Abstract
Tuberculosis is caused by Mycobacterium tuberculosis (Mtb), a bacterial pathogen which is transmitted via aerosol and establishes a chronic lung infection. In naïve hosts, Mtb grows for several weeks without being restricted by IFNγ-producing T cells, which eventually accumulate and limit Mtb dissemination. In this study, we used a mouse model of Mtb/γ-herpesvirus (γHV) coinfection to test the hypothesis that latent γHV infection alters host resistance to Mtb. γHVs are DNA viruses which elicit a polyclonal T cell response and attenuate some acute bacterial pathogens in mice; whether γHVs modulate infection with Mtb is unknown. Here, mice harboring latent mouse gammaherpesvirus 68 (MHV68)—a γHV genetically and biologically related to human Epstein Barr virus (EBV)—were infected via aerosol with a low dose of virulent Mtb. Mtb burdens and IFNγ+ T cell frequencies in mice with latent MHV68 (MHV68POS mice) were subsequently measured and compared to control mice that did not harbor latent MHV68 (MHV68NEG mice). Relative to MHV68NEG controls, MHV68POS mice more effectively limited Mtb growth and dissemination, and had higher frequencies of CD4+IFNγ+ cells in lung-draining lymph nodes. Collectively, our results support a model wherein latent γHV confers moderate protection against subsequent Mtb infection.
1. Introduction
Tuberculosis (TB) is the leading cause of death from an infectious disease [1]. TB is caused by aerogenic transmission of Mycobacterium tuberculosis (Mtb), a bacterial species which infects alveolar macrophages and can disseminate to extrapulmonary tissues via lymphatic or hematogenous spread [2]. TB has a spectrum of clinical manifestations that vary depending on the host response, including severe, active, chronic, subclinical and latent forms [3]. Improved socioeconomic conditions, public health practices and the use of effective drug treatment have reduced global TB rates; however, these rates fell short of the World Health Organization (WHO) goal of reversing TB incidence by 2015 [4], and are not on target to achieve WHO's goal of ending the TB epidemic by 2030 [5]. For these reasons, it is important to continue research into understanding host responses which restrict Mtb growth and dissemination.
T cells have been recognized for ~50 years as being essential for restricting Mtb growth and dissemination [6]. In the mouse model of TB, Mtb growth in the lung is near-logarithmic for the first 4 weeks post-infection [7]; after this period, CD4+ and CD8+ T cells begin to accumulate in the lung and secrete IFNγ, a cytokine which enhances the bactericidal activities of macrophages and other innate lineages. Relative to many lung infection models, the kinetics of T cell accumulation during TB are delayed [8]; this delay is important, as it provides Mtb a window of opportunity to express genes that are necessary for establishing chronic infection [9]. The only known way of shortening this window is to expedite T cells’ response to Mtb, as achieved through either BCG vaccination or, in animal models, the adoptive transfer of T cells from a previously-infected host [7]. The T cell IFNγ response is accompanied by reductions in lung Mtb burden and the delayed appearance of Mtb in extrapulmonary tissues [10,11].
γ-Herpesviruses (γHVs) elicit a prolonged T cell IFNγ response which may affect Mtb pathogenesis. γHVs are a large family of double-stranded DNA viruses that establish life-long, latent infections in mammals. Epstein-Barr virus (EBV), one of two known human γHVs, infects ~50% of children by age 8, and ~90% of adults after age 18 12,13. Related mouse gammaherpesvirus 68 (MHV68) represents a tractable model of chronic gammaherpesvirus infection and overcomes limitations associated with exquisite species specificity of gammaherpesviruses [14-16]. Latent γHV infection, which occurs during the time of chronic infection (after day 12 of MHV68 infection), causes a polyclonal, antigen-independent activation of B and T cells: infection with MHV68 stimulates a significant increases in the number of splenic B cells, CD4+ and CD8+ T cells which are not specific for MHV68 epitopes [14-16]. The increase in the number of immune cells occurs concurrently with activation, as ~40% of splenic CD4+ T cells are CD62LLo by day 16 post-MHV68 infection, and remain elevated as late as day 90 post-MHV68 infection [17]. In humans, the polyclonal nature of γHV infection is best highlighted by the fact that a clinical diagnosis for a recent EBV infection involves testing for antibodies that are reactive with horse red blood cells (heterophile antibodies test). In addition to polyclonal stimulation of adaptive immunity, γHV infection is also associated with a substantial increase in systemic and organ-specific type I and type II interferon levels, an increase that is sustained throughout latent infection [18-20]. In mice, γHV latency provides IFNγ-dependent protection against subsequent Listeria monocytogenes and Yersinia pestis infection [18]. Whether γHV latency influences TB has not been reported.
Given the variety of innate and adaptive immune responses elicited by γHV [21], we hypothesized that latent γHV infection alters the immune response to Mtb to confer protection. To test this hypothesis, we infected two groups of mice—an experimental group harboring latent MHV68 (i.e. MHV68POS mice), and a control group that was never exposed to MHV68 (i.e. MHV68NEG mice)—with Mtb via aerosol, and subsequently measured Mtb burdens in the lung and spleen on post-infection days 17 and 38. The frequencies of IFNγ-producing CD4+ and CD8 + T cells in the mediastinal lymph node were also determined. Our results support a model wherein γHV latency confers a moderate degree of protection against TB, which has important implications for our understanding of human TB resistance.
2. Methods
2.1. Mice
C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were bred and housed in a specific-pathogen-free barrier facility in accordance with institutional and federal guidelines. All mouse experiments were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
2.2. MHV68 inoculation
Intranasal inoculation of 6–8 week old mice with 104 PFU of wild type murine gammaherpesvirus 68 (MHV68; WUMS strain) or 15 μl of phosphate buffered saline(PBS) was performed under light anesthesia, as previously reported [22]. MHV68-innoculated mice were housed separately from carrier-inoculated mice for the duration of the study.
2.3. Mtb infection and burden assessment
Eighteen days post-MHV68 (or carrier) inoculation, mice were aerosol infected with virulent Mtb H37Rv per our reported protocols [11,23]. For bacterial load determinations, lungs and spleen were removed from euthanized mice and individually homogenized in normal sterile saline; serial dilutions of each homogenate were then plated on 7H11 and colonies counted after ≥2 weeks incubation at 37 °C and 5% CO2. Lungs from control mice were plated on day 1 post-infection to confirm the delivery of ~80 Mtb CFU per lung.
2.4. Flow cytometry
Mediastinal lymph node cells were prepared and stained for surface and intracellular markers per our previous protocols [11,23], using antibodies specific for mouse CD4, CD8 and IFNγ (BD Biosciences). Flow cytometry data files were collected on a Guava easyCyte 8HT cytometer, and analyzed using FlowJo software to determine the frequency of CD4+IFNγ+ and CD8+IFNγ+ cells.
2.5. Statistics
GraphPad Prism software was used for graph preparations and statistical analyses Bars in the figures show means plus standard deviations (SD). Student's t-test was used for all comparisons of MHV68POS and MHV68NEG values. The asterisks shown between MHV68POS and MHV68NEG groups indicate the significance of a comparison, where P ≤ 0.05.
3. Results
3.1. MHV68POS mice are more resistant to Mtb growth and dissemination
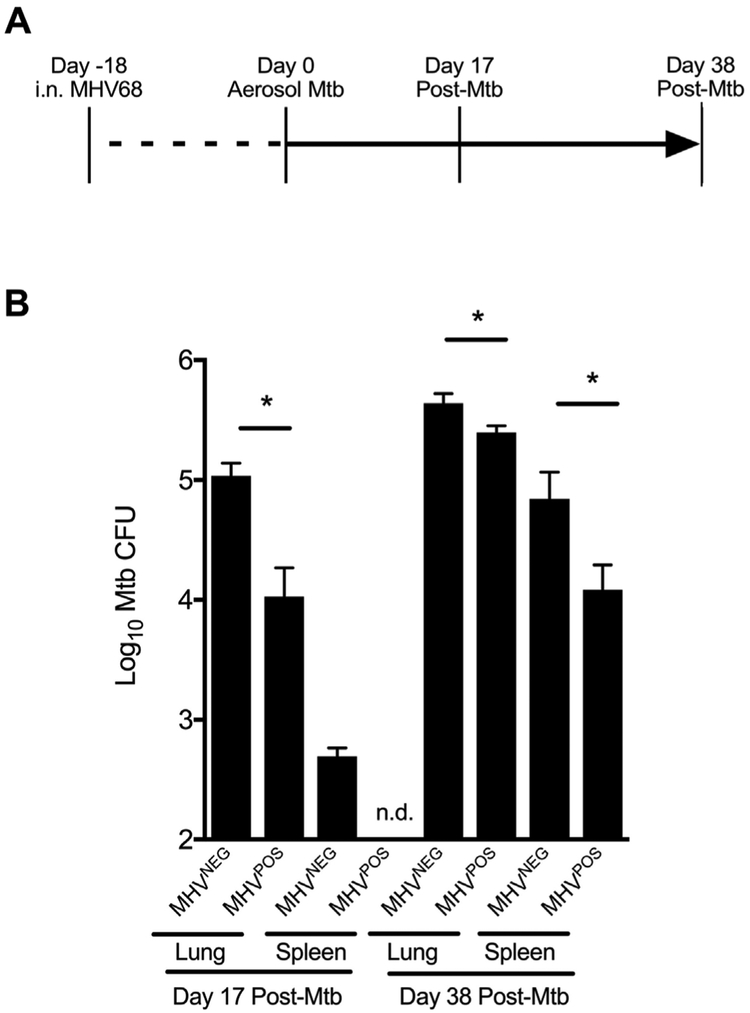
To determine if MHV68 latency affects Mtb growth and dissemination, we inoculated groups of C57BL/6 mice with either MHV68 (104 PFU, MHV68pos mice) or carrier solution (MHV68NEG mice); eighteen days later—a point when viral latency is established in MHV68POS mice [24,25]—all mice were infected via aerosol with ~80 CFU of virulent Mtb (Fig. 1A). MHV68POS and MHV68NEG mice were maintained in separate cages for duration of the experiment. On post-infection days 17 and 38, groups of mice were euthanized and the lungs, spleen and mediastinal lymph nodes (MLNs) were removed and assayed for Mtb CFU burdens or the frequency of IFNγ+ T cells. The results of our Mtb CFU burden assessment are shown in Fig. 1B, and demonstrate that post-infection day 17 lung Mtb burdens are significantly lower in MHV68POS mice compared to MHV68NEG controls. Whereas we detected Mtb CFUs in MHV68NEG spleens on post-infection day 17—a result of lymphatic drainage and hematogenous spread [26]—spleen Mtb CFUs were not detectable in MHV68POS mice at that time. On post-infection day 38, MHV68POS lung and spleen Mtb CFU burdens continued to be significantly lower than MHV68NEG controls (Fig. 1B). Collectively, these data demonstrate that mice that are latently infected with MHV68 are more resistant to Mtb growth and dissemination.
Fig. 1. MHV68POS mice are more resistant to Mtb growth and dissemination.
(A) For this study, adult C57BL/6 mice were intranasally infected with 104 PFU of MHV68, or vehicle alone. Eighteen days later, all mice were aerogenically infected with ~80 CFU of virulent Mtb (H37Rv). On post-Mtb infection days 17 and 38, mice from each group were euthanized and the lungs, spleen and MLNs were removed for either CFU burden assessment or measuring the frequency of IFNγ-producing T cells. (B) Mtb burdens in the lungs and spleen from indicated groups were assessed by plating serial dilutions of homogenized organs on 7H11. Shown for each time is the mean number of CFU (log10) present in 3–4 mice per group per time point. Error bars represent ± SD; asterisks indicate that a significant difference between MHV68POS and MHV68NEG mice was observed at the indicated time point (i.e., P ≤ 0.05 as determined using Student's t-test). Results are representative of three independent experiments.
3.2. Mtb-infected MHV68POS mice have a transient increase in the frequency of IFNγ-producing CD4+ cells
MHV68 elicits an IFNγ+ T cell response that is sustained during viral latency [18-20]. Since IFNγ-producing T cells promote TB resistance [10,11], we next determined if Mtb-infected MHV68POS mice—which have lower CFU burdens than MHV68NEG mice on post-infection day 17—correspondingly have higher frequencies of IFNγ-producing T cells. MLNs were removed from the same mice used for CFU burden assessments (Fig. 1), processed into single cell suspensions, stimulated with PMA/ionomycin in the presence of brefeldin A, and stained for surface CD4, surface CD8 and intracellular IFNγ. The results of our flow cytometry analysis are shown in Fig. 2, and demonstrate that on post-infection day 17 the MLNs of Mtb-infected MHV68POS mice have significantly higher frequencies of CD4+IFNγ+ cells, relative to MHV68NEG controls. There was no significant difference in the frequencies of CD8+IFNγ+ cells between MHV68POS and MHV68NEG mice. By post-infection day 38, no differences in CD4+IFNγ+ frequencies were observed between Mtb-infected MHV68POS and MHV68NEG mice. Collectively, these data demonstrate that Mtb-infected mice with latent MHV68 have transiently higher frequencies of CD4+IFNγ+ cells.
Fig. 2. Mtb-infected MHV68POS mice have a transient increase in the frequency of IFNγ-producing CD4+ cells.
C57BL/6 mice were infected with MHV68 and Mtb H37Rv as depicted in Fig. 1A. On post-Mtb infection days 17 and 38, we determined the frequency of MLN CD4+ and CD8+ cells capable of producing IFNγ following PMA/ionomycin stimulation. Results are representative of three independent experiments; significance values are indicated above the data, as determined using Student's t-test.
4. Discussion
Animal models have been used for over a century to identify host mechanisms which govern TB resistance [27]. Following aerosol infection with Mtb, large outbred animals develop a range of pulmonary disease forms which model the diversity of human TB forms [3]; however, the uniformity and reproducible nature of TB in mice has enabled the discovery of multiple immune mechanisms that regulate TB resistance in mice and humans alike [28]. Regardless of the species used, a difference between animal models and humans is that human immune responses are modified by ubiquitous, life-long viral infections that are absent from most animal research. In the absence of these viruses, animals’ immune responses do not fully reflect the human situation.
γ-herpesviruses (γHV) are one such group of immune-altering viruses, which establish a life-long infection in immunocompetent individuals [21]. In most immunocompetent individuals, the two human γHVs—Kaposi's sarcoma herpesvirus (KSHV), and Epstein-Barr virus (EBV)—infect B cells and establish a latent infection wherein they maintain their genome as a circular episome in the host nucleus and only produce few if any viral proteins that promote B cell proliferation (thus maintaining their cellular reservoir), and occasionally reactivate from latency to lytic cycle (thus maintaining their ability to infect a new host). Although most KSHVPOS and EBVPOS individuals experience mild-to-no clinical disease, a subset develops lymphoproliferative disorders and other malignancies [29]. In lymphopenic individuals, such as those who have HIV/AIDS or transplant-associated immunosuppression, KSHV infection causes malignant diseases of the skin and mucosal surfaces. Communities in sub-Saharan Africa have the highest prevalence of KSHV infection, (seropositivity rates of > 50%), followed by those in the Mediterranean (20–30% seropositivity rates), Europe, Asia and United States (< 10% seropositivity rates) [30]. In contrast to the wide regional variation in KSHV prevalence, EBV infects ~90% of adults worldwide [12,13]. EBV is best known for being the cause of non-malignant, infectious mononucleosis; however, it is also associated with malignant lymphoproliferative diseases such as Burkitt lymphoma and Hodgkin's lymphoma. Importantly, the parameters of γHV infection are further modified by the developmental stage of the host: γHV-driven polyclonal activation and expansion of B and T cells is more subtle in EBV-infected children and MHV68-infected neonatal mice [31]. Unlike adults that clear persistently replicating MHV68, MHV68-infected neonatal mice are also more likely to maintain low levels of persistent MHV68 replication in the lungs [31].
During their initial infection of host cells, KSHV and EBV elicit adaptive, polyclonal immune responses that persists after γHV enters a non-replicative state (i.e. latency). Barton et al. [18] identified a benefit of this response, using the MHV68-infected mouse model: latent γHV-infected mice were resistant to subsequent infection with the bacterial pathogens L. monocytogenes and Y. pestis. Both L. monocytogenes and Y. pestis cause acute diseases (i.e. listeriosis and plague), resistance to which depends on the cytokine IFNγ [32-34]. γHV-driven resistance to L. monocytogenes and Y. pestis is likewise dependent of IFNγ, which is produced by CD4+ and CD8+ T cells during γHV latency [35,36]. The results of Barton et al. were independently reproduced [37], and drove our curiosity whether γHV-driven resistance also applied to TB, which—unlike listeriosis and plague—has both active and latent stages, the latter of which could be considered a chronic bacterial disease. Specifically, we hypothesized that the course of experimental TB would be improved by latent MHV68 infection, since γHVs elicit a polyclonal T cell response that persists in the lung after viral latency is established (12 days in the MHV68 model). Indeed, we observed that latent MHV68 infection reduced the lung and spleen Mtb burdens of mice, and this reduction was associated with elevated numbers of CD4+IFNγ+ cells in the MLN. Mtb infection elicits the clonal expansion of MLN T cells that are specific to Mtb-proteins EsxH, ESAT6 and Ag85, and can transfer immunological memory with varying efficacy [38]. Since Mtb benefits from a suboptimal T cell response in vivo [39], we predict that MHV68 infection expands the pool of Mtb-specific CD4+ T cells prior to Mtb-infection, thus increasing the probability that these cells will encounter Mtb-infected macrophages in the lung and spleen. Additional experiments are needed to test this hypothesis, using Mtb-peptide conjugated MHC tetramers to track the frequency of Mtb-specific T cells in the presence or absence of MHV68.
Finally, our results suggest human γHV-infection may be an important but unrecognized factor which modifies TB outcome, particularly in high TB burden countries where most children acquire EBV by 3 years of age [40,41] (in comparison, only 70% of 12-year-old children are seropositive for EBV in developed countries [42]). Whereas Mtb infection of adults almost uniformly occurs in the setting of chronic γHV-infection, Mtb infection of children more likely occurs in both γHV-naïve and γHV-infected settings. Regarding the TB incidence data of McShane and colleagues [43], who were comparing the relative efficacy of BCG ± MV85A or placebo in children aged 0–3 in South Africa (i.e. the MV85A efficacy trial): in light of our data, it will be important to determine if these two groups also had equivalent numbers of children that were exposed to EBV, as evidenced by the presence of circulating EBV-specific antibodies. The MV85A efficacy trial was important and led to follow-up studies to identify immune correlates of risk in vaccinated children [44]; however, to our knowledge, it has not yet been reported whether there is a correlation between serum anti-EBV titers and TB incidence in these same children. Based on our animal model data, we would predict TB incidence is lower in EBV-infected children. Differences in γHV-infection prevalence may underlie an important clinical difference between adult and pediatric TB: whereas adult TB generally presents as pulmonary disease, widespread dissemination of Mtb is more common in children, resulting in extrapulmonary disease and infection of the brain, bones and other organs. Despite treatment regimens, mortality rates for children with disseminated forms of the disease are 10–20% and over 50% of survivors suffer long term neurological deficits [45]. We hope the results of our study will prompt the design of retrospective or prospective studies to assess whether γHV-infection status associates with TB outcome in adults and children.
Acknowledgments
We wish to acknowledge Chelsea Spurling for her excellent technical assistance with MHV68 infection, as well as the MCW Biomedical Resource Center staff for their excellent care of all the animals used in our study. This work was supported by the Medical College of Wisconsin (MCW) and the MCW Center for Infectious Disease Research (CIDR), as well as National Institutes of Health grants R01 AI121212 (R.T.R.) CA183593, CA203923 [V.L.T.]).
References
- [1].Guinn KM, Rubin EJ. Tuberculosis: just the FAQs. mBio 2017;8 10.1128/mBio.01910-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev 2011;240:252–68. 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lin PL, Flynn JL. The end of the binary era: revisiting the spectrum of tuberculosis. J Immunol 2018;201:2541–8. 10.4049/jimmunol.1800993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Raviglione M, Marais B, Floyd K, Lonnroth K, Getahun H, Migliori GB, Harries AD, Nunn P, Lienhardt C, Graham S, Chakaya J, Weyer K, Cole S, Kaufmann SH, Zumla A. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 2012;379:1902–13. 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- [5].Marais B, Zumla A. Advancing global tuberculosis control after the UNGA-HLM. Lancet 2018;392:1096–7. 10.1016/S0140-6736(18)32361-4. [DOI] [PubMed] [Google Scholar]
- [6].North RJ. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol 1973;7:166–76. [DOI] [PubMed] [Google Scholar]
- [7].Jung YJ, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J Exp Med 2005;201:1915–24. 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol 2011;4:288–93. 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lin MY, Ottenhoff TH. Not to wake a sleeping giant: new insights into host-pathogen interactions identify new targets for vaccination against latent Mycobacterium tuberculosis infection. Biol Chem 2008;389:497–511. [DOI] [PubMed] [Google Scholar]
- [10].Green AM, Difazio R, Flynn JL. IFN-gamma from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J Immunol 2013;190:270–7. 10.4049/jimmunol.1200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miller HE, Robinson RT. Early control of Mycobacterium tuberculosis infection requires il12rb1 expression by rag1-dependent lineages. Infect Immun 2012;80:3828–41. 10.1128/IAI.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Balfour HH Jr., Sifakis F, Sliman JA, Knight JA, Schmeling DO, Thomas W. Age-specific prevalence of Epstein-Barr virus infection among individuals aged 6-19 years in the United States and factors affecting its acquisition. J Infect Dis 2013;208:1286–93. 10.1093/infdis/jit321. [DOI] [PubMed] [Google Scholar]
- [13].Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6-19, 2003-2010. PLoS One 2013; 8:e64921. 10.1371/journal.pone.0064921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Evans AG, Moser JM, Krug LT, Pozharskaya V, Mora AL, Speck SH. A gamma-herpesvirus-secreted activator of Vbeta4+ CD8+ T cells regulates chronic infection and immunopathology. J Exp Med 2008;205:669–84. 10.1084/jem.20071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stevenson PG, Doherty PC. Non-antigen-specific B-cell activation following murine gammaherpesvirus infection is CD4 independent in vitro but CD4 dependent in vivo. J Virol 1999;73:1075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Usherwood EJ, Ross AJ, Allen DJ, Nash AA. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J Gen Virol 1996;77(Pt 4):627–30. 10.1099/0022-1317-77-4-627. [DOI] [PubMed] [Google Scholar]
- [17].Stevenson PG, Doherty PC. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J Virol 1998;72:943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL. Virgin HWt. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007;447:326–9. 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- [19].Mandal P, Krueger BE, Oldenburg D, Andry KA, Beard RS, White DW, Barton ES. A gammaherpesvirus cooperates with interferon-alpha/beta-induced IRF2 to halt viral replication, control reactivation, and minimize host lethality. PLoS Pathog 2011;7:e1002371 10.1371/journal.ppat.1002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barton ES, Lutzke ML, Rochford R. Virgin HWt. Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J Virol 2005;79:14149–60. 10.1128/JVI.79.22.14149-14160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell 2009;138:30–50. https://doi.Org/10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- [22].Darrah EJ, Kulinski JM, Mboko WP, Xin G, Malherbe LP, Gauld SB, Cui W, Tarakanova VL. B cell-specific expression of ataxia-telangiectasia mutated protein kinase promotes chronic gammaherpesvirus infection. J Virol 2017;91 10.1128/JVI.01103-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ray AA, Fountain JJ, Miller HE, Cooper AM, Robinson RT. IL12Rbeta1DeltaTM is a secreted product of il12rb1 that promotes control of extrapulmonary tuberculosis. Infect Immun 2015;83:560–71. 10.1128/IAI.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Barton E, Mandal P, Speck SH. Pathogenesis and host control of gamma-herpesviruses: lessons from the mouse. Annu Rev Immunol 2011;29:351–97. 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- [25].Sunil-Chandra NP, Efstathiou S, Arno J, Nash AA. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J Gen Virol 1992;73(Pt 9):2347–56. 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- [26].Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun 2002;70:4501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Meade GM. Edward livingston trudeau, M.D. Tubercle 1972;53:229–50. [DOI] [PubMed] [Google Scholar]
- [28].Cooper AM. Mouse model of tuberculosis. Cold Spring Harb. Perspect. Med 2014;5:a018556 10.1101/cshperspect.a018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cesarman E. Gammaherpesviruses and lymphoproliferative disorders. Annu Rev Pathol 2014;9:349–72. 10.1146/annurev-pathol-012513-104656. [DOI] [PubMed] [Google Scholar]
- [30].Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of kaposi sarcoma. Cancer Lett 2011;305:150–62. 10.1016/j.canlet.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ptaschinski C, Rochford R. Infection of neonates with murine gammaherpesvirus 68 results in enhanced viral persistence in lungs and absence of infectious mononucleosis syndrome. J Gen Virol 2008;89:1114–21. 10.1099/vir.0.83470-0. [DOI] [PubMed] [Google Scholar]
- [32].Parent MA, Wilhelm LB, Kummer LW, Szaba FM, Mullarky IK, Smiley ST. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect Immun 2006;74:3381–6. 10.1128/IAI.00185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity 1995;3:109–17. [DOI] [PubMed] [Google Scholar]
- [34].Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science 1993;259:1742–5. [DOI] [PubMed] [Google Scholar]
- [35].Flano E, Woodland DL, Blackman MA, Doherty PC. Analysis of virus-specific CD4(+) t cells during long-term gammaherpesvirus infection. J Virol 2001;75:7744–8. 10.1128/JVI.75.16.7744-7748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Christensen JP, Doherty PC. Quantitative analysis of the acute and long-term CD4(+) T-cell response to a persistent gammaherpesvirus. J Virol 1999;73:4279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yager EJ, Szaba FM, Kummer LW, Lanzer KG, Burkum CE, Smiley ST, Blackman MA. gamma-Herpesvirus-induced protection against bacterial infection is transient. Viral Immunol 2009;22:67–72. 10.1089/vim.2008.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang JD, Mott D, Sutiwisesak R, Lu YJ, Raso F, Stowell B, Babunovic GH, Lee J, Carpenter SM, Way SS, Fortune SM, Behar SM. Mycobacterium tuberculosis-specific CD4+ and CD8+ T cells differ in their capacity to recognize infected macrophages. PLoS Pathog 2018;14:e1007060. 10.1371/journal.ppat.1007060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog 2011;7:e1002063. 10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Martro E, Bulterys M, Stewart JA, Spira TJ, Cannon MJ, Thacher TD, Bruns R, Pellett PE, Dollard SC. Comparison of human herpesvirus 8 and Epstein-Barr virus seropositivity among children in areas endemic and non-endemic for Kaposi's sarcoma. J Med Virol 2004;72:126–31. 10.1002/jmv.10548. [DOI] [PubMed] [Google Scholar]
- [41].Biggar RJ, Henle W, Fleisher G, Bocker J, Lennette ET, Henle G. Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int J Cancer 1978;22:239–43. [DOI] [PubMed] [Google Scholar]
- [42].Svahn A, Berggren J, Parke A, Storsaeter J, Thorstensson R, Linde A. Changes in seroprevalence to four herpesviruses over 30 years in Swedish children aged 9-12 years. J Clin Virol 2006;37:118–23. https://doi.Org/10.1016/j.jcv.2006.07.012. [DOI] [PubMed] [Google Scholar]
- [43].Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, Team MATS. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013;381:1021–8. 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fletcher HA, Snowden MA, Landry B, Rida W, Satti I, Harris SA, Matsumiya M, Tanner R, O'Shea MK, Dheenadhayalan V, Bogardus L, Stockdale L, Marsay L, Chomka A, Harrington-Kandt R, Manjaly-Thomas ZR, Naranbhai V, Stylianou E, Darboe F, Penn-Nicholson A, Nemes E, Hatherill M, Hussey G, Mahomed H, Tameris M, McClain JB, Evans TG, Hanekom WA, Scriba TJ, McShane H. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun 2016;7:11290 10.1038/ncomms11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Principi N, Esposito S. Diagnosis and therapy of tuberculous meningitis in children. Tuberculosis (Edinb) 2012;92:377–83. https://doi.Org/10.1016/j.tube.2012.05.011. [DOI] [PubMed] [Google Scholar]