Abstract
Out-of-hospital sudden unexpected deaths are non-accidental deaths that occur without obvious underlying causes and may account for 10% of natural deaths before age 65. Short-term exposure to ambient air pollution is associated with all-cause (non-accidental) and cause-specific (e.g., cardiovascular) mortality, and with immediate exposures often yielding the highest magnitude risk estimates. Few studies have focused on short-term exposure to air pollution and sudden unexpected deaths. Using the University of North Carolina Sudden Unexpected Death in North Carolina population, we examine associations between short-term criteria air pollutant exposures with sudden unexpected deaths using a time-stratified case-crossover design, with data on criteria air pollutants from the Environmental Protection Agency’s Air Quality System. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using conditional logistic regression with air pollutant exposures scaled to roughly inter-quartile ranges; models were adjusted for average temperature and relative humidity on event day and preceding 3 days. Potential for confounding by co-pollutants were examined in two pollutant models. ORs for PM2.5 at lag day 1 were elevated (adjusted OR for 5 μg/m3 increase: 1.17 (0.98, 1.40)), and were robust to co-pollutant adjustment. Elevated odds were observed for SO2 at lag day 0, and reduced odds for O3 at lag day 0; however, these associations were somewhat attenuated towards the null (SO2) or were not robust (O3) to co-pollutant adjustment. This analysis in a racially and socioeconomically diverse cohort, with a more inclusive definition of sudden unexpected death than is typically employed offers evidence that PM2.5 may be a clinically relevant trigger of sudden unexpected deaths in susceptible individuals.
Keywords: sudden unexpected death, air pollution, particulate matter, environmental epidemiology
1. Introduction
The adverse health effects of air pollution are notable for cardiovascular and respiratory morbidity and mortality1,2. Specifically, an extensive body of evidence exists showing that exposure to ambient particulate matter (PM) causes mortality, and this evidence remains robust when studies are conducted in different countries, over the range of PM concentrations evaluated, and for various statistical and analytic methods used to evaluate this relationship2–12. Other criteria pollutants (ozone, nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO)) also exhibit positive associations with mortality across a variety of conditions8,13–18. While the associations reported across this large body of evidence are well established and considered causal6,14,17, cause-specific subtypes of deaths may have different etiologies and relationships with ambient air pollution19,20.
Out-of-hospital sudden unexpected deaths (SUD) are non-accidental deaths that occur without obvious underlying causes, and may account for 10% of natural deaths under age 6521. Definitions of SUD vary across studies, countries, and usage22 leading to uncertainty in estimates of SUD incidence. Many definitions, including the World Health Organization’s, restrict identification of events based on timing of death (e.g., within 1 hour of witnessed or 24 hours of unwitnessed events) or cardiac causes23–25. While it is believed that sudden cardiac deaths make up the majority of SUD26,27, this may be due, in part, to the definitional restrictions which often exclude unwitnessed deaths or specifically focus on sudden cardiac deaths. However, a broader definition of SUD without timing restriction, may capture more deaths/events which share similar risk factors and be more appropriate to assess population based risk.
The definitional requirements of timing and witnessing of death may lead to under-reporting of SUD in economic and racially diverse populations21. Because of this and the fact that previous studies have largely been conducted among affluent or middle-class white populations26–28, generalizability from these studies may be limited. The University of North Carolina at Chapel Hill’s Sudden Unexpected Death in North Carolina (SUDDEN) project29 was designed to address these concerns by collecting data on SUD occurring in a racially and socioeconomically diverse population, with a broader definition of SUD than previously employed.
Short-term (i.e., from hours up to 4 weeks) exposure to ambient air pollution is associated with all-cause (non-accidental) and cause-specific (e.g., cardiovascular) mortality, and with immediate exposures often yielding the highest magnitude risk estimates8,30–33. Fewer studies have focused on short-term exposure to air pollution and SUD as opposed to specifically sudden cardiac arrest. Additionally, methods to identify sudden cardiac death are more likely to exclude low income and minority populations. Using the UNC SUDDEN pilot population, and therefore a more inclusive definition of SUD than has been previously employed, we examine associations between short-term “criteria pollutant” concentrations with SUD using a time-stratified case-crossover design.
2. Methods
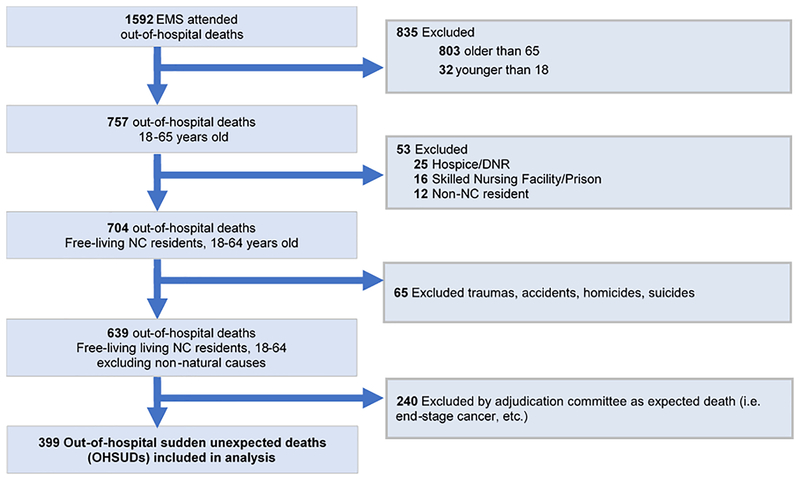
In order to better understand the causes and contributors to SUD, UNC at Chapel Hill researchers developed a project to collect detailed information on SUD cases occurring in Wake County, North Carolina (Figure 1). For this case registry, deaths could be either witnessed or unwitnessed with no time constraint, and SUD is defined as sudden pulseless condition in the absence of terminal disease or overdose at the time of death. All emergency management systems (EMS) attended out-of-hospital deaths from March 1, 2013 to February 28, 2015 (n = 1,592) were reported to the study coordinator, using an electronic query of the Emergency Medical Services (EMS) patient care reporting software (ESO Solutions V 4.8, Austin, Texas). Two research assistants then used EMS scene, medical examiner, toxicology, and autopsy reports when available to sequentially restrict the population to adults aged 18 to 64 (n = 757), “community dwelling” NC residents (i.e., not in institutions such as hospice, prison, etc.) (n = 704), and non-violent deaths (n = 639). Medical records within the last 5 years were systematically obtained for all cases, and cases were then adjudicated to ascertain sudden unexpected deaths using all available information by a majority decision of 3 independent cardiologists using medical and post-mortem records (also used for individual characteristics), leaving a final analytic population of n = 399. The population and selection procedures are described in further detail in Nanavati, et al.28, Mirzaei, et al.34, Patel, et al.35.
Figure 1:
Flow chart of SUDDEN project participant inclusion
To study the temporal effects of air pollution on SUD, we employed a case-crossover design using a time-stratified referent selection approach. In this design, individuals serve as their own controls and time-invariant variables are controlled for by design36–39. Time-stratified referent selection is done so that long (e.g., yearly and seasonal) and short-term (e.g., day of week) trends are accounted for37. In this analysis, referent days were selected within the same month and calendar year of the SUD event, and on all the same days of week. For example, if an individual experienced SUD on March 14, 2014 (event day), a Friday, then all other Fridays within March 2014 (7th, 21st, and 28th) become referent days. Single day lags of 0 to 3 days, and the average of lag 0–1 day, before event and referent periods were chosen as exposure windows. The lag days selected for inclusion are based off the extensive epidemiologic literature reporting evidence of immediate effects of air pollution on mortality (i.e., within the first few days after exposure, 0 to 3 days)6,14,17.
We acquired hourly measurements of PM2.5, temperature, and relative humidity for 2013 – 2015 from the Wake County central site monitor though the EPA’s Air Quality System (AQS) data mart40; and daily 8-hr maximums for ozone (O3) and carbon monoxide (CO), and daily 1-hr maximums for nitrogen dioxide (NO2) and sulfur dioxide (SO2) were obtained from the EPA’s Air Data site41. For PM2.5, temperature, and relative humidity, we calculated daily values by averaging over 24-hour periods (midnight to midnight). Pollutant and weather data were then linked to health data via date. Note that NO2 data were only available from 2014 onward, therefore analyses including NO2 will be restricted to those dates.
Odds ratios (OR) and 95% confidence intervals (CI) were estimated using conditional logistic regression, conditioning on participant ID42–44. All exposures were examined as continuous variables, with a roughly IQR across lag days increase for air pollutants used as exposure contrast: single day lags PM2.5 = 5 μg/m3, CO = 0.02 ppm, NO2 = 15 ppb, SO2 = 1 ppb, O3 = 0.016 ppm (i.e., 16 ppb); for lag 0–1: PM2.5 = 4 μg/m3, CO = 0.15 ppm, NO2 = 12 ppb, SO2 = 1 ppb, O3 = 0.016 ppm. Models were performed individually for exposure to air pollutant assigned to the day of the SUD event, as well as the first, second, or third day before the event (lag days 1, 2, 3), and the combined 0–1 day lag. When examining air pollutant concentrations as the main exposures, we adjusted for average temperature and average relative humidity for day of death/referent day (lag 0) and preceding lag days (lags 1 to 3) (natural cubic splines), this model was selected a priori based on previous work45. We also estimated effects using co-pollutant models that included two criteria air pollutants, to examine robustness of single-pollutant ORs.
Sensitivity analyses were conducted using an unconstrained distributed lag model, all lags entered into the model simultaneously, to examine the cumulative versus single lag effects. In addition, we also stratified deaths by flu season (October to May) and non-flu season (June to September) to examine the potential influence of seasonal variation.
This research was approved by the University of North Carolina at Chapel Hill’s Office of Human Research Ethics as exempt from review. The EPA’s Human Subjects Research Officer similarly reviewed this work and declared it non-human subjects research as all individuals were deceased at time of data collection.
3. Results
3.1. Variable distribution and descriptive statistics
Daily ambient air pollutant concentrations were generally low for gaseous pollutants over the two-year study period compared to average US levels46, while PM2.5 levels were more typical of nation-wide average concentrations (Table 1). Daily co-pollutant correlations were generally low to moderate, with 8 hour maximum CO and 1 hour maximum NO2 being the highest (Pearson correlation coefficient: 0.66) (Table 2). Partial correlations for air pollutants, adjusted for weather variables, were similar to unadjusted correlations (Supplemental Table S.1); with the exception of SO2 which had substantially reduced correlations with both CO and NO2. For assigned exposure concentrations, adjacent lag days had moderate correlations, which were attenuated with increasing time for PM2.5, NO2, SO2, CO, O3, and relative humidity, while adjacent days for temperature were highly correlated.
Table 1:
Descriptive statistics for daily air pollutant concentrations and weather variables for Wake County NC from March 1 2013 to February 28 2015
| Exposure | Time averaging | Mean | STD | Min | 25th | Median | 75th | Max | IQR |
|---|---|---|---|---|---|---|---|---|---|
| PM2.5 (ug/m3) | Daily average | 10.93 | 4.33 | 1.82 | 8.03 | 10.25 | 13.22 | 31.14 | 5.20 |
| CO (ppm) | 8 hr maximum | 0.36 | 0.18 | 0.20 | 0.20 | 0.30 | 0.40 | 1.30 | 0.20 |
| NO2 (ppb) | 1 hr maximum | 15.80 | 9.74 | 1.10 | 7.90 | 13.70 | 23.30 | 42.60 | 15.40 |
| SO2 (ppb) | 1 hr maximum | 1.08 | 1.30 | −0.10 | 0.20 | 0.65 | 1.40 | 9.50 | 1.20 |
| O3 (ppb) | 8 hr maximum | 37.51 | 11.94 | 0 | 29 | 37 | 46 | 71 | 17 |
| Temperature (°F) | Daily average | 59.38 | 16.53 | 14.54 | 44.92 | 62.33 | 74.29 | 85.67 | 29.38 |
| Relative humidity (%) | Daily average | 65.56 | 15.57 | 3.08 | 55.46 | 66.96 | 77.04 | 96.00 | 21.58 |
CO: carbon monoxide
IQR: inter-quartile range
NO2: nitrogen dioxide
O3: ozone
PM2.5: particulate matter under 2 μg/m3 in aerodynamic diameter
SO2: sulfur dioxide
STD: standard deviation
Table 2:
Correlations between daily air pollutant and weather variables for Wake County NC from March 1 2013 to February 28 2015
| CO | NO2 | O3 | PM25 | SO2 | Temperature | Relative humidity | |
|---|---|---|---|---|---|---|---|
| CO | 1 | 0.66 | −0.10 | 0.45 | 0.40 | −0.33 | −0.10 |
| NO2 | 0.66 | 1 | −0.00 | 0.36 | 0.40 | −0.44 | −0.40 |
| O3 | −0.10 | −0.00 | 1 | 0.22 | −0.06 | 0.37 | −0.42 |
| PM25 | 0.44 | 0.36 | 0.22 | 1 | 0.18 | −0.11 | −0.11 |
| SO2 | 0.40 | 0.40 | −0.06 | 0.18 | 1 | −0.53 | −0.37 |
| Temperature | −0.33 | −0.44 | 0.37 | −0.11 | −0.53 | 1 | 0.27 |
| Relative humidity | −0.10 | −0.40 | −0.42 | −0.11 | −0.37 | 0.27 | 1 |
CO: carbon monoxide
NO2: nitrogen dioxide
O3: ozone
PM2.5: particulate matter under 2 μg/m3 in aerodynamic diameter
SO2: sulfur dioxide
The study population is approximately two-thirds male, and two-thirds white, with the majority of the deaths occurring after age 45 years (>80%) and a mean age of 54 years (Table 3). The study population has a higher proportion of black individuals compared to Wake County in general (35.1% in study population v. 20.7% Wake County47). Hypertension, hypercholesterolemia, diabetes, coronary artery disease, and pulmonary disease were all highly prevalent in the study population35.
Table 3:
Demographic characteristics of the SUDDEN project population
| Characteristic | N | % |
|---|---|---|
| Total | 399 | 100 |
| Sex | ||
| Female | 126 | 31.58 |
| Male | 273 | 68.42 |
| Race | ||
| White | 249 | 62.41 |
| Black | 140 | 35.09 |
| Asian or other | 10 | 2.51 |
| Age group | 5 | 1.25 |
| <30 | 12 | 3.01 |
| 30 – 39 | 31 | 7.77 |
| 40 – 49 | 69 | 17.29 |
| 50 – 59 | 181 | 45.36 |
| 60 – 64 | 101 | 25.31 |
| Coronary artery disease | ||
| Yes | 94 | 23.56 |
| No | 277 | 69.42 |
| Missing | 28 | 7.02 |
| History of hypertension | ||
| Yes | 224 | 56.14 |
| No | 147 | 36.84 |
| Missing | 28 | 7.02 |
| Diabetes mellitus | ||
| Yes | 109 | 27.32 |
| No | 262 | 65.66 |
| Missing | 28 | 7.02 |
| Dyslipidemia | ||
| Yes | 146 | 36.59 |
| No | 225 | 56.39 |
| Missing | 28 | 7.02 |
| Heart Failure | ||
| Yes | 51 | 12.78 |
| No | 320 | 80.2 |
| Missing | 28 | 7.02 |
| Smoker | ||
| Yes | 173 | 43.36 |
| No | 114 | 28.57 |
| Missing or unknown | 112 | 28.07 |
| History of stroke | ||
| Yes | 26 | 6.52 |
| No | 345 | 86.47 |
| Missing | 28 | 7.02 |
| Chronic kidney disease | ||
| Yes | 44 | 11.03 |
| No | 327 | 81.95 |
| Missing | 28 | 7.02 |
| Chronic respiratory disorder | ||
| Yes | 123 | 30.83 |
| No | 248 | 62.16 |
| Missing | 28 | 7.02 |
3.2. Results for single air pollutant models
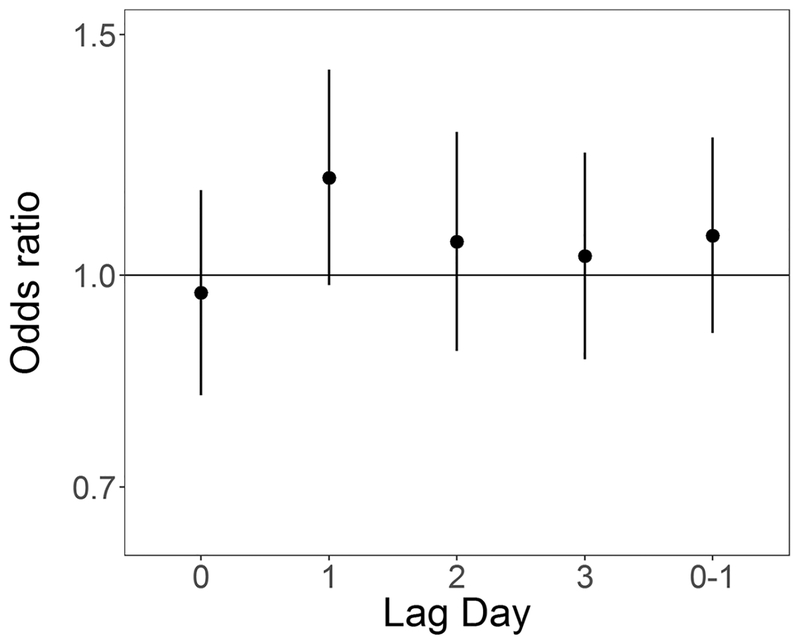
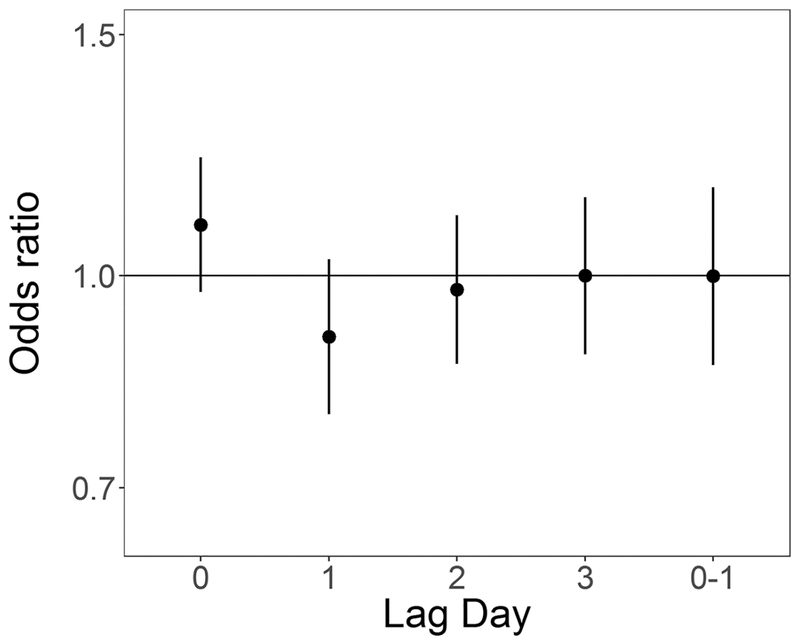
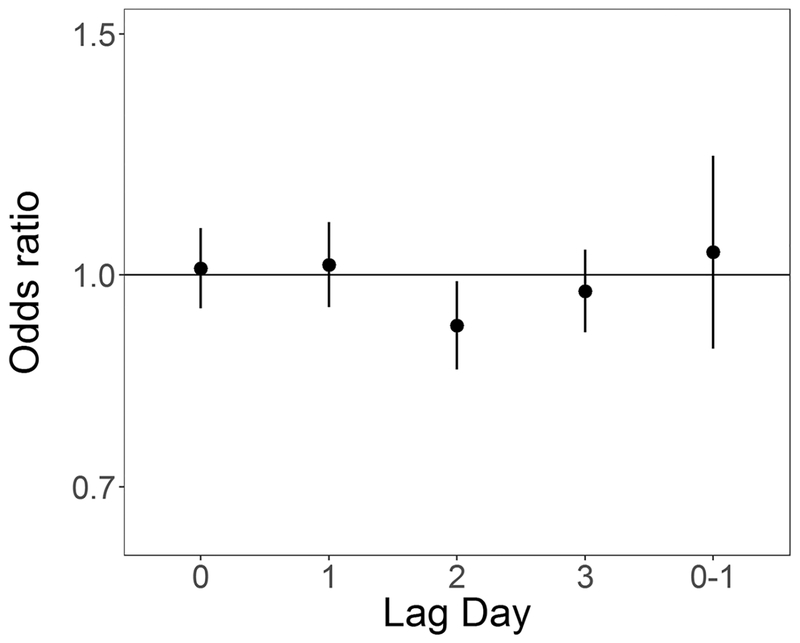
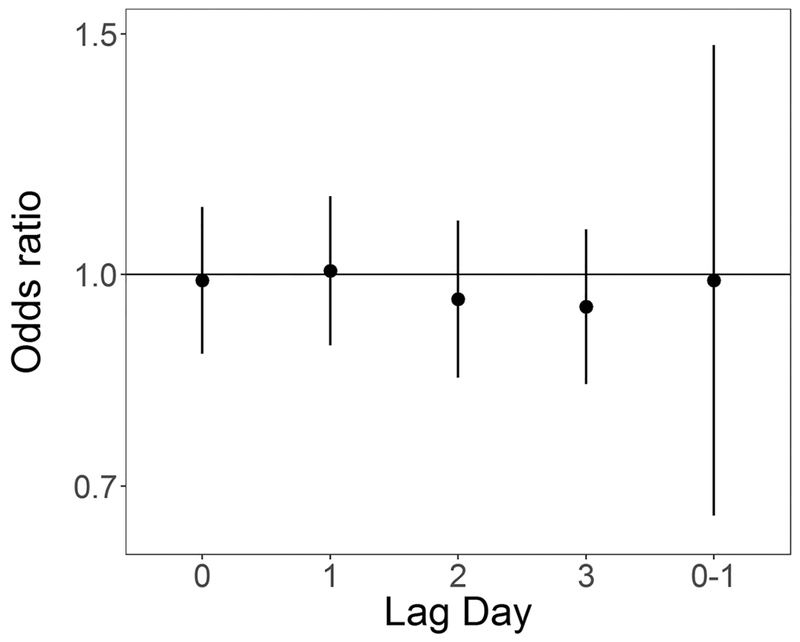
In unadjusted analyses ORs were elevated from the null for ambient 24-hour average PM2.5 at lag days 1 and 2 (OR for 5 μg/m3 increase: 1.12 (0.97, 1.30) and 1.11 (0.96, 1.28) respectively), and null for lag days 0 and 3 and lag 0–1 (Table 4). In models adjusted for temperature and relative humidity ORs for PM2.5 at lag day 1 remained elevated (OR for 5 μg/m3 increase: 1.17 (0.98, 1.40)) (Table 4, Figure 2). In both unadjusted and adjusted models, effect estimates for SO2 exposure were elevated at lag day 0 (adjusted OR for 1 ppb increase: 1.09 (0.97, 1.22)) and negative at lag day 1 (adjusted OR for 1 ppb increase: 0.90 (0.79, 1.03)) (Table 4, Figure 3). ORs for CO and NO2 were generally null in both unadjusted and adjusted models (Table 4, Figures 4 and 5 respectively). For O3, effect estimates from unadjusted models were null, however in adjusted models the lag day 0 effect estimate moved away from the null in an negative direction (OR for 0.016 ppm increase: 0.90 (0.74, 1.09)) (Table 4, Figure 6).
Table 4:
Odds ratios and 95% confidence intervals for air pollutant and out of hospital sudden unexpected death, unadjusted and adjusted for temperature and relative humidity
| Exposure | Lag Day | Unadjusted | Adjusted for temperature and relative humidity |
|---|---|---|---|
| Temperature | 0 | 1.00 (0.98 ,1.01) | |
| Temperature | 1 | 0.99 (0.98 ,1.01) | |
| Temperature | 2 | 1.00 (0.99 ,1.02) | |
| Temperature | 3 | 1.00 (0.99 ,1.02) | |
| PM2.5 | 0 | 0.97 (0.84 ,1.12) | 0.97 (0.82 ,1.15) |
| PM2.5 | 1 | 1.12 (0.97 ,1.30) | 1.18 (0.98 ,1.41) |
| PM2.5 | 2 | 1.11 (0.96 ,1.28) | 1.06 (0.88 ,1.27) |
| PM2.5 | 3 | 1.05 (0.91 ,1.20) | 1.03 (0.87 ,1.23) |
| PM2.5 | 0–1 | 1.05 (0.92 ,1.19) | 1.07 (0.91 ,1.26) |
| NO2 | 0 | 0.99 (0.90 ,1.08) | 0.99 (0.88 ,1.12) |
| NO2 | 1 | 0.98 (0.89 ,1.08) | 1.01 (0.89 ,1.14) |
| NO2 | 2 | 0.93 (0.84 ,1.03) | 0.96 (0.84 ,1.10) |
| NO2 | 3 | 0.91 (0.82 ,1.01) | 0.95 (0.83 ,1.08) |
| NO2 | 0–1 | 0.94 (0.71 ,1.25) | 0.99 (0.67 ,1.47) |
| SO2 | 0 | 1.09 (0.99 ,1.20) | 1.09 (0.97 ,1.22) |
| SO2 | 1 | 0.92 (0.82 ,1.02) | 0.90 (0.79 ,1.03) |
| SO2 | 2 | 0.96 (0.87 ,1.07) | 0.98 (0.86 ,1.11) |
| SO2 | 3 | 0.97 (0.86 ,1.08) | 1.00 (0.88 ,1.14) |
| SO2 | 0–1 | 1.01 (0.89 ,1.14) | 1.00 (0.86 ,1.16) |
| O3 | 0 | 0.97 (0.85 ,1.11) | 0.90 (0.74 ,1.09) |
| O3 | 1 | 1.01 (0.88 ,1.15) | 1.03 (0.86 ,1.25) |
| O3 | 2 | 1.06 (0.93 ,1.20) | 1.05 (0.87 ,1.26) |
| O3 | 3 | 1.00 (0.87 ,1.14) | 0.95 (0.79 ,1.13) |
| O3 | 0–1 | 0.98 (0.77 ,1.25) | 0.92 (0.65 ,1.32) |
| CO | 0 | 0.97 (0.85 ,1.11) | 0.90 (0.74 ,1.09) |
| CO | 1 | 1.01 (0.88 ,1.15) | 1.03 (0.86 ,1.25) |
| CO | 2 | 1.06 (0.93 ,1.20) | 1.05 (0.87 ,1.26) |
| CO | 3 | 1.00 (0.87 ,1.14) | 0.95 (0.79 ,1.13) |
| CO | 0–1 | 0.98 (0.77 ,1.25) | 0.92 (0.65 ,1.32) |
CO: carbon monoxide
NO2: nitrogen dioxide
O3: ozone
PM2.5: particulate matter under 2 μg/m3 in aerodynamic diameter
SO2: sulfur dioxide
Figure 2:
Odds ratios and 95% confidence intervals for 5 μg/m3 (4 μg/m3 for lag 0–1) increase in daily average PM2.5 and out-of-hospital sudden unexpected death in Wake County North Carolina March 1, 2013 to February 28, 2015, adjusted for average temperature and relative humidity, across single day lags from 0 to 3 days and multiday lag of 0 to 1 days.
Figure 3:
Odds ratios and 95% confidence intervals for 16 ppb increase in 8-hr maximum ozone and out-of-hospital sudden unexpected death in Wake County North Carolina March 1, 2013 to February 28, 2015, adjusted for average temperature and relative humidity, across single day lags from 0 to 3 days and multiday lag of 0 to 1 days.
Figure 4:
Odds ratios and 95% confidence intervals for 0.2 ppm (0.15 ppm for lag 0–1) increase in 8-hr maximum CO and out-of-hospital sudden unexpected death in Wake County North Carolina March 1, 2013 to February 28, 2015, adjusted for average temperature and relative humidity, across single day lags from 0 to 3 days and multiday lag of 0 to 1 days.
Figure 5:
Odds ratios and 95% confidence intervals for 1 ppm increase in 1-hr maximum SO2 and out-of-hospital sudden unexpected death in Wake County North Carolina March 1, 2013 to February 28, 2015, adjusted for average temperature and relative humidity, across single day lags from 0 to 3 days and multiday lag of 0 to 1 days.
Figure 6:
Odds ratios and 95% confidence intervals for 15 ppm (12 ppm for lag 0–1) increase in 1-hr maximum NO2 and out-of-hospital sudden unexpected death in Wake County North Carolina March 1, 2013 to February 28, 2015, adjusted for average temperature and relative humidity, across single day lags from 0 to 3 days and multiday lag of 0 to 1 days.
3.3. Results for co-pollutant models
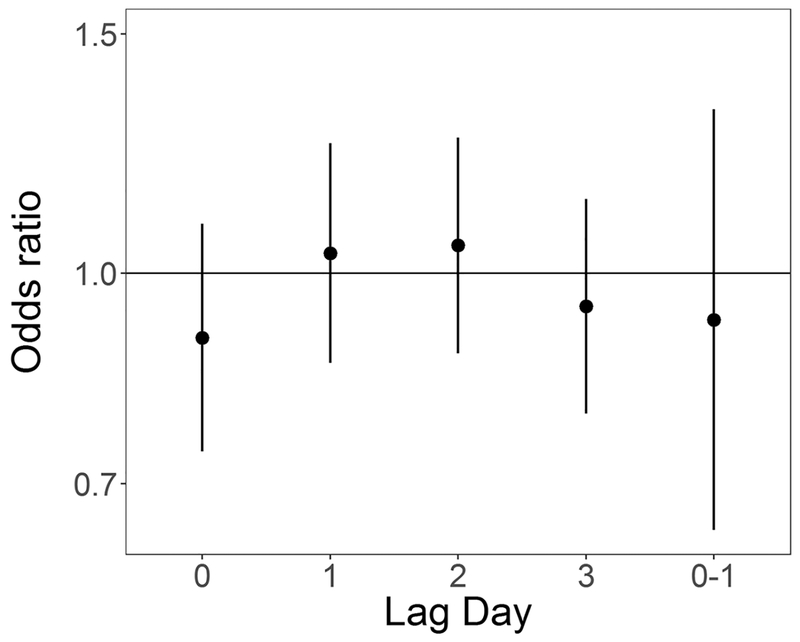
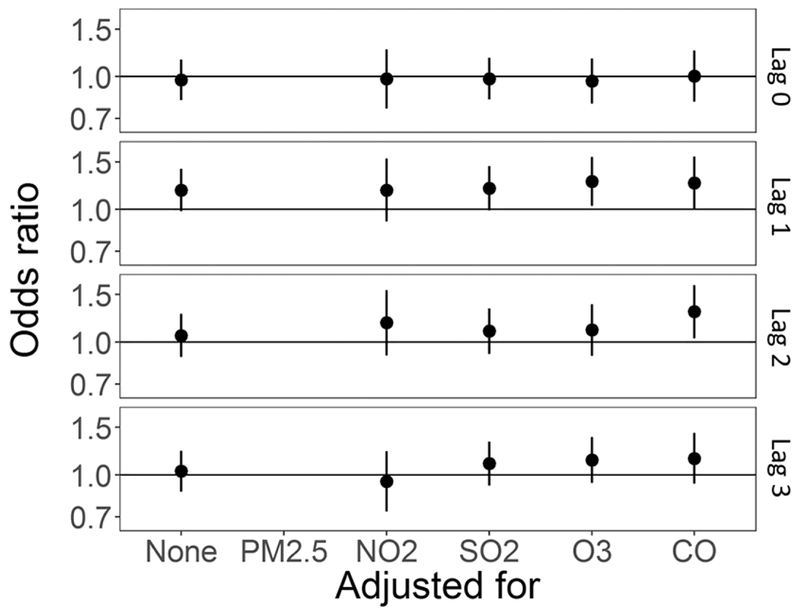
In co-pollutant models, also adjusting for temperature and relative humidity, the effect estimate for exposure to PM2.5 at lag day 1 was robust to inclusion of any of the other air pollutants (Table 5, Figure 7). Interestingly, the OR for lag day 2 does move away from the null with co-pollutant adjustment (Table 5, Figure 7). The effect estimate for SO2 lag day 0 was somewhat attenuated towards the null with adjustment for same-day NO2 and CO (Table 5, Supplemental Figure S.1). The effect estimate for O3 at lag day 0 was highly unstable with co-pollutant adjustment, moving substantially toward the null with PM2.5 and CO adjustment, no movement with SO2 adjustment, and crossing the null and becoming elevated with NO2 adjustment (Table 5, Supplemental Figure S.2). Associations between NO2 and CO, and SUD remained null (Supplemental Figure S.3 and S.4, respectively).
Table 5:
Odds ratios and 95% confidence intervals for air pollutant and out of hospital sudden unexpected death, co-pollutant adjusted models
| Lag Day | Adjusted* for | PM2.5 | NO2 | SO2 | O3 | CO |
|---|---|---|---|---|---|---|
| 0 | None | 0.97 (0.82 ,1.15) | 0.99 (0.88 ,1.12) | 1.09 (0.97 ,1.22) | 0.90 (0.74 ,1.09) | 1.01 (0.95 ,1.08) |
| PM2.5 | 0.98 (0.86 ,1.13) | 1.08 (0.97 ,1.21) | 0.97 (0.79 ,1.21) | 1.01 (0.94 ,1.09) | ||
| NO2 | 0.98 (0.76 ,1.26) | 1.04 (0.89 ,1.21) | 1.21 (0.85 ,1.72) | 1.06 (0.94 ,1.20) | ||
| SO2 | 0.98 (0.82 ,1.17) | 0.98 (0.87 ,1.12) | 0.91 (0.74 ,1.11) | 1.01 (0.94 ,1.08) | ||
| O3 | 0.96 (0.80 ,1.17) | 0.97 (0.84 ,1.11) | 1.08 (0.96 ,1.21) | 1.00 (0.93 ,1.07) | ||
| CO | 1.00 (0.81 ,1.25) | 0.95 (0.79 ,1.13) | 1.06 (0.91 ,1.24) | 0.97 (0.77 ,1.22) | ||
| 1 | None | 1.18 (0.98 ,1.41) | 1.01 (0.89 ,1.14) | 0.90 (0.79 ,1.03) | 1.03 (0.86 ,1.25) | 1.02 (0.95 ,1.09) |
| PM2.5 | 0.99 (0.87 ,1.13) | 0.89 (0.78 ,1.02) | 0.97 (0.79 ,1.19) | 0.99 (0.91 ,1.07) | ||
| NO2 | 1.18 (0.90 ,1.54) | 0.92 (0.78 ,1.10) | 1.12 (0.81 ,1.56) | 1.02 (0.90 ,1.16) | ||
| SO2 | 1.20 (0.99 ,1.45) | 0.99 (0.87 ,1.13) | 1.04 (0.86 ,1.27) | 1.05 (0.97 ,1.13) | ||
| O3 | 1.27 (1.03 ,1.56) | 0.97 (0.83 ,1.12) | 0.91 (0.80 ,1.04) | 1.00 (0.92 ,1.08) | ||
| CO | 1.25 (1.00 ,1.57) | 1.01 (0.85 ,1.21) | 0.87 (0.71 ,1.06) | 1.16 (0.92 ,1.47) | ||
| 2 | None | 1.06 (0.88 ,1.27) | 0.96 (0.84 ,1.10) | 0.98 (0.86 ,1.11) | 1.05 (0.87 ,1.26) | 0.92 (0.85 ,0.99) |
| PM2.5 | 0.93 (0.80 ,1.08) | 0.96 (0.83 ,1.10) | 1.03 (0.84 ,1.27) | 0.89 (0.81 ,0.96) | ||
| NO2 | 1.18 (0.89 ,1.56) | 1.08 (0.91 ,1.29) | 0.98 (0.73 ,1.32) | 0.96 (0.84 ,1.10) | ||
| SO2 | 1.10 (0.90 ,1.33) | 0.94 (0.82 ,1.09) | 1.09 (0.89 ,1.32) | 0.91 (0.84 ,0.99) | ||
| O3 | 1.11 (0.89 ,1.38) | 0.97 (0.83 ,1.12) | 0.97 (0.85 ,1.11) | 0.91 (0.84 ,0.98) | ||
| CO | 1.30 (1.04 ,1.63) | 1.01 (0.84 ,1.23) | 1.02 (0.85 ,1.22) | 1.07 (0.85 ,1.34) | ||
| 3 | None | 1.03 (0.87 ,1.23) | 0.95 (0.83 ,1.08) | 1.00 (0.88 ,1.14) | 0.95 (0.79 ,1.13) | 0.97 (0.91 ,1.04) |
| PM2.5 | 0.97 (0.83 ,1.12) | 1.02 (0.88 ,1.17) | 0.92 (0.76 ,1.13) | 0.95 (0.88 ,1.03) | ||
| NO2 | 0.95 (0.73 ,1.22) | 1.00 (0.85 ,1.19) | 1.08 (0.79 ,1.47) | 1.03 (0.92 ,1.16) | ||
| SO2 | 1.10 (0.91 ,1.33) | 0.97 (0.85 ,1.11) | 0.98 (0.81 ,1.19) | 0.96 (0.89 ,1.04) | ||
| O3 | 1.14 (0.93 ,1.38) | 1.00 (0.86 ,1.16) | 1.01 (0.88 ,1.16) | 0.97 (0.90 ,1.04) | ||
| CO | 1.15 (0.93 ,1.43) | 0.91 (0.76 ,1.09) | 1.06 (0.88 ,1.28) | 0.98 (0.78 ,1.23) |
all models are adjusted for temperature and relative humidity
CO: carbon monoxide
NO2: nitrogen dioxide
O3: ozone
PM2.5: particulate matter under 2 μg/m3 in aerodynamic diameter
SO2: sulfur dioxide
Figure 7:
Co-pollutant adjusted odds ratios and 95% confidence intervals for 5 μg/m3 (4 μg/m3 for lag 0–1) increase in daily average PM2.5 and out-of-hospital sudden unexpected death in Wake County North Carolina March 1, 2013 to February 28, 2015, also adjusted for average temperature and relative humidity. Each panel is for a single day lag: 0, 1, 2, and 3 from top to bottom, odds ratio unadjusted for other co-pollutants is furthest to the left, followed by odds ratio adjusted for NO2, SO2, O3, and CO.
3.4. Results for sensitivity analyses
Unconstrained distributed lag models show similar effect estimates to single day lag models, with a cumulative OR of 1.09 (0.84, 1.42) for PM2.5 (Supplemental Table S.2). In analyses stratified by flu (267 deaths) and non-flu (132 deaths) season, there is some suggestion of separation (suggestive of modification) or potential shifting (suggestive of confounding) of effects (Supplemental Table S.2). However, the stratified odds ratios are fairly unstable due to smaller sample size and should be examined with caution. Odds ratios for PM2.5 lag 1 remain robust to different analyses.
4. Discussion
In this study, we examined associations between the U.S. Clean Air Act’s “criteria pollutants” and SUD, using a more inclusive definition of SUD than has been previously employed. We observed increased odds of SUD with increased daily average ambient PM2.5 exposures 1 day before recorded death, and with daily 1-hour maximum SO2 on day of recorded death, and a negative association with daily 8-hour maximum O3 on day of recorded death. The increased odds of death with increased PM2.5 exposures were robust to adjustment by co-pollutants, while ORs for SO2 exposure were slightly attenuated toward the null and were unstable for O3 exposure. Instability of the estimate for O3 exposure may reflect sensitivities to how temperature is treated48, while SO2 concentrations at lag day 0 may actually reflect PM2.5 concentrations at lag day 149,50. It is also possible that exposures occurring on the recorded event day (lag 0) may occur after death, as exact timing of actual death is uncertain in this study population. Concentrations for gaseous pollutants in Wake County, NC were generally low for the period under study; it is possible that these lower concentrations are more likely to be related to either subclinical health effects or potentially be more relevant for long-term exposures.
Previous studies have not examined SUD with the exact definition used here, however, many studies have established associations with mortality in general, especially in older populations3,5,6,9–11,14,17, and several have examined sudden or out-of-hospital cardiac death (SCD/OHCD) or arrest (SCA/OHCA). There are also a few studies that examine out-of-hospital mortality or mortality in populations restricted to ages less than 65 years. While not all studies reported positive associations, in a meta-analysis of 15 studies of short-term air pollution exposure and OHCA, Zhao, et al.18 found positive pooled associations with increased PM10, PM2.5, and ozone, while findings for SO2 and NO2 were inconsistent. Heterogeneity was large for all exposures and there was evidence of publication bias in studies of ozone, adjustment of which attenuated the pooled effect estimated but did not nullify it. For PM2.5, pooled effect estimates were strongest with cumulative exposure on lag days 0 and 118. The studies included in this analysis (and that were earlier reviewed by Teng, et al.51), included individuals over 65, and typically had mean ages in the 60s18. In a time-series study of OHCD in Shanghai from 2006–2011, Dai, et al.4 observed positive associations with same day PM10, PM2.5, SO2, NO2, CO and temperature changes above or below thresholds, but no association between NO2 or ozone levels; no associations were observed with in-hospital cardiac death. Pollutant levels in this area were high, and all the pollutants were highly correlated with one another except for ozone4. In an Italian study of over 5,000 cases of OHCD, Forastiere, et al.7 observed associations with particle number (a proxy of ultrafine PM) at lag days 0, 1, 2, and 0–1 with a lessening of effect estimate magnitude at the lags further from event day. They also observed associations with PM10 exposure on lag days 0 and 1, though PM10 levels were relatively high (mean 52.1 μg/m3) in the study area.
For out-of-hospital mortality, a study of those over 65 in 8 Italian cities reported a 1.80% (95% CI: 0.83, 2.77) change in mortality per 10 μg/m3 increase in PM2.552. However, an earlier study found no evidence of association between PM10 increases and OHCD in those aged 35–64 in the same cities12. In two US based studies, Schwartz et al. examined out of hospital deaths with: total suspended particles in Philadelphia, PA finding increased risk of death in those with the 35–64 year age categories10; and PM10 in 11 US cities finding a percent change in mortality of 0.89 (0.67, 1.10) per 10 μg/m3 increase11.
Outcomes, population ages, area, air pollutants examined, and timing vary across these studies. Outcomes reported are typically categorized within a more restricted period (within 24 hours unwitnessed or 1 hour witnessed) than used for SUD, so we might expect differences in timing between our study and previous work. However, despite these differences, our observed effect estimates are generally congruent with those reported for PM2.5 in previous studies.
The main mechanisms by which short-term air pollutant exposure may lead to mortality in general, and PM in particular, are through perturbations of the cardiovascular and respiratory systems and mainly rely on oxidative stress or inflammatory response6,53–55. Inflammatory responses and oxidative stress can cause local damage in the respiratory system and become systemic, potentially leading to vascular dysfunction or alterations in lung function and response to allergens6,56,57. Local inflammatory response in the lung can alter the balance between pro- and anti-coagulants, which increases potential for thrombosis55. Soluble PM components can also be translocated across the lung membrane to act more directly on other organ systems53. There are also pathways that may lead to activation of the autonomic nervous system, potentially leading to vascular dysfunction and plaque rupture6,53,58. The people experiencing SUD in this population are generally a vulnerable population in terms of health conditions; there is a high prevalence of: diabetes, hypertension, dyslipidemia, and coronary artery disease. These underlying conditions are likely to lead to increased susceptibility to the effects of air pollutant exposure53.
In terms of exposure assessment and the potential for spatial and temporal variability in air pollutant concentrations to lead to exposure measurement error, PM2.5 is relatively stable both spatially and temporally, lending itself to this type of analysis/exposure ascertainment. Conversely, gaseous pollutants like SO2 tend to be more spatially and temporally heterogeneous; SO2 in the environment travels in plumes and concentrations peak over very short time periods. For SO2 those peak concentrations may be a more relevant exposure metric for a health effect, as opposed to the general low background levels captured though the single county monitor. NO2 is highly source dependent, which in this case is traffic, and the single monitor will only capture general background levels. Related to NO2 exposure, in a spatial analysis of the effects on greenspace, major road density was not observed to have an association within this population59, which suggests that NO2 may not be associated with SUD for long-term exposures in this population. There may be some aspect of exposure misclassification both spatial and temporal, acting in the observed associations with SO2 and NO2. One way to address this would be to examine other metrics and indicators of air pollution exposure, such as using a more spatially refined model to estimate exposure concentrations for individuals rather than a single monitor for the study area. However, this may also introduce the possibility of spatially-related confounding. High correlations between temperature lags may affect adjusted results, but air pollution effect estimates adjusted for temperature were similar to the unadjusted ORs.
The UNC SUDDEN study definition of SUD is broader than previously used sudden cardiac death and WHO-defined SUD, which lends itself to both strengths and limitations. A major strength of this pilot study is that the population is racially and economically diverse, much more so than previous cohorts in which the use of time and witnessing restrictions are likely to systematically exclude many individuals21,28,60. Other strengths of the study include: the case-crossover design employed, such that confounding by time-invariant and long-term trending factors are controlled for by the design and residual or unmeasured confounding is unlikely; and the case ascertainment, which given its thoroughness leaves less opportunity for selection bias to occur.
A corresponding limitation to the broader definition of SUD is that the exact timing of death may be unknown due to the inclusion of unwitnessed deaths, and time of death may be estimated to the time an individual was found. As this analysis is of a temporally based exposure, this likely adds an element of exposure misclassification which may attenuate effect estimates toward the null. While the county used in this study is racially and socioeconomically diverse, it is still only a single county with a moderate population for the US. As such, case numbers of SUD are limited, which may affect our ability to observe effects, particularly with environmental exposures where effect estimates may be expected to be small. In addition, in Wake County, NC, air pollution levels are generally low (with the exception of PM2.5 which is around the national average), leaving the possibility of stronger associations between air pollutants and SUD at higher concentrations.
Another potential limitation is that there may be different underlying etiologies of SUD across age groupings. The cause of a sudden death at age 25 is likely to differ from a cause at age 45. These differences could potentially be explored through cluster analysis, or through stratified or interaction analysis with a population of sufficient size. Relatedly, effects of air pollution exposure may be greater in those aged over 656,61 and observed effect estimates for an adult population below this threshold may be reduced compared to what would be observed in older adults. However, the CDC and CMS have identified the sub-population of adults aged 34–64 years as a high priority group because of increasing mortality, and this study may offer new insight.
The goal of the University of North Carolina at Chapel Hill’s SUDDEN project is to better define the clinical, social, economic, and environmental determinants of SUD so that more effective SUD prevention strategies can be developed. The present study provides further evidence that environmental conditions can modify the risk for SUD. In the present study SUD is associated with short-term exposure to ambient PM2.5 suggesting that exposure to particle pollution can serve as a clinically relevant trigger of SUD in susceptible individuals. In an independent analysis of the same SUD registry, we previously showed that both greenway density and the percentage of forest in Wake County, NC were inversely associated with SUD59. Taken together these findings suggest that modification of environmental conditions might offer a way to decrease the overall risk of SUD in a population.
Several approaches to decrease an individual’s risk for sudden death can be envisioned that include specific guidance on avoidance of exposure to air particle pollution and these are championed by the Center for Medicare and Medicaid Services (CMS) and Centers for Disease Control and Prevention (CDC) joint initiative, Million Hearts® (URL: millionhearts.hhs.gov)62. First, individuals at risk for SUD should optimize healthy behaviors and clinical management of health conditions giving rise to increased susceptibility to air pollution. Individuals can reduce air pollutant exposures by adjusting outdoor activities, or taking more specific actions like using in-home HEPA filtration and wearing an N-95 respirator outdoors when air particle pollution is high. At the population level, efforts should be made to bring airsheds into attainment with the U.S. Environmental Protection Agency’s National Ambient Air Quality Standards. Increasing awareness of the association between air pollution and adverse health outcomes among the general population, and the utility of the Air Quality Index to guide responses to graded levels of air pollution for healthy and at-risk people is anticipated to improve health and decrease adverse clinical events. Approaches for this endeavor would be to connect health care professionals with information about air quality, and consider strategies to better inform the public about risks of air pollution, particularly if they are individuals who have clinical conditions or at risk for cardiopulmonary conditions, e.g., older adults, that may make them particularly vulnerable to the adverse effects of air pollution. Further studies of SUD in economically and socially diverse populations could offer more information about relevant exposure timing, examine potential modifying factors such as underlying disease, and potentially further specify components of air pollution and particulate matter of particular concern.
Supplementary Material
Highlights.
Environmental causes of out-of-hospital sudden unexpected death are largely unknown
Paper presents a case-crossover study of air pollution and sudden unexpected death
Increased odds of death seen with day before death fine particulate matter exposure
Association with air pollution in young, racially, and economically diverse population
Particulate matter may be a clinically relevant trigger of sudden unexpected death
Acknowledgements
The SUDDEN project is funded by private donations, The Heart and Vascular Division of the University of North Carolina at Chapel Hill, and the McAllister Heart Institute, Chapel Hill, North Carolina. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant Award Number 1UL1TR001111.
We would like to thank Jason Sacks and Thomas Luben for their insight and comments.
Abbreviations:
- SUD
Out-of-hospital sudden unexpected deaths
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Publisher's Disclaimer: Disclaimers
Publisher's Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The Wake County EMS Data System supports, maintains, and monitors EMS service delivery, patient care, and disaster preparedness for the Wake County, NC community at large. This manuscript has been reviewed by Wake County EMS Data System investigators for scientific content and consistency of data interpretation with previous Wake County EMS Data System publications
The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, US EPA, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
Computational code may be requested by emailing the corresponding author at rappazzo.kristen@epa.gov. Subject data may be requested through the UNC SUDDEN project page at https://www.med.unc.edu/medicine/cardiology/sudden/contact-us/data-requests. Air pollutant concentrations are available through the AQS Datamart at https://aqs.epa.gov/api.
References
- 1.Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environmental health : a global access science source 2013;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pope CA, Dockery DW. Health effects of fine particulate air pollution: Lines that connect. J Air Waste Manage 2006;56:709–742. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson R, Kang S, Anderson H, Mills I, Walton H. Epidemiological time series studies of PM2. 5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax 2014:thoraxjnl-2013–204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai J, Chen R, Meng X, Yang C, Zhao Z, Kan H. Ambient air pollution, temperature and out-of-hospital coronary deaths in Shanghai, China. Environmental pollution 2015;203:116–121. [DOI] [PubMed] [Google Scholar]
- 5.Dockery DW, Pope CA 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr., Speizer FE. An association between air pollution and mortality in six U.S. cities. The New England journal of medicine 1993;329:1753–1759. [DOI] [PubMed] [Google Scholar]
- 6.U.S. EPA. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2009). In: Agency USEP, ed. Washington, DC, 2009. [Google Scholar]
- 7.Forastiere F, Stafoggia M, Picciotto S, Bellander T, D’ippoliti D, Lanki T, von Klot S, Nyberg F, Paatero P, Peters A. A case-crossover analysis of out-of-hospital coronary deaths and air pollution in Rome, Italy. American journal of respiratory and critical care medicine 2005;172:1549–1555. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal FS, Kuisma M, Lanki T, Hussein T, Boyd J, Halonen JI, Pekkanen J. Association of ozone and particulate air pollution with out-of-hospital cardiac arrest in Helsinki, Finland: evidence for two different etiologies. Journal of Exposure Science and Environmental Epidemiology 2013;23:281. [DOI] [PubMed] [Google Scholar]
- 9.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 US cities, 1987–1994. New England journal of medicine 2000;343:1742–1749. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz J. What are people dying of on high air pollution days? Environmental research 1994;64:26–35. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz J. Assessing confounding, effect modification, and thresholds in the association between ambient particles and daily deaths. Environmental health perspectives 2000;108:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serinelli M, Vigotti MA, Stafoggia M, Berti G, Bisanti L, Mallone S, Pacelli B, Tessari R, Forastiere F. Particulate matter and out-of-hospital coronary deaths in eight Italian cities. Occupational and environmental medicine 2010;67:301–306. [DOI] [PubMed] [Google Scholar]
- 13.Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987–2000. Jama-J Am Med Assoc 2004;292:2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. EPA. Integrated Science Assessment (ISA) of Ozone and Related Photochemical Oxidants (Final Report, Feb 2013). In: Agency USEP, ed. Washington, DC, 2013. [Google Scholar]
- 15.Jhun I, Fann N, Zanobetti A, Hubbell B. Effect modification of ozone-related mortality risks by temperature in 97 US cities. Environ Int 2014;73:128–134. [DOI] [PubMed] [Google Scholar]
- 16.Madrigano J, Jack D, Anderson GB, Bell ML, Kinney PL. Temperature, ozone, and mortality in urban and non-urban counties in the northeastern United States. Environmental health : a global access science source 2015;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. EPA. Integrated Science Assessment (ISA) for Oxides of Nitrogen – Health Criteria (Final Report, 2016). In: Agency USEP, ed. Washington, DC, 2016. [Google Scholar]
- 18.Zhao R, Chen S, Wang W, Huang J, Wang K, Liu L, Wei S. The impact of short-term exposure to air pollutants on the onset of out-of-hospital cardiac arrest: A systematic review and meta-analysis. International journal of cardiology 2017;226:110–117. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Roux AVD, Gassett AJ, Jacobs DR Jr. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. The Lancet 2016;388:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Sampson PD, Sheppard LE, Stein JH, Vedal S, Kaufman JD. Long-Term Exposure to Ambient Ozone and Progression of Subclinical Arterial Disease: The Multi-Ethnic Study of Atherosclerosis and Air Pollution. Environmental health perspectives 2019;127:057001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis ME, Lin F-C, Nanavati P, Mehta N, Mounsey L, Nwosu A, Pursell I, Chung EH, Mounsey JP, Simpson RJ. Estimated incidence and risk factors of sudden unexpected death. Open heart 2016;3:e000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al-Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol 2011;57:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ICD9Data.com. 2013. ICD-9-CM Diagnosis Code 798.2: Death occurring in less than 24 hours from onset of symptoms, not otherwise explained. Available: http://www.icd9data.com/2013/Volume1/780-799/797-799/798/798.2.htm [accessed May 22 2018].
- 24.Organization WH. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO Version for 2016: Chapter IX Diseases of the circulatory system (I00-I99) Available: http://apps.who.int/classifications/icd10/browse/2016/en#/I46.1 [accessed May 22 2018].
- 25.Organization WH. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO Version for 2016: Chapter XVIII Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified (R00-R99) Available: http://apps.who.int/classifications/icd10/browse/2016/en#/R96 [accessed May 22 2018].
- 26.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol 2004;44:1268–1275. [DOI] [PubMed] [Google Scholar]
- 27.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, Van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990s: a population-based study in the Maastricht area on incidence, characteristics and survival. Journal of the American College of Cardiology 1997;30:1500–1505. [DOI] [PubMed] [Google Scholar]
- 28.Nanavati PP, Mounsey JP, Pursell IW, Simpson RJ Jr., Lewis ME, Mehta ND, Williams JG, Bachman MW, Myers JB, Chung EH, Massey M, Lackland D, Evans J, Wagner M. Sudden Unexpected Death in North Carolina (SUDDEN): methodology review and screening results. Open heart 2014;1:e000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.UNC School of Medicine. 2018. SUDDEN Project. Available: https://www.med.unc.edu/medicine/cardiology/sudden [accessed May 22 2018].
- 30.Dennekamp M, Akram M, Abramson MJ, Tonkin A, Sim MR, Fridman M, Erbas B. Outdoor air pollution as a trigger for out-of-hospital cardiac arrests. Epidemiology 2010;21:494–500. [DOI] [PubMed] [Google Scholar]
- 31.Ensor KB, Raun LH, Persse D. A case-crossover analysis of out-of-hospital cardiac arrest and air pollution. Circulation 2013:CIRCULATIONAHA.113.000027. [DOI] [PubMed] [Google Scholar]
- 32.Silverman RA, Ito K, Freese J, Kaufman BJ, De Claro D, Braun J, Prezant DJ. Association of ambient fine particles with out-of-hospital cardiac arrests in New York City. American journal of epidemiology 2010;172:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Straney L, Finn J, Dennekamp M, Bremner A, Tonkin A, Jacobs I. Evaluating the impact of air pollution on the incidence of out-of-hospital cardiac arrest in the Perth Metropolitan Region: 2000–2010. J Epidemiol Community Health 2013:jech-2013–202955. [DOI] [PubMed] [Google Scholar]
- 34.Mirzaei M, Joodi G, Bogle B, Chen S, Simpson RJ Jr. Years of Life and Productivity Loss Because of Adult Sudden Unexpected Death in the United States. Medical care 2019;57:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel S, Conover MM, Joodi G, Chen S, Simpson RJ Jr, Deyo ZM. Medication Use in Women and Men With Sudden Unexpected Death. Annals of Pharmacotherapy 2018:1060028018771061. [DOI] [PubMed] [Google Scholar]
- 36.Jaakkola JJ. Case-crossover design in air pollution epidemiology. The European respiratory journal Supplement 2003;40:81s–85s. [DOI] [PubMed] [Google Scholar]
- 37.Janes H, Sheppard L, Lumley T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology 2005;16:717–726. [DOI] [PubMed] [Google Scholar]
- 38.Maclure M, Mittleman MA. Should we use a case-crossover design? Annual review of public health 2000;21:193–221. [DOI] [PubMed] [Google Scholar]
- 39.Maclure M, Mittleman MA. Case-crossover designs compared with dynamic follow-up designs. Epidemiology 2008;19:176–178. [DOI] [PubMed] [Google Scholar]
- 40.U.S. EPA. 2015. Query Air Data. Available: https://aqs.epa.gov/api [accessed May 22 2018].
- 41.U.S. EPA. 2018. Air Data: Air Quality Data Collected at Outdoor Monitors Across the US. Available: https://www.epa.gov/outdoor-air-quality-data [accessed May 22 2018].
- 42.Fung KY, Krewski D, Chen Y, Burnett R, Cakmak S. Comparison of time series and case-crossover analyses of air pollution and hospital admission data. International Journal of Epidemiology 2003;32:1064–1070. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Zeger SL. On the equivalence of case-crossover and time series methods in environmental epidemiology. Biostatistics 2006;8:337–344. [DOI] [PubMed] [Google Scholar]
- 44.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. American journal of epidemiology 1991;133:144–153. [DOI] [PubMed] [Google Scholar]
- 45.Baxter LK, Dionisio K, Pradeep P, Rappazzo K, Neas L. Human exposure factors as potential determinants of the heterogeneity in city-specific associations between PM 2.5 and mortality. Journal of exposure science & environmental epidemiology 2019;29:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.U.S. EPA. 2017. National Air Quality: Status and Trends of Key Air Pollutants. Available: https://www.epa.gov/air-trends [accessed May 22 2018].
- 47.U.S. Census Bureau. 2010. United States Census Bureau: American FactFinder: Community Facts Wake County, North Carolina. Available: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=CF [accessed May 22 2018].
- 48.Ren C, Williams GM, Mengersen K, Morawska L, Tong S. Does temperature modify short-term effects of ozone on total mortality in 60 large eastern US communities?—An assessment using the NMMAPS data. Environ Int 2008;34:451–458. [DOI] [PubMed] [Google Scholar]
- 49.Alkezweeny A, Powell D. Estimation of transformation rate of SO2 to SO4 from atmospheric concentration data. Atmospheric Environment (1967) 1977;11:179–182. [Google Scholar]
- 50.Lippmann M, Thurston GD. Sulfate concentrations as an indicator of ambient particulate matter air pollution for health risk evaluations. Journal of exposure analysis and environmental epidemiology 1996;6:123–146. [PubMed] [Google Scholar]
- 51.Teng T-HK, Williams TA, Bremner A, Tohira H, Franklin P, Tonkin A, Jacobs I, Finn J. A systematic review of air pollution and incidence of out-of-hospital cardiac arrest. J Epidemiol Community Health 2014;68:37–43. [DOI] [PubMed] [Google Scholar]
- 52.Alessandrini ER, Stafoggia M, Faustini A, Berti G, Canova C, De Togni A, Di Biagio K, Gherardi B, Giannini S, Lauriola P. Association between short-term exposure to PM2. 5 and PM10 and mortality in susceptible subgroups: A multisite case-crossover analysis of individual effect modifiers. American journal of epidemiology 2016:1–11. [DOI] [PubMed] [Google Scholar]
- 53.Cascio WE. Proposed pathophysiologic framework to explain some excess cardiovascular death associated with ambient air particle pollution: Insights for public health translation. Biochimica et Biophysica Acta (BBA)-General Subjects 2016;1860:2869–2879. [DOI] [PubMed] [Google Scholar]
- 54.Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environmental health perspectives 2001;109:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiordelisi A, Piscitelli P, Trimarco B, Coscioni E, Iaccarino G, Sorriento D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart failure reviews 2017;22:337–347. [DOI] [PubMed] [Google Scholar]
- 56.Budinger GS, McKell JL, Urich D, Foiles N, Weiss I, Chiarella SE, Gonzalez A, Soberanes S, Ghio AJ, Nigdelioglu R. Particulate matter-induced lung inflammation increases systemic levels of PAI-1 and activates coagulation through distinct mechanisms. PloS one 2011;6:e18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiarella SE, Soberanes S, Urich D, Morales-Nebreda L, Nigdelioglu R, Green D, Young JB, Gonzalez A, Rosario C, Misharin AV. β 2-Adrenergic agonists augment air pollution–induced IL-6 release and thrombosis. The Journal of clinical investigation 2014;124:2935–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zanobetti A, Gold DR, Stone PH, Suh HH, Schwartz J, Coull BA, Speizer FE. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environmental health perspectives 2010;118:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J, Rappazzo KM, Simpson RJ, Joodi G, Pursell IW, Mounsey JP, Cascio WE, Jackson LE. Exploring links between greenspace and sudden unexpected death: a spatial analysis. Environ Int 2018;113:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mounsey LA, Lin F-C, Pursell I, Joodi G, Lewis ME, Nwosu A, Hodonsky C, Simpson RJ, Mounsey JP. Relation of household income to incidence of sudden unexpected death in Wake County, North Carolina. American journal of cardiology 2017;119:1030–1035. [DOI] [PubMed] [Google Scholar]
- 61.Wettstein ZS, Hoshiko S, Fahimi J, Harrison RJ, Cascio WE, Rappold AG. Cardiovascular and Cerebrovascular Emergency Department Visits Associated With Wildfire Smoke Exposure in California in 2015. Journal of the American Heart Association 2018;7:e007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.CDC. 2018. Million Hearts: Particle Pollution and Heart Disease. Available: https://millionhearts.hhs.gov/tools-protocols/tools/particle-pollution.html [accessed 22 May].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.