Abstract
Objectives:
To describe the placental histology and autopsy findings in pregnancies where fetal demise occurred before a gestational age of 22 weeks.
Study design:
This study was a subset of a larger study where the effect of alcohol exposure during pregnancy on stillbirths was studied. In a prospective cohort, 7,010 singleton pregnancies were followed from the first antenatal visit until infant one year of age visit. Gestational age was assessed by ultrasound, preferably at the first antenatal visit. All pregnancy losses were identified and when the fetuses delivered at or after a gestation of 20 weeks, the mother or parents were approached for consent for autopsy. This study describes the placental pathology and findings at autopsy in losses before 22 weeks gestation (late second trimester miscarriages).
Results:
Fourteen cases were identified in which 13 had an autopsy and 12 had a histological examination of the placenta. The most prevalent histological abnormality was placental abruption which was seen in 6 miscarriages, occasionally on its own, or in combination with maternal vascular malperfusion or acute chorioamnionitis. The second most frequent finding was maternal vascular malperfusion, as found in five placentas, alone or in combination with other pathology. The third most frequent pathology was acute chorioamnionitis, found in four placentas, in combination or alone. Other causes were diffuse chronic villitis due to cytomegalovirus infection and early amnion rupture with anhydramnios and cord obstruction.
Conclusions:
Causes of fetal demise at the end of the second trimester differ little from causes of stillbirth. There is value in using placental histology in late second trimester miscarriages to try to identify the cause of demise.
Keywords: Autopsy, Placental histology, Second trimester miscarriage, Late miscarriage
Introduction
There is very little information on rates and common causes of spontaneous second trimester miscarriage, possibly due to the global lack of reporting systems. In the United States, fetal mortality from the National Vital Statistics System is presented only for fetal deaths at 20 weeks gestation or more [1]. In the United Kingdom(UK) the cut-off point is 24 weeks [2] while 22 weeks is recommended by the World Health Organization [3]. Although the miscarriage rate in human conception is very high in early pregnancy, it improves to less than 10% after six weeks [4]. In the UK, fetal loss at 12–24 weeks gestational age is defined as a late miscarriage [5,6]; and the prevalence rates vary between 0.7 and 3%. After a gestation of 16 weeks, the likelihood of losing a viable pregnancy is only 1%.Simpson et al found a spontaneous miscarriage rate of only 0.4% between 15 and 21 weeks in 264,653 pregnancies [7]. Little is known about the underlying causes of second trimester miscarriage as 51% are classified as idiopathic [6].
There is usually no community based information on perinatal mortality rates based on gestational age at demise rather than birthweight in many countries. Gestational age is preferable to birthweight, as the lower cut off points for birthweight may exclude growth restricted fetuses born at, or shortly after, a gestational age of 22 or 28 weeks depending on the definition used [8].
As the aetiology of second trimester miscarriages and early stillbirths may be similar, information on the causes and rates of miscarriage may provide valuable information in the effort to reduce stillbirths. In addition, it is necessary to analyse stillbirth and perinatal mortality rates regularly to project whether the millennium goals for 2030 will be reached. [8] Although there has been a 25.5% decline in global stillbirth rates from 2000 to 2016, the slow improvement in sub-Saharan Africa is a cause for concern [9]. Every opportunity should therefore be used to obtain additional information regarding stillbirth.
A unique opportunity was afforded South Africa when the Safe Passage Study, undertaken in three populations, chose the low-income population in Cape Town as one of the clinical sites. The primary and secondary hypotheses, design and the schedule of evaluations and events have been described [10]. Precise information on all pregnancies, from the first antenatal visit until the infant one year of age visit, allowed accurate documentation of pregnancy losses and death in the first year of life. As a gestational age of 20 weeks or more was one of the inclusion criteria, in contrast to the 22 week lower limit for the definition of stillbirth in South Africa [11], valuable information became available as autopsies were done in stillbirths from 20 weeks onwards. In the subsequent analyses of these stillbirths, the high prevalence of mid-trimester miscarriage became apparent, precipitating this study.
Methods
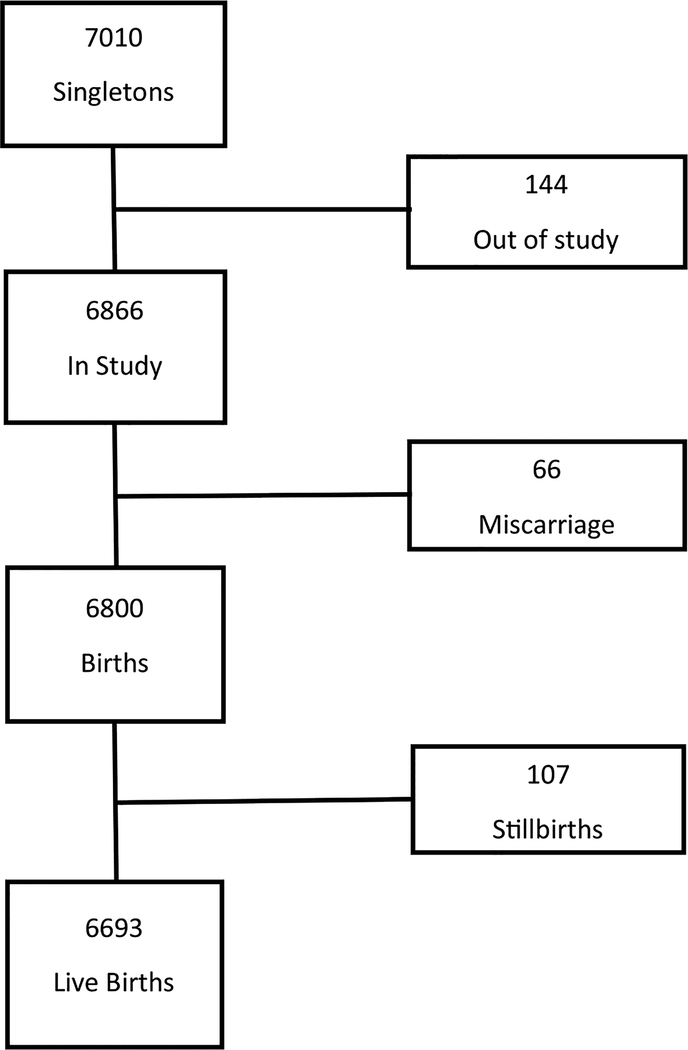
After approval of the Health Research Ethics Committee of Stellenbosch University, 7060 pregnant women were recruited at the antenatal clinics in Bishop Lavis from August 2007 to January 2015. From this cohort, 7010 singleton pregnancies were selected for this study (Fig. 1). At the recruitment visit, participants provided informed consent which included collection of the placenta for histological examination after delivery. With a fetal demise, a separate informed consent was obtained for autopsy [12]. Research midwives checked labor ward admissions and deliveries daily to determine if a study participant had delivered. In women with fetal demise the designated social worker and/or senior personnel of the study were alerted to provide support and counselling and, at an appropriate time, discussed consent for autopsy. Examination of the placenta and the autopsy were done according to a standard protocol [13].
Fig. 1.
The study profile of singleton pregnancies.
All demises were presented and discussed at weekly perinatal mortality meetings which were attended by obstetricians, neonatologists, pathologists, and geneticists. The primary cause of death was then determined according to the Perinatal Problem Identification Programme (PPIP) [14] and all deliveries from 22 weeks and later were excluded from the present study. During the discussions at the perinatal meetings it became apparent that in certain women, fetal demise had occurred prior to 22 weeks but the fetus delivered at or after 22 weeks. The gestational age at demise was determined in different ways: i) early ultrasound examination, ii) gestational age when fetus was known to be alive i.e. fetal heart rate monitored or fetal heart activity observed on ultrasound, iii) measurement of the foot length at autopsy / assessment gestational age by the pathologist using organ weights and degree of maceration [15]. The participant was included in the present study only when it was certain that demise had occurred prior to 22 weeks according to the first ultrasound examination unless there was a great discrepancy between the ultrasound derived gestational age and gestational age according to foot length or birthweight. It therefore means that the study consisted of second trimester miscarriages only. As a dual control, the list of fetal demises beyond 20 weeks gestation was verified. Any case where the fetal heart rate was recorded at or after 22 weeks, was regarded as a stillbirth and therefore not included. Terminations of pregnancy were included in the study as the primary obstetric reason for the termination was taken into account.
Results
There were initially 7010 singleton pregnancies, but after a loss of 136 voluntary withdrawals and 8 lost to follow-up at delivery, 6866 pregnancies (97.9%) remained in the study. (Fig. 1). Spontaneous miscarriages occurred in 66 (rate 0.96%) singleton pregnancies of which 19 occurred before 12 weeks and 47 at gestational age 120 to 244 weeks (rate 0.69%). Most of the miscarriages occurred at a gestational age 160/7 to 196/7 weeks (Table 1). Autopsies were done on 13 fetuses where the gestational age at delivery varied between 206/7 and 244/7 weeks. In the 4 pregnancies where the gestational age at delivery was 22 weeks or more, the gestational age calculated according to foot length was less than this (Table 2). Therefore, these 4 were also regarded as second trimester miscarriages. The ultrasound derived gestational ages were done between 81/7 and 173/7 weeks. Associated complications in these 14 cases were suspected urinary tract infection in 2, pregnancy induced hypertension in 1, previous miscarriages in 2, and a previous termination of pregnancy in 1. Seven cases presented with vaginal hemorrhage and one with loss of fetal movements, after which the fetal demise was diagnosed by ultrasound. In five women, fetal demise was diagnosed at a scheduled ultrasound examination and in the remaining one when she presented during labor. Histology of the placenta was available for 12 women (Table 2). The most prevalent histological abnormality was placental abruption which was seen in 6 miscarriages, occasionally on its own, or in combination with maternal vascular malperfusion or acute chorioamnionitis (inflammatory abruption). The second most frequent finding was maternal vascular malperfusion, alone or in combination with other pathology. The third most frequent pathology was acute chorioamnionitis, in combination or alone. Other abnormalities were more rarely seen (Table 2).
Table 1.
Gestational age at delivery/demise.
| Gestational age | Number | % |
|---|---|---|
| <12 weeks | 19 | 28.8 |
| 120/7 – 136/7 | 5 | 7.6 |
| 140/7 – 156/7 | 5 | 7.6 |
| 160/7 – 176/7 | 13 | 19.7 |
| 180/7 – 196/7 | 16 | 24.2 |
| 200/7 – 216/7 | 8 | 12.1 |
| Total | 66 | 100 |
Table 2.
Placental histology and cause of death in late second trimester miscarriages (demise before 22 weeks’ gestation).
| Placental Histology | Autopsy | Maternal age | Gravidy | Parity | GA at delivery | GA according to FL | Birth weight (g) | Placental weight (g) | Histological Findings |
|---|---|---|---|---|---|---|---|---|---|
| Yes | Yes | 17 | 1 | 0 | 21w2d | 21w6d | 408 | 154 | Acute chorioamnionitis, abruption |
| Yes | Yes | 26 | 2 | 1 | 22w0d | 16w3d | 60 | 42 | Maternal vascular malperfusion |
| Yes | Yes | 17 | 1 | 0 | 22w6d | 16w6d | 160 | 140 | Chronic villitis with OFV, CMV |
| Yes | Yes | 20 | 2 | 1 | 24w4d | 17w5d | 148 | 70 | Early amnion rupture with anhydramnios, cord obstruction |
| Yes | Yes | 25 | 2 | 1 | 21w3d | 14w3d | 50 | 41 | Maternal vascular malperfusion |
| No | Yes | 36 | 1 | 0 | 21w1d | 23w1d | 450 | N/A | N/A |
| Yes | Yes | 32 | 2 | 1 | 21w6d | 22w6d | 410 | 104 | Acute chorioamnionitis, abruption |
| Yes | Yes | 17 | 1 | 0 | 20w6d | 20w2d | 331* | 82 | Acute chorioamnionitis |
| Yes | Yes | 23 | 2 | 0 | 20w6d | 14w4d | 103* | 49 | Termination of pregnancy for Turner syndrome |
| Yes | No | 32 | 2 | 3 | 19w3d | N/A | N/A | 282 | Acute chorioamnionitis, abruption, maternal vascular malperfusion |
| Yes | Yes | 31 | 4 | 3 | 21w2d | 22w1d | 336* | 132 | Abruption |
| Yes | Yes | 29 | 2 | 1 | 22w3d | 15w2d | 62* | 31 | Maternal vascular malperfusion, abruption |
| No | Yes | 22 | 2 | 1 | 21w1d | 16w3d | 71* | N/A | N/A |
| Yes | Yes | 23 | 3 | 0 | 21w4d | 22w4d | 400 | 131 | Maternal vascular malperfusion, abruption |
OFV = Obliterative fetal vasculopathy.
No birth weight available, only autopsy weight; CMV = cytomegalo virus; GA = gestational age; FL = foot length; N/A = not available.
Discussion
The survival probability of early human conceptions is very low. At least 73% of natural single conceptions have no real chance of surviving six weeks of gestation [16]. After very early gestation, survival rates improve rapidly, as 90% of the remainder will survive to term. The rate of mid-trimester or late miscarriage (to use the terminology of the authors) is usually given as between 0.72% to 2.9% of pregnancies [5,6,17]. This is very similar to the 1% to 2.9 % in different methods of artificial reproduction [18]. After 16 weeks the rate is reduced to around 1% [8,7]. In our study a rate of 0.96% was found, but it does not truly reflect the rate from a gestational age of 12 weeks onwards, as not all pregnancies were followed up from a gestational age of 12 weeks since the mean gestational age at booking for the study was 20 weeks 6 days and 30% of participants booked after 24 weeks (unpublished data, Safe Passage Study). The real prevalence rate of second trimester miscarriage in our population is therefore probably much higher.
Several researchers have examined possible underlying causes of second trimester miscarriage. Anderson et al. [19] examined the serum of 1676 pregnant women, donated in early pregnancy, before a gestation of 22 weeks, for soluble FMS-like kinase and placental growth factor. Spontaneous abortion occurred in 59 women (3.5%). Increasing continuous concentrations of both FMS-like kinase and placental growth factor were significantly associated with a decreased hazard ratio for spontaneous abortion. Ball et al. [20] examined whether late miscarriage was associated with reduced trophoblast invasion and spiral artery transformation. Compared to normal pregnancy, myometrial spiral arteries in late miscarriage showed reduced endovascular and intramural trophoblast. In an examination on the association of mutations in coagulation factors in women with unexplained fetal loss, both the factor V and the prothrombin mutations were associated with an approximate tripling of the risk of late miscarriages [21]. The authors postulated that placental thrombosis may be the underlying pathogenic mechanism. In another study, of 351 of miscarriages between 12 and 24 weeks of gestation, 51% were classified as idiopathic [6]. Other causes were antiphospholipid syndrome (33%), cervical weakness (7%), uterine anomaly (4%), bacterial vaginosis (3%) and hypothyroidism (1%) as causes of mid trimester pregnancy losses. However, the authors did not refer to abruption or maternal vascular malperfusion.
Heller et al. [22] reviewed medical records and pathologic material of spontaneous second trimester pregnancy losses. From the 67 placentas available, 38 (56.7%) showed histologic acute chorioamnionitis. Another study tried to establish the cause of death in spontaneous abortion in 422 consecutive second trimester abortions [23]. Different degrees of maceration were found which probably indicated different mechanisms. The largest group was the one without maceration in which an explanation was found in 85%. Ascending infection (acute chorioamnionitis) was found although membranes were not ruptured. Another study reported on placental histology in 118 women with second trimester abortions, stillbirths and perinatal deaths, and the highest infection rate (58.2%) was found in second trimester abortions where infection was also the most frequent cause of death (45.5%) [24].
Although gestational age was determined by ultrasound between 81 and 173 weeks, there were major differences between the ultrasound derived gestational age and gestational age according to foot length or birthweight. This probably indicates a severe degree of fetal growth restriction at the time of the first ultrasound examination. These findings also demonstrate how difficult it may be to determine the exact gestational age in growth restricted fetuses when ultrasound examinations are done in the second trimester. From the abovementioned studies, it is clear that placental pathology plays a major role in the etiology of spontaneous second trimester miscarriages. This is also the main finding in our study. Interestingly, placental disease was also the leading cause of antepartum stillbirths (26%) in the 633 stillbirths examined by the Stillbirth Network. However, they did not differentiate between the different placental conditions such as maternal vascular malperfusion or acute chorioamnionitis [25]. In the Dutch Cohort of 750 antepartum stillbirths, 65% were due to placental disease but intrauterine infection was found in only 1.8% of stillbirths [26].
The etiology of second trimester miscarriages in our population is therefore very similar to that of fetal death. More attention should therefore be given to national statistics on second trimester miscarriages and placental histology should be requested in all cases of spontaneous mid-trimester miscarriage as this may assist in establishing the etiology, thereby possibly preventing midtrimester miscarriages and even more significantly perhaps stillbirths and preterm delivery and adverse pregnancy outcome.
Acknowledgement
The study was funded by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute on Deafness and Other Communication Disorders: U01 HD055154, U01 HD045935, U01 HD055155, U01 HD045991, and U01 AA016501.
Footnotes
Declaration of Competing Interest
The authors have no conflict of interest to report
References
- [1].MacDorman MF, Gregory ECW. Fetal and perinatal mortality: United States. Natl Vital Stat Rep 2013;2015(64):1–24. [PubMed] [Google Scholar]
- [2].Gardosi J, Giddings S, Clifford S, Wood L, Francis A. Association between reduced stillbirth rates in England and regional uptake of accreditation training in customized fetal growth assessment. BMJ Open 2013;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aminu M, Unkels R, Mdegela M, Utz B, Adaji S, van den Broek N. Causes of and factors associated with stillbirth in low- and middle-income countries: a systematic literature review. BJOG 2014;121:141–53. [DOI] [PubMed] [Google Scholar]
- [4].Boklage CE. Survival probability of human conceptions from fertilization to term. Int J Fertil 1990;35:75. [PubMed] [Google Scholar]
- [5].Greenwood DC, Alwan N, Boylan S, Cade JE, Charvill J, Chipps KC, et al. Caffeine intake during pregnancy, late miscarriage and stillbirth. Eur J Epidemiol 2010;25:275–80. [DOI] [PubMed] [Google Scholar]
- [6].McNamee KM, Dawood F, Farquharson RG. Mid-trimester pregnancy loss. Obstet Gynecol Clin North Am 2014;41:87–102. [DOI] [PubMed] [Google Scholar]
- [7].Simpson JL. Incidence and timing of pregnancy losses: relevance to evaluating safety of early prenatal diagnosis. Am J Med Genet 1990;35:165–73. [DOI] [PubMed] [Google Scholar]
- [8].Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016;387:587–603. [DOI] [PubMed] [Google Scholar]
- [9].Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2016;(4):e98–e108. [DOI] [PubMed] [Google Scholar]
- [10].Dukes KA, Burd L, Elliott AJ, Fifer WP, Folkerth RD, Hankins GDV, et al. The safe passage study: design, methods, recruitment, and follow-up approach. Paediatr Perinat Epidemiol 2014;28:455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(18)30541-2/fulltext.
- [12].Odendaal HJ, Gebhardt GS, Teron GB. Stillbirth rates in singleton pregnancies in a stable population at Karl Bremer and Tygerberg hospitals over 50 years. S Afr J Obs Gyn 2013;19:67–70. [Google Scholar]
- [13].Boyd TK, Wright CA, Odendaal H, Elliott AJ, Sens MA, Dunn Folkerth R, et al. The stillbirth classification system for the safe passage study: incorporating mechanism, etiology, and recurrence. Pediatr Dev Pathol 2016. (in press). [DOI] [PubMed] [Google Scholar]
- [14]. http://www.ppip.co.za/saving-babies/.
- [15].Geldenhuys E, Coldrey J, Wright C, Schubert P, Nel D, Groenewald C, et al. The accuracy of fetal foot length as measured at autopsy to reflect gestational age at delivery. Int J Gynaecol Obstet 2017(April 8), doi: 10.1002/ijgo.12177 [Epub ahead of print] PMID: 28391625. [DOI] [Google Scholar]
- [16].Boklage CE. Survival probability of human conceptions from fertilization to term. Int J Fertil 1990;35:75–94. [PubMed] [Google Scholar]
- [17].Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol 2000;14:839–54. [DOI] [PubMed] [Google Scholar]
- [18].Buckett WM, Chian R-, Dean NL, Sylvestre C, Holzer HEG, Tan SL. Pregnancy loss in pregnancies conceived after in vitro oocyte maturation, conventional in vitro fertilization, and intracytoplasmic sperm injection. Fertil Steril 2008;90:546–50. [DOI] [PubMed] [Google Scholar]
- [19].Andersen LB, Dechend R, Karumanchi SA, Nielsen J, Joergensen JS, Jensen TK, et al. Early pregnancy angiogenic markers and spontaneous abortion: an Odense Child Cohort study. Am J Obstet Gynecol 2016. ajog.2016.06.07. [DOI] [PubMed] [Google Scholar]
- [20].Ball E, Bulmer J, Ayis S, Lyall F, Robson S. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J Pathol 2006;208:535–42. [DOI] [PubMed] [Google Scholar]
- [21].Martinelli I, Taioli E, Cetin I, Marinoni A, Gerosa S, Villa MV, et al. Mutations in coagulation factors in women with unexplained late fetal loss. New England J Med Surg Collat Branches Sci 2000;343:1015–8. [DOI] [PubMed] [Google Scholar]
- [22].Heller DS, Moorehouse-Moore C, Skurnick J, Baergen RN. Second-trimester pregnancy loss at an urban hospital. Infect Dis Obstet Gynecol 2003;11:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gaillard DA, Paradis P, Lallemand AV, Vernet VM, Carquin JS, Chippaux CG, et al. Spontaneous abortions during the second trimester of gestation. Arch Pathol Lab Med 1993;117:1022–6. [PubMed] [Google Scholar]
- [24].Rudbeck Roge H, Henriques U. Fetal and perinatal infections. A consecutive study. Pathol Res Pract 1992;188:135–40. [DOI] [PubMed] [Google Scholar]
- [25].The Stillbirth Collaborative Research Network Writing Group. Association between stillbirth and risk factors known at pregnancy confirmation. JAMA 2011;306:2469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Korteweg FJ, Erwich JJHM, Holm JP, Ravisé JM, Van Der Meer J, Veeger NJGM, et al. Diverse placental pathologies as the main causes of fetal death. Obstet Gynecol 2009;114:809–17. [DOI] [PubMed] [Google Scholar]