Abstract
Background
Rectal varices are portosystemic collaterals that arise as a complication of portal hypertension. Despite their significant prevalence among cirrhotic patients, clinically important bleeding occurs only in a minority. Various treatment options are available, with endoscopic therapies being widely used, and both interventional radiology and surgery being considered for refractory bleeding rectal varices.
Case
We report the case of a 61-year-old male with hepatic cirrhosis and bleedingrectal varices refractory to endoscopic therapy, successfully managed with a combination of transjugular intrahepatic portosystemic shunt (TIPS) and selective variceal embolization.
Conclusions
Radiological techniques are effective options for refractory bleeding. Adding embolization to TIPS implantation could represent a valid adjunctive measure for haemostasis of recurrent rectal variceal bleeding.
Keywords: Liver cirrhosis, Rectal varices, Transjugular intrahepatic portosystemic shunt, Therapeutic embolization
Introduction
Portal hypertension is a recognized complication related to different nosological entities that alter either portal blood flow or increase vascular resistance, with liver cirrhosis being by far its commonest aetiology.
Rectal varices are portosystemic collaterals that arise as a complication of portal hypertension. Their prevalence has been reported to be between 38 and 56% in patients with cirrhosis [1], and clinically important bleeding may occur in up to 38% of these patients, a higher incidence than previously reported [2]. Although bleeding rectal varices may be fatal without proper treatment, its rarity precludes the establishment of clinical guidelines to define appropriate management strategies. Currently, various treatment options are available, with endoscopic therapies being widely used and both interventional radiology and surgery being considered for refractory bleeding rectal varices.
We report a case of difficult-to-treatrectal varices managed with a combination of endoscopic injection sclerotherapy (EIS), transjugular intrahepatic portosystemic shunt (TIPS), and selective variceal embolization.
Case Report
A 61-year-old man with alcohol-induced hepatic cirrhosis (Child-Pugh score A, Model for End-stage Liver Disease score of 9 points) and portal hypertension with small oesophageal and rectal varices had previously suffered hepatic decompensation with ascites and hepatic encephalopathy 5 years before and had stopped drinking alcohol by that time. Rectal varices were identified in a colonoscopy 2 years afterwards and further characterized by endoscopic ultrasound.
He presented to the emergency department with haematochezia that had persisted for 10 days and fluctuating level of consciousness in the past few hours. On admission, the patient had hepatic encephalopathy grade II (West Haven). His vital signs were within the normal range and the abdomen non-tender without clinical signs of ascites. Digital rectal examination showed fresh blood. He had anaemia (haemoglobin 8.7 g/dL) and thrombocytopenia (platelets 106,000/µL), total bilirubin level of 1.29 mg/dL with the remaining liver, and coagulation profile within the normal range. Upper endoscopy showed small oesophageal varices without evidence of bleeding. A sigmoidoscopy was performed which demonstrated large rectal varices, congestive haemorrhoids, and brown-coloured blood with no bleeding point detectable. An abdominal ultrasound revealed a patent portal vein.
He was admitted to our gastroenterology department, with the diagnosis of acute hepatic encephalopathy precipitated by probable haemorrhoidal bleeding, under prophylactic antibiotic and lactulose treatment.
One day after admission, the patient was alert and coherent, but a massive haematochezia with haemodynamic instability occurred with a decrease in the haemoglobin level to 6.3 g/dL. At that time, colonoscopy showed enlarged and nodular rectal varices >20 mm with a ruptured point, despite no active bleeding (Fig. 1). EIS with 6 ml 1% polidocanol was performed, alongside with intravenous crystalloids, transfusion of red blood cells, and terlipressin. Over the next week, the patient continued to show blood losses per rectum, demanding transfusion of red blood cells. A second EIS with 1% polidocanol, with a total amount of 16 ml, was attempted (Fig. 2). Due to the absence of clinical improvement, with clinically significant bleeding, a TIPS with a metallic polytetrafluoroethylene-covered stent was placed with no significant immediate complications (Fig. 3). Hepatic encephalopathy grade II developed 9 days later, managed with lactulose and rifaximin, resulting in clinical recovery.
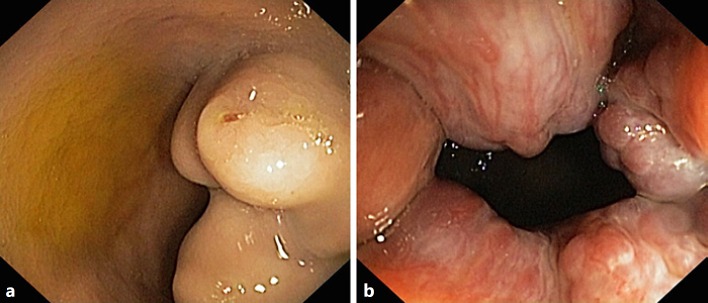
Fig. 1.
Colonoscopy findings after the second bleeding episode. a Enlarged and nodular rectal varices >20 mm with a ruptured point despite no active bleeding. b Congested internal haemorrhoids.
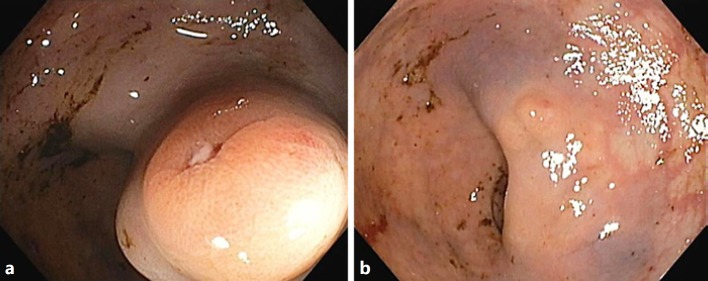
Fig. 2.
Sigmoidoscopy findings 1 week after the first endoscopic injection sclerotherapy. Rectal varices before (a) and after (b) endoscopic injection sclerotherapy with 1% polidocanol.
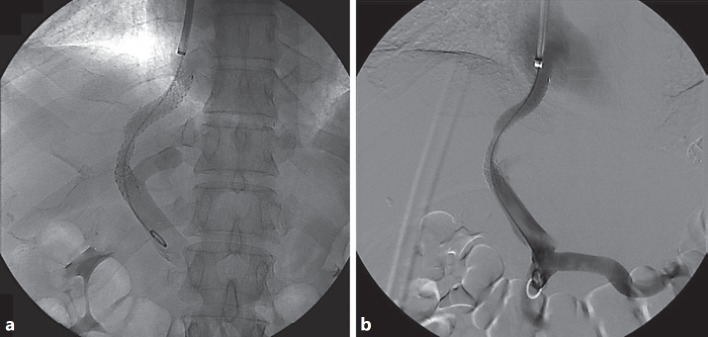
Fig. 3.
Fluoroscopic (a) and digital subtraction angiography (b) control after transjugular intrahepatic portosystemic shunt placement.
Two weeks after TIPS placement, the patient bled again from the rectal varices. Successful haemostasis was achieved with the injection of 15 ml 1% polidocanol. He restarted vasopressor therapy with octreotide (due to previous terlipressin-induced hyponatremia). In the context of refractory rectal variceal bleeding, selective varices embolization was attempted the following day. Portal venous transhepatic angiogram showed hepatofugal flow and a mesenteric-renal shunt. Selective varices catheterization followed by right hypogastric vein occlusion using an occlusion balloon allowed successful retrograde transvenous embolization of rectal varices with ethylene vinyl alcohol and micro-coils. Towards the end of the procedure, the mesenteric-renal shunt was embolized using an Amplatzer vascular plug occlusion device (Fig. 4).
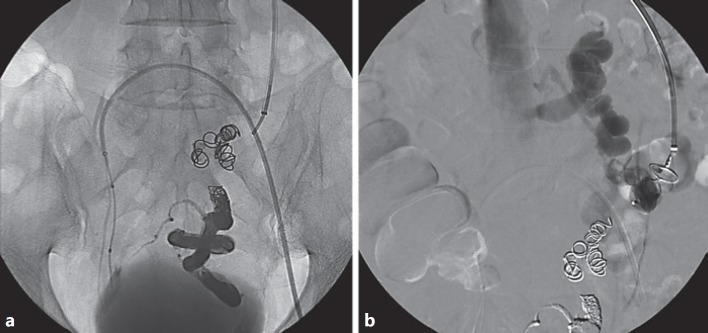
Fig. 4.
a Embolized rectal varices with ethylene vinyl alcohol and coils (procedure made across the transjugular intrahepatic portosystemic shunt tract – transjugular approach). A balloon is placed in the right hypogastric vein (left femoral approach). b Fluoroscopic control during Amplatzer plug deployment in the mesenteric renal shunt.
Four days after obliteration of the rectal varices, no further bleeding occurred, and the patient was discharged after 6 weeks of hospitalization. Rectal endoscopic ultrasound at 6 weeks of follow-up did not identify any varices or collateral circulation (Fig. 5). During 18 months of follow-up, the patient reported no further rectal bleeding, and abdominal ultrasound with Doppler showed a patent TIPS.
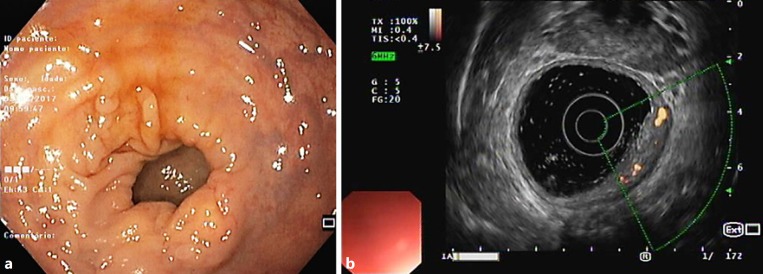
Fig. 5.
Sigmoidoscopy (a) and rectal endoscopic ultrasound (b) at 6 weeks of follow-up with no evidence of rectal varices or collateral circulation.
Discussion
We describe the case of a cirrhotic patient with massive haematochezia due to rectal varices, refractory to vasoactive drugs and several attempts of EIS.
The differential diagnosis of haematochezia in cirrhotic adults may be challenging. While in non-cirrhotic patients the most common source of haematochezia is located in the lower gastrointestinal tract (mostly diverticular disease of the colon), in cirrhotic patients, the proportion of upper gastrointestinal bleeding is significantly higher, with the major cause of haematochezia being oesophageal variceal bleeding, followed by peptic ulcers. Internal haemorrhoid bleeding is the most frequently identified cause of bleeding, originating in the lower gastrointestinal tract among cirrhotic patients [3]. Rectal variceal bleeding accounts for <5% of all varix-related bleeding episodes in Western countries [2], which contrasts with a relatively high prevalence of rectal varices reported in cirrhotic patients – between 38 and 56% [1]. In accordance with this data, our patient had rectal varices identified 3 years before variceal bleeding and was asymptomatic during that period.
Controlling and treating rectal varices can be an even more challenging task. After haemodynamic stabilization, endoscopic therapies are the preferred option since they are readily available and less invasive. EIS has been reported to be more effective in the management of active bleeding from rectal varices with a smaller re-bleeding rate (33.3%) compared to endoscopic band ligation (55.6%) [4]; however, there is no recommendation regarding the concentration and volume of the sclerosant agent to be injected. These high recurrence rates may be a drawback of endoscopic techniques, as portal hypertension remains unchanged. Endoscopic variceal obturation with injection of cyanoacrylate is limited to case reports, most of them guided by endoscopic ultrasound in conjunction with intraluminal placement of embolization coils [5]. In our case, injection sclerotherapy was the preferred endoscopic technique both because of its higher efficacy and because the large size of the rectal varices made band ligation a sub-optimal choice with a potentially increased risk of post-banding bleeding.
TIPS is considered the gold standard for treatment of upper gastrointestinal variceal bleeding refractory to endoscopic therapy in patients with portal hypertension [6], while its therapeutic role in rectal or other ectopic variceal bleeding has only been described in case reports or case series. In 2008, Kochar et al. [7] reported the largest series of patients with bleeding ectopic varices treated by TIPS placement, with 12 out of 28 being due to rectal varices. Despite not reporting the outcomes of rectal variceal bleeding specifically, overall haemostasis was effectively achieved in 67% of the patients. Vangeli et al. [8] published a series of 21 cirrhotic patients who underwent TIPS for bleeding ectopic varices, 9 of which had bleeding rectal varices. Five patients had variceal embolization using the same procedure and none re-bled.
Although TIPS is useful in controlling bleeding from rectal varices, it may not always be successful in controlling massive bleeding from large rectal varices, even after normalization of portal hypertension [7, 8, 9, 10]. One reason for this phenomenon is related to Laplace's equation in which the tension in the varix wall is proportional to the radius of the vessel for any given transmural pressure. Thus, larger varices can bleed at a lower portosystemic gradient, the same holds for gastric varices. Therefore, in some cases as ours, a combination of endoscopic and radiologic procedures, such as TIPS and rectal variceal embolization, is necessary.
Variceal embolization through a radiological approach was first described in gastric varices with a success rate of 89% in controlling bleeding varices [11], although it may induce a significant elevation in portal systemic pressure gradient with worsening of oesophageal varices [12]. Several reports have been published on the successful embolization of rectal varices through distinct endovascular approaches [13, 14, 15, 16, 17, 18], but none addressed the risk of increasing portal venous pressure when used alone.
At present, no evidence-based recommendations support primary prophylaxis to prevent bleeding from ectopic varices. To prevent re-bleeding from rectal varices as well as other ectopic varices, empirical pharmacologic treatment with a beta-blocker is usually tried.
In conclusion, management of rectal varices should be multidisciplinary and on a case-by-case basis. Endoscopic therapies are the preferred approach since they are readily available and less invasive, with radiological techniques such as TIPS being effective options for refractory bleeding. Adding embolization to TIPS implantation could represent a valid adjunctive measure for haemostasis of recurrent rectal variceal bleeding.
Statement of Ethics
This study does not require informed consent nor review/approval by the appropriate Ethics Committee.
Disclosure Statement
The authors have no conflicts of interest to declare.
Author Contributions
Drafting of the manuscript: M.G. Critical revision of the manuscript and final approval of the version to be published: all listed authors.
References
- 1.Al Khalloufi K, Laiyemo AO. Management of rectal varices in portal hypertension. World J Hepatol. 2015 Dec;7((30)):2992–8. doi: 10.4254/wjh.v7.i30.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shudo R, Yazaki Y, Sakurai S, Uenishi H, Yamada H, Sugawara K. Clinical study comparing bleeding and nonbleeding rectal varices. Endoscopy. 2002 Mar;34((3)):189–94. doi: 10.1055/s-2002-20289. [DOI] [PubMed] [Google Scholar]
- 3.Camus M, Khungar V, Jensen DM, Ohning GV, Kovacs TO, Jutabha R, et al. Origin, Clinical Characteristics and 30-Day Outcomes of Severe Hematochezia in Cirrhotics and Non-cirrhotics. Dig Dis Sci. 2016 Sep;61((9)):2732–40. doi: 10.1007/s10620-016-4198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato T, Yamazaki K, Akaike J, Toyota J, Karino Y, Ohmura T. Retrospective analysis of endoscopic injection sclerotherapy for rectal varices compared with band ligation. Clin Exp Gastroenterol. 2010;3:159–63. doi: 10.2147/CEG.S15401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson M, Thompson AI, Hayes PC. The Management of Bleeding from Anorectal Varices. Curr Hepatol Rep. 2017;16((4)):406–15. [Google Scholar]
- 6.Cardenas A, Mendez-Bocanegra A. Report of the Baveno VI Consensus Workshop. Ann Hepatol. 2016 Mar-Apr;15((2)):289–90. doi: 10.5604/16652681.1193729. [DOI] [PubMed] [Google Scholar]
- 7.Kochar N, Tripathi D, McAvoy NC, Ireland H, Redhead DN, Hayes PC. Bleeding ectopic varices in cirrhosis: the role of transjugular intrahepatic portosystemic stent shunts. Aliment Pharmacol Ther. 2008 Aug;28((3)):294–303. doi: 10.1111/j.1365-2036.2008.03719.x. [DOI] [PubMed] [Google Scholar]
- 8.Vangeli M, Patch D, Terreni N, Tibballs J, Watkinson A, Davies N, et al. Bleeding ectopic varices—treatment with transjugular intrahepatic porto-systemic shunt (TIPS) and embolisation. J Hepatol. 2004 Oct;41((4)):560–6. doi: 10.1016/j.jhep.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Sakib SM, Kobayashi K, Jawed M. Potential Pitfalls in Transjugular Portosystemic Shunt Placement for Bleeding Rectal Varices. Case Rep Gastroenterol. 2015 Aug;9((2)):296–301. doi: 10.1159/000439164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Xuan W, Song L. Transjugular intrahepatic portosystemic stent shunt placement and embolization for hemorrhage associated with rupture of anorectal varices. J Int Med Res. 2018 Apr;46((4)):1666–71. doi: 10.1177/0300060517730720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park KS, Kim YH, Choi JS, Hwang JS, Kwon JH, Jang BK, et al. [Therapeutic efficacy of balloon-occluded retrograde transvenous obliteration in patients with gastric variceal bleeding] Korean J Gastroenterol. 2006 May;47((5)):370–8. [PubMed] [Google Scholar]
- 12.Tanihata H, Minamiguchi H, Sato M, Kawai N, Sonomura T, Takasaka I, et al. Changes in portal systemic pressure gradient after balloon-occluded retrograde transvenous obliteration of gastric varices and aggravation of esophageal varices. Cardiovasc Intervent Radiol. 2009 Nov;32((6)):1209–16. doi: 10.1007/s00270-009-9679-3. [DOI] [PubMed] [Google Scholar]
- 13.Hidajat N, Stobbe H, Hosten N, Schroeder RJ, Fauth M, Vogl T, et al. Transjugular intrahepatic portosystemic shunt and transjugular embolization of bleeding rectal varices in portal hypertension. AJR Am J Roentgenol. 2002 Feb;178((2)):362–3. doi: 10.2214/ajr.178.2.1780362. [DOI] [PubMed] [Google Scholar]
- 14.Minamiguchi H, Kawai N, Sato M, Ikoma A, Sanda H, Nakata K, et al. Successful treatment of endoscopically unmanageable rectal varices by balloon-occluded antegrade transvenous sclerotherapy followed by microcoil embolization. J Vasc Interv Radiol. 2013 Sep;24((9)):1399–403. doi: 10.1016/j.jvir.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Kimura T, Haruta I, Isobe Y, Ueno E, Toda J, Nemoto Y, et al. A novel therapeutic approach for rectal varices: a case report of rectal varices treated with double balloon-occluded embolotherapy. Am J Gastroenterol. 1997 May;92((5)):883–6. [PubMed] [Google Scholar]
- 16.Arai H, Kobayashi T, Takizawa D, Toyoda M, Takayama H, Abe T. Transileocolic vein obliteration for bleeding rectal varices with portal thrombus. Case Rep Gastroenterol. 2013 Mar;7((1)):75–81. doi: 10.1159/000348761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Aal AK, Dawoud N, Moustafa AS, Hamed MF, Saddekni S. Percutaneous Transhepatic Embolization of Bleeding Rectal Varices Using A New Embolic And Sclerotic Mixture Augmented By Amplatzer Vascular Plug 2. J Radiol Case Rep. 2016 Sep;10((9)):44–51. doi: 10.3941/jrcr.v10i9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn SS, Kim EH, Kim MD, Lee WJ, Kim SU. Successful hemostasis of intractable rectal variceal bleeding using variceal embolization. World J Gastroenterol. 2015 Feb;21((8)):2558–62. doi: 10.3748/wjg.v21.i8.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]