Abstract
Previous reports show increased incidence of venous thromboembolism [VTE, deep-vein thrombosis (DVT) and pulmonary embolus (PE)] in sickle cell disease (SCD) patients. The incidence, time course, and risk factors for VTE recurrence have been less well described.
We determined the cumulative incidence of first VTE recurrence and bleeding in a cohort of SCD patients with incident VTE. Risk factors for recurrence and bleeding were also determined using multivariable Cox regression models, adjusting for gender, race/ethnicity, era of incident VTE, location and hospitalization-associated status of incident VTE, and SCD-related complications. Results are presented as adjusted hazard ratios (HR) and 95% confidence intervals (CI).
Among 877 SCD patients with an incident VTE, the 1-year and 5-year cumulative incidence of recurrence was 13.2% (95% CI 11.0%-15.5%) and 24.1% (95% CI 21.2%-27.1%). Risk factors for VTE recurrence included more severe SCD (HR=2.41; CI: 1.67-3.47), lower extremity DVT as the incident event (HR=1.64; CI: 1.17-2.30), and pneumonia/acute chest syndrome (HR=1.68; CI: 1.15-2.45). The cumulative incidence of bleeding was 4.9% (CI 3.5%-6.4%) at 6 months and 7.9% (CI: 6.2%-9.8%) at 1 year. More severe SCD (HR=1.61; CI: 1.11-2.35) was associated with bleeding.
The high incidence of VTE recurrence in patients with SCD suggests that extended anticoagulation may be indicated; however, this must be weighed against a relatively high risk of bleeding. Prospective, randomized studies of anticoagulation in SCD patients with VTE are needed.
Keywords: Sickle cell disease, Venous Thromboembolism, Recurrence, Bleeding, Mortality
Introduction
Venous thromboembolism (VTE), which includes pulmonary embolism (PE) and deep vein thrombosis (DVT), has recently been recognized as a common complication of sickle cell disease (SCD). A study of the United States National Hospital Discharge Survey reported the overall prevalence of PE was approximately 4 times higher in SCD patients compared to African Americans without SCD (0.44% versus 0.12% respectively)[1] and a case-control study of hospitalized patients in Pennsylvania reported a prevalence of PE 50- to 100-fold higher in the SCD population compared to the general hospitalized population.[2] In a single-institution cohort study of 404 patients with SCD, the prevalence of VTE was 25%[3] and the same group reported a cumulative incidence of VTE of 11.3% by age 40 years in SCD patients ⩾ 15 years of age. In addition, utilizing the Pediatric Health Information System, 1.7% pf 10,454 pediatric SCD patients developed an incident VTE (median age of VTE was15.9 years) and VTE was independently associated with death.[4]
In our prior work, using a large cohort of SCD patients (n=6237) derived from administrative data in California, we determined the cumulative incidence of VTE by age 40 years was 17.1% (95% CI, 15.2–19.2) for SCD patients with severe disease (hospitalized ⩾3 times a year) and 6.8% (95% CI, 5.7–8.0) for those with less severe disease.[5] We also found that recurrence was greater in those with severe SCD [5-year incidence = 36.8% (95% CI, 32.2–41.5)] than for those with less severe disease (5-year incidence = 19.6% (95% CI, 14.4–25.4)), suggesting that extended anticoagulation might be indicated in SCD patients with severe disease. However, we did not determine the risk factors associated with VTE recurrence, or the incidence and risk factors for bleeding after a diagnosis of VTE in patients with SCD. This information is important to inform decisions about extended anticoagulation therapy for secondary prophylaxis.
Therefore, in the present study, we extend on our findings to describe the cumulative incidence and risk factors associated with VTE recurrence in a larger cohort of SCD patients with incident VTE, using a more stringent definition for VTE recurrence than in our previous report. In addition, we determined the cumulative incidence and risk factors for bleeding in SCD patients subsequent to the incident VTE. In the absence of prospective randomized trials, or even prospective observational studies of SCD patients with VTE, these data may help inform clinical decisions regarding the duration of anticoagulation in patients with SCD and VTE.
Methods
Database
This was a retrospective cohort study. The State of California Office of Statewide Health Planning and Development (OSHPD) maintain records of all patients hospitalized in non-federal hospitals in the state, called the Patient Discharge Database (PDD). Since July 1990, the State of California has required that these hospitals report up to 25 diagnoses and up to 20 procedures associated with each hospitalization, coded using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). Since 2005, an Emergency Department Utilization (EDU) database of all hospital associated ED facilities has also been mandated. Since 1996, a present-on-admission (POA) indicator has been required for essentially all PDD diagnoses. In addition to diagnostic and procedure information, demographic information including age, gender, race/ethnicity, insurance coverage, type of admission and disposition is collected in both PDD and EDU. An encrypted form of the social security number, called the record linkage number (RLN), is used to identify unique individuals, allowing serial linking of multiple hospitalization records over time. This administrative database does not contain laboratory or medication information. All data was linked to the California master death registry providing death information through 2011. This study was approved by the California Health and Human Services Agency Committee for the Protection of Human Subjects, and the University of California, Davis Institutional Review Boards.
Patient identification
As previously reported,[5–7] SCD patients were identified using search algorithms informed by the Registry and Surveillance System for Hemoglobinopathies (RuSH) project and validated by the Public Health Research, Surveillance and Epidemiology in Hemoglobinopathies (PHRESH) study.[8] To be included in this analysis, cases had to have: 1) at least 2 separate admissions with an SCD code in the principal (first position) diagnosis, or 2) at least one admission with an SCD code in the principal position and a SCD code in a secondary position in at least 2 additional admissions. Specific SCD ICD-9-CM codes (282.41, 282.42, 282.60, 282.61, 282.62, 282.63, 282.64, 282.68, 282.69) were used. Additionally, all patients had to be <65 years of age at first encounter to be considered SCD cases. Genotype was deemed unreliable because 67% had multiple genotypes coded across different admissions. The algorithm emphasized specificity over sensitivity and has been used in previous publications.[5–7]
Incident VTE was defined using ICD-9-CM codes shown in Supplemental Table 1. To be defined as an incident acute VTE, a PE or DVT, the code had to be in the principal diagnosis position or a specific VTE location code in a secondary position during a PDD hospitalization or an emergency department visit. In order to capture VTE as a complication of a procedure, we also included patients with a principal diagnosis code of 997.2 or 997.3 plus the corresponding DVT or PE in the second position. An upper extremity (UE) DVT code was allowed in any of the 25 diagnosis codes. The incident VTE event was defined as hospitalization associated if the patient had an inpatient hospitalization within 90 days prior to their incident VTE.
Covariates
Patient sex and race/ethnicity were obtained from the patients’ first PDD or EDU encounter. SCD severity was categorized as severe SCD and less severe SCD. Patients who averaged ≥3 admissions (PDD and EDU combined) per year were defined as severe SCD patients, while all other patients were defined as less severe. We, and others, previously demonstrated the frequency of hospitalization was associated with mortality in SCD patients.[5, 6, 9] SCD related complications, including osteonecrosis of the femoral head (ONFH), stroke, and pneumonia/acute chest syndrome (ACS; coding began in 2003), were included as covariates (Supplemental Table 2). Pneumonia/ACS was defined as any admission with 1) pneumonia in the principal position of an inpatient admission, 2) a SCD code in the principal position and pneumonia in the second position of an inpatient admission, or 3) ACS in any diagnostic position of (PDD or EDU) in year ≥ 2003. We used this combination because ACS was likely coded as pneumonia prior to 2003 and it is clinically difficult to differentiate the two entities.
Outcomes
The primary outcome was recurrent acute VTE [PE, DVT, or UE] at least 30 days after the incident VTE discharge date. In our previous report, the definition of recurrent VTE included subsequent VTE coded in secondary positions and in the EDU. Because some patients with SCD are frequently hospitalized, there is the possibility that a VTE code for an encounter soon after discharge from the incident VTE represented the same event, and not recurrence, as the treating physicians and abstractors would have considered it an active problem. To increase the specificity for VTE recurrence, a VTE code had to be in the principal position (e.g., the primary reason for inpatient admission) or if SCD was coded as the principal diagnosis, the acute VTE code had to be in the second position to be counted as a VTE recurrence. A conservative 30-day cut-off was also used to differentiate between progression of the incident event versus VTE recurrence.
The secondary outcome was bleeding. Bleeding was identified using specific ICD-9-CM codes included in Supplemental Table 3 which were modified from the ATRIA Study to include SCD relevant bleeding events.[10, 11] Patients were classified as having intra-cranial hemorrhage (ICH), gastrointestinal bleeding (GI), menorrhagia, epistaxis, hemophthalmos, gross hematuria, or other bleeding (including renal vascular disorder, hemopericardium, hemoptysis, and hemarthrosis).
Statistical Methods
Univariate descriptive statistics using chi-squared test were used to describe characteristics of the incident VTE SCD cohort by VTE recurrence and bleeding events. The cumulative incidences of VTE recurrence and bleeding, adjusted for the competing risk of death and the alternative outcome, were calculated. Gray’s K-sample test statistic was used to determine whether cumulative incidence of VTE recurrence or bleeding differed by disease severity or incident VTE location.
Multivariable Cox proportional hazards regression models were used to analyze potential risk factors associated with recurrent VTE and bleeding, adjusted for the competing risk of death or the alternative outcome, and accounting for baseline characteristics (gender, race/ethnicity, SCD severity, incident VTE era, age, incident VTE location and hospital associated status, and SCD complications). Event time was calculated from date of incident VTE to date of recurrent VTE or bleeding, date of death or the study cut-off date (12/3½014), whichever occurred first. SCD complications (osteonecrosis of the femoral head, ischemic stroke, and pneumonia/ACS) were included as time dependent covariates. For all regression analyses, the proportional hazard assumption was assessed using Schoenfeld residuals.[12] All covariates included met the proportional hazard assumption.
Information on anticoagulation is not available in the database. A question of significant clinical relevance is the incidence of VTE recurrence after discontinuation of anticoagulation, typically 3-, 6-, or 12-months after the date of the incident VTE during the time period of the study. To indirectly address this question, we estimated the additional one-year, conditional cumulative incidence of VTE recurrence at 15-, 18- and 24-months from the incident VTE among patients that did not have an outcome event (recurrent VTE or bleeding) or the competing risk of death, at 3-, 6-, and 12-months, respectively, after the date of incident VTE. All analyses were performed using SAS version 9.4 (Cary, NC).
Results
Among 6,423 SCD patients identified, 877 patients (13.7%) developed an incident acute VTE (Table 1). The median age of SCD patients with an incident VTE was 31 years and the median follow-up was 6 years. The majority was women, and most incident events occurred within 90 days of an inpatient hospitalization (provoked). Fifty (9.9%) of the 507 women had a pregnancy related incident VTE defined as the incident VTE during their pregnancy or within 6 weeks postpartum. About 85% of patients had a history of pneumonia/ACS or ONFH. Most of the incident VTE events were PE (± DVT) (57.7%), followed by DVT alone (23.5%), and upper extremity DVT alone (18.8%).
Table 1:
Baseline characteristics of California sickle cell disease (SCD) patients with an incident venous thromboembolism (VTE), 1991-2014
| All | Recurrent VTE | No Recurrent VTE | Bleeding | No Bleeding | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | col % | N | col % | P-Value | N | col % | N | col % | P-Value | |
| All | 877 | 100.0% | 257 | 100.0% | 620 | 100.0% | 245 | 100.0% | 632 | 100.0% | ||
| Incident VTE Type | ||||||||||||
| Incident PE | 506 | 57.7% | 133 | 51.8% | 373 | 60.2% | 0.0218 | 127 | 51.8% | 379 | 60.0% | 0.0287 |
| Incident DVT | 206 | 23.5% | 82 | 31.9% | 124 | 20.0% | 0.0002 | 71 | 29.0% | 135 | 21.4% | 0.0169 |
| Incident UE | 165 | 18.8% | 42 | 16.3% | 123 | 19.8% | 0.2279 | 47 | 19.2% | 118 | 18.7% | 0.8616 |
| Incident VTE Age | ||||||||||||
| <10 | 66 | 7.5% | 23 | 8.9% | 43 | 6.9% | 0.3035 | 11 | 4.5% | 55 | 8.7% | 0.0338 |
| 10-19 | 103 | 11.7% | 23 | 8.9% | 80 | 12.9% | 0.0979 | 25 | 10.2% | 78 | 12.3% | 0.3776 |
| 20-29 | 233 | 26.6% | 80 | 31.1% | 153 | 24.7% | 0.049 | 59 | 24.1% | 174 | 27.5% | 0.2993 |
| 30-39 | 228 | 26.0% | 72 | 28.0% | 156 | 25.2% | 0.3804 | 72 | 29.4% | 156 | 24.7% | 0.1541 |
| 40-49 | 128 | 14.6% | 36 | 14.0% | 92 | 14.8% | 0.7511 | 39 | 15.9% | 89 | 14.1% | 0.4896 |
| 50-59 | 94 | 10.7% | 20 | 7.8% | 74 | 11.9% | 0.0703 | 30 | 12.2% | 64 | 10.1% | 0.3629 |
| 60-65 | 17 | 1.9% | 2 | 0.8% | 15 | 2.4% | 0.1086 | 6 | 2.4% | 11 | 1.7% | 0.4947 |
| >65 | 8 | 0.9% | 1 | 0.4% | 7 | 1.1% | 0.2942 | 3 | 1.2% | 5 | 0.8% | 0.5447 |
| Incident VTE Hospitalization Provoked Status | ||||||||||||
| Hospitalized < 90 days prior | 505 | 57.6% | 165 | 64.2% | 340 | 54.8% | 0.0107 | 158 | 64.5% | 347 | 54.9% | 0.0100 |
| Hospitalized > 90 days prior | 372 | 42.4% | 92 | 35.8% | 280 | 45.2% | 0.0107 | 87 | 35.5% | 285 | 45.1% | 0.0100 |
| Era of Incident VTE | ||||||||||||
| 1991-2002 | 333 | 38.0% | 116 | 45.1% | 217 | 35.0% | 0.0049 | 107 | 43.7% | 226 | 35.8% | 0.0303 |
| 2003-2014 | 544 | 62.0% | 141 | 54.9% | 403 | 65.0% | 0.0049 | 138 | 56.3% | 406 | 64.2% | 0.0303 |
| Prior Bleeding | ||||||||||||
| Yes | 182 | 20.8% | 67 | 26.1% | 115 | 18.5% | 0.0124 | 71 | 29.0% | 111 | 17.6% | 0.0002 |
| Gender | ||||||||||||
| Male | 370 | 42.2% | 96 | 37.4% | 274 | 44.2% | 0.0619 | 84 | 34.3% | 286 | 45.3% | 0.0032 |
| Female | 507 | 57.8% | 161 | 62.6% | 346 | 55.8% | 0.0619 | 161 | 65.7% | 346 | 54.7% | 0.0032 |
| Race/Ethnicity | ||||||||||||
| African-American | 812 | 92.6% | 241 | 93.8% | 571 | 92.1% | 0.388 | 226 | 92.2% | 586 | 92.7% | 0.8090 |
| non-African American | 65 | 7.4% | 16 | 6.2% | 49 | 7.9% | 0.388 | 19 | 7.8% | 46 | 7.3% | 0.8090 |
| SCD Severity | ||||||||||||
| Less Severe | 297 | 33.9% | 45 | 17.5% | 252 | 40.6% | <.0001 | 48 | 19.6% | 249 | 39.4% | <.0001 |
| More Severe | 580 | 66.1% | 212 | 82.5% | 368 | 59.4% | <.0001 | 197 | 80.4% | 383 | 60.6% | <.0001 |
| SCD Complications (prior to outcome) | ||||||||||||
| ACS (2003+) | 395 | 45.0% | 103 | 40.1% | 292 | 47.1% | 0.0572 | 109 | 44.5% | 307 | 48.6% | 0.2769 |
| ACS/Pneumonia | 722 | 82.3% | 214 | 83.3% | 508 | 81.9% | 0.6376 | 221 | 90.2% | 520 | 82.3% | 0.0036 |
| AVN | 367 | 41.8% | 103 | 40.1% | 264 | 42.6% | 0.4941 | 107 | 43.7% | 280 | 44.3% | 0.8661 |
| Ischemic Stroke | 65 | 7.4% | 18 | 7.0% | 47 | 7.6% | 0.7666 | 24 | 9.8% | 46 | 7.3% | 0.2171 |
P-value represents bi-variate χ2 test of VTE recurrence vs. no VTE recurrence and bleeding vs. no bleeding
PE- Pulmonary embolism
DVT- Lower extremity deep vein thrombosis
UE- Upper extremity deep vein thrombosis
ONFH- Osteonecrosis of the femoral head
ACS- Acute Chest Syndrome
A total of 257 recurrent VTE events occurred over the study period, with a median time to recurrence of 13.3 months (range 1-208). Of the recurrent events, 65.4% occurred within 90 days of a hospitalization; therefore, these recurrent events could be considered hospitalization associated or provoked and 6 (3.7%) of the 161 women with recurrent VTE had a pregnancy related recurrent VTE. The location of incident and recurrent VTE are shown in Supplemental Table 4. The location of the recurrent events was most often the same as the incident location.
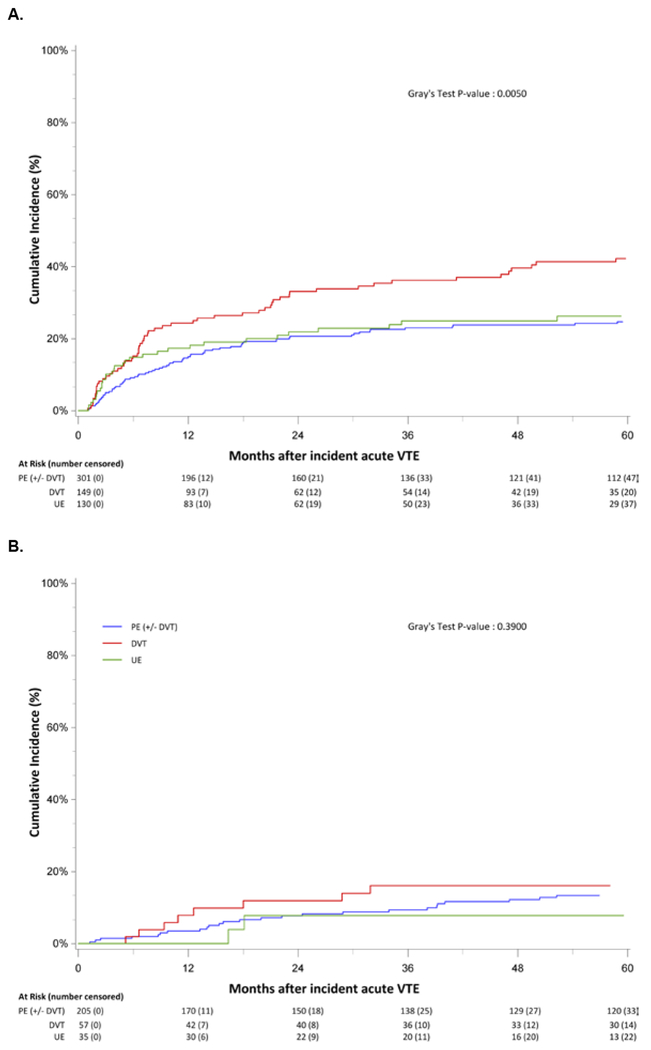
The 1-year and 5-year cumulative incidences of recurrence was 13.2% (95% CI, 11.0%-15.6%) and 24.1% (95% CI, 21.2%-27.1%), respectively. The case fatality rate for VTE recurrence was 3.1% (95% CI, 1.0%-5.2%). Six of the 8 fatal events were PE; the case fatality rate for recurrent PE was 4.5%. The cumulative incidence of recurrence was greater for those with severe SCD [1-year incidence = 17.9% (95% CI, 14.9%-21.2%); 5-year incidence = 28.4% (95% CI, 24.6%-32.2%)] than for those with less severe disease [1-year incidence= 3.9% (95%CI, 2.1%-6.6%); 5-year incidence= 12.2% (95% CI, 8.5%-16.5%)]. The cumulative incidence of recurrence also varied by incident VTE location, with the incidence of VTE recurrence for lower extremity DVT higher than that for PE and UE DVT and incidence curves separating starting at 6 months for both severe and less severe SCD (Figure 1A, 1B).
Figure 1:
Cumulative Incidence of venous thromboembolism (VTE) recurrence among California sickle cell disease (SCD) patients with an incident VTE for severe SCD patients (A) and less severe SCD patients (B) by incident VTE location, 1991-2014
In a multivariable model accounting for the competing risks of death or bleeding, the risk of VTE recurrence was higher among those with more severe SCD (Hazard ratio (HR) = 2.41; 95% confidence interval (CI): 1.67–3.47), when the incident VTE was lower extremity DVT, compared to PE (HR=1.64; 95% CI, 1.17-2.30), and among those with pneumonia/ACS (HR =1.68; 95% CI, 1.15–2.45) (Table 3). In a sub-analysis that included only SCD patients with an incident VTE during the years 2003 to 2014 [when the specific code for acute chest syndrome became available], there was a similar association between history of ACS and recurrent VTE (HR=1.53; 95% CI, 1.04–2.26). There was no association of the risk of recurrence with gender, age, hospitalization associated status of incident VTE, or era of incident VTE diagnosis in this SCD population.
Table 3:
Risk Factors associated with venous thromboembolism (VTE) recurrence and bleeding after incident VTE among California sickle cell disease (SCD) patients
| VTE Recurrence | Bleeding | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-Value | HR | 95% CI | P-Value |
| Gender | ||||||
| Female | 1.23 | (0.93, 1.62) | 0.1458 | 1.00 | (0.69, 1.44) | 0.9988 |
| Male | Ref | - | - | Ref | - | - |
| Race/Ethnicity | ||||||
| African-American | Ref | - | - | 0.84 | (0.38, 1.83) | 0.6549 |
| Non-African American | 0.96 | (0.55, 1.68) | 0.8797 | Ref | - | - |
| SCD Severity | ||||||
| Less Severe | Ref | - | - | Ref | - | - |
| More Severe SCD | 2.33 | (1.63, 3.32) | <.0001 | 2.26 | (1.41, 3.64) | 0.0008 |
| Incident VTE Year | ||||||
| 1991-2002 | Ref | - | - | Ref | - | - |
| 2003-2014 | 0.88 | (0.67, 1.15) | 0.3353 | 1.15 | (0.80, 1.66) | 0.4425 |
| Age at Incident VTE | ||||||
| <25 | Ref | - | - | Ref | - | - |
| 25-39 | 1.09 | (0.81, 1.48) | 0.5692 | 1.13 | (0.72, 1.76) | 0.6004 |
| 40-54 | 0.89 | (0.60, 1.31) | 0.5489 | 1.51 | (0.90, 2.56) | 0.1218 |
| ≥55 | 0.50 | (0.23, 1.06) | 0.0721 | 2.35 | (1.22, 4.55) | 0.0110 |
| Incident VTE Location | ||||||
| PE | Ref | - | - | Ref | - | - |
| DVT | 1.78 | (1.29, 2.46) | 0.0005 | 1.07 | (0.70, 1.63) | 0.7482 |
| UE | 1.07 | (0.73, 1.55) | 0.7399 | 1.24 | (0.78, 1.97) | 0.3691 |
| Incident VTE Hospitalization Associated Status | ||||||
| Yes | 0.83 | (0.61, 1.13) | 0.2285 | 1.14 | (0.78, 1.68) | 0.4950 |
| No | Ref | - | - | Ref | - | - |
| Prior Bleeding | ||||||
| Yes | 1.24 | (0.88, 1.75) | 0.2235 | 1.04 | (0.65, 1.66) | 0.8755 |
| No | Ref | - | - | Ref | - | - |
| SCD Complication prior to outcome | ||||||
| *ONFH | ||||||
| Yes | 1.01 | (0.76, 1.34) | 0.9591 | 0.96 | (0.67, 1.38) | 0.8302 |
| No | Ref | - | - | Ref | - | - |
| *Ischemic Stroke | ||||||
| Yes | 1.23 | (0.73, 2.07) | 0.4298 | 1.24 | (0.65, 2.39) | 0.5170 |
| No | Ref | - | - | Ref | - | - |
| *Pneumonia/ACS | ||||||
| Yes | 1.73 | (1.19, 2.50) | 0.0037 | 1.01 | (0.63, 1.61) | 0.9697 |
| No | Ref | - | - | Ref | - | - |
Multivariable Cox proportional hazard regression model adjusted for the competing risk of other outcome and death
SCD complications were included as time dependent covariates
PE- Pulmonary Embolism
DVT- Lower extremity deep vein thrombosis
UE- Upper extremity deep vein thrombosis
ONFH- osteonecrosis of the femoral head
ACS- Acute chest syndrome
In a sensitivity analysis using a less stringent definition of VTE recurrence (e.g., an acute VTE code in any position at least 30 days after discharge from the index VTE), there were 334 VTE recurrences rather than 257. The results of the Cox model for VTE recurrence again showed that disease severity (HR=1.82; 95% CI, 1.36–2.43) and lower extremity DVT (HR=1.65; 95% CI, 1.24–2.19) as the incident event. In an additional sensitivity analysis, obesity at incident VTE (9.7% of SCD patients) was not associated with recurrent VTE (HR=0.97 95% CI, 0.60-1.59). Due to the low number of pregnancy-associated events, pregnancy was not analyzed as a covariate.
A total of 245 of the 877 SCD patients had a bleeding event after the incident VTE (Table 1). The median time to a bleed was 25.1 months (range 1 day – 230 months) after the incident VTE. Of those with bleeding, ICH occurred in 6.9%, representing 1.9% of all patients with incident VTE. GI bleeding was 40.8% of the bleeds, occurring in 11.4% of all patients with VTE. Epistaxis and menorrhagia were common bleeding types, 22.9% and 12.7%, respectively, among all bleeds, while hemophthalmos, gross hematuria and other bleeds were less common (7.4%, 5.9% and 12.2%, respectively).
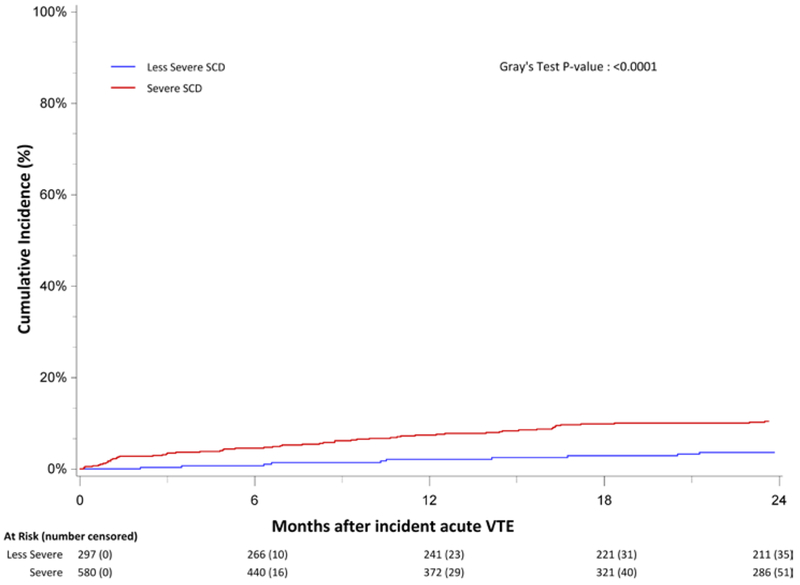
The cumulative incidence of bleeding at 6- and 12-months following the incident VTE was 4.9% (95% CI, 3.6%-6.4%) and 7.9% (95% CI, 6.2%-9.8%). The case fatality rate for bleeding was 7.4% (95% CI, 4.1%-10.6%). Five of the 19 fatal bleeding events were ICH; the case fatality rate for ICH was 29.4%. The incidence of bleeding was greater for those with severe SCD [6-month incidence =6.3% (95% CI, 4.5%-8.5%); 12-month incidence = 10.1% (95% CI, 7.8%-12.7%)] than for those with less severe disease [6-month incidence= 2.1% (95%CI, 1.0%-4.2%); 12-month incidence= 3.5% (95% CI, 1.8%-6.1%)] (Figure 2).
Figure 2:
Cumulative incidence of bleeding among California sickle cell disease (SCD) patients with an incident venous thromboembolism (VTE), by SCD severity, 1991-2014
In a multivariable model accounting for the competing risks of death or recurrent VTE, severe SCD (HR=1.61; 95% CI, 1.11–2.35), older age at incident event (≥55 vs <25 years; p=0.01), and a prior history of bleeding were associated with after VTE bleeding (Table 3). Sex, the type of incident event, and other sickle cell related complications, were not associated with increased risk of bleeding after VTE.
The additional 1-year cumulative incidences of recurrence among severe SCD patients without an outcome event at 3-, 6- and 12-months was 15.2% (15-month), 11.2% (18-month), and 8.7% (24-month), respectively (Supplemental Figure 1A) compared to 4.3%, 5.2%, and 4.3% for less severe disease, respectively. With increasing time, the additional 1-year incidence of VTE recurrence appeared to slowly decline. The additional 1-year cumulative incidences of bleeding among severe SCD patients without an outcome event at 3-, 6- and 12-months was 8.2% (15-month), 8.8% (18-month), and 6.6% (24-month), respectively (Supplemental Figure 1B) compared to 3.5%, 3.2%, and 2.6% for less severe patients, respectively. The incidence of bleeding appeared to be stable.
Discussion
In this large population-based study of SCD patients with incident VTE treated across many facilities in California and followed for a median of 6 years, we found a high incidence of recurrent VTE that varied by SCD severity and location of incident VTE. At 5-years, 28% of SCD patients with severe disease and 12% of patients with less severe disease had a recurrent VTE. In multivariable models, severe disease, lower extremity DVT as the incident event, and history of pneumonia/ACS were independently associated with a recurrent VTE. SCD patients with incident VTE also appear to be at relatively high risk for bleeding (7.9% at 12 months), with severe SCD associated with an increased risk of bleeding.
The primary limitation of our study is our lack of data on the type, intensity, and duration of anticoagulation, which would impact VTE recurrence and bleeding. In order to mitigate this limitation, we performed conditional analyses, using time points that are often used to discontinue time-limited treatment for acute VTE. The additional one-year cumulative incidences of VTE recurrence for the 3-, 6- and 12-month conditional analyses for severe and non-severe SCD patients were close to or over 5%. This is the threshold at which guidelines suggest consideration of indefinite anticoagulation.[13, 14]
However, the suggestion that SCD patients with VTE might benefit from indefinite anticoagulation must be tempered by the surprisingly high incidence of bleeding seen in these patients after an incident VTE. The cumulative incidence of bleeding after VTE was 4.9% at 6-months, which is a period of time when many patients would have been on anticoagulation. This is higher than reported in most clinical trials of anticoagulation for VTE in the general population.[13, 15] and is similar to that seen in patients with cancer-associated VTE.[16, 17]. Many of the bleeding events in the present study may have occurred when patients were presumably no longer on anticoagulation, as the median time to bleeding was 25.1 months.
Most of the bleeding events were from gastrointestinal sources, and an increased risk of GI bleeding is not widely appreciated in patients with SCD. Intracranial hemorrhage represented 6.9% of bleeding events after incident VTE. Intracranial hemorrhage is a recognized and uncommon complication of SCD.[18]
Our findings are consistent with a prior cohort study that also reported a high incidence of VTE recurrence in patients with SCD. In a single-institution study of 104 SCD patients with a history of VTE, 24.8% had a documented recurrence during a median follow-up of 4.8 years.[3] The median time to recurrence was 1.8 years, with 75% of recurrent events occurring after 1 year. Our study significantly expands on their findings by considering the time course and risk factors for VTE recurrence and bleeding.
A recently published single institution retrospective study of 37 SCD patients with VTE treated with anticoagulation revealed a high incidence of recurrence and bleeding.[19] Nine patients developed VTE recurrence within six months of the index event (24%). However, four of the recurrent events occurred ≤ 30 days from the index event and would not have been counted in our analysis. Eliminating the early events results in a 13.5% incidence of VTE recurrence at 6 months; this is still higher than the 8.5% incidence at 6 months seen in our present study utilizing a conservative definition of recurrence. One patient had bleeding (hemopthalmos) and 5 patients had clinically relevant non-major bleeding in their study.
In the present study, the location of incident VTE was highly associated with the location of VTE recurrence, findings similar to what has been reported in the general VTE population. [20] In a patient-level meta-analysis of seven prospective trials of patients with first VTE, Baglin et al. determined the risk of recurrence as PE was three times higher in patients presenting with symptomatic PE than in patients with proximal DVT. [20] The investigators concluded that whilst DVT and PE are manifestations of the same disease, the phenotypic expression may be pre-determined, a finding that appears to be true in SCD patients as well. This information may inform discussions between patients and clinicians about the duration of anticoagulation.
Disease severity was defined by the frequency of hospitalizations and likely correlates with complications that predispose SCD patients to thrombosis. The increased risk of recurrence associated with lower extremity DVT alone has not been previously described in the SCD population. It is possible that vein and valve damage is more profound in SCD patients, in whom leukocyte and coagulation activation are chronic.[21–23] Because the recurrence curves diverge at 6 months, it is also possible that patients with DVT were taken off anticoagulation around that point, whereas those with PE and upper extremity thrombosis (often associated with a permanent central venous catheter) were continued on anticoagulation. There was an association between a history of pneumonia/ACS and VTE recurrence. As recent studies suggest thrombosis may contribute to some episodes of ACS, [24, 25] patients with history of ACS may be predisposed to VTE.
In contrast to the general VTE population,[13] whether an event was classified as hospital-associated was not associated with the risk of VTE recurrence in this study. This may relate to the frequency of admissions in SCD patients. In our study, 66% of SCD patients with incident VTE averaged ≥3 hospitalizations per year and 65% of the recurrent VTE events were within 90 days of a hospitalization. Recurrent hospitalization might be considered a persistent ongoing risk factor for these patients. In addition, the concept of provocation may be more nuanced than in the past. A recent analysis that combined data from several clinical trials suggested that patients with VTE associated with a transient minor or persistent minor provocation had a recurrent VTE as often as those without obvious provocation.[26] SCD itself likely represents a persistent provoking condition. Additional VTE risk factors, such as hormonal contraception, smoking, severe dehydration, and prolonged travel, that could also have played a role in VTE recurrence could not be adequately assessed in the study.
The case-fatality rate for VTE recurrence in this SCD cohort was 3.1% (95% CI, 1.0% - 5.2%) which is somewhat lower, but overlaps with, estimates reported in a meta-analysis of recent clinical trials of secondary prophylaxis with either vitamin K antagonists or DOACs, 5.6% (95% CI, 1.2%–15.4%) and 10.8% (95% CI, 4.4 %-20.9%), respectively.[15] The case-fatality rate for bleeding was 7.35% (95% CI, 4.1%-10.6%) in our study, which also overlaps with rates reported in the meta-analysis: vitamin K antagonists 6.8% (95% CI, 1.4% - 18.6%) and DOACs 0.0% (95% CI, 0.0% - 15.4%). Thus, the acute mortality associated with either VTE recurrence or bleeding in SCD patients is similar to those of the general VTE population.
The high risk of recurrent VTE in patients with SCD raises the question of whether all patients should be considered for indefinite anticoagulation. While this can only be answered by prospective clinical trials, in the absence of this data, one could attempt to risk stratify based on the findings from this study. For example, patients with severe SCD and with a DVT might be considered high risk for recurrence. As patients with SCD may also have liver or renal dysfunction that increase the risk of bleeding, intermittent re-assessment of benefit versus risk of anticoagulation is necessary in SCD patients with VTE.
In the largest cohort study known to date to examine this issue, we show that SCD patients with an incident VTE have a high incidence of recurrent VTE. This high rate of VTE recurrence among SCD patients warrants consideration of extended anticoagulation, but the high rates of bleeding temper this suggestion. Given the lack of knowledge as to the optimal type and duration of anticoagulation for patients with SCD that have suffered an incident VTE, coupled with the frequent nature of these events, a prospective, multicenter randomized trial may be warranted and feasible given the frequency of VTE in this population.
Supplementary Material
Acknowledgements
This work is supported by grants from NIH UL1 TR 00001860 (T.W)
Footnotes
Conflict of Interest
The authors report no relevant conflicts of interest.
References
- 1.Stein PD, Beemath A, Meyers FA, et al. Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease. The American journal of medicine 2006;119:897 e897–811. [DOI] [PubMed] [Google Scholar]
- 2.Novelli EM, Huynh C, Gladwin MT, et al. Pulmonary embolism in sickle cell disease: a case-control study. Journal of thrombosis and haemostasis : JTH 2012;10:760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naik RP, Streiff MB, Haywood C Jr., et al. Venous thromboembolism in adults with sickle cell disease: a serious and under-recognized complication. The American journal of medicine 2013;126:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar R, Stanek J, Creary S, et al. Prevalence and risk factors for venous thromboembolism in children with sickle cell disease: an administrative database study. Blood Adv 2018;2:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunson A, Lei A, Rosenberg AS, et al. Increased incidence of VTE in sickle cell disease patients: risk factors, recurrence and impact on mortality. Br J Haematol 2017;178:319–326. [DOI] [PubMed] [Google Scholar]
- 6.Brunson A, Keegan THM, Bang H, et al. Increased risk of leukemia among sickle cell disease patients in California. Blood 2017;130:1597–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adesina O, Brunson A, Keegan THM, et al. Osteonecrosis of the femoral head in sickle cell disease: prevalence, comorbidities, and surgical outcomes in California. Blood Adv 2017;1:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulukonis ST, Harris WT, Coates TD, et al. Population based surveillance in sickle cell disease: methods, findings and implications from the California registry and surveillance system in hemoglobinopathies project (RuSH). Pediatric blood & cancer 2014;61:2271–2276. [DOI] [PubMed] [Google Scholar]
- 9.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. The New England journal of medicine 1991;325:11–16. [DOI] [PubMed] [Google Scholar]
- 10.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. Journal of the American College of Cardiology 2011;58:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer DE, Chang Y, Fang MC, et al. Should patient characteristics influence target anticoagulation intensity for stroke prevention in nonvalvular atrial fibrillation?: the ATRIA study. Circ Cardiovasc Qual Outcomes 2009;2:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenfeld D Partial Residuals for the Proportional Hazards Regression-Model. Biometrika 1982;69:239–241. [Google Scholar]
- 13.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315–352. [DOI] [PubMed] [Google Scholar]
- 14.Kearon C, Spencer FA, O’Keeffe D, et al. D-dimer testing to select patients with a first unprovoked venous thromboembolism who can stop anticoagulant therapy: a cohort study. Annals of internal medicine 2015;162:27–34. [DOI] [PubMed] [Google Scholar]
- 15.Wu C, Alotaibi GS, Alsaleh K, et al. Case fatality of bleeding and recurrent venous thromboembolism during, initial therapy with direct oral anticoagulants: A systematic review. Thrombosis research 2014;134:627–632. [DOI] [PubMed] [Google Scholar]
- 16.Lee AY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association 2015;314:677–686. [DOI] [PubMed] [Google Scholar]
- 17.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. The New England journal of medicine 2003;349:146–153. [DOI] [PubMed] [Google Scholar]
- 18.Strouse JJ, Lanzkron S, Urrutia V. The epidemiology, evaluation and treatment of stroke in adults with sickle cell disease. Expert review of hematology 2011;4:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts MZ, Gaskill GE, Kanter-Washko J, et al. Effectiveness and safety of oral anticoagulants in patients with sickle cell disease and venous thromboembolism: a retrospective cohort study. Journal of thrombosis and thrombolysis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baglin T, Douketis J, Tosetto A, et al. Does the clinical presentation and extent of venous thrombosis predict likelihood and type of recurrence? A patient-level meta-analysis. Journal of thrombosis and haemostasis : JTH 2010;8:2436–2442. [DOI] [PubMed] [Google Scholar]
- 21.Wun T The Role of Inflammation and Leukocytes in the Pathogenesis of Sickle Cell Disease; Haemoglobinopathy. Hematology 2001;5:403–412. [PubMed] [Google Scholar]
- 22.Wun T, Brunson A. Sickle cell disease: an inherited thrombophilia. Hematology American Society of Hematology Education Program 2016;2016:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ataga KI, Key NS. Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program 2007:91–96. [DOI] [PubMed] [Google Scholar]
- 24.Anea CB, Lyon M, Lee IA, et al. Pulmonary platelet thrombi and vascular pathology in acute chest syndrome in patients with sickle cell disease. American journal of hematology 2016;91:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mekontso Dessap A, Deux JF, Abidi N, et al. Pulmonary artery thrombosis during acute chest syndrome in sickle cell disease. American journal of respiratory and critical care medicine 2011;184:1022–1029. [DOI] [PubMed] [Google Scholar]
- 26.Prins MH, Lensing AWA, Prandoni P, et al. Risk of recurrent venous thromboembolism according to baseline risk factor profiles. Blood Adv 2018;2:788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.