Abstract
Kaposi sarcoma-associated herpesvirus (KSHV) is an emerging pathogen and the causative agent of multiple cancers in immunocompromised patients. To date, there is no licensed prophylactic KSHV vaccine. In this study, we generated a novel subunit vaccine that incorporates four key KSHV envelope glycoproteins required for viral entry in diverse cell types (gpK8.1, gB, and gH/gL) into a single multivalent KSHV-like particle (KSHV-LP). Purified KSHV-LPs were similar in size, shape, and morphology to KSHV virions. Vaccination of rabbits with adjuvanted KSHV-LPs generated strong glycoprotein-specific antibody responses, and purified immunoglobulins from KSHV-LP-immunized rabbits neutralized KSHV infection in epithelial, endothelial, fibroblast, and B cell lines (60–90% at the highest concentration tested). These findings suggest that KSHV-LPs may be an ideal platform for developing a safe and effective prophylactic KSHV vaccine. We envision performing future studies in animal models that are susceptible to KSHV infection, to determine correlates of immune protection in vivo.
Keywords: Kaposi sarcoma-associated human, herpesvirus, Kaposi sarcoma, Prophylactic vaccine, Glycoproteins, Neutralizing antibody titers, Rabbits
1. Introduction
Infection with Kaposi sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus-8 (HHV-8) is estimated to account for >44,000 new cancer cases and 20,000 deaths globally every year [1]. KSHV is associated with the endothelial-based Kaposi sarcoma (KS) and two rare B cell-based lymphomas, primary effusion lymphoma and multicentric Castleman disease, as well as KSHV inflammatory cytokine syndrome [2–5], particularly in immunosuppressed patients. Unlike other HHVs, KSHV is not ubiquitous, but is highly prevalent in certain areas, such as sub-Saharan Africa, where KS is the leading cancer among people living with HIV/AIDS [1,2,6]. Despite the HIV/AIDS pandemic and KSHV endemicity in Africa, the Mediterranean region, and parts of South America, efforts to develop a prophylactic vaccine against KSHV and its associated malignancies have been limited, with no clinical prophylactic vaccine trial ever reported [7].
KSHV etiology provides a window of opportunity for a prophylactic vaccine, even in endemic areas. In Africa, other endemic HHVs achieve widespread transmission and latent infection within the first year of life, infecting up to 76% of children. In contrast, few to no children are infected with KSHV by this age or even by five years of age [8–10]. In other parts of the world, where KSHV exposure typically occurs during adulthood, transmission is so poor that either repeated viral contact, or immunodeficiency, is required to sustain KSHV at > 5% population-wide seroprevalence [11]. KSHV superinfection in immunocompetent people is also limited, suggesting that some immune protection is conferred among adults [11]. Thus, minimal priming of the immune system by a vaccine prior to infection may be all that is required to prevent KSHV infection and associated malignancies in developing countries and to possibly eradicate KSHV and associated malignancies from developed countries.
Like other HHVs, KSHV encodes five conserved glycoproteins: open reading frame [ORF] 8 (gB), ORF22 (gH), ORF47 (gL), ORF39 (gM), and ORF53 (gN) [12,13]. In addition, KSHV also encodes for a unique glycoprotein, gpK8.1, which is the major immunodominant glycoprotein on the virion and is thought to modulate viral tropism for B cells [14]. In the current infection model, KSHV is thought to first attach to host cell receptors through gpK8.1, which then signals gH/gL to activate the gB fusogen [15]. This infection model is supported by experiments that used neutralizing antibodies (nAbs) against gpK8.1 or recombinant KSHV (rKSHV) deletion mutants lacking gpK8.1 [14,16], gB [17], or gH/gL [58] to define the role of each KSHV glycoprotein in infecting various cell types. Because these data suggest that different KSHV glycoproteins modulate viral tropism for different cell types, we reasoned that targeting a single glycoprotein with a prophylactic vaccine might not completely protect against infection of diverse cell types. Thus, we hypothesized that including gpK8.1, gB, and gH/gL complex in a single, potent, multivalent vaccine candidate is necessary for eliciting nAbs that can block KSHV infection of diverse permissive human cell types.
Due to the oncogenic potential of KSHV DNA [3,18], live-attenuated KSHV or any form of inactivated KSHV cannot be used in humans. To circumvent this shortcoming, we herein describe the construction, assembly, and biochemical characterization of a single, multivalent virus-like particle (VLP) that incorporates four key glycoproteins as a candidate KSHV-like particle (KSHV-LP) vaccine. We show that wild-type New Zealand white rabbits immunized with adjuvanted-KSHV-LPs elicited strong nAbs that prevented in vitro KSHV infection of epithelial, endothelial, fibroblast, and B cell lines. These results highlight a novel vaccine approach with potential for clinical application as a prophylactic vaccine against KSHV infection and its associated malignancies.
2. Methods
2.1. Animals and ethic statement
Wild-type New Zealand white rabbits and wild-type BALB/c mice were purchased from Pocono Rabbit Farm and Laboratory Inc. (PR&L) and Jackson Laboratory, respectively. Rabbits and mice used for experiments were housed at PR&L and Beckman Research Institute of City of Hope (BRICOH), respectively. Animal procedures were performed in accordance with PR&L and BRICOH Institutional Animal Care and Use Committee and Institutional Biosafety Committee protocols.
2.2. Cells and virus
Chinese hamster ovary (CHO) cells, human embryonic kidney (HEK-293) cells, HEK-293 cells stably expressing Epstein-Barr virus nuclear protein 1 for enhanced ability to produce recombinant proteins (HEK-293–6E), human umbilical vein endothelial cells (HUVEC), human foreskin fibroblast 1 (HFF-1) cells, mouse myeloma cells (P3X63Ag8.653), and B cells derived from the pleural effusion of a patient with undifferentiated lymphoma (MC116) were purchased from American Type Culture Collection (ATCC, Manassas, VA). Doxycycline-inducible iSLK cells harboring recombinant KSHV.219 expressing enhanced green fluorescent protein from the human elongation factor-1α promoter (iSLK-rKSHV-eGFP.219) [19] were obtained from Dr. D. Dittmer, University of North Carolina–Chapel Hill. CHO, HEK-293, and HFF-1 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM). P3X63Ag8.653 and MC116 cells were cultured in Roswell Park Memorial Institute medium 1640 (RPMI). Hybridomas were cultured in DMEM supplemented with nonessential amino acids (ThermoFisher, Waltham, MA) as described [20]. iSLK-rKSHV-eGFP.219 cells were cultured in DMEM as described [19]. All media were supplemented with 10% heat-inactivated fetal bovine serum (FBS; MilliporeSigma, Burlington, MA), 1% L-glutamine, and 2% penicillin-streptomycin (ThermoFisher). HEK-293–6E cells were cultured in Freestyle F17 Expression media without FBS (Thermo-Fisher). HUVEC cells were cultured in endothelial cell growth ready-to-use media (PromoCell, Edmonton, Alberta). Cell lines were routinely tested for mycoplasma using PCR and shown to be negative.
Virus was produced from iSLK-rKSHV-eGFP.219 cells and purified as described [21]. Purified viruses were quantified by titration in each of the cell types used in infection and neutralization assays.
2.3. Antibodies
Primary mouse monoclonal immunoglobulin G2a (IgG2a) anti-gpK8.1 (clone 4A4; Santa Cruz Biotechnology, Dallas, TX) [22], which detects the gpK8.1 ectodomain (ED), was used for fluorescence-activated cell sorting (FACS), immunoblot, and enzyme-linked immunosorbent assay (ELISA). Primary polyclonal rabbit anti-Newcastle disease virus (NDV) detecting nucleoprotein (NP) was a gift of Dr. T. Morrison, University of Massachusetts Medical School, Worcester. Primary polyclonal goat anti-human IgG Fc or rabbit anti-2A peptide were purchased from ThermoFisher and MilliporeSigma, respectively. Primary monoclonal anti-bodies (mAbs) anti-gpK8.1 (clone 41E7) and anti-gH/gL (clones 54A1, 57C12) were generated and characterized as outlined below and used in immunoblot and ELISA. HRP-conjugated secondary antibodies goat anti-mouse IgG and goat anti-rabbit IgG (MilliporeSigma) were used for immunoblot and ELISA. Goat Fab-2 anti-mouse IgG (H+L) cross-adsorbed secondary antibody, conjugated to Alexa Fluor 488 (AF488) (ThermoFisher) was used for FACS.
2.4. Construction of plasmids to generate KSHV-LPs
To generate polyvalent KSHV-LP vaccine candidates incorporating multiple KSHV proteins (gpK8.1, gB, gL, and gH), two cDNAs were constructed and synthesized (Genewiz, South Plainfield, NJ). Each sequence included the gpK8.1 ED fused to NDV fusion protein (F) transmembrane/cytoplasmic (TM/CT) domains (gpK8.1-F); gB ED fused to NDV F-TM/CT (gB-F); full-length wild-type gL without an F-fusion (WTgL); and gH ED fused to NDV F-TM/CT (gH-F). In one cDNA, each NDV F-TM/CT domain also contained heptad region 2 to support trimerization of the glycoproteins; in the other cDNA, it did not (+/−HR2). To express this complex in its native form, unique 2A-linker sequences (18 amino acids long) from picornavirus [23] were interspersed between individual glycoprotein cDNAs (Table 1). These unique 2A-linkers were recently designed to facilitate expression of a human cytomegalovirus (CMV) envelope glycoprotein pentameric complex in a modified vaccinia Ankara-based vaccine formulation [24]. In the present study, use of 2A-linkers yielded a polycistronic plasmid with cleavage sites that allow gpK8.1-F, gB-F, WTgL, and gH-F to be processed independently after translation and released to natively incorporate on KSHV-LP envelopes. The two synthesized chimeric cDNAs (gpK8.1-F-2A-gB-F-2A-WTgL-2A-gH-F+/−HR2) were cloned into pCAGGS mammalian expression plasmid. Plasmids encoding for chimeric protein of a truncated LANA1 (aa 937–1123) fused to full-length NDV NP and full-length wild type LANA1 (aa 1–1123) were also synthesized and cloned into a pCAGGS vector. All plasmids generated for this study are summarized in Table 2. Plasmids encoding full-length NDV NP and M cloned into pCAGGS have been described [25]. Both NP and M proteins support KSHV-LP formation. Sanger sequencing was used to verify sequence fidelity of all synthesized and cloned constructs (primer sequences listed in Table 3).
Table 1.
2A protein sequence used in expressing polycistronic KSHV glycoproteins.
| 2A position | Nucleotide sequence | Amino acid sequence |
|---|---|---|
| gpK8.1-gB | GCTACTAACTTCAGCCTGCTGAAGCAGGCTGGAGACGTGGAGGAGAACCCTGGACCT | A T N F S L L K Q A G D V E E N P G P |
| gB-gL | GCGACTAACTTCTCATTGTTGAAACAGGCAGGAGATGTCGAAGAGAACCCTGGTCCA | A T N F S L L K Q A G D V E E N P G P |
| gL-gH | GCAACGAATTTCTCCCTTCTAAAGCAAGCCGGTGACGTGGAGGAGAATCCCGGACCC | A T N F S L L K Q A G D V E E N P G P |
Table 2.
Complete list of vectors and plasmids used in the study.
| Plasmid | Protein (s) | Function |
|---|---|---|
| pCAGGS | None | Expression vector |
| pCAGGS-gpK8.1-gB-gL-gH −HR2 | KSHV glycoproteins, K8.1, gB, gH and gL without NDV F protein heptad repeat 2 domain |
VLP production |
| pCAGGS-gpK8.1-gB-gL-gH+HR2 | KSHV glycoproteins, K8.1, gB, gH, gL and NDV F protein heptad repeat 2 domain |
VLP production |
| pCAGGS-NDV NP | NDV nucleocapsid protein | VLP production |
| pCAGGS-NDV M | NDV matrix protein | VLP production |
| pCAGGS-NP LANA1 | Truncated KSHV latent associated nuclear antigen | Immunoblot |
| pCAGGS-LANA1 | KSHV wild type latent associated nuclear antigen | Immunoblot |
| pCAGGS-gpK8.1 | KSHV glycoprotein K8.1 | Stable cell production/ protein production |
| pCAGGS-gB | KSHV glycoprotein gB | Soluble protein production |
| pCAGGS-gH/gL | KSHV glycoproteins gH and gL | Soluble protein production |
| pCMVi-SV40ori-Fc-His | None | Expression vector |
| pCMVi-SV40ori-gpK8.1-Fc-6xHis | KSHV glycoprotein K8.1 | Soluble protein production |
| pCMVi-SV40ori-gB-Fc-6xHis | KSHV glycoprotein gB | Soluble protein production |
| pCMVi-SV40ori-gH/gL- Fc-6xHis | KSHV glycoproteins gH and gL | Soluble protein production |
Table 3.
Primers for construction and sequencing of recombinant KSHV antigen constructs.
| Primer name | Primer sequence (5′−3′) | |
|---|---|---|
| Cloning Primers | KSHV gpK8.1-Fc-His FWD | AAAAAGCGGCCGCGCCACCATGAGTTCCACACAGATTCG |
| KSHV gpK8.1-Fc-His REV | AAAAAACTAGTGTAAAGATGGGTCCGTATTTCTGC | |
| KSHV gB-Fc-His FWD | AAAAAGCGGCCGCGCCACCATGACTCCCAGGTCTAGATTGG | |
| KSHV gB-Fc-His Rev | AAAAAACTAGTTTTAATAAAATTTATGAATCCGGTAAC | |
| KSHV gH-gL Gibson.FOR | TTCTCGAGGATCCGCGGCCGCGCCACCATGGGGATCTTTGCGCTATTTG | |
| KSHV gHgL Gibson.REV | GCATGTGTGAGTTTTGTCACTAGTTGCGCGTCTTCTATACATGCC | |
| Sequencing Primers | FC-Hisseqprimer1 | GTCGAGGTCTCGACGGTATCG |
| FC-Hisseqprimer2 | CTCTATAGGCACACCCCTTTGG | |
| FC-Hisseqprimer3 | CGCGCGCCACCAGACATAATAG | |
| FC-Hisseqprimer4 | GCTTTAATAAGATCTCTAG | |
| FC-Hisseqprimer5 | TGCTGGGCACGGTGGGCATG | |
| FC-Hisseqprimer6 | GGGTCTTTTCTGCAGAAGCTTG | |
| FC-Hisseqprimer7 | CTGACCAAGAACCAGGTCAGC | |
| FC-Hisseqprimer8 | GACGCTCAAGTCAGAGGTGGC | |
| FC-Hisseqprimer9 | CTCCCCGTCGTGTAGATAACTAC | |
| FC-Hisseqprimer1 0 | CAATATTATTGAAGCATTTATC | |
| FC-Hisseqprimer1 1 | TGCGTAAGGAGAAAATACCGC |
2.5. Transfection and generation of stable KSHV-LP-producing cells
To generate gpK8.1-gB-gL-gH KSHV-LPs, 2 µg pCAGGS-gpK8.1-F-2A-gB-F-2A-WTgL-2A-gH-F+/−HR2 plasmid was transiently transfected into CHO or HEK-293 cells using linear polyethylenimine (PEI) transfection reagent (ThermoFisher). After 48 h, cells were stained with anti-gpK8.1 (4A4) primary antibody for 30 min, washed three times with phosphate-buffered saline (PBS), and stained with AF488-goat anti-mouse secondary antibody. FACS was used to detect surface expression of gpK8.1; untransfected and unstained CHO or HEK-293 cells served as negative controls. Upon confirmation of gpK8.1 expression in both CHO and HEK-293 cells, only CHO cells were then co-transfected as above with individual pCAGGS-gpK8.1-F-2A-gB-F-2A-WTgL-2A-gH-F+/−HR2 and pCI-puro plasmids, followed by 5 µg/ml puromycin selection 48 h post-transfection, to generate stable cells expressing the four glycoproteins. Stable CHO cells resistant to puromycin selection were pooled and stained with primary anti-gpK8.1 mAb (4A4), followed by secondary goat Fab-2 anti-mouse IgG (H+L) conjugated to AF488 and sorted five times to generate a colony of CHO cells expressing mainly gpK8.1-gB-WTgL-gH+/−HR2 (~50% anti-gpK8.1-AF488 positive). Stable cells were then co-transfected with 2 µg/µl each pCAGGS-NDV M and pCAGGS-NDV NP plasmids to produce gpK8.1-gB-gH/gL+/−HR2 VLPs (KSHV-LPs +/−HR2). Additional KSHV-LPs were also produced by transiently co-transfecting CHO or HEK-293 cells with pCAGGS-NDV M, pCAGGS-NDV NP, or polycistronic pCAGGS-gpK8.1-F-2A-gB-F-2A-WTgL-2A-gH-F+/−HR2 plasmids.
2.6. Purification and characterization of KSHV-LPs
Supernatants from stable cells releasing KSHV-LPs were collected 24–120 h post-transfection and combined, then cell debris was removed by centrifugation (Corning 500 ml centrifuge tubes, SX4750 rotor, 3,724 xg, 15 min, 4 °C). To pellet KSHV-LPs, the resultant supernatant was ultracentrifuged (Beckman Coulter type-19 fixed-angle aluminum rotor, 9,846 xg, 12 h, 4 °C). The resultant pellet was sucrose-gradient purified as described [26].
2.7. Construction, purification, and characterization of KSHV gpK8.1, gB, and gH/gL proteins
To construct KSHV gpK8.1, gB, and gH/gL Fc-6xHis tagged plasmids, the coding sequence for each protein ED was PCR-amplified with gene-specific primers (Table 3) from previously described pCAGGS-gpK8.1, pCAGGS-gB, and pCAGGS-gH/gL constructs [26]. The upstream (5′ ) and downstream (3′ ) primers contained NotI and SpeI enzyme restriction sites (gpK8.1 and gB), respectively, or Gibson assembly primers (gH/gL) [27], which were used to subclone PCR products into the pCMVi-SV40ori-Fc-His plasmid (Addgene #72065, Watertown, MA), a gift from Dr. W. Wojtowicz, Stanford University, Palo Alto, CA.
Fc-6xHis-tagged recombinant KSHV gpK8.1, gB, and gH/gL proteins were expressed via transient transfection of HEK-293–6E cells using PEI and protein purified as described [28].
2.8. Generation and identification of KSHV-glycoprotein-specific mAbs
mAbs specific to KSHV gpK8.1 and gH/gL were generated using standard techniques [20]. Briefly, we immunized four wild-type BALB/c mice intraperitoneally with 100 µg complete Freund’s adjuvant (MilliporeSigma F5881) mixed with 50 µg purified UV-inactivated rKSHV-eGFP.219 (UV-KSHV) resuspended in 200 µl TNE buffer. Mice were boosted five times with 100 µg incomplete Freund’s adjuvant (MilliporeSigma F5506) and 25 µg UV-KSHV resuspended in 200 µl TNE buffer every two weeks. Once optimal antibody response to gpK8.1 and gH/gL in sera was confirmed using ELISA, a sixth booster immunization was provided intravenously with 25 µg UV-KSHV resuspended in 200 µl TNE buffer. Boosted mice were sacrificed and splenocytes were isolated and fused with a mouse myeloma cell line (P3X63Ag8.653) to generate murine hybridomas, using a standard polyethylene glycol (PEG) method, then cultured and screened for positive (gpK8.1 or gH/ gL) clones as described [20]. Positive hybridomas were expanded, hybridoma secreted antibodies bulk-purified through protein A spin-columns (Clontech, Mountain View, CA), and used for ELISA, FACS, and immunoblot.
2.9. SDS-PAGE, Coomassie staining, and immunoblotting
Cells or KSHV-LPs were lysed in RIPA buffer (MilliporeSigma). Lysed suspensions were vigorously vortexed, incubated for 30 min on ice and processed for immunoblot (i.e., gpK8.1, gB, gH/ gL, NDV NP, and 2A or Fc-tagged protein expression), followed by detection with relevant primary and secondary antibodies as described [26].
2.10. Transmission electron microscopy (TEM)
To assess the composition of KSHV-LPs compared to wild-type KSHV, morphological examination was conducted using TEM. Purified KSHV-LPs +/−HR2 and rKSHV-eGFP.219 virus were fixed in 4% paraformaldehyde, prepared, and analyzed using TEM as described [29].
2.11. Immunization of rabbits and collection and processing of sera
Rabbits (8–10 weeks-old) were immunized subcutaneously (Days 0, 21, 42) with 50 µg total protein of purified KSHV-LPs +/−HR2 (n = 6/group) in 0.5 ml TNE buffer adsorbed to 500 µg aluminum hydroxide (alum) mixed with 50 µg monophosphoryl lipid A from Salmonella enterica serotype Minnesota Re595 (MPL) as adjuvants; alum/MPL-adjuvanted UV–KSHV (n = 6) or alum/MPL-adjuvanted TNE (n = 6) served as positive and negative controls, respectively. Rabbits were bled before immunization (pre-bleed baseline) and on Days 14, 35, 49, 70, and 90 (terminal bleed).
2.12. Measurement of anti-gpK8.1-, gB-, and gH/gL-specific antibody titers in sera from immunized rabbits
Using sera collected from rabbits, antibodies titers were assessed by ELISA, using individual purified glycoproteins as targets as described [26].
2.13. Purification of IgG antibodies from rabbit sera and determination of anti-gpK8.1-, gB-, and gH/gL-specific antibody titers
Purification of IgG antibodies was conducted by pooling Day 49 sera (highest antibody titer) from immunized rabbits (n = 6) from each of the four treatment/control groups. Pooled sera were diluted 1:10 in equilibration/binding/wash buffer (1.5 M glycine, 3 M NaCl, pH 9.0) and purified through protein A spin-columns. Purified IgG concentration was determined using nanodrop. IgGs were separated using SDS-PAGE and stained with Coomassie to determine purity. ELISA was used to determine titers of anti-gpK8.1-, gB-, and gH/gL-specific antibodies from purified IgGs at various concentrations (25, 12.5, 6.25, 3.125, and 1.56 µg/ml). IgGs were stored at 4 °C until use in subsequent experiments.
2.14. In vitro neutralization assay
In vitro neutralization was performed using various cell types of human origin, namely epithelial, fibroblast, endothelial, or B cells, as described [26].
2.15. Statistics
Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA). Multiple comparisons between groups were calculated using Kruskal-Wallis, whereas differences between groups were computed using Dunn’s post-hoc test and Mann-Whitney U test. All statistical tests were two-tailed; p-values ≤ 0.05 were considered significant. IC50 (µg/ml) values were calculated based on neutralization of KSHV.219 by purified IgGs in HEK-293, HFF-1, HUVEC, and MC116 cells using nonlinear, dose-response regression analysis.
3. Results
3.1. Construction, purification, and characterization of KSHV-LP vaccine candidates that incorporate four KSHV glycoproteins
To make multivalent KSHV-LPs, we constructed two versions of a single polycistronic plasmid encoding sequences for four glycoproteins, designated pCAGGS-gpK8.1-F-2A-gB-F-2A-WTgL-2A-gH-F+/−HR2 (Fig. 1a). We fused the EDs of three KSHV glycoproteins (gpK8.1, gB, and gH) to the structural protein NDV F with or without its HR2 domain, which supports protein trimerization and increases the number of immunogens per KSHV-LP [30]. We incorporated full-length wild-type KSHV gL because it does not have a transmembrane domain and depends on gH for transport to the virion surface [31]. The glycoproteins were interspersed with a picornavirus 2A self-cleaving peptide for efficient expression and cleavage under a single CMV promoter [24,32].
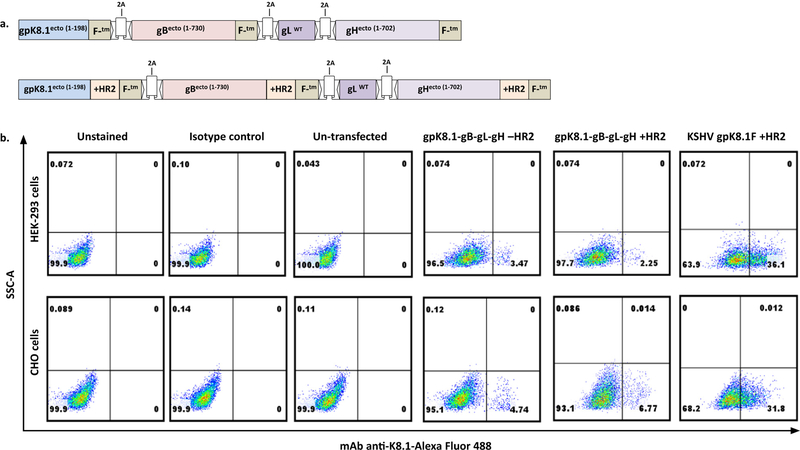
Fig. 1.
Construction and expression of polycistronic plasmids expressing four KSHV glycoproteins. (a) Schematic representation of pCAGGS-gpK8.1-F-2A-gB-F-2A-WTgL-2A-gH-F−/+HR2 plasmids (not drawn to scale). A single transcript was synthesized to express the gpK8.1 ectodomain (ED) fused to NDV fusion protein (F) transmembrane/cytoplasmic (TM/CT) domains (gpK8.1-F), the gB ED fused to NDV F-TM/CT (gB-F), and the gH ED fused to NDV F-TM/CT (gH-F), each with or without heptad region 2 (+/−HR2), and with full-length wild-type gL (WTgL) inserted between the gB and gH sequences. Sequence fidelity was confirmed using Sanger sequencing. The numbers shown represent corresponding amino acid numbers of the EDs. (b) Expression of gpK8.1-gB-WTgL-gH−/+HR2 in HEK-293 and CHO cells. 106 cells from HEK-293 or CHO cells were seeded in a six-well plate and transfected with 2 µg pCAGGS-gpK8.1-F-2A-gB-F-2A-WTgL-2A-gH-F−/+HR2 or pCAGGS-gpK8.1-F+HR2 (positive control). Transfection efficiency was evaluated 48 h post-transfection by staining transfected HEK-293 and CHO cells with gpK8.1 mAb, followed by secondary antibody goat anti-mouse IgG conjugated to AF488. Unstained cells, cells stained with secondary antibody alone (isotype control), and un-transfected cells served as negative controls, and gpK8.1-transfected cells served as positive control. Cells were analyzed using FACS by acquiring 10,000 events.
To confirm plasmid DNA transfection efficiency and expression of various glycoproteins from the polycistronic plasmids, we transiently transfected each plasmid into CHO or HEK-293 cells, two epithelial cell lines widely used for protein expression. To confirm gpK8.1 cell surface expression, we stained transfected cells with anti-gpK8.1 primary antibody and AF488-conjugated secondary antibody, then used FACS to detect gpK8.1 expression. We used the previously constructed pCAGGS-gpK8.1-F+HR2 plasmid as a positive control for gpK8.1 expression [26]; untransfected cells and unstained transfected cells served as negative controls. Transfected CHO and HEK-293 cells expressed gpK8.1 (Fig. 1b). However, gpK8.1 was expressed in fewer total cells transfected with polycistronic plasmids compared to cells transfected with plasmid encoding for a single protein; for example, in CHO cells transfected with gpK8.1-F-2A-gB-F2A-WTgL-2A-gH-F −HR2 or +HR2, gpK8.1 expression was 4.74% and 6.77%, respectively, compared to 31.8% in CHO cells transfected with pCAGGS-gpK8.1-F+HR2. This occurred despite optimization of transfection efficiency using various ratios of DNA:PEI or other transfection reagents (data not shown), and is probably due to the large cDNAs cloned into the plasmids.
To overcome poor transfection efficiency and improve KSHV-LP production for full characterization and immunogenicity testing, we established CHO cells that stably expressed each multicistronic plasmid. We used only CHO cells because > two-thirds of recombinant therapeutic agents on the market are generated in CHO cell lines [33], and because no adventitious human pathogen, a major concern to regulatory authorities, has been reported for CHO cells, in contrast to cell lines of human origin [34]. We co-transfected CHO cells with pCAGGS-gpK8.1-F-2A-gB-F-2A-WTgL-2A-gH-F+/−H R2 and pCI-puro. We selected transfected cells in puromycin, stained puromycin-resistant cells with anti-gpK8.1-AF488 antibody, and performed FACS sorting five times to enrich for gpK8.1-expressing cells (Fig. 2a). Selection was based on gpK8.1 due to lack of well-validated mAbs against other KSHV glycoproteins at the time we made the stable cell lines.
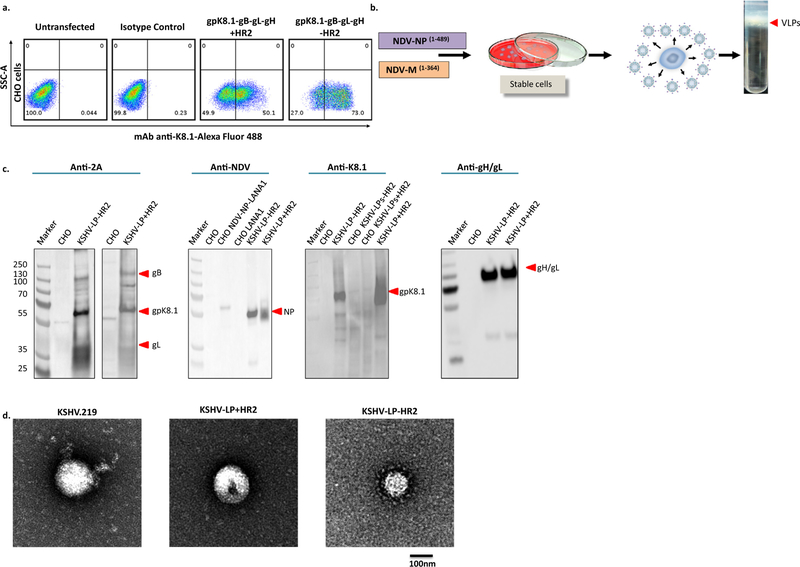
Fig. 2.
Generation of stable CHO cells expressing gpK8.1-gB-WTgL-gH+/−HR2 proteins and production and characterization of purified KSHV-LPs. (a) Generation of stable CHO gpK8.1-gB-WTgL-gH+/−HR2 cells. CHO cells were co-transfected with 2 µg pCAGGS-gpK8.1[…] and pCI-puro plasmids. Forty-eight h post-transfection, cells were maintained in selection media supplemented with puromycin (5 µg/ml) until colonies of puromycin-resistant cells were formed. Stable cells were harvested, re-suspended in 1 × PBS and stained as in Fig. 1b. Stained cells were live-sorted using FACS five times to a purity of ~50% of gpK8.1-expressing cells. (b) Schematic showing the production of KSHV-LPs. KSHV gpK8.1-gB-WTgL-gH+/−HR2 stable CHO cells were co-transfected with pCAGGS NDV NP and pCAGGS NDV M plasmids. Supernatants from transfected cells were collected and clarified and KSHV-LPs were pelleted and sucrose-gradient purified as described in Methods. (c) Immunoblot analysis of purified KSHV-LPs. Un-transfected CHO cells, KSHV gpK8.1-gB-WTgL-gH+/−HR2 stable CHO cells (CHO KSHV-LPs+/−HR2), and purified KSHV-LPs+/−HR2 were resolved on a 4–12% SDS polyacrylamide gel, transferred to a polyvinylidene fluoride membrane, and analyzed by immunoblot using rabbit polyclonal (anti-2A or anti-NDV) or mouse monoclonal (anti-gpK8.1 or anti-gH/gL) antibodies as indicated. pCAGGS encoding for NDV NP fused to LANA1 and pCAGGS LANA1 were used as negative controls. (d) Structural and morphological characterization of KSHV-LPs by TEM. rKSHV-eGFP.219 and KSHV-LPs+/−HR2 were fixed in 4% paraformaldehyde and absorbed to glow-discharged, carbon-coated, 200-mesh EM grids. EM images were collected using an FEI Tecnai 12 TEM and recorded with a Gatan 2 × 2 k CCD camera at a magnification of 21,000X and a defocus value of ~1.5 µm.
To produce KSHV-LPs, we co-transfected enriched stable cells with both pCAGGS-NDV M and pCAGGS-NDV NP, then collected supernatants from transfected cells daily, from 24 to 120 h post-transfection. We purified KSHV-LPs from supernatants using sucrose-gradient sedimentation, followed by floatation gradients (Fig. 2b), then fully characterized their composition and structure as described [26]. In brief, we used anti-2A, anti-NDV, anti-gpK8.1, and anti-gH/gL antibodies in immunoblot to show that all components (i.e., KSHV glycoproteins and NDV structural proteins) were incorporated and packaged into KSH-VLPs (Fig. 2c). Because the 2A sequence between WTgL and gH-F is cleaved and remains with WTgL, it is not possible to use the anti-2A antibody to detect gH. Thus, to further confirm the specificity of the gpK8.1 protein bands (55 and 70 kDa) and also detect gH/gL protein complex (130 kDa), we used the specific mAbs anti-gpK8.1 (41E7) and anti-gH/gL (57C12), generated in our laboratory, to detect individual proteins (Fig. 2c). To our knowledge, there is no commercially available KSHV anti-gB antibody. Thus, it was not feasible for us to unequivocally prove the presence of gB (110 kDa) in the KSHV-LPs. However, the molecular weights we identified for each individual protein in the KSHV subunit vaccines agreed with published data [13], providing strong evidence that KSHV-LPs generated from a polycistronic plasmid are faithful to the composition of the subunits. We used electron microscopy to confirm that the size, shape, and morphology of KSHV-LPs were similar to rKSHV-eGFP.219 virus: KSHV-LP diameters were 100 nm, with a spherical shape and spike-like structures, resembling glycoproteins incorporated into the virions (Fig. 2d).
3.2. Rabbits immunized with KSHV-LPs +/−HR2 elicited high-titer KSHV-glycoprotein-specific antibody responses
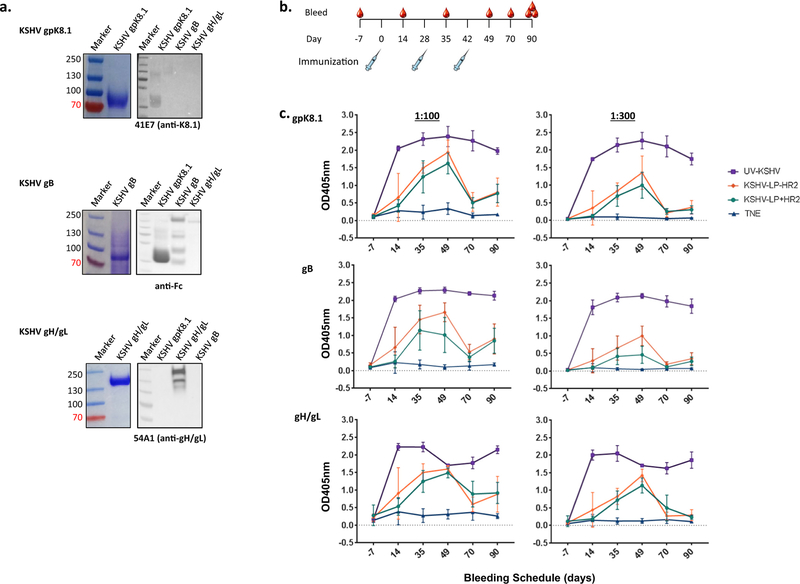
To demonstrate the ability of multivalent KSHV-LPs to induce KSHV-glycoprotein-specific antibody responses, we immunized rabbits with alum/MPL-adjuvanted KHSV-LPs +/−HR2, then used ELISA to determine antibody titers and kinetics over time. Adjuvants were used to improve humoral and cellular immunogenicity [38,39];selection of the alum/MPL formulation was based on AS04, an adjuvant licensed for human use currently being used in two licensed VLP-based vaccines, the human papillomavirus vaccine Cervarix® (GlaxoSmithKline) [35] and the hepatitis B vaccine FEN-Drix® (GlaxoSmithKline) [36]. Because there is no gold standard for measuring IgGs against KSHV infection in humans or immunized animals, due to the inconsistent detection of KSHV-specific protein IgGs in KSHV-infected individuals, we used purified proteins as binding targets for use in ELISA, as described [37]. To do this, we used our recently constructed plasmids that individually encode for soluble gpK8.1, gB, or gH/gL proteins to produce the proteins and confirmed their authenticity using Coomassie and immunoblot (Fig. 3a).
Fig. 3.
KSHV-glycoprotein-specific IgG titers in immunized New Zealand white rabbits. (a) Coomassie stain (left) and immunoblot (right) of KSHV gpK8.1, gB, and gH/gL recombinant Fc-His tagged proteins. Fc-6xHis-tagged recombinant KSHV gpK8.1, gB, and gH/gL proteins were expressed by transiently transfecting HEK-293–6E cells. Culture media was harvested six days post-transfection by centrifugation and filtration through a 0.22 µM. Fc-6xHis-tagged KSHV proteins in the media were purified using protein A spin-columns, concentrated in PBS using Amicon Ultra 15 centrifugal filter units, and quantified using a nanodrop spectrophotometer. To confirm the specificity of the proteins, the concentrated proteins were separated on a 4–12% SDS-PAGE and detected by Coomassie blue stain (for molecular weight) or transferred to a polyvinylidene fluoride membrane for immunoblot analysis using monoclonal anti-gpK8.1 or anti-gH/gL or polyclonal goat anti-human Fc (gB) antibodies as indicated. (b) Immunization and bleeding schedules of wild-type New Zealand white rabbits. Eight- to 10-week-old rabbits (n = 6/treatment) were immunized subcutaneously at Days 0, 28 and 42 with 50 µg purified KSHV-LPs−/+HR2, UV-inactivated KSHV, or TNE buffer, all adsorbed to alum and MPL as adjuvants. Immunized rabbits were bled seven days pre-immunization (−7) and on Days 14, 35, 49, 70, and 90 (terminal bleed). (c) Serum KSHV glycoprotein-specific antibody responses. KSHV-glycoprotein IgG-specific antibody titers in diluted (1:300 and 1:900) sera from immunized rabbits were determined using ELISA with recombinant tagged gpK8.1, gB, and gH/gL proteins; results of quadruplicate replicates for each of the six animals per group are expressed as mean ± standard deviation (SD). Differences in antibody titers between all groups were analyzed using a Kruskal-Wallis test; differences between the −HR2 and+HR2 vaccine were assessed using a Mann-Whitney test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To characterize antibody immune responses, rabbits were immunized subcutaneously three times (Days 0, 21, 42) with 50 µg purified KSHV-LPs +/−HR2 suspended in 0.5 ml TNE buffer adsorbed to 500 µg alum/50 µg MPL. Rabbits immunized with 50 µg purified UV-KSHV or TNE, adjuvanted with alum/MPL, served as positive and negative controls, respectively. To obtain enough sera to support complete characterization of antibody responses, we used 6 rabbits/group. To assess short- and long-term antibody responses, rabbits were bled to obtain sera seven days before the first immunization (pre-bleed) and on Days 14, 28, 35, 49, 70, and 90 (terminal bleed) (Fig. 3b). We used ELISA to determine KSHV-glycoprotein-specific antibody titers from individual animals over time using the purified proteins described above as the binding targets. All rabbits immunized with KSHV-LPs +/−HR2 or UV-KSHV generated KSHV-glycoprotein-specific IgG antibody responses that increased following booster immunizations, compared to TNE-immunized rabbits or pre-bleeds (negative controls) (Fig. 3c). The increase in KSHV glycoprotein-specific antibody response peaked by Day 49, after the second booster immunization. There was no difference in the titers of glycoproteins (K8.1, gB, or gH/gL) between rabbits immunized with KSHV-LPs+HR2 or −HR2. Compared to either KSHV-LP +/−HR2, UV-KSHV immunization elicited significantly higher titers of KSHV-glycoprotein-specific antibodies, which remained high through Day 90. In contrast, antibody titers from animals immunized with either KSHV-LP +/−HR2 dropped by Day 70 (Mann-Whitney test; gpK8.1 Day 49 vs Day 70: KSHV-LPs −HR2 p = 0.0022, KSHV-LPs +HR2 p = 0.0022; gB Day 49 vs Day 70: KSHV-LPs −HR2 p = 0.0022, KSHV-LPs+HR2 p = 0.0022; gH/gL Day 49 vs Day 70: KSHV-LPs −HR2 p = 0.0043, KSHV-LPs+HR2 p = 0.0303), suggesting that long-term antibody responses in KSHV-LP +/−HR2-immunized rabbits are weaker than those of UV-KSHV-immunized rabbits.
3.3. KSHV-LPs +/−HR2 elicit robust nAbs that prevent rKSHV-eGFP.219 infection in diverse cell types
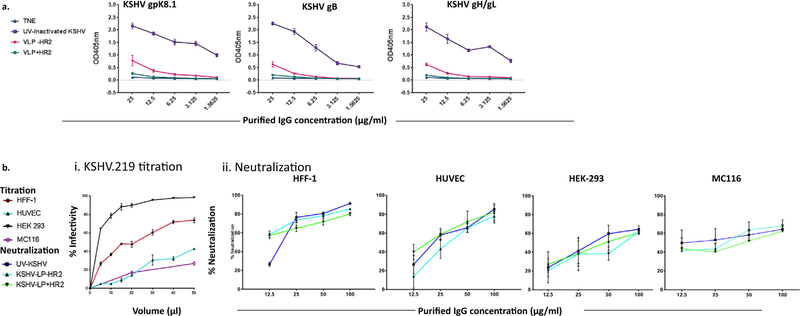
To measure the ability of KSHV-glycoprotein-specific polyclonal antibodies in sera from rabbits immunized with multivalent KSHV-LPs to neutralize viral infection and to reduce the previously observed serum effect [26,29,40], we first pooled (6 rabbits/group) and purified IgGs from Day 49 samples (highest titers), then quantified KSHV-glycoprotein-specific IgGs using ELISA (Fig. 4a). Similar to unpurified sera, titers for gpK8.1-, gB-, and gH/gL-specific IgGs were significantly higher in rabbits immunized with purified KSHV-LPs +/−HR2 compared to TNE (negative control) using a non-parametric Kruskal-Wallis test (gpK8.1, p = 0.0003; gB, p = 0.0002; gH/gL, p = 0.0012). KSHV-specific IgG titers were higher in rabbits immunized with KSHV-LPs-HR2 than KSHV-LPs+HR2; for example, at 25 µg/ml, gpK8.1, gB, and gH/gL IgG titers were 4-fold higher. However, titers were highest in rabbits immunized with UV-KSHV. For example, at 25 µg/ml, gpK8.1, gB and gH/gL IgG titers for UV-KSHV were 3-fold and 10-fold higher than in KSHV-LP-HR2 and KSHV-LP+HR2 IgG-purified samples, respectively (Fig. 4a).
Fig. 4.
In vitro neutralization capabilities of purified IgGs from rabbits immunized with KSHV-LPs+/−HR2 in diverse cell types. (a) Titration of purified IgGs specific to KSHV glycoproteins by ELISA. Purification of IgG antibodies was conducted by pooling equal amounts of Day 49 sera from immunized rabbits (n = 6) from each of the four treatment/control, groups. KSHV-glycoprotein IgG-specific antibody titer was determined using ELISA with recombinant tagged proteins gpK8.1, gB, and gH/gL as targets; results of quadruplicate replicates are expressed as mean ± SD. (b) rKSHV-eGFP.219 titration and neutralization assays in epithelial, fibroblast, endothelial, and B cells. Panel i. rKSHV-eGFP.219 virus titration in diverse cell types. Individual cell lines were seeded overnight at a density of 5 × 105 in quadruplicate in 48-well-plates. The individual cell lines were then incubated with 5, 10, 20, 30, or 50 ml of the purified virus in a total volume of 100 µl of virus plus serum-free media for 24 h at 37°C. Infected cells (eGFP+) were quantified using FACS by acquiring a total of 10,000 events. Panel ii. Neutralization activity in HEK-293 cells, HFF-1 cells, HUVEC, and MC116 B cell lines. Neutralization activities were determined by incubating rKSHV-eGFP.219 virus and purified IgGs of varying concentrations (12.5, 25, 50, or 100 µg/ml) from all groups of test animals for 1 h at 37 °C, before being added to previously seeded cells in a 48-well plate. After 1 h incubation at 37 °C, the cells were thoroughly washed three times with 1 × PBS before adding growth media. The level of neutralization was determined in the cells after 24 h using FACS to quantify the number of eGFP+cells. Cells incubated with virus or with media alone served as positive and negative controls, respectively. Results of quadruplicate replicates are expressed as mean ± SD.
After confirming the presence and titers of KSHV-glycoprotein specific antibodies in purified sera, we performed neutralization activity assays in diverse cell types that represent most of the typespermissive to KSHV infection in vivo. First, we titrated purified rKSHV-eGFP.219 in four permissive cell types of human origin to determine volumes that ensured > 20% rKSHV-eGFP.219 infectivity for in vitro neutralization (Fig. 4b, Panel i). To conduct in vitro neutralization assays, we incubated serially diluted purified IgGs (12.5, 25, 50, or 100 µg/ml) with rKSHV-eGFP.219. We measured the percentage of eGFP+ cells (i.e., KSHV-infected cells) using FACS and defined an effective neutralization titer as 50% inhibition of infection, after normalization to control IgG samples from TNE–immunized rabbits. When rKSHV-eGFP.219 virus was pre-incubated with serially diluted concentrations of total purified IgG antibodies (12.5, 25, 50, or 100 µg/ml) from TNE-immunized rabbits (negative control), all concentrations achieved ~8% neutralizing activity (serum effect) in a non-dose-dependent manner in HEK-293 cells (data not shown). Thus, we used 8% as the normalization percentage to account for the serum effect in all subsequent neutralization assays. In contrast, rKSHV-eGFP.219 pre-incubated with serially diluted concentrations of pooled purified IgGs from rabbits immunized with KSHV-LPs +/−HR2 or UV-KSHV resulted in a dose-dependent reduction of KSHV infection in an endothelial cell line (HUVEC), foreskin fibroblast cell line (HFF-1), and B cell line (MC116) (Fig. 4b, Panel ii). Interestingly, despite UV-KSHV-immunized rabbits having higher titers of KSHV-glycoprotein-specific IgGs than KSHV-LP-immunized rabbits (Fig. 4a), the purified IgGs showed no statistical differences in nAb activities in any cell line tested (Fig. 4b). At the highest dilution analyzed (100 µg/ml), UV-KSHV and both KSHV-LPs +/−HR2 neutralized KSHV infection by 60–90% in all cell types tested (Fig. 4b, Panel ii). Importantly, even at the lowest concentration, IgGs from any of the vaccine candidates neutralized KSHV infection by 12–55%, in all cell types tested, with the highest neutralization activity observed in HFF-1 cells. The calculated concentration required to block 50% of infection (IC50; Table 4) varied between cell lines and vaccine candidates: UV-KSHV, 14.07–28.89 µg/ml; KSHV-LPs −HR2, 26.56–37.97 µg/ml; and KSHV-LPs+HR2, 29.57–49.64 µg/ml. Our results confirm that a select group of KSHV glycoproteins incorporated on the surface of KSHV-LPs, and administered with appropriate adjuvants, can stimulate nAbs that block KSHV infection of diverse cell types in vitro.
Table 4.
IC50 values for UV-KSHV, KSHV-LPs −/+ HR2 purified IgGs in various cell types.
| IC50 (g/ml) | |||
|---|---|---|---|
| Cell-line | UV-KSHV | KSHV-LPs–HR2 | KSHV-LPs+HR2 |
| HEK-293 | 27.36 | 33.49 | 39.9 |
| HFF-1 | 21.41 | 26.56 | 36.24 |
| HUVEC | 28.89 | 28.2 | 29.57 |
| MC116 | 14.07 | 37.97 | 49.64 |
4. Discussion
Envelope glycoproteins that mediate viral entry into permissive cells are recognized as key targets for eliciting nAbs for most licensed subunit vaccines [41], and thus offer an ideal strategy for developing protective subunit prophylactic vaccines against oncogenic KSHV. In this study, we generated multivalent vaccine candidates that incorporate four key KSHV envelope glycoproteins (gpK8.1, gB, and gH/gL) known to mediate viral infection into a single KSHV-LP to elicit potent nAbs in immunized animals [14,17,42]. We showed our newly generated multivalent KSHV-LPs to be immunogenic, eliciting moderate titers of antibodies specific to gpK8.1, gB, and gH/gL. Importantly, we showed for the first time that purified IgGs from pooled sera of rabbits immunized with KSHV-LPs neutralized KSHV infection in four permissive cell types (epithelial, endothelial, fibroblasts, and B cells), comparable to purified IgGs from UV-KSHV immunized rabbits.
The co-expression of a single construct comprising four KSHV glycoproteins, interspersed with 2A self-cleaving peptide for efficient expression and cleavage under a single promoter [32], and NDV NP and M proteins resulted in the successful self-assembly and release of KSHV-LPs. Purified KSHV-LPs from CHO cells structurally resembled purified KSHV virions in size, shape, and morphology. In addition, we confirmed that all four KSHV glycoproteins were incorporated into the KSHV-LPs. These data confirm that we generated a bona fide multivalent KSHV-LP expressing four glycoproteins as a reagent for immunization of animals.
To confirm the immunogenicity of the newly developed subunit KSHV-LP vaccine candidates and to measure their ability to elicit robust and potent broadly nAbs, we immunized rabbits with alum/MPL-adjuvanted KSHV-LPs+/−HR2 and compared antibody response kinetics over time with rabbits immunized with alum/ MPL-adjuvanted UV-KSHV. We detected IgG antibody titers specific to all four KSHV glycoproteins in sera from rabbits immunized with KSHV-LPs +/−HR2, which peaked by Day 49 after the second boost, but dropped significantly by Day 70. This was in contrast to sera from UV-KSHV-immunized rabbits, which maintained high titers throughout all time-points tested, suggesting that the durability of antibodies in KSHV-LP-immunized animals is sub-optimal. The current findings of low immunogenicity of KSHV-LPs relative to UV-KSHV are consistent with our previous studies in KSHV and Epstein-Barr virus [26,29], and other reported studies in foot-and-mouth disease virus, Chikungunya, and Zika virus [43–45], comparing VLP-based vaccines with inactivated virus.
As a first step in dissecting the ability of our candidate vaccine to elicit robust nAbs, we performed in vitro neutralization in epithelial cells, endothelial cells, fibroblasts, and B cells. To reduce the serum effect that has been observed when using total serum to perform neutralization [26,29], we purified total IgGs, and showed that incubation of purified IgGs from KSHV-LP+/−HR2- or UV-KSHV-immunized rabbits with rKSHV-eGFP.219 reduced infection in a dose-dependent manner in all cell types tested. Surprisingly, despite purified IgGs from UV-KSHV immunized rabbits having higher titers of KSHV-glycoprotein-specific antibodies in sera or in purified IgGs, the neutralization activities between KSHV-LPs +/−HR2 and UV-KSHV were comparable at Day 49. The mechanism behind this observation remains under investigation in our laboratory. We predict that future optimization of vaccine parameters, including vaccine dose, frequency of immunization, immunization route, or the use of other robust vectors (e.g., modified vaccinia Ankara vector or adenovirus vector) to deliver KSHV-LPs or wild-type KSHV glycoproteins as live-attenuated vaccines, will elicit high titers of potent nAbs that could block infection in both in vitro and in vivo infection models better than those found in UV-KSHV immunized rabbits.
Despite the public health burden of KSHV infection globally, to date, no KSHV subunit vaccine candidate has been tested in preclinical animal models, apart from our single study [26], or in clinical trials, making it difficult to compare our current results with other studies. Therefore, to assess the efficacy of our vaccine and allow comparison with previous studies for other viruses, we analyzed neutralization activity based on IC50 values for KSHV-LPs in all cell types tested (Table 4). Despite the observed neutralization, the potency of the purified KSHV IgGs was low compared to other neutralizing IgGs raised in various species against different viruses and pathogens [46,47]. For example, HIV IgGs targeting the major immunodominant envelope gp120 trimer, purified from either infected individuals or immunized cows blocked infection with IC50 <1 µg/ml [46,47]. Similar results have been observed for Plasmodium falciparum [48], hepatitis C virus [49], Zika virus [50], and CMV [51]. However, it is important to note that in the case of KSHV-LPs, we used total purified IgGs to calculate IC50, whereas in the other reported cases, IC50 was calculated using antigen-specific IgGs. Future studies should include purifying or isolating KSHV glycoprotein-specific nAbs from animals immunized with KSHV-LPs for fair comparison.
Previous studies have shown that the trimerization of HIV envelope glycoproteins (gp120 and gp41), respiratory syncytial virus F glycoprotein, or influenza hemagglutinin glycoprotein improved the stability of these proteins and enhanced their immunogenicity as subunit vaccines, resulting in the induction of potent broadly nAbs [52–54]. In the current study, the use of the NDV F HR2 domain, which supports trimerization of the proteins (gpK8.1, gB and gH) and increases the number of immunogens per individual KSHV-LP, was not advantageous, as both KSHV-LPs +/−HR2 neutralized KSHV equally in all cell types tested, with - HR2 performing somewhat better than+HR2.
In nature, KSHV only infects humans; however, reports indicate that a humanized mouse model harboring functional human immune system components, and the common marmoset (Callithrix jacchus), a non-human primate model, can be infected with KSHV [55,56]. These two animal models open up potential new frontiers for elucidating KSHV biology, including latent infection, associated diseases, and B-cell- and T–cell-mediated immune responses in vivo [55–57]. Thus, as a pre-requisite for performing Phase I clinical trials, we envision that future studies to elucidate correlates of immune protection in these two models will allow optimization of various vaccine parameters and define correlates of immune protection. In summary, our results demonstrate the successful generation and characterization of multivalent subunit KSHV-LPs as immunogenic vaccine candidates that produce antibody responses in rabbits capable of neutralizing KSHV in vitro in diverse cell types.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) K01 CA184388–02 (J.G.O.) and St. Baldrick’s International Scholar grant (D.H.M.). Research reported in this publication included work performed in City of Hope Core Facilities including Analytical Cytometry, Electron Microscopy, Integrative Genomics, Bioinformatics, Small Animal Studies, and Drug Discovery & Structural Biology supported by the National Cancer Institute of the NIH under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agencies had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish. We thank Ms. Supriya Bautista for her help with the organization of figures and Dr. Sarah T. Wilkinson for editing the manuscript and offering insightful feedback and discussion.
Footnotes
Conflicts of interest
The Authors declare no conflicts of interest.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018. [DOI] [PubMed]
- [2].Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer 2010;10:707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186–91. [DOI] [PubMed] [Google Scholar]
- [4].Uldrick TS, Wang V, O’Mahony D, Aleman K, Wyvill KM, Marshall V, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin Infect Dis 2010;51:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995;86:1276–80. [PubMed] [Google Scholar]
- [6].Wabinga HR, Parkin DM, Wabwire-Mangen F, Mugerwa JW. Cancer in Kampala, Uganda, in 1989–91: changes in incidence in the era of AIDS. Int J Cancer 1993;54:26–36. [DOI] [PubMed] [Google Scholar]
- [7].Wu TT, Qian J, Ang J, Sun R. Vaccine prospect of Kaposi sarcoma-associated herpesvirus. Curr Opin Virol 2012;2:482–8. [DOI] [PubMed] [Google Scholar]
- [8].Minhas V, Brayfield BP, Crabtree KL, Kankasa C, Mitchell CD, Wood C. Primary gamma-herpesviral infection in Zambian children. BMC Infect Dis 2010;10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Minhas V, Crabtree KL, Chao A, M’Soka TJ, Kankasa C, Bulterys M, et al. Early childhood infection by human herpesvirus 8 in Zambia and the role of human immunodeficiency virus type 1 coinfection in a highly endemic area. Am J Epidemiol 2008;168:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gantt S, Orem J, Krantz EM, Morrow RA, Selke S, Huang ML, et al. Prospective characterization of the risk factors for transmission and symptoms of primary human herpesvirus infections among ugandan infants. J Infect Dis 2016;214:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dittmer DP, Damania B. Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy. J Clin Investig 2016;126:3165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bechtel JT, Winant RC, Ganem D. Host and viral proteins in the virion of Kaposi’s sarcoma-associated herpesvirus. J Virol 2005;79:4952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhu FX, Chong JM, Wu L, Yuan Y. Virion proteins of Kaposi’s sarcoma-associated herpesvirus. J Virol 2005;79:800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dollery SJ, Santiago-Crespo RJ, Chatterjee D, Berger EA. Glycoprotein K8.1A of Kaposi’s sarcoma-associated herpesvirus is a critical B cell tropism determinant, independent of its heparan sulfate binding activity. J Virol 2018. [DOI] [PMC free article] [PubMed]
- [15].Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery: a structural view of herpesvirus entry machinery. Nat Rev Microbiol 2011;9:369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Luna REZF, Baghlan A, Chouljenko V, Forghani B, Gao SJ, Kousoulas KG. Kaposi’s sarcoma-associated herpesvirus glycoprotein K8.1 is dispensable for virus entry. J Virol 2004;78:6389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krishnan HH, Sharma-Walia N, Zeng L, Gao SJ, Chandran B. Envelope glycoprotein gB of Kaposi’s sarcoma-associated herpesvirus is essential for egress from infected cells. J Virol 2005;79:10952–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994;266:1865–9. [DOI] [PubMed] [Google Scholar]
- [19].Jinjong MaG D. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: maintenance of tight latency with efficient reactivation upon induction. J Virol Methods 2011;174:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Broering TJ, Garrity KA, Boatright NK, Sloan SE, Sandor F, Thomas WD, et al. Identification and characterization of broadly neutralizing human monoclonal antibodies directed against the E2 envelope glycoprotein of hepatitis C virus. J Virol 2009;83:12473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Myoung J, Ganem D. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: maintenance of tight latency with efficient reactivation upon induction. J Virol Methods 2011;174:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhu L, Puri V, Chandran B. Characterization of human herpesvirus-8 K8.1A/B glycoproteins by monoclonal antibodies. Virology 1999;262:237–49. [DOI] [PubMed] [Google Scholar]
- [23].Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 2011;6:e18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chiuppesi F, Nguyen J, Park S, Contreras H, Kha M, Meng Z, et al. Multiantigenic modified vaccinia virus ankara vaccine vectors to elicit potent humoral and cellular immune reponses against human cytomegalovirus in mice. J Virol 2018;92. [DOI] [PMC free article] [PubMed]
- [25].Pantua HD, McGinnes LW, Peeples ME, Morrison TG. Requirements for the assembly and release of Newcastle disease virus-like particles. J Virol 2006;80:11062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Barasa A, Ye P, Phelps M, Ganapathiram A, Tison T, Ogembo JG. BALB/c mice immunized with a combination of virus-like particles incorporating Kaposi sarcomaassociated herpesvirus (KSHV) envelope glycoproteins gpK8.1, gB, and gH/gL induced comparable serum neutralizing antibody activity to UV-inactivated. KSHV Oncotarget 2017;8:34481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 2009;6:343–5. [DOI] [PubMed] [Google Scholar]
- [28].Sashihara J, Hoshino Y, Bowman JJ, Krogmann T, Burbelo PD, Coffield VM, et al. Soluble rhesus lymphocryptovirus gp350 protects against infection and reduces viral loads in animals that become infected with virus after challenge. PLoS Pathog 2011;7:e1002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ogembo JG, Muraswki MR, McGinnes LW, Parcharidou A, Sutiwisesak R, Tison T, et al. A chimeric EBV gp350/220-based VLP replicates the virion B-cell attachment mechanism and elicits long-lasting neutralizing antibodies in mice. J Transl Med 2015;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Morrison TG. Structure and function of a paramyxovirus fusion protein. Biochimica et Biophysica Acta 2003;1614:73–84. [DOI] [PubMed] [Google Scholar]
- [31].Hahn A, Birkmann A, Wies E, Dorer D, Mahr K, Sturzl M, et al. Kaposi’s sarcoma-associated herpesvirus gH/gL: glycoprotein export and interaction with cellular receptors. J Virol 2009;83:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ryan MD, Drew J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. The EMBO J 1994;13:928–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Walsh G Biopharmaceutical benchmarks 2006. Nat Biotechnol 2006;24:769–76. [DOI] [PubMed] [Google Scholar]
- [34].Hesse F, Wagner R. Developments and improvements in the manufacturing of human therapeutics with mammalian cell cultures. Trends Biotechnol 2000;18:173–80. [DOI] [PubMed] [Google Scholar]
- [35].Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, et al. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention a pooled analysis of 11 clinical trials. Hum Vacc 2009;5:332–40. [DOI] [PubMed] [Google Scholar]
- [36].Kundi M New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vacc 2007;6:133–40. [DOI] [PubMed] [Google Scholar]
- [37].Labo N, Miley W, Marshall V, Gillette W, Esposito D, Bess M, et al. Heterogeneity and breadth of host antibody response to KSHV infection demonstrated by systematic analysis of the KSHV proteome. PLoS Pathog 2014;10:e1004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 2006;314:1936–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 2009;183:6186–97. [DOI] [PubMed] [Google Scholar]
- [40].Zhao B, Zhang X, Krummenacher C, Song S, Gao L, Zhang H, et al. Immunization with Fc-based recombinant epstein-barr virus gp350 elicits potent neutralizing humoral immune response in a BALB/c mice model. Front Immunol 2018;9:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010;17:1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hahn AS, Kaufmann JK, Wies E, Naschberger E, Panteleev-Ivlev J, Schmidt K, et al. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus. Nat Med 2012;18:961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Boigard H, Alimova A, Martin GR, Katz A, Gottlieb P, Galarza JM. Zika virus-like particle (VLP) based vaccine. PLoS NeglTrop Dis 2017;11:e0005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Metz SW, Gardner J, Geertsema C, Le TT, Goh L, Vlak JM, et al. Effective chikungunya virus-like particle vaccine produced in insect cells. PLoS NeglTrop Dis 2013;7:e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Clarke BE, Newton SE, Carroll AR, Francis MJ, Appleyard G, Syred AD, et al. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature 1987;330:381–4. [DOI] [PubMed] [Google Scholar]
- [46].Cheeseman HM, Olejniczak NJ, Rogers PM, Evans AB, King DF, Ziprin P, et al. Broadly neutralizing antibodies display potential for prevention of HIV-1 infection of mucosal tissue superior to that of nonneutralizing antibodies. J Virol 2017;91. [DOI] [PMC free article] [PubMed]
- [47].Sok D, Le KM, Vadnais M, Saye-Francisco KL, Jardine JG, Torres JL, et al. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature 2017;548:108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cheru L, Wu Y, Diouf A, Moretz SE, Muratova OV, Song G, et al. The IC(50) of anti-Pfs25 antibody in membrane-feeding assay varies among species. Vaccine 2010;28:4423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Urbanowicz RA, McClure CP, Brown RJ, Tsoleridis T, Persson MA, Krey T, et al. A diverse panel of hepatitis C virus glycoproteins for use in vaccine research reveals extremes of monoclonal antibody neutralization resistance. J Virol 2015;90:3288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Robbiani DF, Bozzacco L, Keeffe JR, Khouri R, Olsen PC, Gazumyan A, et al. Recurrent potent human neutralizing antibodies to zika virus in brazil and mexico. Cell 2017;169(597–609):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bootz A, Karbach A, Spindler J, Kropff B, Reuter N, Sticht H, et al. Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus. PLoS Pathog 2017;13:e1006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015;349:1301–6. [DOI] [PubMed] [Google Scholar]
- [53].Lu Y, Welsh JP, Swartz JR. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. PNAS 2014;111:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013;342:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chang H, Wachtman LM, Pearson CB, Lee JS, Lee HR, Lee SH, et al. Non-human primate model of Kaposi’s sarcoma-associated herpesvirus infection. PLoS Pathog 2009;5:e1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McHugh D, Caduff N, Barros MHM, Ramer PC, Raykova A, Murer A, et al. Persistent KSHV infection increases EBV-associated tumor formation in vivo via enhanced EBV lytic gene expression. Cell Host Microbe 2017;22(61–73):e7. [DOI] [PubMed] [Google Scholar]
- [57].Wang LX, Kang G, Kumar P, Lu W, Li Y, Zhou Y, et al. Humanized-BLT mouse model of Kaposi’s sarcoma-associated herpesvirus infection. PNAS 2014;111:3146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Muniraju Murali, Mutsvunguma Lorraine Z, Foley Joslyn, Escalante Gabriela M, Rodriguez Esther, Nabiee Romina, Totonchy Jennifer, Mulama David H, Nyagol Joshua, Wussow Felix, Barasa Anne K, Brehm Michael, Ogembo Javier Gordon. Kaposi Sarcoma-associated Herpesvirus Glycoprotein H is Indispensable for Infection of Epithelial, Endothelial, and Fibroblast Cell Types. J Virol 2019. 10.1128/JVI.00630-19. in press. [DOI] [PMC free article] [PubMed]