Abstract
Background
Multiple myeloma is a bone marrow‐based hematological malignancy accounting for approximately two per cent of cancers. First‐line treatment for transplant‐ineligible individuals consists of multiple drug combinations of bortezomib (V), lenalidomide (R), or thalidomide (T). However, access to these medicines is restricted in many countries worldwide.
Objectives
To assess and compare the effectiveness and safety of multiple drug combinations of V, R, and T for adults with newly diagnosed transplant‐ineligible multiple myeloma and to inform an application for the inclusion of these medicines into the World Health Organization's (WHO) list of essential medicines.
Search methods
We searched CENTRAL and MEDLINE, conference proceedings and study registries on 14 February 2019 for randomised controlled trials (RCTs) comparing multiple drug combinations of V, R and T for adults with newly diagnosed transplant‐ineligible multiple myeloma.
Selection criteria
We included RCTs comparing combination therapies of V, R, and T, plus melphalan and prednisone (MP) or dexamethasone (D) for first‐line treatment of adults with transplant‐ineligible multiple myeloma. We excluded trials including adults with relapsed or refractory disease, trials comparing drug therapies to other types of therapy and trials including second‐generation novel agents.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias of included trials. As effect measures we used hazard ratios (HRs) for overall survival (OS) and progression‐free survival (PFS) and risk ratios (RRs) for adverse events. An HR or RR < 1 indicates an advantage for the intervention compared to the main comparator MP. Where available, we extracted quality of life (QoL) data (scores of standardised questionnaires). Results quoted are from network meta‐analysis (NMA) unless stated.
Main results
We included 25 studies (148 references) comprising 11,403 participants and 21 treatment regimens. Treatments were differentiated between restricted treatment duration (treatment with a pre‐specified amount of cycles) and continuous therapy (treatment administered until disease progression, the person becomes intolerant to the drug, or treatment given for a prolonged period). Continuous therapies are indicated with a "c". Risk of bias was generally high across studies due to the open‐label study design.
Overall survival (OS)
Evidence suggests that treatment with RD (HR 0.63 (95% confidence interval (CI) 0.40 to 0.99), median OS 55.2 months (35.2 to 87.0)); TMP (HR 0.75 (95% CI 0.58 to 0.97), median OS: 46.4 months (35.9 to 60.0)); and VRDc (HR 0.49 (95% CI 0.26 to 0.92), median OS 71.0 months (37.8 to 133.8)) probably increases survival compared to median reported OS of 34.8 months with MP (moderate certainty). Treatment with VMP may result in a large increase in OS, compared to MP (HR 0.70 (95% CI 0.45 to 1.07), median OS 49.7 months (32.5 to 77.3)), low certainty).
Progression‐free survival (PFS)
Treatment withRD (HR 0.65 (95% CI0.44 to 0.96), median PFS: 24.9 months (16.9 to 36.8)); TMP (HR 0.63 (95% CI 0.50 to 0.78), median PFS:25.7 months (20.8 to 32.4)); VMP (HR 0.56 (95% CI 0.35 to 0.90), median PFS: 28.9 months (18.0 to 46.3)); and VRDc (HR 0.34 (95% CI 0.20 to 0.58), median PFS: 47.6 months (27.9 to 81.0)) may result in a large increase in PFS (low certainty) compared to MP (median reported PFS: 16.2 months).
Adverse events
The risk of polyneuropathies may be lower with RD compared to treatment with MP (RR 0.57 (95% CI 0.16 to 1.99), risk for RD: 0.5% (0.1 to 1.8), mean reported risk for MP: 0.9% (10 of 1074 patients affected), low certainty). However, the CIs are also compatible with no difference or an increase in neuropathies. Treatment with TMP (RR 4.44 (95% CI1.77 to 11.11), risk: 4.0% (1.6 to 10.0)) and VMP (RR 88.22 (95% CI 5.36 to 1451.11), risk: 79.4% (4.8 to 1306.0)) probably results in a large increase in polyneuropathies compared to MP (moderate certainty). No study reported the amount of participants with grade ≥ 3 polyneuropathies for treatment with VRDc.
VMP probably increases the proportion of participants with serious adverse events (SAEs) compared to MP (RR 1.28 (95% CI 1.06 to 1.54), risk for VMP: 46.2% (38.3 to 55.6), mean risk for MP: 36.1% (177 of 490 patients affected), moderate certainty). RD, TMP, and VRDc were not connected to MP in the network and the risk of SAEs could not be compared.
Treatment with RD (RR 4.18 (95% CI 2.13 to 8.20), NMA‐risk: 38.5% (19.6 to 75.4)); and TMP (RR 4.10 (95% CI 2.40 to 7.01), risk: 37.7% (22.1 to 64.5)) results in a large increase of withdrawals from the trial due to adverse events (high certainty) compared to MP (mean reported risk: 9.2% (77 of 837 patients withdrew)). The risk is probably slightly increased with VMP (RR 1.06 (95% CI 0.63 to 1.81), risk: 9.75% (5.8 to 16.7), moderate certainty), while it is much increased with VRDc (RR 8.92 (95% CI 3.82 to 20.84), risk: 82.1% (35.1 to 191.7), high certainty) compared to MP.
Quality of life
QoL was reported in four studies for seven different treatment regimens (MP, MPc, RD, RMP, RMPc, TMP, TMPc) and was measured with four different tools. Assessment and reporting differed between studies and could not be meta‐analysed. However, all studies reported an improvement of QoL after initiation of anti‐myeloma treatment for all assessed treatment regimens.
Authors' conclusions
Based on our four pre‐selected comparisons of interest, continuous treatment with VRD had the largest survival benefit compared with MP, while RD and TMP also probably considerably increase survival. However, treatment combinations of V, R, and T also substantially increase the incidence of AEs, and lead to a higher risk of treatment discontinuation. Their effectiveness and safety profiles may best be analysed in further randomised head‐to‐head trials. Further trials should focus on consistent reporting of safety outcomes and should use a standardised instrument to evaluate QoL to ensure comparability of treatment‐combinations.
Plain language summary
Multiple drug combinations of bortezomib, lenalidomide and thalidomide for initial treatment of adults with transplant‐ineligible multiple myeloma
Background
Multiple myeloma is a type of blood cancer. It accounts for approximately 2% of all cancers and is still considered incurable. For people with newly diagnosed multiple myeloma (NDMM), who are unsuitable for a procedure where damaged blood cells are replaced with healthy ones (stem‐cell transplant), treatment is usually a multiple drug combination of bortezomib, lenalidomide, or thalidomide, plus melphalan and prednisolone (MP) or dexamethasone (D). Multiple drug combinations are approved for initial anti‐myeloma therapy, however, access to these medicines is restricted in many countries worldwide.
Aim of the review
To compare the benefits and harms of selected anti‐myeloma drugs (bortezomib (V), lenalidomide (R), thalidomide (T)) for transplant‐unsuitable NDMM.
Study characteristics
We searched selected medical databases and trial registries until 14th February 2019. We included studies comparing multiple drug combinations of V, R, and T for the treatment of people with NDMM who were unsuitable for a stem‐cell transplant. We differentiated between fixed treatment duration and continuous therapy. Fixed therapy is a pre‐specified number of cycles, while a continuous therapy is given until the disease gets worse, the person finds the drug hard to tolerate, or when the treatment is given for a prolonged period. Continuous therapies are indicated with a "c".
Key results
We identified 25 studies involving 11,403 transplant‐unsuitable adults with NDMM, and comparing 21 different treatment regimens.
Survival
People who had the standard treatment, MP, lived for an average of 35 months. People treated with RD, TMP, and VRDc probably live for much longer (moderate certainty). Treatment with VMP may also lead to much longer survival, compared to MP (low certainty). People treated with RD lived for an additional 20.4 months; with TMP an additional 11.6 months; with VRDc an additional 36.2 months, and with VMP an additional 14.9 months.
Harms
On average, 0.9% (9 out of 1000) of people treated with MP experienced peripheral nerve damage (polyneuropathies).The evidence was inconclusive whether treatment with RD decreases the risk of developing a polyneuropathy, compared to MP. The estimated risk of polyneuropathies with RD was 0.5%. Treatment with TMP and VMP probably increases the risk of experiencing polyneuropathies compared to MP (moderate certainty). The estimated risk with TMP was 4.0%, and with VMP 79.4%. No VRDc treatment study reported the number of participants with severe polyneuropathies.
On average, 36.1% (361 out of 1000) of people on MP‐treatment experienced at least one serious adverse event (SAE). VMP probably increases the proportion of participants with SAEs compared to MP to 46.2% (moderate certainty).
On average, 9.2% (92 out of 1000) of people treated with MP stop the treatment because of adverse events (AEs). Treatment with RD, TMP, and VRDc leads to a much higher proportion of people stopping treatment because of AEs than MP (high certainty). The risk of stopping treatment with RD is 38.5%; with TMP 37.7%, with VRDc 82.1%. Treatment with VMP probably increases the risk of stopping treatment because of AEs compared to MP (9.75%, moderate certainty).
Quality of life
Quality of life (QoL) was reported in four studies for seven different treatments and was measured with four different tools. Assessment and reporting differed between studies and could not be meta‐analysed. However, all studies reported an improvement in QoL after anti‐myeloma treatment was started for all assessed treatments.
Conclusions
VRDc showed the highest overall survival benefits, compared to MP. RD and TMP also improved OS compared to MP. However, these combinations of drugs also led to more adverse events compared to MP, and led to more people stopping treatment. More trials are needed that look carefully at both harms and QoL.
Summary of findings
Summary of findings 1. Summary of findings.
| Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first‐line treatment in transplant‐ineligible multiple myeloma patients | |||||
|
Patient or population: newly diagnosed, transplant‐ineligible adults with symptomatic multiple myeloma Settings: mostly outpatient; mostly multi‐centre studies across Europe, Asia, North‐ and South America, Australia, and the Pacific region Intervention: lenalidomide plus dexamethasone (RD), thalidomide plus melphalan and prednisone (TMP), bortezomib plus melphalan and prednisone (VMP), continuous bortezomib plus lenalidomide plus dexamethasone (VRDc) Comparison: melphalan and prednisone (MP) | |||||
| Outcomes | Effects and 95% confidence intervals in the effects. Main comparator is MP* | ||||
| Risk with MP | Risk with RD | Risk with TMP | Risk with VMP | Risk with VRDc | |
| Overall survival | Median overall survival over all studies in the network1: 34.8 months | NMA‐median OS: 55.2 (35.2 to 87.0) months | NMA‐median OS: 46.4 (35.9 to 60.0) months | NMA‐median OS: 49.7 (32.5 to 77.3) months | NMA‐median OS: 71.0 (37.8 to 133.8) months |
| NMA‐HR: 0.63 (95% CI 0.40 to 0.99) | NMA‐HR: 0.75 (95% CI 0.58 to 0.97) | NMA‐HR: 0.70 (95% CI 0.45 to 1.07) | NMA‐HR: 0.49 (95% CI 0.26 to 0.92) | ||
| ⊕⊕⊕⊝ moderate confidence in estimates due to inconsistency of I² = 53.9% (downgrade minus 1) | ⊕⊕⊕⊝ moderate confidence in estimates due to inconsistency of I² = 53.9% (downgrade minus 1) | ⊕⊕⊝⊝ low confidence in estimates due to inconsistency of I² = 53.9% (downgrade minus 1), imprecision (downgrade minus 1) | ⊕⊕⊕⊝ moderate confidence in estimates due to inconsistency of I² = 53.9% (downgrade minus 1) | ||
| Progression‐free survival | Median progression‐free survival over all studies included in the network1: 16.2 months | NMA‐median PFS: 24.9 (16.9 to 36.8) months | NMA‐median PFS: 25.7 (20.8 to 32.4) months | NMA‐median PFS: 28.9 (18.0 to 46.3) months | NMA‐median PFS: 47.6 (27.9 to 81.0) months |
| NMA‐HR: 0.65 (95% CI 0.44 to 0.96) | NMA‐HR: 0.63 (95% CI 0.50 to 0.78) | NMA‐HR: 0.56 (95% CI 0.35 to 0.90) | NMA‐HR: 0.34 (95% CI 0.20 to 0.58) | ||
| ⊕⊕⊝⊝ low confidence in estimates due to high risk of bias (downgrade minus 1) and inconsistency of I² = 55.3% (downgrade minus 1). | ⊕⊕⊝⊝ low confidence in estimates due to high risk of bias (downgrade minus 1) and inconsistency of I² = 55.3% (downgrade minus 1). | ⊕⊕⊝⊝ low confidence in estimates due to high risk of bias (downgrade minus 1) and inconsistency of I²=55.3% (downgrade minus 1). | ⊕⊕⊝⊝ low confidence in estimates due to high risk of bias (downgrade minus 1) and inconsistency of I² = 55.3% (downgrade minus 1). | ||
| Polyneuropathies | Mean risk over all studies included in the network²: 0.9% (10/1074) | NMA‐risk: 0.5% (0.1 to 1.8) | NMA‐risk: 4.0% (1.6 to 10.0) | NMA‐risk: 79.4% (4.8 to 1306.0) | No study reported the amount of participants with grade ≥ 3 polyneuropathies for treatment with VRDc. |
| NMA‐RR: 0.57 (95% CI 0.16 to 1.99) | NMA‐RR: 4.44 (95% C: 1.77 to 11.11) | NMA‐RR: 88.22 (95% CI 5.36 to 1451.11) | |||
| ⊕⊕⊝⊝ low confidence in estimates. Downgrade minus 1 for imprecision and minus 1 for high risk of bias. | ⊕⊕⊕⊝ moderate confidence in estimates. Downgrade minus 1 for high risk of bias. | ⊕⊕⊕⊝ moderate confidence in estimates. Downgrade minus 1 for high risk of bias. | |||
| Serious adverse events | Mean risk over all studies included in the network²: 36.1% (177/490) | Risk not available, because RD is not connected to MP in the network. | Risk not available, because TMP is not connected to MP in the network. | NMA‐risk: 46.2% (38.3 to 55.6) | Risk not available, because VRDc is not connected to MP in the network. |
| NMA‐RR not available, because RD is not connected to MP in the network. | NMA‐RR not available, because TMP is not connected to MP in the network. | NMA‐RR: 1.28 (95% CI 1.06 to 1.54) | NMA‐RR not available, because VRDc is not connected to MP in the network. | ||
| Confidence in estimates can not be assessed, because RD is not connected to MP in the network. | Confidence in estimates can not be assessed, because TMP is not connected to MP in the network. | ⊕⊕⊕⊝ moderate confidence in estimates. Downgrade minus 1 for high risk of bias. | Confidence in estimates can not be assessed, because VRDc is not connected to MP in the network. | ||
| Withdrawals due to adverse events | Mean risk over all studies included in the network²: 9.2% (77/837) | NMA‐risk: 38.5% (19.6 to 75.4) | NMA‐risk: 37.7% (22.1 to 64.5) | NMA‐risk: 9.75% (5.8 to 16.7) | NMA‐risk: 82.1% (35.1 to 191.7) |
| NMA‐RR: 4.18 (95% CI 2.13 to 8.20) | NMA‐RR: 4.10 (95% CI 2.40 to 7.01) | NMA‐RR: 1.06 (95% CI 0.63 to 1.81) | NMA‐RR: 8.92 (95% CI 3.82 to 20.84) | ||
| ⊕⊕⊕⊕ high confidence in estimates. | ⊕⊕⊕⊕ high confidence in estimates. | ⊕⊕⊕⊝ moderate confidence in estimates. Downgrade minus 1 for imprecision. | ⊕⊕⊕⊕ high confidence in estimates. | ||
| Quality of life | One study reported that health‐related QoL scores increased steadily from baseline until completion of cycle 10 | One study reported that global health status of patients improved from baseline over the duration of the study and disease symptoms decreased | One study reported that global health status of patients improved from baseline over the duration of the study and disease symptoms decreased | No study reported the outcome QoL for treatment with VMP. | No study reported the outcome QoL for treatment with VRDc. |
| RD was not compared to MP in this study | TMP was not compared to MP in this study | ||||
| *Basis for the assumed risks: 1: Median OS/PFS over all studies in the network were estimated, calculating the mean of all available MP‐medians (OS and PFS, respectively). 2: mean risk over all studies included in the network was estimated, dividing the total events under MP‐therapy by the total of patients treated with MP. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; MP: Melphalan and Prednisone; RD: Lenalidomide and Dexamethasone; TMP: Thalidomide, Melphalan and Prednisone; VMP: Bortezomib, Melphalan and Prednisone; VRDc: continuous Bortezomib, Lenalidomide and Dexamethasone | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Background
Description of the condition
Multiple myeloma, also known as plasma cell myeloma, is a bone marrow‐based hematological malignancy. Myeloma arises from asymptomatic premalignant monoclonal plasma cells via a multistep process of genetic and microenvironmental changes (Palumbo 2011). In contrast to other hematological malignancies, multiple myeloma is usually preceded by an age‐progressive benign condition called monoclonal gammopathy of undetermined significance (MGUS), which further progresses to smouldering (asymptomatic) myeloma and finally to symptomatic myeloma (Anderson 2011; Kuehl 2012).
Early diagnosis of the condition is complicated by widely varying symptoms. Some patients might be symptom‐free, while others present with common symptoms like fatigue, bone pain (mostly in the back, or hips), bone fractures, symptoms of light chain amyloidosis, or high calcium levels in the blood. Amongst others, the latter might lead to kidney problems, abdominal pain, or extreme thirst (The American Cancer Society 2018). The criteria for myeloma diagnosis have been revised in 2014 by the International Myeloma Working Group. These criteria are: presence of at least 10% clonal plasma cells in the bone marrow or biopsy‐proven or extramedullary plasmacytoma and one (or more) myeloma‐defining events (Table 2) (Rajkumar 2014).
1. Myeloma defining events.
| Myeloma defining events (Rajkumar 2014) | |
| CRAB criteria | |
| Hypercalcaemia | > 2.75 mmol/L (> 11 mg/dL) |
| Renal insufficiency | creatine clearance < 40 mL per min or serum creatine > 177 µmol/L (> 2 mg/dL) |
| Anaemia | haemoglobin value < 100 g/L |
| Bone lesions | 1 or more oseolytic lesions on skeletal radiography, computer tomography, or positron emission tomography‐computer tomography |
| Biomarkers of malignancy (SLiM criteria) | |
| clonal bone marrow plasma cell %‐age | ≧ 60 (Sixty)% |
| Involved to uninvolved serum free Light chain ratio | ≧ 100 |
| focal lesions on Magnetic resonance imaging (MRI) studies | < 1 |
To date, multiple myeloma is still considered incurable and accounts for approximately ten per cent of all hematological malignancies and two per cent of all cancers (Cancer Research UK 2018). Globally, there were 138,509 incident cases in 2016 (Cowan 2018). The incident rate increased worldwide by 126% between 1990 and 2016 and is strongly related to age (Cancer Research UK 2018; Cowan 2018). Based on the latest statistics in the USA, the median age of myeloma diagnosis across all races and both genders is 69 years of age (National Cancer Institute 2018). The prognosis of five‐year survival differs widely between high‐income countries and low‐ and middle‐income countries. In the UK, 47% of people diagnosed with multiple myeloma are predicted to survive for at least five years (32.5% for at least 10 years). The reported five‐year survival for men is 49.8%, and for women 43.8% (Cancer Research UK 2018). Later diagnosis and limited access to specialised health care reduces the survival period. In comparison, a recent study in Nigeria reported a five‐year survival rate of only 7.6% (Nwabuko 2017).
Description of the intervention
First‐line treatment for people with newly diagnosed multiple myeloma
The recommended first‐line standard treatment for people with newly diagnosed multiple myeloma (NDMM) in good clinical condition consists of induction chemotherapy followed by high‐dose chemotherapy with autologous stem cell transplantation (Moreau 2017). Some individuals with myeloma might be unsuitable for transplantation because of co‐morbidities, frailty, or limited financial resources (Anderson 2015; Cowan 2018). The combination of melphalan and prednisone (MP) was the former standard treatment for individuals unsuitable for transplantation. This was modified after the introduction of so‐called "novel agents" in the late 1990s to early 2000s. Since then, the preferred first‐line therapy regimens for transplant‐ineligible individuals, consists of two‐, three‐ or multiple‐drug combinations of novel agents. These agents belong to different drug classes including: proteasome inhibitors, e.g. bortezomib; immunomodulatory drugs, e.g. lenalidomide and thalidomide; and corticosteroids, e.g. dexamethasone and prednisone (Anderson 2015; Kumar 2018; Moreau 2017).
The introduction of immunomodulatory drugs and proteasome inhibitors in the treatment of transplant‐ineligible individuals with NDMM has shown a major improvement in overall and progression‐free survival (OS and PFS, respectively). Adding thalidomide (T) and lenalidomide (R) to the melphalan and prednisone (MP) standard regimen led to an increase of OS (40 months versus 31 months, respectively) (Wijermans 2010), and PFS in 65 to 75 year old adults (median PFS with RMP: (lenalidomide,melphalan and prednisone) 15 months versus median PFS with MP: 12 months) (Palumbo 2012), respectively. Extending the standard regimen with bortezomib (V) likewise increased OS (median OS with MP alone: 43.1 months versus 56.4 months with added bortezomib), resulting in a 31% death‐risk reduction following VMP versus MP (San Miguel 2013). Furthermore, various two‐ and three‐drug combinations of bortezomib, thalidomide, lenalidomide, dexamethasone, and cyclophosphamide are recommended options for the treatment of transplant‐ineligible individuals with NDMM after showing highly satisfying results in OS and PFS (Anderson 2015; Moreau 2017). Selecting the best first‐line myeloma treatment when faced with multiple effective drug regimens is challenging. Evidence‐based, as well as consensus‐based clinical practice guidelines recommend multiple treatment combinations, without stating a clear ranking of the options (Kumar 2018; Moreau 2017).
Fixed and continuous treatments
Nowadays anti‐myeloma therapies are either administered as fixed or continuous therapies. Fixed therapy usually refers to a treatment with a fixed or pre‐specified number of cycles. Continuous therapies in transplant‐ineligible myeloma refers to: a therapy which is administered until progressive disease or emerging intolerances, a therapy where the treatment is given for a prolonged period but is still limited (e.g. until a plateau in response), or a therapy where an initial therapy is followed by maintenance treatment (Ludwig 2017b). The aim of continuous therapies is to prolong PFS and OS through improving the depth of the response and suppression of minimal residual disease (Richardson 2018). In the early days, anti‐myeloma therapies were administered for a fixed duration of cycles, because long‐term therapy with conventional chemotherapy agents led to an accumulating, indefensible toxicity. Introducing immunomodulatory drugs and proteasome inhibitors allowed the exploration of continuous therapies, as toxicities of these agents are less severe (Ludwig 2017b). However, although these agents are less toxic, accumulating toxicities of these drug classes are expected to be important. We therefore wanted to focus on drug‐specific adverse events (polyneuropathy, neutropenia, anaemia, thrombocytopenia, thromboembolism, and infections) in this review.
Immunomodulatory drugs and proteasome inhibitors can reduce bone marrow activity (myelosuppression), which may lead to a decrease in red blood cells (anaemia), white blood cells (neutropenia), and platelets (thrombocytopenia) (Miceli 2008). Myelosuppression, especially severe neutropenia can substantially increase the risk of infections. Thalidomide decreases the neutrophil count during the treatment period (days one to 11), whereas lenalidomide decreases the platelet count. During the rest period (days 12 to 21), blood counts return to baseline. During bortezomib treatment thrombocytopenia is the most common haematological toxicity, occurring in around one third of patients, whereas significant neutropenia and anaemia are uncommon.
Bortezomib‐induced peripheral neuropathy usually occurs within the first five courses of bortezomib administration and shows a significant dose‐limiting toxicity of approximately 30 mg/m². Thereafter, typically it does not appear to increase (Argyriou 2008; Windebank 2008).
Generally, the risk for venous thromboembolism is increased in adults with myeloma. Single agent thalidomide or lenalidomide treatment does not increase this risk further. Hovewer, combining immunomodulatory drugs with steroids substantially increases the risk of venous thromboembolism (Palumbo 2008). Most events occur during the first six months of treatment (Kimpton 2016).
In this context, we decided to divide treatment regimens between fixed and continuous therapies to not only compare the effectiveness of treatment regimens, but also of different treatment durations, and to assess whether toxicity of continuous therapies is tolerable.
How the intervention might work
Proteasome‐inhibitors and immunomodulatory drugs belong to a new generation of anti‐cancer agents that work by targeting the microenvironment of the tumour, including specific cell receptors, proteins and signalling pathways (Bianchi 2015).
Approved proteasome inhibitors for myeloma treatment include the first‐in‐class agent bortezomib, and the second‐generation agents carfilzomib, and ixazomib. The mode of action in the treatment of multiple myeloma is generally similar for all proteasome inhibitors. It is based on the supreme sensitivity of myeloma cells to the inhibition of the 26S proteasome. This is a critical complex of the ubiquitin‐proteasome system and responsible for regulation and degradation of the majority of intracellular proteins. These proteins are involved in cell cycle progression, cell growth, and survival. In multiple myeloma, the ubiquitin‐proteasome system is dysregulated, resulting in increased activity of proteasome 26S. This leads to a reduction in the levels of important proteins, like the tumour suppressor p53 and the inhibitor of the anti‐apoptotic protein nuclear factor‐κB, IκB. The continuous activity of the nuclear factor‐κB transcription pathway enables myeloma cells to proliferate rapidly and drive tumour progression. Inhibition of the 26S proteasome leads to multiple downstream effects, resulting in growth arrest and cell death. As cancer cells have an increased level of proteasome activity in general, the pro‐apoptotic effects of proteasome inhibitors can therefore be directly targeted (Gandolfi 2017; Moreau 2012).
Immunomodulatory drugs are glutamic acid derivatives presenting a wide range of biological activities. Their anti‐myeloma properties consist of their immunomodulatory, anti‐angiogenic, anti‐inflammatory, and anti‐proliferative properties, though their exact mechanism of action remains unclear. The molecular target of immunomodulatory drugs is Cereblon (reviewed in Asatsuma‐Okumura 2019) and their binding to Cereblon leads to reduction of Ikaros zinc finger family proteins (IKZF) 1 and 3 by ubiquintination and degradation. IKFZ1 and 3 are critical for myeloma cell survival and downregulation of IKFZ1 and 3 suppressed myeloma cell lines in vitro (Fink 2015; Lu 2014). Three immunomodulatory drugs are clinically used for the treatment of multiple myeloma: thalidomide and its analogues, lenalidomide and pomalidomide (the latter only used in relapsed or refractory myeloma) (Quach 2010). Despite their similar structure, thalidomide, lenalidomide, and pomalidomide differ in their pharmacological properties (Holstein 2017). The immunomodulatory activities are based on the upregulation of T‐cell (CD4+ and CD8+) and natural killer T‐cell production and downregulation of regulatory T‐cells, leading to an increased proliferation of natural killer cells and raised cytotoxicity (Bianchi 2015; Quach 2010). The T‐cell proliferative effects of lenalidomide are 50 to 2000 times higher than that of thalidomide and the effectiveness of T‐cell IL‐2 and IFNγ production augmentation is 300 to more than 1200 times higher (Quach 2010). Likewise, lenalidomide is more effective in decreasing the production of TNF‐α, IL‐1β, IL‐6, and IL‐12 than thalidomide (Holstein 2017). Increasing natural killer cell proliferation results in enhanced death of myeloma cell lines. In addition to the potentiation of natural killer cell proliferation, lenalidomide (not thalidomide) enhances antibody‐dependent cellular cytotoxicity and natural cytotoxicity of natural killer cells, resulting in induced myeloma cell death (Quach 2010). The cytotoxic capacities of lenalidomide originate from multiple mechanisms, comprising inter alia the inhibition of nuclear factor‐κB, downregulation of C/EBPβ (resulting in a decrease of interferon regulatory factor 4 production), activation of caspases, augmented expression of pro‐apoptotic factors and likewise, deduction of anti‐apoptotic factors, and the interruption of the PI3K/Akt pathway (Holstein 2017).
Combination therapies of proteasome inhibitors, immunomodulatory drugs and corticosteroids result in synergistic or enhanced activity of the anti‐cancer agents on anti‐myeloma properties (Gandolfi 2017). Corticosteroids probably enhance anti‐cancer activity through the downregulation of IL‐6‐induced signalling pathways. Synergistic effects of proteasome inhibitors and immunomodulatory drugs are probably due to the combined effects of overlapping activation of caspase pathways, the activation of the proapoptotic BH‐3 protein BIM, the downregulation of interferon regulatory factor 4, the proto‐oncogene MYC, and the apoptosis regulator MCL1 in myeloma cells, and the inhibition of myeloma cell migration and angiogenesis (Gandolfi 2017).
Why it is important to do this review
Double‐ and triple‐drug combinations of novel agents, such as bortezomib, lenalidomide, and thalidomide, are commonly used in high‐income countries for first‐line treatment of adults with multiple myeloma who are unsuitable for transplantation. However, high prices of these anti‐myeloma medicines are limiting their availability in low‐ and middle‐income countries (LMICs). According to the World Health Organization (WHO), about 150 countries worldwide use the WHO's list of essential medicines (EML) as a guidance for the development of national EMLs and reimbursement lists (IMS Institute for Healthcare Informatics 2015; World Health Organization 2019). However, currently anti‐myeloma medicines are not in the EML. We therefore decided to prepare an application for the '22nd WHO Expert Committee on the Selection and Use of Essential Medicines' for the inclusion of anti‐myeloma medicines into the EML. In several discussions with the respective WHO working group, we decided to focus on bortezomib, lenalidomide, and thalidomide, which belong to the first generation of proteasome‐inhibitors and immunomodulatory drugs and are all approved for first‐line treatment. In HICs, a second‐generation of novel agents (e.g. the monoclonal antibody daratumumab) have already been introduced into treatments, however, they are much more expensive. We have therefore concentrated on bortezomib, lenalidomide and thalidomide only, as they more likely to be affordable and available in LMICs.
As there are few randomised controlled trials (RCTs) that perform direct head‐to‐head comparisons of different drug combinations, a systematic review and network meta‐analysis was needed to evaluate the cumulative clinical evidence for the effectiveness and safety of these combinations and to create a clinically meaningful ranking of the treatments. The aim of our systematic review and network meta‐analysis was to provide a comprehensive overview on the effectiveness and harms of novel agents for non‐transplant first‐line multiple myeloma treatment. The network meta‐analysis allowed a hierarchy of the therapeutic options, in particular, if the benefits of one option compared to another translated into a clinically important difference. This comprehensive overview was necessary for clinical decision making, and has the potential to affect international guidelines, clinical pathways, and decision support systems for treatment strategies.
The results of this network meta‐analysis will be published in the Cochrane Library and presented at national and international expert meetings and conferences (e.g. European Hematology Association). The results of the network meta‐analysis have the potential to influence the design of new RCTs on novel agents for myeloma treatment. As patient‐related outcomes were evaluated, a direct impact on patient care and treatment is expected.
Objectives
To compare the effectiveness of different combinations of one or more novel agents (bortezomib, lenalidomide, thalidomide) as treatment of adults with transplant‐ineligible newly diagnosed multiple myeloma (NDMM) to generate a clinically meaningful treatment ranking according to their efficacy and safety and to inform an application for the inclusion of these medicines into the WHO's list of essential medicines.
Methods
Criteria for considering studies for this review
Types of studies
The protocol for this review was registered with PROSPERO (Piechotta 2018). We included studies if they were individually randomised controlled trials (RCTs). We included both full‐text and abstract publications if sufficient information on study design, characteristics of participants and interventions provided. There was no limitation with respect to the length of follow‐up.
We excluded studies that were cluster‐randomised, non‐randomised, case reports and clinical observations.
Types of participants
We included trials involving adults according to the definition in the studies (usually ≧ 18 years of age), with a newly confirmed diagnosis of multiple myeloma, irrespective of type and stage of cancer and gender. We assumed that participants who fulfil the inclusion criteria were equally eligible to be randomised to any of the interventions we planned to compare.
We excluded trials including adults with relapsed or refractory multiple myeloma.
Types of interventions
We included trials that included participants receiving a combination therapy of selected immunomodulatory drugs and/or proteasome inhibitors (bortezomib (V)*, lenalidomide (R)**, thalidomide (T)) in combination with a glucocorticoid (dexamethasone (D), or prednisone (P)) or a glucocorticoid and alkylating agent (cyclophosphamide (C)), or melphalan (M)) in at least one treatment arm for first‐line treatment of transplant‐ineligible myeloma patients.
* V stands for 'Velcade®', the proprietary name of bortezomib.
** R stands for 'Revlimid®', the proprietary name of lenalidomide.
Nowadays, the recommended treatment for adults with transplant‐ineligible newly diagnosed multiple myeloma (NDMM) is either a double‐ or a triple‐drug combination therapy (Kumar 2018; Moreau 2017), consisting of:
immunomodulatory drug (R or T) in combination with a glucocorticoid (D, P) or glucocorticoid and alkylating agent (CD, CP or MP)
proteasome inhibitor (V) in combination with a glucocorticoid (D, P) or glucocorticoid and alkylating agent (CD, CP or MP)
immunomodulatory drug (R or T) in combination with proteasome inhibitor (V), and a glucocorticoid (D, P) or glucocorticoid and alkylating agent (CD, CP or MP)
Regimens, which include either an immunomodulatory drug or a proteasome inhibitor will be referred to as double‐drug combinations. We will refer to triple‐drug combinations, when a immunomodulatory drug and a proteasome inhibitor is included in the regimen.
Combinations of these interventions at any dose and by any route were compared to each other in a full network. We included trials with any treatment duration and divided treatment regimens between fixed and continuous therapies (Description of the intervention).To increase the amount of potential comparisons and to strengthen the network, we also included studies comparing the described double‐ or triple‐drug combinations to the former standard treatment MP. As the aim of this review was to inform an application for the WHO list of essential medicines, the focus of this review was to compare the effectiveness and safety of V, R, and T. Therefore, if we identified additional new drugs for first‐line treatment of multiple myeloma, these were excluded from the network and this review.
We assumed that any participant that meets the inclusion criteria was, in principle, equally likely to be randomised to any of the eligible interventions. We planned to group interventions by evaluating different drug doses together as one drug of interest, according to the product characteristics.
We excluded trials including participants receiving neither V, R, or T (triple or double combination) in at least one treatment arm, and trials including participants receiving no corticosteroid. We excluded trials evaluating the effectiveness and safety of the interventions of interest for supportive treatment during stem cell transplantation, or maintenance therapy, or both. Medications used to treat myeloma in subsequent lines of therapy might be the same as for first‐line treatment, but to ensure that the assumption that participants within the included trials were similar, we focused on first‐line treatment.
Comparison of direct interest
There are few randomised controlled trials comparing directly the effectiveness and safety of double‐ and triple‐combination therapies of the agents of interest (V, R, T) for first‐line treatment of adults with multiple myeloma. Therefore, there is a high degree of uncertainty about whether their effectiveness is comparable, and if not, which one is more effective or safer or both.
Additional interventions to supplement the analysis
Included trials should be comparable in terms of clinical and methodological criteria to ensure the transitivity assumption has been met (Chaimani 2017). Therefore, we did not include any additional interventions.
Types of outcome measures
We included all trials fitting the inclusion criteria mentioned above, irrespective of reported outcomes. We estimated the relative ranking of the competing interventions according to each of the following outcomes.
Overall survival (OS): we used the longest follow‐up available
Progression‐free survival (PFS): we used the longest follow‐up available
Grade 3 or 4 adverse events (AEs)* (with a special focus on polyneuropathy, neutropenia, anaemia, thrombocytopenia, thromboembolism, and infections): number of participants with at least one event
Serious adverse events (SAEs): number of participants with at least one event
Withdrawals due to AEs: number of participants
-
Quality of life (QoL) measured at certain time points with a validated instrument (e.g. EORTC QLQ‐C30)
short (one to three months)
medium (six to nine months)
long (12 months and longer
*The Grades are referring to the severity of AEs according to the Common Terminology Criteria for Adverse Events (CTCAE): Grade 1 refers to mild AEs; Grade 5 to death related to AEs
Search methods for identification of studies
Electronic searches
We searched the following databases without language restrictions. We searched for all possible comparisons formed by the interventions of interest. Thalidomide was the first of our agents of interest, which was used for anti‐myeloma therapy. Therefore, we started the search in 1998, as thalidomide had been mentioned for the first time for myeloma treatment in 1999 (Singhal 1999).
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2) in the Cochrane Library
MEDLINE Ovid (1946 to 14 February 2019)
Medical subject headings (MeSH) or equivalent and text word terms were used. There were no language restrictions. Searches were tailored to individual databases. The search strategy for CENTRAL is in Appendix 1, the search strategies for MEDLINE in Appendix 2. No member of the author team had access to Embase at the time of the search, so we did not include Embase in our search strategy. We will search Embase in any future updates of this review.
Searching other resources
In addition, we searched the following databases/sources.
-
Study registries (up to 14 February 2019, search criteria in Appendix 3)
EU clinical trials register: https://www.clinicaltrialsregister.eu/ctr‐search/search
World Health Organization: http://apps.who.int/trialsearch/
Clinicaltrials.gov: https://clinicaltrials.gov/
ISRCTN: http://www.isrctn.com/
-
Conference proceedings of annual meetings of the following societies for abstracts, were hand searched for abstracts published since 1 January 2010 up to 14 February 2019. Search criteria in Appendix 4).
American Society of Hematology
American Society of Clinical Oncology
European Hematology Association
MEDLINE Ovid (1946 to 14 February 2019) for systematic reviews (Appendix 2): Additionally to searching MEDLINE for randomised controlled trials, we searched for systematic reviews and meta analyses on multiple myeloma. We screened all records to identify systematic reviews and meta analyses investigating immunotherapies for transplant‐ineligible newly diagnosed multiple myeloma. We screened the included studies reference lists and compared them with the records of our Electronic searches.
We checked reference lists of reviews and retrieved articles for additional studies and we performed citation searches on key articles.
We contacted experts in the field for unpublished and ongoing trials.
We contacted authors where necessary for additional information.
Data collection and analysis
Selection of studies
Two review authors (VP, NS) independently screened the results of the search strategies for eligibility for this review by reading the abstracts using Covidence software (Covidence systematic review software). We coded the abstracts as either 'include' or 'exclude'. In the case of disagreement, or if it was unclear whether we should retrieve the abstract or not, we obtained the full‐text publication for further discussion. Independent review authors eliminated studies that clearly did not satisfy the inclusion criteria, and obtained full‐text copies of the remaining studies. Two review authors read these studies independently to select relevant studies, and in the event of disagreement, a third author adjudicated. We did not anonymise the studies before assessment. We included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (Figure 1) in the full review that shows the status of identified studies (Moher 2009) as recommended in Part 2, Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We included studies in the review irrespective of whether measured outcome data were reported in a ‘usable’ way.
1.
Study flow diagram.
Data extraction and management
Two review authors (VP, BS) extracted data using a standardised data extraction form developed in Covidence (Covidence systematic review software). If the review authors were unable to reach a consensus, we consulted a third review author (NS) for final decision. If required, we contacted the authors of specific studies for supplementary information (Higgins 2011a). We contacted Prof. Jesús San Miguel (contact author of the VISTA trial (San Miguel 2008)) and asked how the primary endpoint time‐to‐progression was defined. The author responded that he is currently travelling, and suggested to search for the definition in the supplemental material of the paper. After agreement has been reached, we entered data into Review Manager (RevMan 2014). We extracted the following information.
-
General information:
author, title, source, publication date, country, language, duplicate publications.
-
Quality assessment:
sequence generation, allocation concealment, blinding (participants, personnel, outcome assessors), incomplete outcome data, selective outcome reporting, other sources of bias.
-
Study characteristics:
trial design, aims, setting and dates, source of participants, inclusion/exclusion criteria, comparability of groups, subgroup analysis, treatment cross‐overs, compliance with assigned treatment, length of follow‐up.
-
Participant characteristics:
newly diagnosed individuals, cytogenetic subtype, additional diagnoses, age, gender, ethnicity, number of participants recruited/allocated/evaluated, participants lost to follow‐up, type of treatment (multi‐agent standard treatment (intensity of regimen, number of cycles)).
-
Interventions:
type, dose and cycles of treatment; duration of follow‐up.
-
Outcomes:
overall survival (OS), progression‐free survival (PFS), grade 3 and 4 adverse events, serious adverse events(SAEs), polyneuropathies, neutropenia, anaemia, thrombocytopenia, thromboembolisms, infections, quality of life, withdrawals due to adverse events.
-
Notes:
sponsorship/funding for trial and notable conflict of interest of review authors.
We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to populate a Characteristics of included studies table in the full review.
Data on potential effect modifiers
We extracted from each included study data on the following.
Intervention and population characteristics that may act as effect modifiers (age, stage of disease, performance score, treatment duration, duration of follow‐up, region)
Year of publication
Assessment of risk of bias in included studies
Two review authors (VP, BS) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), with any disagreements resolved by discussion. In order to rate the certainty of the evidence, risk of bias was assessed per endpoint rather than per study only. We completed a 'Risk of bias' table for each included study using the 'Risk of bias' tool in RevMan (RevMan 2014).
We assessed the following for each study.
Random sequence generation (checking for possible selection bias): we assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). Studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number) were excluded.
Allocation concealment (checking for possible selection bias): the method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). Studies that do not conceal allocation (e.g. open list) were excluded.
Blinding of participants and personnel (checking for possible performance bias). we assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, such as identical tablets matched in appearance or smell, or a double‐dummy technique); unclear risk of bias (study states that it was blinded but did not provide an adequate description of how it was achieved). Studies that were not double‐blinded are considered to have high risk of bias.
-
Blinding of outcome assessment (checking for possible detection bias). we assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study has a clear statement that outcome assessors were unaware of treatment allocation, and ideally describes how this was achieved); unclear risk of bias (study states that outcome assessors were blind to treatment allocation, but lacks a clear statement on how it was achieved). Studies where outcome assessment were not blinded were considered as having a high risk of bias. We assessed blinding of outcome assessment in three separate outcome‐categories:
not dependent on outcome assessor: OS;
partly dependent on outcome assessor: PFS;
dependent on outcome assessor: safety outcomes.
Selective reporting (checking for reporting bias): we assessed whether primary and secondary outcome measures were pre‐specified and whether these were consistent with those reported: low risk of bias ( study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that were of interest in the review have been reported in the pre‐specified way, or the study protocol was not available but it was clear that the published reports include all expected outcomes, including those that were pre‐specified); unclear risk of bias (insufficient information to permit judgement of ‘low risk’ or ‘high risk'); high risk of bias (not all of the study’s pre‐specified primary outcomes have been reported or one or more primary outcomes were reported using measurements, analysis methods or subsets of the data that were not pre‐specified or one or more reported primary outcomes were not pre‐specified or one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis, or the study report fails to include results for a key outcome that would be expected to have been reported for such a study).
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data): we assessed the methods used to deal with incomplete data in two separate outcome categories:
time to event data: low risk (censored Kaplan‐Meier curves were provided and study discontinuations were described and balanced between arms); unclear risk (neither Kaplan‐Meier curves, nor flow‐charts were accessible); high risk (‘last observation carried forward’ analysis);
safety data: low risk (safety data was only reported for patients, who received at least one study drug); high risk (intention‐to‐treat population was used to report safety data, however it was stated that participants changed to the other study arm or stopped the study before they received the first dose); unclear risk (it was not described, which population was used to report safety outcomes.
Measures of treatment effect
Relative treatment effect
We used intention‐to‐treat data. For binary outcomes, we extracted number of patients and number of events per arm and calculated risk ratios (RRs) with 95% confidence intervals (CIs) for each trial. For time‐to‐event outcomes, we extracted hazard ratios (HRs) from published data according to Parmar 1998 and Tierney 2007. We checked whether the study authors had checked the proportional hazards assumption. For quality of life (QoL) data, we had planned to calculate continuous outcomes as mean differences (MDs) when assessed with the same instruments; otherwise we had planned to calculate standardised mean differences (SMDs) with 95% CIs. As reporting of QoL data was scarce, network meta‐analysis was not possible and MDs and SMDs were not calculated. We will do as described in an update of this review.
Relative treatment ranking
We obtained a treatment hierarchy using P scores (Rücker 2015). P scores allow ranking treatments on a continuous zero to one scale (higher P score is indicating the better treatment) in a frequentist network meta‐analysis.
Unit of analysis issues
Studies with multiple treatment groups
As recommended in Chapter 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), for studies with multiple treatment groups we combined arms as long as they could be regarded as subtypes of the same intervention.
When arms could not be pooled this way, we included multi‐arm trials using an network meta‐analysis approach that accounted for the within‐study correlation between the effect sizes by re‐weighting all comparisons of each multi‐arm study (Rücker 2012; Rücker 2014). For pairwise meta‐analysis, we treated multi‐arm studies as multiple independent comparisons and did not combine these data in any analysis.
Dealing with missing data
As suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), we took the following steps to deal with missing data.
Whenever possible, we contacted the original investigators to request relevant missing data. We contacted Prof. Jesús San Miguel (contact author of the VISTA trial (San Miguel 2008)) and asked how the primary endpoint time‐to‐progression was defined. The author responded that he is currently travelling, and suggested to search for the definition in the supplemental material of the paper. There we could also find data on PFS. If the number of participants evaluated for a given outcome was not reported, we used the number of participants randomised per treatment arm as the denominator. If only percentages but no absolute number of events were reported for binary outcomes, we calculated numerators using percentages. If estimates for mean and standard deviations were missing, we calculated these statistics from reported data whenever possible, using approaches described in Chapter 7.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If data were not reported numerically but graphically, we estimated missing data from figures. We addressed in the Discussion section the potential impact of missing data on findings of the review.
Assessment of heterogeneity
Assessment of clinical and methodological heterogeneity within treatment comparisons
To evaluate the presence of clinical heterogeneity, we generated summary statistics for the important clinical and methodological characteristics across all included studies. Within each pairwise comparison, we assessed the presence of clinical heterogeneity by visually inspecting the similarity of these characteristics.
Assessment of transitivity across treatment comparisons
To ensure that the assumption of transitivity was justified, we assessed whether the characteristics of included studies were similar; for example inclusion criteria, study period, dosing, route of administration, co‐medication. Furthermore, we assessed whether the patient characteristics were similar across treatment comparisons; for example age, gender, stage of disease, Eastern Cooperative Oncology Group (ECOG) performance score.
Assessment of statistical heterogeneity and inconsistency
To evaluate the presence of heterogeneity and inconsistency in the entire network, we gave the generalised heterogeneity statistic Qtotal and the generalised I2 statistic, as described in Schwarzer 2015. We used the decomp.design command in the R package netmeta (R 2018; Rücker 2018) for decomposition of the heterogeneity statistic into a Q statistic for assessing the heterogeneity between studies with the same design and a Q statistic for assessing the designs inconsistency to identify the amount of heterogeneity/inconsistency within as well as between designs.
To evaluate the presence of inconsistency locally, we compared direct and indirect treatment estimates of each treatment comparison. This can serve as a check for consistency of a network meta‐analysis (Dias 2010). For this purpose, we used the netsplit command in the R package netmeta, which enables the splitting of the network evidence into direct and indirect contributions (R 2018; Rücker 2018). For each treatment comparison, we presented direct and indirect treatment estimates plus the network estimate using forest plots. In addition, for each comparison we gave the z‐value and P value of test for disagreement (direct versus indirect). It should be noted that in a network of evidence there may be many loops and with multiple testing there is an increased likelihood to find an inconsistent loop by chance. Therefore, we were cautious deriving conclusions from this approach.
If we found substantive heterogeneity and/or inconsistency, we explored possible sources by performing pre‐specified sensitivity and subgroup analyses (see below). In addition, we reviewed the evidence base, reconsidered inclusion criteria as well as discussed the potential role of unmeasured effect modifiers to identify further sources.
Assessment of reporting biases
In pairwise comparisons with at least 10 trials, we examined the presence of small‐study effects graphically by generating funnel plots. As we had no such comparison, we were unable to assess small‐study effects. In future updates, we will use linear regression tests (Egger 1997) to test for funnel plot asymmetry. A P value less than 0.1 will be considered significant for this test (Sterne 2011). We will examine the presence of small‐study effects for the OS only.
Moreover, we searched study registries, to identify completed, but not published trials.
Data synthesis
Methods for direct treatment comparisons
Pairwise comparisons were part of the network meta‐analysis, thus we did not plan to perform pairwise meta‐analysis in addition. In order to outline available direct evidence, we provided forest plots for pairwise comparisons, without giving an overall estimate. Only when data could not combined in a network meta‐analysis, e.g. due to inconsistency, did we performed pairwise meta‐analyses according to recommendations provided in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We used random‐effects models. We used the R package meta (R 2018; Schwarzer 2018) for statistical analyses. When trials were clinically too heterogenous to be combined, we performed only subgroup analyses without calculating an overall estimate.
Methods for indirect and mixed comparisons
Where we considered the data to be sufficiently similar to be combined, we performed a network meta‐analysis using the frequentist weighted least squared approach described by Rücker 2012. We used a random‐effects model, taking into account the correlated treatment effects in multi‐arm studies. We assumed a common estimate for the heterogeneity variance across the different comparisons. To evaluate the extent to which treatments were connected, we gave a network plot for all outcomes. For each comparison, we gave the estimated treatment effect along with its 95% CI. We graphically presented the results using forest plots, with melphalan/prednisone as reference. We used the R package netmeta (R 2018; Rücker 2018) for statistical analyses.
GRADE
Quality of the evidence
Two review authors independently rated the certainty of the evidence for pre‐selected outcomes (OS, PFS, SAEs, withdrawals due to AEs, polyneuropathies, QoL) and comparisons (RD, TMP, VMP, and VRDc, respectively compared to MP), respectively. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system to rank the certainty of the evidence using the GRADEprofiler Guideline Development Tool software (McMaster 2015), and the guidelines provided in Chapter 12.2 of the CochraneHandbook for Systematic Reviews of Interventions (Schünemann 2011a) and specifically for network meta‐analyses (Puhan 2014).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence.
High = we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate = we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low = our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low = we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The GRADE system uses the following criteria for assigning a certainty level to a body of evidence (Chapter 12, Schünemann 2011a).
High: randomised trials; or double‐upgraded observational studies.
Moderate: downgraded randomised trials; or upgraded observational studies.
Low: double‐downgraded randomised trials; or observational studies.
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
We decreased grade if:
serious (‐1) or very serious (‐ 2) limitation to study quality;
important inconsistency (‐ 1);
some (‐1) or major (‐ 2) uncertainty about directness;
imprecise or sparse data (‐ 1);
high probability of reporting bias (‐ 1).
'Summary of findings' table
We included a 'summary of findings' table to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the certainty of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes OS, PFS, SAEs, withdrawals due to AEs, polyneuropathies and QoL for the treatment combinations of RD, TMP, VMP, and VRDc, respectively compared to MP.
To obtain the median survival times (OS and PFS, respectively) for each of our selected comparisons, we applied the network meta‐analysis hazard ratio (NMA‐HR) to the assumed median survival times of our comparator melphalan and prednisone as recommended in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019) and by Tierney 2007, e.g.:
median OS for patients treated with intervention x = median survival for patients treated with comparator/ corresponding NMA‐HR (intervention x versus comparator).
Subgroup analysis and investigation of heterogeneity
We considered performing subgroup analyses using the following characteristics.
Follow‐up (short‐term (< 5 years) versus long‐term >= 5 years)
Multiple myeloma international staging system (I, II, III)
Age (<75 versus >75)
Region (low‐ and middle‐income versus high‐income)
Subgroup analysis could not be performed due to the characteristics of included studies. The detailed reasons for incomplete subgroup analysis have been outlined in the Differences between protocol and review section of this review.
Sensitivity analysis
To test the robustness of the results, we conducted fixed‐effect pairwise and network meta‐analyses. We reported the estimates of the fixed‐effect only if they showed a difference to the random‐effects model. We had planned to explore the influence of quality components with regard to low and high risk of bias.
Risk of bias sensitivity analysis could not be performed for each outcome due to overall high risk of included studies. The detailed reasons for incomplete sensitivity analysis have been outlined in the 'Differences between protocol and review' section of this review.
Results
Results
Description of studies
Results of the search
The primary electronic searches performed in June 2018 and February 2019 yielded a total of 9416 potentially relevant references related to the treatment of multiple myeloma. Of these, we identified 1706 as duplicates and excluded 7509 at the initial stage of screening because they did not fulfil our predefined inclusion criteria. The remaining 209 publications were retrieved as full‐text publications or abstract publications for detailed evaluation, out of which 61 (33 studies) were excluded. Reasons for exclusion included: regimen included novel agents which were excluded for our review; comparator not of interest; induction regimen not of interest and/or followed by transplantation; transplant‐setting; prior therapy or relapsed/refractory myeloma; not randomised controlled trial ( RCT); and sequential versus alternating regimens (no evaluation of individual drug combination possible). Five additional records were identified through the screening of reference lists of relevant studies. So finally, 25 studies (148 references) with 11,403 patients were formally included in this review. One study did not report any of our pre‐specified outcomes and could therefore not be included in the main analyses of this review. Overall, we could include 24 studies with 11,337 patients in the main analysis.
The overall number of trials screened, identified, selected, excluded and included are documented in a PRISMA flow diagram (Figure 1).
Included studies
A total of 25 trials met our pre‐specified eligibility criteria. The trials included a total of 11,403 randomised participants from Europe, Asia, North‐ and South America, and the Pacific region, with a median age ranging from 52 to 78.5 years. The first patient enrolment started in August 2001 (Ludwig 2009), and two trials are still ongoing (Magarotto 2016; Pawlyn 2017). Twenty trials were published as full texts, four trials were only published as an abstract (Katsuoka 2013; Kim 2007; Mookerjee 2017; Pawlyn 2017), and one trial has only been published as a letter to the editor (Jacobus 2016). Individual patient data of six trials comparing thalidomide plus melphalan plus prednisone (TMP) versus melphalan plus prednisone (MP) has also been meta‐analysed (Fayers 2011; Palumbo 2013). Presuming higher comparability between the single studies, we extracted all available data from this meta‐analysis as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The characteristics of the included studies are summarised in the table Characteristics of included studies.
Design
All included trials were RCTs. The majority of trials were open‐label, however two studies (Palumbo 2012; Waage 2010) were double‐blind and placebo‐controlled. Of the 25 included trials, 20 were two‐armed RCTs; and five were three‐armed RCTs (Bahlis 2017; Hungria 2016; Magarotto 2016; Niesvizky 2015; Palumbo 2012). The duration of treatment varied in each study based on differences in the duration and number of treatment cycles and the possibility of continuous treatment or maintenance treatment.
In seven trials, participants received a previously defined amount of treatment cycles (Beksac 2011; Facon 2007; Hulin 2009; Hungria 2016; Jacobus 2016; Sacchi 2011; San Miguel 2008).
In seven trials, all participants received maintenance treatment (Durie 2017;Ludwig 2009; Magarotto 2016; Mateos 2014; Niesvizky 2015; Stewart 2015; Zweegman 2016).
-
In five trials, only participants in one arm of the study received maintenance treatment:
in the trial of Palumbo 2012, one arm received lenalidomide maintenance treatment, two arms received placebo maintenance;
in two trials one arm received maintenance treatment and one arm did not; whereas both arms received initial treatment until a plateau in response was reached (Waage 2010; Wijermans 2010);
In two trials the experimental arm received maintenance treatment and the control arm did not (Palumbo 2006; Palumbo 2014).
In the trial of Bahlis 2017, one arm received continuous treatment, and two arms received a fixed amount of treatment cycles.
In two trials, participants were randomised between maintenance and observation regardless of previous therapy (Morgan 2011; Pawlyn 2017).
Three trials did not provide any information regarding maintenance treatment (Katsuoka 2013; Kim 2007; Mookerjee 2017).
Sample sizes
The smallest trial had a sample size of 18 participants (Katsuoka 2013), while the largest trial had a sample size of 1852 participants (Pawlyn 2017).
Location
The majority of trials were multi‐centre trials and included participants from Europe, Asia, North‐ and South America, Australia, and the Pacific region. Seventeem trials included participants from European Countries (Bahlis 2017; Beksac 2011; Facon 2007; Hulin 2009; Ludwig 2009; Magarotto 2016; Mateos 2014; Morgan 2011; Palumbo 2006; Palumbo 2012; Palumbo 2014; Pawlyn 2017; Sacchi 2011; San Miguel 2008; Waage 2010; Wijermans 2010; Zweegman 2016), six trials from northern America (Bahlis 2017; Durie 2017; Jacobus 2016; Niesvizky 2015; San Miguel 2008; Stewart 2015), and Asia (Bahlis 2017; Katsuoka 2013; Kim 2007; Palumbo 2012; San Miguel 2008; Stewart 2015), respectively; two trials from southern America (Hungria 2016; San Miguel 2008), and one trial from Australia (Palumbo 2012) and the Pacific region (Bahlis 2017), respectively.
Participants
All trials included adults (at least 18 years old) with newly diagnosed multiple myeloma (NDMM) with measurable disease according to either the Durie‐Salmon staging system or International Staging System (ISS). Participants were either ineligible for transplant because they were too old (more than 65 years old) or because of significant co‐morbidities or because stem cell transplantation was not planned. Women of childbearing age had to agree to use adequate contraception.
Interventions
In addition to the differentiation between drug combinations, treatment regimens were differentiated into fixed therapy (first‐line therapy was stopped after a pre‐specified amount of therapy cycles) and continuous therapy (first‐line treatment followed by maintenance therapy, continuous first‐line therapy, or continuous first‐line therapy until a plateau phase (response) was reached). Accordingly, the included participants were randomised to a total of 21 different treatment regimens.
Melphalan and prednisone (MP)
Continuous melphalan and prednisone (MPc)
Lenalidomide plus cyclophosphamide plus dexamethasone (RCD)
Continuous lenalidomide plus cyclophosphamide plus prednisone (RCPc)
Lenalidomide and dexamethasone (RD)
Continuous lenalidomide and dexamethasone (RDc)
Lenalidomide plus melphalan plus prednisone (RMP)
Continuous lenalidomide plus melphalan plus prednisone (RMPc)
Thalidomide plus cyclophosphamide plus dexamethasone (TCD)
Continuous thalidomide and dexamethasone (TDc)
Thalidomide plus melphalan plus prednisone (TMP)
Continuous thalidomide plus melphalan plus prednisone (TMPc)
Bortezomib and dexamethasone (VD)
Continuous bortezomib and dexamethasone (VDc)
Bortezomib plus melphalan plus prednisone (VMP)
Continuous bortezomib plus melphalan plus prednisone (VMPc)
Bortezomib plus lenalidomide plus dexamethasone (VRD)
Continuous bortezomib plus lenalidomide plus dexamethasone (VRDc)
Continuous bortezomib plus thalidomide plus dexamethasone (VTDc)
Continuous bortezomib plus thalidomide plus melphalan plus prednisone (VTMPc)
Continuous bortezomib plus thalidomide plus prednisone (VTPc)
Outcome measures
Survival outcomes
Out of the 25 trials, included in this review, 24 reported overall survival (OS) (Bahlis 2017; Beksac 2011; Durie 2017; Facon 2007; Hulin 2009; Hungria 2016; Jacobus 2016; Kim 2007; Ludwig 2009; Magarotto 2016; Mateos 2014; Mookerjee 2017; Morgan 2011; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Pawlyn 2017; Sacchi 2011; San Miguel 2008; Stewart 2015; Waage 2010; Wijermans 2010; Zweegman 2016) and progression‐free survival (PFS) (Bahlis 2017; Beksac 2011; Durie 2017; Facon 2007; Hulin 2009; Hungria 2016; Jacobus 2016; Kim 2007; Ludwig 2009; Magarotto 2016; Mateos 2014; Mookerjee 2017; Morgan 2011; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Pawlyn 2017; Sacchi 2011; San Miguel 2008; Stewart 2015; Waage 2010; Wijermans 2010; Zweegman 2016).
The Cox proportional hazards model was used by all study authors to calculate hazard ratios (HRs) of survival outcomes. However, it is not clear whether all of the trials checked the assumption of proportional hazards.
Safety outcomes
Grade 3 or 4 adverse events were reported in nine studies (Durie 2017; Katsuoka 2013; Ludwig 2009; Magarotto 2016; Niesvizky 2015; Palumbo 2006; Stewart 2015; Wijermans 2010; Zweegman 2016). Reporting of individual grade 3 and 4 adverse events (AEs) was more frequent: polyneuropathy was reported in 18 trials (Bahlis 2017; Beksac 2011; Facon 2007; Hulin 2009; Hungria 2016; Katsuoka 2013; Magarotto 2016; Mateos 2014; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Pawlyn 2017; Sacchi 2011; San Miguel 2008; Stewart 2015; Waage 2010; Zweegman 2016); infections in 15 trials (Bahlis 2017; Beksac 2011; Durie 2017; Facon 2007; Magarotto 2016; Mateos 2014; Morgan 2011; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Sacchi 2011; Waage 2010; Wijermans 2010; Zweegman 2016); thromboembolism in 14 trials (Facon 2007; Hulin 2009; Hungria 2016; Mateos 2014; Mookerjee 2017; Morgan 2011; Palumbo 2006; Palumbo 2014; Sacchi 2011; San Miguel 2008; Stewart 2015; Waage 2010; Wijermans 2010; Zweegman 2016); neutropenia in 14 trials (Bahlis 2017; Facon 2007; Hulin 2009; Hungria 2016; Magarotto 2016; Mateos 2014; Niesvizky 2015; Palumbo 2006; Palumbo 2014; Pawlyn 2017; Sacchi 2011; San Miguel 2008; Stewart 2015; Zweegman 2016); anaemia in 14 trials (Bahlis 2017; Facon 2007; Hungria 2016; Ludwig 2009; Magarotto 2016; Mateos 2014; Mookerjee 2017; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; San Miguel 2008; Stewart 2015; Zweegman 2016); and thrombocytopenia in 12 trials (Bahlis 2017; Facon 2007; Hungria 2016; Ludwig 2009; Magarotto 2016; Mateos 2014; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; San Miguel 2008; Zweegman 2016). Serious adverse event (SAE) data were available in eight studies (Bahlis 2017; Durie 2017; Jacobus 2016; Mateos 2014; Niesvizky 2015; Palumbo 2012; San Miguel 2008; Stewart 2015). The outcome “withdrawals due to adverse events” was defined in retrospect and reported in 16 studies (Bahlis 2017; Durie 2017; Hulin 2009; Hungria 2016; Ludwig 2009; Magarotto 2016; Mateos 2014; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Sacchi 2011; San Miguel 2008; Waage 2010; Wijermans 2010; Zweegman 2016).
Quality of Life
Quality of life (QoL) was assessed and reported in four trials (Bahlis 2017; Palumbo 2012; Waage 2010; Wijermans 2010).
See Characteristics of included studies for further details.
Excluded studies
After the screening of abstracts we excluded 7509 references that obviously did not match the inclusion criteria.
In the full text stage, 33 studies, comprising 26 references, were excluded after detailed evaluation due to the following main reasons.
Twelve trials included a novel agent in the treatment regimen, which was excluded for our review (Facon 2017; Facon 2018; Ludwig 2017a; Mateos 2018; NCT01850524; NCT01863550; NCT02586038; NCT03710603; Palumbo 2016; San Miguel 2014; Takezako 2017; Usmani 2014)
Eight trials included interventions not of interest (Anonymous 2003; Facon 2006; Hernández 2004; Niesvizky 2003; Rajkumar 2006b; Rajkumar 2008; Merz 2015; Zonder 2010)
Three trials, the induction regimens were not of interest or followed by transplantation or both (Hejlova 2000; NCT00522392; Morgan 2002)
Four studies were conducted in a transplant setting (Brioli 2014; Kumar 2012; NCT00734877; Harousseau 2003)
Three trials, participants received a prior therapy or were relapsed/refractory (Barlogie 2004; Foa 2007; Offidani 2004)
Two trials were non‐randomised (Montefusco 2013; White 2007)
One trial reported sequential versus alternating regimens; no evaluation of individual drug combinations was possible ( Mateos 2016)
These publications we described under Characteristics of excluded studies.
Risk of bias in included studies
The summary of the methodological quality of the included studies for all assessed domains across included studies and per included study are presented in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In total, 14 of the 25 included studies (56%) reported a central randomisation process and were therefore judged at low risk of bias (Bahlis 2017; Beksac 2011; Durie 2017; Hulin 2009; Ludwig 2009; Magarotto 2016; Mateos 2014; Morgan 2011; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Sacchi 2011; Stewart 2015; Waage 2010). The remaining 11 studies (44%) did not provide enough information to assess sequence generation and allocation concealment and risk of selection bias was therefore judged to be unclear (Facon 2007; Hungria 2016; Jacobus 2016; Katsuoka 2013; Kim 2007; Mookerjee 2017; Palumbo 2014; Pawlyn 2017; San Miguel 2008; Wijermans 2010; Zweegman 2016).
Blinding
As, in general, RCTs in oncology are performed as open‐label trials, we assumed participants and personnel were not blinded unless blinding was explicitly stated. Two of the 25 included trials were (12%) double‐blind trials and were therefore judged to be at low risk of bias (Palumbo 2012; Waage 2010). The remaining 23 studies (88%), were either stated as, or assumed to be open‐label trials and were therefore judged to be at high risk of bias.
Blinding of outcome assessment was assessed in three outcome categories: overall survival (OS), progression‐free survival (PFS), and safety outcomes. The outcome OS is not dependent on the outcome assessor as participants are either alive or dead at the time of OS assessment. Therefore the risk of bias in this category was judged to be low for all studies.
Progression‐free survival (PFS) and safety outcomes can be dependent on the outcome assessor. Studies were presumed not to be blinded for outcome assessment, if not otherwise specified. As mentioned above, blinding was described in two of 25 studies (12%) and risk of bias was therefore judged as low (Palumbo 2012; Waage 2010). Twenty‐three of the remaining studies were judged to be at high risk of bias .
Incomplete outcome data
Missing outcome data were assessed in two categories: time to event data, and safety outcomes. Study discontinuation was reported in 22 of 25 studies (88%) and balanced between arms. Furthermore, Kaplan‐Meier curves were provided. Risk of bias for incomplete survival data was judged to be at low risk for these studies. Three studies (12%) did not provide Kaplan‐Meier curves or study‐flow‐charts (Katsuoka 2013; Kim 2007; Mookerjee 2017). As information was insufficient, risk of bias for incomplete survival data was judged to be unclear for these studies.
Safety outcomes were judged to be at low risk of bias, if all reported participants received at least one assigned study drug. This was described in twenty‐two of the included studies. For three of the included studies (Katsuoka 2013; Kim 2007; Mookerjee 2017), only an abstract was available. As it was not described in the abstract if all reported patients received at least one assigned study drug, the risk of bias was judged to be unclear.
Selective reporting
In total, 16 of 25 included (64%) studies reported all primary and secondary outcomes as pre‐specified in their protocol (Bahlis 2017; Durie 2017; Facon 2007; Hulin 2009; Ludwig 2009; Magarotto 2016; Morgan 2011; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Sacchi 2011; Stewart 2015; Waage 2010; Wijermans 2010; Zweegman 2016). In these studies, risk of bias for selective reporting was judged as low.
However, primary and secondary outcomes were not reported as pre‐specified in three studies and risk of bias for selective reporting was judged to be high. Pre‐specified primary and secondary outcomes have been altered in one study (Beksac 2011). Response rate was supposed to be evaluated after 12 months, but was reported after four six‐week cycles. Furthermore, time to progression was supposed to be evaluated, but disease‐free survival was reported instead. One study conducted an unplanned interim analysis (Hungria 2016). As interim results suggested poorer outcomes in one arm compared to the other two arms, only descriptive results were further reported. Furthermore, more than 10% of data were missing, without explanations in one arm. One study did not report PFS, overall response rate, and quality of life, although pre‐specified (San Miguel 2008). Risk of bias for selective reporting was unclear in five studies, four of which were only reported as abstracts and the other as a letter to the editor.
For five of the included studies, no protocol was available and full‐text publications are still pending (Jacobus 2016; Katsuoka 2013; Kim 2007; Mookerjee 2017; Pawlyn 2017). Therefore, potential reporting bias could not be assessed and was judged to be unclear. The remaining study did not report duration of response, although previously specified (Mateos 2014). As response rate and complete response have been reported as pre‐specified, risk of bias was judged to be unclear.
Other potential sources of bias
Other bias was detected in two of 25 studies (8%) and judged to be high. One study closed enrolment prematurely due to slow accrual (Jacobus 2016), one study conducted an unplanned interim analysis (Hungria 2016) and further altered analysis of results. In the 23 remaining studies no other bias was assumed and therefore risk judged to be low.
Effects of interventions
See: Table 1
Overall survival (OS)
Overall survival was reported in 24 studies (19 two‐arm studies, five three‐arm studies), and analysis was conducted with 22 studies. Two studies (Kim 2007; Mookerjee 2017) and two direct comparisons of a three‐arm study (Hungria 2016) were excluded because the hazard ratio (HR) was not reported and could not be estimated. Briefly, Kim 2007 did not find a significant difference in OS betweenTD (thalidomide and dexamethasone) and TCD (thalidomide plus cyclophosphamide plus dexamethasone). Mookerjee 2017 reported a median OS of 30.2 months (95% CI 28.2 to 32.2) for patients treated with VRD (bortezomib plus lenalidomide plus dexamethasone) and a median OS of 28.6 months (95% confidence interval (CI) 26 to 31.3) for patients treated with RD (lenalidomide plus dexamethasone). Hungria 2016 only reported Kaplan‐Meier curves and a corresponding HR for the comparison of TMP (thalidomide plus melphalan plus prednisone) versus TCD. The HR for the comparisons TMP versus TD and TCD versus TD could not be estimated. Median OS for patients treated with TMP was 42 months; for patients treated with TCD 32.4 months and for patients treated with TD 54.6 months, respectively.
All 21 treatment regimens and a total of 11,071 participants could be included in the network meta‐analysis (NMA). However, the network was not fully connected and consists of three subnetworks comprising 30 pairwise comparisons (Figure 3). We performed NMA for OS‐subnetworks 1 and 2 (OS‐subnetwork 3 consisted of only two treatment regimens). Results for all network comparisons, including the ranking of treatments are shown in Table 3, and Figure 4, per OS‐subnetwork. Moderate heterogeneity (I² = 53.9%) was observed between studies in OS‐subnetwork 1. Continuous VRDc (hazard ratio (HR) 0.49, 95% CI 0.26 to 0.92); VTMPc (HR 0.49, 95% CI 0.26 to 0.93); fixed treatment withRD (HR 0.63, 95% CI 0.40 to 0.99); and TMP (HR 0.75, 95% CI 0.58 to 0.97) showed a clinically meaningful improvement of OS compared to MP, respectively. According to OS, VRDc and VTMPc were the best treatment options in OS‐subnetwork 1 (P score 0.90 and 0.89, respectively), whilst TDc was worst (P score 0.07).
3.

Network graph for outcome overall survival. Any two treatments are connected by a line when there is at least one study comparing the two treatments. Line width: number of patients
2. Results of NMA for the outcome OS.
|
League tables for the outcome overall survival. Direct and network estimates with 95% CIs are given. Treatments are ordered by P score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². OS‐ Subnetwork 1 No. of studies: 19. No. of treatments: 15. No. of pairwise comparisons: 25. No. of designs: 14 Qtotal= 17.36, df = 8, P = 0.027 / Qwithin= 7.54, df = 5, P = 0.18 / Qbetween= 12.35, df = 3, P = 0.006; I² = 53.9%, Tau² = 0.0381 Treatment Effects: | ||||||||||||||
| VRDc | . | . | 0.71 (0.44, 1.15) | . | . | . | . | . | . | . | . | . | . | . |
| 1.01 (0.41, 2.48) | VTMPc | . | . | 0.70 (0.43, 1.13) | . | . | . | . | . | . | . | . | . | . |
| 0.79 (0.42, 1.48) | 0.78 (0.35, 1.71) | RD | 0.98 (0.65, 1.49) | . | . | 0.77 (0.51, 1.16) | . | . | . | . | . | . | . | . |
| 0.71 (0.44, 1.15) | 0.70 (0.33, 1.50) | 0.90 (0.60, 1.35) | RDc | . | 1.07 (0.70, 1.63) | 0.78 (0.52, 1.18) | 0.98 (0.59, 1.64) | . | . | . | . | . | . | . |
| 0.71 (0.33, 1.51) | 0.70 (0.43, 1.13) | 0.90 (0.48, 1.69) | 1.00 (0.55, 1.79) | VMP | . | . | . | . | . | . | 0.70 (0.45, 1.07) | . | . | . |
| 0.67 (0.35, 1.26) | 0.66 (0.29, 1.50) | 0.85 (0.49, 1.48) | 0.94 (0.63, 1.41) | 0.94 (0.48, 1.84) | RCPc | . | 1.16 (0.69, 1.95) | . | . | . | . | . | . | . |
| 0.65 (0.36, 1.20) | 0.65 (0.32, 1.30) | 0.83 (0.56, 1.24) | 0.92 (0.64, 1.32) | 0.93 (0.56, 1.53) | 0.98 (0.59, 1.62) | TMP | . | . | . | 1.08 (0.49, 2.40) | 0.66 (0.49, 0.88) | . | . | . |
| 0.59 (0.31, 1.11) | 0.59 (0.28, 1.23) | 0.75 (0.45, 1.25) | 0.84 (0.56, 1.25) | 0.84 (0.48, 1.47) | 0.89 (0.55, 1.42) | 0.91 (0.61, 1.35) | RMPc | 0.95 (0.67, 1.33) | . | . | 0.95 (0.54, 1.67) | . | 0.79 (0.45, 1.38) | . |
| 0.53 (0.27, 1.03) | 0.52 (0.25, 1.10) | 0.67 (0.39, 1.16) | 0.75 (0.47, 1.18) | 0.75 (0.42, 1.33) | 0.79 (0.46, 1.35) | 0.81 (0.53, 1.24) | 0.89 (0.66, 1.21) | TMPc | 0.92 (0.66, 1.28) | . | 1.06 (0.64, 1.75) | . | . | . |
| 0.49 (0.23, 1.03) | 0.48 (0.21, 1.09) | 0.62 (0.33, 1.17) | 0.68 (0.39, 1.21) | 0.69 (0.35, 1.33) | 0.73 (0.39, 1.37) | 0.74 (0.43, 1.28) | 0.82 (0.52, 1.29) | 0.92 (0.66, 1.28) | MPc | . | . | . | . | 0.65 (0.38, 1.11) |
| 0.49 (0.24, 1.00) | 0.48 (0.23, 1.02) | 0.62 (0.35, 1.10) | 0.69 (0.40, 1.17) | 0.69 (0.39, 1.22) | 0.73 (0.39, 1.36) | 0.74 (0.48, 1.14) | 0.82 (0.49, 1.37) | 0.92 (0.54, 1.56) | 1.00 (0.54, 1.87) | TCD | 1.12 (0.73, 1.72) | 0.92 (0.61, 1.39) | . | . |
| 0.49 (0.26, 0.92) | 0.49 (0.26, 0.93) | 0.63 (0.40, 0.99) | 0.69 (0.47, 1.03) | 0.70 (0.45, 1.07) | 0.74 (0.44, 1.23) | 0.75 (0.58, 0.97) | 0.83 (0.58, 1.19) | 0.93 (0.64, 1.35) | 1.01 (0.61, 1.67) | 1.01 (0.69, 1.48) | MP | . | 0.82 (0.47, 1.44) | . |
| 0.45 (0.20, 1.02) | 0.44 (0.19, 1.04) | 0.57 (0.28, 1.15) | 0.63 (0.32, 1.23) | 0.63 (0.31, 1.28) | 0.67 (0.32, 1.42) | 0.68 (0.38, 1.24) | 0.75 (0.39, 1.46) | 0.85 (0.43, 1.65) | 0.92 (0.44, 1.95) | 0.92 (0.61, 1.39) | 0.91 (0.52, 1.60) | RCD | . | . |
| 0.43 (0.20, 0.94) | 0.43 (0.19, 0.98) | 0.55 (0.29, 1.07) | 0.61 (0.34, 1.12) | 0.62 (0.31, 1.21) | 0.65 (0.34, 1.27) | 0.67 (0.38, 1.16) | 0.73 (0.44, 1.22) | 0.82 (0.47, 1.44) | 0.90 (0.47, 1.72) | 0.89 (0.47, 1.69) | 0.89 (0.53, 1.48) | 0.97 (0.46, 2.08) | RMP | . |
| 0.31 (0.12, 0.79) | 0.31 (0.12, 0.83) | 0.40 (0.17, 0.92) | 0.44 (0.20, 0.97) | 0.44 (0.19, 1.04) | 0.47 (0.20, 1.08) | 0.48 (0.22, 1.03) | 0.53 (0.26, 1.07) | 0.59 (0.31, 1.12) | 0.65 (0.38, 1.11) | 0.64 (0.28, 1.47) | 0.64 (0.31, 1.33) | 0.70 (0.28, 1.76) | 0.72 (0.31, 1.68) | TDc |
|
OS‐ Subnetwork 2 No. of studies: 2. No. of treatments: 4. No. of pairwise comparisons: 4. No. of designs: 2 Qtotal=0, df = 0, p = n.a./ Qwithin= 0, df = 0, p = n.a./ Qbetween=0, df = 0, p = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | ||||||||||||||
| VMPc | 0.92 (0.65, 1.29) | 0.89 (0.64, 1.25) | 0.67 (0.49, 0.91) | |||||||||||
| 0.92 (0.65, 1.29) | VTDc | 0.98 (0.70, 1.38) | . | |||||||||||
| 0.89 (0.64, 1.25) | 0.98 (0.69, 1.38) | VDc | . | |||||||||||
| 0.67 (0.49, 0.91) | 0.73 (0.46, 1.16) | 0.75 (0.47, 1.18) | VTPc | |||||||||||
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
4.

Forest plot for the outcome OS. (a) OS‐ Subnetwork 1. Reference treatment: MP. (b) OS‐ Subnetwork 2. Reference treatment: VMPc. Treatments are ordered by P‐Score (descending)
Results of OS‐subnetwork 2 suggest a survival advantage for VMPc compared to VTPc (HR 0.67, 95% CI 0.49 to 0.9). No other treatment combinations within OS‐subnetwork 2 were favoured over another (Table 3; Figure 4).
Only one study was included in OS‐subnetwork 3. Jacobus 2016 reported a median OS of 69.22 months (95% CI 59.70 to not estimable (NE)) for participants treated with VRD and 60.22 months (95% CI 37.06 to NE) for participants treated with VD (HR 0.77 (95% CI 0.35 to 1.70).
We rated the certainty of the evidence for OS according to the GRADE system for RD, TMP, bortezomib plus melphalan plus prednisone (VMP), and VRDc. Moderate‐certainty evidence indicates that the use of RD, TMP, and VRDc for first‐line treatment of multiple myeloma patients probably results in a large increase in OS. Low‐certainty evidence indicates that the use of VMP as initial myeloma therapy may result in a large increase in OS (Table 1).
The test for disagreement showed statistically significant disagreement between direct and indirect estimates in closed loops for RCPc‐RDc‐RMPc (P = 0.0148) and MP‐TMP (P = 0.0453) (Figure 5). The only closed loop in OS‐subnetwork 2 consists of a multi‐arm study, so there is no indirect evidence.
5.

Local approach to check inconsistency ‐ comparison of direct and indirect estimate for closed loops in OS‐Subnetwork 1
Progression‐free survival (PFS)
Twenty‐four studies (19 two‐arm studies, five three‐arm studies) reported the outcome PFS, of which 22 studies were included in the analysis. We had to exclude two studies (Kim 2007; Mookerjee 2017) and two direct comparisons of a three‐arm study (Hungria 2016) from the analysis, as no HRs were reported and there were insufficient data to estimate the missing value. Briefly, Kim 2007 did report a PFS of 8.6 (± 1.2) months for patients treated with TD and 19.4 (± 4.8) months for patients treated with TCD (P < 0.05). Mookerjee 2017 reported a median PFS of 27.8 months (95% CI 25.4 to 30.2) for patients treated with VRD and a median PFS of 28 months (95% CI 24.6 to 31.4) for patients treated with RD. Hungria 2016 only reported Kaplan‐Meier curves and a corresponding HR for the comparison of TMP versus TCD. The HR for the comparisons TMP versus TD and TCD versus TD could not be estimated. Median OS for patients treated with TMP was 24.1 months; for patients treated with TCD 25.9 months and for patients treated with TD 21.5 months, respectively.
All 21 treatment regimens and a total of 11,071 participants could be included in NMA. The network was not fully connected and consists of three subnetworks comprising 31 pairwise comparisons (figure 6 in Appendix 5). NMA was conducted for PFS‐subnetworks 1 and 2 (subnetwork 3 consisted of only two treatment regimens). Results, per PFS‐subnetwork, are shown in Table 4 for all network comparisons. Between‐study heterogeneity was moderate (I² = 55.3%) in PFS‐subnetwork 1. In general, continuous treatment regimens were superior to fixed therapy with MP, and nine out of 13 compared bortezomib, lenalidomide, or thalidomide combinations showed a substantial improvement of PFS compared to MP, respectively (figure 7 in Appendix 5). VRDc and VTMPc achieve best effects on PFS outcomes in PFS‐subnetwork 1 (P score: 0.92 and 0.92, respectively). The treatments with the smallest effects on PFS in this subnetwork are RCD (P score: 0.08), TCD (P score: 0.09) and MP (P score 0.13).
3. Results of NMA for the outcome PFS.
|
League tables for the outcome progression‐free survival. Direct and network estimates with 95% CIs are given. Treatments are ordered by P score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². PFS‐ Subnetwork 1 No. of studies: 19. No. of treatments: 15. No. of pairwise comparisons: 25. No. of designs: 14 Qtotal=17.89, df=8, P = 0.022 / Qwithin= 6.06, df = 5, P = 0.30 / Qbetween=11.80, df = 3, P = 0.008; I² = 55.3%, Tau² = 0.0269 Treatment Effects: | |||||||||||||||||
| VRDc | . | . | 0.71 (0.48, 1.06) | . | . | . | . | . | . | . | . | . | . | . | |||
| 1.06 (0.48, 2.36) | VTMPc | . | . | . | . | 0.58 (0.40, 0.85) | . | . | . | . | . | . | . | . | |||
| 0.77 (0.46, 1.29) | 0.73 (0.37, 1.44) | RMPc | 0.80 (0.54, 1.20) | 1.00 (0.76, 1.32) | 0.80 (0.53, 1.19) | . | . | . | . | . | 0.49 (0.31, 0.80) | 0.40 (0.25, 0.64) | . | . | |||
| 0.71 (0.48, 1.06) | 0.67 (0.34, 1.34) | 0.92 (0.67, 1.27) | RDc | . | 1.00 (0.71, 1.40) | . | . | 0.69 (0.48, 0.98) | 0.70 (0.49, 1.00) | . | . | . | . | . | |||
| 0.70 (0.40, 1.20) | 0.66 (0.33, 1.30) | 0.90 (0.70, 1.15) | 0.98 (0.67, 1.42) | TMPc | . | . | 0.84 (0.63, 1.11) | . | . | . | . | 0.62 (0.41, 0.93) | . | . | |||
| 0.67 (0.40, 1.13) | 0.64 (0.30, 1.33) | 0.87 (0.60, 1.26) | 0.95 (0.68, 1.32) | 0.97 (0.63, 1.49) | RCPc | . | . | . | . | . | . | . | . | . | |||
| 0.61 (0.30, 1.24) | 0.58 (0.40, 0.85) | 0.79 (0.45, 1.39) | 0.86 (0.48, 1.54) | 0.88 (0.50, 1.55) | 0.91 (0.49, 1.71) | VMP | . | . | . | . | . | 0.56 (0.35, 0.90) | . | . | |||
| 0.58 (0.31, 1.08) | 0.55 (0.26, 1.15) | 0.75 (0.52, 1.10) | 0.82 (0.51, 1.31) | 0.84 (0.63, 1.11) | 0.87 (0.52, 1.45) | 0.95 (0.51, 1.78) | MPc | . | . | 0.77 (0.49, 1.21) | . | . | . | . | |||
| 0.55 (0.33, 0.90) | 0.52 (0.27, 0.99) | 0.71 (0.51, 0.98) | 0.77 (0.57, 1.04) | 0.79 (0.55, 1.12) | 0.81 (0.54, 1.22) | 0.89 (0.53, 1.50) | 0.94 (0.60, 1.47) | TMP | 1.01 (0.71, 1.43) | . | . | 0.56 (0.44, 0.72) | 0.89 (0.44, 1.78) | . | |||
| 0.53 (0.31, 0.89) | 0.50 (0.24, 1.02) | 0.68 (0.45, 1.04) | 0.74 (0.52, 1.04) | 0.76 (0.48, 1.18) | 0.78 (0.49, 1.23) | 0.86 (0.46, 1.58) | 0.90 (0.53, 1.53) | 0.96 (0.68, 1.35) | RD | . | . | . | . | . | |||
| 0.45 (0.21, 0.96) | 0.42 (0.18, 1.00) | 0.58 (0.32, 1.04) | 0.63 (0.33, 1.21) | 0.64 (0.38, 1.10) | 0.67 (0.34, 1.32) | 0.73 (0.34, 1.58) | 0.77 (0.49, 1.21) | 0.82 (0.43, 1.55) | 0.85 (0.43, 1.71) | TDc | . | . | . | . | |||
| 0.41 (0.22, 0.77) | 0.39 (0.19, 0.80) | 0.53 (0.35, 0.81) | 0.57 (0.35, 0.94) | 0.59 (0.37, 0.93) | 0.61 (0.35, 1.04) | 0.67 (0.36, 1.25) | 0.70 (0.41, 1.20) | 0.75 (0.48, 1.18) | 0.78 (0.45, 1.34) | 0.91 (0.45, 1.84) | RMP | 0.80 (0.51, 1.24) | . | . | |||
| 0.34 (0.20, 0.58) | 0.32 (0.18, 0.59) | 0.44 (0.33, 0.60) | 0.48 (0.35, 0.67) | 0.49 (0.36, 0.67) | 0.51 (0.34, 0.77) | 0.56 (0.35, 0.90) | 0.59 (0.39, 0.89) | 0.63 (0.50, 0.78) | 0.65 (0.44, 0.96) | 0.76 (0.41, 1.41) | 0.84 (0.56, 1.26) | MP | 0.82 (0.57, 1.17) | . | |||
| 0.32 (0.17, 0.57) | 0.30 (0.15, 0.59) | 0.41 (0.27, 0.63) | 0.44 (0.28, 0.69) | 0.45 (0.29, 0.70) | 0.47 (0.28, 0.78) | 0.51 (0.29, 0.91) | 0.54 (0.32, 0.91) | 0.58 (0.40, 0.83) | 0.60 (0.37, 0.98) | 0.70 (0.35, 1.40) | 0.77 (0.46, 1.30) | 0.92 (0.67, 1.27) | TCD | 0.96 (0.68, 1.35) | |||
| 0.30 (0.15, 0.60) | 0.29 (0.13, 0.62) | 0.39 (0.23, 0.68) | 0.43 (0.24, 0.74) | 0.44 (0.25, 0.76) | 0.45 (0.24, 0.83) | 0.49 (0.25, 0.96) | 0.52 (0.28, 0.97) | 0.55 (0.34, 0.91) | 0.58 (0.32, 1.04) | 0.68 (0.31, 1.46) | 0.74 (0.40, 1.38) | 0.88 (0.55, 1.41) | 0.96 (0.68, 1.35) | RCD | |||
|
PFS‐ Subnetwork 2 No. of studies: 2. No. of treatments: 4. No. of pairwise comparisons: 4. No. of designs: 2 Qtotal= 0, df = 0, P = n.a./ Qwithin= 0, d f= 0, P = n.a./ Qbetween= 0, df=0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | |||||||||||||||||
| VTPc | . | 0.83 (0.61, 1.15) | . | ||||||||||||||
| 0.87 (0.56, 1.34) | VTDc | 0.96 (0.72, 1.28) | 0.89 (0.67, 1.19) | ||||||||||||||
| 0.83 (0.61, 1.15) | 0.96 (0.72, 1.28) | VMPc | 0.93 (0.71, 1.23) | ||||||||||||||
| 0.78 (0.51, 1.19) | 0.89 (0.67, 1.19) | 0.93 (0.71, 1.23) | VDc | ||||||||||||||
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
Evidence of PFS‐subnetwork 2 suggests no advantages for any treatment combinations within this subnetwork (Table 4; figure 7 in Appendix 5).
Only one study was included in PFS‐subnetwork 3. Jacobus 2016 reported a median PFS of 20 months (95% CI 11 to 43) for participants treated with VRD and 18 months (95% CI 9 to 37) for participants treated with VD (HR 0.96, 95% CI 0.53 to 1.75).
We rated the certainty of the evidence for PFS according to the GRADE system for RD, TMP, VMP, and VRDc. We rated the evidence for all of these comparisons as low certainty, meaning that the use of RD, TMP, VMP, and VRDc for first‐line treatment of MM patients may result in a large increase in PFS (Table 1).
The test for disagreement showed almost statistically significant disagreement between direct and indirect estimates in closed loops for MP‐TMP (P = 0.0594) (figure 8 in Appendix 5). The only closed loop in PFS‐subnetwork 2 consists of a multi‐arm study, so there is no indirect evidence.
Adverse events
Adverse events (AEs) were not consistently reported across studies. To be able to meta‐analyse results, we could only consider AEs when the number of participants with at least one event of at least grade 3 was reported. We could not consider cumulated events, subgrouping by degrees of severity, or other subgroups.
Adverse events grade 3 and 4
Adverse events grade 3 and 4 were reported in nine studies (Durie 2017; Katsuoka 2013; Ludwig 2009; Magarotto 2016; Niesvizky 2015; Palumbo 2006; Stewart 2015; Wijermans 2010; Zweegman 2016) (seven two‐arm studies, two three‐arm studies), for 13 treatment regimens and a total of 3318 participants. However, the included studies did not fulfil the similarity assumption and are therefore not comparable in NMA. Decisive effect modifiers to withdraw the assumption for similarity were different supportive therapies and various reporting styles of grade 3 and 4 AEs (e.g. different length of treatment, AEs occurring in > 5% or > 10% of the patients, differentiation between non‐haematological and haematological AEs). Pairwise comparisons are illustrated in (figure 9 in Appendix 5), per trial.
Polyneuropathy
Polyneuropathies were reported in 18 studies (Bahlis 2017; Beksac 2011; Facon 2007; Hulin 2009; Hungria 2016; Katsuoka 2013; Magarotto 2016; Mateos 2014; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Pawlyn 2017; Sacchi 2011; San Miguel 2008; Stewart 2015; Waage 2010; Zweegman 2016) (13 two‐arm studies, five three‐arm studies), for 19 treatment regimens and a total of 8978 patients. However, the network was not fully connected and consists of two subnetworks comprising 28 pairwise comparisons (figure 10 in Appendix 5).
We performed NMA for both polyneuropathy‐subnetworks. Results for all network comparisons, including the ranking of treatments are shown in Table 5, per polyneuropathy‐subnetwork. Low heterogeneity (I² = 3.4 %) was observed between studies for polyneuropathy‐subnetwork 1.
4. Results of NMA for the outcome polyneuropathy.
|
League tables for the outcome polyneuropathy. Direct and network estimates with 95% CIs are given. Treatments are ordered by P score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². Polyneuropathy‐subnetwork 1 No. of studies: 16. No. of treatments: 15. No. of pairwise comparisons: 24. No. of designs: 12 Qtotal= 6.21, df = 6, P = 0.40/ Qwithin= 4.10, df = 4, p=0.39/ Qbetween= 2.11, df = 2, P = 0.35; I² = 3.4%, Tau² = 0.0248 Treatment Effects: | |||||||||||||||||
| RCD | . | . | 0.38 (0.23, 0.62) | . | . | . | . | . | . | . | . | . | . | . | |||
| 0.50 ( 0.05, 5.07) | RD | 0.99 (0.31, 3.16) | . | . | . | . | . | . | . | 0.12 (0.05, 0.30) | . | . | . | . | |||
| 0.39 ( 0.04, 3.85) | 0.78 ( 0.26, 2.40) | RDc | . | . | 0.86 (0.26, 2.90) | . | . | 0.83 (0.25, 2.79) | . | 0.12 (0.05, 0.30) | . | . | . | . | |||
| 0.38 ( 0.23, 0.62) | 0.77 ( 0.08, 7.38) | 0.98 ( 0.10, 9.13) | TCD | . | . | . | . | . | 0.19 (0.02, 1.71) | 0.17 (0.02, 1.34) | . | . | . | . | |||
| 0.28 ( 0.03, 2.90) | 0.57 ( 0.16, 1.99) | 0.72 ( 0.26, 2.04) | 0.74 ( 0.08, 7.20) | MP | . | . | 0.66 (0.23, 1.90) | 0.53 (0.19, 1.48) | . | 0.33 (0.11, 0.96) | 0.05 (0.00, 0.83) | 0.01 (0.00, 0.19) | . | . | |||
| 0.26 ( 0.02, 3.16) | 0.52 ( 0.11, 2.38) | 0.66 ( 0.21, 2.09) | 0.68 ( 0.06, 7.85) | 0.91 ( 0.25, 3.34) | RCPc | . | . | 0.96 (0.30, 3.05) | . | . | . | . | . | . | |||
| 0.25 ( 0.01, 6.67) | 0.51 ( 0.04, 6.88) | 0.65 ( 0.06, 7.55) | 0.67 ( 0.03, 16.79) | 0.90 ( 0.08, 9.76) | 0.98 ( 0.08, 12.13) | MPc | . | . | . | . | 0.10 (0.01, 0.80) | . | . | . | |||
| 0.22 ( 0.02, 2.63) | 0.45 ( 0.10, 1.98) | 0.57 ( 0.17, 1.96) | 0.58 ( 0.05, 6.54) | 0.79 ( 0.29, 2.12) | 0.86 ( 0.22, 3.43) | 0.87 ( 0.08, 9.79) | RMP | 0.81 (0.33, 2.00) | . | . | . | . | . | . | |||
| 0.20 ( 0.02, 2.14) | 0.40 ( 0.10, 1.50) | 0.51 ( 0.19, 1.36) | 0.52 ( 0.05, 5.31) | 0.70 ( 0.30, 1.64) | 0.77 ( 0.25, 2.33) | 0.78 ( 0.08, 7.45) | 0.89 ( 0.37, 2.15) | RMPc | . | . | 0.14 (0.05, 0.36) | . | . | . | |||
| 0.07 ( 0.01, 0.69) | 0.14 ( 0.03, 0.69) | 0.18 ( 0.04, 0.85) | 0.19 ( 0.02, 1.71) | 0.25 ( 0.05, 1.24) | 0.28 ( 0.04, 1.74) | 0.28 ( 0.02, 4.58) | 0.32 ( 0.05, 1.93) | 0.36 ( 0.07, 1.91) | TD | 0.89 (0.24, 3.25) | . | . | . | . | |||
| 0.06 ( 0.01, 0.54) | 0.13 ( 0.05, 0.31) | 0.16 ( 0.07, 0.37) | 0.17 ( 0.02, 1.34) | 0.23 ( 0.09, 0.56) | 0.25 ( 0.07, 0.90) | 0.25 ( 0.02, 2.95) | 0.29 ( 0.08, 0.98) | 0.32 ( 0.11, 0.91) | 0.89 ( 0.24, 3.25) | TMP | . | . | . | . | |||
| 0.03 ( 0.00, 0.32) | 0.05 ( 0.01, 0.25) | 0.07 ( 0.02, 0.24) | 0.07 ( 0.01, 0.80) | 0.09 ( 0.03, 0.30) | 0.10 ( 0.02, 0.41) | 0.10 ( 0.01, 0.80) | 0.11 ( 0.03, 0.40) | 0.13 ( 0.05, 0.32) | 0.36 ( 0.06, 2.31) | 0.40 ( 0.10, 1.54) | TMPc | . | . | . | |||
| 0.00 ( 0.00, 0.12) | 0.01 ( 0.00, 0.14) | 0.01 ( 0.00, 0.16) | 0.01 ( 0.00, 0.31) | 0.01 ( 0.00, 0.19) | 0.01 ( 0.00, 0.27) | 0.01 ( 0.00, 0.50) | 0.01 ( 0.00, 0.28) | 0.02 ( 0.00, 0.30) | 0.04 ( 0.00, 1.12) | 0.05 ( 0.00, 0.96) | 0.13 ( 0.01, 2.62) | VMP | 0.48 (0.23, 0.97) | 0.20 (0.01, 3.70) | |||
| 0.00 ( 0.00, 0.06) | 0.00 ( 0.00, 0.07) | 0.00 ( 0.00, 0.08) | 0.00 ( 0.00, 0.16) | 0.01 ( 0.00, 0.10) | 0.01 ( 0.00, 0.14) | 0.01 ( 0.00, 0.25) | 0.01 ( 0.00, 0.15) | 0.01 ( 0.00, 0.16) | 0.02 ( 0.00, 0.57) | 0.02 ( 0.00, 0.50) | 0.06 ( 0.00, 1.35) | 0.48 ( 0.23, 0.97) | VTMPc | . | |||
| 0.00 ( 0.00, 0.07) | 0.00 ( 0.00, 0.09) | 0.00 ( 0.00, 0.11) | 0.00 ( 0.00, 0.17) | 0.00 ( 0.00, 0.13) | 0.00 ( 0.00, 0.17) | 0.00 ( 0.00, 0.28) | 0.00 ( 0.00, 0.19) | 0.00 ( 0.00, 0.20) | 0.01 ( 0.00, 0.69) | 0.01 ( 0.00, 0.64) | 0.03 ( 0.00, 1.69) | 0.20 ( 0.01, 3.70) | 0.42 ( 0.02, 8.45) | VD | |||
|
Polyneuropathy‐subnetwork 2 No. of studies: 2. No. of treatments: 4. No. of pairwise comparisons: 4. No. of designs: 2 Qtotal= 0, df =n0, P = n.a./ Qwithin=0, df = 0,P = n.a./ Qbetween= 0, df = 0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | |||||||||||||||||
| VMPc | 0.88 (0.57, 1.33) | 0.75 (0.33, 1.72) | 0.72 (0.48, 1.08) | ||||||||||||||
| 0.88 (0.57, 1.33) | VDc | . | 0.82 (0.56, 1.21) | ||||||||||||||
| 0.75 (0.33, 1.72) | 0.86 (0.34, 2.17) | VTPc | . | ||||||||||||||
| 0.72 (0.48, 1.08) | 0.82 (0.56, 1.21) | 0.96 (0.38, 2.42) | VTDc | ||||||||||||||
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
The risk ratio (RR) for polyneuropathies is highest for patients receiving bortezomib‐based therapies (RR 88.22, 95% CI 5.36 to 1451.110 to (RR 441.08, 95% CI 7.74 to 25,145.52) compared to MP) (figure 11 in Appendix 5). Furthermore, the RR for polyneuropathy appears to be smaller for patients receiving lenalidomide‐based therapies, compared to patients receiving thalidomide‐based therapies (Table 5). Polyneuropathies are less prevalent in fixed and continuous treatment with lenalidomide plus melphalan plus prednisone (RMP) compared to fixed and continuous treatment with thalidomide plus melphalan plus prednisone (TMP) (RR 0.11, 95% CI 0.03 to 0.40) to (RR 0.32, 95% CI 0.11 to 0.91) ,and less prevalent in fixed and continuous treatment with RD compared to TD (RR 0.14, 95% CI 0.03 to 0.69) to (RR 0.18, 95% CI 0.04 to 0.85). According to polyneuropathies, RCD (P score 0.91), RD (P score 0.83), and RDc (P score 0.78) are the treatments within subnetwork 1 with the smallest incidence whereas, VD (P score 0.04), VTMPc (P score 0.06), and VMP (P score 0.14) are the treatments with the highest incidence.
Evidence of polyneuropathy‐subnetwork 2 suggests no advantages for any treatment combinations within this subnetwork (Table 5; (figure 11 in Appendix 5).
We rated the certainty of the evidence for polyneuropathies according to the GRADE system for RD, TMP, and VMP. VRDc was not included in NMA as no study reported the amount of participants with grade ≥ 3 polyneuropathies. Therefore we did not have an estimate for which we could rate the certainty. Low‐certainty evidence suggests that participants treated with RD may have a smaller risk for polyneuropathies, compared to participants treated with MP. However, the CIs are also compatible with no difference or an increase in neuropathies. Moderate‐certainty evidence suggests treatment with TMP and VMP probably results in a large increase in polyneuropathies, respectively compared to MP.
The test for disagreement showed no statistically significant difference between direct and indirect estimates (figure 12 in Appendix 5). The only closed loop in subnetwork 2 consists of a multi‐arm study, so there is no indirect evidence.
Neutropenia
A total of 14 studies (Bahlis 2017; Facon 2007; Hulin 2009; Hungria 2016; Magarotto 2016; Mateos 2014; Niesvizky 2015; Palumbo 2006; Palumbo 2014; Pawlyn 2017; Sacchi 2011; San Miguel 2008; Stewart 2015; Zweegman 2016) (10 two‐arm studies, four three‐arm studies) reported neutropenia. These studies comprised 16 treatment regimens (22 pairwise comparisons), a total of 8046 participants and were connected to two subnetworks (figure 13 in Appendix 5).
For both neutropenia‐subnetworks NMA could be performed. Table 6 shows the results for all network comparisons, including the ranking of treatments, per neutropenia‐subnetwork. Moderate heterogeneity (I² = 40 %) was observed between studies for neutropenia‐subnetwork 1.
5. Results of NMA for the outcome neutropenia.
|
League tables for the outcome neutropenia. Direct and network estimates with 95% CIs are given. Treatments are ordered by P score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². Neutropenia‐subnetwork 1 No. of studies: 12. No. of treatments: 12. No. of pairwise comparisons: 18. No. of designs: 9 Qtotal= 6.67, df = 4, P = 0.15/ Qwithin= 4.53, df = 3, P = 0.21/ Qbetween= 2.14, df = 1, P = 0.14; I² = 40%, Tau² = 0.0465 Treatment Effects: |
||||||||||||||
| TD | . | . | . | 0.30 (0.04, 2.37) | . | . | . | . | . | 0.16 (0.02, 1.20) | . | |||
| 0.31 (0.04, 2.39) | MP | 0.95 (0.60, 1.51) | . | . | . | 1.07 (0.53, 2.15) | . | . | . | 0.48 (0.33, 0.72) | . | |||
| 0.29 (0.04, 2.39) | 0.95 (0.60, 1.51) | VMP | . | . | . | . | . | 0.73 (0.45, 1.20) | . | . | . | |||
| 0.29 (0.04, 2.29) | 0.94 (0.54, 1.65) | 0.99 (0.48, 2.05) | RD | . | 0.87 (0.55, 1.38) | . | . | . | . | 0.58 (0.37, 0.91) | . | |||
| 0.30 (0.04, 2.37) | 0.96 (0.34, 2.68) | 1.01 (0.32, 3.12) | 1.01 (0.35, 2.93) | TCD | . | . | . | . | 0.63 (0.40, 0.98) | 0.55 (0.21, 1.43) | . | |||
| 0.27 (0.03, 2.08) | 0.86 (0.52, 1.44) | 0.91 (0.46, 1.81) | 0.91 (0.58, 1.44) | 0.90 (0.32, 2.58) | RDc | . | 0.85 (0.49, 1.49) | . | . | 0.67 (0.42, 1.05) | 0.35 (0.21, 0.58) | |||
| 0.26 (0.03, 2.11) | 0.84 (0.48, 1.46) | 0.88 (0.43, 1.82) | 0.89 (0.46, 1.72) | 0.88 (0.29, 2.70) | 0.97 (0.56, 1.69) | TMPc | . | . | . | . | 0.45 (0.29, 0.72) | |||
| 0.25 (0.03, 2.04) | 0.80 (0.40, 1.58) | 0.84 (0.37, 1.92) | 0.85 (0.43, 1.68) | 0.84 (0.26, 2.66) | 0.93 (0.54, 1.59) | 0.95 (0.51, 1.77) | RCPc | . | . | . | 0.41 (0.25, 0.67) | |||
| 0.22 (0.03, 1.85) | 0.69 (0.35, 1.36) | 0.73 (0.45, 1.20) | 0.73 (0.31, 1.77) | 0.73 (0.21, 2.49) | 0.80 (0.34, 1.87) | 0.83 (0.34, 1.98) | 0.87 (0.33, 2.27) | VTMPc | . | . | . | |||
| 0.19 (0.02, 1.56) | 0.60 (0.19, 1.85) | 0.63 (0.19, 2.13) | 0.64 (0.20, 2.01) | 0.63 (0.40, 0.98) | 0.70 (0.22, 2.18) | 0.71 (0.21, 2.39) | 0.75 (0.22, 2.60) | 0.86 (0.23, 3.21) | RCD | . | . | |||
| 0.16 (0.02, 1.20) | 0.52 (0.36, 0.75) | 0.55 (0.30, 0.99) | 0.55 (0.35, 0.86) | 0.55 (0.21, 1.43) | 0.60 (0.40, 0.91) | 0.62 (0.35, 1.10) | 0.65 (0.34, 1.24) | 0.75 (0.35, 1.62) | 0.87 (0.30, 2.51) | TMP | . | |||
| 0.11 (0.01, 0.85) | 0.34 (0.19, 0.61) | 0.36 (0.17, 0.75) | 0.36 (0.20, 0.67) | 0.36 (0.12, 1.09) | 0.40 (0.25, 0.63) | 0.41 (0.27, 0.62) | 0.43 (0.26, 0.70) | 0.49 (0.20, 1.20) | 0.57 (0.17, 1.89) | 0.66 (0.38, 1.14) | RMPc | |||
|
Neutropenia‐subnetwork 2 No. of studies: 2. No. of treatments: 4. No. of pairwise comparisons: 4. No. of designs: 2 Qtotal= 0, df = 0, P = n.a./ Qwithin= 0, df = 0,P = n.a./ Qbetween= 0, df = 0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | ||||||||||||||
| VDc | 0.72 (0.16, 3.16) | . | 0.10 (0.03, 0.31) | |||||||||||
| 0.72 (0.16, 3.16) | VTDc | . | 0.13 (0.05, 0.37) | |||||||||||
| 0.17 (0.05, 0.57) | 0.23 (0.08, 0.70) | VTPc | 0.57 (0.39, 0.84) | |||||||||||
| 0.10 (0.03, 0.31) | 0.13 (0.05, 0.37) | 0.57 (0.39, 0.84) | VMPc | |||||||||||
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
For neutropenia, the RR appears to be similar for medications and treatment durations (figure 14 in Appendix 5). Seven out of 11 compared treatment options have substantially less neutropenia than RMPc (RR 0.11, 95% CI 0.01 to 0.85) to (RR 0.43, 95% CI 0.26 to 0.70), out of which four also have substantially less neutropenia than TMP (RR 0.52, 95% CI 0.36 to 0.75) to (RR 0.60, (95% CI 0.40 to 0.91) (Table 6). Within neutropenia‐subnetwork 1, TD (P‐score: 0.91) is the treatment with the least neutropenia‐incidence; incidence is highest for RMPc (P‐score: 0.03).
Within neutropenia‐subnetwork 2, evidence suggests the highest risk of neutropenia for patients treated with VMPc (P score: 0.00) and the smallest risk of neutropenia for patients treated with VDc (P score: 0.89) (Table 6; figure 14 in Appendix 5).
There is no statistically significant difference between direct and indirect estimate (figure 15 in Appendix 5). The only closed loop in neutropenia‐subnetwork 2 consists of a multi‐arm study, so there is no indirect evidence.
Anaemia
Anaemia was reported in 14 studies (Bahlis 2017; Facon 2007; Hungria 2016; Ludwig 2009; Magarotto 2016; Mateos 2014; Mookerjee 2017; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; San Miguel 2008; Stewart 2015; Zweegman 2016), (nine two‐arm studies, five three‐arm studies), for 19 treatment regimens and a total of 6713 participants. The network was not fully connected, resulting in three subnetworks, comprising 24 pairwise comparisons (figure 16 in Appendix 5).
We performed NMA for anaemia‐subnetworks 1 and 2 (anaemia‐subnetwork 3 consisted of only two treatment regimens). Results, per anaemia‐subnetwork and for all network comparisons, including the ranking of treatments are shown in Table 7. Between‐study heterogeneity (I²= 59 %) was moderate for anaemia‐subnetwork 1.
6. Results of NMA for the outcome anaemia.
|
League tables for the outcome anaemia. Direct and network estimates with 95% CIs are given. Treatments are ordered by P score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². Anaemia‐subnetwork 1 No. of studies: 11. No. of treatments: 13. No. of pairwise comparisons: 19. No. of designs: 10 Qtotal=7.31, df = 3, P = 0.063/ Qwithin=5.38, df = 1, P = 0.020/ Qbetween= 1.93, df = 2, P = 0.38; I² = 59%, Tau² = 0.1287 Treatment Effects: |
||||||||||||||||
| VRD | 0.31 (0.01, 8.11) | . | . | . | . | . | . | . | . | . | . | . | ||||
| 0.31 (0.01, 8.11) | RD | . | 0.84 (0.40, 1.78) | . | 0.84 (0.40, 1.77) | . | . | . | . | . | . | . | ||||
| 0.29 (0.01, 9.54) | 0.94 (0.27, 3.24) | VMP | . | 0.99 (0.41, 2.38) | . | . | . | 0.67 (0.31, 1.43) | . | . | . | . | ||||
| 0.28 (0.01, 7.90) | 0.90 (0.43, 1.87) | 0.96 (0.31, 3.01) | RDc | . | 1.00 (0.47, 2.10) | 0.67 (0.23, 1.96) | . | . | . | . | . | 0.28 (0.10, 0.76) | ||||
| 0.29 (0.01, 10.51) | 0.93 (0.20, 4.24) | 0.99 (0.41, 2.38) | 1.03 (0.24, 4.34) | VTMPc | . | . | . | . | . | . | . | . | ||||
| 0.24 (0.01, 6.86) | 0.78 (0.38, 1.62) | 0.83 (0.28, 2.45) | 0.87 (0.44, 1.70) | 0.84 (0.21, 3.39) | TMP | . | . | 0.98 (0.40, 2.41) | 0.33 (0.03, 3.39) | 0.28 (0.02, 3.21) | . | . | ||||
| 0.22 (0.01, 7.07) | 0.71 (0.21, 2.33) | 0.75 (0.20, 2.78) | 0.79 (0.29, 2.15) | 0.76 (0.16, 3.68) | 0.90 (0.30, 2.72) | RCPc | . | . | . | . | . | 0.42 (0.17, 1.06) | ||||
| 0.19 (0.01, 5.93) | 0.61 (0.20, 1.86) | 0.65 (0.21, 1.95) | 0.67 (0.26, 1.77) | 0.66 (0.16, 2.69) | 0.78 (0.29, 2.08) | 0.86 (0.30, 2.47) | TMPc | 0.79 (0.18, 3.44) | . | . | . | 0.56 (0.30, 1.03) | ||||
| 0.19 (0.01, 5.86) | 0.63 (0.23, 1.67) | 0.67 (0.31, 1.43) | 0.70 (0.30, 1.63) | 0.68 (0.21, 2.16) | 0.80 (0.37, 1.72) | 0.89 (0.31, 2.56) | 1.03 (0.46, 2.30) | MP | . | . | 0.52 (0.23, 1.20) | 0.56 (0.24, 1.31) | ||||
| 0.08 (0.00, 4.73) | 0.26 (0.02, 2.96) | 0.28 (0.02, 3.59) | 0.29 (0.03, 3.24) | 0.28 (0.02, 4.20) | 0.33 (0.03, 3.39) | 0.37 (0.03, 4.80) | 0.43 (0.03, 5.33) | 0.42 (0.04, 4.78) | TCD | 0.84 (0.13, 5.28) | . | . | ||||
| 0.07 (0.00, 4.26) | 0.22 (0.02, 2.79) | 0.23 (0.02, 3.36) | 0.24 (0.02, 3.05) | 0.24 (0.01, 3.91) | 0.28 (0.02, 3.21) | 0.31 (0.02, 4.50) | 0.36 (0.03, 5.00) | 0.35 (0.03, 4.50) | 0.84 (0.13, 5.28) | TD | . | . | ||||
| 0.10 (0.00, 3.07) | 0.31 (0.10, 0.99) | 0.33 (0.11, 0.99) | 0.34 (0.12, 0.97) | 0.33 (0.08, 1.36) | 0.40 (0.14, 1.10) | 0.44 (0.14, 1.39) | 0.51 (0.20, 1.28) | 0.49 (0.23, 1.09) | 1.19 (0.09, 14.97) | 1.41 (0.10, 19.72) | RMP | 1.09 (0.49, 2.40) | ||||
| 0.10 (0.00, 3.04) | 0.32 (0.12, 0.88) | 0.35 (0.13, 0.95) | 0.36 (0.16, 0.81) | 0.35 (0.09, 1.33) | 0.41 (0.18, 0.97) | 0.46 (0.19, 1.13) | 0.53 (0.30, 0.94) | 0.52 (0.26, 1.01) | 1.24 (0.10, 14.68) | 1.47 (0.11, 19.39) | 1.04 (0.49, 2.23) | RMPc | ||||
|
Anaemia‐subnetwork 2 No. of studies: 2. No. of treatments: 4. No. of pairwise comparisons: 4. No. of designs: 2 Qtotal= 0, df = 0, P = n.a./ Qwithin=0, df =0, P = n.a./ Qbetween= 0, df = 0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | ||||||||||||||||
| VDc | . | 0.29 (0.08, 1.02) | 0.25 (0.07, 0.86) | |||||||||||||
| 0.37 (0.09, 1.60) | VTPc | . | 0.67 (0.31, 1.43) | |||||||||||||
| 0.29 (0.08, 1.02) | 0.78 (0.25, 2.36) | VTDc | 0.86 (0.38, 1.93) | |||||||||||||
| 0.25 (0.07, 0.86) | 0.67 (0.31, 1.43) | 0.86 (0.38, 1.93) | VMPc | |||||||||||||
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
The RR for anaemia appears to be similar for medications and treatment durations (figure 17 in Appendix 5). Five out of 12 compared treatment options have a clinically meaningful smaller incidence of anaemia than RMPc (RR 0.32, 95% CI 0.12 to 0.88) to (RR 0.53, 95% CI 0.30 to 0.94), out of which, three also have substantially less neutropenia than RMP (RR 0.31, 95% CI 0.10 to 0.99) to( RR 0.34, 95% CI 0.12 to 0.97) (Table 7). According to the treatment ranking of anaemia‐subnetwork 1, VRD (P‐score: 0.82), RD (P score: 0.73), and VMP (P score: 0.69) are the therapy regimens, with the smallest incidence of anaemia, compared to all other therapy regimens, included in this subnetwork. The therapy regimens with the highest incidence of anaemia in anaemia‐subnetwork 1 are RMPc (P score 0.17), and RMP (P score 0.17), and TD (P score 0.22).
Results of anaemia‐subnetwork 2 suggest a smaller risk of anaemia for VDc compared to VMPc (RR 0.25, 95% CI 0.07 to 0.86). No other treatment combinations within anaemia‐subnetwork 2 were favoured over another (Table 7; figure 17 in Appendix 5).
Only one study was included in anaemia‐subnetwork 3 (Ludwig 2009). Anaemia was experienced by six of 134 participants treated with TDc and 15 of 134 participants treated with MPc (P = 0.067).
There is no statistically significant difference between direct and indirect estimate (figure 18 in Appendix 5). The only closed loop in anaemia‐subnetwork 2 consists of a multi‐arm study, so there is no indirect evidence.
Thrombocytopenia
Thrombocytopenia was reported in 12 studies (Bahlis 2017; Facon 2007; Hungria 2016; Ludwig 2009; Magarotto 2016; Mateos 2014; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; San Miguel 2008; Zweegman 2016), (seven two‐arm studies, five three‐arm studies), and could be analysed with data from 11 studies. One three‐arm study was excluded from the analysis due to zero events in all arms (Hungria 2016). Thrombocytopenia was reported for 16 treatment regimens and a total of 6190 patients. However, the network was not fully connected and consists out of three subnetworks comprising 19 pairwise comparisons (figure 19 in Appendix 5).
We performed NMA for thrombocytopenia‐subnetwork 1 and 2 (thrombocytopenia‐subnetwork 3 consisted of only two treatment regimens). Results for all network comparisons, including the ranking of treatments are shown in Table 8, per thrombocytopenia‐subnetwork. No heterogeneity (I² = 0 %) was observed between studies for thrombocytopenia‐subnetwork 1.
7. Results of NMA for the outcome thrombocytopenia.
|
League tables for the outcome thrombocytopenia. Direct and network estimates with 95% CIs are given. Treatments are ordered by P score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². Thrombocytopenia‐subnetwork 1 No. of studies: 8. No. of treatments: 10. No. of pairwise comparisons: 14. No. of designs: 8 Qtotal= 0.01, df = 2, P = 0.99/ Qwithin= 0.00, df = 0, Pm=mnot available/ Qbetween=m0.01, df = 2, P = 0.99; I² = 0%, Tau² = 0.0 Treatment Effects: | ||||||||||||
| TMPc | 0.79 (0.21, 2.87) | . | . | . | . | . | . | 0.26 (0.17, 0.39) | . | |||
| 0.77 (0.46, 1.29) | MP | . | . | 0.82 (0.66, 1.01) | . | . | 0.72 (0.39, 1.33) | 0.34 (0.22, 0.50) | 0.31 (0.21, 0.46) | |||
| 0.75 (0.38, 1.49) | 0.97 (0.55, 1.72) | RD | 0.88 (0.60, 1.31) | . | . | . | 0.72 (0.49, 1.04) | . | . | |||
| 0.66 (0.36, 1.21) | 0.86 (0.52, 1.40) | 0.88 (0.60, 1.29) | RDc | . | . | 0.82 (0.43, 1.57) | 0.81 (0.57, 1.17) | 0.40 (0.23, 0.71) | . | |||
| 0.63 (0.36, 1.10) | 0.82 (0.66, 1.01) | 0.84 (0.46, 1.54) | 0.95 (0.56, 1.64) | VMP | 0.90 (0.64, 1.26) | . | . | . | . | |||
| 0.57 (0.30, 1.08) | 0.73 (0.49, 1.10) | 0.75 (0.37, 1.51) | 0.86 (0.45, 1.62) | 0.90 (0.64, 1.26) | VTMPc | . | . | . | . | |||
| 0.53 (0.28, 1.02) | 0.69 (0.38, 1.26) | 0.71 (0.36, 1.42) | 0.81 (0.44, 1.48) | 0.85 (0.45, 1.61) | 0.94 (0.46, 1.95) | RCPc | . | 0.49 (0.29, 0.83) | . | |||
| 0.54 (0.29, 1.01) | 0.70 (0.43, 1.14) | 0.72 (0.50, 1.04) | 0.82 (0.59, 1.15) | 0.86 (0.51, 1.46) | 0.96 (0.51, 1.79) | 1.02 (0.53, 1.93) | TMP | . | . | |||
| 0.26 (0.18, 0.39) | 0.34 (0.24, 0.48) | 0.35 (0.20, 0.61) | 0.40 (0.25, 0.63) | 0.41 (0.27, 0.63) | 0.46 (0.27, 0.79) | 0.49 (0.29, 0.82) | 0.48 (0.29, 0.79) | RMPc | 0.92 (0.73, 1.16) | |||
| 0.24 (0.15, 0.38) | 0.31 (0.22, 0.45) | 0.32 (0.18, 0.58) | 0.36 (0.22, 0.60) | 0.38 (0.25, 0.58) | 0.42 (0.25, 0.73) | 0.45 (0.26, 0.79) | 0.44 (0.26, 0.75) | 0.92 (0.73, 1.16) | RMP | |||
|
Thrombocytopenia‐subnetwork 2 No. of studies: 2. No. of treatments: 4. No. of pairwise comparisons: 4. No. of designs: 2 Qtotal= 0, df = 0, P = n.a./ Qwithin= 0, df = 0,P = n.a./ Qbetween= 0, df = 0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | ||||||||||||
| VDc | 0.64 (0.18, 2.22) | . | 0.16 (0.06, 0.46) | |||||||||
| 0.64 (0.18, 2.22) | VTDc | . | 0.26 (0.11, 0.61) | |||||||||
| 0.36 (0.11, 1.16) | 0.56 (0.20, 1.57) | VTPc | 0.46 (0.27, 0.78) | |||||||||
| 0.16 (0.06, 0.46) | 0.26 (0.11, 0.61) | 0.46 (0.27, 0.78) | VMPc | |||||||||
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
Patients receiving any other treatment option of thrombocytopenia‐subnetwork 1 than fixed or continuous treatment with RMP have a clinically meaningful smaller risk for thrombocytopenia (RR 0.26, 95% CI 0.18 to 0.39) to (RR 0.49, 95% CI 0.29 to 0.82) (Table 8). According to the ranking of treatments TMPc (P score: 0.93) is the treatment within thrombocytopenia‐subnetwork 1 with the least thrombocytopenia‐incidence and RMP (P score 0.03), and RMPc (P score 0.09), are the treatments with the highest incidence (figure 20 in Appendix 5).
Within thrombocytopenia‐subnetwork 2, evidence suggests the highest risk of thrombocytopenia for participants treated with VMPc (P score 0.00) and the smallest risk of thrombocytopenia for participants treated with VDc (P score 0.91) (Table 8; figure 20 in Appendix 5).
Only one study was included in thrombocytopenia‐subnetwork 3 (Ludwig 2009). Thrombocytopenia was experienced by two of 134 participants treated with TDc and 16 of 134 participants treated with MPc (P < 0.001).
There is no statistically significant difference between direct and indirect estimates (figure 21 in Appendix 5). The only closed loop in thrombocytopenia‐subnetwork 2 consists of a multi‐arm study, so there is no indirect evidence.
Thromboembolism
A total of 14 studies (Facon 2007; Hulin 2009; Hungria 2016; Mateos 2014; Mookerjee 2017; Morgan 2011; Palumbo 2006; Palumbo 2014; Sacchi 2011; San Miguel 2008; Stewart 2015; Waage 2010; Wijermans 2010; Zweegman 2016), (13 two‐arm studies, one three‐arm study) reported thromboembolism. We had to exclude one study from the analysis due to zero events (Morgan 2011), and therefore the outcome could be analysed with data from 13 studies. Thromboembolisms were reported for 13 treatment regimens and a total of 4277 participants. The network was not fully connected and consists of three subnetworks comprising 15 pairwise comparisons (figure 22 in Appendix 5).
We performed NMA for thromboembolism‐subnetwork 1 (thromboembolism‐subnetworks 2 and 3 consisted of only two treatment regimens). Results for all network comparisons, including the ranking of treatments are shown in Table 9. Low heterogeneity (I² = 26.9 %) was observed between studies for thromboembolism‐subnetwork 1.
8. Results of NMA for the outcome thromboembolism.
|
League table for the outcome thromboembolism. Direct and network estimates with 95% CIs are given. Treatments are ordered by P score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². Thromboembolism‐subnetwork 1 No. of studies: 11. No. of treatments: 9. No. of pairwise comparisons: 13. No. of designs: 7 Heterogeneity / inconsistency: Q = 5.47, df = 4, P = 0.24; I² = 26.9%, Tau² = 0.1107 Treatment Effects: | ||||||||
| MP | 0.67 (0.10, 4.49) | 0.36 (0.16, 0.81) | . | . | . | 0.14 (0.03, 0.67) | . | . |
| 0.67 (0.10, 4.49) | VMP | . | 0.38 (0.11, 1.27) | . | . | . | . | . |
| 0.36 (0.16, 0.81) | 0.54 (0.07, 4.23) | TMP | . | . | . | . | . | . |
| 0.26 (0.03, 2.43) | 0.38 (0.11, 1.27) | 0.71 (0.06, 7.69) | VTMPc | . | . | . | . | . |
| 0.19 (0.03, 1.17) | 0.28 (0.02, 3.90) | 0.52 (0.07, 3.83) | 0.73 (0.04, 13.36) | MPc | . | 0.73 (0.29, 1.80) | . | . |
| 0.15 (0.03, 0.87) | 0.23 (0.02, 2.98) | 0.43 (0.06, 2.86) | 0.60 (0.04, 10.29) | 0.83 (0.27, 2.53) | RMPc | 0.88 (0.46, 1.69) | . | . |
| 0.14 (0.03, 0.67) | 0.20 (0.02, 2.41) | 0.37 (0.06, 2.24) | 0.53 (0.03, 8.39) | 0.73 (0.29, 1.80) | 0.88 (0.46, 1.69) | TMPc | 0.67 (0.11, 4.20) | 0.56 (0.08, 4.09) |
| 0.09 (0.01, 1.04) | 0.13 (0.01, 2.96) | 0.25 (0.02, 3.25) | 0.35 (0.01, 9.77) | 0.48 (0.06, 3.77) | 0.59 (0.08, 4.14) | 0.67 (0.11, 4.20) | TCDc | 0.84 (0.14, 5.18) |
| 0.08 (0.01, 0.98) | 0.11 (0.00, 2.72) | 0.21 (0.01, 3.05) | 0.30 (0.01, 8.94) | 0.41 (0.05, 3.62) | 0.50 (0.06, 4.00) | 0.56 (0.08, 4.09) | 0.84 (0.14, 5.18) | TDc |
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
The RR for thromboembolism appears to be similar for medications and treatment durations (Table 9). Incidence of thromboembolism is substantially smaller in MP‐treated patients than patients treated with RMPc (RR 0.15, 95% CI 0.03 to 0.87)); TMPc (RR 0.14, 95% CI 0.03 to 0.67); and TDc (RR 0.08, 95% CI 0.01 to 0.98)) (figure 23 in Appendix 5). In terms of the risk of thromboembolism, MP (P score: 0.93), followed by VMP (P score 0.80), are the best treatment options within subnetwork 1 and TDc (P score: 0.21), TCDc (P score: 0.25), and TMPc (P score: 0.32) the worst.
Only one study was included in thromboembolism‐subnetwork 2 (Mateos 2014). Thromboembolism were experienced by 1 of 130 participants treated with VMPc and 3 of 130 participants treated with VTPc (P= 0.5). Also, only one study was included in thromboembolism‐subnetwork 3 (Mookerjee 2017). Thromboembolism were experienced by 1 of 74 participants treated with VRD and 0 of 69 participants treated with RD.
As the only closed loop in thromboembolism‐subnetwork 1 consists of multi‐arm studies, there is no indirect evidence.
Infections
Infections were reported in 15 studies (Bahlis 2017; Beksac 2011; Durie 2017; Facon 2007; Magarotto 2016; Mateos 2014; Morgan 2011; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Sacchi 2011; Waage 2010; Wijermans 2010; Zweegman 2016) (11 two‐arm studies, four three‐arm studies), for 17 treatment regimens and a total of 7470 participants. However, the network was not fully connected and consists of three subnetworks comprising 23 pairwise comparisons (figure 24 in Appendix 5).
We performed NMA for infections‐subnetworks 1 and 2 (infections‐subnetwork 3 consisted out of two treatment regimens, only). Results for all network comparisons, including the ranking of treatments are shown in Table 10, per infections‐subnetwork. Moderate heterogeneity (I² = 34.8 %) was observed between studies for infections‐subnetwork 1.
9. Results of NMA for outcome infections.
|
League tables for the outcome infections. Direct and network estimates with 95% CIs are given. Treatments are ordered by P score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². Infections‐subnetwork 1 No. of studies: 12. No. of treatments: 11. No. of pairwise comparisons: 18. No. of designs: 9 Qtotal= 7.67, df = 5, P = 0.18/ Qwithin= 2.72, df=3, P = 0.44/ Qbetween= 4.95, df = 2, P = 0.08; I² = 34.8%, Tau² = 0.0713 Treatment Effects: | |||||||||||||
| MP | 0.50 (0.26, 0.96) | . | 0.57 (0.29, 1.11) | . | . | 0.72 (0.29, 1.79) | . | . | 0.48 (0.20, 1.12) | 0.17 (0.04, 0.82) | |||
| 0.60 (0.34, 1.07) | TMP | . | . | . | 0.77 (0.43, 1.37) | . | 0.53 (0.30, 0.94) | . | . | . | |||
| 0.60 (0.23, 1.54) | 1.00 (0.40, 2.48) | RCPc | . | . | . | 0.58 (0.26, 1.33) | 0.71 (0.30, 1.65) | . | . | . | |||
| 0.57 (0.29, 1.11) | 0.94 (0.39, 2.27) | 0.94 (0.30, 3.00) | TCD | . | . | . | . | . | . | . | |||
| 0.50 (0.19, 1.30) | 0.83 (0.31, 2.26) | 0.84 (0.28, 2.49) | 0.89 (0.28, 2.84) | MPc | . | . | . | . | . | 0.65 (0.39, 1.08) | |||
| 0.50 (0.24, 1.07) | 0.83 (0.47, 1.46) | 0.83 (0.32, 2.17) | 0.89 (0.32, 2.44) | 1.00 (0.34, 2.92) | RD | . | 0.69 (0.39, 1.21) | . | . | . | |||
| 0.40 (0.21, 0.77) | 0.66 (0.33, 1.34) | 0.66 (0.30, 1.47) | 0.70 (0.27, 1.80) | 0.79 (0.37, 1.70) | 0.79 (0.36, 1.76) | RMPc | 1.22 (0.56, 2.65) | . | 0.66 (0.30, 1.48) | 0.91 (0.49, 1.67) | |||
| 0.37 (0.19, 0.73) | 0.61 (0.37, 1.03) | 0.62 (0.27, 1.39) | 0.65 (0.25, 1.70) | 0.74 (0.28, 1.95) | 0.74 (0.43, 1.28) | 0.93 (0.49, 1.78) | RDc | 0.95 (0.48, 1.88) | . | . | |||
| 0.35 (0.13, 0.92) | 0.58 (0.25, 1.38) | 0.58 (0.20, 1.69) | 0.62 (0.19, 2.01) | 0.70 (0.21, 2.30) | 0.70 (0.29, 1.68) | 0.88 (0.34, 2.26) | 0.95 (0.48, 1.88) | VRDc | . | . | |||
| 0.34 (0.16, 0.75) | 0.57 (0.23, 1.39) | 0.57 (0.20, 1.64) | 0.60 (0.21, 1.69) | 0.68 (0.24, 1.94) | 0.68 (0.25, 1.84) | 0.86 (0.40, 1.82) | 0.92 (0.37, 2.28) | 0.98 (0.31, 3.04) | RMP | . | |||
| 0.33 (0.15, 0.73) | 0.54 (0.23, 1.29) | 0.54 (0.21, 1.44) | 0.58 (0.20, 1.65) | 0.65 (0.39, 1.08) | 0.65 (0.25, 1.68) | 0.82 (0.46, 1.46) | 0.88 (0.38, 2.04) | 0.94 (0.32, 2.76) | 0.96 (0.38, 2.41) | TMPc | |||
|
Infections‐subnetwork 2 No. of studies: 2. No. of treatments: 4. No. of pairwise comparisons: 4. No. of designs: 2 Qtotal= 0, df = 0, P = n.a./ Qwithin= 0, df = 0, P = n.a./ Qbetween= 0, df = 0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | |||||||||||||
| VTPc | . | 0.11 (0.01, 0.86) | . | ||||||||||
| 0.12 (0.02, 1.03) | VTDc | 0.89 (0.55, 1.45) | 0.75 (0.47, 1.19) | ||||||||||
| 0.11 (0.01, 0.86) | 0.89 (0.55, 1.45) | VMPc | 0.84 (0.54, 1.30) | ||||||||||
| 0.09 (0.01, 0.76) | 0.75 (0.47, 1.19) | 0.84 (0.54, 1.30) | VDc | ||||||||||
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
The RR for infections appears to be similar for medications and treatment durations (Table 10). MP‐treated patients have substantially fewer infections than patients treated with RMPc (RR 0.40, 95% CI 0.21 to 0.77); RDc (RR 0.37, 95% CI 0.19 to 0.73); VRDc (RR 0.35, (95% CI 0.13 to 0.92); RMP (RR 0.34, 95% CI 0.16 to; 0.75);, and TMPc (RR 0.33, 95% CI 0.15 to 0.73); (figure 25 in Appendix 5). Within infections‐subnetwork 1, and according to the ranking of treatments, incidence of infections is the smallest for MP (P score 0.96), and most frequent for TMPc (P score 0.21), followed by RMP (P score 0.27), VRDc and RDc (P score 0.29, respectively).
Results of infections‐subnetwork 2 suggest a smaller risk of infections for VTPc compared to VMPc (RR 0.11, 95% CI 0.01 to 0.86); and VDc (RR 0.09, 95% CI 0.01 to 0.76), respectively. No other treatment combinations within infections‐subnetwork 2 were favoured over another (Table 10; figure 25 in Appendix 5).
Only one study was included in infections‐subnetwork 3 (Palumbo 2014). Infections were experienced by 32 of 250 participants treated with VTMPc and 23 of 253 participants treated with VMP (P = 0.18).
The test for disagreement showed almost statistically significant disagreement between direct and indirect estimates in closed loops for RMP‐MP‐RMPc (P = 0.0660) (figure 26 in Appendix 5). The only closed loop in infections‐subnetwork 2 consists of a multi‐arm study, so there is no indirect evidence.
Serious adverse events (SAEs)
Serious adverse events were not consistently reported across studies. To be able to meta‐analyse results we could only consider harms when the number of participants with at least one SAE was reported. We could not consider cumulated events or perform subgroup analyses.
Serious adverse events were reported in eight studies (Bahlis 2017; Durie 2017; Jacobus 2016; Mateos 2014; Niesvizky 2015; Palumbo 2012; San Miguel 2008; Stewart 2015) (five two‐arm studies, three three‐arm studies) and were measured for 14 treatment regimens and a total of 7306 participants. However, the network was not fully connected and consists of three subnetworks comprising 14 pairwise comparisons (figure 27 in Appendix 5).
NMA was performed for all SAE‐subnetworks. Results for all network comparisons, including the ranking of treatments are shown in Table 11. Global approach to check for inconsistency and heterogeneity between studies was not applicable.
10. Results of NMA for the outcome SAEs.
|
League tables for the outcome serious adverse events. Direct and network estimates with 95% CIs are given. Treatments are ordered by P‐Score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². SAE‐subnetwork 1 No. of studies: 3. No. of treatments: 5. No. of pairwise comparisons: 5. No. of designs: 3 Qtotal= 0, df = 0, P = n.a./ Qwithin= 0, df = 0, P = n.a./ Qbetween= 0, d f= 0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | ||||
| TMP | 0.88 (0.78, 0.98) | . | 0.70 (0.64, 0.78) | . |
| 0.88 (0.78, 0.98) | RD | 0.98 (0.65, 1.49) | 0.80 (0.73, 0.88) | . |
| 0.86 (0.56, 1.32) | 0.98 (0.65, 1.49) | VRD | . | . |
| 0.70 (0.64, 0.78) | 0.80 (0.73, 0.88) | 0.82 (0.53, 1.26) | RDc | 0.90 (0.75, 1.09) |
| 0.63 (0.51, 0.78) | 0.73 (0.59, 0.89) | 0.74 (0.46, 1.18) | 0.90 (0.75, 1.09) | VRDc |
|
SAE‐subnetwork 2 No. of studies: 2. No. of treatments: 4. No. of pairwise comparisons: 4. No. of designs: 2 Qtotal= 0, df = 0, P = n.a./ Qwithin= 0, df = 0, P = n.a./ Qbetween= 0, df = 0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | ||||
| VMPc | 0.95 (0.78, 1.18) | 0.87 (0.72, 1.07) | 0.50 (0.31, 0.81) | VMPc |
| 0.95 (0.78, 1.18) | VDc | 0.92 (0.75, 1.11) | . | 0.95 (0.78, 1.18) |
| 0.87 (0.72, 1.07) | 0.92 (0.75, 1.11) | VTDc | . | 0.87 (0.72, 1.07) |
| 0.50 (0.31, 0.81) | 0.52 (0.31, 0.88) | 0.57 (0.34, 0.96) | VTPc | 0.50 (0.31, 0.81) |
|
SAE‐subnetwork 3 No. of studies: 3. No. of treatments: 5. No. of pairwise comparisons: 5. No. of designs: 3 Qtotal= 0, df = 0, P = n.a./ Qwithin= 0, df=0, P = n.a./ Qbetween= 0, df = 0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | ||||
| MP | 0.90 (0.68, 1.19) | 0.83 (0.63, 1.10) | 0.78 (0.65, 0.94) | . |
| 0.90 (0.68, 1.19) | RMP | 0.93 (0.71, 1.21) | . | . |
| 0.83 (0.63, 1.10) | 0.93 (0.71, 1.21) | RMPc | . | 0.79 (0.67, 0.93) |
| 0.78 (0.65, 0.94) | 0.87 (0.62, 1.22) | 0.94 (0.68, 1.31) | VMP | . |
| 0.66 (0.48, 0.91) | 0.73 (0.54, 1.00) | 0.79 (0.67, 0.93) | 0.84 (0.58, 1.21) | TMPc |
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
Relative risk for patients to experience at least one SAE appears to be similar across therapy regimens (figure 28 in Appendix 5). In SAE‐subnetwork 1, according to the ranking of treatments SAEs are most likely for VRDc (P score: 0.06) and least likely for TMP (P score 0.94). In SAE‐subnetwork 2, SAEs are most likely for VTPc (P score 0.01) and least likely for VMPc (P score 0.86). In SAE‐subnetwork 3, SAEs are most likely for TMPc (P score 0.05) and least likely for MP (P score 0.92).
The only closed loops in the SAE‐subnetworks consists of multi‐arm studies, so there is no indirect evidence.
We could only rate the certainty of the evidence for SAEs for VMP, as RD, TMP, and VRDs are not connected to MP in the network. Moderate‐certainty evidence suggests that VMP as initial therapy for MM probably slightly increases occurrence of SAEs (Table 1).
Withdrawals due to adverse events (AEs)
The number of participants who stopped assigned therapy and terminated their participation in the study due to AEs, were reported in 16 studies (Bahlis 2017; Durie 2017; Hulin 2009; Hungria 2016; Ludwig 2009; Magarotto 2016; Mateos 2014; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Sacchi 2011; San Miguel 2008; Waage 2010; Wijermans 2010; Zweegman 2016) (11 two‐arm studies, five three‐arm studies), for 19 treatment regimens and a total of 7052 participants. However, the network was not fully connected and consists of two subnetworks comprising 26 pairwise comparisons (figure 29 in Appendix 5).
We performed NMA for both withdrawals due to AEs‐subnetworks. Results for all network comparisons, including the ranking of treatments, are shown in Table 12. Low heterogeneity (I² = 25.5 %) was observed between studies for withdrawals due to AEs‐ subnetwork 1.
11. Results of NMA for the outcome withdrawals due to AEs.
|
League tables for the outcome withdrawals due to adverse events. Direct and network estimates with 95% CIs are given. Treatments are ordered by P score (descending). The treatment with the highest probability of being the best is in the upper left corner. Direct estimates are presented in the upper triangle; network estimates are presented in the lower triangle Global approach to check inconsistency/heterogeneity: Q‐statistics, I². Withdrawals due to AEs‐ Subnetwork 1 No. of studies: 14. No. of treatments: 15. No. of pairwise comparisons: 22. No. of designs: 12 Qtotal= 5.37, df= 4, P = 0.25/ Qwithin= 0.93, df = 2, P = 0.62/ Qbetween= 4.44, df = 2, P = 0.11; I² = 25.5%, Tau² = 0.0392 Treatment Effects: | |||||||||||||||||
| MPc | . | . | 0.76 (0.16, 3.48) | . | . | . | . | 0.29 (0.18, 0.48) | . | . | . | . | . | . | |||
| 0.83 (0.37, 1.85) | MP | 0.94 (0.55, 1.60) | . | . | 0.38 (0.16, 0.91) | 0.33 (0.14, 0.78) | . | 0.25 (0.09, 0.71) | . | . | . | 0.32 (0.17, 0.61) | . | . | |||
| 0.78 (0.30, 2.04) | 0.94 (0.55, 1.60) | VMP | . | 0.82 (0.48, 1.39) | . | . | . | . | . | . | . | . | . | . | |||
| 0.76 (0.16, 3.48) | 0.91 (0.16, 5.10) | 0.97 (0.16, 5.88) | TDc | . | . | . | . | . | . | . | . | . | . | . | |||
| 0.64 (0.21, 1.92) | 0.77 (0.36, 1.63) | 0.82 (0.48, 1.39) | 0.85 (0.13, 5.56) | VTMPc | . | . | . | . | . | . | . | . | . | . | |||
| 0.39 (0.16, 0.97) | 0.47 (0.22, 0.99) | 0.50 (0.20, 1.25) | 0.52 (0.09, 3.06) | 0.61 (0.21, 1.76) | RMP | 0.87 (0.45, 1.69) | . | . | . | . | . | . | . | . | |||
| 0.37 (0.19, 0.72) | 0.45 (0.25, 0.78) | 0.48 (0.22, 1.03) | 0.49 (0.09, 2.60) | 0.58 (0.23, 1.49) | 0.95 (0.50, 1.80) | RMPc | . | 0.85 (0.52, 1.39) | 0.59 (0.33, 1.05) | . | 0.47 (0.27, 0.83) | . | . | . | |||
| 0.30 (0.07, 1.38) | 0.37 (0.09, 1.43) | 0.39 (0.09, 1.69) | 0.40 (0.05, 3.47) | 0.48 (0.10, 2.27) | 0.78 (0.17, 3.45) | 0.82 (0.20, 3.28) | TD | . | . | 0.76 (0.21, 2.75) | . | 0.67 (0.19, 2.34) | . | . | |||
| 0.29 (0.18, 0.48) | 0.35 (0.19, 0.67) | 0.38 (0.16, 0.86) | 0.39 (0.08, 1.94) | 0.46 (0.17, 1.23) | 0.75 (0.35, 1.61) | 0.79 (0.50, 1.24) | 0.97 (0.23, 4.06) | TMPc | . | . | . | . | . | . | |||
| 0.25 (0.11, 0.59) | 0.30 (0.15, 0.61) | 0.32 (0.13, 0.78) | 0.33 (0.06, 1.92) | 0.40 (0.14, 1.11) | 0.65 (0.28, 1.47) | 0.68 (0.39, 1.18) | 0.83 (0.20, 3.41) | 0.86 (0.43, 1.72) | RCPc | . | 0.80 (0.48, 1.34) | . | . | . | |||
| 0.23 (0.06, 0.84) | 0.28 (0.09, 0.84) | 0.30 (0.09, 1.01) | 0.31 (0.04, 2.27) | 0.36 (0.10, 1.38) | 0.59 (0.17, 2.09) | 0.62 (0.20, 1.94) | 0.76 (0.21, 2.75) | 0.79 (0.24, 2.59) | 0.92 (0.29, 2.94) | TCD | . | 0.88 (0.33, 2.31) | . | . | |||
| 0.22 (0.10, 0.50) | 0.27 (0.15, 0.48) | 0.28 (0.13, 0.63) | 0.29 (0.05, 1.65) | 0.35 (0.13, 0.91) | 0.57 (0.26, 1.22) | 0.60 (0.36, 0.98) | 0.73 (0.19, 2.77) | 0.75 (0.40, 1.43) | 0.88 (0.53, 1.45) | 0.96 (0.33, 2.79) | RDc | 0.76 (0.46, 1.26) | 0.81 (0.49, 1.35) | 0.42 (0.23, 0.77) | |||
| 0.20 (0.09, 0.47) | 0.24 (0.14, 0.42) | 0.26 (0.12, 0.55) | 0.27 (0.05, 1.54) | 0.32 (0.13, 0.80) | 0.52 (0.23, 1.16) | 0.55 (0.30, 0.98) | 0.67 (0.19, 2.34) | 0.69 (0.34, 1.38) | 0.80 (0.42, 1.53) | 0.87 (0.33, 2.31) | 0.92 (0.58, 1.45) | TMP | 1.07 (0.65, 1.75) | . | |||
| 0.20 (0.08, 0.49) | 0.24 (0.12, 0.47) | 0.25 (0.11, 0.60) | 0.26 (0.04, 1.56) | 0.31 (0.11, 0.85) | 0.51 (0.21, 1.21) | 0.54 (0.28, 1.03) | 0.65 (0.17, 2.51) | 0.68 (0.32, 1.45) | 0.79 (0.40, 1.57) | 0.86 (0.29, 2.53) | 0.90 (0.55, 1.47) | 0.98 (0.61, 1.59) | RD | . | |||
| 0.09 (0.03, 0.25) | 0.11 (0.05, 0.26) | 0.12 (0.04, 0.32) | 0.12 (0.02, 0.77) | 0.15 (0.05, 0.45) | 0.24 (0.09, 0.63) | 0.25 (0.11, 0.55) | 0.31 (0.07, 1.33) | 0.32 (0.13, 0.76) | 0.37 (0.17, 0.81) | 0.40 (0.12, 1.37) | 0.42 (0.23, 0.77) | 0.46 (0.22, 0.98) | 0.47 (0.21, 1.02) | VRDc | |||
| Withdrawals due to AEs‐ Subnetwork 2 No. of studies: 2. No. of treatments: 4. No. of pairwise comparisons: 4. No. of designs: 2 Qtotal= 0, df = 0, P = n.a./ Qwithin= 0, df=0, P = n.a./ Qbetween= 0, df = 0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects: | |||||||||||||||||
| VDc | 0.89 (0.67, 1.18) | 0.86 (0.65, 1.14) | . | ||||||||||||||
| 0.89 (0.67, 1.18) | VMPc | 0.97 (0.74, 1.27) | 0.68 (0.37, 1.25) | ||||||||||||||
| 0.86 (0.65, 1.14) | 0.97 (0.74, 1.27) | VTDc | . | ||||||||||||||
| 0.60 (0.31, 1.18) | 0.68 (0.37, 1.25) | 0.70 (0.36, 1.37) | VTPc | ||||||||||||||
c: continuous; C: cyclophosphamide; CI: confidence interval; df: degrees of freedom; D: dexamethasone; M: melphalan; NMA: network meta‐analysis; n.a.: not applicable; OS: overall survival; P: prednisone; R: revlimid (lenalidomide); T: thalidomide; V: velcade (bortezomib)
Patients who received fixed or continuous MP‐therapy had a substantially lower risk of withdrawing from the study due to AEs than nine out of 13 treatments withdrawals due to AEs‐ subnetwork 1 (RR 0.09, 95% CI 0.03 to 0.25) to (RR 0.47 (95% CI 0.22 to 0.99); (figure 30 in Appendix 5). Participants treated with VRDc had a substantially higher risk of withdrawing from the study due to AEs than11 of the compared treatments of withdrawals due to AEs‐ subnetwork 1 (RR 0.09. 95% CI 0.03 to 0.25) to (RR 0.46 (95% CI 0.22 to 0.98) (Table 12). Treatment discontinuation due to AEs is most frequent for VRDc (P score 0.01), followed by RD (P score 0.22), and TMP (P score 0.22), and least frequent for MPc (P score 0.91), followed by MP (P score 0.86), and VMP (P score 0.84), in withdrawals due to AEs‐subnetwork 1.
Evidence of withdrawals due to AEs‐subnetwork 2 suggests no advantages for any treatment combinations within this subnetwork (Table 12; (figure 30 in Appendix 5).
We rated the certainty of the evidence for withdrawals due to AEs according to the GRADE system for RD, TMP, VMP, and VRDc. High‐certainty evidence suggests that treatment with RD, TMP, and VRDc for first‐line treatment of MM result in a large increase in withdrawals due to AEs. Moderate‐certainty evidence suggest that the use of VMP as initial therapy for MM probably results in little to no difference in withdrawals due to AEs, compared to MP (Table 1).
The test for disagreement showed no statistically significant difference between direct and indirect estimates (figure 31 in Appendix 5). The only closed loop in withdrawals due to AEs‐ subnetwork 2 consists of a multi‐arm study, so there is no indirect evidence.
Quality of life (QoL)
Quality of life was reported in four of the 25 included trials (Bahlis 2017; Palumbo 2012; Waage 2010; Wijermans 2010), and measured for seven treatment regimens and a total of 2260 participants. All trials used the validated European Organization of Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)‐C30 to evaluate QoL. Participants self‐assessed QoL at multiple time points, which could be assigned to three different periods: short (one to three months after start of treatment), medium (six to nine months after start of treatment), long (12 months and longer, after start of treatment). Bahlis 2017 and Palumbo 2012 further applied the EORTC QLQ‐MY20, and Wijermans 2010 the EORTC QLQ‐MY24; questionnaires, which were specifically designed for adults with multiple myeloma. Bahlis 2017 additionally evaluated QoL using the EuroQoL EQ‐5D questionnaire. As the treatment regimens used in the trials did not correspond with each other, no network could be built, and we could therefore not perform NMA on this outcome.
In summary, data from Bahlis 2017* showed that the global health status of patients treated with RD and TMP improved from baseline over the duration of the study and disease symptoms decreased (mean change from baseline after three months: RD: + 4.5, TMP: + 4; mean change from baseline after six months: RD: + 5, TMP: + 5.5; mean change from baseline after 12 months: RD: + 3.5, TMP: + 5; mean change from baseline after 18 months: RD: + 5, TMP: + 4). Evaluated on the basis of EORTC QLQ‐MY20, disease symptoms were substantially lower in patients receiving RD compared to TMP three months after start of treatment (mean change from baseline after three months: RD: ‐10, TMP: ‐7; P < 0.05), and side effects of treatment were lower in patients receiving RDc compared to TMP, continuously from cycle one until 12 months post‐baseline (mean change from baseline after three months: RD: + 1.0, TMP: + 4; mean change from baseline after six months: RD: + 0.5, TMP: + 3.5; mean change from baseline after 12 months: RD: + 1.5, TMP: + 5; P < 0.05, respectively), indicating a better health‐related QoL.
An improvement of QoL, evaluated on the basis of EORTC QLQ‐C30, of patients receiving TMPc and MPc improved after the initiation of anti‐myeloma treatment (Waage 2010; Wijermans 2010). Wijermans 2010 reported a mean QLQ‐C30 global health score of 49 (95% CI 45 to 52) for MPc and a mean score of 54 (95% CI50 to 58) for TMPc at baseline, respectively and increased after treatment induction (P time < 0.001; P arm = 0.05):
eight months: MPc: 65 (95% CI 57 to 73); TMPc: 66 (95% CI 59 to 73)
12 months: MPc: 62 (95% CI 58 to 66); TMPc: 63 (95% CI 58 to 67)
18 months: MPc: 64 (95% CI 57 to 70); TMPc: 70 (95% CI 65 to 75)
after re‐treatment/relapse/progression: MPc: 54 (95% CI 51 to 58); TMPc 63 (95% CI 59 to 68)
Waage 2010* reported a mean QLQ‐C30 global health score of 48 (95% CI 43 to 52) for MPc and a mean score of 47 (95% CI 43 to 53) for TMPc at baseline, respectively and increased after treatment induction.
12 months: MPc: 65 (95% CI 60 to 70); TMPc: 63 (95% CI 58 to 68)
24 months: MPc: 60 (95% CI 52 to 68); TMPc: 62 (95% CI 56 to 70)
36 months: MPc: 72 (95% CI 66 to 81); TMPc: 62 (95% CI 51 to 76)
From baseline until completion of cycle, 10 health‐related QoL scores increased steadily for RMPc (+ 12.2 (standard deviation (SD): 25.4), P < 0.001), RMP (+ 8.8 (SD; 24.7), P < 0.001), and MP (+ 6.2 (SD: 24.6), P < 0.05) and the difference was statistically significant for all groups (Palumbo 2012). The largest increase in global health scores was reported for RMPc (baseline score: 49.6 (SD: 23.5)). However, the baseline‐score was lower in this group compared to RMP (baseline score: 53.2 (SD: 23.5)) and MP (baseline score: 52.8 (SD: 22.8)), respectively.
*These data were read from graphs.
Discussion
Summary of main results
The aim of this systematic review and network meta‐analysis was to synthesise all available evidence on first‐line treatment options for adults with multiple myeloma in non‐transplant settings. We identified 25 eligible randomised controlled trials (RCTs) that included 11,403 participants. A total of 21 different treatment regimens were investigated in these studies. All studies could be included in network meta‐analyses. Overall risk of bias was generally high across studies because they were open‐label studies. The certainty of the evidence for pre‐selected treatment comparisons was low to moderate for survival outcomes, and moderate to high for safety outcomes. The main outcomes and comparisons are summarised in the Table 1.
Survival
Evidence suggests that treatment with RD, TMP, and VRDc probably results in a large increase in overall survival (OS), compared to MP. The use of VMP as initial myeloma therapy may result in a large increase in OS, compared to MP. Furthermore, treatment with RD, TMP, VMP, and VRDc may result in a large increase in progression‐free survival ( PFS) compared to MP.
Harms
The evidence suggests that the risk for polyneuropathies may be smaller for treatment with RD compared to treatment with MP. However, the confidence intervals (CIs) are also compatible with no difference or an increase in neuropathies. Treatment with TMP and VMP probably results in a large increase in polyneuropathies compared to MP. No study reported the outcome polyneuropathies for participants treated with VRDc. By comparing all included drug combinations with each other, the evidence suggests a lower risk for polyneuropathies for patients receiving lenalidomide‐ and thalidomide‐based therapies compared to bortezomib‐based therapies. Serious adverse events (SAEs) are probably higher in participants treated with VMP compared to MP. RD, TMP, and VRDc were not connected to MP in the network, therefore the risk ofSAEs could not be compared for these comparisons. By comparing the drug combinations with each other, the evidence suggests no discernible difference for any treatment combination. Treatment with RD, TMP, and VRDc results in a large increase in withdrawals due to AEs compared to MP. For participants treated with VMP, the risk of withdrawal due to adverse events is probably slightly increased, compared to MP.
Quality of life (QoL)
Quality of life was reported in four of the included studies for seven different treatment regimens (MP, MPc, RD, RMP, RMPc, TMP, TMPc) and was measured with four different tools. Assessment and reporting of QoL differed between studies and could not be meta‐analysed. The comparison to MP is therefore not possible. However, all studies reported an improvement of health‐related QoL after the start of anti‐myeloma treatment for all assessed treatment regimens.
When interpreting the results of this network meta‐analysis, it is important to consider that network meta‐analyses are no substitute for direct head‐to‐head comparisons. Therefore results may best be confirmed by additional RCTs of multiple drug combinations. Furthermore, it should be considered that results lacking statistical significance do not necessarily discredit disparities, which may be clinically relevant for some individuals.
Application for the inclusion of bortezomib, lenalidomide, or thalidomide into the WHO's list of essential medicines
Based on the effects of the interventions an application for the '22nd WHO Expert Commitee on the Selection and Use of Essential Medicines' for the inclusion of bortezomib, lenalidomide, or thalidomide into the EML was prepared. The full application can be accessed here: https://www.who.int/selection_medicines/committees/expert/22/applications/myeloma/en/
The Expert committee recommended to include all three of the proposed medicines (World Health Organization 2019a). The 21st list can be accessed here: https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf?ua=1
Overall completeness and applicability of evidence
In this systematic review and network meta‐analysis, we could include 25 published RCTs, comparing first‐line treatment options including combinations of bortezomib, lenalidomide, and thalidomide for adults with transplant‐ineligible multiple myeloma. Twenty of these studies were published as full texts and provided detailed information on the study design, participants, methods and outcomes (Bahlis 2017; Beksac 2011; Durie 2017; Facon 2007; Hulin 2009; Hungria 2016; Ludwig 2009; Magarotto 2016; Mateos 2014; Morgan 2011; Niesvizky 2015; Palumbo 2006; Palumbo 2012; Palumbo 2014; Sacchi 2011; San Miguel 2008; Stewart 2015; Waage 2010; Wijermans 2010; Zweegman 2016). Five trials were either published as an abstract (Katsuoka 2013; Kim 2007; Mookerjee 2017; Pawlyn 2017) or letter (Jacobus 2016), and therefore did not provide all relevant information. Most studies provided hazard ratios (HRs) for survival outcomes, or enough information to calculate HRs, so that we could include all 21 treatment options in network meta‐analyses. However, it was not clear whether all of the trials checked the assumption of proportional hazards. If this assumption is not met and cox proportional hazards model was used, it is not clear what impact this would have on the meta‐analysis. Also, networks were not fully connected, so that only treatments which were included in the same subnetwork could be compared to each other. Reporting of AEs differed between studies and led to a limited comparability. At the protocol stage, we decided to use the most frequently reported way for network meta‐analysis (amount of participants with at least one event grade ≥ 3 (or at least one SAE)). Therefore, to be able to include reported data into network meta‐analyses, we could only consider AEs, when the amount of participants with at least one event grade ≥ 3 (or at least one SAE) were reported. We could not consider cumulated events or breakdowns in degrees of severity or further subgroups. As a result, network meta‐analysis was not possible for the outcome grade 3 and 4 AEs due to severe inconsistency. Furthermore, not all trials reported the amount of participants with at least one event. As a result, data were not available for every treatment combination, reducing the informative value of our results. We determined moderate inconsistency within the networks for the outcomes OS, PFS, neutropenia anaemia, and infections. The inconsistency could not be explained statistically and originates probably from the fact that our included trials slightly differ on some effect modifiers (definition of transplant‐ineligibility, subsequent therapies, duration of follow‐up, age, stage of disease, performance score, study start date, prophylaxis, and regions). Nevertheless, from a clinical point of view, our included studies remain largely comparable as these differences are generally small. As previously described, only four trials reported data on health‐related quality of life and network meta‐analysis was not possible for this outcome. Considering the points outlined, we conclude that the completeness and applicability of evidence in this systematic review and network meta‐analysis ranged from high for the outcome withdrawal for adverse events to low for PFS (Table 1).
We did not aim to evaluate adequate prophylactic treatments for the prevention of common adverse events which are associated to the investigated treatments in this review. Therefore we did not analyse whether or which prophylaxis was administered to the participants in our included trials. Adequate prophylaxis may not be universally available. However, co‐administration of appropriate evidence‐based prophylaxis is highly important and may reduce the most frequent adverse events (e.g. infections, thromboembolism) (Key 2019; Taplitz 2018).
Disparities in myeloma incidences by racial ethnicity have been largely explored in recent years and higher incidence rates for black people compared to white people have been discovered (Waxman 2010). Based on these findings, Kirtane 2017 retrospectively explored survival disparities among racial ethnicities. Data suggested that black, Hispanic, and Asians achieve lower survival improvements with immunotherapies compared to white. However, the authors concluded that these differences may rather occur from restricted access to effective treatment modalities. The interaction of participants racial ethnicities and response to anti‐myeloma therapy was further explored with patient‐level data from nine clinical trials with newly diagnosed multiple myeloma (NDMM) (Ailawadhi 2018). Efficacy analysis did not identify statistically significant differences in OS, PFS, or response rates between race‐ethnicity groups. Considering these findings, we conclude that the evidence in this systematic review and network meta‐analysis is equally applicable for low‐ and middle‐income countries(LMICs) and high‐income countries (HICS).
In addition to the studies, included in this review, we are aware of a further two completed and 10 ongoing studies, comparing multiple drug combinations of novel agents for first‐line treatment of transplant‐ineligible multiple myeloma patients. All of these trials included novel second‐generation agents: daratumumab (Jacobus 2016; Facon 2018; Mateos 2018; NCT03710603), siltuximab (San Miguel 2014), elotuzumab (Takezako 2017; Usmani 2014), pembrolizumab (Palumbo 2016), carfilzomib (Facon 2017; Ludwig 2017a; NCT01863550), and ixazomib (NCT01850524; NCT02586038). As outlined in the Why it is important to do this review section of this review, treatment combinations including these agents have already been introduced into anti‐myeloma therapy in HICs. However, as our review was prepared for a specific guidance, we included treatment combinations with novel agents of the first generation, only. Please refer to the following sections for more details: Why it is important to do this review and Methods. Besides these trials, we identified no further ongoing trials matching our inclusion criteria for this review.
Quality of the evidence
The risk of bias was assessed in 25 studies and ten sub‐categories. We decided to expand the 'Risk of bias' criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). In order to be able to rate the certainty in the evidence for our pre‐selected outcomes, we assessed each category whether a judgement would apply for the study in general or whether the judgement could vary between endpoints. We decided that detection bias and attrition bias are endpoint‐dependent 'Risk of bias' categories. Therefore, we assessed the blinding of outcome assessment in three subcategories (not dependent on outcome assessor: OS; partly dependent on outcome assessor:PFS; dependent on outcome assessor: safety outcomes) and incomplete outcome data in two subcategories (time‐to‐event data: survival outcomes; safety data: safety outcomes). A full‐text publication was available for 20 studies, for four studies an abstract only, and for one study a letter only. For the five studies without full‐text publications, we were unable to assess the full risk of bias. All trials described their participants as being randomly assigned to the respective treatment options. However, the process of random sequence generation and allocation concealment was only described in 14 trials. For the remaining 11 trials, the risk of selection bias was unclear. Most of the included studies had an open‐label study design, resulting in an overall high performance and detection bias. Blinding in cancer settings is usually difficult as treatment durations differ, depending on assigned drug combinations. Moreover, the included drugs are differently administered by default: lenalidomide and thalidomide as an daily oral dose; bortezomib once to twice weekly subcutaneous. Detection bias for OS was generally judged as low, as the occurrence and the assessment of this outcome is independent from blinding of participants and personnel. Attrition bias was generally low between studies and judged as unclear for the studies with a published abstract only. We compared whether reported outcomes corresponded with the outcomes, which have been defined at protocol stage, to detect potential reporting bias. This was not the case for three studies (Beksac 2011; Hungria 2016; San Miguel 2008). Beksac 2011 and San Miguel 2008 altered some of their pre‐specified outcomes. Hungria 2016 did not carry‐on one arm of the trial due to unsatisfying interim results. Risk of reporting bias was judged to be high for these three trials. As described in the results, we could identify other bias in three of the included trials. Overall, we judged the risk of bias within and across studies to be high.
We rated the certainty in the evidence for four pre‐selected treatment‐regimens (RD, TMP, VMP, VRDc), respectively compared to MP, and the six most patient‐relevant outcomes (OS, PFS, SAEs, Withdrawals due to AEs, polyneuropathies, QoL) (see Table 1). The certainty of OS estimates for RD, TMP, and VRDc was rated to be moderate. We downgraded by one level for inconsistency due to moderate heterogeneity in OS subnetwork 1 (Figure 3). The certainty of OS estimates for VMP was rated to be low, as we downgraded by one level for imprecision due to wide confidence intervals in addition to the downgrade due to inconsistency. The certainty of all pre‐selected treatment regimens was rated as low for PFS. We downgraded by one level due to high risk of bias and another level for inconsistency due to moderate heterogeneity in PFS subnetwork 1. For the outcome SAE, we could only rate the certainty in the evidence for VMP, as RD, TMP, and VRDc were not connected to MP in the network. We rated the certainty of the SAE estimates for VMP as moderate, as we downgraded by one level for high risk of bias. For the outcome withdrawals due to AEs, we rated the certainty of the estimates to be high for RD, TMP, and VRDc and wish to emphasise the large (negative) effect for all estimates. The certainty of the estimates for VMP was rated to be moderate. We downgraded by one level for imprecision because of wide confidence intervals which included no effect. For the outcome polyneuropathies, we rated the certainty of the estimates to be low for RD and moderate for TMP and VMP and wish to emphasise the large (negative) effect for TMP and VMP. No study reported the outcome polyneuropathies for the treatment with VRDc and we therefore had no estimates for which we could assess the certainty in the evidence. Meta‐analysis was not possible for the outcome QoL and we could not assess the certainty in the evidence.
Potential biases in the review process
To avoid potential bias in the review, we only included RCTs and conducted all review steps in duplicate by two independent review authors (study selection, data extraction, 'Risk of bias' assessment, and GRADE of the evidence). Any conflicts were discussed until consensus could be reached. An experienced Information Specialist developed a sensitive search strategy. We searched all relevant databases, trial registries, conference proceedings, and reference lists to minimise potential publication bias. We did not identify any publication bias and are confident that we identified all relevant studies. Overall, we followed Cochrane guidelines and recommendations in every stage of our review and are not aware of any deficiencies in our review process. However, in our opinion, both the 'Risk of bias' assessment and GRADE, are subjective tools in general and therefore are done in duplicate to avoid or minimise subjective influences. Nonetheless, the assessment of one author team can be more rigorous than from another author team and therefore may lead to a different overall judgement of the quality of the included studies and the certainty in the evidence.
Agreements and disagreements with other studies or reviews
To our knowledge this is the first comprehensive review with network meta‐analysis comparing safety and effectiveness of multiple drug combinations as initial treatment for adults with newly diagnosed, transplant‐ineligible multiple myeloma. Furthermore, this is the first network meta‐analysis distinguishing not only between different drug combinations, but also between treatment durations.
We identified four systematic reviews and network meta‐analyses that have been conducted to evaluate the effectiveness of different first‐line treatment options for adults with transplant‐ineligible multiple myeloma. Kuhr 2016 performed a network meta‐analysis indirectly comparing VMP and TMP over the common comparator MP. Seven trials were included in this network meta‐analysis, all of which are also included in our review. Results showed that VMP and TMP were superior to MP in terms of OS (VMP: hazard ratio (HR) 0.70 (95% confidence interval (CI) 0.57 to 0.86); TMP: HR 0.83 (95% CI 0.66 to 1.03); and PFS (VMP: HR 0.56 (95% CI 0.39 to 0.79); TMP: HR 0.67 (95% CI 0.56 to 0.81); and that VMP may not be superior to TMP (OS: HR 0.85 (95% CI 0.63 to 1.14); PFS: HR 0.83 (95% CI 0.56 to 1.23). These findings are in agreement with our larger network meta‐analysis. Furthermore, Kuhr 2016 and colleagues found a statistically significantly lower risk for the incidence of grade 3 and 4 AEs for MP compared to VMP risk ratio (RR 1.13 (95% CI 1.06 to 1.20), and TMP (RR 2.06 (95% CI 1.43 to 2.98), respectively. Network estimates indicated a lower risk for grade 3 and 4 AEs for VMP compared to TMP (RR 0.55 (95% CI 0.38 to 0.80)). Due to strong variations between reporting and defining grade 3 and 4 AEs in our included studies, we could not accept the similarity assumption and therefore not perform network meta‐analysis for this outcome.
Additionally to the three compared treatment regimens, which have been compared by Kuhr 2016, and Weisel 2017 also included lenalidomide‐based regimens (MPR (in our review referred to as RMP), MPR‐R (in our review referred to as RMPc), and RD) in their network meta‐analysis. All studies, included by Weisel 2017, were included in our review. Analysis of survival data showed statistically significant superiority of RD compared to all treatment combinations for OS (MP HR 0.46 (95% CI 0.34 to 0.60); MPT: HR 0.75 (95% CI 0.62 to 0.90); MPR: HR 0.38 (95% CI 0.23 to 0.60); MPR‐R: HR 0.47 (95% CI 0.31 to 0.71); MPT‐T: HR 0.46 (95% CI 0.33 to 0.64); VMP: HR 0.66 (95% CI 0.46 to 0.93)), and PFS (MP: HR 0.39 (95% CI 0.3 to 0.50); MPT: HR 0.69 (95% CI 0.59 to 0.80); MPR: HR 0.39 (95% CI 0.26 to 0.58); MPR‐R: HR 0.64 (95% CI 0.46 to 0.89); MPT‐T: HR 0.60 (95% CI 0.45 to 0.79), VMP: HR 0.70 (95% CI 0.49 to 0.99)). These results do not completely align with our results. However, according to the P score ranking of our network meta‐analysis, results show that fixed and continuous treatment with RD do have the highest benefit on OS out of the treatment combinations, which have been compared by Weisel 2017. Our ranking of treatments according to the P score suggests a higher benefit for continuous RMP for the outcome PFS than RD.
Liu 2017 and colleagues compared first‐line treatment options for elderly people with transplant‐ineligible myeloma. Despite these inclusion criteria, two trials, analysing reduced‐intensity stem cell transplantation (STC) in one arm, were included in their review (Facon 2007; Palumbo 2004). One of these trials was a three‐arm study, also comparing MP versus TMP (Facon 2007). We included this study in our review, but excluded the STC‐arm from our analysis. Furthermore, one study, evaluating the efficacy of the monoclonal antibody siltuximab plus VMP, was included in the review of Liu 2017, which was not included in ours. PFS results showed superiority of continuous lenalidomide plus dexamethasone (abbreviated to Ld in the article) (HR 0.41 (95% CI 0.21 to 0.79)); MPR‐R (HR 0.58 (95% CI 0.41 to 0.84)), MPT (HR 0.57 (95% CI 0.38 to 0.84)) and MPT‐T (HR 0.72 (95% CI 0.55 to 0.95)) compared to MP, respectively. and correspond with our results. OS results showed superiority of fixed treatment with RD (given for 18 treatment cycles) (HR 0.55 (95% CI 0.41 to 0.74)); RDc (HR 0.49 (95% CI 0.37 to 0.66)), MPT (HR 0.63 (95% CI 0.51 to 0.78)), and VMP (HR 0.7 (95% CI 0.54 to 0.9)), and inferiority of TD (HR 1.55 (95% CI 1.06 to 12.27)), compared to MP, respectively. In comparison, only the statistically significant OS superiority of RD compared to MP could be confirmed by our results (Table 3).
Blommestein 2019 included 24 trials in their systematic review and network meta‐analysis, out of which four were not included in our review (Facon 2006; Mateos 2018; Rajkumar 2008; Zonder 2010). These trials included treatment combinations, which were excluded for our review (dexamethasone alone, dexamethasone + melphalan, dexamethasone + interferon alpha, daratumumab + VMP). Furthermore, Blommestein 2019 also included the STC‐arm of Facon 2007 in their analysis. They did not include five trials, which we additionally included, because no full‐text publication was available (Jacobus 2016; Katsuoka 2013; Kim 2007; Mookerjee 2017; Pawlyn 2017). Despite these differences, results for the (single reported) outcome PFS align with our network meta‐analysis. According to the P score ranking, Blommestein 2019 identified Dara‐VMP as the best treatment option, followed by VMPT‐VT, VRd, MPR‐R. However, we did not include Dara‐VMP in our analysis, VTMPc, VRDc, and RMPc took the first three places in our PFS treatment ranking.
In summary, consensus in all network meta‐analyses can be reached that drug combinations including one or more novel agents are superior to MP in terms of survival outcomes.
Authors' conclusions
Implications for practice.
Considering all 21 comparisons in this network meta‐analysis, continuous treatment with VRD (bortezomib plus lenalidomide plus dexamethasone) or VTMP (bortezomib plus thalidomide melphalan and prednisone) showed the highest survival benefits, compared to MP (melphalan and prednisone). RD ( lenalidomide and dexamethasone) and TMP (thalidomide melphalan and prednisone) also considerably improved overall survival (OS), respectively compared to MP. However, treatment combinations of bortezomib, lenalidomide and thalidomide also substantially increase incidence of adverse events (AEs), and lead to a higher risk of treatment discontinuation. We included the outcome “withdrawals due to adverse events” so that clinicians in the field can be informed by data on expected AEs and consequential treatment discontinuations for their choice of therapy. Clinicians in the field should individually evaluate, with their patients, whether the increase in OS achieved with the novel drug combinations is outweighed by the increase in harms including the increase in risk of polyneuropathies.
Implications for research.
Substantial clinical evidence from randomised controlled trials (RCTs) support the effectiveness of multiple drug combinations of bortezomib, lenalidomide and thalidomide as first‐line treatment for multiple myeloma. Their effectiveness and safety profiles may best be analysed in further randomised head‐to‐head trials. Further trials should focus on consistent reporting of safety outcomes and should use a standardised instrument to evaluate QoL to ensure comparability of treatment combinations.
What's new
| Date | Event | Description |
|---|---|---|
| 14 August 2020 | Amended | Following correspondence between the editorial base and the funding institution of one of the authors, the internal sources of support and the acknowledging statement was updated. |
History
Review first published: Issue 11, 2019
| Date | Event | Description |
|---|---|---|
| 25 November 2019 | Amended | Minor formatting edit to improve readability of the Summary of Findings table in the published PDF. |
Acknowledgements
This review was published in collaboration with the Cochrane Fast‐Track Service. We particularly thank Helen Wakeford (Fast‐Track Managing Editor), Newton Opiyo (Cancer Network Associate Editor and Sign‐off Editor), Toby Lasserson (Sign‐off Editor), Kerry Dwan (Statistical Editor), and Heather Maxwell (Copy Editor) for their support. The Fast‐Track Service team made comments on the review and managed the editorial process.
We thank Lorenzo Moja and Nicola Magrini, Secretary of the World Health Organization Expert Committee on the Selection and Use of Essential Medicines, for their support and guidance during the planning and conduct of this review.
We thank Robin Featherstone for commenting on the search strategy and Gerald Gartlehner for commenting on the methodology of this review.
We thank the consumer editors Caroline Healy and Céline Fournier who supported the improvement of the comprehensibility of this review.
We thank all external peer‐reviewers who read and commented on this review. We thank Fergus Macbeth, Lynda Ware, Anne Eisinga, Angela Dispenzieri, and Giada Bianchi who greatly helped to improve this review.
The research was supported by NHS Blood and Transplant and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
ID Search
#1 MeSH descriptor: (Multiple Myeloma) explode all trees
#2 myelom*
#3 MeSH descriptor: (Plasmacytoma) explode all trees
#4 plasm*cytom*
#5 plasmozytom*
#6 plasm* cell myelom*
#7 myelomatosis
#8 MeSH descriptor: (Leukemia, Plasma Cell) explode all trees
#9 (plasma* near/3 neoplas*)
#10 kahler*
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 with Cochrane Library publication date between Jan 1998 and Feb 2019
Appendix 2. MEDLINE/Ovid search strategy
| MEDLINE/Ovid search strategy for RCTs | |
| # | searches |
| 1 | exp MULTIPLE MYELOMA/ |
| 2 | myelom$.tw,kf,ot. |
| 3 | exp PLASMACYTOMA/ |
| 4 | plasm?cytom$.tw,kf,ot. |
| 5 | plasmozytom$.tw,kf,ot. |
| 6 | plasm$ cell myelom$.tw,kf,ot. |
| 7 | myelomatosis.tw,kf,ot. |
| 8 | LEUKEMIA, PLASMA CELL/ |
| 9 | (plasma$ adj3 neoplas$).tw,kf,ot. |
| 10 | kahler*.tw,kf,ot. |
| 11 | or/1‐10 |
| 12 | randomized controlled trial.pt. |
| 13 | controlled clinical trial.pt. |
| 14 | randomi?ed.ab. |
| 15 | placebo.ab. |
| 16 | clinical trials as topic.sh. |
| 17 | randomly.ab. |
| 18 | trial.ti. |
| 19 | or/12‐18 |
| 20 | exp ANIMALS/ not HUMANS/ |
| 21 | 19 not 20 |
| 22 | 11 and 21 |
| 23 | limit 22 to dt=19980101‐20190214 |
| MEDLINE/Ovid search strategy for Systematic Reviews | |
| # | searches |
| 1 | exp MULTIPLE MYELOMA/ |
| 2 | myelom$.tw,kf,ot. |
| 3 | exp Plasmacytoma/ |
| 4 | plasm?cytom$.tw,kf,ot. |
| 5 | plasmozytom$.tw,kf,ot. |
| 6 | plasm$ cell myelom$.tw,kf,ot. |
| 7 | myelomatosis.tw,kf,ot. |
| 8 | LEUKEMIA, PLASMA CELL/ |
| 9 | (plasma$ adj3 neoplas$).tw,kf,ot. |
| 10 | kahler*.tw,kf,ot. |
| 11 | or/1‐10 |
| 12 | (MEDLINE or systematic review).tw. or meta analysis.pt. |
| 13 | 11 and 12 |
| 14 | limit 11 to systematic reviews |
| 15 | 13 or 14 |
| 16 | from 15 keep 1‐818 |
| 17 | limit 16 to dt=19980101‐20190214 |
Appendix 3. Search criteria study registries
We applied the following search criteria (adjusted according to available search options):
phase: ≥ 2
study type: interventional studies
condition: multiple myeloma
interventions: bortezomib, velcade, lenalidomide, revlimid, thalidomide
date of registration or study start: as of 01 January 1998
Appendix 4. Search criteria conference proceedings
We searched for the following keywords:
multiple myeloma or plasmozytoma in combination with newly diagnosed, first‐line or initial therapy
transplant‐ineligible, non transplant
bortezomib, velcade, lenalidomide, revlimid, thalidomide
random
Appendix 5. Additional figures
Figure 6; Figure 7; Figure 8; Figure 9; Figure 10; Figure 11; Figure 12; Figure 13; Figure 14; Figure 15; Figure 16; Figure 17; Figure 18; Figure 19; Figure 20; Figure 21; Figure 22; Figure 23; Figure 24; Figure 25; Figure 26; Figure 27; Figure 28; Figure 29; Figure 30; Figure 31
6.

Network graph for the outcome PFS. Any two treatments are connected by a line when there is at least one study comparing the two treatments. Line width: number of patients
7.

Forest plot for the outcome PFS. (a) PFS‐subnetwork 1. Reference treatment: MP. (b) PFS‐subnetwork 2. Reference treatment: VMPc. Treatments are ordered by P‐Score (descending)
8.
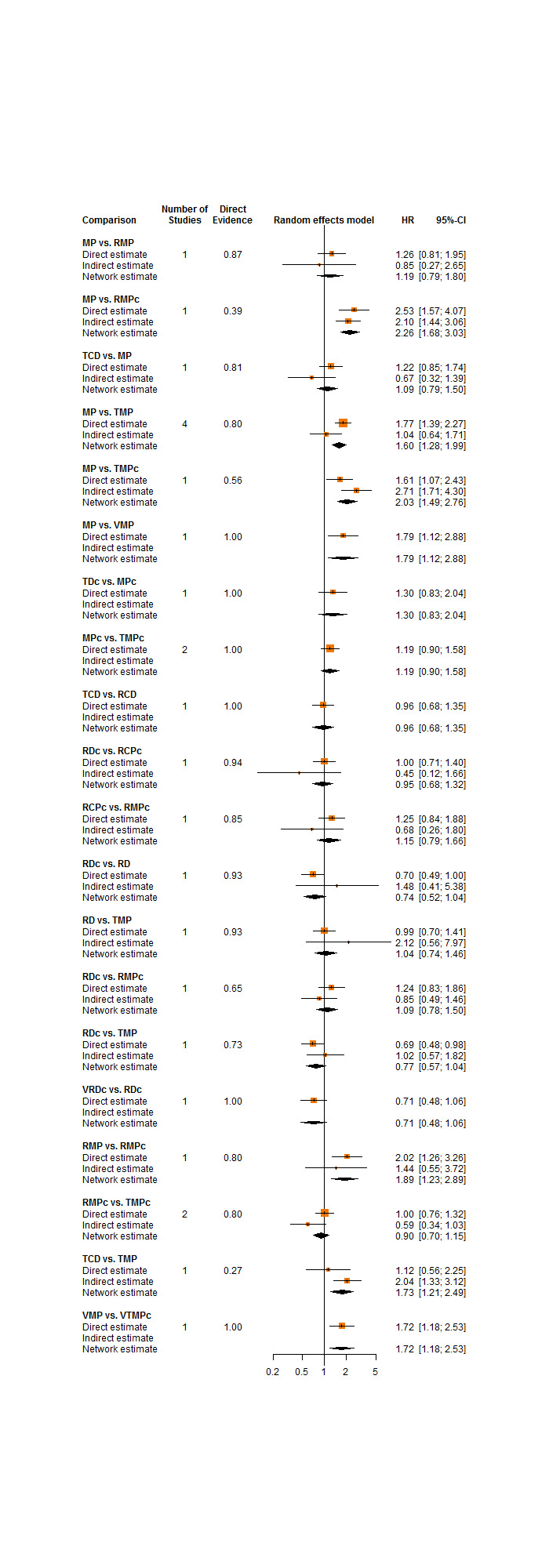
Local approach to check inconsistency ‐ comparison of direct and indirect estimate for closed loops in PFS‐subnetwork 1
9.

Pairwise comparisons for the outcome grade 3 or 4 adverse events.
The first‐named interventions are referred to as "intervention I". The last‐named interventions are referred to as "intervention II".
A RR < 1 indicates an advantage for the experimental intervention and correspondingly a smaller risk for AEs grade 3 or 4.
10.

Network graph for the outcome polyneuropathy. A line connects any two treatments when there is at least one study comparing the two treatments. Line width: number of patients
11.

Forest plot for outcome polyneuropathy. (a) polyneuropathy‐ sSubnetwork 1. Reference treatment: MP. (b) polyneuropathy‐subnetwork 2. Reference treatment: VMPc. Treatments are ordered by P‐Score (descending).
12.

Local approach to check inconsistency ‐ comparison of direct and indirect estimate for closed loops in polyneuropathy‐subnetwork 1
13.

Network graph for the outcome neutropenia. A line connects any two treatments when there is at least one study comparing the two treatments. Line width: number of patients
14.

Forest plot for outcome neutropenia. (a) neutropenia‐subnetwork 1. Reference treatment: MP. (b) neutropenia‐subnetwork 2. Reference treatment: VMPc. Treatments are ordered by P‐Score (descending).
15.

Local approach to check inconsistency ‐ comparison of direct and indirect estimate for closed loops in neutropenia‐subnetwork 1
16.

Network graph for the outcome anaemia. A line connects any two treatments when there is at least one study comparing the two treatments. Line width: number of patients
17.

Forest plot for outcome anaemia. (a) anaemia‐subnetwork 1. Reference treatment: MP. (b) anaemia‐subnetwork 2. Reference treatment: VMPc. Treatments are ordered by P‐Score (descending).
18.

Local approach to check inconsistency ‐ comparison of direct and indirect estimate for closed loops in anaemia‐subnetwork 1
19.

Network graph for the outcome thrombocytopenia. A line connects any two treatments when there is at least one study comparing the two treatments. Line width: number of patients
20.

Forest plot for outcome thrombocytopenia. (a) thrombocytopenia‐subnetwork 1. Reference treatment: MP. (b) thrombocytopenia‐subnetwork 2. Reference treatment: VMPc. Treatments are ordered by P‐Score (descending).
21.

Local approach to check inconsistency ‐ comparison of direct and indirect estimate for closed loops in thrombocytopenia‐subnetwork 1
22.

Network graph for the outcome thromboembolism. A line connects any two treatments when there is at least one study comparing the two treatments. Line width: number of patients
23.

Forest plot for outcome thromboembolism. thromboembolism‐subnetwork 1. Reference treatment: MP. Treatments are ordered by P‐Score (descending).
24.

Network graph for the outcome infections. A line connects any two treatments when there is at least one study comparing the two treatments. Line width: number of patients
25.

Forest plot for outcome infections. (a) infections‐subnetwork 1. Reference treatment: MP. (b) infections‐subnetwork 2. Reference treatment: VMPc. Treatments are ordered by P‐Score (descending).
26.

Local approach to check inconsistency ‐ comparison of direct and indirect estimate for closed loops in infections‐subnetwork 1
27.

Network graph for the outcome SAEs. A line connects any two treatments when there is at least one study comparing the two treatments. Line width: number of patients
28.

Forest plot for outcome serious adverse events. (a) SAE‐subnetwork 1. Reference treatment: RD. (b) SAE‐subnetwork 2. Reference treatment: VMPc. SAE‐subnetwork 3. Reference treatment: MP. Treatments are ordered by P‐Score (descending).
29.

Network graph for the outcome withdrawals due to adverse events. A line connects any two treatments when there is at least one study comparing the two treatments. Line width: number of patients
30.

Forest plot for outcome withdrawals due to adverse events. (a) withdrawals due to AEs‐subnetwork 1. Reference treatment: MP. (b) withdrawals due to AEs‐subnetwork 2. Reference treatment: VMPc. Treatments are ordered by P‐Score (descending).
31.

Local approach to check inconsistency ‐ comparison of direct and indirect estimate for closed loops in withdrawals due to AEs‐subnetwork 1
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bahlis 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "validated interactive voice‐response system" |
| Allocation concealment (selection bias) | Low risk | Comment: central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: "open‐label" |
| Blinding of safety assessment (detection bias) | High risk | Quote: "open‐label" |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol and results for all pre‐specified primary and secondary outcomes available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Beksac 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised centrally" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised centrally" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | High risk | Comment: protocol available; pre‐specified primary and secondary outcomes have been altered. RR was supposed to be evaluated after 12 months, was reported after four 6‐week cycles; TTP was supposed to be evaluated, DFS was reported |
| Other bias | Low risk | Quote: "Data were monitored by an independent contract research organization (CRO; OMEGA, Ankara, Turkey), who also performed statistical analyses (Dr Z. Arat). spss 15.0 (SPSS Inc., Chicago, IL, USA) software was used for data analysis"; Comment: the study appear to be free of other sources of bias |
Durie 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "dynamic allocation algorithm" |
| Allocation concealment (selection bias) | Low risk | Comment: central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"no masking to treatment interventions" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote:"no masking to treatment interventions" |
| Blinding of safety assessment (detection bias) | High risk | Quote:"no masking to treatment interventions" |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol and results for all pre‐specified primary and secondary outcomes available |
| Other bias | Low risk | Quote: "An independent data and safety monitoring committee reviewed unblinded safety data twice a year." |
Facon 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol and results for all pre‐specified primary and secondary outcomes available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Hulin 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned patients centrally" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly assigned patients centrally" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curve |
| Incomplete safety data (attrition bias) | Low risk | Comment: all patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not available, but report includes all expected outcomes |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Hungria 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: " open‐label" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: " open‐label" |
| Blinding of safety assessment (detection bias) | High risk | Quote: " open‐label" |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | High risk | Quote:"an unplanned interim analysis was conducted and suggested that the TD arm displayed inferior efficacy in terms of PFS, when compared with MPT and CTD (...). Therefore, only descriptive results for the TD arm will be presented"; Comment: >10% missing data, without explanations in one arm |
| Other bias | High risk | Quote:"an unplanned interim analysis was conducted and suggested that the TD arm displayed inferior efficacy in terms of PFS, when compared with MPT and CTD (...). Therefore, only descriptive results for the TD arm will be presented"; Comment: >10% missing data, without explanations in one arm |
Jacobus 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information, only letter available |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information, only letter available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: insufficient information, only letter available, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: insufficient information, only letter available, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: insufficient information, only letter available, probably unblinded |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information, only letter available |
| Other bias | High risk | Quote: "closed enrollment prematurely due to slow accural" |
Katsuoka 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Incomplete survival data (attrition bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Incomplete safety data (attrition bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Kim 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Incomplete survival data (attrition bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Incomplete safety data (attrition bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Ludwig 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"centralized and computerized randomisation system" |
| Allocation concealment (selection bias) | Low risk | Quote:"centralized and computerized randomisation system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curve |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: pre‐specified secondary outcome QoL not reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Magarotto 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised based on a computer‐generated randomisation schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised based on a computer‐generated randomisation schedule" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: Patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available; pre‐specified primary and most secondary outcomes reported; pre‐specified secondary outcomes TTP and TTNT not reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Mateos 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerised random number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "computerised random number generator" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants, those giving the interventions, those assessing outcomes, and those analysing the data were not masked to group assignment." |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: Patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: "Participants, those giving the interventions, those assessing outcomes, and those analysing the data were not masked to group assignment." |
| Blinding of safety assessment (detection bias) | High risk | Quote: "Participants, those giving the interventions, those assessing outcomes, and those analysing the data were not masked to group assignment." |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol available; pre‐specified outcome "duration of response" not reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Mookerjee 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Incomplete survival data (attrition bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Incomplete safety data (attrition bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Morgan 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "automated 24‐hour telephone system" |
| Allocation concealment (selection bias) | Low risk | Quote: "automated 24‐hour telephone system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: not described, probably unblinded |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: 2 pathways in 1 trial; relevant pre‐specified primary and secondary results reported, other pre‐specified results regarding the other pathway reported in other publications |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Niesvizky 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "interactive voice response system on the basis of a computer‐generated randomisation schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "interactive voice response system on the basis of a computer‐generated randomisation schedule" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: " open‐label" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: " open‐label" |
| Blinding of safety assessment (detection bias) | High risk | Quote: " open‐label" |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol and results for all pre‐specified primary and secondary outcomes available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Palumbo 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation sequence was generated by a centralised computer" |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomly allocated to treatments by use of an automated assignment procedure concealed from the investigators" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Open Label" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: "Open Label" |
| Blinding of safety assessment (detection bias) | High risk | Quote: "Open Label" |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol and results for all pre‐specified primary and secondary outcomes available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Palumbo 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "procedure utilising a validated interactive voice response system" |
| Allocation concealment (selection bias) | Low risk | Quote: "procedure utilising a validated interactive voice response system" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients and treating physicians were unaware of the treatment assignments" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | Low risk | |
| Blinding of safety assessment (detection bias) | Low risk | Quote: "Patients and treating physicians were unaware of the treatment assignments" |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐specified outcomes reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Palumbo 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "OPEN LABEL" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: Patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: "OPEN LABEL" |
| Blinding of safety assessment (detection bias) | High risk | Quote: "OPEN LABEL" |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol and results for all pre‐specified primary and secondary outcomes available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Pawlyn 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Blinding of safety assessment (detection bias) | High risk | Comment: insufficient information, only abstracts available, probably unblinded |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information, only abstracts available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Sacchi 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "centrally randomised" |
| Allocation concealment (selection bias) | Low risk | Quote "centrally randomised" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: Patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: "open label" |
| Blinding of safety assessment (detection bias) | High risk | Quote: "open label" |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available; pre‐specified primary and most secondary outcomes reported; pre‐specified outcome DR not reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
San Miguel 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: " open‐label" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are death or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: " open‐label" |
| Blinding of safety assessment (detection bias) | High risk | Quote: " open‐label" |
| Incomplete survival data (attrition bias) | Low risk | Comment: censored Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received study drug |
| Selective reporting (reporting bias) | High risk | Comment: protocol available; not all pre‐specified primary and secondary endpoints are reported; missing: ORR, QoL |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Stewart 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatments were assigned using permuted blocks within strata with dynamic balancing within main institution and their affiliate networks" |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatments were assigned using permuted blocks within strata with dynamic balancing within main institution and their affiliate networks" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: Patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: "open label" |
| Blinding of safety assessment (detection bias) | High risk | Quote: "open label" |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol and results for all pre‐specified primary and secondary outcomes available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Waage 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"centralized and performed by telephone call or fax" |
| Allocation concealment (selection bias) | Low risk | Quote:"centralized and performed by telephone call or fax" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"neither patient nor doctor know which of these are given"; Comment: double‐blind |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | Low risk | Quote:"neither patient nor doctor know which of these are given"; Comment: double‐blind |
| Blinding of safety assessment (detection bias) | Low risk | Quote:"neither patient nor doctor know which of these are given"; Comment: double‐blind |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available; 2‐phase study. Pre‐specified objectives regarding maintenance therapy (Time to 2. response, Frequency of 2. response, Time to 2. progression) not reported in this publication; not sure whether frequency of response stands for PR, VGPR, PR, MR, NR |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Wijermans 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: " open‐label" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: Patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: " open‐label" |
| Blinding of safety assessment (detection bias) | High risk | Quote: " open‐label" |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | Comment: protocol and results for all pre‐specified primary and secondary outcomes available |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Zweegman 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: " open‐label" |
| Blinding of overall survival assessment (detection bias) | Low risk | Comment: Patients are dead or alive |
| Blinding of progression free survival assessment (detection bias) | High risk | Quote: " open‐label" |
| Blinding of safety assessment (detection bias) | High risk | Quote: " open‐label" |
| Incomplete survival data (attrition bias) | Low risk | Comment: Kaplan Meier curves |
| Incomplete safety data (attrition bias) | Low risk | Comment: all reported patients received at least one study drug |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available; study ongoing. Pre‐specified objectives regarding maintenance therapy not yet reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
ECOG: Eastern Cooperative Oncology Group i.v.: intravenous MM: multiple myeloma NCI: National Cancer Institute NDMM: newly diagnosed multiple myeloma s.c.subcutaneous SGOT: serum glutamic‐oxaloacetic transaminase ( AST ‐ aspartate aminotransferase) SGPT: Serum glutamic pyruvic transaminase (ALT, ‐ alanine aminotransferase) WHO: Worlsd Health Organization
Units dL: decilitre kg: kilogram mg: milligram m²: square metre mm3: cubic millimetre μL: microlitre
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 2003 | Comparator not of interest, dexamethasone alone |
| Barlogie 2004 | Patients with relapsed or progressive disease after at least one autologous transplant |
| Brioli 2014 | Patients who are candidates to receive double autologous transplantation |
| Facon 2006 | Comparator not of interest, dexamethasone alone |
| Facon 2017 | Includes a novel agent, which was excluded from our review |
| Facon 2018 | Includes a novel agent, which was excluded from our review |
| Foa 2007 | Relapsed/refractory multiple myeloma patients |
| Harousseau 2003 | Transplant setting |
| Hejlova 2000 | The protocol consisted of 4 cycles of induction therapy using VAD followed by autologous transplantation. Patients were then randomised into two groups receiving interferon‐alpha or interferon‐alpha plus dexamethasone as maintenance treatment. |
| Hernández 2004 | Comparator not of interest, dexamethasone combined with melphalan |
| Kumar 2012 | A total of 150 patients were included in the study, out of which 75 received a stem cell mobilisation after cycle 2 and 59 received a stem cell transplantation after cycle 4. |
| Ludwig 2017a | Includes a novel agent, which was excluded from our review |
| Mateos 2016 | Sequential versus alternating VMP/Rd: no results after first cycle |
| Mateos 2018 | Includes a novel agent, which was excluded from our review |
| Merz 2015 | Comparator not of interest, bortezomib+doxorubicin+dexamethasone |
| Montefusco 2013 | Non‐randomised trial |
| Morgan 2002 | Standard conventional‐dose combination chemotherapy with adriamycin, carmustine, cyclophosphamide, and melphalan (ABCM) or a sequence of treatment of cyclophospamide, vincristine, adriamycin, and methyl‐prednisolone (C‐VAMP) followed by high‐dose therapy (HDT) |
| NCT00522392 | Consolidation therapy, patients received 1‐6 prior cycles of dexamethasone‐based regimens |
| NCT00734877 | Transplant‐setting |
| NCT01850524 | Includes a novel agent, which was excluded from our review |
| NCT01863550 | Includes a novel agent, which was excluded from our review |
| NCT02586038 | Includes a novel agent, which was excluded from our review |
| NCT03710603 | Includes a novel agent, which was excluded from our review |
| Niesvizky 2003 | Comparator not of interest, dexamethasone alone |
| Offidani 2004 | Relapsed/refractory multiple myeloma patients |
| Palumbo 2016 | Includes a novel agent, which was excluded from our review |
| Rajkumar 2006b | Comparator not of interest, dexamethasone alone |
| Rajkumar 2008 | Comparator not of interest, dexamethasone alone |
| San Miguel 2014 | includes a novel agent, which was excluded from our review |
| Takezako 2017 | Includes a novel agent, which was excluded from our review |
| Usmani 2014 | Includes a novel agent, which was excluded from our review |
| White 2007 | Non‐randomised trial |
| Zonder 2010 | Comparator not of interest, dexamethasone alone |
Differences between protocol and review
The protocol for this review was registered with PROSPERO (Piechotta 2018). After further discussions with the WHO EML Cancer Medicines Workung Group and the perusal of the characteristics of included studies, we had to revise some elements of the methodology. These elements are outlined below.
Outcomes
Adequate prophylaxis of treatment‐related adverse events is very important, but not easily available in low‐ and middle‐income countries (LMICs). As the aim of this review was to inform an application for the WHO's list of essential medicines, we decided that beside focusing on the efficacy and safety of the studied drug combinations, we should also compare the adherence to the assigned therapies. We added the outcome "withdrawals due to adverse events" after discussions with the WHO EML Cancer Medicines Working Group.
Subgroup analyses
Follow‐up (short term (< 5 years) versus long term >= 5 years)
Considering the treatment durations of the included studies, we decided to adapt the planned follow‐up subgroup analysis. Instead of comparing a follow‐up time of < 1 year to >= 1 year, we decided to compare a follow‐up time of < 5 years versus >= 5 years to better focus on the long‐term efficacy of the compared treatment combinations.
Subgroup analyses for a follow‐up period >= 5 years could not be performed for overall survival (OS) and progression‐free survival (PFS) as only two studies (Jacobus 2016; Mateos 2014) reported a median follow‐up over five years. Treatment regimens of these two studies were not connected in a network.
Multiple myeloma international staging system (I, II, III)
Subgroup analyses for myeloma disease staging could not be performed as all studies included disease stages I, II, and III.
Age (< 75 versus > 75)
Subgroup analyses for age groups (<75 versus. >=75 years) could not be performed, as only one study (Hulin 2009) included only patients >75 years.
Region (low‐ and middle‐income countries versus high‐income countries)
Subgroup analyses for regions (low‐ and middle‐income versus high‐income countries) could not be performed, as all trials which included patients from low‐ and middle‐income regions were multicentre studies and also included patients from high‐income settings.
Sensitivity analysis
Risk of bias (low versus high)
For OS, robustness of results for VRDc and VTMPc could not be tested in sensitivity analysis for low risk of bias, as all studies which included these treatments (Durie 2017; Palumbo 2014) had an unclear or high risk of bias.
Furthermore, risk of bias sensitivity analysis could not be performed for all other outcomes as Palumbo 2012 and Waage 2010 were the only studies with a low risk of bias. For PFS, polyneuropathies and infections, sensitivity analysis without high risk of bias could not be performed as all studies reporting these outcomes, except Palumbo 2012 and Waage 2010 had a high risk of bias and treatment regimens of these two studies were not connected in a network. For anaemia, thrombocytopenia, severe adverse events (SAEs), withdrawals due to AEs, sensitivity analysis without high risk of bias could not be performed as all studies reporting these outcomes, except Palumbo 2012, which ad a high risk of bias. For thromboembolism, sensitivity analysis without high risk of bias could not be performed as all studies reporting this outcome, except Waage 2010, which had a high risk of bias. For neutropenia, sensitivity analysis without high risk of bias could not be performed as all studies reporting this outcome had a high risk of bias.
Contributions of authors
| Drafted the protocol | VP, NS, TJ, PL, LE |
| Clinical expertise and advice | CS, SO, ST |
| Developed and ran the search strategy | IM |
| Obtained copies of studies | VP |
| Selected which studies to include (2 people) | NS, VP |
| Extracted data from studies (2 people) | VP, BS |
| Entered data into RevMan | VP |
| Carried out the analyses | AA, KK |
| Interpreted the analyses | VP, TJ, CS |
| Drafted the final review | VP, NS, LE |
| Will update the review | NS, VP |
Sources of support
Internal sources
-
University Hospital Cologne, Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, Germany
Provision of the offices, including technical equipment
NHS Blood and Transplant, UK
External sources
-
Cochrane, UK
Cochrane Review Support Programme (Award of £5000 after publication of the review)
Declarations of interest
NS: none known.
VP: none known.
PL: none known.
TJ: none known.
IM: none known.
CS: The author has received honoraria and travel support from Janssen, Celgene, Novartis, Bristol Myers Squibb and Takeda
SO: none known.
LE: none known.
ST: none known.
KK: none known.
BS: none known.
AA: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Bahlis 2017 {published data only}
- Avet-Loiseau H, Hulin C, Benboubker L, Dimopoulos MA, Belch A, Reece D, et al. Impact of cytogenetics on outcomes of transplant-ineligible patients with newly diagnosed multiple myeloma treated with continuous lenalidomide plus low-dose dexamethasone in the first (MM-020) trial. In: Blood. Vol. 126. 2015:730.
- Bahlis N, Corso A, Mugge LO, Shen ZX, Desjardins P, Stoppa AM, et al. Impact of response in patients (pts) with stem cell transplant (SCT)-ineligible newly diagnosed multiple myeloma (NDMM) treated with continuous lenalidomide + low-dose dexamethasone (Rd) in the first trial. In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 15. 2015:e56.
- Bahlis N, Corso A, Mugge Lo, Shen ZX, Desjardins P, Stoppa AM, et al. Assessing the benefit of continuous treatment in the first trial (MM-020): impact of response in patients with transplant-ineligible newly diagnosed multiple myeloma. In: Haematologica. Vol. 100. 2015:85-6.
- Bahlis NJ, Corso A, Mugge LO, Shen ZX, Desjardins P, Stoppa AM, et al. Benefit of continuous treatment for responders with newly diagnosed multiple myeloma in the randomized FIRST trial. Leukemia 2017;31(11):2435-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. New England Journal of Medicine 2014;371(10):906-17. [DOI] [PubMed] [Google Scholar]
- Delforge M, Minuk L, Eisenmann JC, Arnulf B, Canepa L, Fragasso A, et al. Health-related quality of life (HRQOL) in patients (pts) with newly diagnosed multiple myeloma (NDMM): the first trial. In: Haematologica. Vol. 99. 2014:109-10. [DOI] [PMC free article] [PubMed]
- Delforge M, Minuk L, Eisenmann JC, Arnulf B, Canepa L, Fragasso A, et al. Health-related quality-of-life in patients with newly diagnosed multiple myeloma in the FIRST trial: lenalidomide plus low-dose dexamethasone versus melphalan, prednisone, thalidomide. Haematologica 2015;100(6):826-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos M, Cheung M, Roussel M, Liu T, Gamberi B, Kolb B, et al. Impact of renal impairment on outcomes after treatment with lenalidomide and low-dose dexamethasone in patients with newly diagnosed multiple myeloma: first trial results. In: Haematologica. Vol. 100. 2015:83-4.
- Dimopoulos MA, Cheung MC, Roussel M, Liu T, Gamberi B, Kolb B, et al. Impact of renal impairment on outcomes with lenalidomide and dexamethasone treatment in the FIRST trial, a randomized, open-label phase 3 trial in transplant-ineligible patients with multiple myeloma. Haematologica 2016;101(3):363-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facon T, Dimopoulos MA, Dispenzieri A, Catalano JV, Belch A, Cavo M, et al. Final analysis of overall survival from the first trial. In: Blood. Vol. 128. 2016.
- Facon T, Dimopoulos MA, Dispenzieri A, Catalano JV, Belch A, Cavo M, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood 2018;131(3):301-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facon T, Dimopoulos MA, Dispenzieri A, Catalano JV, Belch A, Hulin C, et al. Lenalidomide+low-dose dexamethasone (RD) VS. melphalanprednisone-thalidomide (MPT) in newly diagnosed multiple myeloma (NDMM) patients (PTS): the first trial. In: Haematologica. Vol. 99. 2014:220-1.
- Facon T, Dimopoulos MA, Hulin C, Benboubker L, Belch A, Ludwig H, et al. Updated overall survival (OS) analysis of the FIRST study: lenalidomide plus low-dose dexamethasone (Rd) continuous vs melphalan, prednisone, and thalidomide (MPT) in patients (Pts) With newly diagnosed multiple myeloma (NDMM). In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 15. 2015:e134.
- Facon T, Dimopoulos MA, Hulin C, Benboubker L, Belch A, Ludwig H, et al. Updated overall survival analysis of the first study: continuous lenalidomide plus low-dose dexamethasone vs melphalan, prednisone, and thalidomide in patients with newly diagnosed multiple myeloma. In: Haematologica. Vol. 100. 2015:3. [DOI] [PMC free article] [PubMed]
- Facon T, Hulin C, Dimopoulos MA, Belch A, Meuleman N, Mohty M, et al. A frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated with continuous lenalidomide plus low-dose dexamethasone in the first (MM-020) trial. In: Haematologica. Vol. 101. 2016:258-9.
- Facon T, Hulin C, Dimopoulos MA, Belch A, Meuleman N, Mohty M, et al. A frailty scale predicts outcomes of patients with newly diagnosed multiple myeloma who are ineligible for transplant treated with continuous lenalidomide plus low-dose dexamethasone on the first trial. In: Blood. Vol. 126. 2015:4239.
- Facon T, Hulin C, Dimopoulos MA, Belch A, Reece DE, Catalano JV, et al. FIRST study: updated overall survival (OS) in stem cell transplant (SCT)-ineligible newly diagnosed multiple myeloma (NDMM) patients (pts) treated with continuous lenalidomide plus low-dose dexamethasone (Rd) vs melphalan, prednisone, and thalidomide (MPT). In: Journal of Clinical Oncology. Vol. 33. 2015.
- Facon T. Continuous therapy for elderly newly diagnosed multiple myeloma. In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 15. 2015:e28.
- Hulin C, Belch A, Shustik C, Petrucci MT, Dührsen U, Lu J, et al. Updated outcomes and impact of age with lenalidomide and low-dose dexamethasone or melphalan, prednisone, and thalidomide in the randomized, phase III FIRST trial. Journal of Clinical Oncology 2016;34(30):3609-17. [DOI] [PubMed] [Google Scholar]
- Hulin C, Shustik C, Belch A, Petrucci M, Duhrsen U, Lu J, et al. Effect of age on efficacy and safety outcomes in patients with newly diagnosed multiple myeloma receiving lenalidomide and low-dose dexamethasone (RD): the first trial. In: Haematologica. Vol. 100. 2015:152-3.
- Hulin C, Shustik C, Belch A, Petrucci MT, Duhrsen U, Lu J, et al. Continuous treatment with lenalidomide and low-dose dexamethasone for patients with transplant-ineligible newly diagnosed multiple myeloma in the first trial: impact of age. In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 15. 2015:e130-1.
- Lu J, Lee JH, Huang SY, Qiu L, Lee JJ, Liu T, et al. Continuous treatment with lenalidomide and low-dose dexamethasone in transplant-ineligible patients with newly diagnosed multiple myeloma in Asia: subanalysis of the first trial. In: Blood. Vol. 126. 2015:4240. [DOI] [PMC free article] [PubMed]
- Lu J, Lee JH, Huang SY, Qiu L, Lee JJ, Liu T, et al. The first trial: analysis of the Asian subgroup of transplant-ineligible patients with newly diagnosed multiple myeloma treated with continuous lenalidomide and low-dose dexamethasone. In: Haematologica. Vol. 101. 2016:548-9.
- Weisel K, Vogl D, Corso A, Delforge M, Song K, Dimopoulos MA, et al. Health resource utilization with continuous lenalidomide treatment (TX) in elderly patients with newly diagnosed multiple myeloma (NDMM). In: Haematologica. Vol. 100. 2015:24-5.
Beksac 2011 {published data only}
- Beksac M, Haznedar R, Firatli-Tuglular T, Ozdogu H, Aydogdu I, Konuk N, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. European Journal of Haematology 2011;86(1):16-22. [DOI] [PubMed] [Google Scholar]
Durie 2017 {published data only}
- Durie B, Hoering A, Rajkumar SV, Abidi MH, Epstein J, Kahanic SP, et al. Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (PTS) with previously untreated multiple myeloma without an intent for immediate Autologous Stem Cell Transplant (ASCT): results of the randomized phase III trial SWOG S0777. In: Blood. Vol. 126. 2015:25. [DOI] [PMC free article] [PubMed]
- Durie BG, Hoering A Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 2017;389:519-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Facon 2007 {published data only}
- Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Harousseau JL, et al. Randomized clinical trial comparing melphalan-prednisone (MP), MP-thalidomide (MP-THAL) and high-dose therapy using melphalan 100 MG/Mý (MEL100) for newly diagnosed myeloma patients Aged 65-75 years. Interim analysis of the IFM 99-06 Trial on 350 patients. In: Blood. Vol. 104. 2004:63.
- Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet 2007;370(9594):1209-18. [DOI] [PubMed] [Google Scholar]
Hulin 2009 {published data only}
- Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. Journal of Clinical Oncology 2009;27(22):3664-70. [DOI] [PubMed] [Google Scholar]
- Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Melphalan-prednisone-thalidomide (MP-T) demonstrates a significant survival advantage in elderly patients >=75 years with multiple myeloma compared with melphalan-pPrednisone (MP) in a randomized, double-blind, placebo-controlled trial, IFM 01/01. In: Blood. Vol. 110. 2007.
- Hulin C, Virion J, Leleu X, Rodon P, Pegourie B, Benboubker L, et al. Comparison of melphalan-prednisone-thalidomide (MP-T) to melphalan-prednisone (MP) in patients 75 years of age or older with untreated multiple myeloma (MM). Preliminary results of the randomized, double-blind, placebo controlled IFM 01-01 trial. In: Journal of Clinical Oncology. Vol. 25. 2007:441.
Hungria 2016 {published data only}
- Anonymous. Iberoamerican protocol with thalidomide in patients with symptomatic newly diagnosed multiple myeloma over 65 years. available at: https://clinicaltrials.gov/show/nct01532856 (Accessed 06 May 2019).
- Crusoe E, Maiolino A, Bittencourt R, Fantl DB, MacIel J, Sampaio M, et al. A phase III study comparing thalidomide/cyclophosphamide/dexa vs thalidomide/dexa vs thalidomide/melphalan/prednisone in de novo multiple myeloma patients not eligible for ASCT. In: Blood. Vol. 118. 2011.
- Hungria V, Crusoe E, Maiolino A, Bittencourt R, Fantl D, Quero A, et al. A phase III study comparing thalidomide/cyclophosphamide/ dexa vs thalidomide/dexa vs thalidomide/melphalan/prednisone in de novo multiple myeloma patients not eligible for ASCT: preliminary analysis. In: Haematologica. Vol. 92. 2010:148.
- Hungria VT, Crusoe EQ, Maiolino A, Bittencourt R, Fantl D, Maciel JF, et al. Phase 3 trial of three thalidomide-containing regimens in patients with newly diagnosed multiple myeloma not transplant-eligible. Annals of Hematology 2016;95(2):271-8. [DOI] [PubMed] [Google Scholar]
Jacobus 2016 {published data only}
- Fonseca R, Rajkumar SV. Consolidation therapy with bortezomib/lenalidomide/ dexamethasone versus bortezomib/dexamethasone after a dexamethasone-based induction regimen in patients with multiple myeloma: a randomized phase III trial. Clinical Lymphoma and Myeloma 2008;8(5):315-7. [DOI] [PubMed]
- Jacobus SJ, Rajkumar SV, Weiss M, Stewart AK, Stadtmauer EA, Callander NS, et al. Randomized phase III trial of consolidation therapy with bortezomib-lenalidomide-Dexamethasone (VRd) vs bortezomib-dexamethasone (Vd) for patients with multiple myeloma who have completed a dexamethasone based induction regimen. Blood Cancer Journal 2016;6(7):e448. [DOI] [PMC free article] [PubMed]
Katsuoka 2013 {published data only}
- Katsuoka Y, Kato Y, Omoto E, Sasaki O, Kimura H, Meguro K, et al. Phase ii trial of bortezomib based regimen for transplant-ineligible multiple myeloma-tomato study. In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 13. 2013:S148.
Kim 2007 {published data only}
- Kim YK, Lee JJ, Sohn SK, Shin HJ, Lee SR, Shim HJ, et al. Efficacy and safety of thalidomide and dexamethasone combination with or without cyclophosphamide in patients with newly diagnosed multiple myeloma. In: Haematologica. Vol. 92. 2007:411-2.
Ludwig 2009 {published data only}
- Ludwig H, Drach J, Tothova E, Gisslinger H, Linkesch W, Jaksic B, et al. Thalidomide-dexamethasone (TD) versus melphalan-prednisolone (MP) as first line treatment in elderly patients with multiple myeloma (MM): an interim analysis. In: Onkologie. Vol. 28. 2005:14-5.
- Ludwig H, Hajek R, Tóthová E, Drach J, Adam Z, Labar B, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood 2009;113(15):3435-42. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Tothova E, Hajek R, Drach J, Adam Z, Labar B, et al. Thalidomide-dexamethasone vs. melphalan-prednisone as first line treatment and thalidomide-interferon vs. interferon maintenance therapy in elderly patients with multiple myeloma. In: Blood. Vol. 110. 2007:163a.
- Ludwig H, Tothova E, Hajek R, Drach J, Labar B, Egyed M, et al. Thalidomide-dexamethasone versus melphalan-prednisolone as first line treatment in elderly patients with multiple myeloma: second interim analysis. In: Haematologica. Vol. 92. 2007:166.
- Ludwig HL, Drach J, Tothova E, Gisslinger H, Linkesch W, Jaksic B, et al. Thalidomide-dexamethasone versus melphalan-prednisolone as first line treatment in elderly patients with multiple myeloma: an interim analysis. In: European Hematology Association. 2005.
- Ludwig HL, Drach J, Tothova E, Gisslinger H, Linkesch W, Jaksic B, et al. Thalidomide-dexamethasone versus melphalan-prednisolone as first line treatment in elderly patients with multiple myeloma: an interim analysis. In: Haematologica. Vol. 90. 2005:158.
Magarotto 2016 {published data only}
- Bringhen S, Offidani M, Musto P, Liberati AM, Benevolo G, Cascavilla N, et al. Long term outcome of lenalidomide-dexamethasone (RD) vs melphalan-lenalidomide-prednisone (MPR) vs cyclophosphamide-prednisone-lenalidomide (CPR) as induction followed by. In: Blood. Vol. 130. 2017.
- Gay F, Bringhen S, Offidani M, Liberati A, Cellini C, Magarotto V, et al. Efficacy and safety of 3 lenalidomide-based combinations in elderly newly diagnosed multiple myeloma patients: results from the phase 3 community based emn01 trial. In: Haematologica. Vol. 98. 2013:95-6.
- Gentile M, Magarotto V, Offidani M, Musto P, Bringhen S, Teresa Petrucci M, et al. Lenalidomide and low-dose dexamethasone (Rd) versus bortezomib, melphalan, prednisone (VMP) in elderly newly diagnosed multiple myeloma patients: A comparison of two prospective trials. American Journal of Hematology 2017;92(3):244-50. [DOI] [PubMed] [Google Scholar]
- Larocca A, Bringhen S, Petrucci MT, Offidani M, Benevolo G, Galli M, et al. Early death in elderly patients with newly diagnosed multiple myeloma treated with new drugs: a pooled analysis of two randomized phase 3 trials. In: Haematologica. Vol. 100. 2015:51-2.
- Larocca A, Bringhen S, Petrucci MT, Offidani M, Paoli L, Pietrantuono G, et al. Early mortality in elderly newly diagnosed multiple myeloma patients treated with novel agents: a pooled analysis of two large randomized phase III trials. In: Haematologica. Vol. 100. 2015:81-2.
- Larocca A, Magarotto V, Offidani M, Pour L, Liberati AM, Benevolo G, et al. Lenalidomide-dexamethasone vs melphalan-prednisone-lenalidomide vs cyclophosphamide-prednisone-lenalidomide in ndmm. In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 13. 2013:S113-s114.
- Larocca A, Offidani M, Ciccone G, Guglielmelli T, Cangialosi C, Salvini M, et al. Impact of bortezomib-or lenalidomide-based induction treatment on high risk cytogenetic transplant-ineligible patients with newly diagnosed multiple myeloma enrolled in the gimema-MM-03-05 and EMN01 trials. In: Blood. Vol. 130. 2017.
- Magarotto V, Bringhen S, Hajek R, Musto P, Goldaniga MC, Ledda A, et al. Lenalidomide-dexamethsone (RD) vs melphalan-lenalidomide-prednisone (MPR) vs cyclophosphamide-prednisone-lenalidomide (CPR) in elderly community-based newly diagnosed myeloma patients. In: Haematologica. Vol. 99. 2014:103-4.
- Magarotto V, Bringhen S, Musto P, Benevolo G, Ledda A, Genuardi M, et al. Feasibility of lenalidomide maintenance therapy in frail patients with multiple myeloma: subgroup analysis of a randomized phase 3 trial. In: Haematologica. Vol. 100. 2015:100-1.
- Magarotto V, Bringhen S, Musto P, Benevolo G, Ledda A, Genuardi M, et al. Lenalidomide-dexamethasone or melphalan-lenalidomide-prednisone (MPR) or cyclophosphamide-prednisone-lenalidomide (CPR) for initial treatment of real life elderly patients with newly diagnosed multiple myeloma. In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 15. 2015:e69.
- Magarotto V, Bringhen S, Offidani M, Benevolo G, Patriarca F, Mina R, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood 2016;127(9):1102-8. [DOI] [PubMed] [Google Scholar]
- Magarotto V, Bringhen S, Offidani M, Benevolo G, Patriarca F, Palladino C, et al. Maintenance therapy with lenalidomide in elderly patients with newly diagnosed multiple myeloma: a Post-HOC analysis of the EMN01 trial. In: Haematologica. Vol. 100. 2015:82.
- Magarotto V, Bringhen S, Offidani M, Liberati AM, Benevolo G, Patriarca F, et al. Lenalidomide plus dexamethasone (RD) vs melphalan-lenalidomide-prednisone (MPR) or cyclophosphamide-prednisone-lenalidomide (CPR) in elderly comunity-based newly diagnosed multiple myeloma patients: efficacy and safety results from a phase 3 trial. In: Haematologica. Vol. 98. 2013:50.
Mateos 2014 {published data only}
- Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, López de la Guía A, et al. Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial. In: Blood. Vol. 120. 2012:2581-8. [DOI] [PubMed]
- Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, Paz R, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncology 2010;11(10):934-41. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Oriol A, Martínez-López J, Teruel AI, López de la Guía A, López J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood 2014;124(12):1887-93. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Oriol A, Martinez-Lopez J, Gutierrez N, Teruel AI, Paz R, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncology 2010;11(10):934-41. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Oriol A, Martinez-Lopez J, Teruel AI, Lopez de la Guia A, Blanchard MJ, et al. Do we still need the alkylators as part of the up front treatment of elderly newly diagnosed multiple myeloma patients? Updated follow-up of GEM2005MAS65 Spanish trial comparing VMP vs VTP as induction. In: Haematologica. Vol. 99. 2014:221.
Mookerjee 2017 {published data only}
- Mookerjee A, Gupta R, Jasrotia S, Sahoo R, Kumar R, Thulkar S, et al. Bortezomib, lenalidomide and low-dose dexamethasone (VRD) versus lenalidomide and low-dose dexamethasone (LD) for newly-diagnosed multiple myeloma-a randomized phase III study. In: Blood. Vol. 130. 2017.
Morgan 2011 {published data only}
- Morgan GJ, Davies FE, Gregory WM, Russell NH, Bell SE, Szubert AJ, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood 2011;118(5):1231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niesvizky 2015 {published data only}
- Moon MA. Triple therapy added toxicity without survival benefit in multiple myeloma. Journal of Clinical Oncology 2015;11(7):3-4.
- Niesvizky R, Flinn I, Rifkin R, Charu V, Essell J, Gaffar Y, et al. Efficacy and safety of three bortezomib-based induction regimens followed by weekly bortezomib maintenance therapy in newly diagnosed multiple myeloma (MM) patients (PTS) ineligible for transplant: results of the phase 3B upfront study. In: Haematologica. Vol. 96. 2011:S98.
- Niesvizky R, Flinn I, Rifkin R, Gabrail N, Charu V, Clowney B, et al. Patient-reported quality of life (QOL) in previously untreated, elderly multiple myeloma (MM) patients treated with bortezomib-based regimens: results from the phase 3B upfront study. In: Haematologica. Vol. 96. 2011:123-4.
- Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B, et al. Community-based phase IIIB trial of three UPFRONT bortezomib-based myeloma regimens. Journal of Clinical Oncology 2015;33(33):3921-9. [DOI] [PubMed] [Google Scholar]
- Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B. Patient-reported quality of life (QOL) in elderly, newly diagnosed multiple myeloma (MM) patients receiving bortezomib-based combinations: results from all randomized patients in the community-based, phase 3b UPFRONT study. In: Blood. Vol. 118. 2011.
- Niesvizky R, Flinn IW, Rifkin RM, Gabrail N, Charu V, Gaffar Y, et al. Impact of baseline characteristics on efficacy and safety after bortezomib-based induction and maintenance in newly diagnosed multiple myeloma (MM) patients ineligible for transplant in the phase IIIb UPFRONT study. In: Journal of Clinical Oncology. Vol. 29. 2011.
- Niesvizky R, Flinn IW, Rifkin RM, Gabrail NY, Charu V, Clowney B, et al. Phase 3b UPFRONT study: safety and efficacy of weekly bortezomib maintenance therapy after bortezomib-based induction regimens in elderly, newly diagnosed multiple myeloma patients. In: Blood. Vol. 116. 2010.
- Niesvizky R, Reeves J, Flinn I, Gabrail N, Rifkin R, Charu V, et al. Phase 3b upfront study: interim results from a community based prospective randomized trial evaluating three bortezomib-based regimens in elderly, newly diagnosed multiple myeloma patients. In: Haematologica. Vol. 92. 2010:144.
Palumbo 2006 {published data only}
- Palumbo A, Bringhen S, Caravita T, Callea V, Zambello R, Pregno P, et al. A prospective randomized controlled trial of oral melphalan, prednisone, thalidomide versus oral melphalan, prednisone in elderly newly diagnosed myeloma patients. In: Haematologica. Vol. 91. 2006.
- Palumbo A, Bringhen S, Caravita T, Falcone A, Callea V, Cangialosi C, et al. Oral melphalan, prednisone, thalidomide versus oral melphalan, prednisone in elderly newly diagnosed myeloma patients. In: Haematologica. Vol. 92. 2007:147.
- Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet 2006;367(9513):825-31. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone and thalidomide in elderly multiple myeloma patients: updated results of a randomized controlled trial. In: Haematologica. Vol. 93. 2008:363-5. [DOI] [PubMed]
- Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. In: Blood. Vol. 112. 2008:3107-14. [DOI] [PubMed]
Palumbo 2012 {published data only}
- Cavo M, Dimopoulos M, Palumbo A, Weisel K, Delforge M, Blade J, et al. Impact of renal impairment on the efficacy and safety of melphalan-prednisone-lenalidomide (LEN) induction followed by LEN maintenance in newly diagnosed multiple myeloma: mM-015 post-hoc analysis. In: Haematologica. Vol. 98. 2013:330-1.
- Dimopoulos MA, Delforge M, Hájek R, Kropff M, Petrucci MT, Lewis P, et al. Lenalidomide, melphalan, and prednisone, followed by lenalidomide maintenance, improves health-related quality of life in newly diagnosed multiple myeloma patients aged 65 years or older: results of a randomized phase III trial. Haematologica 2013;98(5):784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Palumbo A, Hajek R, Kropff M, Petrucci MT, Lewis P, et al. Factors that influence health-related quality of life in newly diagnosed patients with multiple myeloma aged? 65 years treated with melphalan, prednisone and lenalidomide followed by lenalidomide maintenance: results of a randomized trial. Leukemia and Lymphoma 2014;55(7):1489-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay F, Cavallo F, Raimondo F, Hardan I, Nagler A, Petrucci MT, et al. Impact of continuous treatment vs fixed duration of therapy in newly diagnosed myeloma patients: pFS1, PFS2, OS endpoints. In: Haematologica. Vol. 99. 2014:519.
- Palumbo A, Adam Z, Kropff M, Foa R, Catalano J, Gisslinger H. A phase 3 study evaluating the efficacy and safety of lenalidomide (len) combined with melphalan and prednisone followed by continuous lenalidomide maintenance (MPR-R) in patients (pts) = 65 Years (yrs) with newly diagnosed multiple myeloma (NDMM): updated results for pts aged 65-75 yrs enrolled in MM-015. In: Blood. Vol. 118. 2011.
- Palumbo A, Delforge M, Catalano J, Hajek R, Kropff M, Petrucci MT, et al. A phase 3 study evaluating the efficacy and safety of lenalidomide combined with melphalan and prednisone in patients 65 years with newly diagnosed multiple myeloma (NDMM): continuous use of lenalidomide vs fixed-duration regimens. In: Blood. Vol. 116. 2010.
- Palumbo A, Dimopoulos M, Delforge M, Hajek R, Kropff M, Petrucci M, et al. A phase 3 study to determine the efficacy and safety of lenalidomide combined with melphalan and prednisone in patients = 65 years with newly diagnosed multiple myeloma (NDMM). In: Haematologica. Vol. 92. 2010:234-5.
- Palumbo A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. Journal of Clinical Oncology 2015;33(30):3459-66. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma.. New England Journal of Medicine 2012;366(19):1759-69. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Kropff M, Hajek R, Delforge M, Catalano J, Petrucci MT, et al. Incidence of second primary malignancy (SPM) in melphalan-prednisone-lenalidomide combination followed by lenalidomide maintenance (MPR-R) in newly diagnosed multiple myeloma (MM) patients=65 years. In: Haematologica. Vol. 96. 2011:218.
Palumbo 2014 {published data only}
- Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood 2010;116(23):4745-53. [DOI] [PubMed] [Google Scholar]
- Bringhen S, Larocca A, Rossi D, Romano A, Genuardi M, Ria R, et al. Efficacy and safety of once weekly bortezomib in multiple myeloma patients. In: Blood. Vol. 116. 2010. [DOI] [PubMed]
- Bringhen S, Magarotto V, Rossi D, Ria R, Offidani M, Patriarca F, et al. Bortezomib, melphalan, prednisone and thalidomide followed by maintenance with bortezomib and thalidomide (VMPT-VT) vs bortezomib, melphalan, prednisone (VMP) alone as first line treatment of multiple myeloma: updated follow-up and impact of prognostic factors. In: Haematologica. Vol. 96. 2011:67-8.
- Corso A, Raimondo F, Mangiacavalli S, Ria R, Offidani M, Pascutto C, et al. Bortezomib-based therapy overcomes the prognostic role of ISS score in multiple myeloma patients not eligible for transplant. In: Haematologica. Vol. 96. 2011:128.
- Gay F, Cavallo F, Raimondo F, Hardan I, Nagler A, Petrucci MT, et al. Impact of continuous treatment vs fixed duration of therapy in newly diagnosed myeloma patients: pFS1, PFS2, OS endpoints. In: Haematologica. Vol. 99. 2014:519.
- Gay F, Larocca A, Petrucci MT, Musto P, Liberati AM, Callea V, et al. Achievement of complete remission is a strong prognostic factor in 895 elderly myeloma patients treated with melphalan-prednisone based-regimens: results of 3 multicenter Italian trials. In: Haematologica. Vol. 92. 2010:236.
- Gentile M, Magarotto V, Offidani M, Musto P, Bringhen S, Petrucci MT, et al. Lenalidomide and low-dose dexamethasone (Rd) versus bortezomib, melphalan, prednisone (VMP) in elderly newly diagnosed multiple myeloma patients: A comparison of two prospective trials. American Journal of Hematology 2017;92(3):244-50. [DOI] [PubMed] [Google Scholar]
- Genuardi M, Bringhen S, Rossi D, Spadano A, Pisani F, Nozza A, et al. Evaluation of the efficacy and safety of weekly infusion bortezomib in elderly patients with newly diagnosed multiple myeloma. In: Haematologica. Vol. 95. 2010:S104-s105.
- Larocca A, Bringhen S, Petrucci MT, Offidani M, Paoli L, Pietrantuono G, et al. Early mortality in elderly newly diagnosed multiple myeloma patients treated with novel agents: a pooled analysis of two large randomized phase III trials. In: Haematologica. Vol. 100. 2015:81-2.
- Larocca A, Bringhen S, Petrucci Mt, Offidani M, Benevolo G, Galli M, et al. Early death in elderly patients with newly diagnosed multiple myeloma treated with new drugs: a pooled analysis of two randomized phase 3 trials. In: Haematologica. Vol. 100. 2015:51-2.
- Larocca A, Offidani M, Ciccone G, Guglielmelli T, Cangialosi C, Salvini M, et al. Impact of bortezomib-or lenalidomide-based induction treatment on high risk cytogenetic transplant-ineligible patients with newly diagnosed multiple myeloma enrolled in the gimema-MM-03-05 and EMN01 trials. In: Blood. Vol. 130. 2017.
- Morabito F, Gentile M, Mazzone C, Rossi D, Di Raimondo F, Bringhen S, et al. Safety and efficacy of bortezomib-melphalan-prednisone-thalidomide followed by bortezomib-thalidomide maintenance (VMPT-VT) versus bortezomib-melphalan-prednisone (VMP) in untreated multiple myeloma patients with renal impairment. Blood 2011;118(22):5759-66. [DOI] [PubMed] [Google Scholar]
- Morabito F, Gentile M, Mazzone C, Rossi D, Ria R, Offidani M, et al. VMPT (bortezomib, melphalan, prednisone and thalidomide) followed by maintenance with bortezomib-thalidomide (VT) is active and well tolerated in newly diagnosed patients with multiple myeloma (MM) with moderate renal impairment (RI) ineligible for autologous stem cell transplantation: results from a cohort analysis of a phase III randomized controlled trial. In: Haematologica. Vol. 96. 2011:S98-s99.
- Palumbo A, Bringhen S, Cavalli M, Ria R, Offidani M, Patriarca F, et al. Bortezomib, melphalan, prednisone and thalidomide followed by maintenance with bortezomib and thalidomide (VMPT-VT) for initial treatment of elderly multiple myeloma patients: updated follow-up and impact of prognostic factors. In: Blood. Vol. 116. 2010.
- Palumbo A, Bringhen S, Larocca A, Rossi D, Di Raimondo F, Magarotto V, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. Journal of Clinical Oncology 2014;32(7):634-40. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, et al. Bortezomib-melphalan-prednisone-thalidomide followed by continuous bortezomib-thalidomide for initial therapy of elderly multiple myeloma patients: a prospective randomized trial. Journal of Clinical Oncology 2010;95:S101-s102. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. Journal of Clinical Oncology 2010;28(34):5101-9. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. Journal of Clinical Oncology 2010;28(34):5101-9. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Bringhen S, Rossi D, Cavalli M, Ria R, Gentilini S, et al. Overall survival benefit for bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide (VMPT-VT) versus bortezomib-melphalan-prednisone (VMP) in newly diagnosed multiple myeloma patients. In: Blood. Vol. 120. 2012.
- Palumbo A, Bringhen S, Rossi D, Cavalli M, Ria R, Offidani M, et al. A prospective randomized trial of bortezomib-melphalan-prednisone-thalidomide followed by continuous bortezomib-thalidomide for initial therapy of multiple myeloma: effect of age and co-morbidities. In: Haematologica. Vol. 92. 2010:235-6.
- Palumbo A, Bringhen S, Rossi D, Magarotto V, Raimondo F, Ria R, et al. A prospective, randomized, phase iii study of bortezomib, melphalan, prednisone and thalidomide (VMPT) versus bortezomib, melphalan and prednisone (VMP) in elderly newly diagnosed myeloma patients. In: Blood. Vol. 112. 2009:243.
- Palumbo A, Bringhen S, Rossi D, Ria R, Magarotto V, Offidani M, et al. Bortezomib, melphalan, prednisone and thalidomide followed by bortezomib and thalidomide (VMPT-VT) as initial treatment of multiple myeloma (MM) patients older than 65 years: updated follow-up and prognostic factors. In: Haematologica. Vol. 96. 2011:S80-s81.
- Palumbo A, Bringhen S, Rossi D, Ria R, Magarotto V, Offidani M, et al. Prognostic factors in elderly myeloma patients receiving bortezomib, melphalan, prednisone and thalidomide followed by bortezomib and thalidomide (VMPT-VT) as first line treatment. In: Haematologica. Vol. 96. 2011:376.
- Palumbo A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. Journal of Clinical Oncology 2015;33(30):3459-66. [DOI] [PubMed] [Google Scholar]
- Palumbo AB, Bringhen S, Rossi D, Berretta S, Montefusco V, Peccatori J, et al. A phase III study of VMPT versus VMP in newly diagnosed elderly myeloma patients. In: Journal of Clinical Oncology. Vol. 27. 2009:437.
Pawlyn 2017 {published data only}
- Brioli A, Davies F, Gregory W, Hinsley S, Marshall S, Pawlyn C, et al. Low rate of secondary primary malignancies (SPMs) in newly diagnosed multiple myeloma (MM) patients treated with lenalidomide: first results from the MRC MM XI trial. In: Haematologica. Vol. 98. 2013:104.
- Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide is a highly effective maintenance therapy in myeloma patients of all ages; Results of the phase III myeloma XI study. In: Blood. Vol. 128. 2016.
- Jones JR, Cairns D, Gregory W, Sigsworth R, Collett C, Pawlyn C, et al. The NCRI Myeloma XI trial for newly diagnosed symptomatic multiple myeloma (NDMM); second primary malignancy (SPM) incidence when lenalidomide is used as an induction and maintenance treatment option. In: British Journal of Haematology. Vol. 173. 2016:86.
- Jones JR, Cairns DA, Gregory WM, Collett C, Pawlyn C, Sigsworth R, et al. Second malignancies in the context of lenalidomide treatment: an analysis of 2732 myeloma patients enrolled to the Myeloma XI trial. Blood Cancer Journal 2016;6(12):e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JR, Cairns DA, Sigsworth R, Collett C, Pawlyn C, Striha A, et al. Myeloma XI trial for newly diagnosed multiple myeloma (NDMM); a report of second primary malignancy (SPM) rates and the importance of review of reported cases. In: Blood. Vol. 126. 2015:1847.
- Pawlyn C, Davies FE, Cairns D, Striha A, Best P, Sigsworth R, et al. Continuous treatment with lenalidomide improves outcomes in newly diagnosed myeloma patients not eligible for autologous stem cell transplant: results of the myeloma xi trial. In: Blood. Vol. 130. 2017.
- Pawlyn C, Davies FE, Gregory WM, Szubert AJ, Bell SE, Ouzman J, et al. Sequential immunomodulatory drug (IMID) and proteosome inhibitor therapy improves response rates in newly diagnosed multiple myeloma: preliminary results from the myeloma XI trial. In: Blood. Vol. 120. 2012.
- Pawlyn C, Kaiser MF, Cairns D, Striha A, Jones JR, Shah V, et al. Factors predicting poor outcomes for myeloma patients at different ages: results from 3894 patients in the myeloma XI trial. In: Blood. Vol. 130. 2017. [DOI] [PMC free article] [PubMed]
- Tute RM, Rawstron AC, Cairns D, Pawlyn C, Davies F, Collett C, et al. Minimal residual disease predicts outcome in transplant ineligible myeloma patients: results from the UK NCRI Myeloma XI trial. In: British Journal of Haematology. Vol. 173. 2016:105.
- Tute RM, Rawstron AC, Cairns DA, Pawlyn C, Davies FE, Collett C, et al. Impact of minimal residual disease in transplant ineligible myeloma patients: results from the UK NCRI myeloma XI trial. In: Blood. Vol. 128. 2016.
Sacchi 2011 {published data only}
- Sacchi S, Marcheselli R, Lazzaro A, Morabito F, Fragasso A, Di Renzo N, et al. A randomized trial with melphalan and prednisone versus melphalan and prednisone plus thalidomide in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplant. Leukemia and Lymphoma 2011;52(10):1942-8. [DOI] [PubMed] [Google Scholar]
San Miguel 2008 {published data only}
- Delforge M, Terpos E, Richardson PG, Shpilberg O, Khuageva NK, Schlag R, et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. European Journal of Haematology 2011;86(5):372-84. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Mateos MV, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. European Journal of Haematology 2011;86(1):23-31. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kastritis E, et al. VMP (Bortezomib, Melphalan, and Prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. Journal of Clinical Oncology 2009;27(36):6086-93. [DOI] [PubMed] [Google Scholar]
- Harousseau JL, Palumbo A, Richardson PG, Schlag R, Dimopoulos MA, Shpilberg O, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood 2010;116(19):3743-3750. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. Journal of Clinical Oncology 2010;28(13):2259-66. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Schlag R, Khuageva N, Dimopoulos M, Kropff M, Spicka I, et al. Prolonged therapy with bortezomib plus melphalan-prednisone (VMP) results in improved quality and duration of response in the phase III vista study in previously untreated multiple myeloma (MM). In: Haematologica. Vol. 93. 2008.
- Richardson P, Schlag R, Khuageva N, Dimopoulos M, Shpilberg O, Kropff M, et al. Characterization of haematological parameters with bortezomib-melphalan-prednisone versus melphalan-prednisone in newly diagnosed myeloma, with evaluation of long-term outcomes and risk of thromboembolic events with use of erythropoiesis-stimulating agents: analysis of the VISTA trial. British Journal of Haematology 2011;153:212-21. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Schlag R, Khuageva N, Shpilberg O, Dimopoulos M, Kropff M, et al. MMY-3002: a phase 3 study comparing Bortezomib-Melphalan-Prednisone (VMP) with Melphalan-Prednisone (MP) in newly diagnosed multiple myeloma. In: Blood. Vol. 110. 2007.
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. New England Journal of Medicine 2008;359(9):906-17. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M. Continued overall survival benefit after 5 years' follow-up with bortezomib-melphalan-prednisone (VMP) versus melphalan-prednisone (MP) in patients with previously untreated multiple myeloma, and no increased risk of second primary malignancies: final results of the phase 3 VISTA trial. In: Blood. Vol. 118. 2011.
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M. Superior efficacy with bortezomib plus melphalan-prednisone (VMP) versus melphalan-prednisone (MP) alone in previously untreated multiple myeloma (MM): results of the phase III MMY-3002 VISTA study. In: Haematologica. Vol. 93. 2008.
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M. Updated follow-up and results of subsequent therapy in the phase III vista trial: bortezomib plus melphalan-prednisone versus melphalan-prednisone in newly diagnosed multiple myeloma. In: Blood. Vol. 112. 2009:242.
- Spicka I, Mateos MV, Redman K, Dimopoulos MA, Richardson PG. An overview of the VISTA trial: newly diagnosed, untreated patients with multiple myeloma ineligible for stem cell transplantation. Immunotherapy 2011;3(9):1033-40. [DOI] [PubMed] [Google Scholar]
Stewart 2015 {published data only}
- Stewart AK, Jacobus S, Fonseca R, Weiss M, Callander NS, Chanan Khan AA, et al. E1A06: a phase III trial comparing melphalan, prednisone, and thalidomide (MPT) versus melphalan, prednisone, and lenalidomide (MPR) in newly diagnosed multiple myeloma MM). In: Haematologica. Vol. 99. 2014:220.
- Stewart AK, Jacobus S, Fonseca R, Weiss M, Callander NS, Chanan-Khan AA, et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood 2015;126(11):1294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waage 2010 {published data only}
- Waage A, Gimsing P, Fayers P, Abildgaard N, Ahlberg L, Björkstrand B, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood 2010;116(9):1405-12. [DOI] [PubMed] [Google Scholar]
- Waage A, Gimsing P, Juliusson G, Turesson I, Fayers P. Melphalan-prednisone-thalidomide to newly diagnosed patients with multiple myeloma: a placebo controlled randomised phase 3 trial. In: Blood. Vol. 110. 2007:32a.
Wijermans 2010 {published data only}
- Beurden-Tan C, Blommestein H, Zweegman S, Sonneveld P. The relationship of response on time to next treatment based on evidence from two RCTs in newly diagnosed stem cell transplantation ineligible multiple myeloma patients. In: Blood. Vol. 128. 2016:(no pagination).
- Verelst S, Termorshuizen F, Uyl-De Groot C, Sonneveld P, Wijermans P. Effect of thalidomide with melphalan and prednisone on health-related quality of life (HRQOL) in elderly patients with newly diagnosed multiple myeloma: a prospective analysis in a randomized trial (HOVON 49). In: Haematologica. Vol. 96. 2011:S135. [DOI] [PubMed]
- Verelst S, Termorshuizen F, Uyl-de Groot C, Schaafsma MR, Ammerlaan R, Wittebol S. Effect of thalidomide with melphalan and prednisone on health-related quality of life (HRQOL) in elderly patients with newly diagnosed multiple myeloma: a prospective analysis in a randomized trial. In: Blood. Vol. 116. 2010. [DOI] [PubMed]
- Verelst SG, Termorshuizen F, Uyl-de Groot CA, Schaafsma MR, Ammerlaan AH, Wittebol S, et al. Effect of thalidomide with melphalan and prednisone on health-related quality of life (HRQoL) in elderly patients with newly diagnosed multiple myeloma: a prospective analysis in a randomized trial. Annals of Hematology 2011;90(12):1427-39. [DOI] [PubMed] [Google Scholar]
- Wijermans P, Schaafsma M, Norden Y, Ammerlaan R, Sonneveld P, Wittebol S, et al. Melphalan + prednisone vs melphalan + prednisone + thalidomide in induction therapy for multiple myeloma in elderly patients: first interim results of the Dutch cooperative group HOVON. In: Haematologica. Vol. 93. 2008:177.
- Wijermans P, Schaafsma M, Norden Y, Ammerlaan R, Wittebol S, Sinnige H, et al. Melphalan + prednisone versus melphalan + prednisone + thalidomide in induction therapy for multiple myeloma in elderly patients: final analysis of the Dutch Cooperative Group HOVON 49 study. Blood 2009;112(11):241-2. [Google Scholar]
- Wijermans P, Schaafsma M, Termorshuizen F, Ammerlaan R, Wittebol S, Sinnige H, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. Journal of Clinical Oncology 2010;28(19):3160-6. [DOI] [PubMed] [Google Scholar]
Zweegman 2016 {published data only}
- Beurden-Tan C, Blommestein H, Zweegman S, Sonneveld P. The relationship of response on time to next treatment based on evidence from two RCTs in newly diagnosed stem cell transplantation ineligible multiple myeloma patients. In: Blood. Vol. 128. 2016:(no pagination).
- Kongsgaard Nielsen L, Stege C, Witte B, Holt B, Mellqvist UH, Salomo M, et al. Health-related quality of life in non-transplant eligible newly diagnosed multiple myeloma patients treated with melphalan/prednisolone plus either thalidomide or lenalidomide; results of the HOVON87/NMSG18 study. In: Quality of Life Research. Vol. 26. 2017:56-7.
- Stege C, Holt B, Mellqvist UH, Levin MD, Salomo M, Abildgaard N, et al. Frailty predicts survival and toxicity in newly diagnosed multiple myeloma patients ineligible for autologous stem cell transplantation; report of the HOVON-87/NMSG-18 study group. In: Blood. Vol. 130. 2017:(no pagination).
- Stege C, Kongsgaard Nielsen L, Witte B, Holt B, Mellqvist UH, Salomo M, et al. Quality of life with melphalan/prednisone plus either thalidomide (MPT-T) or lenalidomide (MPR-R) in non-transplant eligible newly diagnosed multiple myeloma; Results of the Hovon87/NMSG18 study. In: Haematologica. Vol. 102. 2017:190.
- Zweegman S, Holt B, Mellqvist UH, Salomo M, Bos GM, Levin MD, et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood 2016;127(9):1109-16. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 2003 {published data only}
- Anonymous. A multicenter parallel-group, placebo controlled, randomized, double-blind study of combination thalidomide plus glucocorticoid therapy versus glucocorticoid therapy alone as induction therapy for previously untreated subjects with multiple myeloma. available at: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00500364/full (accessed 06 May 2019).
Barlogie 2004 {published data only}
- Anonymous. Study of bortezomib and Revlimid ™ for patients relapsing or progressing on total therapy II. available at: https://clinicaltrials.gov/show/nct00093028 (Accessed 28 May 2019).
- Barlogie B, Rasmussen E, Tricot G, Crowley J. Management of patients with multiple myeloma (MM) failing total therapy 2 (TT 2) according to thalidomide (THAL) randomization. In: Blood. Vol. 104. 2004:414a.
Brioli 2014 {published data only}
- Brioli A, Galli M, Tacchetti P, Crippa C, Spadano T, Pezzi A, et al. A combination therapy with bortezomib (BOR) and thalidomide in newly diagnosed myeloma patients is associated with a low incidence of second primary malignancies (SPMS). In: Haematologica. Vol. 99. 2014:373.
Facon 2006 {published data only}
- Facon T, Mary JY, Attal M, Pegourie B, Harousseau JL, Sadoun A. Melphalan-prednisone versus dexamethasone-based regimens for newly diagnosed myeloma patients aged 65-75 years. Final analysis of the IFM 95-01 trial on 489 patients. In: Blood. Vol. 102. 2003:147a.
- Facon T, Mary JY, Pégourie B, Attal M, Renaud M, Sadoun A, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood 2006;107(4):1292-8. [DOI] [PubMed]
Facon 2017 {published data only}
- Anonymous. Phase 3 study of carfilzomib, melphalan, prednisone vs bortezomib, melphalan, prednisone in newly diagnosed multiple myeloma. available at: ttps://clinicaltrials.gov/show/nct01818752 (accessed 03 July 2019).
- Facon T, Lee JH, Moreau P, Niesvizky R, Dimopoulos MA, Hajek R, et al. Phase 3 Study (CLARION) of carfilzomib, melphalan, prednisone (KMP) v bortezomib, melphalan, prednisone (VMP) in newly diagnosed multiple myeloma (NDMM). Clinical Lymphoma, Myeloma and Leukemia 2017;17(1):e26-7. [Google Scholar]
Facon 2018 {published data only}
- Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. LBA-2 phase 3 randomized study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant (MAIA). In: Blood. Vol. 132. 2018:LBA-2.
Foa 2007 {published data only}
- Foa R, Weber D, Dimopoulos M, Olesnyckyj M, Yu Z, Zeldis J, et al. Lenalidomide/dexamethasone improves response and prolongs time to progression, even in patients with IgA multiple myeloma: a sub-analysis of the MM-009/010 studies. In: Blood. Vol. 110. 2007:284b.
Harousseau 2003 {published data only}
- Harousseau JL, Attal M. The IFM-90 trial. In: Hematology Journal. Vol. 4. 2003:S55.
Hejlova 2000 {published data only}
- Hejlova N, Hajek R, Adam Z, Scudla Z, Bacovsky J, Koza V, et al. Report of the "4W" trial of the Czech myeloma group. In: Hematology Journal. Vol. 1. 2000:168-9.
Hernández 2004 {published data only}
- Hernández JM, García-Sanz R, Golvano E, Bladé J, Fernandez-Calvo J, Trujillo J, et al. Randomized comparison of dexamethasone combined with melphalan versus melphalan with prednisone in the treatment of elderly patients with multiple myeloma. British Journal of Heamatology 2004;127(2):159-64. [DOI] [PubMed] [Google Scholar]
Kumar 2012 {published data only}
- Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012;119(19):4375-82. [DOI] [PubMed] [Google Scholar]
Ludwig 2017a {published data only}
- Anonymous. Patients with newly diagnosed multiple myeloma comparing KTd vs. KRd induction therapy and investigating a K-mono maintenance strategy. Available at: https://clinicaltrials.gov/show/nct02891811 (accessed 03 July 2019).
- Ludwig H. AGMT-MM2 A randomized phase II study in transplant ineligible patients with newly diagnosed multiple myeloma (NDMM) comparing Carfilzomib + Thalidomide + Dexamethasone(KTd) with Carfilzomib + Lenalidomide + Dexamethasone (KRd) induction therapy followed by Carfilzomib (K) maintenance or control. In: Magazine of European Medical Oncology. Vol. 10. 2017:S44-s45.
Mateos 2016 {published data only}
- Mateos MV, Gutierrez NC, Martin ML, Martinez-Lopez J, Hernandez MT, Martinez R, et al. Bortezomib plus melphalan and prednisone (VMP) followed by lenalidomide and dexamethasone (RD) in newly diagnosed elderly myeloma patients overcome the poor prognosis of high-risk cytogenetic abnormalities (CA) detected by fluorescence in situ hibridization (FISH). In: Blood. Vol. 126. 2015:4243.
- Mateos MV, Martínez-López J, Hernández MT, Ocio EM, Rosiñol L, Martínez R, et al. Sequential vs alternating administration of VMP and Rd in elderly patients with newly diagnosed MM. Blood 2016;127(4):420-5. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Martinez-Lopez J, Hernandez M, Martinez R, Rosinol L, Ocio EM, et al. Depth of response as surrogate marker for progression-free survival and overall survival in elderly newly diagnosed myeloma patients treated with VMP and Rd: gEM2010MAS65. In: Haematologica. Vol. 102. 2017:143.
- Mateos MV, Martinez-Lopez J, Hernandez MT, Ocio EM, Rosinol L, Martinez R, et al. Bortezomib, melphalan, prednisone (VMP) and lenalidomide plus dexamethasone (RD) is the optimal combination for patients with newly diagnosed multiple myeloma (MM) patients between 65 and 80 years. In: Blood. Vol. 126. 2015:1848.
Mateos 2018 {published data only}
- Facon T, Cavo M, Jakubowiak A, San Miguel J, Kumar S, Orlowski RZ, et al. Two randomized open-label studies of daratumumab (DARA) plus standard of care treatment versus standard of care alone in patients with previously untreated multiple myeloma (MM) ineligible for high-dose therapy: 54767414MMY3007 (Alcyone) and 54767414MMY3008 (Maia). In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 15. 2015:e294-5.
- Mateos MV, Cavo M, Jakubowiak AJ, Carson RL, Qi M, Bandekar R, et al. A randomized open-label study of bortezomib, melphalan, and prednisone (VMP) versus daratumumab (DARA) plus VMP in patients with previously untreated multiple myeloma (MM) who are ineligible for high-dose therapy: 54767414MMY3007 (Alcyone). In: Journal of Clinical Oncology. Vol. 33. 2015:supplement 1 (no pagination).
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone (D-VMP) versus bortezomib, melphalan, and prednisone (VMP) in newly diagnosed multiple myeloma (NDMM) patients (PTS) ineligible for transplant: a phase 3 ran-domized study (alcyone). In: Haematologica. Vol. 103. 2018:22.
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. New England Journal of Medicine 2018;378(6):518-28. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak AJ, Knop S, et al. Phase 3 randomized study of daratumumab plus bortezomib, melphalan, and prednisone (D-VMP) versus bortezomib, melphalan, and prednisone (VMP) in newly diagnosed multiple myeloma (NDMM) patients (Pts) ineligible for transplant (ALCYONE). In: Blood. Vol. 130. 2017:Supplement 1 (no pagination).
- Stirrups R. Daratumumab for untreated multiple myeloma. Lancet Oncology 2018;19(2):e80. [DOI] [PubMed]
Merz 2015 {published data only}
- Merz M, Salwender HJ, Haenel M, Bertsch U, Kunz C, Hielscher T, et al. Clinical risk factors for peripheral neuropathy in patients treated with subcutaneous or intravenous bortezomib for newly diagnosed multiple myeloma. In: BLood. Vol. 126. 2015:4233.
Montefusco 2013 {published data only}
- Montefusco V. First-line treatment in elderly patients. In: European journal of cancer. Vol. 49. 2013:S19.
Morgan 2002 {published data only}
- Morgan G, Davies FE, Hawkins K, Brown J, Drayson MT. The MRC Myeloma VII Trial of standard versus intensive treatment in patients <65 years of age with multiple myeloma. In: Blood. Vol. 100. 2002:178a.
NCT00522392 {published data only}
- Anonymous. Bortezomib and dexamethasone with or without lenalidomide in treating patients with multiple myeloma previously treated with dexamethasone. available at: https://clinicaltrials.gov/show/nct00522392 (accessed 06 May 2019).
NCT00734877 {published data only}
- Anonymous. UARK 2013-13, Total Therapy 4B - Formerly 2008-01 - A phase III trial for low risk myeloma. available at: https://clinicaltrials.gov/show/nct00734877 (accessed 06 May 2019).
NCT01850524 {published data only}
- Anonymous. IXAZOMIB plus lenalidomide and dexamethasone versus placebo plus lenalidomide and dexamethasone in adult patients with newly diagnosed multiple myeloma. available at: https://clinicaltrials.gov/show/nct01850524 (accessed 03 July 2019).
NCT01863550 {published data only}
- Anonymous. Bortezomib or carfilzomib With lenalidomide and dexamethasone in treating patients with newly diagnosed multiple myeloma. available at: https://clinicaltrials.gov/show/nct01863550 (accessed 03 July 2019).
NCT02586038 {published data only}
- Anonymous. Study that compares 3 arm: MLN9708 dexamethasone, MLN9708 cyclophosphamide and dexamethasone, MLN9708 thalidomide and dexamethasone followed by maintenance with MLN9708 in newly diagnosed elderly multiple myeloma patients. available at: https://clinicaltrials.gov/show/nct02586038 (accessed 03 July 2019).
NCT03710603 {published data only}
- Anonymous. A study comparing daratumumab, VELCADE (bortezomib), lenalidomide, and dexamethasone (D-VRd) with VELCADE, lenalidomide, and dexamethasone (VRd) in participants with untreated multiple myeloma and for whom hematopoietic stem cell transplant is not planned as initial therapy. available at: https://clinicaltrials.gov/show/nct03652064 (accessed 03 July 2019).
- Anonymous. Daratumumab, VELCADE (bortezomib), lenalidomide and dexamethasone compared to VELCADE, lenalidomide and dexamethasone in subjects with previously untreated multiple myeloma. available at: https://clinicaltrials.gov/show/nct03710603 (accessed 03 July 2019).
Niesvizky 2003 {published data only}
- Niesvizky R, Pekle K, Lyons L, Pearse RN, Bergsagel PL, Schuster MW. Dexamethsone alone, or in combination with low-dose thalidomide as induction therapy for advanced multiple myeloma, and the effect of the addition of clarithromycin (Biaxin?) on response rate. Interim results of a prospective, sequential, randomized trial. In: Blood. Vol. 102. 2003:237a.
Offidani 2004 {published data only}
- Offidani M, Corvatta L, Marconi M, Olivieri A, Catarini M, Mele A, et al. Thalidomide plus oral melphalan compared with thalidomide alone for advanced multiple myeloma. Hematology Journal 2004;5(4):312-7. [DOI] [PubMed] [Google Scholar]
Palumbo 2016 {published data only}
- Lonial S, Ribeiro De Oliveira M, Yimer H, Mateos MV, Rifkin R, Schjesvold F, et al. KEYNOTE-185: a randomized, open-label phase 3 study of pembrolizumab in combination with lenalidomide and lowdose dexamethasone in newly diagnosed and treatmentnaive multiple myeloma (MM). In: Annals of Oncology. Vol. 27. 2016:viii16.
- Palumbo A, Mateos MV, San Miguel J, Shah J, Thompson S, Marinello P, et al. Pembrolizumab in combination with lenalidomide and low-dose dexamethasone in newly diagnosed and treatment-naive multiple myeloma (MM): randomized, phase 3 KEYNOTE-185 study. In: Annals of Oncology. Vol. 27. 2016:(no pagination).
- Shah J, Lonial S, Oliveira MR, Yimer H, Mateos MV, Rifkin R, et al. KEYNOTE-185: randomized, open-label, phase III study of pembrolizumab in combination with lenalidomide and low-dose dexamethasone in patients with newly diagnosed and treatment-naive multiple myeloma (MM). In: Journal for Immunotherapy of Cancer. Vol. 4. 2016.
Rajkumar 2006b {published data only}
- Greipp PR. Eastern Cooperative Oncology Group E1A00: phase III randomized study of dexamethasone with or without thalidomide in patients with newly diagnosed multiple myeloma. Clinical Advances in Hematology & Ooncology 2003;1(3):188-9. [PubMed] [Google Scholar]
- Rajkumar SV, Blood E , Vesole D, Fonseca R, Greipp PR, Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2006;24(3):431-6. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Vesole DH, Shepard PR, Greipp PR. A randomised phase III trial of thalidomide plus dexamethasone versus dexamethasone in newly diagnosed multiple myeloma (E1A00): a trial coordinated by the Eastern Cooperative Oncology Group. In: Journal of Clinical Oncology. Vol. 22. 2004:558. [DOI] [PubMed]
- Rajkumar SV, Vesole DH, Shepard R, Greipp PR. Thalidomide plus dexamethasone versus dexamethasone alone in newly diagnosed multiplemMyeloma (E1A00): results of a phase III trial coordinated by the Eastern Cooperative Oncology Group. In: Blood. Vol. 104. 2004:63.
Rajkumar 2008 {published data only}
- Rajkumar SV, Hussein M, Catalano J, Jedrzejcak W, Sirkovich S, Olesnyckyj M, et al. A multicenter, randomized, double-blind, placebo-controlled trial of thalidomide plus dexamethasone versus dexamethasone alone as initial therapy for newly diagnosed multiple myeloma. In: Journal of Clinical Oncology. Vol. 24. 2006:426. [DOI] [PMC free article] [PubMed]
- Rajkumar SV, Hussein M, Catalano J, Jedrzejczak W, Sirkovich S, Olesnyckyj M, et al. A randomized, double-blind, placebo-controlled trial of thalidomide plus dexamethasone versus dexamethasone alone as primary therapy for newly diagnosed multiple myeloma. In: Blood. Vol. 108. 2006:238.
- Rajkumar SV, Rosiñol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. Journal of Clinical Oncology 2008;26(13):2171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
San Miguel 2014 {published data only}
- San Miguel J, Blade J, Samoilova O, Shpilberg O, Grosicki S, Maloisel F, et al. Randomized, open-label, phase 2 study of siltuximab (an anti-il-6 mab) and bortezomib-melphalan-prednisone versus bortezomib-melphalan-prednisone in patients with previously untreated multiple myeloma. In: Haematologica. Vol. 98. 2013:97.
- San Miguel J, Blade J, Shpilberg O, Grosicki S, Maloisel F, Min CK, et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood 2014;123(26):4136-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takezako 2017 {published data only}
- Anonymous. PH III study of lenalidomide and dexamethasone with or without elotuzumab to treat peviously untreated multiple myeloma (ELO 1 substudy). available at: https://clinicaltrials.gov/show/nct01891643 (accessed 03 July 2019).
- Anonymous. Phase III study of lenalidomide and dexamethasone with or without elotuzumab to treat newly diagnosed, previously untreated multiple myeloma. available at: https://clinicaltrials.gov/show/nct01335399 (accessed 03 July 2019).
- Takezako N, Ohta K, Handa H, Hori M, Kinoshita G, Shelat S, et al. Elotuzumab plus lenalidomide/dexamethasone (ELD) vs LD in patients with newly diagnosed multiple myeloma: phase 2, randomized, open-label study in Japan. In: Blood. Vol. 130. 2017:supplement 1 (no pagination).
Usmani 2014 {published data only}
- Anonymous. S1211 Bortezomib, Dexamethasone, and Lenalidomide With or Without Elotuzumab in Treating Patients With Newly Diagnosed High-Risk Multiple Myeloma. available at: https://clinicaltrials.gov/show/nct01668719 (accessed 03 July 2019).
- Usmani SZ, Hoering A, Sexton R, Ailawadhi S, Shah JJ, Fredette S, et al. SWOG 1211: a randomized phase I/II study of optimal induction therapy for newly diagnosed high-risk multiple myeloma (HRMM). In: Journal of Clinical Oncology. Vol. 32. 2014:15 suppl.1 (no pagination).
White 2007 {published data only}
- White DJ, Kovacs MJ, Belch A, Stewart K, Chen C, Rubin S, et al. Phase II testing of lenalidomide plus melphalan for previously untreated older patients with multiple myeloma: toxicity data from the NCIC CTG MY.11 trial. In: Blood. Vol. 110. 2007:63a.
- White DJ, Paul N, Macdonald DA, Meyer RM, Shepherd LE. Addition of lenalidomide to melphalan in the treatment of newly diagnosed multiple myeloma: the National Cancer Institute of Canada Clinical Trials Group MY.11 trial. Current Oncology 2007;14(2):61-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zonder 2010 {published data only}
- Zonder JA, Crowley J, Hussein MA, Bolejack V, Moore DF, Whittenberger BF, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232). Blood 2010;116(26):5838-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonder JA, Crowley J, Hussein MA, Bolejack V, Moore DF, Whittenberger BF, et al. Superiority of lenalidomide (Len) plus high-dose dexamethasone (HD) compared to HD alone as treatment of newly-diagnosed multiple myeloma (NDMM): results of the randomized, double-blinded, placebo-controlled SWOG trial S0232. In: Blood. Vol. 110. 2007:32a.
- Zonder JA, Crowley JJ, Bolejack V, Hussein MA, Moore DF, Whittenberger BF. A randomized Southwest Oncology Group study comparing dexamethasone (D) to lenalidomide + dexamethasone (LD) as treatment of newly-diagnosed multiple myeloma (NDMM): impact of cytogenetic abnormalities on efficacy of LD, and updated overall study results. In: Journal of Clinical Oncology. Vol. 26. 2008:459.
- Zonder JA. Phase III randomized study of dexamethasone with or without CC-5013 in patients with newly diagnosed multiple myeloma. http://cochranelibrary-wiley.com/o/cochrane/clcentral/articles/578/CN-00501578/frame.html 2003.
Additional references
Ailawadhi 2018
- Ailawadhi S, Jacobus S, Sexton R, Stewart AK, Dispenzieri A, Hussein MA, et al. Disease and outcome disparities in multiple myeloma: exploring the role of race/ethnicity in the Cooperative Group clinical trials. Blood Cancer Journal 2018;8(7):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2011
- Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annual Review of Pathology 2011;6:249-74. [DOI] [PubMed] [Google Scholar]
Anderson 2015
- Anderson KC, Alsina M, Atanackovic D, Biermann JS, Chandler JC, Costello C, et al. Multiple Myeloma, version 2.2016: Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2015;13(11):1398-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Argyriou 2008
- Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 2008;112(5):1593-9. [DOI] [PubMed] [Google Scholar]
Asatsuma‐Okumura 2019
- Asatsuma-Okumura T, Ito T, Handa H. Molecular mechanisms of cereblon-based drugs. Pharmacology and Therapeutics 2019;202:132-9. [DOI] [PubMed] [Google Scholar]
Bianchi 2015
- Bianchi G, Richardson PG, Anderson KC. Promising therapies in multiple myeloma. Blood 2015;126(3):300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blommestein 2019
- Blommestein HM, Beurden-Tan CHY, Franken MG, Uyl-de Groot CA, Sonneveld P, Zweegman S. Efficacy of first-line treatments for multiple myeloma patients not eligible for stem cell transplantation - A Network Meta-analysis. Haematologica 2019;104(5):1026-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cancer Research UK 2018
- Cancer Research UK . Myeloma statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma (accessed 27 August 2018).
Chaimani 2017
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. [DOI] [PubMed] [Google Scholar]
Covidence systematic review software [Computer program]
- Veritas Health Innovation Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, Available at www.covidence.org.
Cowan 2018
- Cowan AJ, Allen C, Barac A, Basaleem H, Bensenor I, Curado MP, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncology 2018;4(9):1221-7. [DOI: 10.1001/jamaoncol.2018.2128] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses on behalf of the Cochrane Statistical Methods Group. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dias 2010
- Dias S, Welten NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis.. Statistics in Medicine 2010;29(7-8):932-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fayers 2011
- Fayers P M, Palumbo A, Hulin C, Waage A, Wijermans P, Beksac M, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood 2011;118(5):1239-47. [DOI] [PubMed] [Google Scholar]
Fink 2015
- Fink EC, Ebert BL. The novel mechanism of lenalidomide activity. Blood 2015;126(21):2366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandolfi 2017
- Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer and Metastasis Reviews 2017;36(4):561-84. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA (editors. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org..
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG (editors. Chapter 16: Special topics in statistics. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holstein 2017
- Holstein SA, McCarthy PL. Immunomodulatory drugs in multiple myeloma: mechanisms of action and clinical experience. Drugs 2017;77(5):505-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
IMS Institute for Healthcare Informatics 2015
- IMS Institute for Healthcare Informatics. Understanding the Role and Use of Essential Medicines Lists,. Available at: http://apps.who.int/medicinedocs/documents/s21980en/s21980en.pdf (accessed 30 August 2019) 2015.
Key 2019
- Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. Journal of Clinical Oncology 2019;[Epub ahead of print]. [DOI: 10.1200/JCO.19.01461] [DOI] [PubMed] [Google Scholar]
Kimpton 2016
- Kimpton M, Buyting R, Corsi DJ, Rupani N, Carrier M, McCurdy A. Incidence and risk factors of venous thromboembolism in patients with multiple myeloma receiving immunomodulatory agents. Blood 2016;128(22):2624. [Google Scholar]
Kirtane 2017
- Kirtane K, Lee SJ. Racial and ethnic disparities in hematologic malignancies. Blood 2017;130(15):1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuehl 2012
- Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. Journal of Clinical Investigation 2012;122(10):3456-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuhr 2016
- Kuhr K, Wirth D, Srivastava K, Lehmacher W, Hellmich M. First-line therapy for non-transplant eligible patients with multiple myeloma: direct and adjusted indirect comparison of treatment regimens on the existing market in Germany. European Journal of Clinical Pharmacology 2016;72(3):257-65. [DOI] [PubMed] [Google Scholar]
Kumar 2018
- Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Castillo J, et al. NCCN guidelines insights: multiple myeloma, version 3.2018. Journal of the National Comprehensive Cancer Network 2018;16(1):11-20. [DOI] [PubMed] [Google Scholar]
Liu 2017
- Liu X, Chen J, He YA, Meng X, Li K, He CK, et al. Comparing efficacy and survivals of initial treatments for elderly patients with newly diagnosed multiple myeloma: a network meta-analysis of randomized controlled trials. OncoTargets and Therapy 2017;10:121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2014
- Lu G, Middleton RE, Sun H, Naniong M, Ott C J, Mitsiades CS, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014;343(6168):305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ludwig 2017b
- Ludwig H, Zojer N. Fixed duration vs continuous therapy in multiple myeloma. ASH Education Program 2017;Hematology 2017(1):212-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMaster 2015 [Computer program]
- GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org, 2015.
Miceli 2008
- Miceli T, Colson K, Gavino M, Lilleby K. Myelosuppression associated with novel therapies in patients with multiple myeloma: consensus statement of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing 2008;12(Supplement 3):13-20. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreau 2012
- Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012;120(5):947-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreau 2017
- Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2017;28 Suppl 4:iv52-61. [DOI] [PubMed] [Google Scholar]
National Cancer Institute 2018
- National Cancer Institute. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html (accessed 27 August 2018).
Nwabuko 2017
- Nwabuko OC, Igbigbi EE, Chukwuonye II, Nnoli MA. Multiple myeloma in Niger Delta, Nigeria: complications and the outcome of palliative interventions. Cancer Management and Research 2017;9:189-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palumbo 2004
- Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood 2004;104(10):3052-7. [DOI] [PubMed] [Google Scholar]
Palumbo 2008
- Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008;22(2):414-23. [DOI] [PubMed] [Google Scholar]
Palumbo 2011
- Palumbo A, Anderson K. Multiple myeloma. New England Journal of Medicine 2011;364(11):1046-60. [DOI] [PubMed] [Google Scholar]
Palumbo 2013
- Palumbo A, Waage A, Hulin C, Beksac M, Zweegman S, Gay F, et al. Safety of thalidomide in newly diagnosed elderly myeloma patients: a meta-analysis of data from individual patients in six randomized trials. Haematologica 2013;98(1):87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clinical research ed.) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Quach 2010
- Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010;24(1):22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
R 2018 [Computer program]
- R Foundation for Statistical Computing R: A language and environment for statistical computing. R Core Team. Vienna, Austria: R Foundation for Statistical Computing, 2018.
Rajkumar 2014
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncology 2014;15(12):e538-48. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 2018
- Richardson PG, Laubach J, Gandolfi S, Facon T, Weisel K, O'Gorman P. Maintenance and continuous therapy for multiple myeloma. Expert Review of Anticancer Therapy 2018;18(8):751-64. [DOI] [PubMed] [Google Scholar]
Rücker 2012
- Rücker G. Network meta-analysis, electrical networks and graph theory. Research Synthesis Methods 2012;3(4):312-24. [DOI] [PubMed] [Google Scholar]
Rücker 2014
- Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Statistics in Medicine 2014;33(25):4353-69. [DOI] [PubMed] [Google Scholar]
Rücker 2015
- Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Medical Research Methodology 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rücker 2018 [Computer program]
- http://CRAN.R-project.org/package=netmeta netmeta: Network Meta-Analysis using Frequentist Methods. R package version 0.9-8. Rücker G, Schwarzer G, Krahn U, König J. http://CRAN.R-project.org/package=netmeta, 2018.
San Miguel 2013
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. Journal of Clinical Oncology 2013;31(4):448-55. [DOI] [PubMed] [Google Scholar]
Schwarzer 2015
- Schwarzer G, Carpenter JR, Rücker G. Chapter 8: Network Meta-Analysis. In: netmeta: Network Meta-Analysis using Frequentist Methods. Springer International Publishing Switzerland, 2015. [Google Scholar]
Schwarzer 2018 [Computer program]
- http://CRAN.R-project.org/package=meta meta: General Package for Meta-Analysis. R package version 4.9-2. Schwarzer G. http://CRAN.R-project.org/package=meta, 2018.
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings tables'. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S editor(s). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2019
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl AE, Skoetz N, Guyatt GH. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane, Draft Version (29 January 2019). [Google Scholar]
Singhal 1999
- Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. New England Journal of Medicine 1999;341(21):1565-71. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Taplitz 2018
The American Cancer Society 2018
- The American Cancer Society. Signs and symptoms of multiple myeloma. https://www.cancer.org/cancer/multiple-myeloma/detection-diagnosis-staging/signs-symptoms.html (accessed 27 August 2018).
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waxman 2010
- Waxman AJ, Mink PJ, Devesa SS, Anderson WF, Weiss BM, Kristinsson SY, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood 2010;116(25):5501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisel 2017
- Weisel K, Doyen C, Dimopoulos M, Yee A, Lahuerta JJ, Martin A, et al. A systematic literature review and network meta-analysis of treatments for patients with untreated multiple myeloma not eligible for stem cell transplantation. Leukemia & Lymphoma 2017;58(1):153-61. [DOI] [PubMed] [Google Scholar]
Windebank 2008
- Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. Journal of the Peripheral Nervous System 2008;13(1):27-46. [DOI] [PubMed] [Google Scholar]
World Health Organization 2019
- World Health Organization. WHO updates global guidance on medicines and diagnostic tests to address health challenges, prioritize highly effective therapeutics, and improve affordable access.. Available at: https://www.who.int/news-room/detail/09-07-2019-who-updates-global-guidance-on-medicines-and-diagnostic-tests-to-address-health-challenges-prioritize-highly-effective-therapeutics-and-improve-affordable-access (accessed: 30 August 2019) 2019.
World Health Organization 2019a
- World Health Organization. Executive Summary. The Selection and Use of Essential Medicines 2019. Report of the 22nd WHO Expert Committee on the 2019 Selection and Use of Essential Medicines.. Available at: https://apps.who.int/iris/bitstream/handle/10665/325773/WHO-MVP-EMP-IAU-2019.05-eng.pdf?ua=1 (accessed 30 August 2019).
References to other published versions of this review
Piechotta 2018
- Piechotta V, Jakob T, Scheckel B, Langer P, Monsef I, Scheid, C, et al. Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis. PROSPERO 2018 CRD42018118092 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018118092. [DOI] [PMC free article] [PubMed]


