Abstract
We report the cardioprotective effects of moderate aerobic exercise from parallel pediatric murine models of doxorubicin (Doxo) exposure in non-tumor-bearing immune competent (NTB-IC) mice and tumor-bearing nude mice (TB-NM). In both models, animals at four weeks of age underwent Doxo treatment with or without two weeks of simultaneous exercise. In sedentary NTB-IC or TB-NM mice, Doxo treatment resulted in a statistically significant decrease in ejection fraction (EF) and fractional shortening (FS) compared to control animals. Interestingly, moderate aerobic exercise during Doxo treatment significantly mitigated decreases in EF and FS. In contrast, these protective effects of exercise were not observed when exercise was started after completion of Doxo treatments. Moreover, in the TB-NM model, Doxo caused a decrease in heart mass:tibia length and in body weight that was prevented by exercise, while NTB-IC mice exhibited no change in these measurements. Doxo delivery to the hearts of TB-NM was decreased by consistent moderate aerobic exercise prior to Doxo injection. These findings demonstrate the important but subtle differences in cardiotoxicity observed in different mouse models. Collectively, these results also strongly suggest that aerobic exercise during early-life doxorubicin exposure mitigates cardiotoxicity, possibly through altered delivery of doxorubicin to myocardial tissue.
Keywords: Doxorubicin, Cardiotoxicity, Aerobic Exercise
INTRODUCTION
Anthracycline chemotherapies, such as Doxo, are among the most effective anticancer agents utilized in regimens for over half of all childhood cancer patients (1). Unfortunately, exposure to Doxo during childhood is unequivocally linked to increased rates of cardiac disease decades later (1–7). Recent epidemiologic studies indicate that the cumulative incidence of heart failure in long-term pediatric cancer survivors may be as high as 20% by age 50 (8). Because the number of childhood cancer survivors in the United States is expected to surpass half a million by 2020 (9), it is imperative that novel strategies to mitigate anthracycline-induced cardiac damage are evaluated in relevant preclinical models, as a precursor to logically designed clinical trials.
A widely accepted mechanism by which Doxo induces damage to cell populations that comprise myocardium is through the production of reactive oxygen species that damage mitochondria and induce apoptosis (1, 10–13). There are a variety of potential therapeutic strategies that could be beneficial in reducing the amount of ROS produced in heart tissue when Doxo accumulates. Among these strategies is aerobic exercise, which is known to decrease oxidative stress in myocytes and other cells in the heart (14, 15). Importantly, reduction of oxidative stress and cardioprotection by aerobic exercise has already been validated in various preclinical models of cardiac injury including myocardial infarction, ischemia reperfusion, and heart failure (16).
With respect to Doxo-induced cardiotoxicity, aerobic exercise has been previously shown to mitigate cardiac dysfunction in adult rat and mouse models (17–28). However, these studies do not adequately reflect the clinical scenario in pediatric oncology, where patients are exposed to Doxo early in life when the heart is actively growing and still undergoing development. The vast majority of prior animal studies have all focused on the effects of exercise in Doxo-treated adult animals and the protective effects of exercise on Doxo-induced cardiotoxicity have not been previously evaluated in young mice with a developing heart. Importantly our experiments began at 4-weeks of age with tumor cell injection. At one week post establishment of tumor xenografts, exercise and doxorubicin commenced and continued in TB-NM and NTB-IC through seven weeks of age. This time period corresponds with the established timeline of mouse heart development that continues until approximately 9-10 weeks of age. For example, Piquereau and colleagues(29) studied postnatal mouse heart growth and found that heart mass roughly doubles in size between 3 and 9 weeks of age, a time period that corresponds with the time period assessed in our studies.
In addition, the presence of tumor and the role of the immune system in response to Doxo have not previously been investigated in young mice. Here, we demonstrate the cardioprotective potential of aerobic exercise in clinically relevant pediatric murine models of Doxo-induced cardiotoxicity with and without tumor burden and with and without a functional immune system, when exercise is used during the time frame of Doxo exposure.
MATERIALS AND METHODS
Animals
Animal experiments using non-tumor-bearing immune competent (NTB-IC) mice were performed at Greehey Children’s Cancer Research Institute (GCCRI). Experiments with tumor-bearing nude mice (TB-NM) were performed at MD Anderson Cancer Center (MDACC). All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the institution where they were performed (GCCRI or MDACC).
NTB-IC
C57Bl/6J mice, at 4 weeks of age, were purchased from Jackson Labs. Mice were randomly assigned into four groups, with a total of 32 mice (n = 8): sedentary control mice that received drug delivery vehicle (Control), sedentary mice that received doxorubicin (Doxo), exercised mice that received drug delivery vehicle (Exer), and exercised mice that received doxorubicin (Doxo + Exer).
TB-NM
Athymic Swiss nude male mice were purchased from MDACC’s institutional animal resource. When animals were 4 weeks of age, all mice received subcutaneous injections of 5×106 A673 Ewing’s sarcoma cells (American Type Culture Collection) in 200 μl PBS. 7 days later, mice were randomly assigned to one of four groups (32 mice, n = 8 per group): sedentary control mice that received drug delivery vehicle (Control), sedentary mice that received doxorubicin (Doxo), exercised mice that received drug delivery vehicle (Exer), and exercised mice that received doxorubicin (Doxo + Exer) using the same treatment schema as for NTB-IC.
Exercise and doxorubicin treatment
Treadmill exercise protocol
For both protocols, mice were exercised 5 days a week, giving them 2 consecutive off-days for a total of 2 weeks (10 total days of exercise). For TB-NM, exercise was begun 7 days after tumor cell injection, when palpable tumors were present. Exercise for both TB-NM and NTB-IC mice was administered with a standardized treadmill protocol, which consisted of 45 minutes of constant walking at a rate of 12 m/min at 0% slope with 2 minutes of ramp up from 0 m/min and 2 minutes of cool down to 0 m/min at the beginning and end of the protocol. Once exercise was concluded, mice were immediately placed back in their cages and allowed to eat and drink freely.
Doxorubicin treatment
Mice received intraperitoneal (IP) injections of 2.5 mg/kg doxorubicin, twice a week for two weeks. Doxo was given on day 3, 5, 10, and 12 after exercise was begun (days 10, 15, 17, 19 after tumor cell inoculation). For graphical representation, please see Fig 1A.
Figure 1. Doxorubicin with or without exercise causes weight loss in nude mice.
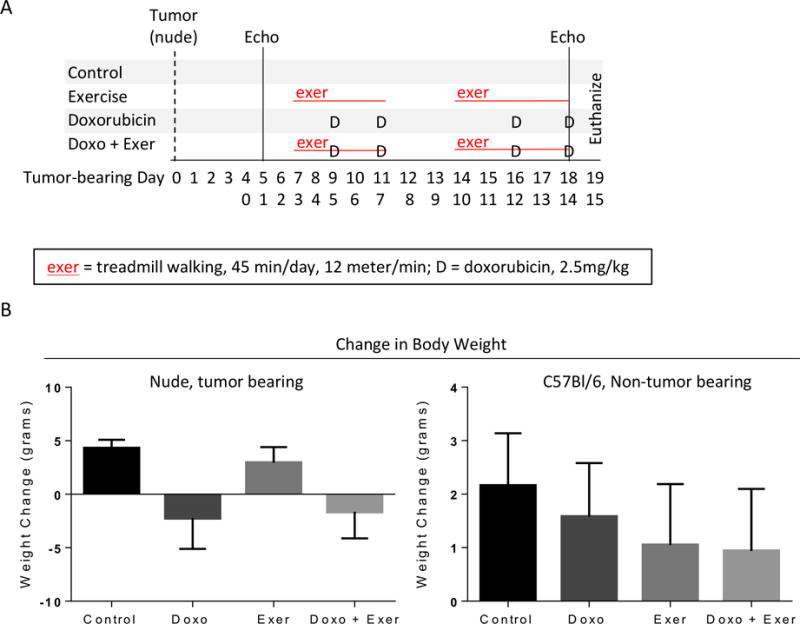
A) Schematic of study design. Parallel experiments were performed using either A673 tumor-bearing nude mice (TB-NM) or non-tumor bearing C57Bl6/J mice (NTB-IC). For TB-NM, tumor cells were injected subcutaneously and tumors were allowed to establish. After this point, study design was identical in both models. Echocardiograms were performed pre-treatment and after the final doxorubicin dose. Animal weight was monitored over time. Treatment groups included control (saline injection, no exercise); exercise alone (treadmill exercise 5 days per week, 45 minutes per day, 12 meters/min); Doxo alone (4 total doses of 2.5mg/kg doxorubicin given on days 9, 11, 16, 18); combination Doxo and exercise. B) Animal weight was tracked over time. Graphs show the mean difference in starting and final animal weight per treatment group +/− standard deviation, separated by TB-NM or NTB-IC mice, respectively. N = 6-12 per group.
Selection of doxorubicin dosing and equivalence to humans
The doxorubicin dosing regimen selected for this study is roughly equivalent to that used in pediatric oncology patients. Nair et al.(30) describe a method to easily to convert animal dosing (in mg/kg) to human equivalents (in mg/kg) by dividing the mouse dose (in mg/kg) by 12.3. Thus, for our model, four doses over 2 weeks at 2.5 mg/kg/per dose equals a human equivalent dose of 0.813 mg/kg; 10 mg/kg divided by 12.3. From CDC growth charts, we then calculated the surface area for an average 5-year old male based on 50th percentile for height and weight as follows: Weight (18 kg) × height (109 cm)/3600 = 0.55 m2. Using the dose of doxorubicin delivered during delayed intensification in the current Children’s Oncology Group high risk ALL study (AALL1131) of 25 mg/m2, the absolute dose of doxorubicin given is 13.75 mg/dose; 25 mg/m2 × 0.55 m2 = 13.8 mg. This compares to a mouse dose converted to human equivalent dosing of 14.6 mg; 0.813 mg/kg × 18 kg = 14.6 mg.”
Exercise delivered after completion of doxo exposure
(Fig S1) Juvenile NTB-IC mice were given a cumulative dose of 25 mg/kg Doxo over 5 weeks, then were divided into groups that performed 8 weeks of exercise (3 days per week using the parameters noted above) or unexercised mice (control). Animals were not pre-trained. This was an intentional choice because pre-training animals (prior to tumor inoculation or doxorubicin exposure) may cause changes to cardiac fitness that would skew our results. The average pediatric patient in the United States does not meet national guidelines for weekly physical activity, meaning that the patient population that we are trying to model is not pre-trained prior to a cancer diagnosis. Thus, mice that are not pre-trained make the most appropriate model to study the impact of exercise during doxorubicin therapy. Because we used a moderate intensity exercise regimen (12 meters/min, equivalent to ~65% VO2 max for mice, ~brisk walk for humans) and not a high-intensity or exhaustive regimen, pre-training was not needed. We did, however, allow animals to acclimate to the treadmill each session by beginning the treadmill at a low speed and slowly increasing to the exercise speed that was used for each 45 minute session.
Echocardiography
Echocardiography was performed prior to the first bout of exercise in all mice, and again on the final day of the experiment prior to euthanasia (day 18 after tumor cell inoculation; day 12 after the first day of exercise for all mice).
NTB-IC Mice (GCCRI)
Echocardiography was performed with the Vevo 2100 system (Visualsonics, Inc., Toronto, Canada) with a MS400 linear array transducer (30 MHz). The device’s transducer was positioned perpendicularly with a stand. Settings for the instrument are described in Table 1. Frame rate for both B-mode and M-mode was set to >200 frames per minute. B-mode long-axis parasternal images were recorded when optimal views of the aorta, papillary muscle, and endocardium were visible. M-mode short-axis images were recorded at the level of the papillary muscles and the LV was bisected to obtain the optimal M-Mode selection. At least 3 B-mode and M-mode images were captured for each mouse. All images were saved to a local computer and analyzed off line by a technician who was blinded to the animal’s age and group. Conventional echocardiographic measurements of the LV included EF, FS, end-diastolic dimension (EDD), end-systolic dimension (ESD), anterior and posterior wall thickness, and mass. For long-axis B-mode measurements, the endocardium was traced semi automatically beginning from the mitral valve and excluding the papillary muscle. EF and FS were calculated by software using standard computational methods.
Table 1.
Vevo 2100 Instrument Settings
| Parameter | Value |
|---|---|
| Transmit | |
| Frequency (Mhz) | 30 |
| Power (%) | 100 |
| Acquisition | |
| Gain (dB) | 27 |
| Display | |
| Dynamic range (dB) | 65 |
| Display Map | C5 |
| Brightness | 50 |
| Contrast | 50 |
| B-mode | |
| Frame rate (fps) | 235 |
| Depth (mm) | 13.00 |
| Width (mm) | 10.36 |
| M-mode | |
| Sweep speed (Hz) | 1200 |
TB-NM (MDACC)
Cardiac function was measured in anaesthetized mice using transthoracic echocardiography (Vevo 770 echocardiography with a 30 MHz linear signal transducer and 707B probe, Visual Sonics, Toronto, CA). The heart was imaged when optimal views of the aorta, papillary muscle, and endocardium were visible in the two-dimensional mode (B-mode) in the parasternal long axis view. M-mode short-axis images were recorded at the level of the papillary muscles and the LV was bisected to obtain the optimal M-Mode selection. The measurements of intra-ventricular septal (IVS) thickness, left ventricular posterior wall thickness (LVPW), and left ventricular internal diameters (LVID) were made in systole and diastole. The endocardium was traced manually beginning from the mitral valve and excluding the papillary muscle. Left ventricular percent fractional shortening, ejection fraction, chamber volume and mass were calculated by Vevo 770 software.
Necropsy and tissue collection
Within 4 hours after the final bout of exercise and doxorubicin injection, animals were euthanized by C02 and cervical dislocation. The intraperitoneal cavity was exposed and inspected visually before the heart and the tibia were removed. Excess blood was squeezed from the heart before obtaining wet whole heart weight (mg), and tibia was thoroughly cleaned before obtaining tibia length (mm). Heart was then sectioned for histological evaluation, and one piece was snap frozen for future use.
Histology and immunohistochemistry
One portion of each heart was fixed in 4% PFA at 4 degrees overnight before being embedded in paraffin and sectioned for immunohistochemical analysis. Hematoxylin & Eosin staining was performed by the MDACC core facility, and sections were analyzed for cardiomyocyte size (diameter of 100 cells per heart were averaged to obtain one value). To evaluate heart vasculature, slides were rehydrated by sequential xylene and ethanol, then heat mediated citrate buffer antigen retrieval was performed. Slides were incubated with anti-CD31 (Abcam, Cambridge, MA) followed by fluorescent secondary antibody.
Quantitation of Doxorubicin in Myocardial Tissue
Tumor-bearing nude mice performed 10 days of exercise as described above but received no doxorubicin during these two weeks. Mice were injected with 10 mg/kg doxorubicin via tail vein 72 hours after the last exercise session. Twenty minutes later, mice were euthanized and hearts were immediately harvested. A roughly 40 mg portion was collected, weighed, and homogenized in acid isopropanol/Triton-X. Doxorubicin fluorescence was read using a spectrophotometer (OD488) and compared to a standard curve, then normalized against the weight of heart tissue input and the background reading of a heart from a mouse that received no doxorubicin.
Statistical Analyses
Statistical differences were evaluated using GraphPad Prism 6 software and Two-way ANOVA or Unpaired Student’s T Test. For each experimental condition, cardiac functional outcomes were compared between control and doxo treated animals. To examine the effects of exercise doxo and doxo + exercise were compared. For all comparisons, statistical significance was defined as p < 0.05.
RESULTS
Exercise counteracts Doxo-induced body weight and heart weight loss
We first evaluated the use of exercise after Doxo exposure to determine whether exercise could mitigate cardiac toxicity. Juvenile NTB-IC mice were given a cumulative dose of 25 mg/kg Doxo over 5 weeks, then were divided into groups that performed 8 weeks of exercise or unexercised mice (control). Exercise after Doxo exposure afforded no recovery of Fractional Shortening (FS) or Ejection Fraction (EF) as measured by echocardiogram relative to Doxo-treated animals that did not perform exercise (Fig S1). Thus, we next evaluated whether exercise during the time period of Doxo exposure can be cardioprotective. We used Doxo given twice weekly for two weeks to induce cardiac toxicity in two parallel mouse models. Nude mice bearing A673 Ewing’s sarcoma tumors (Tumor-bearing nude mice; TB-NM) or C57Bl/6 mice without tumors (Non-tumor bearing immune competent mice; NTB-IC) were treated with 2.5mg/kg doxorubicin twice weekly for two weeks to induce acute cardiac toxicity. Mice in the exer or Doxo + exer group performed treadmill exercise five days per week (Fig 1A).
Treatment with Doxo caused significant weight loss in nude mice, with or without exercise (Fig 1B). In order to model juvenile exposure to Doxo and to evaluate the consequences of exposure during the period of heart growth and development, mice were only four weeks old at the beginning of the experiment. Thus, animals in control and exercise only groups gained weight due to normal growth during the experiment. It is also important to note that tumor shrinkage in response to chemotherapy represented a negligible component of weight loss. In contrast to TB-NM, NTB-IC mice exhibited no significant difference in the amount of weight gained between groups (Fig 1B).
There was a significant reduction in heart weight:tibia length in Doxo treated TB-NM,but not in Doxo treated NTB-IC mice (Fig 2a). To account for the reduction in heart size in nude mice, we examined cardiomyocyte size on histological sections. There was no difference in cardiomyocyte size (cross-sectional diameter) between hearts from different treatment groups in TB-NM or NTB-IC mice (Fig 2b and data not shown). Similarly, there was no obvious difference in the structure of hearts from mice in any group (Fig 2b and data not shown).
Figure 2. Doxorubicin induced heart weight reduction is rescued by exercise.
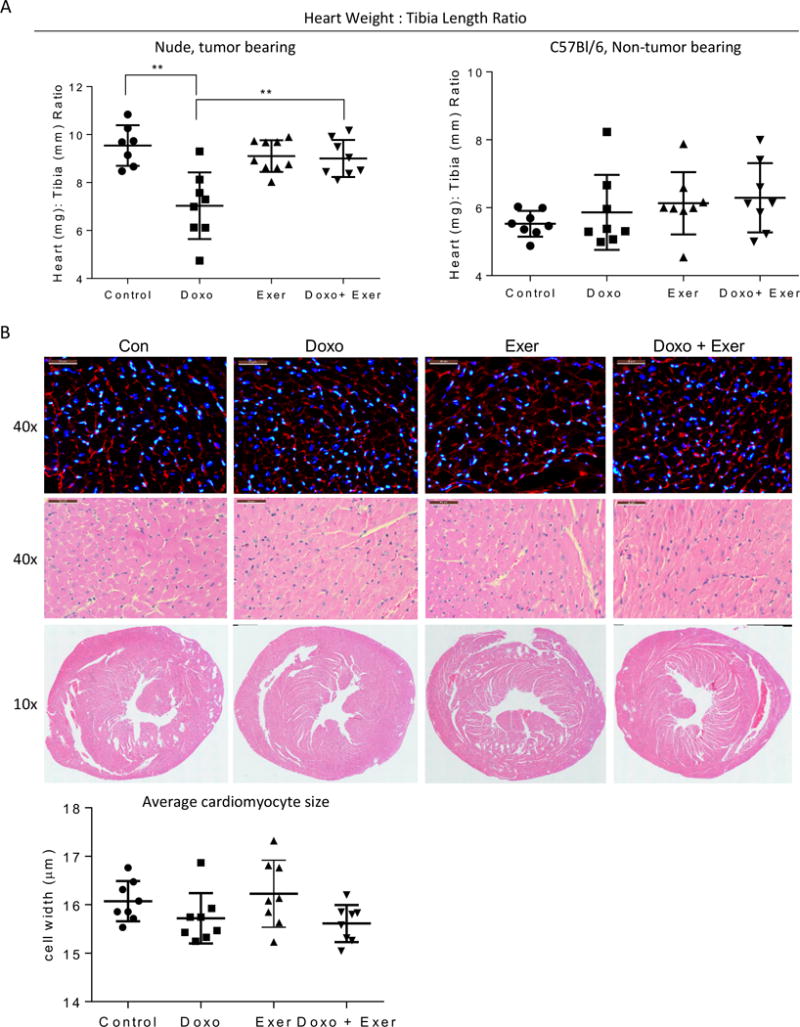
A) At euthanasia, wet heart weight (mg) and tibia length (mm) was measured for each animal. Graphs show the mean heart weight: tibia length +/− standard deviation per treatment group, graphed as nude, tumor-bearing and C57Bl/6 mice. Each symbol represents the value for one mouse. B) Representative H & E staining and DAPI/WGA staining of heart sections from each treatment group of nude, tumor-bearing mice at high magnification (40×) and low magnification (10×). The average width of 100 cardiomyocytes per heart were used to obtain one value per heart, depicted in the graph.
Doxo-induced reduction in cardiac function is mitigated by exercise
Echocardiograms were performed at baseline before exposure to Doxo or Exer, and on the day before euthanasia (Fig 1a). Fractional shortening (FS), ejection fraction (EF), and left ventricular posterior wall thickness in systole and diastole were evaluated (Fig 3a). In TB-NM and in NTB-IC mice, FS and EF were significantly reduced after Doxo exposure compared to baseline, while no change was observed in these functional measures over time in control or exercised animals (Fig 3b, c). Importantly, performing exercise in the same time period as Doxo exposure prevented the loss in cardiac function induced by Doxo alone (Fig 3b, c). Left ventricular posterior wall thickness during systole or diastole were not significantly changed by Doxo or Exer in either model (data not shown).
Figure 3. Doxorubicin reduced heart function is rescued by exercise as detected by echocardiogram.
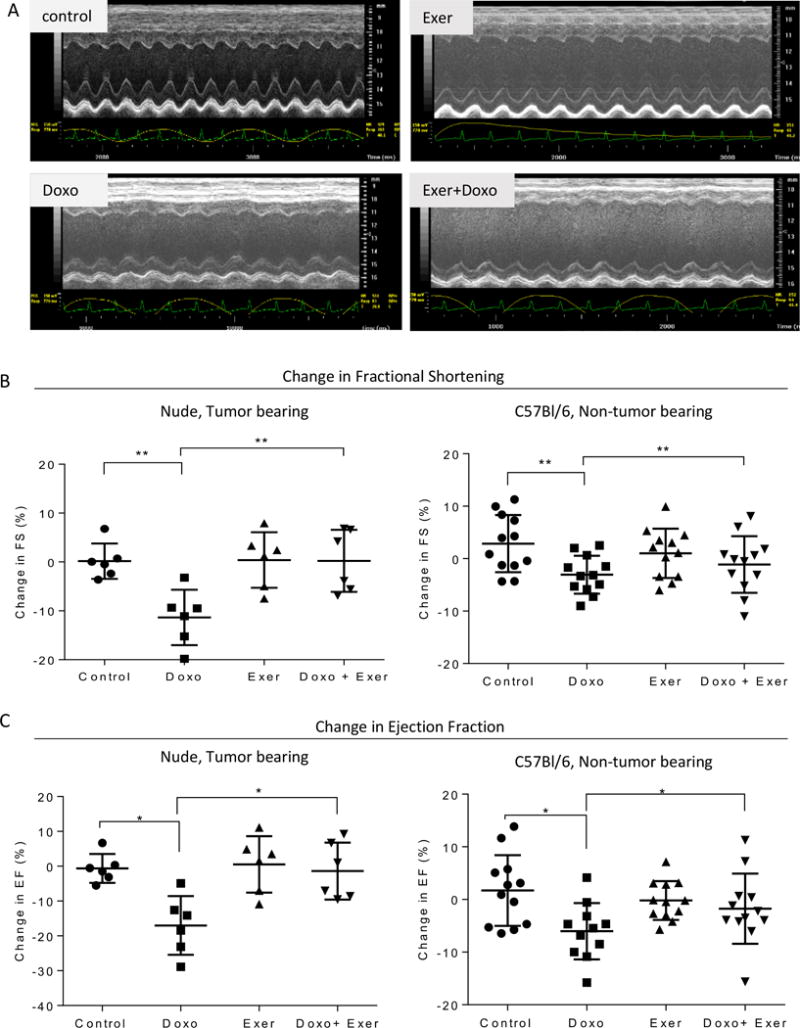
A) Representative echocardiograms performed on hearts of animals from each treatment group of TB-NM. B) Graphs show the mean difference in starting and final fractional shortening per treatment group +/− standard deviation, separated by TB-NM or NTB-IC, respectively. N = 6-12 per group. Each symbol represents the value for one mouse. C) Graphs show the mean difference in starting and final ejection fraction per treatment group +/− standard deviation, separated by TB-NM or NTB-IC, respectively. N = 6-12 per group. Each symbol represents the value for one mouse.
Exercise decreases Doxo accumulation in cardiac tissue
The prevention of Doxo-induced cardiotoxicity by exercise suggested that exercise protects the heart cells from Doxo exposure. To explore this possibility, we used A673 TB-NM. Animals were unexercised or performed treadmill running five days per week for two weeks. Seventy-two hours after the final day of exercise, mice were given a bolus dose of Doxo before the hearts were harvested and Doxo in the tissue was quantified by spectrophotometry. Hearts from animals in the exercise group had significantly less Doxo than those from control mice (Fig 4a). To determine whether the reduced doxorubicin in the hearts of exercised mice was due to a reduction in vascular density, we performed CD31 staining. However, there was no difference in microvessel density between groups (Fig 4b).
Figure 4. Doxorubicin in cardiac tissue is reduced by exercise independent of a decrease in microvessel density.
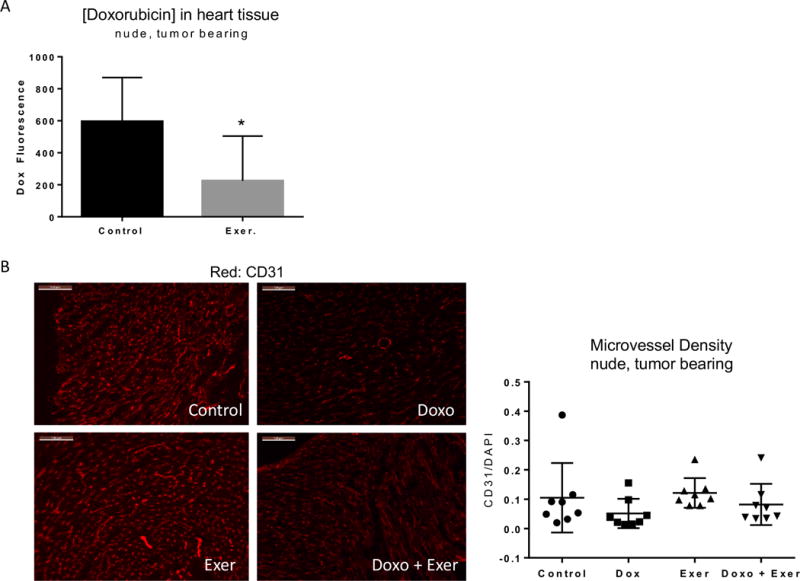
A) Doxorubicin concentration in cardiac tissue was measured by spectrophotometry. Graphs show the mean +/− standard deviation for hearts from non-exercised vs. exercised tumor-bearing mice. B) Representative CD31 (red, endothelial cells) staining of heart sections from each treatment group of nude, tumor-bearing mice. Graphs show the mean +/− standard deviation of CD31:DAPI (endothelial cell: total nuclei) ratio for 5 sections per animal.
DISCUSSION
Doxo is a highly efficacious cytotoxic chemotherapy agent that has been unequivocally associated with acute and chronic cardiac side effects (1–8, 11). With respect to the development of late organ toxicities, pediatric patients face potentially worse outcomes in comparison to adult patients treated for cancer, as pediatric patients are still undergoing organ development and remodeling during the time of treatment exposures. Here, we have identified moderate aerobic exercise as an effective intervention in mitigating acute cardiac side effects associated with Doxo exposure in pediatric mice, which implicates exercise as an attractive beneficial adjuvant to chemotherapy in pediatric cancer patients.
Exercise prevented the reduction in FS and EF induced by Doxo in both models. Importantly, Doxo caused a reduction in body weight and in heart:tibia size ratio that was rescued by exercise in TB-NM. Doxo did not cause reductions in body weight or heart weight:tibia length ratio in NTB-IC mice, emphasizing the differences in the two models and the importance of utilizing appropriate models for evaluation of Doxo induced cardiotoxicity. The differential responses between the two models suggest that either the immune system or the presence of tumor cells contribute to cardiotoxic response and future studies are needed to delineate the underlying mechanisms that mediate these observed differences. TB-NM are more sensitive to Doxo, perhaps secondary to their immunodeficiency, whereas NTB-IC mice were much hardier. In both models, changes in function were mitigated by exercise with a bigger effect noted in the TB-NM. To date, the role that the immune system and tumor cells play in doxo-induced cardiotoxicity in juvenile mice has not been adequately modeled, but our data indicate that further studies to identify the roles of both (and thus the most appropriate model of juvenile Doxo exposure) are warranted.
Less Doxo was delivered to the hearts of TB-NM that had performed 2 weeks of aerobic exercise. Our observations suggest that exercise during Doxo exposure may increase the therapeutic index for Doxo by simply reducing the level of drug penetrating heart tissue.
We utilized aerobic exercise as an intervention because it has a myriad of cardioprotective effects on the heart. The American Heart Association recommends regular exercise to promote cardiac health in healthy individuals and the American Society of Sports Medicine recommends regular exercise for cancer survivors of all ages (31). However, no specialized recommendations exist that are specifically designed for pediatric cancer patients and childhood cancer survivors. Research in animal models suggests that exercise promotes various adaptations in the heart and vascular system that make the heart more resilient against various cellular stressors, including oxidative and proteostatic stressors (32). Notably, each of these has been previously described among the important downstream consequences of Doxo exposure in the heart. Therefore, exercise is a potential intervention that may be a beneficial strategy to reduced treatment-induced cardiac damage in childhood cancer patients.
Current clinical and basic research on how exercise affects the outcome of Doxo exposure have centered on exercise at three different time points: before exposure (preconditioning), during treatment, and after treatment (rehabilitation). There is strong animal model evidence that supports the benefit of exercise in pediatric populations and rehabilitation is important in individuals who have developed heart failure (17–28). Exercise during chemotherapy has been shown to be safe (33–35), but the cardioprotective benefits of exercise when delivered during the active treatment phase are still inconclusive. This controversy exists for many reasons, including the difficulty of diagnosing subclinical cardiac dysfunction, persistence of a low grade sub-clinical cardiac abnormality for several years after anthracycline exposure, and unknown pathologic mechanisms contributing to the development of heart failure in up to 20% of anthracycline-treated cancer patients (2). As more sensitive clinical measures of cardiac dysfunction are developed and validated, such as strain echocardiography and MRI, we will begin to develop a better understanding of subclinical cardiac dysfunction and how exercise during anthracycline treatment alters cardiac function throughout a patient’s lifetime. Importantly, most previous studies have not examined how exercise affects Doxo tissue distribution in mice and thus building on the observations presented here could provide novel perspective on how exercise influences Doxo cardiotoxicity and even toxicity in other normal tissue.
Future studies in mice need to focus on fully understanding the pharmacokinetics of Doxo during exercise, as well as how acute Doxo accumulation in the heart can modify the development of cardiac dysfunction throughout the lifespan. Moreover, it is imperative that these evaluations are conducted in relevant preclinical mouse models that account for animal age at exposure and the presence or absence of a tumor and the immune system. Further, a model delivering exercise during Doxo exposure followed by a prolonged latent period before evaluating changes in cardiac function would have the most relevance with respect to pediatric cancer survivors.
In summary, this study performed in pediatric mice suggests that exercise during Doxo exposure may be a clinically relevant strategy to mitigate Doxo-induced acute cardiac toxicity. These findings provide a framework for logically designed clinical studies of exercise to prevent the significant morbidity associated with late onset cardiotoxicity in the growing population of childhood cancer survivors.
Supplementary Material
Figure S1. Exercise Initiated After Early Doxorubicin Exposure Fails to Mitigate Cardiotoxicity After Eight Weeks. A) Treatment and Exercise Schema. C57BL6/J mice were treated weekly for five weeks with intraperitoneal doxorubicin (1 mg/kg/dose or 5 mg/kg/dose). At the conclusion of the treatments, animals were divided into 4 groups (n = 8) corresponding to sedentary and exercise for both doxorubicin doses administered. Mice in the exercise groups then completed consistent aerobic exercise as denoted. B). Change from Baseline in Ejection Fraction (EF) and Fractional Shortening (FS) for mice treated according to the schema noted in A. For endpoint analysis, n = 8 for all groups except the 5 mg/kg DOX-Exercise. For that group n = 4 due to 4 deaths that occurred during the exercise period.
Acknowledgments
We acknowledge the Center for Energy Balance in Cancer Prevention and Survivorship for input and intellectual support (MDACC). We additionally acknowledge Peter Houghton, PhD and Anthony Infante, MD, PhD for their helpful comments in preparation of the manuscript (UTHSCSA).
Funding
NIH P30CA016672 (MDACC core grant)
GJA: St. Baldrick’s Foundation Scholar (Career Development Award) and Hyundai Hope on Wheels.
References
- 1.Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin: the good, the bad and the ugly effect. Current medicinal chemistry. 2009;16(25):3267–85. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Cochran TR, Franco VI, et al. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;10(12):697–710. doi: 10.1038/nrclinonc.2013.195. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Adams MJ, Colan SD, et al. Long-term Cardiovascular Toxicity in Children, Adolescents, and Young Adults Who Receive Cancer Therapy: Pathophysiology, Course, Monitoring, Management, Prevention, and Research Directions: A Scientific Statement From the American Heart Association. Circulation. 2013;128(17):1927–95. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 4.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27(14):2339–55. doi: 10.1200/JCO.2008.21.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125(1):47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738–43. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 8.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD. Jemal A Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 10.Sterba M, Popelova O, Vavrova A, et al. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid Redox Signal. 2013;18(8):899–929. doi: 10.1089/ars.2012.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawyer DB. Anthracyclines and heart failure. N Engl J Med. 2013;368(12):1154–6. doi: 10.1056/NEJMcibr1214975. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 13.Bertazzoli C, Bellini O, Magrini U, et al. Quantitative experimental evaluation of adriamycin cardiotoxicity in the mouse. Cancer Treat Rep. 1979;63(11–12):1877–83. [PubMed] [Google Scholar]
- 14.Narasimhan M, Rajasekaran NS. Exercise, Nrf2 and Antioxidant Signaling in Cardiac Aging. Front Physiol. 2016;7:241. doi: 10.3389/fphys.2016.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerreiro LF, Rocha AM, Martins CN, et al. Oxidative status of the myocardium in response to different intensities of physical training. Physiol Res. 2016;65(5):737–49. doi: 10.33549/physiolres.933185. [DOI] [PubMed] [Google Scholar]
- 16.Powers SK, Smuder AJ, Kavazis AN, et al. Mechanisms of exercise-induced cardioprotection. Physiology (Bethesda) 2014;29(1):27–38. doi: 10.1152/physiol.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sturgeon K, Schadler K, Muthukumaran G, et al. Concomitant low-dose doxorubicin treatment and exercise. Am J Physiol Regul Integr Comp Physiol. 2014;307(6):R685–92. doi: 10.1152/ajpregu.00082.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen BT, Lien CY, Hydock DS, et al. Exercise mitigates cardiac doxorubicin accumulation and preserves function in the rat. J Cardiovasc Pharmacol. 2013;62(3):263–9. doi: 10.1097/FJC.0b013e3182982ce0. [DOI] [PubMed] [Google Scholar]
- 19.Dolinsky VW, Rogan KJ, Sung MM, et al. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am J Physiol Endocrinol Metab. 2013;305(2):E243–53. doi: 10.1152/ajpendo.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirinbayan V, Roshan VD. Pretreatment effect of running exercise on HSP70 and DOX-induced cardiotoxicity. Asian Pac J Cancer Prev. 2012;13(11):5849–55. doi: 10.7314/apjcp.2012.13.11.5849. [DOI] [PubMed] [Google Scholar]
- 21.Hayward R, Lien CY, Jensen BT, et al. Exercise training mitigates anthracycline-induced chronic cardiotoxicity in a juvenile rat model. Pediatr Blood Cancer. 2012;59(1):149–54. doi: 10.1002/pbc.23392. [DOI] [PubMed] [Google Scholar]
- 22.Calve A, Haddad R, Barama SN, et al. Cardiac response to doxorubicin and dexrazoxane in intact and ovariectomized young female rats at rest and after swim training. Am J Physiol Heart Circ Physiol. 2012;302(10):H2048–57. doi: 10.1152/ajpheart.01069.2011. [DOI] [PubMed] [Google Scholar]
- 23.Ashraf J, Roshan VD. Is short-term exercise a therapeutic tool for improvement of cardioprotection against DOX-induced cardiotoxicity? An experimental controlled protocol in rats. Asian Pac J Cancer Prev. 2012;13(8):4025–30. [PubMed] [Google Scholar]
- 24.Hydock DS, Lien CY, Jensen BT, et al. Exercise preconditioning provides long-term protection against early chronic doxorubicin cardiotoxicity. Integr Cancer Ther. 2011;10(1):47–57. doi: 10.1177/1534735410392577. [DOI] [PubMed] [Google Scholar]
- 25.Wonders KY, Hydock DS, Schneider CM, et al. Acute exercise protects against doxorubicin cardiotoxicity. Integr Cancer Ther. 2008;7(3):147–54. doi: 10.1177/1534735408322848. [DOI] [PubMed] [Google Scholar]
- 26.Hydock DS, Lien CY, Schneider CM, et al. Exercise preconditioning protects against doxorubicin-induced cardiac dysfunction. Med Sci Sports Exerc. 2008;40(5):808–17. doi: 10.1249/MSS.0b013e318163744a. [DOI] [PubMed] [Google Scholar]
- 27.Chicco AJ, Schneider CM, Hayward R. Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J Cardiovasc Pharmacol. 2006;47(2):182–9. doi: 10.1097/01.fjc.0000199682.43448.2d. [DOI] [PubMed] [Google Scholar]
- 28.Chicco AJ, Hydock DS, Schneider CM, et al. Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. J Appl Physiol (1985) 2006;100(2):519–27. doi: 10.1152/japplphysiol.00148.2005. [DOI] [PubMed] [Google Scholar]
- 29.Piquereau J, Novotova M, Fortin D, et al. Postnatal development of mouse heart: formation of energetic microdomains. J Physiol. 2010;588(Pt 13):2443–54. doi: 10.1113/jphysiol.2010.189670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 32.Vega RB, Konhilas JP, Kelly DP, et al. Molecular Mechanisms Underlying Cardiac Adaptation to Exercise. Cell Metab. 2017;25(5):1012–26. doi: 10.1016/j.cmet.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(32):3643–50. doi: 10.1200/JCO.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braam KI, van der Torre P, Takken T, et al. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2013;4:CD008796. doi: 10.1002/14651858.CD008796.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–80. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Exercise Initiated After Early Doxorubicin Exposure Fails to Mitigate Cardiotoxicity After Eight Weeks. A) Treatment and Exercise Schema. C57BL6/J mice were treated weekly for five weeks with intraperitoneal doxorubicin (1 mg/kg/dose or 5 mg/kg/dose). At the conclusion of the treatments, animals were divided into 4 groups (n = 8) corresponding to sedentary and exercise for both doxorubicin doses administered. Mice in the exercise groups then completed consistent aerobic exercise as denoted. B). Change from Baseline in Ejection Fraction (EF) and Fractional Shortening (FS) for mice treated according to the schema noted in A. For endpoint analysis, n = 8 for all groups except the 5 mg/kg DOX-Exercise. For that group n = 4 due to 4 deaths that occurred during the exercise period.


