Abstract
Since almost 30 years, lung transplantation is a considerable therapeutic option in patients suffering from end-stage lung disease. Up to now, the impact of donor-specific antibodies directed against donor HLA (human leukocyte antigen) before and after transplantation is still a matter of debate. As histocompatibility testing is not required for each patient according to the current national guidelines and Eurotransplant recommendations for lung transplantation, each transplantation unit has to establish a local protocol together with the tissue typing laboratory how to implement an immunological risk assessment strategy for their patients while enabling access to transplantation. Desensitization regimens might help in case of highly alloimmunized patients waiting for urgent transplantation.
Keywords: HLA antibodies, Lung transplantation, Alloimmunization, Antibody-mediated rejection
Introduction
In the last few years, several authors were able to elucidate the impact of de novo donor-specific antibodies directed against donor HLA (human leukocyte antigen) after transplantation of solid organs on graft and patient survival [1, 2, 3]. In renal transplantation, there is no doubt that anti-HLA antibodies directed against donor HLA and antibodies which cause a positive crossmatch test may lead to acute rejection of the transplant [4]. In heart and lung transplantation, the presence of HLA antibodies before transplantation also seems to be important because their detection in traditional functional assays as complement-dependent cytotoxicity (CDC) is associated with decreased survival [5]. Since the introduction of solid-phase immunoassay (SPI) technology, the clinical impact especially of preformed HLA antibodies in transplantation of solid organs is still a matter of debate [6, 7, 8].
With this review, we would like to describe the impact, screening, and therapy of HLA antibodies in patients before and after lung transplantation while presenting the actual Munich approaches always intending access to transplantation.
Lung Transplantation
Since the late 1980s, lung transplantation has become an option for patients with end-stage lung disease to retain lung function and to improve survival and quality of live [9]. Individuals living with end-stage lung disease are severely restricted with respect to normal daily exercises, dependent on oxygen substitution, and threatened by death.
Hence, the number of lung transplantations is continuously increasing. The International Thoracic Organ Transplant Registry from the International Society of Heart and Lung Transplantation (ISHLT) reports about 4,122 lung transplantations in 2015 [10, 11]. According to the Eurotransplant (ET) annual report (2017), lung transplantation is also increasingly practiced in Europe (www.eurotransplant.org). Unfortunately, long-term survival is still lower compared to other solid organ transplants with a current 5-year survival of 50.3%, although it has improved over the last few years [12, 13]. Since the first years of single lung transplantation, bilateral lung transplantation has become the standard procedure due to the better long-term outcome [10]. Certainly, the operation procedure is dependent on the underlying disease [14]. Patients with cystic fibrosis or pulmonary hypertension are transplanted bilaterally, and single lung transplantation is possible in few patients with interstitial fibrosis or chronic obstructive pulmonary disease exposed to a higher mortality risk after transplantation.
In the past, lung allocation was based on waiting time and urgency within ET. In 2011, the Lung Allocation Score was implemented following the allocation principle in North America [15, 16, 17]. Urgency and prospect of success were taken into account for the Lung Allocation Score. Until now, the results showed a decrease in mortality in patients on the waiting list and a better 1-year graft survival after transplantation [18, 19]. Histocompatibility in terms of HLA matching is only considered in kidney allocation, but it does not play a role in nonrenal transplantation. So far, HLA typing and antibody screening is not required for each patient according the current national guidelines and ET recommendations for lung transplantation. Thus, a local clinical protocol has to be established taking histocompatibility testing into account. Furthermore, in case of immunized patients on the waiting list, it is always challenging which donor HLA should be avoided, and the decision should be based on patient characteristics by a multidisciplinary team. In this review, we evaluate different strategies.
After transplantation, patients are not only at risk of severe infections due to intensive immunosuppressive therapy but also of malignancies. Beside infections, chronic lung allograft dysfunction (CLAD) is the main factor limiting long-term survival [20]. The bronchiolitis obliterans syndrome (BOS) is the obstructive manifestation of CLAD [21, 22]. In a recent study by, Kulkarni et al. [23], most recipients died or developed BOS within 4 years, and very few remained alive and free from BOS 10 years after transplantation. Risk factors for developing BOS are various: (1) primary graft dysfunction, (2) acute cellular rejection, (3) antibody-mediated rejection (AMR), (4) lymphocytic bronchiolitis, (5) infections, or (6) gastroesophageal reflux [24]. BOS is the main course for transplantation failure and retransplantation. However, not all patients suffer from the obstructive form of CLAD. In some individuals, a restrictive allograft syndrome is prevalent [25]. Recently, de novo donor-specific HLA antibodies (dnDSA) have been found to increase the risk of CLAD and to accelerate its progression [26, 27, 28, 29].
Antibody-Mediated Rejection
During an allogeneic lung transplantation, the recipient will be exposed to foreign leukocyte antigens (HLA) derived from the donor and is at risk of developing antibodies. These antibodies will induce a cellular and humoral rejection in the recipient, which may lead to the loss of the transplanted organ. To reduce the risk of rejection, an optimal immunological match between the donor and the recipient would be favorable. Nevertheless, due to the ongoing scarcity of donor organs and the persistent discrepancies between the high number of needed organs and the small number of organs offered, immunological “mismatches” have to be accepted. As a result, patients after “mismatched” lung transplantation often develop DSA which are able to trigger an AMR [27, 30]. AMR is well described in renal transplantation, where it has compromised graft survival [31, 32]. However, in lung transplantation, a standardized definition of AMR regarding histopathology as well as the impact of HLA antibodies is still discussed. In 2016, a working group created by the ISHLT published a proposal with the aim to determine criteria for pulmonary AMR [33]. The authors divided AMR in clinical (presence of allograft dysfunction) and subclinical (absence of allograft dysfunction) classes, grading the classes in possible, probable, and definite rejection episodes. The degree of certainty of the diagnosis depends on the demonstration of whether multiple criteria are present or absent. “Define” AMR reveals all criteria such as allograft dysfunction, lung histology, C4d+, and DSA, while all other causes are excluded. Nevertheless, some open questions remain, and according to the authors consensus definitions are dynamic, and more efforts should be made to develop effective strategies for the prevention, diagnosis, and particularly the management of AMR.
Histocompatibility Testing
There is no doubt that the polymorphic HLA system is presently the most important biological barrier in the setting of organ transplantation. Patients awaiting kidney and/or pancreas transplantation have to be typed for their individual HLA profile before entering the waiting list. Up to now, they are typed for HLA-A, -B, -C (class I) and -DRB1 and -DQB1 (class II) according to national and international guidelines. Is has to be mentioned that there are additional immunogenic HLA loci as HLA-DQA1, -DPA1, and -DPB1 (class II) which are not taken into account during the allocation process. Besides HLA typing, the patient is to be screened regularly for the presence of HLA antibodies.
HLA Antibody Detection
Concerning screening for HLA antibodies, the traditional cell-based CDC method revealing complement-binding HLA antibodies of IgG and IgM type is still a valuable tool for HLA antibody detection and specification, especially class I. Due to the famous work of Patel and Terasaki [4] in 1969, it is well known that cytotoxic HLA antibodies are strongly associated with graft rejection, but the CDC assay is not sensitive and specific enough to detect these deleterious antibodies in a sufficient and reliable manner.
Meanwhile the microparticle-based technology using flow cytometry (SPI/Luminex® technique) has been successfully applied [34]. Different kinds of multi- or single-antigen beads enable screening or specification of HLA class I and/or II antibodies also against HLA-DQA and -DP antigens. After incubation of patient serum with Luminex microbeads, alloantibody binding to its antigenic target is made detectable by adding a fluorescent-conjugated antihuman IgG. The mean fluorescence intensity (MFI) helps to estimate the amount of IgG antibody in patient sera in a semiquantitative way. In case of pretransplantation depending on the detected HLA antibodies, the HLA of a prospective donor is either defined as unacceptable antigen (UNAG) or as a risk antigen. If several HLA antibodies are detectable, a panel-reactive antibody (PRA) value (%) is calculated based on the frequency of the defined UNAG within a pool of HLA-typed deceased donors (Fig. 1). The higher the immunization the higher the PRA value. There is no doubt that the Luminex technique is more sensitive and more specific than the CDC technique, but the interpretation of the results is still challenging both before and after transplantation. In their review, Gebel and Bray [35] summarized that there are several factors significantly influencing HLA antibody detection and specification results, e.g., the threshold level (MFI), the kinds of antigens (native or recombinant), and their density on the beads, denatured HLA epitopes expressed on single-antigen beads (especially class I), and the prozone effect or desensitization agents like intravenous immunoglobulin (ivIg). Using C1q, C3d, or C4d might help to determine which of the Luminex-detected HLA antibodies are able to bind complement. It has to be kept in mind that the ability to bind complement is associated with DSA MFI and antibody titer [36]. In kidney transplantation, it has been shown that in contrast to post-dnDSA, the risk of pretransplant DSA for AMR seems to be identical independent of the antibody capacity for fixing complement [37].
Fig. 1.
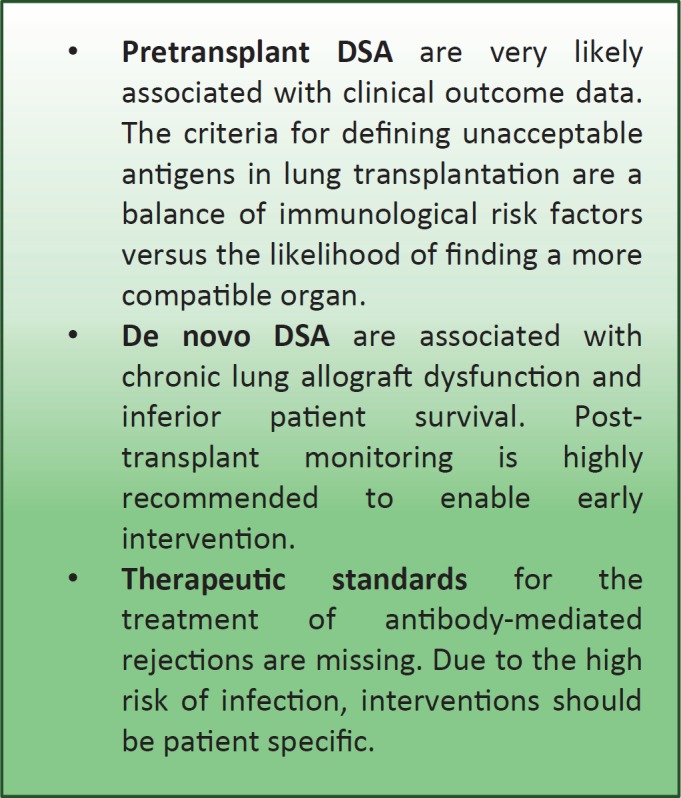
Key points.
Crossmatch
Within ET, the prospectively performed CDC crossmatch (CDC XM) is still the golden standard [4]. Donor lymphocytes prepared from peripheral blood or a piece of spleen are attacked by donor-specific patient alloantibody in the presence of complement. Thus, this technique is limited to the detection of complement-binding HLA specificities.
In contrast, the flow-cytometric crossmatch (flow XM) is sensitive for the detection of complement and non-complement-binding HLA class I and II antibodies [38]. As donor lymphocytes, recipient serum, and a fluorescent-labeled antihuman immunoglobulin are incubated together, the flow XM enables a perfect correlation to the Luminex antibody identification results [39]. Another advantage compared to CDC XM is the independence of personal visual interpretation. It is important to mention that the International Antibody Consensus Group did neither vote for CDC XM nor for flow XM [7].
Based on the Luminex technology and the definition of UNAG or risk antigens, a less time-consuming virtual crossmatch (VXM) can be performed in case of an organ offer [40]. This useful tool enables patients awaiting heart and lung transplantation immunological risk assessment and an extended donor pool.
Impact of Preformed HLA Antibodies on Clinical Outcome Data
The groups of Hadjiliadis et al. [41]and Shah et al. [5] could impressively demonstrate the deleterious role of complement-binding preformed DSA on graft and patient survival after lung transplantation.
But the impact of Luminex-detected HLA antibodies before transplantation on graft function is still a matter of debate. As mentioned before, patients awaiting lung transplantation do not have to be screened for the presence of HLA antibodies according to national regulatory bodies. Thus, most of the studies focusing on the role of preformed Luminex-detected HLA antibodies are performed retrospectively, mostly in small patient cohorts. With the Luminex technique, HLA antibodies have been identified in up to 30–40% of the recipients following lung transplantation. Several data from the literature help to assume that preformed Luminex-detected HLA antibodies – whether specific to donor antigens or not – precede chronic rejection leading to organ failure.
In 2014, Smith et al. [8] demonstrated a 1-year survival for 51.9% of DSA-positive patients while patients with complement-binding DSA revealed a worse 1-year survival of 12.5%. Patients transplanted against DSA and surviving the first year were not more at risk to develop BOS than patients without immunological risk. Another study published in the same year reported a significant association of pretransplant HLA antibodies with higher AMR rates, whereas 1-year survival was not different. In contrast to these data, Chin et al. [42] could not show an association between pretransplant DSA and worse 1-year survival or medium-term (up to 60 months) clinical outcomes after transplantation. In their retrospectively performed study including 56 patients, Brugière et al. [43] found a lower freedom from BOS and a higher rate of mortality for patients with preformed class II DSA. They used an unusual MFI threshold of 300 in their study while among reports on kidney transplantation HLA specificities with MFI below 1,000 are widely considered negative [44]. Up to now, there is no consensus regarding the appropriate MFI threshold stating which preformed HLA antibodies have to be considered as clinically relevant leading to define HLA of a potential donor as unacceptable or as risk factor for lung transplantation candidates (Table 1).
Table 1.
Recent literature on the impact of Luminex-detected pretransplant HLA antibodies (ab) on lung transplant outcome
| Reference | Patients,n | Study period | MFI | Anti-HLA ab | DSA | AMR | BOS | 1-year survival | XM | Risk predictor | Patient selection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Smithet al.[8], 2014 | 425 | 1991–2003 | >5,000 | 63 | 12/27 | na | ns | ↓ | CDC | DSA (MFI >5,000) and DSA+C4d | Retrospective, single center |
| C4d+ | 9/27 | ↓↓ | |||||||||
| >2,000–5,000 | 7/27 | ns | |||||||||
| <2,000 | 8/27 | ns | |||||||||
| Kimet al. [66], 2014 | 224 (waiting list), 126 (transplanted) | 2008–2012 | >3,000 | 77 | na | ↑ | na | ns | CDC | DSA (MFI >3,000) for AMR | Retrospective, single center |
| >1,000–3,000 | 54 | ns | na | ns | |||||||
| Chinet al. [42], 2015 | 195 | 2008–2012 | >500 | 87 | 56 | ns | ns | ns | CDC | – | Retrospective, single center |
| Brugière et al. [43], 2013 | 56 | Before 2008 | >300 | 47 | 18 | na | ↑ (for class II ab) | na | – | DSA class II | Retrospective, single center |
DSA, donor-specific HLAantibodies;AMR, antibody-mediated rejection; BOS, bronchiolitis obliterans syndrome; MFI, mean fluorescence intensity; ns, nonsignificant difference; na, not applicable; XM, crossmatch.
In 2013, Tait et al. [7] published consensus guidelines on pre- and posttransplantation monitoring of HLA antibodies in solid organ transplantation demanding that the presence of preformed DSA should be avoided in heart and lung transplantation. Ideally, all DSA are considered, but this is often not practical in the setting of organ scarcity and recipient allosensitization. This recommendation opens the discussion on the technique-based definition of DSA. Should only CDC+/Luminex+ HLA antibodies be taken into account? What about CDC–/ Luminex+ HLA specificities, and which mean fluorescence threshold is to be used? Can we dispose the traditional prospective CDC XM only heading for a VXM in case of an organ offer? As there are no guidelines, each HLA laboratory has to establish a local protocol together with the transplantation unit how to implement an immunological risk assessment strategy for their patients while still enabling access to transplantation (Table 2). If HLA antibodies are detectable in patient serum, the information about immunizing events like former transplantations, blood transfusions, and pregnancies is very important for the definition of UNAG versus risk antigens.
Table 2.
Immunological risk assessment strategy: current approach of the Munich Lung Transplant Group (MLTP)
| Risk group | I low risk | II risk | III high risk | IV very high risk |
|---|---|---|---|---|
| HLA alloimmunization | – | + | + | + |
| CDC ab | – | – | – | + |
| Luminex ab (PRA) | – | <50% | >50% | >85% |
| Unacceptable antigen | – | + | + | + |
| Risk HLA | – | – | + | + |
| Crossmatch | – | Virtual | Virtual | CDC |
ab, antibodies; PRA, panel-reactive antibodies; CDC, complement-dependent cytotoxicity.
Patients belonging to risk group I are not immunized, and in case of an organ offer, each donor is suitable from the immunological point of view. Patients of risk groups II–IV are HLA alloimmunized. The classification UNAG and risk antigen is based on the knowledge of immunizing events and the Luminex results. UNAG are an explicit contraindication in case of an organ offer. Donors with risk HLA can be accepted while taking the higher immunological risk into account and intensifying the immunosuppressive therapy. In risk groups II and III, the HLA profile of the donor is accepted based on VXM. Very highly immunized patients belonging to risk category IV are discussed for desensitization protocols before transplantation. These patients should get a prospective CDC XM based on T cells. If there is a need for an extended donor pool while a prospective CDC XM is not possible, a perioperative CDC XM in the recipient laboratory is highly recommended to adapt the immunosuppressive regime.
Finally, with the recently published study showing that allosensitized patients awaiting lung transplantation had longer waiting times, a decreased likelihood of transplantation, and at last a reduced overall survival, the definition of UNAG still remains a daily challenge [45].
In the last years, there have been several attempts to substitute the HLA-based matching of donor and recipient towards epitope matching for transplantation, but up to now this has not been implemented in daily routine [46]. Another approach in predicting the immunogenicity of donor HLA is the PIRCHE II (Predicted Indirectly Recognizable HLA Epitopes presented by recipient HLA class II) algorithm. Geneugelijk et al. [47] previously demonstrated that donor-derived PIRCHE II seems to play a role in dnDSA formation after kidney transplantation. Whether this can also be demonstrated for patients awaiting another solid organ transplantation has to be awaited until significant studies have been published.
Impact of dnDSA
A possible impact of de novo HLA antibodies against the donor, occurring after lung transplantation, on graft survival was reported more than 10 years ago by Girnita et al. [48] in 2005. Patients with dnDSA had a higher rate of acute organ rejections and a higher rate of BOS than patients without de novo formation of antibodies. These retrospective data indicated that de novo HLA antibodies induce graft failure. In the course of the years, other groups confirmed these data (Table 3) [26, 49, 50]. Moreover, there is evidence that dnDSA are associated with decreased survival following lung transplantation [26, 27, 51]. Several data indicate that patients suffering from AMR had significantly higher incidence rates of dnDSA [30, 52]. dnDSA appear frequently (20–50%) after lung transplantation. However, the pathophysiological impact is still not completely understood due to the fact that some patients developing dnDSA show a rapid decline in lung function and an impaired survival, but others do not. The majority of dnDSA appear early after transplantation, mostly in the first year after transplantation, and are directed against HLA-DQ [52, 53]. In recent years, there was growing interest in persistence as opposed to spontaneous dnDSA clearance. Especially, persistent DSA seem to be more harmful [54, 55, 56]. Verleden et al. [50] recommend to distinguish transient and persistent dnDSA. We found that patients with persistent dnDSA not disappearing in the first year after transplantation had a significantly higher risk of mortality, with 60% of these patients not surviving the first year [54]. It would, therefore, be advisable to screen patients regularly after transplantation.
Table 3.
Association of de novo donor-specific HLA antibodies (dnDSA) with chronic allograft dysfunction (CLAD) and survival
| Reference | Recent literature on the impact of posttransplant dnDSA on lung transplant outcome |
|||||||
|---|---|---|---|---|---|---|---|---|
| patients/DSA, n/n | follow-up | AMR | ACR | CLAD | survival | risk factors | patient selection | |
| Schmitzeret al.[54], 2018 | 72/23 | 18 months | na | ns | ↑ | ↓ only persistent DSA | Persistence of DSA | Prospective, single center |
| Hachemet al. [67], 2018 | 119/43 | 4 months | na | ↑ | na | ns | LAS DSA >3,000 MFI | Prospective, multicenter |
| Roux et al. [52], 2017 | 206/105 | 2.8 years | ↑ only DQ-DSA | na | ↑ only DQ-DSA | ↓ only DQ-DSA | DQ-DSA | Prospective, single center |
| Verleden et al. [50], 2017 | 362/61 | 3.9 years | na | na | ↑ | ↓ | Persistence of DSA | Single center |
| Kauke et al. [26], 2015 | 120/27 | 3.5 years | na | ns | ↑ | ↓ | DSA >3,000 MFI | Retrospective, single center |
| Morrellet al. [27], 2014 | 445/58 | 2 years | na | na | ↑ | ↓ | Prospective, single center | |
| Safavi et al. [49], 2014 | 148/38 | 3.5 years | na | na | ↑ | ↓ | Single center | |
| lus et al. [51], 2014 | 546/100 (early DSA) | 1.9 years | na | na | na | ↓ only early DSA | Retransplantation, PGF, preformed HLA ab | Retrospective, single center |
| Lobo et al. [30], 2013 | 44/13 | na | ↑ | (↑) | ↑ | ↓ | Cystic fibrosis | Retrospective, single center |
AMR, antibody-mediated rejection;ACR, acutecellularrejection;ns, nonsignificant difference;LAS, lungallocation score; MFI, meanfluorescenceintensity;PGF, primary graft dysfunction;ab, antibody;na, notapplicable.
In our Munich approach, we screen lung transplant patients continuously after transplantation (after 3 weeks, after 3, 6, 9, 12, 18, and 24 months, and then yearly) and on demand in case of lung dysfunction. Another crucial point in posttransplant monitoring of HLA antibodies is the sensitivity of the applied detection assays. It is well known that Luminex screening beads are less sensitive than single-antigen beads. Other HLA loci e.g., HLA-C, -DQA, -DPA, and -DPB besides the known HLA loci A, B, DRB1, and DQB1, might be underestimated because donors are rarely typed for all loci. To validate these important findings and to be able to predict the risk to develop acute graft failure and BOS, multicenter prospective studies are requested.
Therapeutic Options
Sensitization to HLA is an obstacle to the success of lung transplantation. As already described, both preformed and de novo DSA are very likely to be associated with acute rejection, CLAD, and decreased survival. Several lung transplant centers practice avoidance of DSA at the time of organ allocation. Concerning lung transplant candidates with a high PRA and a small number of compatible donors, waiting time and risk of death on the waiting list should be kept in mind. Desensitization regimens aim to reduce the HLA antibody titer in patient sera to enlarge the donor pool and to improve posttransplant outcome. Many centers have employed therapeutic approaches to desensitize the immunized patients prior to transplantation, but only few data are available [57, 58]. Multicenter randomized trials are missing. Most of the protocols failed because treatment has been individualized. All of the data are retrospectively collected in single centers. To remove antibodies, plasma exchange or immunoabsorption are mainly used. Due to rare side effects, ivIg for immunmodulation are frequently given alone or after plasma exchange. Drugs like rituximab (anti-CD20 antibody) for B-cell depletion or bortezomib (proteasome inhibitor) for plasma cell depletion are administered to the patient to inhibit DSA production (Table 4). In 2015, the Toronto Lung Transplant Program published a protocol for the management of sensitized patients [57]. Pretransplant DSA were identified, and, based on the clinical situation of the patient and the likelihood of more compatible organ offers, a treatment strategy has been settled. The authors demonstrated that lung transplantation is safely performed in DSA/PRA-positive patients with similar outcomes to unsensitized patients. One year before, Snyder et al. [58] published successful transplantation of 9 immunized patients after desensitization, but they summarized that not all treated highly immunized patients could benefit from their multimodal desensitization protocol. Recently, Roux et al. [59] reported on issues of the Banff Lung Conference 2017. The working group discussed actual knowledge of diagnosis and treatment of pulmonary AMR. Consensus was reached that specific treatments depend on the clinical course and response to first-line interventions. This circumstance makes it rather difficult to favor any specific regime. Nevertheless, plasma exchange, ivIg, and B-cell depletion seem to be therapeutic options for AMR treatment in lung transplant candidates as well as in kidney or heart transplant recipients [60, 61, 62, 63]. The largest study presents 4-year experience of preemptive treatment of early DSA with IgA- and IgM-enriched ivIg. Ius et al. [64] reported on similar 4-year graft survival in treated patients with early DSA compared to patients without antibodies. In contrast to other studies, they showed a high antibody clearance. This phenomenon might be explained by the early start of intervention before allograft dysfunction occurred. Nevertheless, it has to be kept in mind that some patients might be “overtreated.” Sullivan et al. [65] pointed out that clearance of DSA correlated significantly with improved outcomes. Unfortunately, response to treatment was poor, with only 12 of 47 patients demonstrating clearance of DSA and worse survival in the DSA-positive group that never cleared DSA.
Table 4.
Desensitization and antibody-mediated rejection (AMR) treatment: efficacy in lung transplant patients
| Reference | Recent literature on desensitization in lung transplant candidates and treatment of pulmonary AMR |
||||||
|---|---|---|---|---|---|---|---|
| patients/DSA/PRA,n/n/n | treated patients,n | cessation of treatment | clearance of DSA/HLA ab | 1-year survival | therapy | side effects | |
| Preformed DSA Tinckamet al.[56], 2015 | 340/53/93 | 53 | 43% | MFI f | ns | Plasma exchange, ivIg, ATG | No |
| Snyderet al.[58], 2014 | –/2/9 | 18 | 44% | ns | ns | Plasma exchange, ivIg, bortezomib, rituximab | Thrombocytopenia |
| dn DSA Iuset al.[64], 2018 | 452/128 (only early DSA) | 128 | 9% | 90% | ns | ivIg enriched with IgA and IgM± plasma exchange and rituximab | Anemia, abdominal pain |
| Sullivanet al.[65], 2018 | 194/124 | 47 | na | 25% | f | ivIg± plasma exchange, rituximab or bortezomib | na |
| Vachaet al. [62], 2017 | –/16 | 16 | 35% | 27% | 56.2% | Plasma exchange, ivIg, bortezomib, rituximab | Thrombocytopenia |
| Rouxet al. [63], 2016 | 206/22 | 22 | 33% | na | 54% | ivIg, plasma exchange, rituximab | na |
ab, antibodies; ATG, anti-T-lymphocyte globulins; dnDSA, de novo donor-specific HLA antibodies; PRA, panel-reactive ab; MFI, mean fluorescence intensity; ns, nonsignificant difference; ivIg, intravenous immunoglobulins; na, not applicable.
In summary, up to now, there are no evidence-based recommendations for desensitization protocols and treatment of AMR.
Conclusion
HLA-alloimmunized lung transplant recipients remain a high-risk group of patients demanding for peculiar attention during waiting time. With the available sensitive Luminex technique, more HLA antibodies are detectable, but their impact especially during waiting time still remains a matter of debate. Identification of risk antigens enables the clinician to make more evidence-based decisions regarding whether or not to accept an organ for an immunized patient and whether an intensified immunosuppression is indicated. Monitoring for DSA posttransplant identifies recipients at a greater risk for pulmonary AMR and can guide treatment. Clinical trials do not provide conclusive evidence to support any specific treatment.
Outlook
As mentioned before, up to now histocompatibility testing is not required in the current national guidelines and ET recommendations for lung transplantation. National/international guidelines demanding for HLA typing and HLA antibody screening before and after transplantation would help to standardize the immunological information for each lung recipient. The knowledge about HLA typing of donor and recipient and the HLA immunological profile of the patient could facilitate performing multicenter studies in order to define UNAG for the patient and to establish desensitization protocols and AMR treatment strategies. Finally, a commonly accepted immunological risk assessment is highly requested for all patients to improve long-term graft survival.
Disclosure Statement
There are no conflicts of interest. The authors have no funding to disclose.
References
- 1.Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009 May;87((10)):1505–13. doi: 10.1097/TP.0b013e3181a44206. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira A, Felipe C, Cristelli M, Viana L, Basso G, Stopa S, et al. Donor-Specific Anti-Human Leukocyte Antigens Antibodies, Acute Rejection, Renal Function, and Histology in Kidney Transplant Recipients Receiving Tacrolimus and Everolimus. Am J Nephrol. 2017;45((6)):497–508. doi: 10.1159/000475888. [DOI] [PubMed] [Google Scholar]
- 3.Rose ML. De novo production of antibodies after heart or lung transplantation should be regarded as an early warning system. J Heart Lung Transplant. 2004 Apr;23((4)):385–95. doi: 10.1016/j.healun.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969 Apr;280((14)):735–9. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 5.Shah AS, Nwakanma L, Simpkins C, Williams J, Chang DC, Conte JV. Pretransplant panel reactive antibodies in human lung transplantation: an analysis of over 10,000 patients. Ann Thorac Surg. 2008 Jun;85((6)):1919–24. doi: 10.1016/j.athoracsur.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Süsal C, Ovens J, Mahmoud K, Döhler B, Scherer S, Ruhenstroth A, et al. No association of kidney graft loss with human leukocyte antigen antibodies detected exclusively by sensitive Luminex single-antigen testing: a Collaborative Transplant Study report. Transplantation. 2011 Apr;91((8)):883–7. doi: 10.1097/TP.0b013e3182100f77. [DOI] [PubMed] [Google Scholar]
- 7.Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013 Jan;95((1)):19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 8.Smith JD, Ibrahim MW, Newell H, Danskine AJ, Soresi S, Burke MM, et al. Pre-transplant donor HLA-specific antibodies: characteristics causing detrimental effects on survival after lung transplantation. J Heart Lung Transplant. 2014 Oct;33((10)):1074–82. doi: 10.1016/j.healun.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JD, Patterson GA, Grossman R, Maurer J. Double-lung transplant for advanced chronic obstructive lung disease. Am Rev Respir Dis. 1989 Feb;139((2)):303–7. doi: 10.1164/ajrccm/139.2.303. [DOI] [PubMed] [Google Scholar]
- 10.Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb SB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report—2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant. 2015 Oct;34((10)):1264–77. doi: 10.1016/j.healun.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Chambers DC, Yusen RD, Cherikh WS, Goldfarb SB, Kucheryavaya AY, Khusch K, et al. International Society for Heart and Lung Transplantation The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant. 2017 Oct;36((10)):1047–59. doi: 10.1016/j.healun.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. International Society for Heart and Lung Transplantation The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report—2013; focus theme: age. J Heart Lung Transplant. 2013 Oct;32((10)):965–78. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. International Society of Heart and Lung Transplantation The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012 Oct;31((10)):1073–86. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Neurohr C, Huppmann P, Thum D, Leuschner W, von Wulffen W, Meis T, et al. Munich Lung Transplant Group Potential functional and survival benefit of double over single lung transplantation for selected patients with idiopathic pulmonary fibrosis. Transpl Int. 2010 Sep;23((9)):887–96. doi: 10.1111/j.1432-2277.2010.01071.x. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb J, Greer M, Sommerwerck U, Deuse T, Witt C, Schramm R, et al. Introduction of the lung allocation score in Germany. Am J Transplant. 2014 Jun;14((6)):1318–27. doi: 10.1111/ajt.12752. [DOI] [PubMed] [Google Scholar]
- 16.Davis SQ, Garrity ER., Jr Organ allocation in lung transplant. Chest. 2007 Nov;132((5)):1646–51. doi: 10.1378/chest.07-0011. [DOI] [PubMed] [Google Scholar]
- 17.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6((5 Pt 2)):1212–27. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb J, Smits J, Schramm R, Langer F, Buhl R, Witt C, et al. Lung Transplantation in Germany Since the Introduction of the Lung Allocation Score. Dtsch Arztebl Int. 2017 Mar;114((11)):179–85. doi: 10.3238/arztebl.2017.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant. 2016 Apr;35((4)):433–9. doi: 10.1016/j.healun.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2007 Jul;26((7)):681–6. doi: 10.1016/j.healun.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014 Feb;33((2)):127–33. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Verleden SE, Sacreas A, Vos R, Vanaudenaerde BM, Verleden GM. Advances in Understanding Bronchiolitis Obliterans After Lung Transplantation. Chest. 2016 Jul;150((1)):219–25. doi: 10.1016/j.chest.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni HS, Cherikh WS, Chambers DC, Garcia VC, Hachem RR, Kreisel D, et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: An International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J Heart Lung Transplant. 2019 Jan;38((1)):5–16. doi: 10.1016/j.healun.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, Wilson KC, et al. ISHLT/ATS/ERS BOS Task Force Committee. ISHLT/ATS/ERS BOS Task Force Committee An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014 Dec;44((6)):1479–503. doi: 10.1183/09031936.00107514. [DOI] [PubMed] [Google Scholar]
- 25.Van Herck A, Verleden SE, Sacreas A, Heigl T, Vanaudenaerde BM, Dupont LJ, et al. Validation of a post-transplant chronic lung allograft dysfunction classification system. J Heart Lung Transplant. 2019 Feb;38((2)):166–73. doi: 10.1016/j.healun.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Kauke T, Kneidinger N, Martin B, Dick A, Schneider C, Schramm R, et al. Bronchiolitis obliterans syndrome due to donor-specific HLA-antibodies. Tissue Antigens. 2015 Sep;86((3)):178–85. doi: 10.1111/tan.12626. [DOI] [PubMed] [Google Scholar]
- 27.Morrell MR, Pilewski JM, Gries CJ, Pipeling MR, Crespo MM, Ensor CR, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014 Dec;33((12)):1288–94. doi: 10.1016/j.healun.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Morrell MR, Patterson GA, Trulock EP, Hachem RR. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2009 Jan;28((1)):96–100. doi: 10.1016/j.healun.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Witt CA, Gaut JP, Yusen RD, Byers DE, Iuppa JA, Bennett Bain K, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013 Oct;32((10)):1034–40. doi: 10.1016/j.healun.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobo LJ, Aris RM, Schmitz J, Neuringer IP. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J Heart Lung Transplant. 2013 Jan;32((1)):70–7. doi: 10.1016/j.healun.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Feucht HE. Complement C4d in graft capillaries— the missing link in the recognition of humoral alloreactivity. Am J Transplant. 2003 Jun;3((6)):646–52. doi: 10.1034/j.1600-6143.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 32.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003 Jun;3((6)):665–73. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 33.Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2016 Apr;35((4)):397–406. doi: 10.1016/j.healun.2016.01.1223. [DOI] [PubMed] [Google Scholar]
- 34.Lachmann N, Todorova K, Schulze H, Schönemann C. Luminex(®) and its applications for solid organ transplantation, hematopoietic stem cell transplantation, and transfusion. Transfus Med Hemother. 2013 Jun;40((3)):182–9. doi: 10.1159/000351459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gebel HM, Bray RA. HLA antibody detection with solid phase assays: great expectations or expectations too great? Am J Transplant. 2014 Sep;14((9)):1964–75. doi: 10.1111/ajt.12807. [DOI] [PubMed] [Google Scholar]
- 36.Schaub S, Hönger G, Koller MT, Liwski R, Amico P. Determinants of C1q binding in the single antigen bead assay. Transplantation. 2014 Aug;98((4)):387–93. doi: 10.1097/TP.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 37.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013 Sep;369((13)):1215–26. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 38.Lindemann M, Nyadu B, Heinemann FM, Kribben A, Paul A, Horn PA, et al. High negative predictive value of an amplified flow cytometry crossmatch before living donor kidney transplantation. Hum Immunol. 2010 Aug;71((8)):771–6. doi: 10.1016/j.humimm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Peräsaari JP, Jaatinen T, Merenmies J. Donor-specific HLA antibodies in predicting crossmatch outcome: comparison of three different laboratory techniques. Transpl Immunol. 2018 Feb;46:23–8. doi: 10.1016/j.trim.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Bielmann D, Hönger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S. Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant. 2007 Mar;7((3)):626–32. doi: 10.1111/j.1600-6143.2007.01667.x. [DOI] [PubMed] [Google Scholar]
- 41.Hadjiliadis D, Chaparro C, Reinsmoen NL, Gutierrez C, Singer LG, Steele MP, et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant. 2005 Jul;24((7 Suppl)):S249–54. doi: 10.1016/j.healun.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Chin N, Paraskeva M, Paul E, Cantwell L, Levvey B, Williams T, et al. Comparative analysis of how immune sensitization is defined prior to lung transplantation. Hum Immunol. 2015 Oct;76((10)):711–6. doi: 10.1016/j.humimm.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Brugière O, Suberbielle C, Thabut G, Lhuillier E, Dauriat G, Metivier AC, et al. Lung transplantation in patients with pretransplantation donor-specific antibodies detected by Luminex assay. Transplantation. 2013 Mar;95((5)):761–5. doi: 10.1097/TP.0b013e31827afb0f. [DOI] [PubMed] [Google Scholar]
- 44.Schinstock CA, Gandhi MJ, Stegall MD. Interpreting Anti-HLA Antibody Testing Data: A Practical Guide for Physicians. Transplantation. 2016 Aug;100((8)):1619–28. doi: 10.1097/TP.0000000000001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tague LK, Witt CA, Byers DE, Yusen RD, Aguilar PR, Kulkarni HS, et al. Association between Allosensitization and Waiting List Outcomes among Adult Lung Transplant Candidates in the United States. Ann Am Thorac Soc. 2019 Jul;16((7)):846–52. doi: 10.1513/AnnalsATS.201810-713OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duquesnoy RJ. Are We Ready for Epitope-Based HLA Matching in Clinical Organ Transplantation? Transplantation. 2017 Aug;101((8)):1755–65. doi: 10.1097/TP.0000000000001667. [DOI] [PubMed] [Google Scholar]
- 47.Geneugelijk K, Niemann M, Drylewicz J, van Zuilen AD, Joosten I, Allebes WA, et al. PIRCHE-II Is Related to Graft Failure after Kidney Transplantation. Front Immunol. 2018 Mar;9:321. doi: 10.3389/fimmu.2018.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girnita AL, Duquesnoy R, Yousem SA, Iacono AT, Corcoran TE, Buzoianu M, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005 Jan;5((1)):131–8. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 49.Safavi S, Robinson DR, Soresi S, Carby M, Smith JD. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2014 Dec;33((12)):1273–81. doi: 10.1016/j.healun.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Verleden SE, Vanaudenaerde BM, Emonds MP, Van Raemdonck DE, Neyrinck AP, Verleden GM, et al. Donor-specific and -nonspecific HLA antibodies and outcome post lung transplantation. Eur Respir J. 2017 Nov;50((5)):1701248. doi: 10.1183/13993003.01248-2017. [DOI] [PubMed] [Google Scholar]
- 51.Ius F, Sommer W, Tudorache I, Kühn C, Avsar M, Siemeni T, et al. Early donor-specific antibodies in lung transplantation: risk factors and impact on survival. J Heart Lung Transplant. 2014 Dec;33((12)):1255–63. doi: 10.1016/j.healun.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Roux A, Bendib Le Lan I, Holifanjaniaina S, Thomas KA, Picard C, Grenet D, et al. Characteristics of Donor-Specific Antibodies Associated With Antibody-Mediated Rejection in Lung Transplantation. Front Med (Lausanne) 2017 Oct;4:155. doi: 10.3389/fmed.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tikkanen JM, Singer LG, Kim SJ, Li Y, Binnie M, Chaparro C, et al. De Novo DQ Donor-Specific Antibodies Are Associated with Chronic Lung Allograft Dysfunction after Lung Transplantation. Am J Respir Crit Care Med. 2016 Sep;194((5)):596–606. doi: 10.1164/rccm.201509-1857OC. [DOI] [PubMed] [Google Scholar]
- 54.Schmitzer M, Winter H, Kneidinger N, Meimarakis G, Dick A, Schramm R, et al. Persistence of de novo donor specific HLA-Antibodies after lung transplantation: a potential marker of decreased patient survival. HLA. 2018 Jun;92((1)):24–32. doi: 10.1111/tan.13306. [DOI] [PubMed] [Google Scholar]
- 55.Le Pavec J, Suberbielle C, Lamrani L, Feuillet S, Savale L, Dorfmüller P, et al. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant. 2016 Sep;35((9)):1067–77. doi: 10.1016/j.healun.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Tinckam KJ, Keshavjee S, Chaparro C, Barth D, Azad S, Binnie M, et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am J Transplant. 2015 Feb;15((2)):417–26. doi: 10.1111/ajt.13076. [DOI] [PubMed] [Google Scholar]
- 57.Islam AK, Sinha N, DeVos JM, Kaleekal TS, Jyothula SS, Teeter LD, et al. Early clearance vs persistence of de novo donor-specific antibodies following lung transplantation. Clin Transplant. 2017;31((8)) doi: 10.1111/ctr.13028. short title: HLA-antibodies in lung transplantation. [DOI] [PubMed] [Google Scholar]
- 58.Snyder LD, Gray AL, Reynolds JM, Arepally GM, Bedoya A, Hartwig MG, et al. Antibody desensitization therapy in highly sensitized lung transplant candidates. Am J Transplant. 2014 Apr;14((4)):849–56. doi: 10.1111/ajt.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roux A, Levine DJ, Zeevi A, Hachem R, Halloran K, Halloran PF, et al. Banff Lung Report: current knowledge and future research perspectives for diagnosis and treatment of pulmonary antibody-mediated rejection (AMR) Am J Transplant. 2019 Jan;19((1)):21–31. doi: 10.1111/ajt.14990. [DOI] [PubMed] [Google Scholar]
- 60.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011 Jul;365((4)):318–26. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 61.Jackups R, Jr, Canter C, Sweet SC, Mohanakumar T, Morris GP. Measurement of donor-specific HLA antibodies following plasma exchange therapy predicts clinical outcome in pediatric heart and lung transplant recipients with antibody-mediated rejection. J Clin Apher. 2013 Aug;28((4)):301–8. doi: 10.1002/jca.21270. [DOI] [PubMed] [Google Scholar]
- 62.Vacha M, Chery G, Hulbert A, Byrns J, Benedetti C, Finlen Copeland CA, et al. Antibody depletion strategy for the treatment of suspected antibody-mediated rejection in lung transplant recipients: does it work? Clin Transplant. 2017 Mar;31((3)):e12886. doi: 10.1111/ctr.12886. [DOI] [PubMed] [Google Scholar]
- 63.Roux A, Bendib Le Lan I, Holifanjaniaina S, Thomas KA, Hamid AM, Picard C, et al. Foch Lung Transplantation Group Antibody-Mediated Rejection in Lung Transplantation: Clinical Outcomes and Donor-Specific Antibody Characteristics. Am J Transplant. 2016 Apr;16((4)):1216–28. doi: 10.1111/ajt.13589. [DOI] [PubMed] [Google Scholar]
- 64.Ius F, Verboom M, Sommer W, Poyanmehr R, Knoefel AK, Salman J, et al. Preemptive treatment of early donor-specific antibodies with IgA- and IgM-enriched intravenous human immunoglobulins in lung transplantation. Am J Transplant. 2018 Sep;18((9)):2295–304. doi: 10.1111/ajt.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan D, Ahn C, Gao A, Lacelle C, Torres F, Bollineni S, et al. Evaluation of current strategies for surveillance and management of donor-specific antibodies: single-center study. Clin Transplant. 2018 Jul;32((7)):e13285. doi: 10.1111/ctr.13285. [DOI] [PubMed] [Google Scholar]
- 66.Kim M, Townsend KR, Wood IG, Boukedes S, Guleria I, Gabardi S, et al. Impact of pretransplant anti-HLA antibodies on outcomes in lung transplant candidates. Am J Respir Crit Care Med. 2014 May;189((10)):1234–1239. doi: 10.1164/rccm.201312-2160OC. [DOI] [PubMed] [Google Scholar]
- 67.Hachem RR, Kamoun M, Budev MM, Askar M, Ahya VN, Lee JC, et al. Human leukocyte antigens antibodies after lung transplantation Primary results of the HALT study. Am J Transplant. 2018 Sep;18((9)):2285–2294. doi: 10.1111/ajt.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]


