Abstract
Introduction:
Long-term outcomes after curative resection in patients with germline RET mutations and medullary thyroid cancer (MTC) are highly variable and mutation specific oncologic outcomes are not well described.
Methods:
Sixty-six patients identified from 1986 to 2017 from a single institution cancer database were assessed for recurrence and survival using Kaplan-Meier estimates and correlated with clinicopathologic features using log-rank or Cox proportional hazards.
Results:
Median follow-up was 9.3 years (0.3-31.5), median tumor diameter was 1.5 cm (Range 0.1-7.5), preoperative calcitonin (Ct) was known in 41 patients (median 636, [0-9600]). Overall survival (OS) of the cohort was 94% at 10 years. Cumulative incidence of locoregional recurrence (LR) was 38% at 10 years and 19/24 (79%) underwent repeat neck operation. Cumulative incidence of distant recurrence was 27% at 10 years. Predictors of distant recurrence were tumor size, positive lymph nodes, and pre and postoperative CEA, but not Ct. M918T mutation-bearing patients had 10-year distant recurrence free survival of 0% compared to 83% in all others (p<0.001), but equivalent 10-year OS (100% vs 92%, p=0.49).
Conclusion:
Structural and metastatic recurrence is common in patients with germline RET mutations and MTC and can occur 20 years after initial treatment; however, survival remains high. Management should focus on optimal surveillance strategies and long-term control of structural disease.
INTRODUCTION
The association between germline mutations of the RET proto-oncogene and familial medullary thyroid carcinoma (fMTC) has been established for 25 years1–4. Several studies have investigated long term oncologic outcomes associated with medullary thyroid cancer, but there is a paucity of literature in regards to outcomes of patients with familial disease5,6.
Current American Thyroid Association (ATA) guidelines designate specific mutations within categories of “moderate”, “high”, and “highest” risk which is based primarily on the age of development of MTC7–9. MTC patients with ATA high-risk codon 634 mutations have similar rates of distant metastatic disease and overall survival as compared to patients with MEN2A moderate-risk mutations10. These findings indicate that although specific mutations confer differential risk of developing MTC at a young age, the relationship between mutation and tumor behavior, aggressiveness, and long term oncologic outcomes is unclear, especially with MEN2B patients and the ATA highest-risk M918T mutation.
We investigate the long-term oncologic outcomes for patients with germline RET mutations and medullary thyroid cancer from a single institution. We report the rates of local and distant recurrence, mortality, factors associated with recurrence, and outcome differences in patients with the ATA highest risk M918T mutation.
METHODS
Following institutional review board approval, we reviewed the cancer registry records at Memorial Sloan-Kettering Cancer Center (MSKCC) for patients with medullary thyroid cancer. We identified 340 patients who underwent thyroidectomy for medullary thyroid cancer between 1986 and 2017. Three hundred and sixteen had thyroidectomy with curative intent with no metastatic or persistent gross disease in the neck. Sixty-six patients were identified as having familial disease based on clinical criteria and/or an identified germline mutation in the RET proto-oncogene. Clinical, pathologic, and follow-up data were obtained by review of the medial record. Postoperative lab values were recorded as the first postoperative level within 30 days of surgery. RET mutation risk category was assigned according to the ATA guidelines8.
Lymph node dissections were defined according to levels of the neck recorded in the operative and pathology reports. Levels II-V were classified as the lateral neck and designated as ipsilateral or contralateral to the primary site in the thyroid. The central neck was defined as levels VI and VII.
All recurrences were defined as structural disease rather than biochemical recurrence. Local recurrence was defined as in the thyroid bed. Nodal recurrence was defined as recurrence in the neck in any lymph node basin. Distant recurrence was defined as recurrence outside of the neck including the mediastinum. Locoregional and distant recurrences were recorded independently and the time to recurrence or death was calculated from the date of surgery.
Patients, disease and treatment characteristics were summarized using the frequency and percentages for categorical variables, and the median and range for continuous variables. Overall survival (OS) and recurrence-free survival (RFS) were calculated from date of procedure until date of death (for OS), or until the first recurrence or death of any cause (for RFS), or censored at the time of the last available follow-up. OS and RFS were estimated using Kaplan-Meier methods and compared between ATA risk (M918T vs all others) using log-rank test. Cox proportional hazards model was used to examine the univariate association between potential risk factors such as age, tumor size, lymph node positivity, and preop and postop biomarkers CEA and calcitonin and developed of any nodal recurrence (NR) with OS. Preop and postop biomarkers were transformed using natural logarithm transformation. Development of any LRR was treated as time-dependent covariate in the Cox regression model.
Cumulative incidence function was used to estimate the probability of any nodal recurrence (NR) and probability of any distance recurrence (DR). Patients who experienced another event without the event of interest (death or developed a LR or DR) were treated as competing events. Gray’s test was used to compare the cumulative incidence of any DR according to ATA risk (M918T vs all others). Fine and Gray univariate regression methods was used to examine the association between potential risk factors and the risk of LR or DR. Multivariate analysis was not performed due to the limited number of events in this series.
All statistical analyses were performed using SAS Version 9.4 (SAS Institute, INC., Cary, NC, USA) or R Version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). All P-values were two-sided and P-values of <0.05 were considered to indicate statistical significance.
RESULTS
Clinicopathologic characteristics
The median age of 66 patients who underwent thyroidectomy with curative intent for medullary thyroid cancer and had a germline RET mutation was 35.2 years (range 3.4-72.3). The most common RET mutations in our cohort were M918T (n=8), C634X (n=8), and V804M (n=6). Other mutations identified were Y791F, C618X, C611X, T618C, C620R, C786O, and L790F. Nine patients had family history of MEN2A and 2 MEN2B, as well as 23 patients with clinically familial disease without a specific mutation identified.
Clinicopathologic variables are listed in table 1. Median follow up for survivors was 9.3 years (range 0.3-31.5). Structural disease was defined as known macroscopic disease in the lymph nodes of the neck at the time of thyroidectomy. Ten patients (15%) had structural disease, 1 patient in the central compartment, 6 in the ipsilateral lateral compartment, and 3 in both the central and ipsilateral lateral. Structural disease was detected by ultrasound in 4 patients, physical exam in 3 patients, CT in 2 patients, and in one patient on a PET scan. All patients with known structural disease underwent lymphadenectomy of the involved compartment.
Table 1.
Clinicopathologic variables
| Variable | Median (range) |
|---|---|
| Age (years) | 35.2 (3.4,72.3) |
| Follow up (years) | 9.3 (3,31.5) |
| Gender (Female) | 37 (56%) |
| Prophylactic intent | 14 (21%) |
| Known Structural disease | 10 (15%) |
| Tumor size (cm) | 1.5 (0.1,7.5) cm |
| Node positive | 36 (55%) |
| Number of positive nodes | 1 (0,49) |
| Persistent biochemical disease* | 36 (55%) |
| Preop calcitonin (pg/ml), n=41 | 636 (0,9600) |
| Preop CEA (ng/ml), n=17 | 29.4 (0.9,179) |
| Post op calcitonin (pg/ml), n=61 | 16 (0,9750) |
| Post op CEA (ng/ml), n=45 | 8.4 (0.8,1207) |
Persistent biochemical disease was defined as an elevation in the first postoperative calcitonin level
Surgeon preference for prophylactic neck dissection in the absence of structural disease is variable. Of 66 patients including the 10 patients with structural disease, 52 (79%) underwent lymphadenectomy at the time of thyroidectomy. Central lymphadenectomy was performed in 44 patients (67%), ipsilateral lateral in 28 patients (42%), and contralateral lateral in 13 (20%). In the entire cohort, 36 (55%) patients had node positive disease on final pathology with the number of positive nodes ranging from 1 to 49. The most commonly positive compartment was the central neck (n=26, 39%), followed by the ipsilateral lateral (n=23, 35%), and contralateral lateral (n=4, 6%) and almost half the patients with positive nodes had multiple positive compartments (n=17).
Basal serum calcitonin levels were known preoperatively in 41 patients (62%) with a median level of 636 pg/ml (Range 0-9750), normal < 10 pg/ml. Preoperative CEA levels were obtained in 17 patients (26%) with a median of 29.4 ng/ml (range 0.8-1207), normal < 2.5 ng/ml. Postoperative calcitonin levels were known in 61 patients (92%) with a median value of 16 mg/dl (range 0-3750). Persistent biochemical disease was defined as an elevation in the basal calcitonin above the upper limit of normal on the first postoperative level. Thirty-six patients (55%) had persistent biochemical disease, including 28 of 36 (78%) of patients with positive lymph nodes. Postoperative CEA level was known in 45 patients (68%) with a median level of 8.4 ng/ml (Range 0.8-1207).
Recurrence
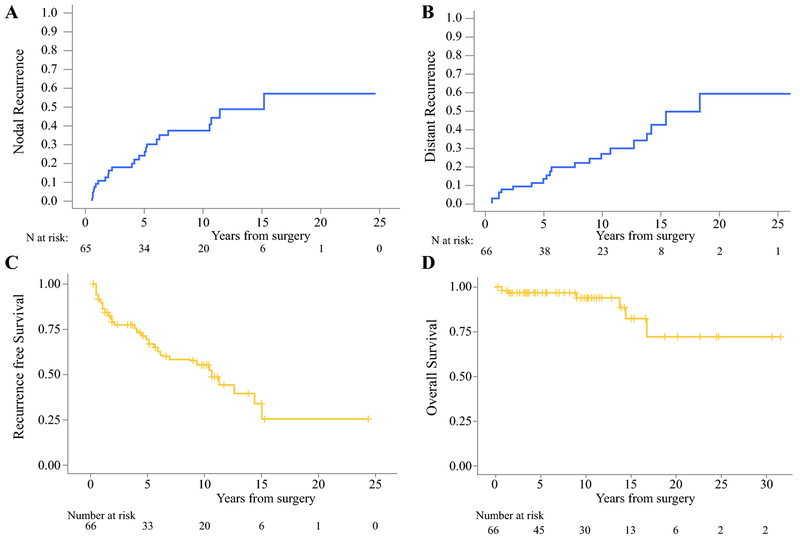
Twenty-four patients developed a nodal recurrence (NR), Figure 1A. Six recurrences were in the central neck, 14 were in the ipsilateral lateral neck, 2 in the contralateral neck, 1 in the ipsilateral and contralateral neck, and one in the central and contralateral neck. The 5-year cumulative incidence of NR was 26% and the 10-year incidence is 37%. Of these recurrences 19 (79%) were managed with reoperation and neck dissection. Factors associated with NR on univariate analysis are listed in table 2. Age, tumor size, number of positive lymph nodes, preoperative calcitonin, and persistent biochemical disease were associated with NR.
Figure 1:
Cumulative incidence of A) locoregional recurrence and B) Distant recurrence in 66 patients. (c) Recurrence-free survival and (d) overall survival
Table 2.
Univariate analysis of factors associated with recurrence and survival
| Factor | NR | DR | OS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (years) | 1.03 (1,1.05) | 0.03 | 1.02 (0.99,1.04) | 0.16 | 1.03 (0.98,1.09) | 0.18 |
| Gender (male) | 1.08 (0.48,2.39) | 0.86 | 1.50 (0.65,3.45) | 0.34 | 1.76 (0.32,9.75) | 0.52 |
| Tumor size (cm) | 1.22 (1.04,1.42) | 0.01 | 1.26 (1.06,1.51) | 0.01 | 1.48 (1.03,2.13) | 0.03 |
| Number of positive lymph nodes | 1.06 (1.02,1.1) | 0.001 | 1.06 (1.02,1.12) | 0.008 | 1.02 (0.93,1.10) | 0.70 |
| LN positivity (yes/no) | 2.29 (0.98,5.38) | 0.06 | 2.83 (1.13,7.09) | 0.03 | 1.90 (0.34,10.52) | 0.46 |
| Ct pre-op*1 | 1.6 (1.18,2.17) | 0.003 | 1.19 (0.93,1.53) | 0.17 | 2.00 (0.59,6.61) | 0.26 |
| CEA pre-op*2 | 2.13 (0.74,6.17) | 0.16 | 7.57 (2.38,24.13) | 0.001 | n/a | |
| Ct post-op*3 | 1.39 (1.13,1.70) | 0.002 | 1.15 (0.9,1.46) | 0.26 | 1.27 (0.80,2.01) | 0.30 |
| CEA post-op*4 | 1.17 (0.92,1.5) | 0.21 | 1.71 (1.26,2.33) | 0.001 | 1.85 (1.12,3.09) | 0.02 |
| Structural disease | 2.93 (1.19,7.2) | 0.09 | 1.89 (0.68,5.25) | 0.22 | 1.38 (0.16,12.03) | 0.77 |
| Persistent biochemical disease | 16.34 (2.29,116.72) | 0.005 | 4.19 (0.99,17.59) | 0.05 | 2.34 (0.26,21.12) | 0.45 |
CEA and Ct levels were log-transformed.
n missing = 25
n missing = 49
n missing = 5
n missing = 21
Twenty-one patients (32%) developed a distant recurrence (DR), Figure 1B. Location and management of distant metastasis are detailed in table 3. The cumulative incidence of DR was 14% at 5 years and 27% at 10 years. Factors associated with distant recurrence are listed in table 2. Statistically significant associations with DR were tumor size, number of positive lymph nodes, preoperative CEA, and postoperative CEA.
Table 3.
Management of Patients with Distant Recurrence
| Patient | Mutation | Time (months) | Location | Detection | Management |
|---|---|---|---|---|---|
| 1 | M918T | 13.5 | Bone | CT | cabozantanib, vandetanib, Dacarbazine/5FU |
| 2 | Positive NOS | 169.6 | Bone | MRI | cabozantinib, RT (to symptomatic bone met) |
| 3 | M918T | 91.6 | Liver | PET | hepatic embolization, LOXO-292 |
| 4 | C634Y | 184.5 | Lung, liver | PET | Liver wedge resection, cabozantinib |
| 5 | V804M | 62.3 | Liver | MRI | LOXO-292 |
| 6 | Positive NOS | 118.8 | Liver | CT | RFA liver X2 |
| 7 | Positive NOS | 6.5 | Lung, liver, bone | CT | RT (neck, sacrum) |
| 8 | M918T | 134.7 | lung | CT | RT (lung hilum (hemoptysis)), sorafenib, cabozantanib, vandetanib |
| 9 | C618F | 67.2 | Liver | CT | RT (neck), vandetanib, RT C4, everolimus |
| 10 | Positive NOS | 13.3 | lung/liver/bone/breast | CT | RT, imatinib, vandetanib, |
| 11 | M918T | 99.3 | Lung | PET | RT(neck), Lung resection, Vandetanib |
| 12 | Positive NOS | 106.2 | Lung, liver, bone | CT | RT (neck), vandetanib, Loxo-292 |
| 13 | M918T | 65.8 | Liver, lungs | CT | RT (neck), vandetanib, cabozantanib, regorafenib, sunitinib |
| 14 | M918T | 51.6 | bone | CT | RT (neck), carbo/taxol, RT(rib), vandetanib, LOXO-292 |
| 15 | V804M | 6.4 | Liver, Brain | MRI | Vandetanib, Temozolomide/capecit abine, RT(brain) |
| 16 | MEN2B NOS | 356.5 | Lung | CT | Vandetanib, RT (neck) |
| 17 | M918T | 47.0 | Liver | MRI | Cabozantanib, LOXO-292 |
| 18 | C618F | 152.1 | Liver | MRI | Surveillance |
| 19 | Positive NOS | 199.6 | Bone | CT | Surveillance |
| 20 | C620 | 220.0 | Liver, Lung | CT | Surveillance |
| 21 | C634Y | 128.0 | Liver | CT | Surveillance |
NOS: Not otherwise specified, RT: Radiation, RFA: radiofrequency ablation
Sixteen patients (24%) had both NR and DR. Isolated nodal recurrences (without DR) was seen in 8 patients (12%) and isolated DR in 5 (8%). Recurrence free survival is shown in Figure 1C. Disease free survival was 69% at 5 years, and 55% at 10-years.
Survival
Six patients (9%) died over the study period, Figure 1D. Four of the 6 deaths (67%) were from disease, which was present in the liver, lungs, or both, and 2 patients died of causes unrelated to MTC. No patients died from invasive disease in the neck. Five-year overall survival is 97% and 10-year overall survival was 94%. Excluding the 14 patients with prophylactic intent thyroidectomy, five-year overall survival is 96% and 10-year overall survival 93% in the 52 patients who underwent thyroidectomy with known MTC. Factors associated with overall survival are tumor size and postoperative CEA, table 2. LRR was analyzed as a time dependent covariate and was not associated with survival (HR 0.70, 95% CI 0.09-5.41, p=0.74). Of note, persistent biochemical disease and recurrence in the neck were not associated with survival.
Prophylactic intent Patients
Fourteen patients (21%) underwent thyroidectomy with prophylactic intent and were found to have MTC on pathology. Median age of these patients was 11 years (3.4-37) and tumor size was 0.3 cm (0.1-1.3). Four (29%) had a positive lymph node. Patients were followed for a median of 9.6 years (0.8-20.2) and there was observed 1 NR at 10.7 years from resection and 2 distant recurrences at 10.7 and 12.7 years. Five and 10-year overall survival was 100% in prophylactic intent patients.
Outcomes by RET mutation
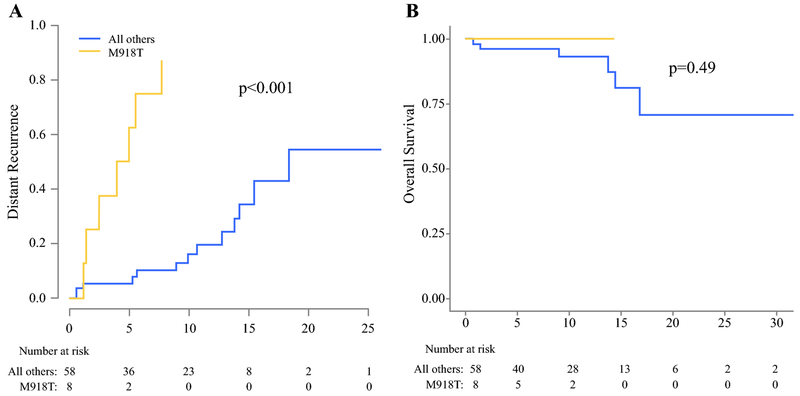
We compared the 8 patients with ATA highest risk M918T mutation to the remaining 58 patients. Median follow up for the M918T patients was 8.9 years. Cumulative incidence of distant recurrence and overall survival (OS) are shown in Figure 2. The 10-year distant recurrence free survival in patients with M918T mutations was 0% compared to 83% in all other patients, (p<0.001). However, this increase in distant recurrence did not correspond to increased mortality with 10-year overall survival of 100% in patients with M918T mutations compared to 92% in patients with other mutations, p=0.49.
Figure 2:
A) Cumulative incidence of distant recurrence and B) Overall survival for patients with the M918T mutation compared to all other patients.
DISCUSSION
In this study, we report the long term oncologic outcomes for 66 patients with familial medullary thyroid carcinoma (fTMC) treated with curative intent thyroidectomy. We report low rates of long-term disease-free survival, with initial recurrences up to 20 years from thyroidectomy. The risk for both locoregional and distant recurrences do not appear to plateau with a median follow up of 10 years. These findings indicate that patients with fMTC require life-long surveillance for recurrent disease. Current ATA guidelines for surveillance involve Ct and CEA every 6 months and cross-sectional imaging depending on physical exam findings and Ct levels8.
There are several limitations to this study. It is retrospective and spans a 30-year period in which an effective systemic therapy has been developed. Additionally, preoperative and postoperative calcitonin and CEA were missing in a significant number of patients limiting the analysis of those predictive biomarkers. Finally, the median follow-up of 10 years in this study might be insufficient to characterize true long term survival in these patients.
Whereas recurrence was common, death was uncommon with our patients having 10-year overall survival of 94%. It is important to note that this study included 14 patients for which the thyroidectomy was performed with prophylactic intent without diagnosis of the underlying MTC prior to surgical pathology. This early treatment could have contributed to the excellent observed overall survival, and to the young median age of 35 years in this study. We did not observe a relationship between age and mortality, which could be due to a low number of events, but older age has previously been reported as a risk factor for death in patients with MTC10–12.
The distinction between recurrence and survival is best illustrated in patients with the ATA highest risk M918T mutation. All patients with this mutation had a distant recurrence by 10 years, but all were still alive at last follow up. Although we did not demonstrate a difference in survival in patients with M918T mutations, our median follow up of 8.9 years could be insufficient for a difference to manifest. These findings indicate a disease biology that is difficult to eradicate but manageable with associated long-term survival. Voss et al. found no difference in distant recurrence free survival or overall survival between patients with high risk codon 634 mutations and patients with moderate risk mutations10. That study, however, was limited to MEN2A patients and did not contain patients with the M918T mutation. Similarly, we found no differences in outcomes between high and moderate risk mutations but did show a significant difference in the incidence of distant recurrence in highest risk M918T patients, which, to our knowledge is the first report of a cancer specific outcome effected by a specific mutation.
One active question in the management of MTC is the role of lateral neck dissection for elevated preoperative basal serum calcitonin in the absence of identified structural disease in the lateral neck13. Machens and colleagues have investigated ways to predict lymph node metastasis in different lymph node compartments based on quantifying lymph node involvement in the central compartment and serum calcitonin levels14–16. These studies have focused on rates of identified microscopic disease and postoperative serum calcitonin levels, and less on oncologic outcomes of recurrence and survival. Increased postoperative calcitonin levels have been associated with recurrence and reoperation17–20 and we found that pre and postoperative calcitonin and persistent biochemical disease were associated with increased risk of locoregional recurrence. Interestingly these associations did not hold for distant recurrence and survival which could be due to a limited number of events.
Thirty-nine years ago Norton and Brennan reported that in node positive patients biochemical “cure” was extremely rare and highly dependent upon the method of detection of calcitonin levels. Patients with normal basal peripheral calcitonin frequently had elevated levels of peripheral calcitonin when stimulated with pentagastrin and essentially all patients when undergoing internal jugular catheterization with stimulation would have residual biochemical disease, despite normal peripheral levels21.
The relationship between aggressive surgery, normalization of biochemical levels, and subsequent effects on recurrence and survival has not been well characterized. Further investigation is needed, both in familial and sporadic MTC, to characterize differences in oncologic outcomes resulting from lymph node dissection in the absence of structural disease and identify patients that might benefit from more aggressive upfront surgery. Our results demonstrate a disease biology with high recurrence rates, but good long-term survival. With overall survival of 94% at 10 years it is difficult to justify a highly morbid intervention with the goal of improving long-term outcomes.
Familial medullary thyroid carcinoma is characterized by high rates of locoregional and distant recurrence, the risk of which persists for 20 years after diagnosis. However, despite high rates of structural recurrence, mortality is rare. More study is needed to determine the impact of aggressive surgery for biochemical disease, particularly in the neck lymph nodes at the time of initial thyroidectomy, although it is unlikely this could improve survival above what was observed in our study. Management strategies for fMTC should focus on optimizing approaches to surveillance and long-term control of structural disease.
SYNOPSIS.
Familial medullary thyroid cancer is characterized by high rates of local and distant recurrence with continued risk of recurrence at 20 years, but excellent overall survival. Patients with the ATA highest risk M918T mutation have higher rates of distant recurrence compared to other mutations.
ACKNOWLEDGEMENTS
The work was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
The authors have no relevant financial interests to disclose
Presented as an oral presentation at the Society of Surgical Oncology 72nd Annual Cancer Symposium, San Diego, CA, March 2019
REFERENCES
- 1.Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. September 1985;42(2):581–588. [DOI] [PubMed] [Google Scholar]
- 2.Donis-Keller H, Dou S, Chi D, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. July 1993;2(7):851–856. [DOI] [PubMed] [Google Scholar]
- 3.Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. January 27 1994;367(6461):375–376. [DOI] [PubMed] [Google Scholar]
- 4.Mulligan LM, Eng C, Healey CS, et al. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet. January 1994;6(1):70–74. [DOI] [PubMed] [Google Scholar]
- 5.Yip L, Cote GJ, Shapiro SE, et al. Multiple endocrine neoplasia type 2: evaluation of the genotype-phenotype relationship. Arch Surg. April 2003;138(4):409–416; discussion 416. [DOI] [PubMed] [Google Scholar]
- 6.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. November 1 2006;107(9):2134–2142. [DOI] [PubMed] [Google Scholar]
- 7.Skinner MA, Moley JA, Dilley WG, Owzar K, Debenedetti MK, Wells SA Jr. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med. September 15 2005;353(11):1105–1113. [DOI] [PubMed] [Google Scholar]
- 8.Wells SA Jr., Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. June 2015;25(6):567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machens A, Niccoli-Sire P, Hoegel J, et al. Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med. October 16 2003;349(16):1517–1525. [DOI] [PubMed] [Google Scholar]
- 10.Voss RK, Feng L, Lee JE, et al. Medullary Thyroid Carcinoma in MEN2A: ATA Moderate- or High-Risk RET Mutations Do Not Predict Disease Aggressiveness. J Clin Endocrinol Metab. August 1 2017;102(8):2807–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esfandiari NH, Hughes DT, Yin H, Banerjee M, Haymart MR. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J Clin Endocrinol Metab. February 2014;99(2):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho AS, Wang L, Palmer FL, et al. Postoperative Nomogram for Predicting Cancer-Specific Mortality in Medullary Thyroid Cancer. Ann Surg Oncol. August 2015;22(8):2700–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy M, Chen H, Sippel RS. Current understanding and management of medullary thyroid cancer. Oncologist. 2013;18(10):1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machens A, Hauptmann S, Dralle H. Prediction of lateral lymph node metastases in medullary thyroid cancer. Br J Surg. May 2008;95(5):586–591. [DOI] [PubMed] [Google Scholar]
- 15.Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. June 2010;95(6):2655–2663. [DOI] [PubMed] [Google Scholar]
- 16.Dralle H, Machens A. Surgical management of the lateral neck compartment for metastatic thyroid cancer. Curr Opin Oncol. January 2013;25(1):20–26. [DOI] [PubMed] [Google Scholar]
- 17.Barbet J, Campion L, Kraeber-Bodere F, Chatal JF, Group GTES. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. November 2005;90(11):6077–6084. [DOI] [PubMed] [Google Scholar]
- 18.Machens A, Ukkat J, Hauptmann S, Dralle H. Abnormal carcinoembryonic antigen levels and medullary thyroid cancer progression: a multivariate analysis. Arch Surg. March 2007;142(3):289–293; discussion 294. [DOI] [PubMed] [Google Scholar]
- 19.Machens A, Schneyer U, Holzhausen HJ, Dralle H. Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J Clin Endocrinol Metab. April 2005;90(4):2029–2034. [DOI] [PubMed] [Google Scholar]
- 20.Rowland KJ, Jin LX, Moley JF. Biochemical cure after reoperations for medullary thyroid carcinoma: a meta-analysis. Ann Surg Oncol. January 2015;22(1):96–102. [DOI] [PubMed] [Google Scholar]
- 21.Norton JA, Doppman JL, Brennan MF. Localization and resection of clinically inapparent medullary carcinoma of the thyroid. Surgery. June 1980;87(6):616–622. [PubMed] [Google Scholar]