Some bias might explain differences in the results from cross‐sectional surveys and clinical studies. Nevertheless, it must be noted that in clinical studies, the administration of fluoride supplements was supervised and took place through structured programs. Retrospective or cohort studies report data which mainly relate to children who took supplements on an individual basis. In this case, there was no supervision nor control of compliance or doses of fluoride administered. The fluorosis risk from fluoride supplementation could be lower when fluoride supplements are administered within school programs rather than on an individual basis (Banting 1999).
Authors' conclusions
Implications for practice.
This review suggests that the use of fluoride supplements is associated with a reduction in caries increment when used in permanent teeth and when compared with no other preventive fluoride treatment. For children aged 5 to 12 years at baseline, the use of fluoride supplements was associated with a 24% (95% confidence interval 16% to 33%) reduction in decayed, missing and filled surfaces (D(M)FS). When the fluoride supplements were compared with the use of topical fluorides (toothpastes, varnishes, rinses) or with the use of other preventive measures (xylitol lozenges), there was no differential effect. Many of the studies included in the review had been conducted at a time when topical fluorides were not widely used. There is a lack of evidence from the review to make actual good recommendations because, at the present time, the effect of fluoride supplements in children using fluoride toothpastes on a regular basis would probably be limited.
For children aged less than 5 years, there was weak evidence that the use of fluoride supplements prevents dental caries in primary teeth. From one study, no caries‐inhibiting effect was observed while in another study, the use of fluoride supplements was associated with a substantial reduction in caries increment. When fluoride supplements were compared with the use of topical fluorides (toothpastes, varnishes, rinses), there was no differential effect on deciduous teeth. Unfortunately, the review provides little information on the adverse effects such as enamel fluorosis. The ratio benefit/risk of fluoride supplementation was thus unknown for young children (< 6 years). Based on these results, it may not be appropriate to recommend the ingestion of fluoride supplements in children under 6 years as there is considerable uncertainty surrounding the ratio benefit/risk of this preventive intervention.
Implications for research.
The quality of the trials included in this review was generally low and many reports lacked important data or methodological information. This is probably due to the fact that most of the studies were relatively old. Based on the results of this review, several recommendations could thus be made for conducting and reporting clinical trials in order to facilitate future systematic reviews and meta‐analyses. First, study authors should include the numbers, means and standard deviations for all of the group outcome measures. Second, authors should use caries increment data measured at the surface level (DMFS) and give total caries increment calculated for all teeth and all types of surfaces in order to facilitate comparisons between studies. Third, authors should carefully describe the methodology used to ensure the quality of the study concerning randomisation, allocation concealment or blindness in outcome assessment. This information is necessary to provide an objective measure of the internal validity of the included trials, and is critical for making well‐informed interpretations of review findings. Fourth, in case of randomisation based on clusters, this should be clearly reported so that the possibility of bias due to important differences between clusters can be checked.
For older children (> 6 years): Further randomised comparisons of fluoride supplements and placebo on permanent teeth would be impossible to conduct today in developed countries as the vast majority of the children brush their teeth with fluoridated toothpastes. It would not be justifiable to ask children not to use fluoridated toothpastes during the study period as the evidence for their efficacy is high. The situation could be different in developing countries where accessibility to fluoridated toothpastes is not ensured. Comparisons of fluoride supplements with other topically applied fluoride interventions on permanent teeth would not provide more useful information. Comparisons of fluoride supplements with other preventive measures would perhaps be more interesting as we found only one study in this review allowing this kind of comparison. The respective efficacy of the combination of topical fluorides plus fluoride supplements against topical fluorides alone or against topical fluorides plus other preventive measures has not been explored in this review and would also need to be evaluated.
For younger children (< 6 years): Future studies would be useful to determine the relative effectiveness of different fluoride sources such as fluoride supplements, applications of fluoride varnish, daily use of fluoridated toothpaste or a combination of these modalities. On deciduous teeth, there was no clear evidence that fluoride supplements have a caries‐inhibiting effect when compared with no fluoride supplement. There was little evidence from studies which have compared fluoride supplements with other fluoride interventions (toothpastes, varnishes, rinses). There was also little information on the effects of fluoride toothpaste in the deciduous dentition particularly for fluoride concentrations above 1000 ppm (Walsh 2010). It would thus be interesting to evaluate the respective efficacy and safety of these fluoride sources. This kind of study would need a long follow‐up as it would be necessary to assess caries incidence in deciduous teeth as well as dental fluorosis and caries in erupting permanent teeth.
Many countries or international institutions recommend the use of fluoride supplements for children who are at high caries risk. The effect of the different supplementation regimens proposed (doses, age at start, level of risk, modalities of administration) is unknown and would need evaluation. Compliance and adherence of the children and of their families would probably be a crucial factor in determining the efficacy of those regimens in high risk populations. Moreover, the modalities of administration of fluoride supplements are key factors for the future studies. The review did not determine precisely if the effect of fluoride supplements was pre‐ or posteruptive or both. Now the common view is that it is through the posteruptive (topical) effect that fluorides have caries preventive action. In this context, ingestion of the supplements is not necessary nor needed to obtain a preventive effect as the topical application of fluoride compounds is all that is required to provide preventive effect on dental caries.
What's new
| Date | Event | Description |
|---|---|---|
| 25 November 2019 | Review declared as stable | This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future. |
Notes
This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future
Acknowledgements
We would like to thank the following investigators who provided additional information about their trials: Carina Kallestal, Nils Oscarsson, Sven Poulsen and Z Knychalska‐Karwan. We would like to thank those who have helped us in translating some abstracts or manuscripts: Elena Bakutkina (Russian), Melita Vlentic Peruzovic (Croatian), Katalin Karolyhazy (Hungarian), Janusz Lipiecki (Polish), Perrine Cluzel (Italian), Vera Gwizdz (Czech), Naoka Tanda (Japanese), Janjan Jan (Slovenian), Britta Tendal (Swedish), Vassily Vlassov (Russian), Daniel Bereczki (Hungarian), Tomasz Hryszko (Polish), Zongdao Shi, Chunjie Li and Yifan Zhang (Chinese), Dalibora Rako and Livia Puljak (Croatian), Toru Naito (Japanese), Paul Riordan (Norwegian), Sudaduang Krisdapong (Thai). We also would like to warmly thank Valeria Marinho, Anne Littlewood, Helen Worthington, Philip Riley, Luisa Fernandez Mauleffinch and Jan Clarkson from the Cochrane Oral Health Group for their help.
Appendices
Appendix 1. MEDLINE via Ovid search strategy
1. exp Tooth demineralization/ 2. Dental caries activity tests/ 3. Dental caries susceptibility/ 4. Dental enamel solubility/ 5. ((teeth or tooth or dental or dentin or enamel or root$ or rampant or recur$) adj5 (cavit$ or caries or carious or decay$)).mp. 6. (DMF or DFS or DFT or DMFT).ti,ab. 7. DMF Index/ 8. ((tooth or teeth or enamel or dentin or root$) adj5 (deminerali$ or reminerali$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 9. or/1‐8 10. exp Fluorides/ 11. Cariostatic agents/ 12. (fluoride$ or cariostat$).mp. 13. (fluoride$ and (tablet$ or drop$ or lozenge$ or pill$ or "chewing gum$" or supplement$)).mp. 14. or/10‐12 15. 13 and 14 16. 9 and 15
Appendix 2. EMBASE via Ovid search strategy
1. exp Tooth demineralization/ 2. Dental caries activity tests/ 3. Dental caries susceptibility/ 4. Dental enamel solubility/ 5. ((teeth or tooth or dental or dentin or enamel or root$ or rampant or recur$) adj5 (cavit$ or caries or carious or decay$)).mp. 6. (DMF or DFS or DFT or DMFT).ti,ab. 7. DMF Index/ 8. ((tooth or teeth or enamel or dentin or root$) adj5 (deminerali$ or reminerali$)).mp. 9. or/1‐8 10. exp Fluorides/ 11. Cariostatic agents/ 12. (fluoride$ or cariostat$).mp. 13. (fluoride$ and (tablet$ or drop$ or lozenge$ or pill$ or "chewing gum$" or supplement$)).mp. 14. or/10‐12 15. 13 and 14
Appendix 3. LILACS/PanAmerican/WHOLIS/MedCarib/BBO search strategy
teeth or tooth or dental or dentin$ or enamel or root$ or rampant or recur$) [Words] and (cavit$ or caries or carious or decay$) [Words] and (fluoride$ and (tablet$ or drop$ or lozenge$ or pill$ or "chewing gum" or supplement$))
Appendix 4. CENTRAL search strategy
#1 MeSH descriptor Tooth Demineralization explode all trees #2 MeSH descriptor Dental Caries Activity Tests explode all trees #3 MeSH descriptor Dental Caries Susceptibility explode all trees #4 MeSH descriptor Dental Enamel Solubility explode all trees #5 ((tooth in All Text or teeth in All Text or dental* in All Text or dentin* in All Text or enamel in All Text or root* in All Text or rampant in All Text or recur* in All Text) and (cavit* in All Text or caries in All Text or carious in All Text or decay* in All Text)) #6 (DMF in Title, Abstract or Keywords or DFS in Title, Abstract or Keywords or DFT in Title, Abstract or Keywords or DMFT in Title, Abstract or Keywords) #7 ((deminerali* in All Text or reminerali* in All Text) and (tooth in All Text or teeth in All Text or enamel in All Text or dentin* in All Text or root* in All Text)) #8 MeSH descriptor DMF Index explode all trees #9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) #10 MeSH descriptor Fluorides explode all trees #11 MeSH descriptor Cariostatic Agents explode all trees #12 fluoride* in All Text #13 cariostat* in All Text #14 (fluoride* in All Text and (tablet* in All Text or drop* in All Text or lozenge* in All Text or pill* in All Text or "chewing gum" in All Text)) #15 (fluoride* in All Text and supplement* in All Text) #16 (#10 or #11 or #12 or #13) #17 (#14 or #15) #18 (#9 and (#16 and #17))
Appendix 5. Cochrane Oral Health Group's Trials Register search strategy
(fluoride* AND (supplement* or tablet* or drop* or lozenge* or pill* or "chewing gum*"))
Appendix 6. Current Controlled Trials search strategy
(fluoride% and (tablet% or drop% or lozenge% or pill% or "chewing gum"))
Data and analyses
Comparison 1. Fluoride supplements vs no fluoride supplement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS (24‐36 months) | 3 | 1240 | Prevented fraction (Random, 95% CI) | 0.24 [0.16, 0.33] |
| 2 DMFS (55 months) | 1 | 529 | Prevented fraction (Random, 95% CI) | 0.25 [0.12, 0.38] |
| 3 DMFS (72 months) | 1 | 437 | Prevented fraction (Random, 95% CI) | 0.28 [0.16, 0.41] |
| 4 D(M)FT (24‐36 months) | 3 | 1208 | Prevented fraction (Random, 95% CI) | 0.29 [0.19, 0.39] |
| 5 dmfs (24‐36 months) | 1 | 115 | prevented fraction (Random, 95% CI) | 0.73 [0.46, 0.99] |
| 6 dmft (24‐36 months) | 2 | 696 | Prevented fraction (Random, 95% CI) | 0.46 [0.08, 0.83] |
1.1. Analysis.
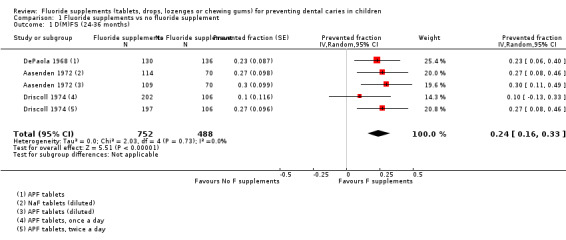
Comparison 1 Fluoride supplements vs no fluoride supplement, Outcome 1 D(M)FS (24‐36 months).
1.2. Analysis.
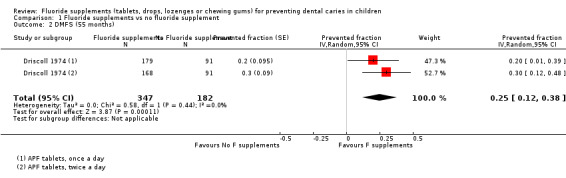
Comparison 1 Fluoride supplements vs no fluoride supplement, Outcome 2 DMFS (55 months).
1.3. Analysis.
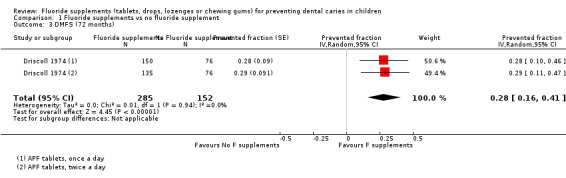
Comparison 1 Fluoride supplements vs no fluoride supplement, Outcome 3 DMFS (72 months).
1.4. Analysis.
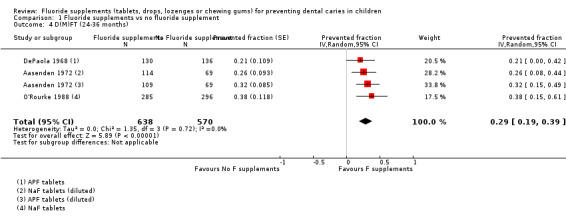
Comparison 1 Fluoride supplements vs no fluoride supplement, Outcome 4 D(M)FT (24‐36 months).
1.5. Analysis.
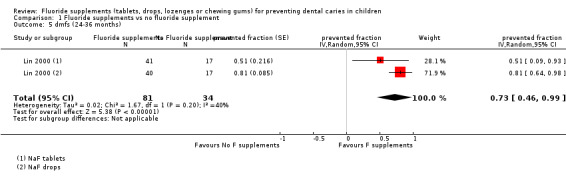
Comparison 1 Fluoride supplements vs no fluoride supplement, Outcome 5 dmfs (24‐36 months).
1.6. Analysis.
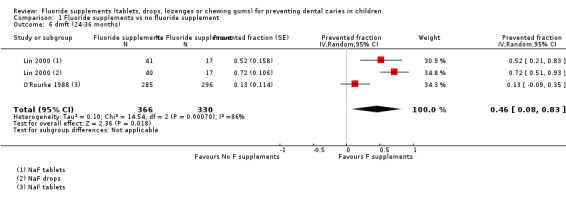
Comparison 1 Fluoride supplements vs no fluoride supplement, Outcome 6 dmft (24‐36 months).
Comparison 2. Fluoride supplements vs Topical Fluoride.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(MF)S (24‐36 months) | 4 | 2047 | Prevented fraction (Random, 95% CI) | ‐0.10 [‐0.25, 0.05] |
| 2 DMFS (48 months) | 1 | 472 | Prevented fraction (Random, 95% CI) | 0.0 [‐0.20, 0.20] |
| 3 DMFS (60 months) | 2 | 971 | Prevented fraction (Random, 95% CI) | 0.06 [‐0.18, 0.31] |
| 4 DMFS (96 months) | 1 | Prevented fraction (Random, 95% CI) | 0.21 [0.04, 0.38] | |
| 5 d(m)fs (24‐36 months) | 2 | 1051 | prevented fraction (Random, 95% CI) | 0.13 [‐0.07, 0.33] |
2.1. Analysis.
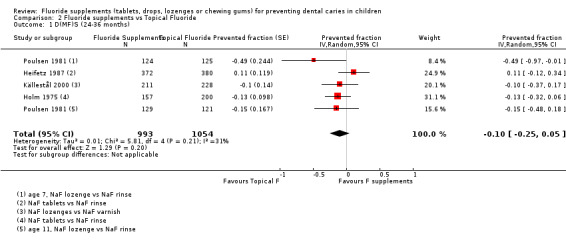
Comparison 2 Fluoride supplements vs Topical Fluoride, Outcome 1 D(MF)S (24‐36 months).
2.2. Analysis.
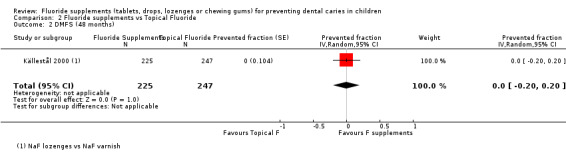
Comparison 2 Fluoride supplements vs Topical Fluoride, Outcome 2 DMFS (48 months).
2.3. Analysis.
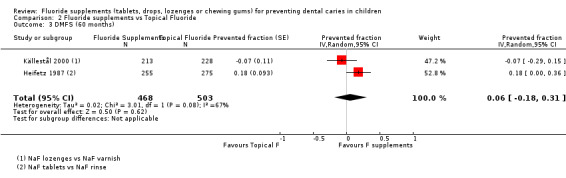
Comparison 2 Fluoride supplements vs Topical Fluoride, Outcome 3 DMFS (60 months).
2.4. Analysis.
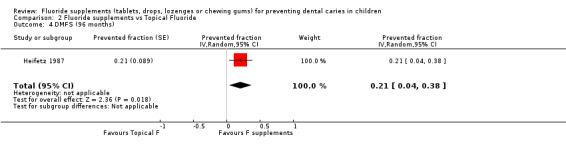
Comparison 2 Fluoride supplements vs Topical Fluoride, Outcome 4 DMFS (96 months).
2.5. Analysis.
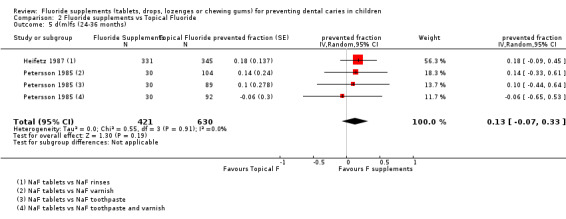
Comparison 2 Fluoride supplements vs Topical Fluoride, Outcome 5 d(m)fs (24‐36 months).
Comparison 3. Fluoride supplements vs other preventive measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DMFS proximal (24‐36 months) | 1 | 115 | Prevented fraction (Random, 95% CI) | 0.0 [‐0.59, 0.59] |
3.1. Analysis.
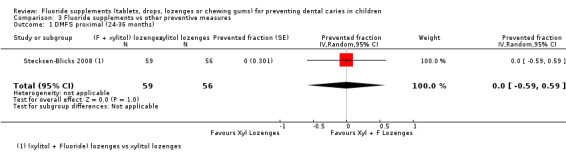
Comparison 3 Fluoride supplements vs other preventive measures, Outcome 1 DMFS proximal (24‐36 months).
Comparison 4. Fluoride supplements vs no fluoride supplement (teeth erupted at baseline or erupting during the study).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 D(M)FS | 2 | Prevented fraction (Random, 95% CI) | Totals not selected | |
| 1.1 teeth erupted at baseline | 2 | Prevented fraction (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 teeth erupting during the study | 2 | Prevented fraction (Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
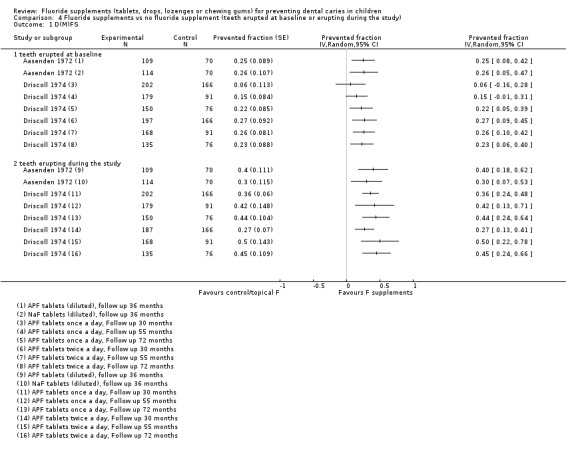
Comparison 4 Fluoride supplements vs no fluoride supplement (teeth erupted at baseline or erupting during the study), Outcome 1 D(M)FS.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aasenden 1972.
| Methods | Random allocation on an individual basis, allocation concealment unknown Double blind Placebo‐controlled APF compared to NaF and placebo tablets Study duration 3 years 33.6% drop out after 3 years; attributable to "reasons unrelated to the program" The number of children attributed to each group is not given. Percentage of drop out per group cannot be calculated |
|
| Participants | 362 children analysed after 3 years (available at final examination) Age at start: 8 to 11 years Surfaces affected at start: 7.32 to 8.58 DFS Exposure to other fluoride: Majority of children with a "history of some kind of topical fluoride". No water fluoridation (0.1 ppm fluoride in the water supply) Year study began: Not reported, before 1972 Location: Massachusetts, USA |
|
| Interventions | Fluoride supplements diluted in a liquid resulting in a solution The liquid contained a surfactant (0.01% polysorbate 80) to favour contact with the tooth surfaces Fluoride: 0.02% F as NaF APF tablets: 0.1 M phosphate at pH 4 Solution administered daily at school (138 to 173 days per year) Children instructed to hold 5 ml in the mouth for 1 min and then swallow No information or data on compliance |
|
| Outcomes | Baseline mean DFS, DFT, number of sound teeth, number of sound surfaces Number of teeth erupted during the study Caries increment (DFS, DFT) reported at 1, 2 and 3 years of follow‐up Mean number of extracted teeth after 3 years Mean DFS increment for surfaces present initially and that erupted during the study DFS increment after 3 years according to oral hygiene status Fluoride concentrations of biopsies from maxillary central incisors and canines |
|
| Notes | Participants randomised (n = 545) Baseline characteristics "balanced": at start, no difference in age, sex ratio, caries prevalence (DFS, DFT), mean number of sound teeth or surfaces between the groups Clinical caries assessment made by one examiner Clinical diagnostic threshold = "surface discontinuity penetrable by explorer" Radiographic assessment using posterior bitewings and radiograph of anterior teeth in cases of doubt Radiographic diagnostic threshold = discontinuity in normal enamel radiolucency Reliability: Not reported Account for reversals: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "three groups were formed by random allocation of the participants" Comment: Not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: "all three solutions were flavoured", "the group affiliations were unknown to dental personnel and participants throughout the study and the examinations were done in random order. Previous findings were not available to the personnel during the examinations" Comment: Blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotes: "all data refer to the 109, 114 and 139 subjects remaining in groups 1,2,3 at the final examination" Drop out for length of follow‐up: 33.6% in 3 years Drop out by group: Unknown Reasons for losses: "due to a variety of reasons unrelated to the dental program" Comments: Numbers lost moderate but % of drop out per group not given. Caries data used in the analysis pertain to continuous participants |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: Baseline mean DFS, DFT, number of sound teeth, number of sound surfaces Number of teeth erupted during the study Caries increment (DFS, DFT) reported at 1, 2 and 3 years of follow‐up. Mean number of extracted teeth after 3 years Mean DFS increment for surfaces present initially and that erupted during the study DFS increment after 3 years according to oral hygiene status Fluoride concentrations of biopsies from maxillary central incisors and canines Comment: All prespecified outcomes (in 'Methods') were reported. Final mean DFS and DFT not available |
| Other bias | Unclear risk | Comment: Initial caries indexes appear balanced (DFS: 7.99 in controls and 7.32 to 8.58 in treated children). Reliability of outcome assessment is not reported. There is no indication of contamination or co‐intervention |
DePaola 1968.
| Methods | Random allocation on an individual basis, allocation concealment unknown Double blind Placebo‐controlled APF compared to placebo tablets Study duration 2 years 18.6% drop out after 2 years; mainly attributable to the moving of families No differential group losses |
|
| Participants | 266 children analysed after 2 years (available at final examination) Age at start: 101 months Surfaces affected at start: 3.90 to 4.45 DFS Exposure to other fluoride: No history of exposure to fluoride supplements or fluoridated water (0.07 ppm fluoride in the water supply) Year study began: Not reported, before 1968 Location: Boston, Massachusetts, USA |
|
| Interventions | Fluoride tablets (NaF 2.2 mg) vs non‐fluoride tablets (all tablets with sodium biphosphate, hexamic acid, mannitol) Tablets administered daily at school Tablets chewed, swished around the mouth and swallowed The mean number of tablets ingested was 149.4 the first year (113 to 159) and 159.5 during the second year (116 to 168) |
|
| Outcomes | Baseline mean DFS, mean number of surfaces available Caries increment reported at 10 and 24 months follow‐ups Mean Crude and net DFS, DFT increments, mean number of teeth and surfaces erupting during the study period for all teeth and surfaces Mean DFS increment for surfaces that erupted during the study |
|
| Notes | Participants randomised (n = 327) Baseline characteristics "balanced": at start, no difference in age, caries prevalence (DFS), number of surfaces available between the groups (no statistical test) Clinical caries assessment made by one examiner, diagnostic threshold = "surface discontinuity penetrable by explorer" Radiographic assessment (2 posterior bitewings) by one examiner; diagnostic threshold = discontinuity in normal enamel radiolucency Reliability: Not reported Account for reversals: Reversal rates in surfaces between the 1st, 2nd and 3rd examination |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "children were assigned at random into two groups" Comment: Not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quotes: "fluoride tablets and tablets without fluoride were similar in taste and appearance", "the identity of agents and the group affiliation of subjects were unknown to dental personnel and participants throughout the program", " the test and control tablets were contained in colour‐coded wide‐mouthed bottles of 500 and were removed with specially made forceps by a dental assistant who gave them to each pupil" Comment: Blind outcome assessment and use of placebo described. Test and control tablets stored in different types of bottles |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotes: "all data presented refer to the 130 treatment and 136 control subjects who were continuous participants in the investigation" Drop out for length of follow‐up: 18.6% in 2 years Drop out by group: 32/162 children missing in fluoride group and 29/165 in non‐fluoride group Reasons for losses: "subjects losses were attributable to the moving of families or other reasons generally unrelated to dental parameters" Comments: Numbers lost were low. No differential loss between groups. Caries data used in the analysis pertain to continuous participants |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: Baseline mean DFS, mean number of surfaces available Caries increment reported at 10 and 24 months follow‐ups Mean crude and net DFS, DFT increments, mean number of teeth and surfaces erupting during the study period for all teeth and surfaces Mean DFS increment for surfaces that erupted during the study Comment: All prespecified outcomes (in 'Methods') were reported. Final mean DFS and DFT not available |
| Other bias | Unclear risk | Comment: Initial caries indexes appear balanced (DFS: 4.09 in controls and 4.41 in treated children). Reliability of outcome assessment is not reported. There is no indication of contamination or co‐intervention |
Driscoll 1974.
| Methods | Random allocation on an individual basis, allocation concealment unknown Double blind Placebo‐controlled: Fluoride tablet once a day compared with placebo tablet once a day and with Fluoride tablet twice a day Study duration 6 years 38.1% drop out after 30 months, 48.8% after 55 months and 57.6% after 6 years Reason for attrition: Not reported No differential group losses |
|
| Participants | 640 children analysed after 30 months Age at start: 6.6 years Surfaces affected at start: 1.07 to 1.40 DMFS Exposure to other fluoride: < 0.3 ppm fluoride in the water Year study began: 1969 Location: Wayne county, NC, USA |
|
| Interventions | Fluoride tablet once a day vs fluoride tablet twice a day vs placebo tablet On schooldays, 115 to 149 days/year Fluoride tablets: APF, 1 mg F, NaF, pH = 4.5, M10 phosphate Tablets chewed, rinsed for 30 seconds with the resulting solution and then swallowed Compliance: 95% of the tablets used during the first year and 86% during the third year, the percentage of tablets used was slightly lower in group C (82.9%) as compared to groups A and B (93.9 and 92.1%) |
|
| Outcomes | Baseline DMFS Caries increment reported at 30 months, 55 months and 6 years (+ evaluation 2 and 4 years after discontinuation of the treatment) DMFS increment (unadjusted and adjusted on baseline DMFS) DMFS increment for teeth present at baseline and teeth erupting during the study DMFS increment per type of surfaces (occlusal, buccolingual, mesiodistal) Surfaces reversals in diagnosis Percentage of tablets used Distribution of the children according to fluorosis classification |
|
| Notes | Participants randomised (n = 1034) Baseline characteristics "balanced": At start, no difference in age and sex but lower mean DMFS in the group receiving fluoride tablets/twice a day Clinical caries assessment made by 2 to 3 examiners, using the ADA's conference on the clinical testing of cariostatic agents (1968) diagnostic criteria Reliability of clinical examination not reported but it is stated that "the examiners continued to calibrate their examining techniques throughout the survey." Account for reversals: Reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "the record forms of the study participants placed into blocks according to race, sex, number of erupted permanent teeth. Within each block, the records were randomly assigned to one of three study groups" Comment: Randomisation process partly described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quotes: "the examiners did not know the group to which any child was assigned" and "the tablets were packaged in colour‐coded bottles so that their identity was unknown to the teachers and students" Comment: Blind outcome assessment and use of placebo described. Test and control tablets stored in different types of bottles |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out for length of follow‐up: 38.1% after 30 months, 48.8% after 55 months and 57.6% after 6 years Drop out by group after 30 months: 134/345 children missing in the group F tablet (1/day), 134/345 in the group F tablet (2/day) and 126/344 the placebo tablet group Reasons for losses: Not reported Comments: Numbers lost were high. No differential loss between groups. Caries data used in the analysis pertain to continuous participants |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: Baseline DMFS Caries increment reported at 30 months, 55 months and 6 years (+ evaluation 2 and 4 years after discontinuation of the treatment) DMFS increment (unadjusted and adjusted on baseline DMFS) DMFS increment for teeth present at baseline and teeth erupting during the study DMFS increment per type of surfaces (occlusal, buccolingual, mesiodistal) Surfaces reversals in diagnosis Percentage of tablets used Ditribution of the children according to fluorosis classification Comment: All prespecified outcomes (in 'Methods') were reported. Final mean DMFS not available |
| Other bias | Unclear risk | Comment: Initial caries scores appear balanced but a lower DMFS was observed in the group which received F tablets twice a day (DMFS at baseline: 1.35 in the placebo tablet group, 1.40 in the F tablet group ‐ once a day and 1.07 in the F tablet group ‐ twice a day). Reliability of outcome assessment is not reported. Surface reversals are reported. There is no indication of contamination or co‐intervention |
Heifetz 1987.
| Methods | Random allocation on an individual basis, allocation concealment unknown Single blind Fluoride tablets compared with fluoride rinses and with both procedures Study duration 8 years 36.3% drop out after 2 years, 51.9% after 5 years and 61% after 8 years Reason for attrition, lost of subjects due to movement of families from the area No differential group losses |
|
| Participants | 1154 children analysed after 2 years Age at start: 5 to 6 years Surfaces affected at start: 0.24 DMFS, 4.73 dmfs Exposure to other fluoride: < 0.3 ppm fluoride in the water, the majority of participants had access to fluoride containing toothpastes Year study began: 1981 Location: Springfield, Ohio, USA |
|
| Interventions | Fluoride tablet vs fluoride rinse vs both procedures on schooldays Rinse: Once a week, 0.2% NaF solution, 0.09% F Tablet: One tablet chewed, rinsed and swallowed daily, neutral NaF, 1 mg F Compliance: "children participated in average in more than 90% of the maximum number of treatment offered. nearly identical in each group" 30 children excluded because they received treatment for less than 4 years on 5 After year 5, more harder tablets have been used |
|
| Outcomes | Baseline mean DMFS, dmfs Caries increment reported at 2, 5 and 8 year follow‐ups DMFS, dmfs increment DMFS, dmfs increment per type of surface (occlusal, bucco‐lingual, mesiodistal) DMFS increment for early erupting and late erupting teeth (+ evaluation of fluorosis 3 years after discontinuation of treatment) |
|
| Notes | Participants randomised (n = 1640) Baseline characteristics "balanced": At start, no difference in age, sex and mean DMFS, dmfs between groups Clinical caries assessment made by 2 to 3 examiners, using the ADA's conference on the clinical testing of cariostatic agents (1968) diagnostic criteria Reliability measured by comparing caries increment mean values obtained by each examiner, no statistical difference was found Account for reversals: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "children were assigned randomly to one of 3 groups" Comment: Not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: "the examiners were not aware of the group assignments of the children" Comment: Blind outcome assessment described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out for length of follow‐up: 29.6% after 2 years, 51.9% after 5 years, 61% after 8 years Drop out by group after 2 years: 164/544 children missing in the F rinse group, 165/537 in the F tablet group and 157/559 in the group with both procedures Reasons for losses: "the predominant reason for loss of subjects was movement of the family from the Springfield area" Comments: Numbers lost were high. No differential loss between groups. Caries data used in the analysis pertain to continuous participants |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: Baseline mean DMFS, dmfs Caries increment reported at 2, 5 and 8 year follow‐ups DMFS, dmfs increment DMFS, dmfs increment per type of surface (occlusal, bucco‐lingual, mesiodistal) DMFS increment for early erupting and late erupting teeth (+ evaluation of fluorosis 3 years after discontinuation of treatment) Comment: All prespecified outcomes (in 'Methods') were reported. Final mean DMFS and dmfs not available |
| Other bias | Unclear risk | Comment: Initial caries indexes appear balanced (DMFS at baseline: 0.22 in F rinse group, 0.30 in F tablet group and 0.19 in F tablet + rinse group for children remaining after two years). Reliability of outcome assessment is not reported. Account for reversals or errors in clinical interpretation are not reported. There is no indication of contamination or co‐intervention |
Holm 1975.
| Methods | School classes randomly divided into 2 groups, cluster‐randomised Allocation concealment: Not reported Single blind Study duration 2 years Fluoride chewable tablet compared with fluoride mouthrinse 11% drop out after 2 years Reason for attrition: Not reported No differential group losses |
|
| Participants | 357 children analysed after 2 years Age at baseline 11 to 12 years Surfaces affected at start: 6.6 to 6.9 DFS Exposure to other fluoride: Many schools at that time got fluoride mouthwash on a weekly basis Year study began: 1971 Location: Public schools in the city of Lund, Sweden |
|
| Interventions | Fluoride chewable tablet vs fluoride mouthrinse Fluoride tablets: on schooldays, 200 days/year, NaF, 0.42 mg F Fluoride mouthrinse: Once a week as routine prevention in Sweden Tablets distributed at school by teachers. No information on compliance |
|
| Outcomes | Baseline: Mean number of surfaces erupted, mean number of decayed or filled surfaces (DFS) Caries increment reported after 2 years DS increment DS increment for occlusal and mesio‐distal surfaces Gingivitis and plaque |
|
| Notes | Participants randomised (n = 401) Baseline characteristics "balanced": At start, no difference between the groups for the number of teeth erupted and the number of carious or filled teeth Clinical and radiographic caries assessment made by 2 examiners, using Koch's (1967) diagnostic criteria Reliability of clinical examination not reported Account for reversals: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "School classes were randomly divided into two groups to make every age group and school equally represented in the two study groups" Comment: Randomisation process partly described, cluster |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: "The assessing dentists at the time of assessment had no knowledge regarding group assignment" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out for length of follow‐up: 11% after 2 years Drop out by group after 2 years: 24/181 children missing in the test group, 20/220 in the control group Reasons for losses: Not reported Comments: Numbers lost were low. No differential loss between groups. Caries data used in the analysis pertain to continuous participants |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: Mean number of surfaces erupted, mean number of decayed or filled surfaces (DFS) Caries increment reported after 2 years DS increment DS increment for occlusal and mesio‐distal surfaces Gingivitis and plaque Comment: Few outcomes were reported. No final data for mean DS and DFS |
| Other bias | Unclear risk | Comment: Initial caries scores appear balanced (DS at baseline: 6.9 in the tablet group, 6.6 in the mouthwash group). Reliability of outcome assessment is not reported. Surface reversals or errors in clinical interpretation are not reported. There is no indication of contamination or co‐intervention |
Källestål 2000.
| Methods | Random allocation on an individual basis, allocation concealment unknown Not blind (field trial) Study duration 5 years Groups with information on toothbrushing, prescription of fluoride lozenges, semi‐annual applications of fluoride varnish, individual preventive appointments were compared 18.4% drop out after 5 years Reason for attrition: Children moved from the area No differential group losses |
|
| Participants | 925 children analysed after 5 years (available at final examination) Age at start: 12 years Children with a predicted high caries risk Surfaces affected at start: 2.5 to 3.07 DMFS (for children who completed the study) Exposure to other fluoride: All toothpastes fluoridated, fluoride consumption evaluated by questionnaire (fluoride in water: > or < 1 ppm, use of F supplements, toothbrushing habits) Year study began: 1995 Location: 26 dental clinics, Sweden |
|
| Interventions | Toothbrushing information vs prescription of fluoride lozenges vs fluoride varnish applications vs individual program Toothbrushing information: Information on toothbrushing once a year at each dental examination Prescription of fluoride lozenges: 0.25 mg F, NaF, 3 to 6 tablets per day, sucking type Fluoride varnish applications: NaF, 2.2% F, applied 3 times a week every 6 months after professional cleansing of the teeth Individual program: evaluation of oral hygiene status and counselling in dental hygiene, oral hygiene and diet checked every 3 months, professional cleaning and fluoride varnish applied every 3 months All high risk children received sealants Compliance was checked every year 31% of the children were judged as having a good compliance the in group "toothbrushing information" , 62% in the group "fluoride lozenges", 76% in the group "fluoride varnish" and 65% in the group "individual program". Criteria used to define compliance varied from one group to another |
|
| Outcomes | At baseline: living area and professional status in parents, mean DMFT, DMFS, DMFSe (enamel), DMFSa (approximal) Caries increment reported at 4 years: DMFS, DMFSe increment Mean DMFS and DMFSe (enamel) annual values for each of the 5 years of study Cost‐effectiveness analysis: Mean treatment time, mean treatment cost, total treatment costs, total patient and family related costs Multivariate analysis of DMFS and DMFSe (enamel) increment as dependant variables with socioeconomic status, ethnicity, participation in earlier programs, sealants use, self‐administered fluoride, eating sweets, toothbrushing interval and preventive regimen as independent variables |
|
| Notes | Participants randomised (n = 1134) Baseline characteristics "balanced": "At start, no difference in mean DMFS between groups (no test)" Clinical and radiographic caries assessment made the dentists from the 26 clinics, using CK assessment diagnostic criteria (Flink 1999, Kallestal 2000) Reliability of caries assessment: Inter‐ and intraexaminer reproducibility tests with Kappa varying from 0.64 to 0.88 Reversals were included in the calculation of caries increment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "Each high‐risk child was randomly assigned to one of four preventive programs" Comment: Not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quotes: "The collaboration with the clinicians and their crucial contribution to the data collection made it impossible to do the caries registration in a blinded fashion" Comment: Examiners not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out for length of follow‐up: 18.4% drop out after 5 years Drop out by group: Number of children in each group at baseline, not reported Reasons for losses: "The most common reason for dropping out was that child had moved from the area. Some of the examination records (30%) of those lost to follow‐up during all years were located and their mean caries incidence over the whole study period was the same as that of the study group." Comments: Numbers lost unclear. Caries data used in the analysis pertain to participants present at the time of each annual examination |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: At baseline: Living area and professional status in parents, mean DMFS and DMFSe (enamel), DMFT, DMFSa (approximal) Caries increment reported at 4 years: DMFS, DMFSe increment Mean DMFS and DMFSe (enamel) annual values for each of the 5 years of study Cost‐effectiveness analysis: Mean treatment time, mean treatment cost, total treatment costs, total patient and family related costs Multivariate analysis of DMFS and DMFSe(enamel) increment as dependant variables with socioeconomic status, ethnicity, participation in earlier programs, sealants use, self administered fluoride, eating sweets, toothbrushing interval and preventive regimen as independent variables Comments: Many data but annual caries increments not available except at 4 years |
| Other bias | Low risk | Quotes: "all previous programmes were discontinued as no preventive programmes including sealant placement were to be conducted other than those randomly assigned within the study. Important factors such as use of fluoride in the development of caries were followed throughout the study by using questionnaires and reports from each clinic." Comment: Contamination by other preventive programs was avoided and potential co‐intervention carefully considered Comment: Initial caries scores appear balanced (2.93 in the information group, 2.50 in the F lozenges group, 3.07 in the F varnish group, 2.71 in the individual program group). Reliability of outcome assessment is reported. Surface reversals are considered in the calculation of net caries increment. There is no indication of contamination or co‐intervention |
Lin 2000.
| Methods | Random allocation on an individual basis, allocation concealment unknown Single blind Study duration 2 years Fluoride drops compared with fluoride tablets and with no treatment 17.8% drop out after 2 years Reason for attrition: Not reported but "children were excluded if a 10‐day dosage remained as a residual amount after each 3 month period" Slightly lower attrition in the fluoride tablet group |
|
| Participants | Children with cleft lip and/or palate 115 children analysed after 2 years (available at final examination) Age at start: 22 to 26 months At start: 0.18 to 0.34 dmft, 0.23 to 0.34 dmfs Exposure to other fluoride: < 0.1 ppm fluoride in the water, toothbrushing without fluoridated toothpastes Year study began: Not reported Location: Orthodontic clinic, Kaohsiung Medical centre, Taiwan |
|
| Interventions | Fluoride drops vs fluoride tablets vs nothing Fluoride drops, NaF, 1 drop = 0.25 mg F, 2 drops per day Fluoride tablets, NaF, 1 tablet = 0.5 mg F, 1 tablet per day All children recalled every 3 months for oral hygiene procedure Tablets and drops administered at home by parents. Compliance was checked at each recall appointment. Subjects were excluded if a 10‐day dosage remained as residual amount after each period. No data provided on compliance |
|
| Outcomes | At baseline: dmft, dmfs Caries increment reported after 2 years dmft, dmfs increment Final dmft, dmfs |
|
| Notes | Participants randomised (n = 140) Baseline characteristics "balanced": At start, no difference between the groups for mean dmft and dmfs Clinical caries assessment made by 2 examiners, using the modified method of Radike's (1972) diagnostic criteria. Reliability: Interexaminer was tested by computing the Pearson correlation coefficient (r = 0.90) Account for reversals: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "subjects were randomly assigned into a control group of no fluoride supplements, a fluoride tablet group and a liquid tablet group" Comment: Not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: "examiners were blind to the assignments" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out for length of follow‐up: 17.8% after 2 years Drop out by group after 2 years: 10/44 children missing in the control group, 5/46 in the F tablet group and 10/50 in the F liquid group Reasons for losses: Not reported Comments: Numbers lost were low. Slight differential loss between groups. Caries data used in the analysis pertain to continuous participants |
| Selective reporting (reporting bias) | Low risk | Outcome reported: At baseline: dmft, dmfs Caries increment reported after 2 years dmft, dmfs increment Final dmft, dmfs Comment: All prespecified outcomes (in 'Methods') were reported |
| Other bias | Unclear risk | Comment: Initial caries levels appear balanced (dmfs at baseline: 0.34 in the tablet group, 0.23 in the drop group and 0.27 in the control group). Reliability of outcome assessment is not reported. Surface reversals or errors in clinical interpretation are not reported. There is no indication of contamination or co‐intervention |
O'Rourke 1988.
| Methods | Pragmatic study evaluating cost appraisal Random allocation on an individual basis, allocation concealment unknown Single blind Study duration 3 years Fluoride tablets compared with no treatment 24.5% drop out after 2 years and 31.2% after 3 years Reason for attrition: 6 withdrawn and others leaving the schools No differential group losses |
|
| Participants | 529 children analysed after 3 years (available at final examination) Age at start: 5 years and 3 months At start: 3.32 to 3.66 dmft Exposure to other fluoride: Not reported Year study began: Not reported, before 1988 Location: 22 primary schools, Manchester, UK |
|
| Interventions | Fluoride tablets vs nothing Fluoride tablets, NaF, 1 mg F, 1 tablet per school day Tablets distributed to the children at school, no information on compliance |
|
| Outcomes | At baseline: dmft, DMFT not available Caries increment reported after 1, 2 and 3 years dmft, DMFT increment Number of children with toothache, having local/general anaesthesia and fear at final examination Evaluation of costs (Resource Related Index) Final examination: dmft, DMFT not available |
|
| Notes | Participants randomised (n = 769) Baseline characteristics "balanced": At start, no difference between the groups for mean dmft Clinical caries assessment made by one examiner, using the Downer's (1979) diagnostic criteria Reliability of caries assessment: Not reported Account for reversals: Not reported Results calculated for all eligible children without considering compliance |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "children paired on socioeconomic factors and randomly allocated to control or test groups within each pair" Comment: Randomisation process partly described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: "the examinations were carried out by an examiner unconnected with the conduct of the trial and were blind" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out for length of follow‐up: 24.5% drop out after 2 years and 31.2% after 3 years Drop out by group: 70/336 children missing in the control group, 60/323 in the F tablet group between year 1 and year 3 Reasons for losses: 6 withdrawn and others leaving the schools Comments: Numbers lost were moderate. No differential loss between groups. Caries data used in the analysis pertain to continuous participants |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: Caries increment after 1, 2 and 3 years dmft, DMFT increment Number of children with toothache, having local/general anaesthesia and fear at final examination Evaluation of costs (Resource Related Index) Comments: Baseline and final dmft and DMFT not available. Caries increment measured at the tooth level only |
| Other bias | Unclear risk | Comment: Initial caries levels appear balanced (dmft at baseline: 3.66 in the tablet group, 3.32 in the control group). Reliability of outcome assessment is not reported. Reversals or errors in clinical interpretation are not reported. There is no indication of contamination or co‐intervention |
Petersson 1985.
| Methods | Random allocation on an individual basis, allocation concealment unknown Single blind Study duration 2 years Administration of "fluoride tablets + placebo dentifrice" compared with "fluoride dentifrice", "fluoride varnish + placebo dentifrice" and "fluoride varnish + fluoride dentifrice" Drop out: 5% after 2 years Reason for attrition: Not reported No differential group losses |
|
| Participants | 357 children analysed after 2 years (available at final examination) Age at start: 3 years Surfaces affected at start: 0.9 dfs Exposure to other fluoride: water 0.2 ppm F Year study began: Before 1978 Location: City of Uddevalla, Sweden |
|
| Interventions | Fluoride tablets + placebo dentifrice vs fluoride dentifrice vs fluoride varnish + placebo dentifrice vs fluoride varnish + fluoride dentifrice Fluoride tablets: 0.25 mg F, NaF, 2 tablets per day, sucking type Fluoride varnish: NaF, 2.2% F, applied every 6 months Fluoride dentifrice: NaF, 0.025% F, used twice a day All groups with information about dental health care, dietary counselling and oral hygiene instructions Products regularly supplied at the dental clinic and administered at home. No data or information on compliance |
|
| Outcomes | At baseline: sex, mean dfs, distribution according to the number of dfs Caries increment reported at 1 and 2 years Caries increment: Mean number of new manifest carious tooth surfaces (ds) (no SD) and distribution according to the number of new ds Mean number of new manifest carious tooth surfaces (ds) (no SD) and distribution according to the number of new ds per type of surface (occlusal, bucco‐lingual, approximal) |
|
| Notes | Participants randomised (n = 376) Baseline characteristics "balanced": At start, no difference between groups for the number of dfs (no SD, no test) Clinical and radiological (if necessary) caries assessment made by 2 examiners, using Koch's (1967) diagnostic criteria. Diagnostic threshold = manifest carious lesion Reliability of caries assessment: Not reported Account for reversals: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "children listed in official population list and numbered I to IV consecutively and in this way 4 groups were formed" Comment: Randomisation process partly described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quotes: "examiners at the clinical examination not aware to which group the child belonged" but "two dentists were responsible for the examinations of the children, introducing the prophylactic programs, necessary restorative treatments and follow‐up" Comment: Blind outcome assessment not clearly ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop out for length of follow‐up: 5% after 2 years Drop out by group after 2 years: 5/96 children missing in F tablet + placebo dentifrice group, 4/85 in F dentifrice group, 6/98 in F varnish + placebo dentifrice group and 4/88 in F varnish + F dentifrice group Reasons for losses: Not reported Comments: Numbers lost were low. No differential loss between groups. Reasons for losses not reported. Caries data used in the analysis pertain to continuous participants. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: At baseline: sex, mean dfs, distribution according to the number of dfs Caries increment reported at 1 and 2 years Caries increment: Mean number of new manifest carious tooth surfaces (ds) (no SD) and distribution according to the number of new ds Mean number of new manifest carious tooth surfaces (ds) (no SD) and distribution according to the number of new ds per type of surface (occlusal, bucco‐lingual, approximal) Comment: Caries increment measured by the "Mean number of new manifest carious tooth surfaces (ds)". Standard deviation not available. Final dfs not available |
| Other bias | Unclear risk | Comment: Initial caries levels appear balanced (dfs at baseline: 0.9 in all 4 groups). Reliability of outcome assessment is not reported. Reversals or errors in clinical interpretation are not reported. There is no indication of contamination or co‐intervention |
Poulsen 1981.
| Methods | Random allocation on an individual basis, allocation concealment unknown Double blind Study duration 3 years Fluoride lozenge and placebo rinse compared with fluoride rinse and placebo lozenge 25.5% drop out after 3 years Reason for attrition: Moved to non‐involved schools No differential group losses |
|
| Participants | 499 children analysed after 3 years (available at final examination) Age at start: 7 and 11 years Surfaces affected at start: 2.01 to 2.27 DMFS (age 7) and 4.73 to 4.90 DMFS (age 11) Exposure to other fluoride: < 0.25 ppm fluoride in the water Year study began: Not reported, before 1981 Location: Region of Aarhus, Denmark |
|
| Interventions | Fluoride lozenge and placebo rinse vs fluoride rinse and placebo lozenge Fluoride rinse: 10 ml, 0.2% neutral NaF, fortnightly, on schooldays (40 rinses per child for the study period) Fluoride lozenges: Chewable, with 536 mg sorbitol, NaF (1.1 mg), one per day, on schooldays (450 lozenges per child for the study period) Lozenges and rinses administered at school Children received 90% of the maximal number of Lozenges and 80% of the rinses (100 weeks or 500 schooldays) |
|
| Outcomes | At baseline: mean DMFS, age, number of erupted surfaces for all teeth (per age group: 7, 11 years at baseline) Caries increment reported at 3 years DMFS increment given separately per age group (7, 11 years) and for teeth erupted at baseline or erupting during the study DMFS increment on proximal surfaces of premolars and molars in older children (age 11) for teeth erupted and erupting during the study Distribution of the children according to age (7, 11 years), baseline DMFS (0, 1‐2, > 3), number of erupted surfaces (< 30, > 30) and caries increment (< 1, > 2) Distribution of the children according to consumption of lozenges and participation in the rinsing program |
|
| Notes | Participants randomised (n = 670) Baseline characteristics "balanced": At start, no differences in mean age, DMFS, number of erupted surfaces between the groups (no statistical test) Clinical and radiological (for older children) caries assessment made by 1 examiner, using Moller & Poulsen (1973) diagnostic criteria. Diagnostic threshold = cavity: loss of surface continuity Reliability of caries assessment: Not reported Account for reversals: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "on the basis of the clinical examination, children were stratified according to DMFS and randomly distributed into 2 groups." Comment: Randomisation process partly described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: "Lozenges and rinsing solutions were coded and nobody knew the code (answer from the author)" Comment: Blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out for length of follow‐up: 25.5% drop out after 3 years Drop out by group: 85/338 children missing after 3 years in fluoride lozenge + placebo rinse group and 86/332 in fluoride rinse + placebo lozenge group Reasons for losses: Children moved to non‐involved schools Comments: Numbers lost were moderate. No differential loss between groups. Caries data used in the analysis pertain to continuous participants |
| Selective reporting (reporting bias) | Low risk | OUtcomes reported: At baseline: Mean DMFS, age, number of erupted surfaces for all teeth (per age group: 7, 11 years at baseline) Caries increment reported at 3 years DMFS increment given separately per age group (7, 11 years) and for teeth erupted at baseline or erupting during the study DMFS increment on proximal surfaces of premolars and molars in older children (age 11) for teeth erupted and erupting during the study Distribution of the children according to age (7, 11 years), baseline DMFS (0, 1‐2, > 3), number of erupted surfaces (< 30, > 30) and caries increment (< 1, > 2) Distribution of the children according to consumption of lozenges and participation in the rinsing program Comment: Caries increment given separately per age group and per status of teeth eruption. Final DMFS not available |
| Other bias | Unclear risk | Comment: Initial caries indexes appear balanced (DMFS in 7 years old children: 2.18 in the F Lozenges + placebo rinse group and 2.01 in the F rinse + placebo lozenges group; DMFS in 11 years old children: 4.73 in the F lozenges + placebo rinse group and 4.81 in the F rinse + placebo lozenges group). Reliability of outcome assessment is not reported. Account for reversals or errors in clinical and radiological interpretation are not reported. There is no indication of contamination or co‐intervention |
Stecksen‐Blicks 2008.
| Methods | Random allocation on an individual basis, allocation concealment described Double blind Fluoride + xylitol compared with non‐fluoride + xylitol lozenges Study duration 2 years 28.1% drop out after 2 years Reason for attrition: Relocation and violence of study protocol No differential group losses |
|
| Participants | 115 children analysed after 2 years (available at final examination) Age at start: 10 to 12 years Children with a predicted high caries risk (computerised risk assessment by the regular dentists) Surfaces affected at start: 2.1 to 2.9 DMFS (approximal) (for children who completed the study) Exposure to other fluoride: Use of fluoride toothpaste encouraged: "all the participants were encouraged to brush their teeth with fluoridated toothpastes two times a day during the entire study period", fluoride in water supply < 0.3 ppm Year study began: 2001 Location: Public dental clinic, city of Umea, Sweden |
|
| Interventions | Fluoride xylitol lozenges (NaF, 0.5 mg) vs non‐fluoride xylitol lozenges (all lozenges with acid malic/malic acid and 422 mg xylitol) Slow melting lozenges Pots of lozenges sent every 3 months 2 lozenges, 3 times a day Lozenges administered at home Compliance was checked every 3 months. Non‐consumed tablets were collected and compliance evaluated by calculating the weight of remaining tablets 41% of the children were classified as having a good, 30% a fair and 29% a poor compliance. Caries incidence did not vary according to compliance. Good compliance was higher (48%) in the group "xylitol" as compared to the group "xylitol + fluoride" (34%) |
|
| Outcomes | At baseline: Mean DMFSa (approximal caries prevalence), DSe (enamel lesions on approximal surfaces) Caries increment reported at 24 months Caries increment: DMFSa (approximal caries prevalence) and DSe (enamel lesions on approximal surfaces) increments Final examination: Mean DMFSa (approximal caries prevalence), DSe (enamel lesions on approximal surfaces) Mean Caries increment and cumulative distribution frequency of caries increment (DMFSa) among the subjects with good compliance |
|
| Notes | Participants randomised (n = 160) but one reference group with high risk children who refused to participate (n = 70) was also studied. This reference group was not considered in the meta‐analysis Baseline characteristics "balanced": At start, no significant difference in mean DMFSa and DSe between the groups Clinical and radiographic caries assessment made by two examiners. Diagnostic threshold = "lesion within enamel (DSe)or passing into dentine (DMFSa)". Caries increment (DMFSa, DSe) assessed from bitewing radiographs Reliability in caries assessment: 50 sets of radiographs re‐examined after 1 month in order to check intra‐ and interexaminer reliability (Kappa = 0.85 to 0.89) Account for errors or reversals: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "the subjects were randomly assigned to one of the two study groups and each patient was given a code number." Comment: Randomisation process partly described |
| Allocation concealment (selection bias) | Low risk | Quotes: "the randomisation was performed at the department for pharmaceutical testing at the University Hospital Pharmacy which kept the code list locked in a safe during the entire project." and "The code was broken when the study was finalized and all data were processed." Comment: Allocation concealment described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: "code list locked in a safe during the entire project." and " the study products were slow‐melting lozenges distributed in identical pots and labelled with the patient's individual code number." and "The pots were packed and labelled at the department for pharmaceutical testing at the University Hospital Pharmacy." Comment: Blind outcome assessment and use of identical lozenges in both groups described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out for length of follow‐up: 28.1% after 2 years Drop out by group: 21/80 children missing after two years in fluoride + xylitol group and 24/80 in xylitol group Reasons for losses: "relocation and violence of study protocol" and "2 children aborted treatment after 1 month because of stomachache." Comments: Numbers lost were moderate. No differential loss between groups. Caries data used in the analysis pertain to continuous participants |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: At baseline: Mean DMFSa (approximal caries prevalence), Mean DSe (enamel lesions on approximal surfaces) At final examination (24 months): DMFSa (approximal caries prevalence) and DSe (enamel lesions on approximal surfaces) increments, mean DMFSa (approximal caries prevalence), mean DSe (enamel lesions on approximal surfaces) Mean caries increment and cumulative distribution frequency of caries increment (DMFSa) among the subjects with good compliance Comments: All prespecified outcomes (in 'Methods') were reported. Caries increment measured only on approximal surfaces |
| Other bias | Unclear risk | Comments: Initial caries scores appear balanced (DMFSa at baseline: 2.1 in xylitol group and 2.9 in xylitol + fluoride group). Intra‐ and interexaminer reproducibility reported and satisfactory. Account for reversals or errors in radiographic interpretation are not reported. There is no indication of contamination or co‐intervention |
ADA = American Dental Association; APF = acidulated phosphate fluoride; DFS/DFT = number of decayed and filled permanent surfaces/teeth; dmfs/dmft/DMFS/DMFT = number of decayed, missing and filled surfaces/teeth (primary/permanent teeth); F = fluoride; n = number; NaF = sodium fluoride; ppm = parts per million; vs = versus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aasenden 1974 | Random allocation not stated or indicated No follow‐up of the children |
| Abary‐Murillo 1952 | Random allocation not stated or indicated Insufficient length of follow‐up (6 months) |
| Adyatmaka 1996 | Random allocation not stated or indicated |
| Allmark 1982 | No random allocation |
| Barmes 1985 | No random allocation |
| Bibby 1955 | Randomisation not ensured Insufficient length of follow‐up (12 to 14 months) |
| Binder 1958 | Random allocation not stated or indicated |
| Blinkhorn 1981 | In the test group home consumption of fluoride tablets was associated to chair side education while no preventive intervention was applied to the control group Insufficient length of follow‐up (18 months) |
| Frankl 1972 | Use of a fluoride solution (rinse) which was ingested Did not fulfil the definition of fluoride supplements Quote: "the swallowing procedure avoided the problem of expectoration in the classroom" |
| Grissom 1964 | No random allocation |
| Hamberg 1971 | Random allocation not stated or indicated |
| Hardwick 1981 | Random allocation not stated or indicated |
| Hennon 1966 | No longitudinal follow‐up of the children Randomisation not ensured |
| Hennon 1972 | No random allocation |
| Hennon 1977 | No longitudinal follow up of the children Randomisation not ensured |
| Hippchen 1965 | Random allocation not stated or indicated |
| Hu 1998 | No random allocation |
| Kessler 1958 | Random allocation not stated or indicated |
| Khambanonda 1983 | No random allocation |
| Knychalsa‐Karwan 1965 | No random allocation |
| Kosenko 1984 | Random allocation not stated or indicated |
| Larsen 1947 | Random allocation not stated or indicated Insufficient length of follow‐up (4 months) |
| Leksell 2003 | Abstract with no data Caries increment and DMF values not given |
| Li 2005 | No random allocation |
| Mann 1989 | No random allocation |
| Margolis 1967 | Random allocation not stated or indicated |
| McCall 1985 | Random allocation not stated or indicated |
| Pashaev 1993 | Random allocation not stated or indicated |
| Pollak 1961 | Random allocation not stated or indicated |
| Stephen 1978 | No random allocation |
| Stephen 1990 | No random allocation |
| Stones 1949 | The number of children in each group is unknown. DMF indexes are not used. Caries increment and PF values cannot be calculated |
| Strean 1946 | Random allocation not stated or indicated Insufficient length of follow‐up (6 to 8 months) |
| Strubig 1982 | Random allocation not stated or indicated |
| Szczygiel 1969 | Random allocation not stated or indicated Insufficient length of follow‐up (19 months) |
| Wan 2000 | Random allocation not stated or indicated |
| Wrzodek 1960 | Random allocation not stated or indicated |
| Ziemnowicz‐Glowacka 1960 | Random allocation not stated or indicated |
DMF = decayed, missing, filled; PF = prevented fraction.
Characteristics of studies awaiting assessment [ordered by study ID]
Horowitz 1994.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Not yet assessed |
Niedenthal 1957.
| Methods | Random allocation: Not described Allocation concealment: Unknown Blindness in outcome assessment NaF tablets compared with no treatment or placebo tablets (not stated) Study duration 6 years 22% drop out after 3 years, 42% after 6 years 345 children attributed to the treatment group and 305 in the control group |
| Participants | 508 children analysed after 3 years (1954) and 205 after 6 years (1957) Age at start: 6 to 7 years Surfaces affected at start: 0.22 DMFT Exposure to other fluoride: not stated Year study began: 1951 Location: Offenbach (Germany) |
| Interventions | Fluoride tablet vs no treatment Tablets administered on schooldays, under teacher supervision Fluoride tablets: 0.5 mg F, 2 tablets per day |
| Outcomes | Caries data at baseline (1951), after 3 years (1954) and 6 years (1957) At baseline: Mean DMFT (no SD) At final examinations: Mean DMFT (no SD) Caries increments: DMFT increment (no SD) |
| Notes | Participants randomised (n = 650) Baseline characteristics "balanced": unknown Clinical caries assessment made by two examiners Diagnostic threshold: Not stated Reliability: Not reported Account for reversals: Not reported |
Prasertsom 1992.
| Methods | Random allocation on an individual basis: The sample was divided into 2 groups with the equal number of children using drawing lots method Double blind Placebo‐controlled NaF tablets compared to placebo tablets Study duration 3 years 20.7% drop out after 3 years Number of children attributed to each group: Unknown |
| Participants | 493 children analysed after 3 years (available at final examination) Age at start: 5 to 6 years and 7 to 8 years Surfaces affected at start: 0.63 to 0.71 DMFS (5 to 6 years) and 1.55 to 1.83 DMFS (7 to 8 years) Exposure to other fluoride: Children used fluoride mouthrinse 0.2% every 2 weeks according to the national oral health promotion program Year study began: 1987 Location: 2 schools in Bangkok, Thailand |
| Interventions | Fluoride tablets (NaF, 2.2 mg) vs non‐fluoride tablets (flour tablets) Tablets administered daily at school Tablets chewed all over the mouth before swallowing |
| Outcomes | Baseline and final DMFS (SD) by study group, age group Percentage of children affected by caries in permanent and deciduous teeth, mean dfs by year of study, study group |
| Notes | Participants randomised (n = 622) Baseline characteristics "balanced": mean DMFS Clinical caries assessment made by 3 examiner teams. Each team consisted of 3 examiners Calibration exercises were carried out for the examiners' teams Diagnostic threshold: Not stated Reliability: Not reported Account for reversals: Not reported |
DMFS/DMFT = number of decayed, missing and filled permanent surfaces/teeth; F = fluoride; n = number; NaF = sodium fluoride; SD = standard deviation; vs = versus.
Differences between protocol and review
The paragraph 'statistical analysis' in the method section (Data collection and analysis) has been rewritten in view of the analyses that were effectively conducted.
Two authors have been added and the order of citation was changed in view of the actual participation of each author.
Contributions of authors
Conceiving, designing and co‐ordinating the review: Stéphanie Tubert‐Jeannin (STJ). Developing search strategy and undertaking searches: Emmanuel Amsallem (EA) and STJ. Screening search results and retrieved papers against inclusion criteria: STJ, Paul Tramini (PT), Andreas Schulte (AS), Martin J Koch, (MJK), Laurent Gerbaud (LG). Assessing risk of bias and extracting data from papers: STJ, PT, AS, MJK, LG, Myriam Rege Walther (MRW). Writing to authors for additional information: STJ, AS, LG. Data management for the review and entering data into RevMan: Candy Auclair (CA), LG. Analysis and interpretation of data: CA, LG, Christiane Ruffieux (CR), STJ. Writing the review: STJ, LG, CA. Providing general advice on the review: Amid Ismail (AI).
Sources of support
Internal sources
No sources of support supplied
External sources
-
British Orthodontic Society (BOS), UK.
The BOS have provided funding for the Cochrane Oral Health Group Global Alliance (see www.ohg.cochrane.org)
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care
Declarations of interest
None of the identified review authors have any financial interests that would present a conflict.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Aasenden 1972 {published data only}
- Aasenden R, DePaola PF, Brudevold F. Effects of daily rinsing and ingestion of fluoride solutions upon dental caries and enamel fluoride. Archives of Oral Biology 1972;17(12):1705‐14. [DOI] [PubMed] [Google Scholar]
DePaola 1968 {published data only}
- DePaola PF, Lax M. The caries‐inhibiting effect of acidulated phosphate‐fluoride chewable tablets: a two‐year double‐blind study. Journal of the American Dental Association 1968;76(3):554‐7. [DOI] [PubMed] [Google Scholar]
Driscoll 1974 {published data only}
- Driscoll WS, Heifetz SB, Brunelle JA. The use of fluoride tablets by school children: treatment and post‐treatment effects on dental caries. Journal of Dental Research 1979;58(Special Issue, IADR Abstracts):294 (Abs 808). [Google Scholar]
- Driscoll WS, Heifetz SB, Korts DC. Effect of acidulated phosphate‐fluoride chewable tablets on dental caries in schoolchildren: results after 30 months. Journal of the American Dental Association 1974;89(1):115‐20. [DOI] [PubMed] [Google Scholar]
- Driscoll WS, Heifetz SB, Korts DC. Effect of chewable fluoride tablets on dental caries in schoolchildren: results after six years of use. Journal of the American Dental Association 1978;97(5):820‐4. [DOI] [PubMed] [Google Scholar]
- Driscoll WS, Heifetz SB, Korts DC, Meyers RJ, Horowitz HS. Effect of acidulated phosphate‐fluoride chewable tablets in schoolchildren: results after 55 months. Journal of the American Dental Association 1977;94(3):537‐43. [DOI] [PubMed] [Google Scholar]
- Shern RJ, Driscoll WS, Korts DC. Enamel biopsy results of children receiving fluoride tablets. Journal of the American Dental Association 1977;95(2):310‐4. [DOI] [PubMed] [Google Scholar]
Heifetz 1987 {published data only}
- Driscoll WS, Nowjack‐Raymer R, Heifetz SB, Li SH, Selwitz RH. Evaluation of the comparative effectiveness of fluoride mouth rinsing, fluoride tablets, and both procedures in combination: interim findings after five years. Journal of Public Health Dentistry 1990;50(1):13‐7. [DOI] [PubMed] [Google Scholar]
- Driscoll WS, Nowjack‐Raymer R, Selwitz RH, Li SH. Additive effects of fluoride tablets and fluoride mouthrinsing: 8‐year findings. Journal of Dental Research 1991;70(Special Issue, IADR Abstracts):358 (Abs 743). [Google Scholar]
- Driscoll WS, Nowjack‐Raymer R, Selwitz RH, Li SH, Heifetz SB. A comparison of the caries‐preventive effects of fluoride mouth rinsing, fluoride tablets, and both procedures combined: final results after eight years. Journal of Public Health Dentistry 1992;52(2):111‐6. [DOI] [PubMed] [Google Scholar]
- Heifetz SB, Horowitz HS, Meyers RJ, Li SH. Evaluation of the comparative effectiveness of fluoride mouth rinsing, fluoride tablets, and both procedures in combination: interim findings after two years. Pediatric Dentistry 1987;9(2):121‐5. [PubMed] [Google Scholar]
- Heifetz SB, Nowjack‐Raymer R, Driscoll WS, Li Nih SH. Evaluation of the additive effectiveness of fluoride tablets and fluoride mouthrinsing: interim results after 5 years. Journal of Dental Research 1988;67(Special Issue, IADR Abstracts):230 (Abs 940). [Google Scholar]
Holm 1975 {published data only}
- Holm GB, Holst K, Koch G, Widenheim J. Fluoride chewing tablets ‐ a new aid in caries prevention. Comparative effect of a weekly mouthrinse with 0.2% NaF and daily chewing of a fluoride tablet (Gostrimant (R)). 2 year clinical test in schoolchildren. Tandläkartidningen 1975;67(6):354‐461. [PubMed] [Google Scholar]
Källestål 2000 {published data only}
- Källestål C. The effect of five years' implementation of caries‐preventive methods in Swedish high‐risk adolescents. Caries Research 2005;39(1):20‐6. [DOI] [PubMed] [Google Scholar]
- Källestål C, Flinck A, Allebeck P, Holm A‐K, Wall S. Evaluation of caries preventive measures. Swedish Dental Journal 2000;24(1‐2):1‐11. [PubMed] [Google Scholar]
- Oscarson N, Källestål C, Fjelddahl A, Lindholm L. Cost‐effectiveness of different caries preventive measures in a high‐risk population of Swedish adolescents. Community Dentistry and Oral Epidemiology 2003;31(3):169‐78. [DOI] [PubMed] [Google Scholar]
Lin 2000 {published data only}
- Lin YT, Tsai CL. Comparative anti‐caries effects of tablet and liquid fluorides in cleft children. Journal of Clinical Dentistry 2000;11(4):104‐6. [PubMed] [Google Scholar]
- Lin YT, Tsai TP. Comparative anti‐caries effects of tablet and liquid fluorides in cleft children. International Journal of Paediatric Dentistry 1999;9(Suppl 1, IAPD Abstracts):90 (Abs P4.25). [PubMed] [Google Scholar]
O'Rourke 1988 {published data only}
- O'Rourke CA, Attrill M, Holloway PJ. Cost appraisal of a fluoride tablet programme to Manchester primary schoolchildren. Community Dentistry and Oral Epidemiology 1988;16(6):341‐4. [DOI] [PubMed] [Google Scholar]
Petersson 1985 {published data only}
- Petersson LG, Koch G, Rasmusson CG, Stanke H. Effect on caries of different fluoride prophylactic programs in preschool children. A two year clinical study. Swedish Dental Journal 1985;9(3):97‐104. [PubMed] [Google Scholar]
Poulsen 1981 {published data only}
- Poulsen S, Gadegaard E, Mortensen B. Cariostatic effect of daily use of a fluoride‐containing lozenge compared to fortnightly rinses with 0.2% sodium fluoride. Caries Research 1980;14:169 (Abs 3). [DOI] [PubMed] [Google Scholar]
- Poulsen S, Gadegaard E, Mortensen B. Cariostatic effect of daily use of a fluoride‐containing lozenge compared to fortnightly rinses with 0.2% sodium fluoride. Caries Research 1981;15(3):236‐42. [DOI] [PubMed] [Google Scholar]
Stecksen‐Blicks 2008 {published data only}
- Stecksén‐Blicks C, Holgerson PL, Twetman S. Effect of xylitol and xylitol‐fluoride lozenges on approximal caries development in high‐caries‐risk children. International Journal of Paediatric Dentistry 2008;18(3):170‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aasenden 1974 {published data only}
- Aasenden R, Peebles TC. Effects of fluoride supplementation from birth on dental caries and fluorosis in teenaged children. Archives of Oral Biology 1978;23(2):111‐5. [DOI] [PubMed] [Google Scholar]
- Aasenden R, Peebles TC. Effects of fluoride supplementation from birth on human deciduous and permanent teeth. Archives of Oral Biology 1974;19(4):321‐6. [DOI] [PubMed] [Google Scholar]
Abary‐Murillo 1952 {published data only}
- Abary‐Murillo J. Fluoride therapy. The Journal of the Philippine Dental Association 1952;5:19‐21. [Google Scholar]
Adyatmaka 1996 {published data only}
- Adyatmaka A, Destri M. Dental caries reduction and inhibitory effects of a fluoride tablet program in West Kalimantan. Journal of Dental Research 1996; Vol. 75, issue Spec issue:2296.
Allmark 1982 {published data only}
- Allmark C, Green HP, Linney AD, Wills DJ, Picton DC. A community study of fluoride tablets for school children in Portsmouth. Results after six years. British Dental Journal 1982;153(12):426‐30. [DOI] [PubMed] [Google Scholar]
Barmes 1985 {published data only}
- Barmes, Barnaud D, Khambonandas J, Infirri JS. Field trials of preventive regimens in Thailand and French Polynesia. International Dental Journal 1985;35(1):66‐72. [PubMed] [Google Scholar]
Bibby 1955 {published data only}
- Bibby BG, Wilkins E, Witol E. A preliminary study of the effects of fluoride lozenges and pills on dental caries. Oral Surgery Oral Medecine and Oral Pathology 1955;8(2):213‐6. [DOI] [PubMed] [Google Scholar]
Binder 1958 {published data only}
- Binder K. [Vorlaufiger bericht uber die bisherigen erfahrungen und resultate der fluortablettenverabfolgung an wiener schulkinder]. Österreichische Zeitschrift für Stomatologie 1958;55(12):653‐7. [Google Scholar]
Blinkhorn 1981 {published data only}
- Blinkhorn AS, Downer MC, Mackie IC, Bleasdale RS. Evaluation of a practice based preventive programme for adolescents. Community Dentistry and Oral Epidemiology 1981;9(6):275‐9. [DOI] [PubMed] [Google Scholar]
Frankl 1972 {published data only}
- Frankl SN, Fleisch S, Diodati RR. The topical anticariogenic effect of daily rinsing with an acidulated phosphate fluoride solution. Journal of the American Dental Association 1972;85(4):882‐6. [DOI] [PubMed] [Google Scholar]
Grissom 1964 {published data only}
- Grissom DK, Dudenbostel RE, Cassel WJ, Murray RT. Comparative study of systemic sodium fluoride and topical stannous fluoride application in preventive dentistry. Journal of Dentistry for Children 1964;31(4):314‐22. [Google Scholar]
Hamberg 1971 {published data only}
- Hamberg L. Controlled trial of fluoride in vitamin drops for prevention of caries in children. Lancet 1971;1(7696):441‐2. [DOI] [PubMed] [Google Scholar]
Hardwick 1981 {published data only}
- Hardwick JL, Bloodworth G, Mason MG. Caries preventive effect of fluoride‐sucrose tablets distributed over 3 years to children aged 12 initially. Caries Research 1981; Vol. 15, issue 2:183‐4.
Hennon 1966 {published data only}
- Hennon DK, Stookey GK, Muhler JC. The clinical anticariogenic effectiveness of supplementary fluoride‐vitamin preparations‐‐results at the end of four years. Journal of Dentistry for Children 1967;34(6):439‐43. [PubMed] [Google Scholar]
- Hennon DK, Stookey GK, Muhler JC. The clinical anticariogenic effectiveness of supplementary fluoride‐vitamin preparations. Results at the end of three years. Journal of Dentistry for Children 1966;33(1):3‐12. [PubMed] [Google Scholar]
- Hennon DK, Stookey GK, Mulher JC. The clinical anticariogenic effectiveness of supplementary fluoride vitamin preparations results at the end of five and a half years. Pharmacology and therapeutics in dentistry 1970;1(1):1‐6. [PubMed] [Google Scholar]
Hennon 1972 {published data only}
- Hennon DK, Stookey GK, Muhler JC. Prophylaxis of dental caries: relative effectiveness of chewable fluoride preparations with and without added vitamins. Journal of Pediatrics 1972;80(6):1018‐21. [DOI] [PubMed] [Google Scholar]
Hennon 1977 {published data only}
- Hennon DK, Stookey GK, Beiswanger BB. Fluoride‐vitamin supplements: effects on dental caries and fluorosis when used in areas with suboptimum fluoride in the water supply. Journal of the American Dental Association 1977;95(5):965‐71. [DOI] [PubMed] [Google Scholar]
Hippchen 1965 {published data only}
- Hippchen P. Caries control with fluorides in children of Dusseldorf. Zahnärztliche Mitteilungen 1965;55(18):897‐900. [PubMed] [Google Scholar]
Hu 1998 {published data only}
- Hu D, Wan H, Li S. The caries‐inhibiting effect of a fluoride drop program: a 3‐year study on Chinese kindergarten children. Chinese Journal of Dental Research 1998;1(3):17‐20. [PubMed] [Google Scholar]
- Hu D, Wan H, Li S, Dogon L. The caries inhibiting effect of a 3‐years fluoride drop program. Journal of Dental Research 1995;74(Special Issue ‐ IADR Abstracts):411 (Abs 82). [Google Scholar]
- Hu D, Wan H, Liu D. Five year experience with a school based fluoride tablet program. Journal of Dental Research 1996;75(Special Issue, IADR Abstracts):361 (Abs 2747). [Google Scholar]
- Wan H, Hu D, Li S, Liu D. 3‐year study of fluoride tablet and sealant on dental caries. Journal of Dental Research 1997;76(1):214 (Abs No 1602). [Google Scholar]
Kessler 1958 {published data only}
- Kessler W, Solth K. [Ergebnisse der Zahnkaries‐Prophylaxe durch interne fluorgaben]. Stomatologia 1958;11:14‐20. [Google Scholar]
Khambanonda 1983 {published data only}
- Khambanonda S, Chandravejjsmarn R, Barmes DE, Sardo Infirri J, Möller I. Prevention of dental caries in Thailand: 3 fluoridated products submitted for comparative tests. Journal de Biologie Buccale 1983;11(3):255‐63. [PubMed] [Google Scholar]
Knychalsa‐Karwan 1965 {published data only}
- Knychalska‐Karwan Z, Laskowska L. Use of fluoride tablets in Polish children. Dental Abstracts 1964;9:53. [Google Scholar]
- Knychalska‐Karwan Z, Laskowska L, Pelcowa M, Wedler A. Remote results of a 3‐year administration of fluorine tablets. Czasopismo Stomatologiczne 1965;18(4):377‐81. [PubMed] [Google Scholar]
Kosenko 1984 {published data only}
- Kosenko KN, Rudinskaia LA, Todorashko OV, Kariachka TZ, Vakulenko LV. Clinical evaluation of the caries‐prophylactic effectiveness of a fluoride‐containing lacquer. Stomatologiia 1984;63(1):77‐9. [PubMed] [Google Scholar]
Larsen 1947 {published data only}
- Larsen NP. Fluorine in control of children's tooth decay. Postgraduate Medicine 1947;2(5):358‐64. [DOI] [PubMed] [Google Scholar]
Leksell 2003 {published data only}
- Leksell E. Evaluation of three caries preventive methods: a 3‐year radiological study in Swedish urban school children with high caries risk. International Journal of Paediatric Dentistry 2003;13(Suppl 1):49‐50. [Google Scholar]
Li 2005 {published data only}
- Li SM, Fan X, Zou J. 2‐year clinic effect of fluoride drop on dental caries prevention of primary teeth. Hua Xi Kou Qiang Yi Xue Za Zhi 2005;23(2):136‐7. [PubMed] [Google Scholar]
Mann 1989 {published data only}
- Mann J, Horesh E, Ran F, Gedalia I. The effect of fluoride drop administration on dental caries increment‐‐a longitudinal study. Israeli Journal of Dental Sciences 1989;2(3):148‐52. [PubMed] [Google Scholar]
Margolis 1967 {published data only}
- Margolis FJ, Macauley J, Freshman E. The effects of measured doses of fluoride. A five‐year preliminary report. American Journal of Diseases of Children 1967;113(6):670‐2. [DOI] [PubMed] [Google Scholar]
- Margolis FJ, Reames HR, Freshman E, Macauley JC, Mehaffey H. Fluoride: Ten‐year prospective study of deciduous and permanent dentition. American Journal of Diseases of Children 1975;129(7):794‐800. [DOI] [PubMed] [Google Scholar]
McCall 1985 {published data only}
- McCall DR, Stephen KW, Sutherland DA. Five years of community fluoride tablets and fissure. Caries Research 1985;19(2):173‐4. [Google Scholar]
Pashaev 1993 {published data only}
- Pashaev ChA, Akhmedbeili RM. The results of comprehensive caries prophylaxis in children under endemic goiter conditions. Stomatologiia 1993;72(4):61‐4. [PubMed] [Google Scholar]
Pollak 1961 {published data only}
- Pollak H. Caries prevention by administration of milgatum fluoride tablets. Dental Abstracts 1961; Vol. 6:119‐20.
Stephen 1978 {published data only}
- Stephen KW, Campbell D. Caries reduction and cost benefit after 3 years of sucking fluoride tablets daily at school. A double‐blind trial. British Dental Journal 1978;144(7):202‐6. [DOI] [PubMed] [Google Scholar]
Stephen 1990 {published data only}
- Stephen KW, Kay EJ, Tullis JI. Combined fluoride therapies. A 6‐year double‐blind school‐based preventive dentistry study in Inverness, Scotland. Community Dentistry and Oral Epidemiology 1990;18(5):244‐8. [DOI] [PubMed] [Google Scholar]
Stones 1949 {published data only}
- Stones HH, Lawton FE, Bransby ER, Hartley HO. The effect of topical applications of potassium fluoride and of the ingestion of tablets containing sodium fluoride on the incidence of dental caries. British Dental Journal 1949;86(11):263‐71. [PubMed] [Google Scholar]
Strean 1946 {published data only}
- Strean LP, Beaudet JP. Dental caries inhibited by fluoride‐vitamin tablets. Dental Survey 1946;22:689‐91. [PubMed] [Google Scholar]
Strubig 1982 {published data only}
- Gülzow HJ, Strübig W. Need for the continuous ingestion of fluoride tablets. Deutsche Zahnärztliche Zeitschrift 1984;39(7):512‐4. [PubMed] [Google Scholar]
- Strübig W, Aeckerle‐Wittern B, Burchard GL. Caries statistics after 2 years of tablet fluoridation. Das Offentliche Gesundheitswesen 1982;44(7):462‐4. [PubMed] [Google Scholar]
Szczygiel 1969 {published data only}
- Szczygiel A, Szczepanska I, Marcinczak S, Konieczna W. Effect of 2 years of administration of fluoride tablets on the state of dentition of rural children. Roczniki Panstwowego Zakladu Higieny 1969;20(3):373‐81. [PubMed] [Google Scholar]
- Szczygiel A, Szczepanska I, Marcinczak S, Konieczna W. The effects of the 2‐year administration of fluorides in tablet form on the state of teeth in rural children. Czasopismo Stomatologiczne 1970;23(3):231‐9. [PubMed] [Google Scholar]
Wan 2000 {published data only}
- Wan H, Hu D, Tong G, Fan X, Li X. A five years study of fluoride tablets on dental caries in Chinese children. Journal of Dental Research 2000; Vol. 79, issue spec issue:210 (Abs 533).
Wrzodek 1960 {published data only}
- Wrzodek G. Fluorine for caries prevention in Hesse, Germany. Dental Abstracts 1960; Vol. 5:716.
Ziemnowicz‐Glowacka 1960 {published data only}
- Ziemnowicz‐Glowacka W. Prevention of caries by means of fluodar tablets. Czasopismo Stomatologiczne 1960;13:719‐28. [Google Scholar]
- Ziemnowicz‐Glowacka W. Sodium fluoride tablets as a mean to reduce the incidence of caries in kindergarten pupils. Dental Abstracts 1961; Vol. 6:397‐8.
References to studies awaiting assessment
Horowitz 1994 {published data only}
- Horowitz HS. Evaluation of the Bermuda dietary fluoride supplement program. Washington, D.C; PAHO 1994; Vol. May (PAHO/BER/HSS/94.O1).
- Watson MR. Evaluation of the Bermuda dietary fluoride supplement program. Washington, D.C; PAHO 1994; Vol. May (PAHO/BER/HSS/94.02).
Niedenthal 1957 {published data only}
- Niedenthal A. [Kariesprophylaxe mit natriumfluorid‐Dragees bei offenbacher Schulkindern]. Zahnärztliche Mitteilungen 1957;17(57):576‐80. [Google Scholar]
Prasertsom 1992 {published data only}
- Prasertsom PA, Apchichatabute VT. The results of caries reduction of using fluoride tablets in first grade primary school children. Journal of the Dental Association of Thailand 1992;42(3):138‐43. [Google Scholar]
Additional references
Adair 1999
- Adair SM. Overview of the history and current status of fluoride supplementation schedules. Journal of Public Health Dentistry 1999;59(4):252‐8. [DOI] [PubMed] [Google Scholar]
Banting 1999
- Banting DW. International fluoride supplement recommendations. Community Dentistry and Oral Epidemiology 1999;27(1):57‐61. [DOI] [PubMed] [Google Scholar]
Binder 1978
- Binder K, Driscoll WS, Schützmannsky G. Caries‐preventive fluoride tablet programs. Caries Research 1978;12 Suppl 1:22‐30. [DOI] [PubMed] [Google Scholar]
Birch 1969
- Birch RC. The role of dietary supplements of fluoride in dental health programs for fluoride‐deficient areas. Journal of Public Health Dentistry 1969;29(3):170‐87. [DOI] [PubMed] [Google Scholar]
Burt 1999
- Burt BA. The case for eliminating the use of dietary fluoride supplements for young children. Journal of Public Health Dentistry 1999;59(4):269‐74. [DOI] [PubMed] [Google Scholar]
CDC 2001
- Centers for Disease Control and Prevention. Recommendations for using fluoride to prevent and control dental caries in the United States. Morbidity and Mortality Weekly Report: Recommandation Report 2001;50(RR‐14):1‐42. [PubMed] [Google Scholar]
Driscoll 1979
- Driscoll WS, Heifetz SB, Brunnelle JA. Treatment and posttreatment effects of chewable fluoride tablets on dental caries: findings after 7 1/2 years. Journal of the American Dental Association 1979;99(5):817‐21. [DOI] [PubMed] [Google Scholar]
Driscoll 1981
- Driscoll WS, Heifetz SB, Brunelle JA. Caries‐preventive effects of fluoride tablets in schoolchildren four years after discontinuation of treatments. Journal of the American Dental Association 1981;103(6):878‐81. [DOI] [PubMed] [Google Scholar]
Dubey 1965
- Dubey SD, Lehnhoff RW, Radike AW. A statistical confidence interval for true per cent reduction in caries‐incidence studies. Journal of Dental Research 1965;44(5):921‐3. [DOI] [PubMed] [Google Scholar]
Espelid 2010
- Espelid I. Caries preventive effect of fluoride in milk, salt and tablets: a literature review. European Archives of Paediatric Dentistry 2009;10(3):149‐56. [DOI] [PubMed] [Google Scholar]
Featherstone 1999
- Featherstone JD. Prevention and reversal of dental caries: role of low level fluoride. Community Dentistry and Oral Epidemiology 1999;27(1):31‐40. [DOI] [PubMed] [Google Scholar]
Fejerskov 2004
- Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Research 2004;38(3):182‐91. [DOI] [PubMed] [Google Scholar]
Gillespie 2007
- Gillespie GM, Marinho VCC, Marthaler TM, Holt R, Poulsen S, Stephen K, et al. Salt fluoridation for preventing dental caries. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006846] [DOI] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ismail 1994
- Ismail AI. Fluoride supplements: current effectiveness, side effects, and recommendations. Community Dental and Oral Epidemiology 1994;22(3):164‐72. [DOI] [PubMed] [Google Scholar]
Ismail 1999
- Ismail AI, Bandekar RR. Fluoride supplements and fluorosis: a meta‐analysis. Community Dental and Oral Epidemiology 1999;27(1):48‐56. [DOI] [PubMed] [Google Scholar]
Ismail 2008
- Ismail AI, Hasson H. Fluoride supplements, dental caries and fluorosis: a systematic review. Journal of the American Dental Association 2008;139(11):1457‐68. [DOI] [PubMed] [Google Scholar]
Limeback 1999
- Limeback H. A re‐examination of the pre‐eruptive and post‐eruptive mechanism of the anti‐caries effects of fluoride: is there any anti‐caries benefit from swallowing fluoride?. Community Dentistry and Oral Epidemiology 1999;27(1):62‐71. [DOI] [PubMed] [Google Scholar]
Marinho 2002a
- Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002280] [DOI] [PubMed] [Google Scholar]
Marinho 2002b
- Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002279] [DOI] [PubMed] [Google Scholar]
Marinho 2003a
- Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD002284] [DOI] [PubMed] [Google Scholar]
Marinho 2003b
- Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2004a
- Marinho VC, Higgins JP, Sheiham A, Logan S. Combinations of topical fluoride (toothpastes, mouthrinses, gels, varnishes) versus single topical fluoride for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002781.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marthaler 2004
- Marthaler TM. Changes in dental caries 1953‐2003. Caries Research 2004;38(3):173‐81. [DOI] [PubMed] [Google Scholar]
McDonagh 2000
- McDonagh MS, Whiting PF, Wilson PM, Sutton AJ, Chestnutt I, Cooper J, et al. Systematic review of water fluoridation. BMJ 2000;321(7265):855‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nowjack‐Raymer 1995
- Nowjack‐Raymer RE, Selwitz RH, Kingman A, Driscoll WS. The prevalence of dental fluorosis in a school‐based program of fluoride mouth rinsing, fluoride tablets, and both procedures combined. Journal of Public Health Dentistry 1995;55(3):165‐70. [DOI] [PubMed] [Google Scholar]
Petersen 2005
- Petersen PE, Bourgeois D, Ogawa H, Estupinan‐Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bulletin of the World Health Organization 2005;83(9):661‐9. [PMC free article] [PubMed] [Google Scholar]
RevMan 2011
- Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Riordan 1993
- Riordan PJ. Fluoride supplements in caries prevention: a literature review and proposal for a new dosage schedule. Journal of Public Health Dentistry 1993;53(3):174‐89. [DOI] [PubMed] [Google Scholar]
Riordan 1996
- Riordan PJ. The place of fluoride supplements in caries prevention today. Australian Dental Journal 1996;41(5):335‐42. [DOI] [PubMed] [Google Scholar]
Riordan 1999
- Riordan PJ. Fluoride supplements for young children: an analysis of the literature focusing on benefits and risks. Community Dentistry and Oral Epidemiology 1999;27(1):72‐83. [DOI] [PubMed] [Google Scholar]
Sohn 2007
- Sohn W, Ismail AI, Taichman LS. Caries risk‐based fluoride supplementation for children. Pediatric Dentistry 2007;29(1):23‐31. [PubMed] [Google Scholar]
Strean 1946
- Strean L, Beaudet J. Dental caries inhibited by fluoride vitamin tablets. Dental Survey 1946;22:689‐91. [PubMed] [Google Scholar]
Swedish Council 2002
- The Swedish Council on technology assessment in health care. Prevention of dental caries. A systematic review. Report number 161 2002. [ISBN:991‐87890‐81‐X]
Thylstrup 1990
- Thylstrup A. Clinical evidence of the role of pre‐eruptive fluoride in caries prevention. Journal of Dental Research 1990;69(Special Issue):742‐50. [DOI] [PubMed] [Google Scholar]
Van Rijkom 1998
- Rijkom HM, Truin GJ, van’t Hof MA. A meta‐analysis of clinical studies on the caries‐inhibiting effect of fluoride gel treatment. Caries Research 1998;32(2):83–92. [DOI] [PubMed] [Google Scholar]
Walsh 2010
- Walsh T, Worthington HV, Glenny AM, Appelbe P, Marinho VC, Shi X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007868.pub2] [DOI] [PubMed] [Google Scholar]
Yeung 2005
- Yeung A, Hitchings JL, Macfarlane TV, Threlfall AG, Tickle M, Glenny AM. Fluoridated milk for preventing dental caries. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003876.pub2] [DOI] [PubMed] [Google Scholar]
Yeung 2011
- Yeung CA. Efficacy of salt fluoridation. Evidence Based Dentistry 2011;12(1):17‐8. [DOI] [PubMed] [Google Scholar]


